Abstract
Background
Aedes aegypti mosquitoes are vectors of a variety of emerging viral pathogens, including yellow fever, dengue, chikungunya, and Zika virus. This species has established endemic populations in all cities across southern New Mexico sampled to date. Presently, control of Aedes-borne viruses relies on deployment of insecticides to suppress mosquito populations, but the evolution of insecticide resistance threatens the success of vector control programs. While insecticide resistance is quite common in Ae. aegypti field populations across much of the U.S., the resistance status of this species in populations from New Mexico has not previously been assessed.
Results
First, we collected information on pesticide use in cities in southern New Mexico and found that the most commonly used active ingredients were pyrethroids. The use of insecticides with the same mode-of-action over multiple years is likely to promote the evolution of resistance. To determine if there was evidence of resistance in some cities in southern New Mexico, we collected Ae. aegypti from the same cities and established laboratory strains to assess resistance to pyrethroid insecticides and, for a subset of populations, to organophosphate insecticides. F2 or F4 generation mosquitoes were assessed for insecticide resistance using bottle test bioassays. The majority of the populations from New Mexico that we analyzed were resistant to the pyrethroids permethrin and deltamethrin. A notable exception to this trend were mosquitoes from Alamogordo, a city that did not report using pyrethroid insecticides for vector control. We screened individuals from each population for known knock down resistance (kdr) mutations via PCR and found a strong association between the presences of the F1534C kdr mutation in the para gene of Ae. aegypti (homologue to F1534C in Musca domestica L.) and pyrethroid resistance.
Conclusion
High-level pyrethroid resistance is common in Ae. aegypti from New Mexico and geographic variation in such resistance is likely associated with variation in usage of pyrethroids for vector control. Resistance monitoring and management is recommended in light of the potential for arbovirus outbreaks in this state. Also, alternative approaches to mosquito control that do not involve insecticides should be explored.
Background
Aedes aegypti is an anthropophilic mosquito species with a worldwide distribution in tropical, subtropical and temperate climate zones [1]. It is the principal vector of yellow fever, dengue fever, chikungunya, and Zika virus [2, 3]. This species originated in Africa and has been transported around the world by humans first in sailing ships and later in trains, automobiles, and airplanes [4]. Ae. aegypti has infested all southern states of the U.S. [5]. In New Mexico, Ae. aegypti is common in cities in the southern counties of the state but is absent in the central portion of the state north of Albuquerque [1]; the distribution of this species in northeastern New Mexico is still under investigation. The populations of Ae. aegypti in New Mexico are closely related to other populations of the species in southwestern North America [6]. As is typical for Ae. aegypti in southwestern deserts, [7–10], Ae. aegypti populations in New Mexico undergo drastic seasonal fluctuations driven by the annual monsoon (authors’ personal observations). While no local transmission of Ae. aegypti-borne diseases have been reported in New Mexico, the high densities of this mosquito in urban areas coupled with frequent introductions of these viruses into the U.S. are a cause for concern [11–14].
Insecticides are the most common and the most successful tool used to control human disease vectors [15]. They are widely used in New Mexico [16]. However, insecticide resistance is a serious problem for mosquito control efforts [17]. Insecticide resistance evolves readily in insect populations when they are repeatedly exposed to the same insecticide or to insecticides with the same mode of action. Such resistance undermines effort to improve public health. For instance, high levels of resistance severely impede malaria control efforts in Africa [18, 19] and interventions to control dengue and Zika virus transmission in South America [20, 21].
The mechanisms of mosquito insecticide resistance fall into two broad categories: enhanced metabolic detoxification of insecticides [22] and mutations in insecticide target proteins that render these proteins less susceptible to corresponding insecticides [23, 24]. As examples of the latter mechanism, knock down resistance (kdr) mutations in the paralytic (para) gene of the house fly Musca domestica and the fruit fly Drosophila melanogaster can render these two dipteran species highly resistant to pyrethroid insecticides [25–27]. The para gene encodes a voltage-gated sodium channel that is expressed in axons of nerve cells and that is the main target of pyrethroids. Pyrethroid-binding prevents these channels from closing, resulting in the complete depolarization of the axonal membrane, paralyzing the insect. Specific point mutations in the para gene that alter sodium channel structure in such a way that there is decreased affinity towards pyrethroids can cause insecticide resistance in mosquitoes by reducing neuronal sensitivity to this class of insecticides. The dipteran PARA protein is a large channel with 24 transmembrane domains organized in four domains (I-IV) each with six membrane spanning segments [28]. Several point mutations in the para gene of Aedes aegypti have been implicated in pyrethroid resistance. In particular the mutations V410L, G923V, L982W, S989P, I1011M, I1011V, V1016G, V1016I, T1520I, F1534C, and D1763Y are known to confer the kdr phenotype [28–34]. The various mutations in the Para protein are numbered differently in different insect species due to variation in the length of the homologous proteins. For example, Ae. aegypti F1565 is the homologous mutation to Musca domestica F1534C and Aedes albopictus F1474C [35]. We use the Musca annotation numbers in this manuscript since this is established practice.
Aedes aegypti KDR mutations with high allele frequency have been found in several countries, including the US. For example, the F1534C KDR mutation was reported to occur at a frequency of 0.41–0.79 in India [36], while F1534C and a second KDR mutation, V1016G, were detected in insecticide resistant Aedes aegypti across Malaysia [37]. The F1534C mutation was detected at high frequency in Ae. aegypti from Brazil and Thailand as well [33, 38]. A DDT and pyrethroid-resistant population of Ae. aegypti from the Grand Cayman islands carried the F1534C mutation at an allele frequency of 0.68 [39]. F1534C is also common in Ae. aegypti from Mexico [40, 41]. Taken together, these studies suggest that the F1534C mutation is one of the most common and widespread kdr mutations in Ae. aegypti.
In the current study, we characterized the pattern of pyrethroid resistance in seven populations of Aedes aegypti established from mosquitoes collected in cities in southern New Mexico and screened for resistance mutations in the para gene, the molecular target of pyrethroids. We found that resistance was widespread in the offspring of the mosquitoes we collected and associated with a high frequency of the kdr mutation F1534C. Importantly, Alamogordo, which was also the only city that did not report use of pyrethroids for mosquito control, was also the only city in which Ae. aegypti did not exhibit strong resistance to pyrethroids.
