Abstract
Background and Purpose:
Candida species are the common opportunistic pathogens during the course of human immunodeficiency virus (HIV) infection. Oropharyngeal candidiasis (OPC) is generally known as the initial sign of HIV infection. The aim of this study was to compare demographic characteristics and frequency of Candida species between HIV/AIDS patients and non-HIV subjects in Kerman, southeast of Iran.
Materials and Methods:
This study was conducted on 143 samples collected from the oral cavity of 81 HIV/AIDS patients and 35 non-HIV subjects. The samples were cultured on Sabouraud dextrose agar and CHROMagar. The identification of Candida species was accomplished using the color of colony and polymerase chain reaction-restriction fragment length polymorphism.
Results:
According to the results, C. albicans (n=25, 69.14%) was the most prevalent species isolated from the HIV/AIDS patients, followed by C. glabrata (n=19, 23.46%). Other isolated species included C. parapsilosis (n=4, 4.94 %), C. krusei (n=1, 1.24%), and C. kefyr (n=1, 1.24%). Out of the 35 Candida species recovered from the oral samples of non-HIV subjects, 23 (65.71%) and 12 (34.29%) cases were C. krusei and C. albicans, respectively. Candida krusei was the only non-albicans species found in the non-HIV subjects that was also the predominant isolated species. Regarding the HIV/AIDS patients, the highest prevalence of OPC was observed in the age group of 41-50 years. However, in the non-HIV subjects, the age group of 31-40 years had the highest prevalence of this infection. Furthermore, no correlation was observed between the gender and number of Candida isolates.
Conclusion:
Consideration of the epidemiologic data showed that the two groups were significantly different in terms of the prevalence of Candida species, which could play a major role in the selection of effective drugs for the treatment of candidiasis.
Key Words: Candida species, HIV infection, Iran, Kerman, Oral cavity
Introduction
Acquired immunodeficiency syndrome (AIDS) is an important dreadful disease in humans. Human immunodeficiency virus (HIV) is an agent, which may cause an infection. AIDS is the advanced condition of HIV infection that could take 10-15 years to develop. HIV is a chronic disease that can be managed, but not treated (1). AIDS weakens the immune system and makes the affected individuals susceptible to a number of conditions, such as renal and cardiovascular diseases, cancer, metabolic bone disease, lipodystrophy, vitamin D deficiency, a variety of opportunistic infections (e.g., viral, parasitic, fungal, and bacterial infections), and malignancies. These opportunistic diseases enhance the risk of mortality and particularly decrease the quality of life and life expectancy (2, 3).
Candidiasis is a prevalent opportunistic infection in HIV/AIDS patients. This infection is caused by various Candida species, especially C. albicans, in the mouth, throat, and esophagus (4-6). Oropharyngeal candidiasis (OPC) is generally known as the initial manifestation of HIV infection. This condition usually develops in HIV-infected patients when the CD4+ T-lymphocyte count declines to > 350 CFU/ml. In the HIV patients with CD4 cell counts of ≤ 200 CFU/ml, thrush diffuses to the esophagus, thereby turning the oral candidiasis to esophageal candidiasis (7).
Various types of OPC manifestations include pseudomembranous (thrush), erythematous, hyperplastic, and angular cheilitis, as well as mucocutaneous candidiasis (8, 9). The patterns of OPC in HIV-positive patients include pseudomembranous candidiasis, erythematous candidiasis, angular cheilitis, linear gingival erythema, ulcerations, oral hairy leukoplakia, and salivary gland swellings (10).
The OPC is generally treated with antifungal agents depending on the severity of the infection. In this regard, the patients with mild to moderate OPC infections are usually managed with the oral administration of miconazole, clotrimazole, or nystatin for 1-2 weeks. Furthermore, the severe infection cases are generally prescribed fluconazole or another type of antifungal drug (11). With this background in mind, the present study was conducted to compare demographic characteristics, strains, and prevalence of Candida species between HIV-infected patients and non-HIV subjects in Kerman, southeast of Iran.
Materials and Methods
Subjects and sample collection
This study was conducted on 81 HIV/AIDS patients and 35 non-HIV subjects from March 2017 to April 2018 (i.e., over 14 months) in Kerman. The HIV/AIDS cases were selected out of the patients referring to the Kerman Counseling Resource Center for Behavioral Disorders for receiving periodic examinations and antiviral drugs or solving various health problems.
