Abstract
Background: Little is known about the nutritional status of patients with peritoneal metastasis (PM), in particular about the evolution of the so-called anorexia-cachexia syndrome in these patients. The objective of the study was to assess nutritional status in PM patients at the end of life, including metabolic aspects.
Methods: Observational study. Prospective nutritional status assessment in 87 PM patients, including Subjective Global Assessment: (SGA), physical examination (body mass index [BMI], bioelectrical impedance analysis [BIA], anthropometry and blood chemistry).
Results: 85 % patients had received previous chemotherapy. Peritoneal carcinomatosis index was 16±11, Karnofsky 81±14 % and ascites volume 1,000±1,690 ml. SGA was reduced with 22.0±9.6 points, BMI normal with 25.3±5.8 kg/m2 and resting metabolism was 1,527±248 kcal/day. Serum total protein and albumin were at the inferior normal limit (6.5±0.8 g/dl, respectively 3.7±0.8 g/dl) and C-reactive protein (CRP) was elevated (2.9±4.1 g/dl). Serum levels of protein (p=0.05), albumin (p=0.003) and transferrin (p=0.001) were higher in gastrointestinal than in ovarian PM patients. When patients were grouped according to time from first assessment to death, serum protein and albumin decreased until end of life, whereas ascites volume, resting metabolism and CRP increased.
Conclusion: Both increased resting metabolism and decreased caloric intake contribute to the development of the cachexia-anorexia syndrome in PM patients. End of life is caused by energetic dysbalance and exhaustion.
Keywords: anorexia, cachexia, cancer, nutrition, peritoneal metastasis
Introduction
Cachexia is a complex metabolic syndrome associated with underlying chronic illness. It is a multifactorial process resulting in progressive weight loss and skeletal muscle atrophy – with or without loss of fat mass. In the case of cancer, cachexia results from adaptation to the disease, creating an environment characterized by inflammation, loss of appetite (anorexia), low levels of anabolic hormones and anemia [1]. Anorexia and decreased food intake result into an energy deficit. In addition, inflammation increases energy demands, cells become insulin resistant, and low levels of anabolic hormones result in muscle wasting (reviewed in [2]). Cachexia and anorexia are forming together the so-called “cachexia-anorexia syndrome”. In cancer, the cachexia-anorexia syndrome is associated with poor quality of life, poor physical function, and poor prognosis [3].
Peritoneal metastasis (PM) is a relatively common spreading pattern of malignant tumors originating in the gastrointestinal tract [4], the ovary [5] and other origins [6]. Very few data on the nutritional state of peritoneal metastasis patients have been published. To our knowledge, there are no specific data available on the evolution of nutritional status of patients with advanced peritoneal metastasis at the end of life.
The aim of this study is to assess the nutritional state of patients with advanced peritoneal metastasis and to determine its evolution until end of life.
Patients and methods
Study design
This is a non-interventional study observing the nutritional status on the basis of clinical and laboratory data collected routinely in peritoneal metastasis patients at our institution.
Patients
Between 4/2014 and 05/2015, 111 patients with advanced peritoneal metastasis were referred to our institution (setting: specialized therapy centre for peritoneal cancer, University hospital, tertiary care center). Before admission, each patient history was discussed in the interdisciplinary tumor board and best therapy was recommended on a case-by-case, individual basis. There were no specific inclusion or exclusion criteria.
Regulatory framework
Nutritional status is recorded routinely in all peritoneal metastasis patients at our institution in order to screen malnutrition and to offer nutritional support and/or drug therapy in these patients. Data are entered into a prospective registry authorized by the Ethics Committee of Ruhr-University Bochum, Germany (Nr. 15–5280). All patients gave their written informed consent for data collection. Beside clinical standard of practice, no additional tests were performed for the present study. Therapy, data collection, and data analysis were performed according to the Declaration of Helsinki (1983), in particular patients were free to refuse collection and/or storage of nutritional data.
Data collection
The day of admission, following data were collected:
-
–
Subjective Global Assessment Score Sheet (SGA) as described elsewhere [7] including medical history (weight and diet changes) and gastrointestinal symptoms.
