Seroepidemiological studies on the prevalence of antibodies to malaria antigens are primarily conducted on individuals from regions of endemicity. It is therefore difficult to accurately correlate the antibody responses to the timing and number of prior malaria infections.
KEYWORDS: CHMI, CSP, MSP-1, malaria, Plasmodium falciparum, Plasmodium vivax, antibodies, heterologous, homologous
ABSTRACT
Seroepidemiological studies on the prevalence of antibodies to malaria antigens are primarily conducted on individuals from regions of endemicity. It is therefore difficult to accurately correlate the antibody responses to the timing and number of prior malaria infections. This study was undertaken to assess the evolution of antibodies to the dominant surface antigens of Plasmodium vivax and P. falciparum following controlled human malaria infection (CHMI) in malaria-naive individuals. Serum samples from malaria-naive adults, collected before and after CHMI with either P. vivax (n = 18) or P. falciparum (n = 18), were tested for the presence of antibodies to the circumsporozoite protein (CSP) and the 42-kDa fragment of merozoite surface protein 1 (MSP-142) of P. vivax and P. falciparum using an enzyme-linked immunosorbent assay (ELISA). Approximately 1 month following CHMI with either P. vivax or P. falciparum, >60% of subjects seroconverted to homologous CSP and MSP-1. More than 50% of the subjects demonstrated reactivity to heterologous CSP and MSP-142, and a similar proportion of subjects remained seropositive to homologous MSP-142 >5 months after CHMI. Computational analysis provides insight into the presence of cross-reactive responses. The presence of long-lived and heterologous reactivity and its functional significance, if any, need to be taken into account while evaluating malaria exposure in field settings.
INTRODUCTION
Antibodies are a key marker of the adaptive immune response. Due to the ease of their detection, the presence of antigen-specific antibodies is used as a biomarker for exposure to specific pathogens. Seroepidemiological analysis is widely used as an indicator of clinical immunity (1–4) as well as endemicity and transmission intensity (1, 5, 6) of malaria caused by both Plasmodium falciparum and P. vivax. Naturally acquired immunity (NAI) studies are usually performed with the goal of understanding clinical (i.e., antidisease) immunity. The general belief is that NAI is acquired after multiple infections, is species and stage specific (7), and is predominantly directed to the blood stages (8) rather than sporozoites (9).
In addition to studying the presence of antibodies for their clinical relevance, several studies have utilized the presence of antibodies as an indicator of exposure in malaria-naive populations. The majority of serosurveillance analyses on malaria-naive individuals have been performed on travelers and soldiers from Europe following travel to countries where malaria is endemic (10–14). Despite the presence of orthologous antigens, few studies have evaluated the presence of cross-reactive antibodies. Sera from regions with a predominance of one species, but not a total absence of the other species, have been shown to have cross-reactivity to some antigens, such as merozoite surface protein 1 (MSP-1) (15), MSP-5 (16), P. falciparum s48/45 (Pfs48/45) or P. vivax s48/45 (Pvs48/45) (17), and PfCLAG9 (18). Using P. falciparum and P. vivax protein microarrays, King and colleagues (3) observed cross-reactivity using sera from “essentially monoexposed” regions of endemicity. There is a paucity of data on the reactivity of sera from malaria-naive individuals to P. vivax antigens. The few reports evaluating P. vivax reactivity in “nonexposed” subjects have been performed on samples from regions in countries of endemicity that were declared malaria-free but experienced brief outbreaks (19, 20). The presence of antibodies in these subjects was attributed to the recent infection outbreak. Detection of antibodies reactive to P. vivax has been used as a marker of exposure in Duffy-negative individuals in Africa (21).
The present study was undertaken to assess the evolution of antibodies to the dominant surface antigens of sporozoites and merozoites of P. vivax and P. falciparum following primary malaria infection in naive individuals. The goals of this study were to determine if naive individuals with no prior exposure to malaria generate antibodies to homologous preerythrocytic and erythrocytic antigens following a single controlled human malaria infection (CHMI) and whether responses to heterologous antigens are detected. For this purpose, two representative antigens that are the dominant antigens on the surface of sporozoites (circumsporozoite protein [CSP]) and merozoites (MSP), as well as leading vaccine candidates, were selected. The results demonstrate the highly immunogenic nature of sporozoites, as evidenced by the detection of anti-CSP antibodies following a single CHMI. As expected, a high proportion of subjects react to the blood-stage antigen MSP-1, and these responses were detectable several months after exposure. The presence of long-lived and heterologous reactivity may confound the determination of time and species of exposure in field settings.
RESULTS AND DISCUSSION
There are a few reports on the evaluation of immune responses after CHMI in malaria-naive individuals. Using different methodologies, these studies looked at responses to PfEMP1 (22), PvCSP, (23), PfCSP, PfAMA-1, PfLSA-1, and lysate (24) and observed various levels of responses. The key goal of our study was to evaluate the immune responses to homologous and heterologous preerythrocytic and erythrocytic antigens generated following P. vivax CHMI (PvCHMI) and P. falciparum CHMI (PfCHMI) of individuals who had no prior exposure to malaria. In this study, using two separate cohorts of malaria-naive individuals who were exposed to CHMI via the bite of mosquitoes infected with either P. falciparum (n = 18) or P. vivax (n = 18), we evaluated the acquisition of antibodies to the dominant surface antigens present in the preerythrocytic and erythrocytic stages of both P. falciparum and P. vivax. Individual IgM and IgG responses of all 36 subjects are shown in Fig. S1 and S2 in the supplemental material.
Homologous responses after P. vivax and P. falciparum CHMI.
