Abstract
Background: Peritoneal metastasis (PM) develop in more than 50 % of gastric cancer (GC) patients. Median survival without treatment is not more than 3–7 months, and 8–12 months after modern combination chemotherapy. Innovative therapeutic approaches are urgently needed.
Methods: Phase-2, open label prospective clinical trial assessing safety and efficacy of bidirectional chemotherapy for treating peritoneal metastasis of gastric cancer (PMGC). Treatment protocol included initial staging laparoscopy or laparotomy, 3–4 courses of systemic chemotherapy (XELOX) followed by Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) procedures every 6 weeks until progression of disease or death. Primary endpoints were overall survival and histological peritoneal regression grading score after rebiopsy.
Results: 31 patients were included (9 men, 22 women, mean age 52 years), 24 with synchronous PM at diagnosis, 7 with metachronous PM after previous chemotherapy. Mean PCI was 13.8 (min-max 6–34). XELOX was administered in all patients and combined with 56 PIPAC procedures. Complete and partial pathological response was found in 60 % of the 15 patients eligible for tumor response assessment (4 and 5 patients, respectively). Median survival was 13 months.
Conclusions: Bidirectional chemotherapy combining XELOX with PIPAC with cisplatin and doxororubicin is well tolerated, can induce objective tumor regression and is associated with a promising survival in PMGC.
Keywords: cisplatin, doxorubicin, gastric cancer, Hyperthermic IntraPeritoneal Chemotherapy (HIPEC), intraperitoneal chemotherapy, peritoneal carcinomatosis, Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC), XELOX
Introduction
Gastric cancer (GC) is the fourth most common cancer and the second cause of cancer-related deaths in the Russian Federation [1]. Peritoneal dissemination is one of the most unfavorable course of progression and recurrence of GC [2]. Up to 40 % of GC patients show synchronous peritoneal metastasis (PM) at time of diagnosis and peritoneal relapse (metachronous metastasis) develop after radical surgery in 10–46 % of cases [3, 4]. Risk factors for peritoneal metastasis in gastric cancer (PMGC) include invasion of serosa, lymph node metastases, presence of free cancer cells in peritoneal lavage, and diffuse type of cancer according to the Lauren classification [5, 6]. With a median survival of 3–5 months, PMGC has long been considered as an end-stage disease without effective therapeutic options [6, 7]. In Russia, the majority of patients with PMGC are considered as incurable, are not subject to specific antitumor treatment and receive best supportive care including repeated paracentesis for ascites.
Nowadays, the standard method of treatment for PMGC is systemic palliative chemotherapy, including combinations such as FLOT (5-fluorouracil, folinic acid, oxaliplatin and doecetaxel) or XELOX (oxaliplatin and capecitabin). In general, modern cytotoxic drugs combinations increase the median survival of patients with metastatic gastric cancer to 8–12 months [6]. Speficially, efficacy of systemic chemotherapy on PMGC is probably lower than on parenchymatous metastases, especially for chemoresistant tumors such as diffuse (or signet-ring) histology according to Lauren. In 10–40 % of patients, PMGC are isolated and other distant metastases are absent [8, 9].
Currently, several potentially effective treatments for patients with isolated PMGC are under development. One is complete cytoreductive surgery (CRS) combined with Hyperthermic IntraPeritoneal Chemotherapy (HIPEC). This method showed a high and moderate effectiveness in several disseminated malignant tumors such as peritoneal pseudomyxoma, ovarian cancer, and colorectal cancer. However, the effectiveness of the HIPEC in PMGC is significantly lower owing to the features of the disease and to its low sensitivity to cytostatic agents [10–12]. A systematic review of 10 published studies showed a median survival of patients with PMGC equal to 7.9 months after CRS and HIPEC for the entire group and 15 months in the cases of complete cytoreduction (CC-0). The 5-year survival was only 13 % [13]. The preventive use of HIPEC after radical gastrectomy showed the best efficiency in patients with high risk for developing metachronous PMGC, in particular in the presence of serosal invasion (T4 tumor category), lymph nodes invasion (N1 category), and presence of free tumor cells in the peritoneal lavage.