-
–
Physical examination including body weight and estimation of body composition by bioelectrical impedance analysis (BIA) according to the instructions of the manufacturer.
-
–
Skinfold measurement using Slim Skinfold Caliper Measure Body Fat Tester (0 to 80 mm, eaglefit GmbH, Ulm-Langenau, Germany) measuring skinfolds to determinate the amount of subcutaneous fat. Then, body density and body fat percentage were predicted according to the instructions of the manufacturer [8].
-
–
Blood chemistry including C-reactive protein (CRP), total protein, albumin and transferrin. Normal range was CRP: <0.5 mg/dl, total protein: 6.6–8.7 g/dl, serum albumin: 3.5–5.2 g/dl and transferrin 200–360 mg/dl.
All these methods are non-invasive and are not inducing any extra physical or psychical stress for the patients. Determination of nutritional status was performed by two specialized, registered study nurses.
Statistical analysis
This is an observational, explorative study intended to generate pilot data for later therapeutic studies. No sample size was calculated beforehand. Usual descriptive statistics are provided, including means and standard deviations. A non-parametric test (Mann-Withney U-test) was used to compare means between ovarian and gastrointestinal cancer patients. A p<0.05 was considered significant. Body fat data were derived from skinfold measurement using a dedicated Microsoft-Excel based calculation tool [9]. We used the software SPSS for Windows (SPSS 20.0, SPSS Inc., Chicago, IL, USA) for statistical analysis and graphical design.
Results
Within the period of time under investigation, 111 consecutive patients with peritoneal metastasis were admitted to our hospital. All of these patients were invited to fill out the SGA questionnaire and to undergo physical examination but 24 refused to do so or gave incomplete information, so that 87 datasets remained for further analysis. A complete set of nutritional data, including anamnestic scores (SGA), physical examination (BMI, bioelectrical impedance analysis, anthropometry) and blood chemistry, could be obtained in these patients.
Patients characteristics are summarized in Table 1. The vast majority of patients (85 %) were in the salvage situation: they had already received at least one line of systemic palliative chemotherapy and the mean time from cancer diagnosis was 36 (±35) months. General condition was moderately impaired with a mean Karnofsky Index of 81±14 %. Peritoneal metastasis was advanced with a mean Peritoneal Carcinomatosis Index (PCI) of 16±11.
Table 1:
Patient characteristics.
| Patients characteristics | All patients (n=87) | % |
| n | ||
| Number of patients | 87 | |
| Gender | ||
| Male | 30 | 34.5 |
| Female | 57 | 65.5 |
| Age, years | ||
| Median (min-max) | 63 (26–83) | |
| Peritoneal Carcinomatosis Index | ||
| (mean±SD) | 16 (±11) | |
| Karnofksy Index | ||
| (mean±SD) | 81 (±14) | |
| Primary tumor | ||
| Ovarian | 29 | 33.3 |
| Gastric | 15 | 17.2 |
| Colorectal | 14 | 16.1 |
| CUP (of unknown origin) | 10 | 11.5 |
| Appendiceal | 4 | 4.6 |
| Gallbladder | 4 | 4.6 |
| Small intestine | 2 | 2.3 |
| Breast | 3 | 3.5 |
| Mesothelioma | 1 | 1.1 |
| Pancreas | 2 | 2.3 |
| Others (neuroendocrine, sarcoma, oesophagus) | 3 | 3.5 |
| Previous chemotherapy | 74 | 85.1 |
Table 2 describes the nutritional status of these 87 patients with PM. At admission, moderate malnourishment was documented with a mean Subjective Global Assessment Score (SGA) of 22.5 (±4) (stage B). Physical examination showed a normal Body Mass Index (BMI), a normal skeletal muscle percentage, a normal visceral fat level and a normal fat percentage according to the bioelectrical impedance analysis (BIA). Skinfold measurement confirmed a normal skeletal muscle percentage and a normal body fat percentage. Laboratory values showed a 6-fold increase of C-Reactive Protein with 2.8 (±4) g/dl as a sign of major and chronic systemic inflammation as a reaction to peritoneal tumor involvement. Total serum protein (6.4±0.9 g/dl), serum albumin (3.6±0.8 g/dl) and transferrin (230±68 mg/dl) levels were at the lower normal limit.