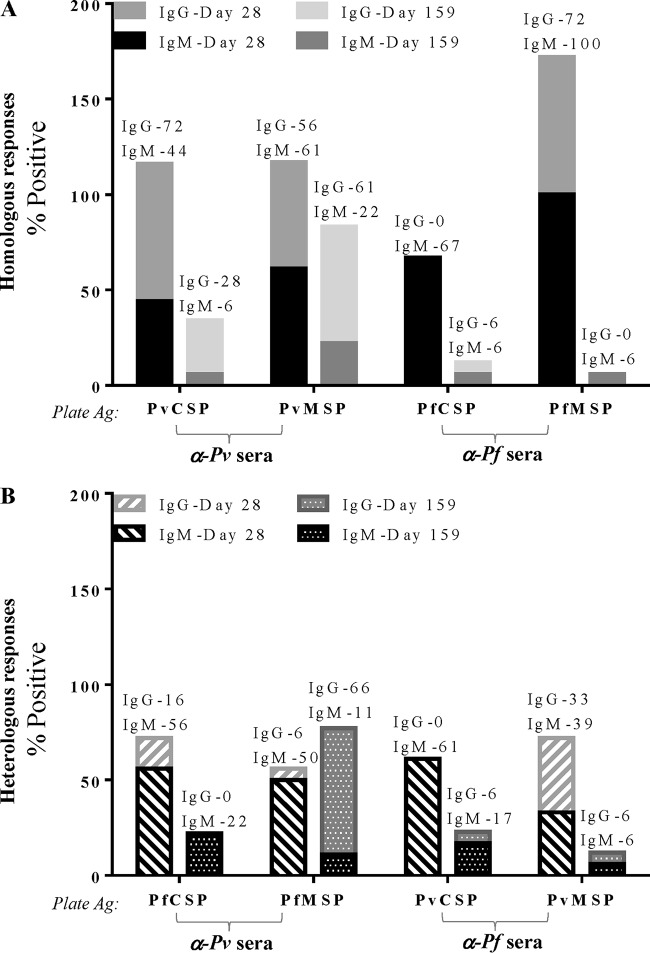
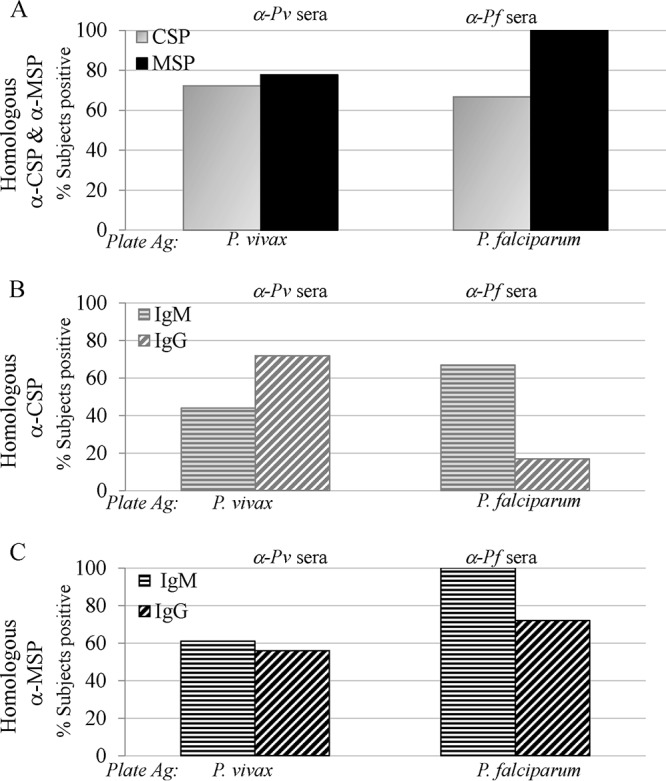
Based on the positivity criteria defined in Materials and Methods, following PvCHMI and PfCHMI, 72% and 67% of the subjects recognized homologous CSP, respectively, while slightly higher proportions, 78% and 100%, of the subjects demonstrated reactivity to homologous MSP, respectively (Fig. 1A), 28 days after CHMI.
FIG 1.
Malaria-naive subjects generate immune responses to homologous antigens (Ag) 1 month after controlled human malaria infection. (A) Following CHMI via the bite of five mosquitoes infected with either P. vivax (α-Pv sera) or P. falciparum (α-Pf sera), more than two-thirds of exposed subjects seroconverted to homologous CSP and MSP. (B and C) IgM and IgG antibodies to both CSP (B) and MSP (C) are detectable.
(i) Homologous preerythrocytic responses. Both P. vivax and P. falciparum sporozoites were able to induce not only IgM but also IgG responses to homologous CSP (Fig. 1B). Seventy-two percent of subjects elicited antibodies to PvCSP, 44% of whom were IgM positive (IgM+); all 72% were anti-PvCSP IgG+ (Fig. 1B). Sixty-seven percent of subjects showed reactivity to PfCSP after PfCHMI, all of whom were IgM+, and a smaller percentage (17%) were IgG+ (Fig. 1B). The repeat region of P. falciparum CSP contains a tetrameric motif (NANP/NVDP), while P. vivax has two different immunologically non-cross-reactive nonameric motifs. The type 1 (which was the strain used for challenge in the present study) motif is defined by the sequence GDR(A/D)GQPA. The lower percentage of IgG and higher percentage of anti-PfCSP IgM responses could be due to the shorter repetitive epitope (NANP), as highly repetitive epitopes have the ability to induce T-cell-independent antibody responses. In murine studies, PfCSP has been shown to generate both T-cell-dependent (25) and T-cell-independent (26) responses. Notably, PvCSP was able to mount IgG responses.
(ii) Homologous erythrocytic responses. Higher frequencies of immune responses to MSP-1 were observed. Seventy-eight percent of subjects were positive for PvMSP (Fig. 1A), with 61% being IgM+ and 56% being IgG+ after PvCHMI (Fig. 1C). All subjects seroconverted to PfMSP after PfCHMI; of these, 100% were IgM+ and 72% were IgG+ (Fig. 1C). Such high frequencies of erythrocytic responses are not surprising, as tens of thousands of merozoites are released during each 48-h cycle, providing an increased bolus of antigen. Several field studies show that as opposed to CSP, high MSP-1 antibody levels are seen in areas of high and low malaria transmission (27).
Heterologous responses after P. vivax and P. falciparum CHMI.
The presence of species-specific responses is used as a biomarker for exposure to malaria. However, with a few exceptions, seroreactivity studies have been performed using samples obtained from the field; thus, the results come with a caveat. This study investigates the presence of heterologous reactivity in unambiguous samples obtained from known malaria-naive individuals. Thus, all observed responses are expected to be due to recent exposure to CHMI or the presence of promiscuous epitopes. Unexpectedly, more than half of the subjects showed heterologous responses: 61% and 50% of the volunteers exposed to P. vivax CHMI and 61% and 67% of the volunteers exposed to P. falciparum CHMI showed seroreactivity to heterologous CSP and MSP, respectively (Fig. 2A), on day 28 after CHMI. Individual responses of all 36 subjects are shown in Fig. S2 in the supplemental material.
FIG 2.
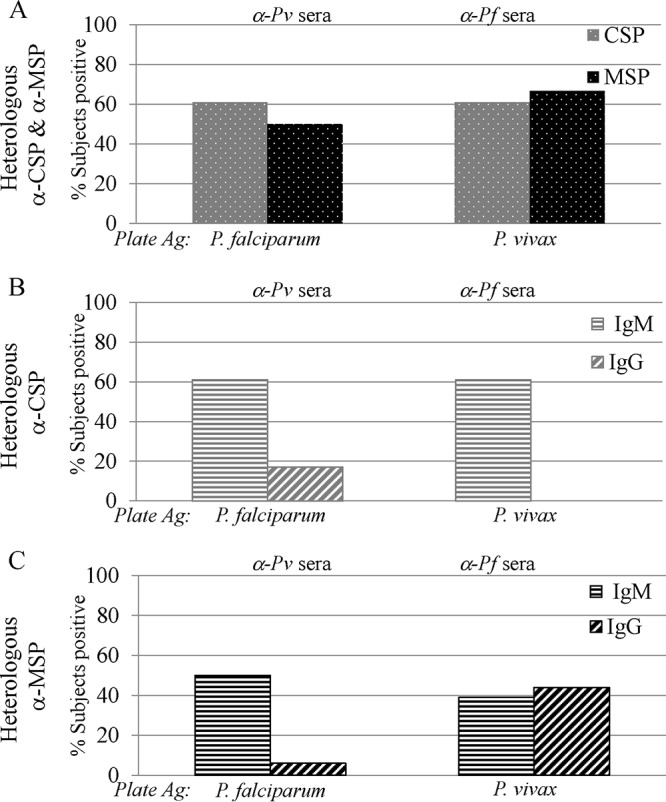
At least half of the subjects in each group recognize heterologous antigens. (A) One month after exposure, ≥50% of the subjects showed reactivity to heterologous CSP and MSP. (B) Almost all the heterologous anti-CSP responses after PvCHMI and PfCHMI were IgM. (C) After PfCHMI, similar numbers of subjects had IgM and IgG antibodies directed against PvMSP, while after PvCHMI, higher anti-PvMSP IgM responses were observed.