Another potentially effective treatment for patients with isolated PMGC is Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC), a procedure first performed in Germany in 2011 [14]. The rationale for PIPAC is based on repeated application of intraperitoneal chemotherapy by taking advantage of the physical properties of gases and of pressure. Low-dose cisplatin and doxorubicin are used (10 % of the usual intraperitoneal doses applied during HIPEC). Preclinical studies showed relative benefits of delivering aerosolized substances under the capnoperitoneum conditions vs. conventional intraperitoneal chemotherapy with liquid solutions. Specifically, benefits included a more even distribution of the drug and a deeper penetration into the peritoneum compared to those in peritoneal lavage. The increased intraperitoneal pressure was shown to increase the drug capture by tumor cells [15]. The first experience of the clinical application of PIPAC in patients with PMGC showed encouraging results. In particular, a complete histological response was observed in 25 % of the patients and partial response or stabilization in further 34 % [16].
The Moscow Oncology Institute (named after P.A. Herzen) is a reference center for cancer patients in the Russian Federation and includes a surgical oncology unit specialized in upper gastrointestinal cancers. Our experience using CRS and HIPEC in PMGC patients confirmed the limited effectiveness of this method in advanced PM (disseminated peritoneal involvement with a Peritoneal Carcinomatosis Index (PCI) > 6). Thus, we were looking for innovative therapy concepts for these patients. In August 2013 we opened the first PIPAC program in Russia. Specifically, we developed a prospective study protocol in PMGC combining PIPAC (low-dose cisplatin and doxorubicin) with systemic combination chemotherapy (oxaliplatin and capecitabin: XELOX protocol). In this article, we now present the safety and efficacy results of this multimodal concept after 56 PIPAC procedures performed in 31 patients with PMGC.
The results of the study were presented at the ESMO meeting 2016 [17].
Materials and methods
Ethical and regulatory framework
The study protocol was approved by the local Ethics Committee and the Academic Council of the Moscow Oncology Institute (Ethics Committee meeting #12 from 07 Feb 2014). Therapy was performed according to the principles of the Declaration of Helsinki. All patients gave their informed consent for therapy. Patient insurance was not provided.
Study design
Open-label, single-arm prospective efficacy and safety study (Phase-2 trial).
Study protocol
The study protocol is illustrated in a chart flow (Figure 1). Diagnosis of PM was confirmed by diagnostic laparoscopy or laparotomy (if PM was not suspected before surgery) in all cases. Only patients with proven PM were included in the study. Peritoneal Carcinomatosis Index (PCI) was evaluated and documented and multiple biopsies of the peritoneum taken. In a first therapy phase, patients were treated with 4 courses of XELOX (Oxaliplatin 130 mg/m2 intravenous q3w combined with Capecitabin 1.000 mg/m2 orally twice a day during 2 weeks, followed by one week therapy pause). Then, PIPAC procedures were performed at 6 weeks interval, two XELOX courses being administered between the PIPAC cycles. Each PIPAC procedure included diagnostic laparoscopy, evaluation of PCI, and peritoneal biopsies. Patients were treated until progression of disease POD or death. Criteria of POD included 50 % and more PCI increase and/or ascitis fluid accumulation and/or distant lymphogenic and hematogenic metastases.
Figure 1:
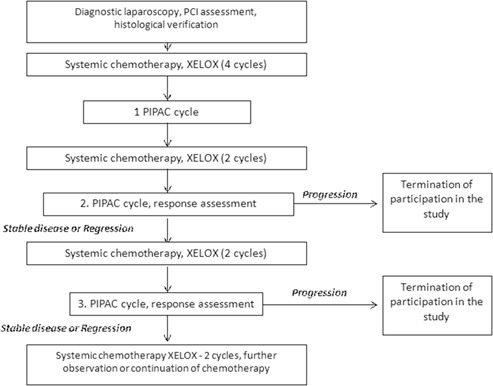
Flow diagram of the study.