Table 2:
Natural evolution of nutritional status in PM patients. Patients are stratified according to the time period between first admission and the end of life.
| Time to death | >120 Days | 61–120 Days | < 60 Days | Normal range |
| (n=51) | (n=25) | (n=11) | ||
| Subjective Global Assessment Score Points (SGA) (median±SD) | 22.7±3.6 | 21.2±4.3 | 22.8±3.2 | >24 |
| Ascites (ml; mean±SD) | 754±1607 | 915±1571 | 1533±1763 | 0 |
| PCI (min 0; max 39) | 11.1±9.2 | 19.1±9.4 | 22.8±12.1 | 0 |
| Karfofsky Index (min 0 %, max 100 %) | 83±15.7 | 80.9±10.2 | 78.9±11,7 | 100% |
| Body mass index, kg/m2 | 26.4±7.2 | 24.3±3.3 | 25.7±6.9 | M: >20 F: >19 |
| Bioelectrical impedance analysis (BIA) | ||||
| Resting metabolism, kcal | 1566±281 | 1466±176 | 1586±340 | # |
| Body fat percentage, % | M: 22.8±8.8 | M: 21.4±6.0 | M: 23.2±10.7 | # |
| F: 30.9±11.7 | F: 30.9±7.6 | F: 31.5± 6.9 | # | |
| Skeletal muscle percentage, % | M: 34.6±3.8 | M: 34.5±2.8 | M: 34.1±5.7 | # |
| F: 29.1±5.0 | F: 29.0±3.6 | F: 26.2±6.2 | # | |
| Visceral fat level | 9.2±5.6 | 7.9±3.5 | 9.2±8.3 | 9-Jan |
| Skinfold measurement | ||||
| Body fat weight, kg | 23.9±11.1 | 20.3±5.6 | 23.7±11.8 | # |
| Fat free mass, kg | M: 62.4±9.6 | M: 59.2±5.9 | M: 64.5±14.1 | # |
| F: 45.9±9.8. | F: 42.4±3.8. | F: 47.4±8.8 | # | |
| Body fat percentage, % | M: 26.2±7.0 | M: 24.4±5.1 | M: 30.3±5.7 | # |
| F: 32.7±8.7. | F: 32.5±5.2 | F: 28.3±7.1 | # | |
| Blood chemistry | ||||
| CRP, mg/dL | 2.6±3.3 | 2.6±3.2 | 4.1±6.2 | < 0.5 |
| Total protein, g/dL | 6.4±0.9 | 6.6±0.8 | 6.2±0.7 | 6.6-8.7 |
| Albumin, g/dL | 3,7±0.7 | 3.6±0.7 | 3.2±1.3 | 3.5-5-2 |
| Transferrin, mg/dl | 231.3±59.2 | 237.9±75.3 | 205±74.5 | 200-360 |
Note: # normal values are depending on the age and on the sex of patients. M, male; F, female.
To determine the natural evolution of nutritional status in PM patients, we stratified the patients according to the time period between first admission and the end of life. C-reactive protein level further deteriorated until death as a sign of massive inflammatory systemic response to peritoneal involvement (Figure 1). As a rule, most nutritional parameters deteriorated until death, as exemplified by albumin and total protein serum levels (Figure 2). Resting metabolism increased in the last months of life (Figure 3), which is explained both by tumor growth and systemic inflammatory response. Ascites volume increased until end of life, contributing to protein loss, hypoproteinemia and skeletal muscle consumption. (Supplementary Material 1). The available protein pool was significantly less in ovarian than in gastrointestinal PM patients (total protein (p=0.05), albumin (p=0.003), as shown in Supplementary Material 2.
Figure 1:
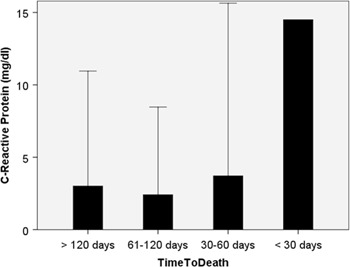
C-reactive protein (CRP) serum levels in 87 peritoneal metastasis patients, grouped according to their survival time.