After making coordination with the Health Department of Kerman University of Medical Sciences, oral samples were collected from the patients. The investigated patients had not received prophylactic antifungal medications. They were checked for OPC signs and symptoms, including erythema with or without soreness or burning sensation, dysphagia, white patches, redness and soreness at the corners of the mouth.
The non-HIV subjects were chosen out of the individuals referring to the Medical Mycology Laboratory of Afzalipoor Faculty of Medicine in Kerman. Samples were obtained from the oral cavity of the HIV-negative subjects that did not display any clinical signs or symptoms of OPC. Informed consent was obtained from all participants. They also filled out a structured questionnaire addressing demographic factors (e.g., age, gender, and personal health status) and duration HIV/AIDS.
The samples were collected by scraping the subject’s oral mucosa and tongue with a sterile swab. All oral swabs were transported to the Mycology Research Laboratory without any delay and prepared for laboratory investigations on the same day. This study was approved by the Ethics Committee of Kerman University of Medical Sciences, Kerman (IR.KMU.REC.1395.146).
Direct microscopic examination, staining, and culturing
All the samples were examined in 10% potassium hydroxide (Merck, Germany). A portion of the samples were inoculated onto CHROMagar Candida medium (HiMedia, Mumbai, India) and Sabouraud dextrose agar (SDA, Merck, Germany), and then incubated at 37°C for at least 1 week.
DNA extraction and molecular identification of different Candida species using polymerase chain reaction-restriction fragment length polymorphism
In the present study, the identification of Candida species was accomplished through polymerase chain reaction-restriction fragment length polymorphism (PCR- RFLP) using a specific restriction enzyme, namely Msp I (Fermentas Life Sciences, Lithuania). Briefly, DNA was extracted from each of the Candida cultures obtained from HIV-infected and non-HIV subjects using Exgene Tissue SV Plus-mini kit (Gene All, General Bio System, South Korea) according to the manufacturer's instruction.
The PCR amplification was performed using the universal fungal primers (i.e., ITS1: 5-TCC-GTAGGTGAA-CCT-GCG-G-3 and ITS4: 5-TCC-TCC-GCT-TAT-TGA-TATGC30; Pishgam Co, Tehran, Iran). Then, RFLP was implemented following the manufacturer's instructions using Msp I for the identification of different Candida species (12, 13). Finally, after the electrophoresis, Candida species were detected based on the size and number of the obtained bands (14).
Statistical analysis
The data were expressed as frequencies and percentages. Data analysis was performed in SPSS software, version 21 (SPSS, Inc. Chicago, Illinois) using Student’s paired t-test.
Results
Demographic characteristic
A total of 116 subjects, including 81 HIV/AIDS patients and 35 non-HIV subjects, were enrolled in this study. Table 1 presents the demographic characteristics of HIV-infected and non-HIV subjects associated with OPC. The mean ages of the HIV/AIDS and non-HIV subjects were 40.8±11.14 and 40.97±10.2 years, respectively. The results revealed no significant difference between the two groups in terms of age.
Table 1.