Technique of pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Punction of the abdomen in the periomphalic area was followed by insufflation of a normothermic capnoperitoneum (12 mmHg). Two 10 mm-sized trocars were inserted and the abdominal cavity inspected, with the assessment and documentation of peritoneal carcinomatosis index (PCI). Multiple biopsies of the peritoneum were taken at suspect locations (not less than in 3 anatomic zones) to assess the pathological response. Ascites fluid was aspirated and the volume documented. The Capnopen® device (Capnomed GmbH, Villingendorf, Germany) was connected with the high-pressure angiographic injector and the device inserted into one of the trocars. Tightness of the abdominal cavity was verified by zero CO2 flow. Cisplatin was administered at a dose of 7.5 mg/m2 in 150 mL of 0.9 % NaCl solution and doxorubicin at a dose of 1.5 mg/m2 in 50 ml of 0.9 % NaCl solution. The drugs were aerosolized into the abdominal cavity at a rate of 30 ml/min, with an upstream pressure of 200 psi and an intraperitoneal pressure of 12 mmHg. Drug administration was controlled by video monitoring. The therapeutic capnoperitoneum was maintained in steady state for 30 minutes at 37 °C. The procedure was performed in an operating room equipped with laminar flow ventilation system and remote-controlled. At the end of the procedure, the aerosol was evacuated by a closed aerosol waste system (CAWS) via two successive microporous filters. Trocars were removed and the puncture sites sutured. The abdominal cavity was not drained.
Inclusion criteria
Verified diagnosis of PMGC; age between 18 and 85 years; good performance status (ECOG 1 or 2).
Exclusion criteria
Patients were ineligible, if they had extraabdominal metastatic disease (not including retroperitoneal disease such as aortic/paraaortic lymph node), underwent chemotherapy or surgery within the last four weeks prior to study enrolment or a previous treatment with the maximum cumulative dose of anthracyclines and anthracenediones; history of allergic reactions to cisplatin or other platinum containing compounds or doxorubicin; severe renal impairment or severe hepatic impairment with organ-specific functional parameters; history of myocardial insufficiency not controlled by concurrent medication, severe cardiac arrhythmia not controlled by concurrent medication, or recent myocardial infarction or myelosuppression; immunocompromised status such as immunosuppressive therapy or a known disease of the immune system; any form of previous intraabdominal chemotherapy or intraabdominal antibody therapy; pregnancy.
Safety assessment
Safety was evaluated on postoperative days 1 and 3 through physical examination and blood collection for routine laboratory tests. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The severity of CTCAE was judged by research team during the patients’ Hospital stay including the admission for next PIPAC. Adverse events were recorded until postoperative day 30 after each PIPAC application.
Therapy response assessment
Laparoscopy and biopsies were carried out at the end of the first 4 courses of systemic chemotherapy with XELOX and later at the beginning each PIPAC procedure. Initial and follow-up examinations included upper gastrointestinal endoscopy, thoracic and abdominal spiral CT, tumor markers (CEA, CA-72-4, CA-125) and routine laboratory checks (CBC, blood chemistry, urinalysis). PIPAC efficacy was evaluated based on two criteria: PCI value and tumor regression grading according to the Peritoneal Regression Grading Score (PRGS) [18].
Statistics
Analysis was by intention to treat. Analysis was performed using nonparametric tests since data were not normally distributed. Survival was modeled in a Kaplan-Meier survival curve. We used the statistical software StatSoft: Statistica 7.0 for statistical analysis.
Results
Thirty-one patients with PMGC were accrued and enrolled between August 2013 and June 2016. Data were locked by July 31st, 2016. All 31 patients received at least one cycle of the study medication and are thus included in the safety and efficacy analyses (intention to treat). Patient characteristics are summarized in Table 1. All patients had PMGC proven by histology, verified in 20 patients by diagnostic laparoscopy, and in 11 by exploratory laparotomy performed in the referring hospital. Laparoscopic non-access rate was zero, because all patients have underwent laparoscopy or laparotomy before inclusion in the study.
Table 1:
Characteristics of 31 patients with PC from gastric origin undergoing pressurized intraperitoneal aerosol chemotherapy (PIPAC).
| Variable | Value | Percentage |
| Number of patients | 31 | |
| Sex (M:F) | 9:22 | 29 %:71 % |
| Mean age, years (min-max) | 52 (25–70) | |
| Histology (Lauren classification) | ||
| Diffuse/signet ring | 30 | 97 % |
| Intestinal | 1 | 3 % |
| Peritoneal Carcinomatosis Index (PCI), (mean min-max) | 16 (6–34) | |
| Peritoneal metastasis | ||
| Synchronous | 7 | 23 % |
| Metachronous | 24 | 77 % |
| Chemotherapy | ||
| Previous | 7 | 23 % |
| Synchronous | 31 | 100 % |
| PIPAC sessions, n | ||
| 1 | 16 | 52 % |
| 2 | 7 | 23 % |
| 3 | 6 | 19 % |
| 4 | 2 | 6 % |
| Histological tumor response (15 patients eligible) | ||
| Complete response (PRGS1) | 4/15 | 27 % |
| Partial response (PRGS2) | 5/15 | 33 % |
| No response (PRGS 3 and 4) | 6/15 | 40 % |
| Median survival (days) | 390 |
PRGS, Peritoneal Regression Grading Score.