Materials & methods
Collection of pesticide usage data
In New Mexico, the entity responsible for vector control varies by county and city. Municipal offices in each city listed in Table A in S1 File or its home county were contacted via email and/or telephone to request the name and contact information of the individual or office that could provide information on pesticides used for mosquito control. We then requested that those individuals provide us with a list of pesticides in use in 2017 as well as those used over the previous five years, if possible. We were unable to obtain contacts for Roswell or Portales, and Lordsburg does not provide vector control. In many cases, vector control professionals were able to provide specific ingredients (active chemicals) in use, but not pesticide product name per se. Of the cities queried, only Alamogordo reported that they did not use chemical pesticides; they utilize the bacterial larvicide Vectobac. We note that this inquiry did not capture vector control contractors or other similar entities.
Establishment of New Mexico Ae. aegypti strains
Mosquito adults and eggs were collected in the summer and early fall of 2017 as part of the SouthWest Aedes Research and Mapping project (SWARM). Adults were collected using BG Sentinel or gravid traps. Adults were transferred into BugDorm-1 Insect Rearing Cage (30 by 30 by 30 cm, BugDorm Store, Taichung, Taiwan) and given 20% sucrose ad libitum. The number of founding individuals and the number of different locations where trapping was performed is shown in Table B in S1 File. Despite vigorous collection efforts, it was not possible to collect a sufficient number of field-derived mosquitoes to immideately test insecticide resistance. Thus propagation of these strains was performed as describe below in the mosquito culture section. Eggs were collected using dark oviposition cups containing water and a wooden spatula [42]. Mosquito eggs were collected, dried, and stored for at least three days before hatching the G0 generation. Strains from different cities were amplified to F2 or, in some cases, F4 generation to produce enough individuals to perform Resistance Bottle Tests (see below). We were careful to keep the number of generations well below the 10 generations required to restore pyrethroid susceptibility in Ae. aegypti [43]. We were able to collect enough adult mosquitoes from eight cities in Southern New Mexico: Alamogordo, Carlsbad, Deming, Las Cruces, Lovington, Roswell, and Sunland Park to establish laboratory strains with sufficient numbers to perform insecticide resistance testing at the F2 (N = 4 cities) or F4 (N = 3 cities). We were not successful in establishing a laboratory strain with mosquitoes collected in Socorro, but were able to genotype these field-collected mosquitoes (below).
Mosquito culture
Mosquito culture protocols were similar to those described in Marquardt, 2004 [44]. Larvae were reared in large pans containing 1.5L of distilled water. The larvae were fed ad libitum with Special Kitty cat food (Amazon). Each pan received approximately 4 pellets and the water was changed every 3 days. This regimen ensured that larvae were not crowded, as high larval densities can suppress insecticide resistance [45]. When pupae started to appear, they were removed daily and placed into small cups with water within BugDorm-1 Insect Rearing Cages (Bugdorm Store, Mega View Science, Taiwan). Cages with the adults were maintained in an insectary at 80% humidity and 27°C with a light/dark cycle of 14/10, respectively. Adult female mosquitoes were blood-fed via an artificial feeding system (Chemglass Life Sciences, Vineland, NJ) with bovine blood purchased from a commercial provider (Hemostat, Dixon, CA). Mosquitoes were offered a blood meal twice a week to ensure high egg yields. Three days after blood feeding a cup with water lined with partially submerged germination paper was put in the cage to serve as an egg laying substrate. Eggs were collected after three days, dried and stored in paper envelopes in an insect chamber at 27°C and 80% humidity.
Insecticide resistance bottle tests
Insecticide resistance was tested using a novel bottle bioassay that we developed based on the standard CDC Bottle Bioassay [46, 47] with major modifications. The protocol for this test was deposited at https://www.protocols.io/ under the title ‘NMSU Mosquito Insecticide Resistance Bottle Test Protocol’. Insecticides were purchased from Sigma-Aldrich (see Table 1). Bottles were prepared 24 hours prior to the experiment tests with one of four insecticides chosen based on the pesticide usage data shown in Table 1: the pyrethroids etofenprox (37.5μg/bottle), permethrin (86μg/bottle), deltamethrin (10.5μg/bottle) and the organophosphate chloropyrifos (40μg/bottle). These concentrations, chosen based on a pilot calibration experiment for each active ingredient, are higher than recommended in the original protocol, and therefore reflect a highly conservative test of resistance. Moreover, these higher concentrations allow effective detection of insecticide resistance in a shorter period than the standard bottle test. Bottles rinsed in acetone were used as a control. Two control mosquito strains were procured from MR4 [48]: the Rockefeller laboratory strain (Rock, MRA-734), which is sensitive to pyrethroids and was used as a negative control and the Puerto Rico strain (Puerto Rico, NR-48830), which is resistant to pyrethroids and was used as a positive control. A 50:50 mixture of individuals of ROCK and Puerto Rico mosquitoes was also used as an additional control to represent populations which contained a mixture of resistant and susceptible mosquitoes. Fifteen unfed mosquitoes at 2–5 days old were added to each bottle. Mortality over time was assessed by visual observation. Mosquitoes that were unable to crawl were counted as dead. Nine replicates were performed for each treatment. Statistical differences between the mortality curves of the control strains and the experimental strains were assayed via Kaplan Meier survival analysis [49] using XLSTAT. Strains that showed significantly delayed mortality (p< = 0.05) were identified as resistant.
Table 1. Pesticides used in this study.
| Name | Sigma catalog # | CAS-No. | Concentration (%) |
|---|---|---|---|
| Etofenprox | 34094 | 80844-07-1 | < = 100 |
| Permethrin | 45614 | 52645-53-1 | 90–100 |
| Deltamethrin | 45423 | 52918-63-5 | 90–100 |
| Chlorpyrifos | 45395 | 2921-88-2 | < = 100 |
Genotyping kdr mutations in the para gene
DNA from individual mosquitoes was extracted using the DNeasy Blood & Tissue Kit following the manufacturer’s instructions (Qiagen, Palo Alto, CA). Homogenization was performed with disposable pellet mixers and a cordless motor (VWR, Radnor, PA). PCRs primers for the sequences surrounding amino acid positions 989, 1534, and 1763 of the Ae. aegypti voltage-gated para-like sodium channel (para) (Genbank accession number >EU399181.1) were designed using Primer Blast software [50] on the website of the National Center for Biotechnology Information (NCBI); primer design was further refined based on primers used in previous studies [35, 51, 52].