Demographic characteristics of HIV/AIDS patients and non-HIV subjects
| Characteristics | Classification |
HIV/AIDS patients (n=81)
|
non-HIV subjects (n=35)
|
||
|---|---|---|---|---|---|
| Number (%) | 95% CI | Number (%) | 95% CI | ||
| Age groups | 0-10 | 1 (1.235) | 0.06177-6.089 | 0 (0.0) | - |
| 11-20 | 4 (4.938 ) | 1.569-11.91 | 0 (0.0) | - | |
| 21-30 | 5 (6.173) | 2.262-13.68 | 6 (17.14) | 8.1-32.68 | |
| 31-40 | 20 (24.69) | 16.59-35.08 | 13 (37.14) | 23.16-53.66 | |
| 41-50 | 38 (46.91) | 36.43-57.67 | 10 (28.57) | 16.33-45.05 | |
| 51-60 | 11 (13.58) | 7.76-22.7 | 6 (17.14) | 8.1-32.68 | |
| 61-70 | 2 (2.47) | 0.68-8.56 | 0 (0.0) | - | |
| Gender | Male | 29 (35.8) | 53.34-73.78 | 27 (77.14) | 60.98-87.93 |
| Female | 52 (64.2) | 26.22-46.66 | 8 (22.86) | 12.07-39.02 | |
| Duration of HIV/AIDS (years) | 0-1 | 6 (7.41) | 3.44-15.24 | 0 (0.0) | |
| 1-5 | 32 (39.51) | 29.57-50.4 | |||
| 5-10 | 22 (27.16) | 18.67-37.71 | |||
| 10-15 | 15 (18.52) | 11.56-28.33 | |||
| ≥ 15 | 6 (7.41) | 3.44-15.24 | |||
Prevalence of Candida species in HIV-infected and non-HIV subjects
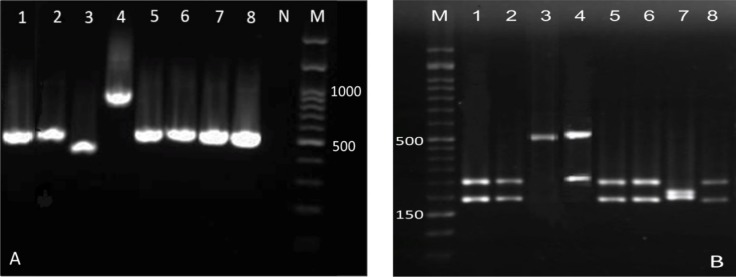
Figure 1 displays the patterns of ITS-RFLP for Candida species before and after digestion with Msp I in HIV/AIDS patients. A total of 81 Candida isolates were obtained from the oral samples of HIV/AIDS patients. Figure 1 also depicts the prevalence of different Candida species among HIV-infected and non-HIV subjects. The analysis led to the identification of five different species, the most common of which was C. albicans.
Figure 1.
Patterns of polymerase chain reaction products of Candida isolates before (A) and after (B) digestion with Msp I in HIV/AIDS patients; in figure B: lanes 1, 2, 5, 6, and 8: C. albicans (297 and 238 bp), lane 3: C. parapsilosis (520 bp), lane 4: C. glabrata (557 and 341 bp), lane 7: C. krusei (261 and 249 bp), and lane M: 50 bp ladder
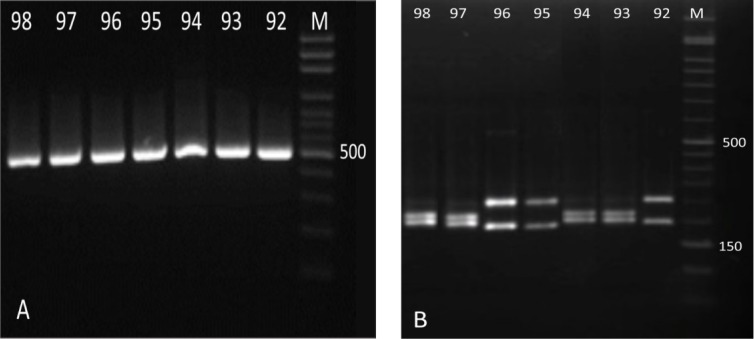
Figure 2 illustrates the patterns of ITS-RFLP for Candida species before and after digestion with Msp I in non-HIV subjects. Candida krusei was the only non-albicans species found in non-HIV subjects and identified as the predominant isolated species. Nevertheless, no C. glabrata, C. parapsilosis, or C. kefyr were detected in these subjects (Figure 2). There was a statistically significant difference between the HIV/AIDS patients and non-HIV subjects in terms of prevalence of identified Candida species (Figure 3).
Figure 2.
Pattern of polymerase chain reaction products of Candida isolates before (A) and after (B) digestion with Msp I in non-HIV subjects; in figure B: lanes 92, 95, and 96: C. albicans (297 and 238 bp), lanes 93, 94, 97, and 98: C. krusei (261 and 249 bp), lane M: 50 bp ladder
Figure 3.
Prevalence of Candida species in HIV/AIDS patients and non-HIV subjects
Table 2 shows the frequency of the Candida species recovered from oral candidiasis in HIV/AIDS patients. Based on the results, the frequency of the Candida species was significantly higher in females compared with that in males (64.2% vs. 35.8%). However, in non-HIV subjects with OPC, the prevalence of Candida species was four times higher in men than in women (80% vs. 20%). No correlation was observed between gender and number of Candida species (Table 2).
Table 2.