(i) Heterologous preerythrocytic responses. Following PvCHMI, 61% of the subjects were positive for anti-PfCSP IgM and 17% were positive for IgG, whereas following PfCHMI, all 61% showed IgM responses to PvCSP. No subjects were positive for anti-PvCSP IgG (Fig. 2B). These results suggest that the highly immunogenic repeat motif of PfCSP may be more efficient in priming cross-reactive IgM that recognizes the heterologous CSP.
(ii) Heterologous erythrocytic responses. Half the subjects demonstrated anti-PfMSP IgM following PvCHMI, while 6% generated an IgG response. Following PfCHMI, a higher number of subjects mounted a heterologous IgG response. Of the 67% of subjects who showed positive MSP heterologous responses (Fig. 2A), 39% were IgM+ and 44% were IgG+ (Fig. 2C). These results parallel the heterologous CSP responses, where PfCHMI is more likely to induce heterologous responses than PvCHMI.
Longevity of the immune response.
In the absence of continuous exposure, responses to malaria are notoriously short-lived (28, 29), and they peak with parasite exposure during the transmission season and ebb to negligible levels at other times. Immunity is known to wane rapidly once the individuals are no longer exposed to the stimuli. We observed the expected decrease in the frequency of individuals responding to homologous and heterologous preerythrocytic antigens on day ∼159 after CHMI.
The percentages of subjects who showed any response (either IgM or IgG) decreased from 72% (IgM, 44%; IgG, 72%) on day 28 postchallenge to 33% (IgM, 6%; IgG, 28%) at day ∼159 postchallenge for homologous PvCSP and from 67% (IgM, 67%; IgG, 0%) to 22% (IgM, 6%; IgG, 6%) for PfCSP (Fig. 3A). However, while the total percentage of individuals showed a small decline (78% versus 67% for homologous PvMSP and 100% to 67% for homologous PfMSP), the percentage of individuals positive for homologous IgG to MSP did not decline (56% and 61% PvMSP positive and 72% and 67% PfMSP positive for IgG on day 28 versus day ∼159) (Fig. 3A). There was a marked decline in the number of subjects positive for a response to heterologous antigens (anti-MSP IgM responses decreased from 33% and 50% for PfMSP and PvMSP, respectively, to 6% and 11%, respectively) (Fig. 3B). This is likely the result of maturation and expansion of higher-affinity antigen-specific antibodies that outcompete the lower-affinity antibodies over time.
FIG 3.
Homologous erythrocytic responses persist over time, while heterologous responses wane. (A) Homologous preerythrocytic (CSP) responses decrease, but similar numbers of subjects demonstrate positive antibody responses to homologous MSP on day 28 versus day 159 after homologous challenge. (B) Heterologous preerythrocytic and erythrocytic responses decrease at day 159 postchallenge. Each bar shows IgM and IgG responses; numerical values denote the percentages positive for each isotype.
Specificity and magnitude of responses.
Overall, the percentages of subjects positive for a response to homologous and heterologous antigens were not substantially different, ranging from a low of 50% positivity for heterologous MSP to a high of 100% positivity for homologous MSP. The specificity of sera for malaria antigens was confirmed by evaluating their reactivity to glutathione S-transferase (GST) from Schistosoma japonicum as an unrelated control antigen. The reactivity of sera at a single serum dilution was evaluated to obtain a comparative assessment of responses, both the frequency and magnitude, to the various antigens. Only one subject was IgM+ and another was IgG+ for the unrelated GST plate antigen, with lower optical density (OD) values than for Plasmodium antigens (Fig. 4A and B).
FIG 4.
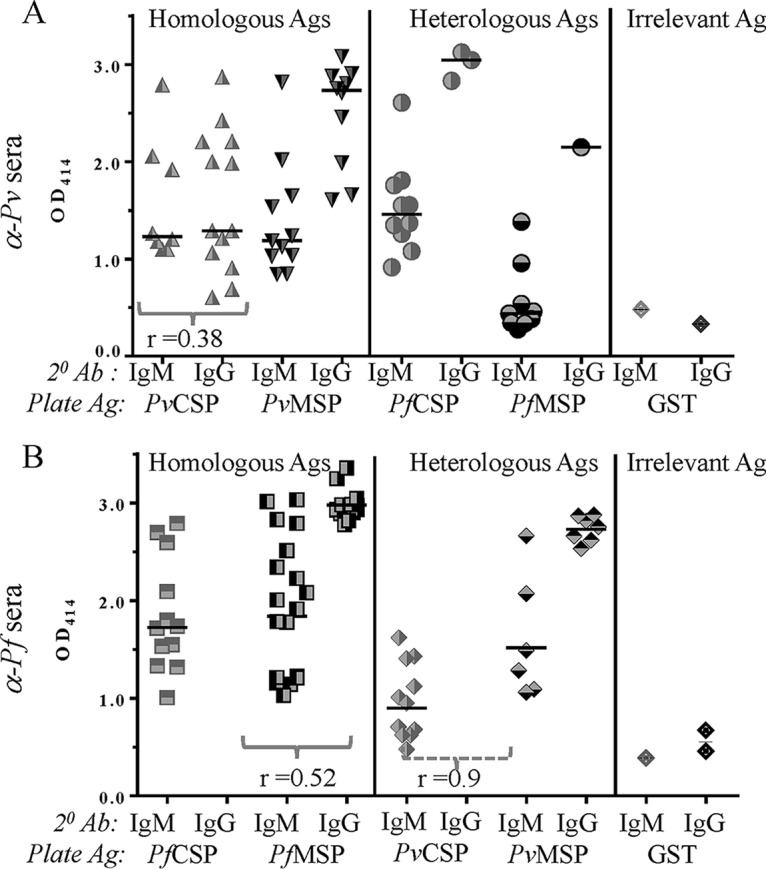
Magnitude of homologous and heterologous responses 1 month after CHMI at a single serum dilution of 1:500 to PvCSP, PvMSP, PfCSP, and PfMSP. (A) P. vivax challenge induces both IgM and IgG responses to both homologous preerythrocytic and erythrocytic antigens. The majority of heterologous responses were IgM. The horizontal line represents the median value. (B) After P. falciparum CHMI, a large percentage of individuals show homologous and heterologous reactivity, but no IgG responses to either homo- or heterologous CSP were seen. The horizontal line represents the median value. There was a positive Spearman correlation between homologous IgM and IgG for PvCSP (r = 0.38) (A) as well as PfMSP (r = 0.52; P = 0.07) (B). The only heterologous correlation was seen between anti-CSP and MSP IgMs: PvCHMI sera showed a weak positive correlation between PfCSP and PfMSP (A), while the PfCHMI sera demonstrated a high correlation between PvCSP and PfMSP IgMs (r = 0.9; P = 0.04) (B). Ab, antibody.