Primers used in this study are shown in Table 2. The PCR amplicon from the first primer pair includes three relevant mutation sites: S989P, I1011M, and L1021F/H/S while the amplicons derived from the other two primer pairs cover one mutation site each: F1534C, and D1763Y. We used the Taq PCR Master Mix Kit (Qiagen, Hilden, Germany). PCR reactions contained 10 to 100 ng of genomic DNA. PCR conditions were identical for all three amplicons: 2 minutes at 95°C, followed by 35 cycles of 30 seconds at 95°C, 30 seconds at 60° C, and 30 seconds at 68° C, followed by the final extension for 10 minutes at 68° C and the final holding temperature of 10° C. PCR products were cleaned using the QIAquick PCR purification kit (Qiagen). DNA fragments were sent to MCLAB (Molecular Cloning Laboratories, South San Francisco, CA) for Sanger sequencing using the forward primers in the table. DNA sequencing files were analyzed using Seqman software (DNAstar, Mdison, WI).
Table 2. Primers used in this study.
| Primer Name | Sequence |
|---|---|
| 989f | 5’ GAC AAT GTG GAT CGC TTC CC 3’ |
| 989r | 5’ TGC CGA CAG CGA GGA TGA ACC 3’ |
| 1534f | 5’ GAG AAC TCG CCG ATG AAC TT 3’ |
| 1534r | 5’ TCT GCT CGT TGA AGT TGT CGA T 3’ |
| 1763f | 5’ TCG AGA AGT ACT TCG TGT CG 3’ |
| 1763r | 5’ AAC AGC AGG ATC ATG CTC TG 3’ |
Results
Pesticide use in New Mexico
We requested information from personnel that were responsible for vector control from twelve cities in New Mexico and received a total of ten answers (Table A in S1 File). Pyrethroids are the most common public health insecticides currently in use in New Mexico, and chlorpyrifos is also a commonly used active ingredient. Only Alamagordo reported that they did not use adulticides, relying instead on VectoBac to target larvae.
Resistance of mosquitoes from New Mexico to permethrin and other insecticides
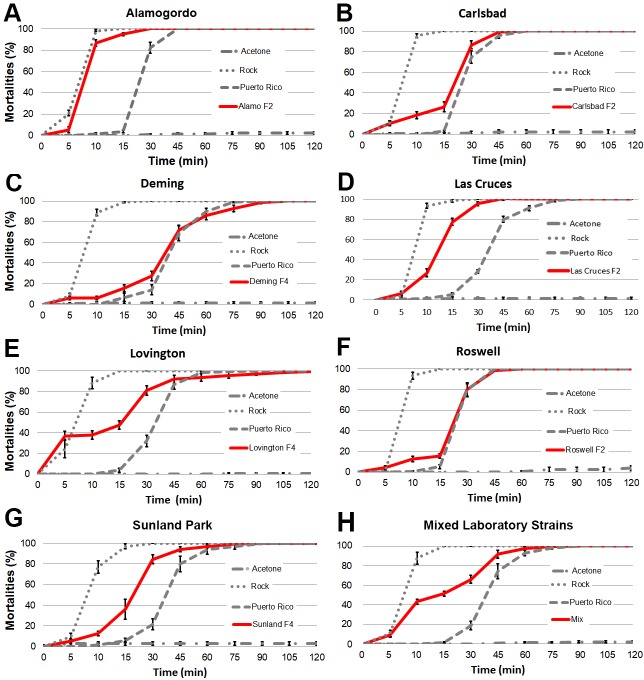
We performed a novel insecticide resistance bottle test to determine mortality curves with field-derived Ae. aegypti strains from New Mexico in order to determine whether they exhibited levels of resistance comparable to a highly resistant strain, the Puerto Rico strain, for permethrin (Fig 1), deltamethrin, and etofenprox (Figs A–F in S1 File). Subsequently we tested pyrethroid-resistant strains for resistance against chlorpyrifos (Figs G—J in S1 File). Due to limitations in the number of mosquitoes available, not every New Mexico strain was tested against every insecticide. The Ae. aegypti laboratory strains Rockefeller (Rock) and Puerto Rico showed, as expected, no resistance and strong resistance, respectively, to pyrethroids (Fig 1, Figs A—F in S1 File). Fig 2 gives and overview of our findings, described below and in Figs A–J in S1 File, for each New Mexico strain. Susceptible Rockefeller mosquitoes showed high mortality rates after ten minutes while the Puerto Rico strain mosquitoes started to die after 30 minutes. There was variation when complete mortality was reached in the Puerto Rico strain ranging from 45 min to 75 minutes. Mosquitoes from Alamogordo (F2 generation) showed some resistance against permethrin and deltamethrin compared to the Rock strain. However, Alamogordo resistance levels were much lower than those found for the Puerto Rico control strain. Mosquitoes from Alamogordo showed no resistance against chlorpyrifos (Fig 2, Fig A and H in S1 File). Mosquitoes from Carlsbad (F2 generation) showed high levels of resistance against permethrin and deltamethrin but were sensitive to chlorpyrifos (Fig 1B, Figs C and I in S1 File). Mosquitoes from Deming (F4 generation) showed high levels of resistance against permethrin (Fig 1C). Because of low mosquito numbers available, we did not perform tests for other insecticides. Mosquitoes from Las Cruces (F2 generation) showed resistance to permethrin, deltamethrin, and etofenprox while there was no resistance against chlorpyrifos. (Fig 1D and Figs B, F, G in S1 File). The levels of resistance against pyrethroids were intermediate to the levels measured for the two control strains (Fig 1D). Mosquitoes from Lovington (F4 generation) were only tested against permethrin. Lovington mosquitoes’ survival curve was bimodal, suggesting that the mosquitoes we tested were a mixture of susceptible and highly resistant mosquitoes (Fig 1E). Supporting this interpretation, a mixture of mosquitoes from the laboratory strains Rockefeller (susceptible) and Puerto Rico (resistant) resulted in a double-sigmoid mortality curve (Fig 1H). Because of low mosquito numbers available, we did not perform tests for other insecticides. Mosquitoes from Roswell (F2 generation) showed strong resistance to permethrin, deltamethrin, and etofenprox while there was no resistance against chlorpyrifos (Fig 1F, and Figs D, E, J in S1 File). Mosquitoes from Sunland Park (F4 generation) showed some resistance against permethrin (Fig 1G). Because of low mosquito numbers available, we did not perform tests for other insecticides.
Fig 1. Permethrin-resistance levels in Aedes aegypti from New Mexico.