Distribution of Candida species in HIV/AIDS patients and non-HIV subjects with oropharyngeal candidiasis based on gender
| Candida species |
Male n (%)
|
Female n (%)
|
Total n (%)
|
95% CI
|
P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| HIV/AIDS patients | non-HIV subjects | HIV/AIDS patients | non-HIV subjects | HIV/AIDS patients | non-HIV subjects | HIV/AIDS patients | non-HIV subjects | ||
| C. albicans | 21 (25.93) | 10 (28.57) | 35 (43.21) | 2 (5.7) | 56 (69.14) | 12 (34.29) | 58.41-7814 | 20.84-50.85 | 0.11 |
| Total | 29 (35.8) | 28 (80) | 52 (64.2) | 7 (20) | 81 (100) | 35 (100) | 100 | 100 | - |
| C. krusei | 0 (0.0) | 18 (51.43) | 1 (1.235) | 5 (14.29) | 1 (1.235) | 23 (65.71) | 0.062-6.089 | 49.15-79.16 | 0.23 |
| C. parapsilosis | 2 (2.47) | 0 (0.0) | 2 (2.47) | 0 (0.0) | 4 (4.938 ) | 0 (0.0) | 1.569-11.91 | - | 0.18 |
| C. glabrata | 5 (6.173) | 0 (0.0) | 14 (17.28) | 0 (0.0) | 19 (23.46) | 0 (0.0) | 15.57-33.76 | - | 0.16 |
| C. kefyr | 1 (1.235) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.235) | 0 (0.0) | 0.062-6.089 | - | 0.42 |
Discussion
The aim of this study was to compare demographic characteristics, strains, and prevalence of Candida species in HIV-infected and non-HIV subjects in Kerman. In Kerman province, 729 cases of AIDS have been identified, 90% of whom are male. About 75% of these patients had been infected via injecting drug, while 19% of them had a history of high-risk sexual activity, and 2% of them had been infected through the mother-to-child transmission.
The prevalence of HIV/AIDS among women has undergone an increase in recent years. In the present study, OPC was more prevalent in females than in males. As our results indicated, the age group of 41-50 years had the maximum Candida species frequency (n=38, 46.91%), rendering a male to female ratio of 1.79:1. Maheshwari et al. (3) reported that the age group of 21-45 years had the highest prevalence of OPC with a male to female ratio of 2.2:1. They suggested that some factors, such as occupation and higher rate of migration, predispose the males to such infection, compared to females.
The OPC has been reported to be prevalent among the HIV/AIDS patients in the age group of 29-39 years (15). Similar to our results, Lar et al. (16) and Awoyeni et al. (15) reported a higher rate of OPC in women than in men. However, unlike our findings, in another study, HIV patients with the age group of 61-70 years had the highest prevalence of OPC. It seems that the high prevalence of OPC in the elderly could be due to their weaker immune system (17).
The difference in OPC prevalence between genders could be related to their lack of awareness about the risk factors for virus transmission from sexual partner, drug injection, or non-use of condom. The weakening of the immune system by the virus sets the condition for the growth of Candida species, especially OPC development. The males reportedly show less tendency to refer to the special health centers for undergoing HIV test, counseling, and routine examinations until the disease becomes symptomatic (15).
Oral carriage of Candida species (62-67%) is prevalent in HIV/AIDS patients (2). Our results showed that 69.14% of the oral samples obtained from these patients were C. albicans and 30.86% of the cases belonged to non-Candida albicans Candida (NCAC) species. These findings are in agreement with the those obtained by Katiraee et al. (18), Li et al. (19), and Mousavi et al. (20). Nonetheless, Kwamin,s et al. (21) and Maheshwari et al. (3) reported lower isolation rates of C. albicans in HIV/AIDS patients than our study.
In line with our research, in a study performed in India, 61.7% of the Candida isolates obtained from HIV/AIDS patients were C. albicans. However, in the mentioned study, the frequency and diversity of NCAC species were different from our results. In contrast to the present study, the common NCAC species were C. guilliermondii (n=14) and C. parapsilosis (n=9) (4). Nonetheless, no cases of C. guilliermondii, C. tropicalis, C. dubliniensis, or C. famata were found in the current study.
The frequency of Candida carriage among the non-HIV subjects was obtained as 40%. Four different Candida species were identified in this group, the most common of which were C. albicans, followed by C. tropicalis (22). Consistent with our results, in Yitayew,s study, HIV/AIDS patients were reported to have a significantly higher frequency of oral Candida species carriage as compared to non-HIV subjects (23). Here, the most common Candida species recovered from non-HIV subjects was C. krusei, followed by C. albicans.