After PvCHMI (Fig. 4A), at a serum dilution of 1:500, 8 of the 18 subjects were positive for anti-PvCSP IgM, and 13 were positive for anti-PvCSP IgG, with median homologous OD values of 1.2 and 1.3 for IgM and IgG, respectively. Both IgM and IgG responses to homologous PvMSP were observed: 11 subjects had positive IgM responses, with a median OD of 1.2, and 10 subjects were positive for IgG, with a median OD of 2.7.
Heterologous responses after PvCHMI (Fig. 4A) were predominantly IgM, with 10 subjects being positive for PfCSP (median OD, 1.5) and 9 being positive for PfMSP (median OD, of 0.4). These results could reflect the presence and expansion of recently recruited B cells that have yet to undergo affinity maturation and, therefore, are generating broadly reactive antibodies. While a lower frequency of heterologous IgG responses was observed (3 subjects positive for PfCSP and 1 positive for PfMSP), the positive subjects had higher ODs (ODs of 3 and 2.1 for IgM and IgG, respectively).
After PfCHMI, equally high numbers of subjects showed a positive response to homologous and heterologous antigens (Fig. 4B). Twelve subjects were positive for a response to PfCSP, with a median OD of 1.7, and 11 were positive for a response to PvCSP (median OD, 0.9). No IgG responses to either homologous or heterologous CSP were seen. The lack of isotype switching from IgM to IgG in subjects was specific for CSP. Both IgM and IgG responses to homologous and heterologous MSP were seen. Eighteen subjects had positive IgM responses to PfMSP (median OD, 1.9), and 13 had positive IgG responses (median, OD 2.9). As with homologous MSP, heterologous MSP responses also demonstrated higher IgG responses. The 6 subjects positive for PvMSP IgM had a median OD of 1.4, while the 7 IgG responders had a median OD of 2.8. As with PvCHMI, low numbers and magnitudes of anti-GST responses were observed after PfCHMI (Fig. 4B).
r values showing correlation and P values showing significance between groups are provided in Fig. 4A and B.
Analysis of predicted epitopes.
The high levels of cross-reactive responses observed in this study were contrary to the data reported in the literature (30). Therefore, to further expand on the observation of cross-reactivity between the orthologous CSPs and MSPs of P. vivax and P. falciparum, computational tools were used to predict potential epitopes on these antigens.
(i) Linear epitope analysis. Several linear B-cell epitopes were predicted for CSP and MSP by different programs, with significant overlaps in P. falciparum and P. vivax proteins. The ABCpred server predicted numerous epitopes for PfCSP and PvCSP that cluster in some areas in the sequence of each protein. The identity and similarity between PfCSP and PvCSP sequences are 27 and 37%, respectively. The top-scoring epitopes cluster in homologous areas where numbers of matching residues are reasonably high. For example, four linear epitopes were predicted by ElliPro in the C-terminal domains of CSP of P. falciparum, and three were predicted for P. vivax, which have higher identity (46%) and similarity (75.9%) than the full-length protein. Among these the top-scoring epitopes, KMEKC (residues 346 to 350) for PfCSP exhibits remarkable similarity to TMDKC (residues 329 to 333) for PvCSP, which maps to the corresponding area of the sequence (Tables 1 and 2). These predicted epitopes may represent sites for binding of cross-reactive antibodies.
TABLE 1.
Sequence of the C-terminal region of P. vivax CSPa
| Predicted linear epitope of P. vivax CSP (C-terminal region) | Start position | End position | Peptide sequenceb | No. of residues | Score |
|---|---|---|---|---|---|
| 1 | 329 | 333 | TMDKC | 5 | 0.844 |
| 2 | 307 | 318 | RVNAANKKPEDL | 12 | 0.653 |
| 3 | 296 | 299 | VTCG | 4 | 0.567 |
The sequence of the C-terminal region of P. vivax CSP (residues 277 to 333) (NCBI accession no. XP_001613068.1) was analyzed for the presence of linear B-cell epitopes using ElliPro (IEDB Analysis Resources). Top-ranking epitopes (using a threshold value of 0.5) are listed.
Residues common between P. falciparum and P. vivax are highlighted in boldface. Similar residues are indicated by italics.
TABLE 2.
Sequence of the C-terminal region of P. falciparum CSPa
| Predicted linear epitope of P. falciparum CSP (C-terminal region) | Start position | End position | Peptide sequenceb | No. of residues | Score |
|---|---|---|---|---|---|
| 1 | 346 | 350 | KMEKC | 5 | 0.834 |
| 2 | 323 | 338 | KPGSANKPKDELDYAN | 16 | 0.631 |
| 3 | 297 | 302 | NKIQNS | 6 | 0.578 |
| 4 | 312 | 315 | VTCG | 4 | 0.57 |
The sequence of the C-terminal region of P. falciparum CSP (residues 287 to 350) (NCBI accession no. CAA33421.1) was analyzed for the presence of linear B-cell epitopes using ElliPro (IEDB Analysis Resources). Top-ranking epitopes (using a threshold value of 0.5) are listed.
Residues common between P. falciparum and P. vivax are highlighted in boldface. Similar residues are indicated by italics.
The amino acid sequences of the MSPs are more similar than those of the CSPs. The PfMSP and PvMSP sequences display ∼38% identity and 45% similarity. Using default options (threshold value of 0.5 and peptide length of 16 residues) in ABCpred for linear epitope prediction, top-scoring epitopes were identified in the corresponding regions in both MSP sequences.