Shown are mortality curves of a permethrin-susceptible control strain (Rock, dotted line), a resistant control strain (Puerto Rico, dashed line), susceptible mosquitoes in acetone-treated bottles (Rock, dotted/dashed line), and strains established from field collections (red line). Please note that the X-axis is not a linear time scale but has five minute intervals until 15 min and then switches to 15 min intervals. In all cases (A-H), the control (Acetone) mortality curves were significantly different (P<0.0001) from the curves for all other treatments and the curves plotted for the Puerto Rico strain and the Rock strain were also significantly different from each other (P < .0001). The curves plotted for the permethrin-exposed Rock strain were also significantly different from the other strains (P < .0001). A. The curve plotted for the Puerto Rico strain (a known resistant strain) is significantly different than that plotted for the Alamogordo strain (P < .0001). B. The curve plotted for the Puerto Rico strain is significantly different than that plotted for the Carlsbad strain (P < .0001). C. The curve plotted for the Puerto Rico strain is not significantly different than that plotted for the Carlsbad strain (P = 0.597). D. The curve plotted for the Puerto Rico strain is significantly different than that plotted for the Las Cruces strain (P < .0001). E. The curve plotted for the Puerto Rico strain is not significantly different than that plotted for the Lovington strain (P < .0001). F. The curve plotted for the Puerto Rico strain is not significantly different than that plotted for the Roswell strain (P = .206). G. The curve plotted for the Puerto Rico strain is not significantly different than that plotted for the Sunland strain (P < .0001). H. There was a significant difference between the curves plotted for the permethrin-exposed mixed strain (50% Rock, 50% Puerto Rico) and the curves plotted for the pure strains (P < .0001). The curve plotted for the Puerto Rico strain is not significantly different than that plotted for the mixed strains (P < .0001).
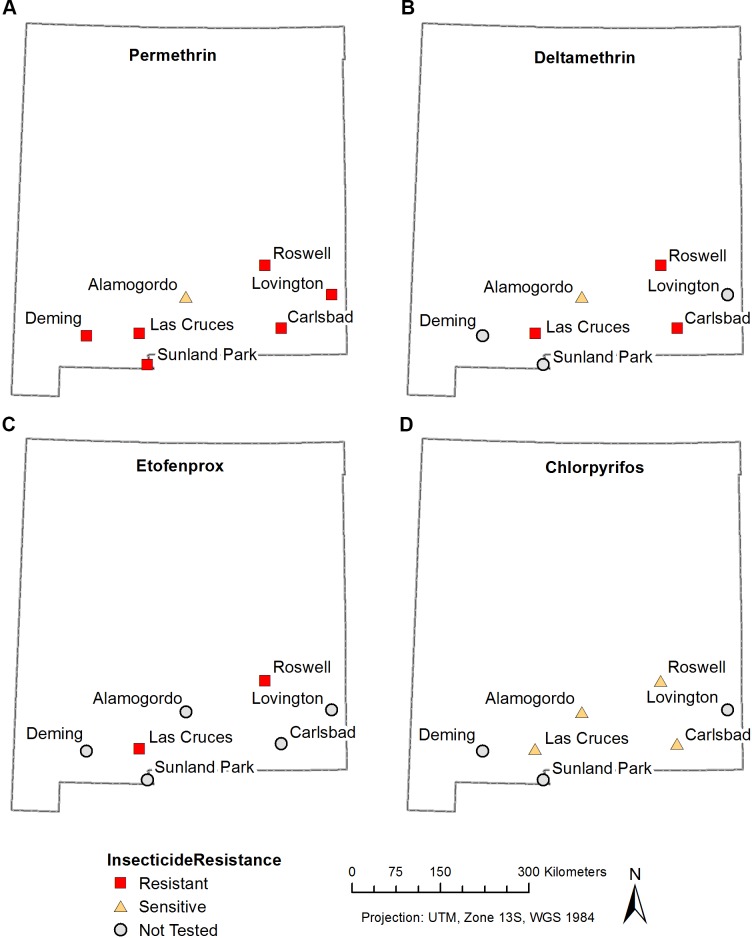
Fig 2. Insecticide resistance in Aedes aegypti from New Mexico.
The resistance status of individual populations was determined via bottle testing (see Fig 1 and Figs A-J in S1 File). Populations were classified as resistant when their mortality curves were significantly shifted towards longer survival compared to the mortality curves of the sensitive Rock strain. Resistant populations are marked with red squares, sensitive populations are marked with yellow triangles. The grey circles represent populations that have not yet been tested for this particular resistance. ALA–Alamogordo, CRL- Carlsbad, DEM-Deming, LC-Las Cruces, LOV-Lovington, ROS-Roswell, SOC-Socorro, SUN-Sunland Park. A. resistance map for permethrin, B. resistance map for deltamethrin, C. resistance map for etofenprox, D. resistance map for chlorpyrifos.
Kdr mutation frequencies in Ae. aegypti from New Mexico
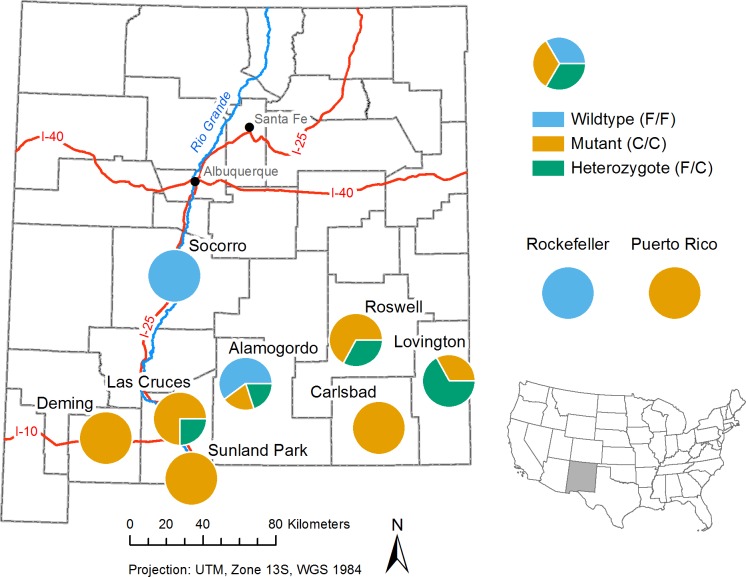
Table 3 shows the genotypes of 50 individual mosquitoes from various locations in southern New Mexico. Mutations were observed only at the 1534 position of the kdr gene. Phenylalanine (F/F) at this position (wildtype) confers susceptibility to pyrethroids while a cysteine (C/C) confers resistance (mutant) in Ae. aegypti [28]. The resistance status of heterozygotes (F/C) is unknown. Out of 46 mosquitoes we analyzed, 29 (60%) were found to have the homozygous mutant phenotype (C/C), 8 (17%) were found to be heterozygous (F/C), and 11 (23%) were found to be wildtype (F/F) (Fig K in S1 File). Fig L in S1 File shows an alignment of the Aedes aegypti and Musca domestica Para proteins. Fig 3 shows a genotype frequency map of the different genotypes we found for the 1534 mutation in different cities in New Mexico.