Source: CRP was already elevated (by a factor 6-fold) at first admission and further increased during the last 4 months of life. Cachexia results from adaptation to the disease, creating an environment characterized in particular by inflammation.
Figure 2:
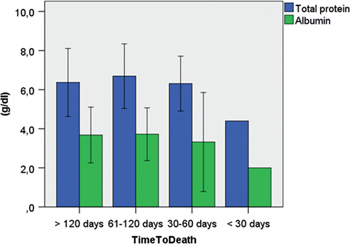
Total serum protein and serum albumin in 87 peritoneal metastasis patients, grouped according to their survival time.
Source: Both parameters deteriorated until end of life, as a manifestation of natural history of disease.
Figure 3:
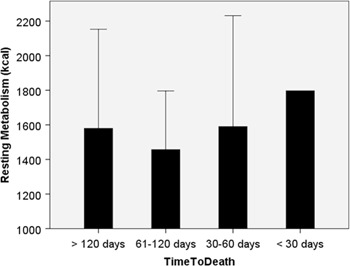
Resting metabolism increased over the last 4 months of life in peritoneal metastasis patients (n=87).
Discussion
Prior work has documented that patients with advanced cancer are generally affected by the so-called cachexia-anorexia syndrome [10]. However, to our knowledge, there is no clinical data describing quantitatively the nutritional status of patients with peritoneal metastasis. The aim of this study was to assess the nutritional status of patients with PM with simple but effective methods by combining anamnestic scores, physical examination and laboratory tests. This assessment included the Subjective Global Assessment Score Sheet (SGA), physical examination including body weight, estimation of body composition by body impendance analysis (BIA), anthropometry (SM) and blood chemistry.
Nutritional screening at admission
The majority of patients with peritoneal metastasis were moderately or severely malnourished at time of first admission (56 % patients with SGA-Index B or C). This finding underscores the crucial role of nutritional assessment in PM patients. A first nutritional screening is indicated at an early point of time, for example just after diagnosis of PM. This first assessment (for example the SGA score) is simple and can be performed by any physician or specialized nurse.
Evolution of nutritional status
When peritoneal metastasis patients were grouped according to the time period between hospital admission and death, we found a progressive deterioration in most subjective, physical and laboratory parameters of nutrition. CRP was already elevated (by a factor 6-fold) at first admission and further increased during the last 4 months of life. Cachexia results from adaptation to the disease, creating an environment characterized in particular by inflammation. This finding is in accordance with previous work showing that, in patients with advanced cancer, the anorexia-cachexia syndrome deteriorates progressively until death [2].
The negative evolution of the nutritional status appears to be significant and rapid in PM patients. Patients reported about a slow but continuous deterioration of the SGA score, confirming quality of life results obtained in a previous study [11]. When gastrointestinal symptoms were determined using the QLQ30 questionnaire of EORTC in PM patients, appetite loss, but also nausea/vomiting, diarrhea and obstipation deteriorated at the end of life. Physical examination and laboratory tests are coherent with the subjective data.
On the basis of the above data, we suggest to repeat nutritional assessment at regular intervals in PM patients, investigating in particular gastrointestinal symptoms and performance status. All patients with SGA score C and probably most patients with SGA score B should be referred to a specialist to determine a nutrition support plan with the hope of delaying and slowing down the development of the cachexia-anorexia syndrome – and prolong life.
Weight loss is a powerful independent variable that predicts mortality in patients with cancer [12]. It is very likely that, in peritoneal metastasis as well as in other cancers, tumor growth increases energy consumption. Cancer cells are programmed to rely on aerobic glycolysis to support their proliferation and anabolic growth [13]. Increased tumor metabolism is harming healthy organs by stealing their energy resources. This process results into modified body metabolism with loss of muscle mass and possibly of fatty tissue [14]. Moreover, peritoneal tumor spreading causes progressive intestinal dysfunction by infiltration of the bowel wall. Patients report about ill-defined abdominal pain, nausea and anorexia which in turn impair an adequate oral nutritional intake [15]. Vomiting is observed when malignant bowel obstruction develops and finally patients have to receive parenteral nutrition [16].