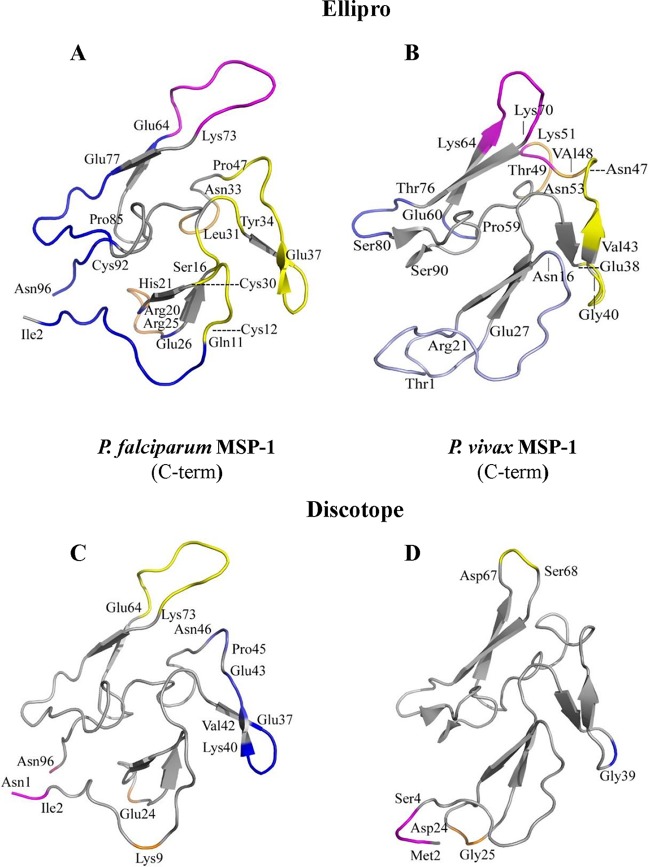
(ii) Discontinuous epitope analysis. A majority of B-cell epitopes are discontinuous (31), i.e., epitopes that are formed when the protein folds into its natural three-dimensional (3D) state. Analysis of such discontinuous epitopes therefore requires 3D structural data of proteins. The crystal structure of the C-terminal domain of PfCSP was reported a few years ago (32). We used the atomic coordinates for the 63 residues of PfCSP from the crystal structure (PDB accession no. 3VDJ) (32) to predict discontinuous (or conformational) epitopes in this domain. We also generated a 3D model for the corresponding domain of P. vivax CSP (residues 271 to 333) using SWISS MODEL and used it for prediction of conformational epitopes. The 5 top-scoring discontinuous epitopes predicted by ElliPro (listed in order from high to low scores, shown in different colors in the figures and described below) contained 8, 4, 13, 4, and 4 residues for the PfCSP C-terminal domain structure (Fig. 5A) (PDB accession no. 3VDJ) and 3, 9, 8, 10, and 3 residues for the PvCSP structural model (Fig. 5B). For both proteins, these epitopes cluster in corresponding regions on the 3D structure of the proteins (Fig. 5A and B). Amino acid residues on the highest-scoring epitope predicted for the PfCSP C-terminal domain (shown in magenta in Fig. 5A) are located in distant areas on the primary sequence but close on the 3D structure (residues Val312-Gly315 and Met347-Cys350). A similar high-scoring discontinuous epitope was also predicted for the PvCSP structural model in the corresponding location (shown in magenta in Fig. 5B) (residues Val296-Gly299 and Thr329-Cys333). The largest discontinuous epitope (in yellow) contains residues from distant areas of the PfCSP primary sequence (residues Ser304-Leu306, Lys323-Gly325, and Ala327-Glu333) (Fig. 5A) but located in nearby structural areas. A high-scoring epitope is also predicted in the corresponding region of the PvCSP structure (residues Gly288-Glu290, Arg307-Asn309, and Thr319-Asp322) (Fig. 5B). The lowest-scoring epitope for PfCSP (residues Leu334-Asn338) is shown in blue (Fig. 5A), and that for PvCSP (residues Ala285-Val287) is depicted in orange (Fig. 5B). The highest-scoring epitope for PvCSP (residues Pro271-Glu273) (Fig. 5B, red) corresponds to the second-highest-scoring epitope in PfCSP (residues Pro287-Lys290) (Fig. 5A, red).
FIG 5.
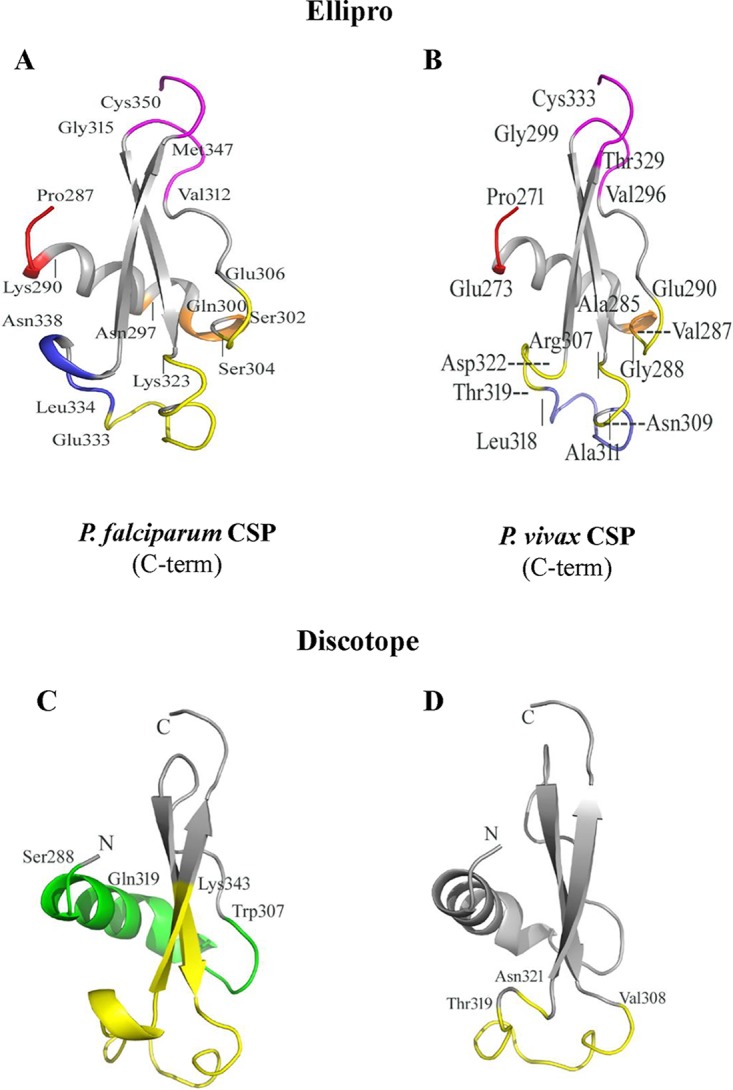
Discontinuous epitopes are predicted in similar regions on the C-terminal domains of P. vivax and P. falciparum CSPs using two separate prediction tools. (A and B) Cartoon diagrams showing the locations of conformational epitopes on the 3D structures of the C-terminal domains of P. falciparum CSP (A) and P. vivax CSP (B) as predicted by ElliPro (31). (A) ElliPro-predicted scores, in descending order, for the five PfCSP epitopes are 0.717 (magenta), 0.699 (red), 0.668 (yellow), 0.668 (orange), and 0.547 (blue). (B) The scores for the five epitopes, ranked in order, for PvCSP are 0.725 (red), 0.721 (magenta), 0.647 (slate), 0.556 (yellow), and 0.503 (orange). Individual residues are marked and described in the text. (C and D) Cartoon diagrams of conformational epitopes predicted by DiscoTope (30) for the C-terminal domains of P. falciparum and P. vivax CSPs, respectively. (C) The two predicted epitopes for PfCSP are highlighted in green for a 19-residue epitope and in yellow for a 24-residue epitope. (D) The single 13-residue predicted epitope for PvCSP is highlighted in yellow.