Table 3. kdr genotypes in mosquitoes from New Mexico–Shown are the genotypes of 50 individual mosquitoes collected at different locations in New Mexico during the 2017 mosquito season.
The letters represent the specific amino acids encoded by the sequenced DNA at the specific locations (location numbers refer to the Musca para protein). Identical letters indicate that mosquitoes were homozygous at this position while different letters show that mosquitoes were heterozygous at this position. Genotypes of the pyrethroid-susceptible control strain Rockefeller and the pyrethroid-resistant Puerto Rico control strain are shown at the end of the table (the genotypes of five individual mosquitoes were identical in these strains). Amino acids are identified using the single-letter amino acid code.
| Mutation | S989P | I1011M | L1021F/H/S | F1534C | D1763Y |
|---|---|---|---|---|---|
| Alamogordo | S/S | I/I | L/L | C/C | - |
| Alamogordo | S/S | I/I | L/L | F/F | D/D |
| Alamogordo | S/S | I/I | L/L | F/F | D/D |
| Alamogordo | S/S | I/I | L/L | F/F | D/D |
| Alamogordo | S/S | I/I | L/L | F/C | D/D |
| Alamogordo | S/S | I/I | L/L | - | - |
| Carlsbad | S/S | I/I | L/L | C/C | D/D |
| Carlsbad | S/S | I/I | L/L | C/C | D/D |
| Carlsbad | S/S | I/I | L/L | - | - |
| Carlsbad | S/S | I/I | - | C/C | - |
| Carlsbad | S/S | I/I | L/L | C/C | D/D |
| Carlsbad | S/S | I/I | L/L | - | - |
| Carlsbad | - | - | L/L | - | - |
| Carlsbad | S/S | I/I | L/L | C/C | D/D |
| Deming | S/S | I/I | L/L | C/C | D/D |
| Deming | S/S | I/I | L/L | C/C | D/D |
| Deming | S/S | I/I | L/L | C/C | D/D |
| Deming | S/S | I/I | L/L | C/C | D/D |
| Deming | S/S | I/I | L/L | C/C | D/D |
| Deming | S/S | I/I | L/L | C/C | D/D |
| Las Cruces | S/S | I/I | - | F/C | D/D |
| Las Cruces | S/S | I/I | L/L | C/C | - |
| Las Cruces | - | - | - | C/C | D/D |
| Las Cruces | S/S | I/I | L/L | C/C | D/D |
| Lovington | S/S | I/I | L/L | C/C | D/D |
| Lovington | - | - | - | F/C | D/D |
| Lovington | S/S | I/I | - | F/C | D/D |
| Lovington | S/S | I/I | - | F/C | D/D |
| Lovington | - | - | - | F/C | D/D |
| Lovington | S/S | I/I | L/L | C/C | D/D |
| Roswell | S/S | I/I | L/L | C/C | D/D |
| Roswell | S/S | I/I | L/L | F/C | D/D |
| Roswell | S/S | I/I | L/L | C/C | D/D |
| Roswell | S/S | I/I | L/L | C/C | D/D |
| Roswell | S/S | I/I | L/L | C/C | D/D |
| Roswell | S/S | I/I | - | F/C | D/D |
| Socorro | S/S | I/I | L/L | F/F | D/D |
| Socorro | S/S | I/I | L/L | F/F | D/D |
| Socorro | S/S | I/I | - | F/F | D/D |
| Socorro | - | I/I | - | F/F | D/D |
| Socorro | S/S | I/I | L/L | F/F | D/D |
| Socorro | S/S | I/I | L/L | F/F | D/D |
| Socorro | S/S | I/I | - | F/F | D/D |
| Sunland | - | - | - | C/C | D/D |
| Sunland | - | - | - | C/C | D/D |
| Sunland | S/S | I/I | L/L | C/C | D/D |
| Sunland | S/S | I/I | L/L | C/C | D/D |
| Sunland | S/S | I/I | - | C/C | D/D |
| Sunland | - | - | - | C/C | D/D |
| Sunland | - | - | - | C/C | D/D |
| Rock (5) | S/S | I/I | L/L | F/F | D/D |
| Puerto Rico (5) | S/S | I/I | L/L | C/C | D/D |
Fig 3. Genotype frequency map.
The pie charts represent the percentage of the three specific kdr F1534C genotypes in mosquitoes collected at different locations. Wildtype, susceptible mosquitoes are shown in blue. Mutant, resistant mosquitoes are shown in yellow. Heterozygotes are shown in green. The genotype frequencies of the laboratory strains, Rockefeller and Puerto Rico, are shown to the upper right of the map. The location of New Mexico within the United States is shown in the lower right of the figure.
Discussion
High levels of insecticide resistance in mosquitoes can severely impede vector control programs [15]. During outbreaks of mosquito-borne diseases, insecticide resistance can become a serious threat to public health. Therefore, monitoring levels of resistance to commonly used insecticides in vector populations is paramount for successful pest management during public health emergencies like the recent Zika virus outbreak [53]. The current study characterized, for the first time, insecticide resistance of the mosquito vector Ae. aegypti across most of its range in New Mexico. The species is largely confined to cities in southern New Mexico, which sit within the Chihuahuan Desert. Its range extends farther north on the eastern edge of the state, and its northeastern range limit is still under investigation. Our unpublished surveys in 2017 and 2018 showed that Ae. aegypti becomes abundant in all cities in southern New Mexico surveyed to date following the onset of the summer monsoonal rains.
We initiated the current project by requesting information on pesticide use from public vector control programs in New Mexican counties or cities that contain populations of Ae. aegypti. The most common active ingredients used by our respondents were the pyrethroids permethrin, deltamethrin, etofenprox, and lambda cyhalothrin and, to a lesser degree, the organophosphate acetylcholine-esterase inhibitors malathion and chlorpyrifos. Based on these findings, we decided to proceed with screening for resistance against three pyrethroids and chlorpyrifos. It should be noted that we only received information from public vector control programs and not privately-owned pest control companies or individuals that may have performed mosquito control in or close to the locations we targeted. Therefore, Table A in S1 File does not provide a comprehensive list of all of the insecticides to which mosquitoes in an area may have been exposed.