Mean Peritoneal Carcinomatosis Index was 16 (min 6, max 34). PCI was low (≤9) in 7 patients (22.6 %), moderate (PCI 10–20) in 16 patients (51.6 %), and high (PCI > 20) in 8 patients (25.8 %). Diffuse cancer (signet-ring carcinoma according to Lauren classification) prevailed in the histology with 30/31 patients, intestinal cancer was diagnosed in the last patient. Twelve patients (38.7 %) had malignant ascites. Five patients (16.1 %) had decompensated tumor stenosis requiring endoscopic stenting.
Altogether, 56 PIPAC procedures were performed in the 31 patients included in this prospective study. By July 31st, 2016, a single PIPAC procedure (reasons for not undergoing more than one PIPAC are presented in Table 2) had been performed in 16 patients (51.6 %), 2 procedures in 7 patients (22.5 %), 3 procedures in 6 patients (19.4 %), and 4 procedures in 2 patients (6.5 %). At the time of the analysis, 8 of 31 patients are still included in the study, waiting for further PIPAC procedures.
Table 2:
Reason for not undergoing more than one PIPAC.
| Case number | Reason for not undergoing more than one PIPAC |
| 1 | Progression of the Carcinomatosis |
| 2 | Progression of the Carcinomatosis |
| 3 | Progression of the Carcinomatosis |
| 4 | Progression of the Carcinomatosis |
| 5 | Progression of the Carcinomatosis |
| 6 | Progression of the Carcinomatosis |
| 7 | Progression of the Carcinomatosis |
| 8 | Progression of the Carcinomatosis |
| 9 | Patient is waiting for the next PIPAC |
| 10 | Patient is waiting for the next PIPAC |
| 11 | Patient is waiting for the next PIPAC |
| 12 | Patient is waiting for the next PIPAC |
| 13 | Patient is waiting for the next PIPAC |
| 14 | Patient was lost to follow-up |
| 15 | Patient was lost to follow-up |
| 16 | Patient was lost to follow-up |
The average hospital stay was 3 days. There was no hospital death (CTCAE 5) and no major complication (CTCAE 4). The reasons for study termination (11 patients) were progression of disease (8 patients) and 3 patients were lost for follow up. Four adverse events were reported in 56 procedures. In one patient, peritoneal biopsy was complicated by diaphragmatic perforation with development of capnothorax requiring drainage (CTCAE 3, 3.2 %). Three (9.7 %) patients reported about postoperative nausea during the first 2 days after PIPAC (CTCAE 2, 6.4 %).
Pathological response was evaluated in 15 patients eligible for response assessment who had received at least two PIPAC procedures and re-biopsy (Figure 2). Four patients (27 %) showed a complete pathologic response (PRGS 1, absence of cancer cells on the fibrous tissue background), 5 patients (33 %) showed major response (PRGS 2, individual cancer cells with marked degenerative changes on the fibrous tissue background), and no significant regression was observed in 6 patients (40 %, PRGS 3 and 4).
Figure 2:
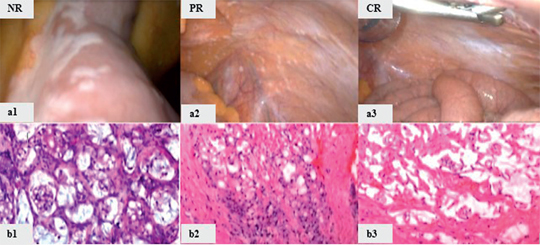
Pathological response after PIPAC.
NR – no response (a1,b1): histology shows vital tumor (PRGS 3 and 4). PR – рartial response (a2, b2): inflammatory reaction with nodular sclerosis (PRGS 2). CR – сomplete response (a3, b3): large areas of devitalized tumor (PRGS 1).
Median survival was 13 months. Survival statistics showed an overall one-year survival of 49.8 % (Figure 3). Fourteen patients died in the course of disease. By July 31st, 2016, 17 patients are alive and continue to participate in this open-label study.