To further validate the results of the ElliPro analysis, an alternate algorithm was used for predicting the discontinuous epitopes on the structure of the PfCSP and PvCSP C-terminal domains. The epitopes identified by ElliPro overlap those predicted by DiscoTope (Fig. 5C and D) (33). While this method predicted 49 B-cell epitope residues out of 71 total residues (PDB accession no. 3VDJ) of the crystal structure for the PfCSP C-terminal domain, only 13 epitope residues were predicted for the PvCSP C-terminal domain model. The predicted epitope (residues Val308-Asn321) (Fig. 5D) for the PvCSP C-terminal domain overlaps one of the epitopes in PfCSP (residues Gln319-Lys343) (Fig. 5C, yellow). PfCSP epitope residues Ser288-Trp307 (highlighted in green in Fig. 5C) do not have a corresponding epitope predicted for PvCSP. Based on these predictions, we can anticipate considerable cross-reactivity with antibodies resulting from heterologous infections, as was observed in our study.
3D structures of MSP-1 have been determined for the C-terminal region (corresponding to residues 1607 to 1702 of P. falciparum strain 3D7 [NCBI accession no. Z35327]); therefore, the prediction of discontinuous epitopes was limited to the C-terminal domain. Nuclear magnetic resonance (NMR) structures of PfMSP-1 (96 residues) and PvMSP-1 (90 residues) were determined (PDB accession no. 1CEJ [34] and 2NPR [35], respectively). We used coordinates reported under PDB accession no. 1CEJ and 2NPR for prediction of discontinuous epitopes using ElliPro and DiscoTope (https://www.iedb.org/). Results of these analyses are shown in Fig. 6. Discontinuous epitopes predicted by ElliPro are shown in different colors on the cartoon drawings for PfMSP-1 (Fig. 6A) and PvMSP-1 (Fig. 6B). Epitopes predicted by DiscoTope are shown in Fig. 6C and D for PfMSP-1 and PvMSP-1, respectively. In general, homologous structural areas are predicted to be the sites for epitopes in both proteins. Interestingly, in the high-resolution crystal structure of a 19-kDa domain of PfMSP-1 (PfMSP-119) in complex with the Fab domain of the monoclonal antibody G17.12, PfMSP-119 residues 8 to 11, 13, 14, 24 to 26, 28, 38, and 39 are located at the antigen-antibody interface (PDB accession no. 1OB1 [36]). Several of these residues are at or near the epitopes predicted by ElliPro and DiscoTope, thus supporting the use of computational tools for prediction of potential epitopes. It should be noted that the structure of this complex reveals the interaction sites for only one monoclonal antibody, G17.12. Several predicted conformational epitopes shown in Fig. 6 are located on corresponding regions on the PfMSP-1 and PvMSP-1 structures. Due to structural homology between the proteins, these sites represent potential epitopes for cross-reactive antibodies.
FIG 6.
Cartoon drawings showing potential antigenic epitopes on the C-terminal domains of PfMSP-1 (96 residues) and PvMSP-1 (90 residues) solution structures. (A and B) Cartoon diagrams showing the locations of conformational epitopes on the 3D structures of the C-terminal domains of P. falciparum MSP-1 (A) and P. vivax MSP-1 (B), as predicted by ElliPro (31). (A) ElliPro-predicted scores, in descending order, for the four PfMSP-1 epitopes are 0.82 (magenta), 0.602 (blue), 0.588 (orange), and 0.533 (yellow). (B) Scores for the five epitopes, ranked in order, for PvCSP are 0.716 (yellow), 0.703 (blue), 0.689 (magenta), 0.65 (light blue), and 0.547 (light orange). Individual residues are marked. The second-highest-scoring epitope for PfMSP-1 (shown in blue in panel A) consists of a total of 27 residues that are distributed in different regions in the amino acid sequence but are spatially close on the 3D structure. The lowest-scoring epitope (yellow in panel A) includes 20 residues that are from different areas of PfMSP-1 sequences but are located in proximity on the 3D structure. (C and D) Cartoon diagrams of conformational epitopes predicted by DiscoTope (30) for the C-terminal domains of P. falciparum and P. vivax MSP-1s, respectively. The predicted epitopes for PfMSP-1 (C) and PvMSP-1 (D) are shown in different colors.
The presence of overlapping conformational epitopes identified by two separate algorithms supports the in vitro observation of the presence of cross-reactive antibodies following exposure to P. vivax and/or P. falciparum.
Antibodies to sporozoites and the central repeat region of CSP have been used as markers of exposure to Plasmodium spp. The repeat sequences of plasmodia are species specific; therefore, anti-CSP antibodies have been used to demonstrate correlation with infection prevalence, transmission intensity, and exposure (1, 37). Blood-stage antigens, such as MSP-1 and AMA-1, are also used for seroepidemiological studies (38). All these studies have been performed with homologous antigens. Few studies have looked at the presence of heterologous responses. In fact, due to the prevalent belief that antibodies are species specific, the presence of anti-PvCSP antibodies has been considered evidence of P. vivax transmission in West Africa (21).
Analysis of sera from the field shows detectable, but low-level, responses to preerythrocytic antigens due to limited sporozoite exposure (39), and the positive responses are attributed to multiple exposures to the parasite (27). These studies did not assess cross-reactive responses to evaluate the presence of heterologous responses following an increase in homologous reactivity. A few studies previously reported the presence of antigen- and stage-transcending antibodies. Cross-reactive antibodies between CSP and blood stages have been shown in humans (40) as well as primates (41). At the antigen level, in a study on acute malaria cases, antibodies to heterologous MSP-1 were observed in patients experiencing either P. vivax or P. falciparum infection (15). However, the presence of submicroscopic infections or the presence of residual antibodies from a previous infection(s) could not be ruled out by those authors. Similarly, there is a strong indication of the presence of cross-reactive responses to other sexual (17) and asexual (3, 16, 18) blood-stage antigens from the field. However, the data cannot be exclusively attributed to cross-reaction, as the presence of heterologous species, while rare, has not been ruled out.
Data from the present study demonstrating the presence of heterologous responses in naive subjects lend credence to anecdotal reports from regions of endemicity. The results were supported by computational analysis, which predicted linear and conformational epitopes on the orthologous proteins in matching areas of the antigens. Currently, a small number of antigen-antibody crystal structures are available, limiting the size of the discontinuous epitope database. Therefore, the predictive ability of the algorithm is insufficient. This means that there is a high likelihood of the presence of many more discontinuous epitopes than predicted by these programs. As seen with the predicted epitopes, these “so far not predicted” epitopes are also likely to be located in structurally homologous areas on the same antigens from P. falciparum and P. vivax.
Our data support additional studies from regions of endemicity with various levels of transmission intensity and endemicity (i.e., regions of holo-, hyper-, or hypoendemicity) to evaluate the presence of heterologous reactivity and its correlation with the amount of parasite exposure. Further analysis, such as performing competitive enzyme-linked immunosorbent assays (ELISAs) and functional assays, will throw additional light on the epitopes being recognized and whether these antibodies play a functional role.
MATERIALS AND METHODS
Study subjects.
Malaria-naive subjects (males and females between the ages of 18 and 55 years) residing in the Washington, DC, metropolitan area were enrolled as the control arm of ongoing studies to test malaria vaccines at the Walter Reed Army Institute of Research (WRAIR).
Ethics statement.