The discrepancy in the reported frequency and diversity of identified Candida species could be related to the difference in sampling methods, sample size, demographic and clinical characteristics of studied population, such as difference in T CD4 count, high-risk behaviors (e.g., high-risk sexual behaviors, improper diet, and smoking), oral hygiene, lifestyle, intravenous drug abuse, use of highly active antiretroviral therapy, having dentures, antifungal therapy, immune system, and geographical locations (19, 23, 24).
One of the limitations of the present study was the non-cooperation of the affected people for sampling or filling the questionnaire. Furthermore, the size of the two studied groups was unequal. Another limitation was the incapability of PCR-RFLP to discriminate some species, such as C. parapsilosis complex and more importantly C. dubliniensis, which is a common identified species in patients with HIV/AIDS.
Conclusion
The OPC is known as a major opportunistic infection among HIV/AIDS patients. This study compared the demographic characteristics, strains, and prevalence of Candida species in HIV-infected and non-HIV subjects in Kerman. Antiretroviral and antifungal therapies could change the frequency and distribution of Candida species.
Acknowledgments
The present study was approved by the Ethics Committee of Kerman University of Medical Sciences. The authors wish to thank the financial support of the Deputy of Research and Technology affiliated to the Kerman University of Medical Sciences.
Author’s contribution
S. S. contributed with study design and data collection, A. H. and P. GH. A. aided in data collection and performed data analysis, and S. S. wrote the manuscript.
Conflicts of interest
No conflicts of interest have been declared by the authors.
Financial disclosure
This study was supported by the Research Deputy of Kerman University of Medical Sciences (Grant No: 950070).
References
- 1.Soumya D, Hima Bindu A. Opportunistic diseases as a consequence of HIV/AIDS. J AIDS Clin Res. 2011;2(6) [Google Scholar]
- 2.Ribeiro AL, de Alencar Menezes TO, de Melo Alves-Junior S, de Menezes SA, Marques-da-Silva SH, Vallinoto AC. Oral carriage of Candida species in HIV-infected patients during highly active antiretroviral therapy (HAART) in Belém, Brazil. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(1):29–33. doi: 10.1016/j.oooo.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Maheshwari M, Kaur R, Chadha S. Candida species prevalence profile in HIV seropositive patients from a major tertiary care hospital in New Delhi, India. J Pathog. 2016:2016:6204804. doi: 10.1155/2016/6204804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anwar KP, Malik A, Subhan KH. Profile of candidiasis in HIV infected patients. Iran J Microbiol. 2012;4(4):204–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Patel PK, Erlandsen JE, Kirkpatrick WR, Berg DK, Westbrook SD, Louden C, et al. The changing epidemiology of oropharyngeal candidiasis in patients with HIV/AIDS in the era of antiretroviral therapy. AIDS Res Treat. 2012:2012:262471. doi: 10.1155/2012/262471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salari S, Khosravi AR, Mousavi SA, Nikbakht-Brojeni GH. Mechanisms of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV-infected patients with oropharyngeal candidiasis. J Mycol Med. 2016;26(1):35–41. doi: 10.1016/j.mycmed.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Gaitán-Cepeda LA, Sánchez-Vargas O, Castillo N. Prevalence of oral candidiasis in HIV/AIDS children in highly active antiretroviral therapy era A literature analysis. Int J STD AIDS. 2015;26(9):625–32. doi: 10.1177/0956462414548906. [DOI] [PubMed] [Google Scholar]
- 8.Moris DV, Melhem MS, Martins MA, Mendes RP. Oral Candida spp colonization in human immunodeficiency virus-infected individuals. J Venom Animals Toxins Includ Trop Dis. 2008;14(2):224–57. [Google Scholar]