We next established Aedes aegypti laboratory strains from field-collected adults from those cities that yielded an adequate number of Ae. aegypti. Because 135 mosquitoes are required to perform nine replicates of insecticide resistance bottle testing, a minimum of 20 adult F0 females were needed to raise enough F2 mosquitoes for insecticide resistance testing. In order to detect resistance, we used a modified bottle test in which mortality over time in response to high concentrations of target insecticides in a New Mexican Ae. aegypti population was compared to a well-documented susceptible (Rockefeller) and resistant (Puerto Rico) strain. Our approach is conservative, because “resistance” is defined in the context of a high concentration of insecticide, efficient because mosquitoes need to be monitored for a shorter period of time than a standard CDC bottle assay, affordable because only one concentration of insecticide is used, and highly repeatable because the within-assay comparison among strains minimizes variation due to variation in the concentrations of active ingredients in different batches of insecticides, pipetting errors, and shifts in environmental conditions during the test itself. Further work is needed to compare the accurateness and efficacy of this novel insecticide resistance bioassay to the standard CDC-bottle test and other assays used to measure insecticide resistance.
By our assay, resistance to pyrethroids was widespread in the F2 and F4 generations of strains we established from isolates from New Mexico, with the interesting exception of mosquitoes from Alamogordo, the only city that did not report using pyrethroids for mosquito control. Resistant strains did show considerable variation in survival curves during exposure to permethrin. For example, the mortality curves for the Roswell and Deming strains closely resembled the highly resistant Puerto Rico strain, whereas Las Cruces mosquitoes exhibited a sigmoid mortality curve that lies between the curves for the susceptible and resistant control strains. These patterns suggest that mosquitoes from Las Cruces possess a different set of resistance mechanisms than the Roswell and Deming strains. The mortality curve found for the Lovington strain was double sigmoid, indicating that this strain is a likely a mixture of susceptible and resistant mosquitoes. This hypothesis is supported by the double sigmoid mortality curve that was observed for an even mixture of susceptible Rockefeller and resistant Puerto Rico mosquitoes. The same is true for the Carlsbad strain, but the number of susceptible mosquitoes in this strain is lower. We were not able to perform insecticide resistance testing with deltamethrin, etofenprox, and chlorpyrifos for all strains because limited numbers of mosquitoes were available. In general, we found that strains that were resistant to permethrin were also resistant to other pyrethroids we tested.
We did not find any resistance against the organophosphate chlorpyrifos in our tests. This confirms findings from an earlier study showing that pyrethroid resistance and resistance to chlorpyrifos are not always correlated [54]. Chlorpyrifos could therefore be considered a viable alternative to pyrethroids; however, the use of chlorpyrifos as a public health insecticide is controversial because of its adverse effects on prenatal human development [55]. Also, chlorpyrifos resistance has been found in wild populations of Culex mosquitoes [56] and it has been experimentally evolved in laboratory experiments [57].
It must be noted that the laboratory strains we established for different cities do not necessarily reflect resistance levels in Aedes aegypti populations in these cities. A more extensive sampling regime would be necessary to study how widespread resistance is geographically and over time.
To explore the genetic basis for pyrethroid resistance in New Mexican mosquitoes, we sequenced fragments of the para gene of 50 mosquitoes from different cities in New Mexico and showed that only one known kdr mutation was present–F1534C. This specific mutation is notorious around the world where pyrethroids have been used to control Ae. aegypti [36, 58, 59]. It has been suggested that this particular mutation confers no or very little fitness reduction [60, 61]. This lack in fitness cost explains the fact that insecticide resistance can develop quickly in Ae. aegypti and it can persist for years, even after the relevant active ingredients are no longer used [62]. The high level of kdr mutations within the mosquitoes we analyzed suggests that alternatives to pyrethroids should be considered for mosquito control in New Mexico.
Two significant caveats pertain to this study. First, we used F2 and F4 mosquitoes for the resistance testing because we were not able to procure enough F0 or F1 mosquitoes from the field. This strategy has been used before [63, 64], however, starting a population from low numbers of individuals bears the risk that their offspring don’t represent all genotypes and phenotypes present in the field populations. In order to preserve field levels of resistance, we used laboratory generations not higher than F4. However, we did capture a wide geographic swath of populations; the two most distant cities in our study, Deming and Lovington, are separated by approximately 300 miles. Second, a relatively low number of mosquitoes from different cities in New Mexico was analyzed genetically (between 4 and 8 individuals), preventing us from correlating the levels of pyrethroid resistance with frequencies of kdr mutations in individual field populations. Nonetheless, the finding that the kdr mutation is common in New Mexico is intriguing and should motivate more extensive studies.
Conclusion
Ae. aegypti from New Mexico showed high levels of pyrethroid resistance and high incidence of the kdr mutation F1534C in the para gene. All strains that we tested were still susceptible to the organophosphate chlorpyrifos. Based on the results of our study, we recommend a statewide resistance monitoring and management program to aid the control of Ae. aegypti and other disease-transmitting mosquitoes that threaten public health. Our results also stress the importance of developing alternative approaches to mosquito control like sterile-insect-technique [65, 66] or the use of Wolbachia, [67, 68].
Supporting information
(PDF)
Acknowledgments
We thank the 2017 BIOL302 class at NMSU for their help with primer development and testing. We thank the New Mexico vector control districts that kindly provided information on the insecticides they used.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was funded under a Memorandum of Agreement between the New Mexico Department of Health and the Regents of New Mexico State University, MOA#: 17/665.0300.20615. Funding was procured by an Epidemiology and Laboratory Capacity for Infectious Diseases (ELC) continuation grant (section M1, under CK14-1401) from the Centers of Disease Control and Prevention (CDC) awarded to the New Mexico Department of Health.