All parent studies were conducted following independent scientific and ethical review by the WRAIR scientific review committee, the WRAIR institutional review board (IRB), and the USAMRMC Human Subjects Research and Review Board; some protocols were additionally reviewed by the Western IRB. Written informed consent was obtained from subjects prior to recruitment. Serum samples used in the present study were coded, deidentified, and used per protocol or following review and “exempt from human use” designation. All methods were performed in accordance with relevant guidelines and regulations.
Controlled human malaria infection.
All subjects used for this analysis served as infectivity controls in studies evaluating the efficacy of malaria vaccines. Eighteen subjects were exposed to P. vivax infection, and another 18 were exposed to P. falciparum infection, as described below.
(i) P. vivax infectivity controls. A total of 18 subjects (three cohorts of 6 subjects each) were exposed to the bites of 5 Anopheles dirus mosquitoes infected with P. vivax (type 1) from Thailand (42; I. Chuang, J. Sattabongkot, A. Yadava, J. Bennett, E. Kamau, D. Fryauff, T. Richie, and C. Ockenhouse, unpublished data). Laboratory-reared A. dirus mosquitoes were membrane fed on blood from three different Thai donors (one for each cohort) infected with P. vivax. The mosquito feeding study was conducted in Thailand under protocols headed by Jetsumon Sattabongkot following approvals by the WRAIR IRB and the Ministry of Public Health, Thailand. Donor blood was screened for coinfection with other plasmodia, and only blood that was microscopically determined to be infected with P. vivax was used to initiate mosquito infection. Membrane feeding was conducted at the satellite clinical laboratory in Mae-Sot province in Thailand, after which the infected mosquitoes were transported to the laboratory in Bangkok. Donor blood was also screened per international blood transfusion guidelines for the presence of blood- and mosquito-borne infections. In addition, blood and infected mosquitoes were screened by PCR and ELISAs to identify parasites to the species level to ensure that they were infected with the type 1 strain (based on CSP genotype and phenotype) of P. vivax. Infected mosquitoes that were singly infected with P. vivax (type 1) and originated from a donor whose blood was ruled transfusion eligible were transported to, and maintained at, the insectary at WRAIR, Silver Spring, MD. A manuscript providing additional details on the screening and permits required for transporting infected mosquitoes is in preparation (Chuang et al., unpublished).
Seventeen to 21 days after an infectious blood meal, malaria-naive subjects were exposed to the bites of 5 infected mosquitoes. Blood smears were performed starting on day 5 postchallenge, and on day 9 postchallenge, volunteers were accommodated in a hotel under the care of trained medical staff. Subjects were monitored daily and received antimalarial therapy as soon as they were diagnosed with malaria based on thick blood smears, and they were treated with both chloroquine and primaquine until they were determined to be malaria free (42).
(ii) P. falciparum infectivity controls. Eighteen subjects who served as infectivity controls in two different studies (6 in one study and 12 in a second study) were used for the purpose of this study. Volunteers were challenged using the standard WRAIR P. falciparum CHMI model, the details of which are available in a review by Spring and colleagues (43). Briefly, laboratory-reared Anopheles stephensi mosquitoes were membrane fed on in vitro-cultured P. falciparum (strain 3D7). Parasites were maintained in transfusion-grade blood and cultured until they developed into gametocytes. Infected mosquitoes were maintained in the WRAIR insectary, and on days 17 to 24 postinfection, mosquitoes were used to bite malaria-naive volunteers for a total of five infected bites. Postexposure protocols for monitoring and treatment were similar to those for the P. vivax challenge described above, except that these volunteers were not treated with primaquine.
All volunteers used in this analysis became parasitemic. The mean prepatent period after PvCHMI was 11.1 ± 1.5 days (42; Chuang et al., unpublished), and that after PfCHMI was 12.3 ± 1.7 days (44, 45).
Antigens.
(i) Circumsporozoite protein. P. vivax CSP (VMP001), produced under current good manufacturing practices, comprises 259 amino acids representing the N-terminal region, a truncated central repeat region based on a Korean isolate, and the C-terminal region of P. vivax CSP and 10 non-CSP amino acids, for a total of 269 amino acids (46, 47).
P. falciparum CSP (FMP013), based on the 3D7 strain of P. falciparum, comprises 278 amino acids representing the N-terminal region, 22 repeat motifs (19 NANP and 3 NVDP motifs), and the C-terminal region of P. falciparum CSP and 23 non-CSP amino acids, for a total of 301 amino acids (48).
(ii) Merozoite surface protein 1. Research-grade protein based on the Sal I strain of P. vivax comprises 362 amino acids denoting the 42-kDa region of P. vivax MSP-1 and 18 non-MSP-1 amino acids, for a total of 380 amino acids (49).
P. falciparum MSP (FMP-1), based on the 3D7 strain, comprises 374 amino acids representing the 42-kDa region of MSP-1 and 17 non-MSP-1 amino acids for a total of 391 amino acids (50).
These proteins are referred to as PvCSP, PfCSP, PvMSP, and PfMSP.
Enzyme-linked immunosorbent assay.
Serum was separated from venous blood drawn from subjects prior to challenge as well as at day 28 (∼1 month) and day 159 (∼5.3 months) postchallenge and stored at −80°C until use. For immunological assessment, Immulon 2HB plates (Dynatech, VA) were coated overnight at 4°C with 1 μg/ml of either PvCSP, PvMSP-1, PfCSP, or PfMSP-1 diluted in phosphate-buffered saline (PBS). Glutathione S-transferase (GST) from Schistosoma japonicum (Abcam, MA), coated at a concentration of 1 μg/ml, was used as an unrelated control antigen in some assays. After blocking with casein buffer, serum was added to the wells and titrated 2-fold starting at a dilution of 1:125 up to a dilution of 1:8,000. Plates were incubated for 2 h, washed, and incubated for an hour with horseradish peroxidase-labeled anti-human IgM or IgG (KPL Inc., MD) secondary antibodies. The reaction was developed using the ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] peroxidase substrate system (KPL Inc., MD) and read after 60 min using a SpectraMax 340PC plate reader (Molecular Devices, CA) at a wavelength of 414 nm.
Serum samples from the vivax malaria cohort (18 pre- and 18 postchallenge) and the falciparum malaria cohort (18 pre- and 18 postchallenge) were tested individually against all four antigens, and positivity criteria were calculated separately for the two cohorts for each of the four antigens. OD at 414 nm (OD414) values for all 18 prebleed samples, at each dilution, were averaged.
A sample was considered positive if the OD414 of the postchallenge serum sample was ≥0.1 and ≥2 standard deviations of the means of the values for the prebleed samples at the corresponding dilution.
A subject was considered positive if at least two consecutive serum dilutions met the positivity criteria defined above. Subjects were designated positive if they met the positivity criteria for either IgM, IgG, or both.
Statistical analysis.
Data were plotted using Microsoft Excel and GraphPad Prism, version 6. Spearman correlation analysis was performed to measure the association between groups at a defined serum dilution using GraphPad Prism.
Computational analysis of B-cell epitopes.