Finally, drug-related side effects: (e. g. caused by chemotherapy, morphine derivatives, antibiotics, etc.) can cause anorexia or interfere with the ingestion of food [17]. Systemic chemotherapy can induce severe gastrointestinal symptoms in a significant proportion of patients. Chemotherapy acts on all rapidly dividing cells including tumor cells but also normal cells lining the gastrointestinal tract, susceptible to damage and growth inhibition. This can result in patients experiencing clusters of gastrointestinal symptoms such as lack of appetite, nausea, vomiting, diarrhea, etc. [18].
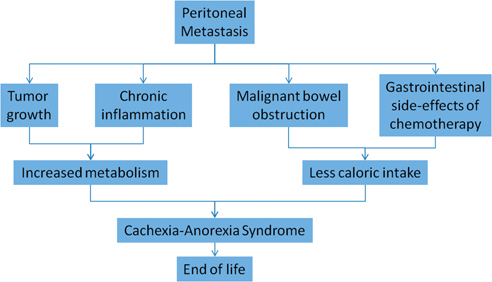
It is most likely that in peritoneal metastasis patients, all these three pathways together contribute to the development of the cachexia-anorexia syndrome and finally to death, as represented in Figure 4. Both increased resting metabolism and decreased caloric intake contribute to the development of the cachexia-anorexia syndrome, which is ultimately incompatible with life. Patients with PM die from energetic dysbalance and exhaustion. Therapeutic approaches should tackle systematically each of these pathways.
Figure 4:
Pathways leading to development of cachexia-anorexia syndrome in peritoneal metastasis patients.
Source: Both increased resting metabolism and decreased caloric intake contribute to the development of the cachexia-anorexia syndrome, which is ultimately incompatible with life.
As the multimodal therapy of PM is increasingly effective, the diagnosis and treatment of cachexia are becoming important goals to improve both life expectancy and quality of life. Providing and ensuring adequate caloric intake to PM patients is indeed necessary to maintain body weight. Unfortunately, adequate nutritional supply is not sufficient, since weight stabilization does not prevent the continuing loss of skeletal muscle mass in PM patients, and does not correct the underlying metabolic abnormalities. Potential pharmacological strategies targeting skeletal muscle wasting might be associated with nutritional support and include for example androgens, selective androgen receptor modulators, antimyostatin drugs, growth hormone and insulin-like growth factor (reviewed in [1]). However data demonstrating effectiveness of these agents is lacking. At the present time, anti-inflammatory trials in cachectic cancer patients have not been successful [19].
To our knowledge, there is no published recommendation for treating the cachexia-anorexia syndrome in PM patients. Since we cannot refer to previous work, we propose to address the problem by tackling systematically the increased tumor metabolism, the progressive intestinal dysfunction and the adverse effects of systemic chemotherapy. Tumor metabolic demands might be best reduced by effective cancer therapy, which has been made possible in some cases by new therapeutic options in PM that appeared in the recent years. These options include potentially curative approaches such as the combination of CytoReductive Surgery (CRS) and Hyperthermic IntraPEritoneal Chemotherapy (HIPEC), and palliative approaches such as personalized palliative systemic chemotherapy [19] or various forms of intraperitoneal chemotherapy [20, 21]. Gastrointestinal side-effects of systemic chemotherapy might be prevented, at least in part, by suspending this therapy early enough in the course of disease and before the development of painful and disabling symptoms. Another alternative might be for example intraperitoneal drug delivery using pressurized aerosols (PIPAC) using low dose chemotherapy since this form of drug delivery has been reported to be effective without causing severe additional gastrointestinal symptoms in the vast majority of patients [11, 22]. There are reasons to be optimistic about the possibility that in the future, cachexia may be treated effectively. A number of drugs have already been developed that target key underlying mechanisms, namely, reduced food intake and altered metabolism and regulation of muscle mass [23]. These potential therapeutic avenues have now to be evaluated in adequate studies in PM from the nutritional perspective.