Figure 3:
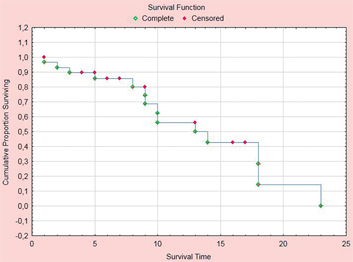
Overall survival of 31 consecutive patients treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin combined with systemic XELOX therapy.
Х-axis: survival in months; У-axis: cumulative survival.
Discussion
Our study shows that PIPAC with intraperitoneal cisplatin and doxorubicin combined with palliative systemic chemotherapy with XELOX is active in patients with PMGC. Histological tumor response was seen in 60 % of patients. In general, therapy was well tolerated. Hospital mortality was zero. There were no serious adverse events (CTCAE > 3).
The upper gastrointestinal unit of the Moscow Oncology Institute was the second center worldwide and the first center in Russia to apply PIPAC in human patients. Our data are important because they are the first to confirm independently previous experience obtained with PIPAC in PMGC. Specifically, the objective histological regression rate observed in our patients (60 % major or complete regression after combination chemotherapy) is somewhat superior to the rate (50 %) reported in a retrospective case series of 24 PMGC patients treated by PIPAC alone [16].
The diagnosis of PMGC is linked with an unfavorable prognosis, with a median reported survival of 3–7 months, and with a virtually zero 5-year survival. PMGC are observed in 14–43 % of the patients with primary GC and constitute 35 % of all synchronous metastases in GC [19]. Until shortly, PMGC was considered a terminal stage of disease due to the low life expectancy and poor response to any kind of therapy. The development of locoregional treatments, including cytoreductive surgery, HIPEC and various options of intraperitoneal chemotherapy is indeed in the process of improving the outcome of PMGC. However, the effectiveness of these methods in PMGC with high PCI and mucinous histology (signet-ring cancer) is still modest. Moreover, cytoreductive surgery combined with HIPEC is an aggressive procedure with significant morbidity and the benefit/risk relationship of this combined therapy in patients with a short life expectancy might be questioned.
The dose of cisplatin and doxorubicin used in PIPAC is only 10 % of the dose applied in HIPEC, which virtually eliminates the development of systemic toxicity. Moreover, since the systemic AUC of doxorubicin is only 1 % of the AUC after intravenous administration, PIPAC can be in principle combined with systemic chemotherapy [20]. Previous studies showed that intraperitoneal application of doxorubicin as PIPAC can reach high drug concentration in tumor nodes and that the drug penetrates in the nuclei of cells located in the submesothelial fat tissue [21]. From the methodological perspective, PIPAC is attractive for designing clinical trials since repeated diagnostic laparoscopy prior to each PIPAC procedure allows objective evaluation of the treatment effectiveness, including the pathologic response. The diagnostic role of laparoscopy appears decisive since CT is not sensitive enough to detect low-volumetric disease in PMGC [22].
The treatment protocol of this study evaluates for the first time the combination of PIPAC with systemic chemotherapy in patients with advanced GC and PM. In these patients with short life expectancy, PIPAC combined with systemic chemotherapy is a simple, minimally invasive, and safe method of palliative treatment. The low incidence of complications and the lack of systemic toxicity allow the use of this method in debilitated patients with low performance status. We have not detected any serious adverse events during and after the PIPAC procedure. In one patient, there was a microperforation of the diaphragm during the peritoneal biopsy. The diaphragm was sutured, the pleural cavity was drained and the patient recovered well. In the future, this complication can be easily prevented by preferring other sites of biopsy.
To date, the follow-up period available ranges from 2 to 18 months. Median overall survival was 13 months, that is higher than historical data in this clinical setting (3–7 months) according to the Russian and foreign literature [6]. Although a selection bias cannot be excluded, the study group included mainly patients with a high PCI, signet-ring histology and/or ascites, all factors being known as linked to a poor prognosis.
As an experimental technique, PIPAC has been used in patients who are quite ill and have already failed multiple treatment regimes, but it may not be limited to that group of patients in the future [23]. Our treatment protocol involves the use of PIPAC combined with systemic chemotherapy as first-line therapy in advanced GC patients with PM, which is a unique experience for this method of treatment. Overall survival appears encouraging with 13 months). This calculated survival might even increase since 17/31 patients are still alive and continue to participate to the study.