- 9.Berenji F, Zabolinejad N, Badiei Z, Kakhi S, Andalib Aliabadi Z, Ganjbakhsh M. Oropharyngeal candidiasis in children with lymphohematopoietic malignancies in Mashhad, Iran. Curr Med Mycol. 2015;1(4):33–6. doi: 10.18869/acadpub.cmm.1.4.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adedigba MA, Ogunbodede EO, Jeboda SO, Naidoo S. Patterns of oral manifestation of HIV/AIDS among 225 Nigerian patients. Oral Dis. 2008;14(4):341–6. doi: 10.1111/j.1601-0825.2007.01384.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Cuesta C, Sarrion-Pérez MG, Bagán JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. 2014;6(5):576–82. doi: 10.4317/jced.51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salari S, Ayatollahi Mousavi SA, Hadizadeh S, Izadi A. Epidemiology of dermatomycoses in Kerman province, southeast Iran: a 10-years retrospective study (2004–2014) Microb Pathog. 2017;110:561–7. doi: 10.1016/j.micpath.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 13.Salari S, Bakhshi T, Sharififar F, Naseri A, Ghasemi Nejad Almani P. Evaluation of antifungal activity of standardized extract of Salvia rhytidea Benth (Lamiaceae) against various Candida isolates. J Mycol Med. 2016;26(4):323–30. doi: 10.1016/j.mycmed.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Bakhshi T, Salari S, Naseri A, Esfandiarpour I, Mohammadi M, Almani PG. Molecular identification of Candida species in patients with candidiasis in Birjand, Iran, using polymerase Chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. J Isfahan Med Sch. 2016;33(359):1986–93. [Google Scholar]
- 15.Awoyeni A, Olaniran O, Odetoyin B, Hassan-Olajokun R, Olopade B, Afolayan D, et al. Isolation and evaluation of Candida species and their association with CD4+ T cells counts in HIV patients with diarrhoea. Afr Health Sci. 2017;17(2):322–9. doi: 10.4314/ahs.v17i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lar PM, Pam KV, Tiri Y, Shola O, Agabi Y, Dashen M, et al. Prevalence and distribution of Candida species in HIV Infected persons on antiretroviral therapy in Jos. J Med Med Sci. 2012;3(4):254–9. [Google Scholar]
- 17.Esebelahie NO, Enweani IB, Omoregie R. Candida colonisation in asymptomatic HIV patients attending a tertiary hospital in Benin City, Nigeria. Libyan J Med. 2013;8(1):20322. doi: 10.3402/ljm.v8i0.20322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katiraee F, Teifoori F, Soltani M. Emergence of azole-resistant Candida species in AIDS patients with oropharyngeal candidiasis in Iran. Curr Med Mycol. 2015;1(3):11–6. doi: 10.18869/acadpub.cmm.1.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li YY, Chen WY, Li X, Li HB, Li HQ, Wang L, et al. Asymptomatic oral yeast carriage and antifungal susceptibility profile of HIV-infected patients in Kunming, Yunnan Province of China. BMC Infect Dis. 2013;13(1) doi: 10.1186/1471-2334-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayatollahi MS, Samira S, Sasan R, Naser Shahabi N, Sanaz H, Hossein K, et al. Identification of Candida species isolated from oral colonization in Iranian HIV-positive patients, by PCR-RFLP method. Jundishapur J Microbiol. 2012;5(1):336–40. [Google Scholar]
- 21.Kwamin F, Nartey NO, Codjoe FS, Newman MJ. Distribution of Candida species among HIV-positive patients with oropharyngeal candidiasis in Accra, Ghana. J Infect Dev Ctries. 2013;7(1):41–5. doi: 10.3855/jidc.2442. [DOI] [PubMed] [Google Scholar]
- 22.Jain PA, Veerabhadrudu K, Kulkarni R, Ajantha G, Shubhada C, Amruthkishan U. Comparative study of adherence of oral Candida albicans isolates from HIV sero-positive individuals and HIV sero-negative individuals to human buccal epithelial cells. Indian J Pathol Microbiol. 2010;53(3):513–7. doi: 10.4103/0377-4929.68300. [DOI] [PubMed] [Google Scholar]
- 23.Yitayew B, Woldeamanuel Y, Asrat D. Oral Candida carriage among HIV infected and non--infected individuals in Tikur Anbesa specialized hospital. Addis Ababa Ethiopia. 2015;4(2):1–8. [Google Scholar]
- 24.Katiraee F, Khosravi A, Khalaj V, Hajiabdolbaghi M, Khaksar A, Rasoolinejad M, et al. Oropharyngeal candidiasis and oral yeast colonization in Iranian Human Immunodeficiency Virus positive patients. J Mycol Med. 2010;20(1):8–14. [Google Scholar]