In summary, this pilot study is providing for the first time baseline and evolution nutritional data in patients with PM and underlines the need for diagnosis and treating the anorexia-cachexia syndrome in these patients. Obviously, the cachexia-anorexia syndrome is deteriorating until end of life in PM patients, with the development of a severe inflammatory syndrome and an increase of resting metabolism. In the next research step, we intend to confirm the value of nutritional status for predicting life expectancy in PM patients in a prospective study. The data presented here will be useful to determine inclusion and exclusion criteria for this study.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Supplemental Material
The online version of this article (DOI: 10.1515/pp-2016-0003) offers supplementary material, available to authorized users.
References
- 1.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–9. [DOI] [PubMed]
- 2.Laviano A, Meguid MM, Inui A, Muscaritoli M, Rossi-Fanelli F. Therapy insight: Cancer anorexia-cachexia syndrome–when all you can eat is yourself. Nat Clin Pract Oncol 2005;2:158–65. [DOI] [PubMed]
- 3.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: Mechanisms and clinical implications. Gastroenterol Res Pract 2011;2011:601434. [DOI] [PMC free article] [PubMed]
- 4.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–63. [DOI] [PubMed]
- 5.Romero I, Bast RC Jr. Minireview: Human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology 2012;153:1593–602. [DOI] [PMC free article] [PubMed]
- 6.Thomassen I, Verhoeven RH, van Gestel YR, van de Wouw AJ, Lemmens VE, de Hingh IH. Population-based incidence, treatment and survival of patients with peritoneal metastases of unknown origin. Eur J Cancer 2014;50:50–6. [DOI] [PubMed]
- 7.www.subjectiveglobalassessment.com, Jeejeebhoy Holdings Inc. Accessed 29 May 2014.
- 8.http://www.omron-healthcare.de/unsere-produkte/gewichtskontrolle/bf511. Accessed 08 Jun 2014.
- 9.http://www.eaglefit.de/berechnung.xls. Accessed 29 May 2014.
- 10.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381-410. Review. [DOI] [PubMed]
- 11.Odendahl K, Solass W, Demtröder C, Giger-Pabst U, Zieren J, Tempfer C et al. Quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J Surg Oncol 2015;41:1379–85. [DOI] [PubMed]
- 12.Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care: correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005;103:2189–98. [DOI] [PubMed]
- 13.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;5930:1029–1033. [DOI] [PMC free article] [PubMed]
- 14.Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia–pathophysiology and management. J Gastroenterol 2013;48:574-94. Review. [DOI] [PMC free article] [PubMed]
- 15.Klein C, Stiel S, Bükki J, Ostgathe C. Pharmacological treatment of malignant bowel obstruction in severely ill and dying patients: a systematic literature review. Schmerz 2012;26:587–99. [DOI] [PubMed]
- 16.O’Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opin Pharmacother 2011;12:2205–14. [DOI] [PubMed]
- 17.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr 2008;27:5–15. [DOI] [PubMed]
- 18.Cherwin CH. Gastrointestinal symptom representation in cancer symptom clusters: a synthesis of the literature. Oncol Nurs Forum 2012;39:157–65. [DOI] [PMC free article] [PubMed]
- 19.Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, Rowland Jr KM, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007;110:1396e403. [DOI] [PubMed]
- 20.Chan CH, Cusack JC, Ryan DP. A critical look at local-regional management of peritoneal metastasis. Hematol Oncol Clin North Am 2015;29:153–8. [DOI] [PubMed]
- 21.Katharina N, Wiebke S, Cedric D, Clemens B. Tempfer and Marc A. Reymond. Intraperitoneal cancer therapy: Principles and practice. CRC press (Taylor and Francis), Boca Raton, FL, 2016.
- 22.Tempfer CB, Winnekendonk G, Solass W, Horvat R, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol Oncol 2015;137:223–8. [DOI] [PubMed]
- 23.Fearon K, Argiles JM, Baracos VE, Bernabei R, Coats A, Crawford J, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015;6:272–4. [DOI] [PMC free article] [PubMed]