Conclusions
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is easy to use, minimally invasive, and an harmless palliative treatment in GC patients with widespread PM. In PMGC, PIPAC combined with systemic XELOX chemotherapy as a first-line treatment induced objective histological regression in 60 % of patients, and overall median overall survival appears encouraging with 13 months. These preliminary results suggest a potential of PIPAC as a method for the palliative treatment of patients with PMGC, at least when cytoreduction and HIPEC is not indicated. A randomized trial is now needed to compare the efficacy and safety of PIPAC with low-dose Cisplatin and Doxorubicin combined with systemic chemotherapy with XELOX vs. chemotherapy alone in PMGC. Moreover, PIPAC is a generic technique with attractive methodological properties for further research, including the optimization of the aerosol formation, dose-finding studies, and the use of new drug combinations.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Vladimir Khomyakov, Email: vladimirkhom@mail.ru.
Andrey Ivanov, Email: ivanov.av.mnioi@gmail.com.
References
- 1.Kaprin A, Starinskii V, Petrova G. Current state of medical service for oncological diseases in Russia in 2014. 2016:62.
- 2.Kaprin A, Sobolev D, Khomiakov V. Experience with hyperthermic intraoperative intraperitoneal chemotherapy in the treatment of locally advanced gastric cancer (Cyt+). Onkologiya Zhurnal imeni PA Gerzena 2015;1:67–70.
- 3.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453–62. [DOI] [PubMed]
- 4.Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg 2014;219:664–75. [DOI] [PubMed]
- 5.Roviello F, Marrelli D, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003;90:1113–19. [DOI] [PubMed]
- 6.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622–8. [DOI] [PubMed]
- 7.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358–63. [DOI] [PubMed]
- 8.Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242–6. [DOI] [PubMed]
- 9.Mori T, Fujiwara Y, Sugita Y, Azama T, Ishii T, Taniguchi K, et al. Application of molecular diagnosis for detection of peritoneal micrometastasis and evaluation of preoperative chemotherapy in advanced gastric carcinoma. Ann Surg Oncol 2004;11:14–20. [DOI] [PubMed]
- 10.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63–8. [DOI] [PubMed]
- 11.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608–18. [DOI] [PubMed]
- 12.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237–42. [DOI] [PubMed]
- 13.Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol 2011;104:692–8. [DOI] [PubMed]
- 14.Solass W, Kerb R, Murdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9. [DOI] [PMC free article] [PubMed]
- 15.Dakwar GR, Shariati M, Willaert W, Ceelen W, De Smedt SC, Remaut K. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis - Mission possible? [Review). Adv Drug Deliv Rev 2016 Jul 13;pii: S0169-409X(16)30212-5. doi: 10.1016/j.addr.2016.07.001. [Epub ahead of print]. [DOI] [PubMed]
- 16.Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016;20:367–73. [DOI] [PMC free article] [PubMed]
- 17.ESMO Meeting 2016. Ann Oncol 2016;27;ii51. doi: 10.1093/annonc/mdw199.166. [DOI]
- 18.Solass W, Sempoux C, Carr NY, Detlefsen S, Bibau F. Peritoneal sampling procedures and histological assessment of therapeutic response: Proposal of the Peritoneal Regression Grading Score (PRGS. Pleura Peritoneum 2016;1:99–107. [DOI] [PMC free article] [PubMed]
- 19.Abbasi SY, Taani HE, Saad A, Badheeb A, Addasi A. Advanced gastric cancer in Jordan from 2004 to 2008: a study of epidemiology and outcomes. Gastrointest Cancer Res 2011;4:122–7. [PMC free article] [PubMed]
- 20.Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol 2016 Apr;29:128. [DOI] [PMC free article] [PubMed]
- 21.Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J, et al. Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol 2014;132:307–11. [DOI] [PubMed]
- 22.Kwee RM, Kwee TC. Modern imaging techniques for preoperative detection of distant metastases in gastric cancer [Review). World J Gastroenterol 2015 Oct 7;21:10502–9. doi: 10.3748/wjg.v21.i37.10502. [DOI] [PMC free article] [PubMed]
- 23.Technology Brief. Health Policy Advisory Committee on Technology. Queensland, Australia. Pressurized IntraPeritoneal Aerosol Chemotherapy. July 2015. https://www.health.qld.gov.au/healthpact/docs/br