References
- 1.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science (New York, NY. 2007;316(5832):1718–23. 10.1126/science.1138878 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements AN, editor. The Biology of Mosquitoes. London: Chapman & Hall; 1992. [Google Scholar]
- 4.Soper FL. Dynamics of Aedes aegypti distribution and density. Seasonal fluctuations in the Americas. Bulletin of the World Health Organization. 1967;36(4):536 [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn MB, Eisen RJ, Eisen L, Boegler KA, Moore CG, McAllister J, et al. Reported distribution of aedes (stegomyia) aegypti and Aedes (stegomyia) albopictus in the United States, 1995–2016 (diptera: culicidae). Journal of medical entomology. 2016;53(5):1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pless E, Gloria-Soria A, Evans BR, Kramer V, Bolling BG, Tabachnick WJ, et al. Multiple introductions of the dengue vector, Aedes aegypti, into California. PLOS Neglected Tropical Diseases. 2017;11(8):e0005718 10.1371/journal.pntd.0005718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker KR, Joy TK, Ellers-Kirk C, Ramberg FB. Human and environmental factors affecting Aedes aegypti distribution in an arid urban environment. Journal of the American Mosquito Control Association. 2011;27(2):135–41. 10.2987/10-6078.1 [DOI] [PubMed] [Google Scholar]
- 8.Landau KI, van Leeuwen WJ. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. Journal of Vector Ecology. 2012;37(2):407–18. 10.1111/j.1948-7134.2012.00245.x [DOI] [PubMed] [Google Scholar]
- 9.Walker KR, Williamson D, Carrière Y, Reyes-Castro PA, Haenchen S, Hayden MH, et al. Socioeconomic and Human Behavioral Factors Associated With Aedes aegypti (Diptera: Culicidae) Immature Habitat in Tucson, AZ. Journal of medical entomology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden MH, Uejio CK, Walker K, Ramberg F, Moreno R, Rosales C, et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. EcoHealth. 2010;7(1):64–77. 10.1007/s10393-010-0288-z [DOI] [PubMed] [Google Scholar]
- 11.Weaver SC. Prediction and prevention of urban arbovirus epidemics: A challenge for the global virology community. Antiviral research. 2018;156:80–4. 10.1016/j.antiviral.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubaugh ND, Ladner JT, Kraemer MU, Dudas G, Tan AL, Gangavarapu K, et al. Genomic epidemiology reveals multiple introductions of Zika virus into the United States. Nature. 2017;546(7658):401 10.1038/nature22400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morens DM, Fauci AS. Chikungunya at the door—Déjà vu all over again? New England Journal of Medicine. 2014;371(10):885–7. 10.1056/NEJMp1408509 [DOI] [PubMed] [Google Scholar]
- 14.Fauci AS, Morens DM. Zika Virus in the Americas—Yet Another Arbovirus Threat. N Engl J Med. 2016;374(7):601–4. Epub 2016/01/14. 10.1056/NEJMp1600297 . [DOI] [PubMed] [Google Scholar]
- 15.Rose RI. Pesticides and public health: integrated methods of mosquito management. Emerging infectious diseases. 2001;7(1):17 10.3201/eid0701.700017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Center SE. A citizens' guide to pesticide use and regulation in New Mexico2004.
- 17.Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerging infectious diseases. 1998;4(4):605 10.3201/eid0404.980410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends in parasitology. 2016;32(3):187–96. 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 19.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends in parasitology. 2011;27(2):91–8. 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Maciel-de-Freitas R, Avendanho FC, Santos R, Sylvestre G, Araújo SC, Lima JBP, et al. Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak. PloS one. 2014;9(3):e92424 10.1371/journal.pone.0092424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yakob L, Walker T. Zika virus outbreak in the Americas: the need for novel mosquito control methods. The Lancet Global Health. 2016;4(3):e148–e9. 10.1016/S2214-109X(16)00048-6 [DOI] [PubMed] [Google Scholar]
- 22.Bariami V, Jones CM, Poupardin R, Vontas J, Ranson H. Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl Trop Dis. 2012;6(6):e1692 Epub 2012/06/22. 10.1371/journal.pntd.0001692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annual review of entomology. 2015;60:537–59. 10.1146/annurev-ento-010814-020828 [DOI] [PubMed] [Google Scholar]
- 24.Hemingway J, Boddington R, Harris J, Dunbar S. Mechanisms of insecticide resistance in Aedes aegypti (L.)(Diptera: Culicidae) from Puerto Rico. Bulletin of Entomological Research. 1989;79(1):123–30. [Google Scholar]
- 25.Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the houseflypara-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Molecular and General Genetics MGG. 1996;252(1–2):51–60. [DOI] [PubMed] [Google Scholar]
- 26.Williamson MS, Denholm I, Bell CA, Devonshire AL. Knockdown resistance (kdr) to DDT and pyrethroid insecticides maps to a sodium channel gene locus in the housefly (Musca domestica). Molecular and General Genetics MGG. 1993;240(1):17–22. [DOI] [PubMed] [Google Scholar]
- 27.Pittendrigh B, Reenan R, Ganetzky B. Point mutations in the Drosophila sodium channel gene para associated with resistance to DDT and pyrethroid insecticides. Molecular and General Genetics MGG. 1997;256(6):602–10. [DOI] [PubMed] [Google Scholar]
- 28.Du Y, Nomura Y, Zhorov BS, Dong K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects. 2016;7(4):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, et al. Pyrethroid and DDT cross‐resistance in Aedes aegypti is correlated with novel mutations in the voltage‐gated sodium channel gene. Medical and Veterinary Entomology. 2003;17(1):87–94. [DOI] [PubMed] [Google Scholar]
- 30.Saavedra‐Rodriguez K, Urdaneta‐Marquez L, Rajatileka S, Moulton M, Flores A, Fernandez‐Salas I, et al. A mutation in the voltage‐gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect molecular biology. 2007;16(6):785–98. 10.1111/j.1365-2583.2007.00774.x [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Shen W-K, Wang T-T, Lin Y-H, Hsu E-L, Dai S-M. A novel amino acid substitution in a voltage-gated sodium channel is associated with knockdown resistance to permethrin in Aedes aegypti. Insect biochemistry and molecular biology. 2009;39(4):272–8. 10.1016/j.ibmb.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 32.Srisawat R, Komalamisra N, Eshita Y, Zheng M, Ono K, Itoh TQ, et al. Point mutations in domain II of the voltage-gated sodium channel gene in deltamethrin-resistant Aedes aegypti (Diptera: Culicidae). Applied Entomology and Zoology. 2010;45(2):275–82. [Google Scholar]
- 33.Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P, Prapanthadara La. High‐throughput assays for detection of the F1534C mutation in the voltage‐gated sodium channel gene in permethrin‐resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Tropical Medicine & International Health. 2011;16(4):501–9. [DOI] [PubMed] [Google Scholar]
- 34.Haddi K, Tomé HV, Du Y, Valbon WR, Nomura Y, Martins GF, et al. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: a potential challenge for mosquito control. Scientific Reports. 2017;7:46549 10.1038/srep46549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasai S, Ng LC, Lam-Phua SG, Tang CS, Itokawa K, Komagata O, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus. Japanese journal of infectious diseases. 2011;64(3):217–21. [PubMed] [Google Scholar]
- 36.Kushwah RBS, Dykes CL, Kapoor N, Adak T, Singh OP. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLOS Neglected Tropical Diseases. 2015;9(1):e3332 10.1371/journal.pntd.0003332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishak IH, Jaal Z, Ranson H, Wondji CS. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasites & Vectors. 2015;8(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linss JGB, Brito LP, Garcia GA, Araki AS, Bruno RV, Lima JBP, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasites & Vectors. 2014;7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris AF, Rajatileka S, Ranson H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. The American journal of tropical medicine and hygiene. 2010;83(2):277–84. 10.4269/ajtmh.2010.09-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez‐Monroy B, Gutierrez‐Rodriguez SM, Villanueva‐Segura OK, Ponce‐Garcia G, Morales‐Forcada F, Alvarez LC, et al. Frequency and intensity of pyrethroid resistance through the CDC bottle bioassay and their association with the frequency of kdr mutations in Aedes aegypti (Diptera: Culicidae) from Mexico. Pest management science. 2018. [DOI] [PubMed] [Google Scholar]
- 41.Saavedra-Rodriguez K, Maloof FV, Campbell CL, Garcia-Rejon J, Lenhart A, Penilla P, et al. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Scientific Reports. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silver JB. Mosquito ecology: field sampling methods: Springer Science & Business Media; 2007. [Google Scholar]
- 43.Grossman MK, Uc-Puc V, Rodriguez J, Cutler DJ, Morran LT, Manrique-Saide P, et al. Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biology letters. 2018;14(6):20180022 10.1098/rsbl.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marquardt WH. Biology of Disease Vectors. 2nd ed Marquardt WH, editor: Academic Press; 2004. [Google Scholar]
- 45.Grossman MK, Uc-Puc V, Flores AE, Manrique-Saide PC, Vazquez-Prokopec GM. Larval density mediates knockdown resistance to pyrethroid insecticides in adult Aedes aegypti. Parasites & Vectors. 2018;11(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Organization WH. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2016.
- 47.Brogdon W, Chan A. Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay/methods in Anopheles research. CDC Atlanta USA: CDC Technical Report. 2010.
- 48.Wu Y, Fairfield A, Oduola A, Cypess R. The Malaria Research and Reference Reagent Resource (MR4) Center—creating African opportunities. African journal of medicine and medical sciences. 2001;30:52–4. [PubMed] [Google Scholar]
- 49.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53(282):457–81. [Google Scholar]
- 50.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith LB, Kasai S, Scott JG. Voltage‐sensitive sodium channel mutations S989P+ V1016G in Aedes aegypti confer variable resistance to pyrethroids, DDT and oxadiazines. Pest management science. 2018;74(3):737–45. 10.1002/ps.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaves LF, Kawashima E, Futami K, Minakawa N, Marin Rodriguez R. Lack of kdr mutations in a population of Asian tiger mosquitoes from Costa Rica. Bulletin of Insectology. 2015;68(1):61–3. [Google Scholar]
- 53.Vasconcelos PF, Powers AM, Hills S. The Emergence of Chikungunya and Zika Viruses in the Americas Chikungunya and Zika Viruses: Elsevier; 2018. p. 215–35. [Google Scholar]
- 54.Lopez B, Ponce G, Gonzalez JA, Gutierrez SM, Villanueva OK, Gonzalez G, et al. Susceptibility to chlorpyrifos in pyrethroid-resistant populations of Aedes aegypti (Diptera: Culicidae) from Mexico. Journal of medical entomology. 2014;51(3):644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–e59. 10.1542/peds.2006-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasteur N, Marquine M, Ben Cheikh H, Bernard C, Bourguet D. A new mechanism conferring unprecedented high resistance to chlorpyrifos in Culex pipiens (Diptera: Culicidae). Journal of medical entomology. 1999;36(6):794–802. [DOI] [PubMed] [Google Scholar]
- 57.Alout H, Labbé P, Berthomieu A, Makoundou P, Fort P, Pasteur N, et al. High chlorpyrifos resistance in Culex pipiens mosquitoes: strong synergy between resistance genes. Heredity. 2016;116(2):224 10.1038/hdy.2015.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarez LC, Ponce G, Saavedra‐Rodriguez K, Lopez B, Flores AE. Frequency of V1016I and F1534C mutations in the voltage‐gated sodium channel gene in Aedes aegypti in Venezuela. Pest management science. 2015;71(6):863–9. 10.1002/ps.3846 [DOI] [PubMed] [Google Scholar]
- 59.Plernsub S, Saingamsook J, Yanola J, Lumjuan N, Tippawangkosol P, Walton C, et al. Temporal frequency of knockdown resistance mutations, F1534C and V1016G, in Aedes aegypti in Chiang Mai city, Thailand and the impact of the mutations on the efficiency of thermal fogging spray with pyrethroids. Acta tropica. 2016;162:125–32. 10.1016/j.actatropica.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 60.Plernsub S, Stenhouse S, Tippawangkosol P, Lumjuan N, Yanola J, Choochote W, et al. Relative developmental and reproductive fitness associated with F1534C homozygous knockdown resistant gene in Aedes aegypti from Thailand. Trop Biomed. 2013;30(4):621–30. [PubMed] [Google Scholar]
- 61.Brito LP, Linss JG, Lima-Camara TN, Belinato TA, Peixoto AA, Lima JBP, et al. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PloS one. 2013;8(4):e60878 10.1371/journal.pone.0060878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Lourdes Macoris M, Martins AJ, Andrighetti MTM, Lima JBP, Valle D. Pyrethroid resistance persists after ten years without usage against Aedes aegypti in governmental campaigns: Lessons from São Paulo State, Brazil. PLOS Neglected Tropical Diseases. 2018;12(3):e0006390 10.1371/journal.pntd.0006390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deming R, Manrique-Saide P, Barreiro AM, Cardeña EUK, Che-Mendoza A, Jones B, et al. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasites & Vectors. 2016;9(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lima JBP, Da-Cunha MP, Junior RCDS, Galarado AKR, Soares SDS, Braga IA, et al. Resistance of Aedes aegypti to organophosphates in several municipalities in the State of Rio de Janeiro and Espirito Santo, Brazil. The American journal of tropical medicine and hygiene. 2003;68(3):329–33. [PubMed] [Google Scholar]
- 65.Hendrichs J, Robinson A. Sterile insect technique Encyclopedia of Insects (Second Edition): Elsevier; 2009. p. 953–7. [Google Scholar]
- 66.Vargas-Terán M, Hofmann H, Tweddle N. Impact of screwworm eradication programmes using the sterile insect technique Sterile insect technique: Springer; 2005. p. 629–50. [Google Scholar]
- 67.Iturbe‐Ormaetxe I, Walker T, O'Neill SL. Wolbachia and the biological control of mosquito‐borne disease. EMBO reports. 2011;12(6):508–18. 10.1038/embor.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann A, Montgomery B, Popovici J, Iturbe-Ormaetxe I, Johnson P, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476(7361):454 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.