Different computational tools were used for the prediction of linear and discontinuous B-cell epitopes. Sequence comparisons were made to determine the presence of cross-reactive B-cell epitopes between orthologs. Analysis of primary sequences was performed using alignment tools in ClustalW2 (http://www.ebi.ac.uk/Tools/psa/). Local similarities were identified using Matcher (EMBOSS), within ClustalW2 for pairwise alignment of CSP sequences of P. vivax CSP (NCBI accession no. XP_001613068.1) and P. falciparum CSP (NCBI accession no. CAA33421.1). A similar analysis was performed for the C-terminal MSP-142 sequences of P. vivax MSP-1 (NCBI accession no. XP_001614842) and P. falciparum MSP-1 (NCBI accession no. CAA84556.1). The C-terminal MSP-119 regions correspond to amino acids 1606 to 1702 for P. falciparum and amino acids 1640 to 1729 for P. vivax.
Linear epitopes were predicted using ABCpred (51), an artificial neural network-based B-cell epitope prediction server. ABCpred relies on training data sets of experimentally determined epitopes obtained from the B-cell epitope database (BCIPEP [52]) for the recognition of real epitopes and a set of nonepitope random peptides generated from the Swiss-Prot database of protein sequences (https://www.ebi.ac.uk/uniprot/).
Discontinuous or conformational B-cell epitopes were predicted using DiscoTope and ElliPro (EDB tools [http://www.iedb.org/]), tools which use 3D structural information of the antigen for epitope prediction. DiscoTope (33) predicts the probability of epitopes based on calculation of the solvent-accessible surface area and contact distances among the residues. ElliPro (53), on the other hand, relies on recognition of structural protrusions, and the predictions by this method are based on solvent accessibility and flexibility of the structural areas. Additionally, ElliPro also provides prediction of linear B-cell epitope sequences included in the structure.
A 3D structural model for the P. vivax CSP domain corresponding to the C-terminal domain of P. falciparum CSP, for which the crystal structure is available (PDB accession no. 3VDJ [32]), was generated using SWISS MODEL (https://swissmodel.expasy.org) (54, 55).
Supplementary Material
ACKNOWLEDGMENTS
We thank Haylee Hollenbeck and Jessica Albaugh for performing some of the ELISAs. We acknowledge Jetsumon Sattabongkot and her team for their pivotal role in executing the P. vivax CHMI study. In addition, we gratefully acknowledge the assistance of the WRAIR clinical research coordinators, members of the Division of Human Subjects Protection, as well as all the study participants who made this study possible.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication.
C.E.H. and L.M.H. performed experiments and tabulated data; E.B.-L. assisted in obtaining necessary approvals for sample use and provided input on the draft manuscript and revisions; D.M.T. served as clinical research coordinator on clinical studies and collated and provided clinical data; J.W.B., J.A.R., and I.C. served as principal investigators on clinical studies; E.A. and S.D. provided antigens; D.C. performed computational analysis and assisted in drafting the manuscript and revisions; and A.Y. conceived and designed the study, analyzed data, and wrote the manuscript. All authors reviewed and approved the manuscript.
We declare that we have no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00541-18.
REFERENCES
- 1.Folegatti PM, Siqueira AM, Monteiro WM, Lacerda MV, Drakeley CJ, Braga EM. 2017. A systematic review on malaria sero-epidemiology studies in the Brazilian Amazon: insights into immunological markers for exposure and protection. Malar J 16:107. doi: 10.1186/s12936-017-1762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franca CT, Li Wai Suen CSN, Carmagnac A, Lin E, Kiniboro B, Siba P, Schofield L, Mueller I. 2017. IgG antibodies to synthetic GPI are biomarkers of immune-status to both Plasmodium falciparum and Plasmodium vivax malaria in young children. Malar J 16:386. doi: 10.1186/s12936-017-2042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King CL, Davies DH, Felgner P, Baum E, Jain A, Randall A, Tetteh K, Drakeley CJ, Greenhouse B. 2015. Biosignatures of exposure/transmission and immunity. Am J Trop Med Hyg 93:16–27. doi: 10.4269/ajtmh.15-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cespedes N, Li Wai Suen CSN, Koepfli C, Franca CT, Felger I, Nebie I, Arevalo-Herrera M, Mueller I, Corradin G, Herrera S. 2017. Natural immune response to Plasmodium vivax alpha-helical coiled coil protein motifs and its association with the risk of P. vivax malaria. PLoS One 12:e0179863. doi: 10.1371/journal.pone.0179863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corran P, Coleman P, Riley E, Drakeley C. 2007. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol 23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Dewasurendra RL, Dias JN, Sepulveda N, Gunawardena GS, Chandrasekharan N, Drakeley C, Karunaweera ND. 2017. Effectiveness of a serological tool to predict malaria transmission intensity in an elimination setting. BMC Infect Dis 17:49. doi: 10.1186/s12879-016-2164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins WE, Jeffery GM, Roberts JM. 2004. A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg 70:642–644. doi: 10.4269/ajtmh.2004.70.642. [DOI] [PubMed] [Google Scholar]
- 8.Mueller I, Galinski MR, Tsuboi T, Arevalo-Herrera M, Collins WE, King CL. 2013. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv Parasitol 81:77–131. doi: 10.1016/B978-0-12-407826-0.00003-5. [DOI] [PubMed] [Google Scholar]
- 9.Boyd M, Kitchen SF. 1936. Is the acquired homologous immunity to P. vivax equally effective against sporozoites and trophozoites? Am J Trop Med 16:317–322. doi: 10.4269/ajtmh.1936.s1-16.317. [DOI] [Google Scholar]
- 10.Orlandi-Pradines E, Rogier C, Koffi B, Jarjaval F, Bell M, Machault V, Pons C, Girod R, Boutin J-P, Pagès F. 2009. Major variations in malaria exposure of travellers in rural areas: an entomological cohort study in western Cote d’Ivoire. Malar J 8:171. doi: 10.1186/1475-2875-8-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobelens FG, Verhave JP, Leentvaar-Kuijpers A, Kager PA. 1998. Testing for anti-circumsporozoite and anti-blood-stage antibodies for epidemiologic assessment of Plasmodium falciparum infection in travelers. Am J Trop Med Hyg 58:75–80. doi: 10.4269/ajtmh.1998.58.75. [DOI] [PubMed] [Google Scholar]
- 12.Molle I, Petersen E, Buhl MR. 1999. Retrospective evaluation of exposure to P. falciparum using antibodies to circumsporozoite protein and to cultured P. falciparum antigens. Scand J Infect Dis 31:69–71. [DOI] [PubMed] [Google Scholar]
- 13.Medina Costa R, de Sousa KP, Atouguia J, Tavira LT, Silva MS. 2013. Prevalence and level of antibodies anti-Plasmodium spp. in travellers with clinical history of imported malaria. J Parasitol Res 2013:247273. doi: 10.1155/2013/247273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jelinek T, Loscher T, Nothdurft HD. 1996. High prevalence of antibodies against circumsporozoite antigen of Plasmodium falciparum without development of symptomatic malaria in travelers returning from sub-Saharan Africa. J Infect Dis 174:1376–1379. doi: 10.1093/infdis/174.6.1376. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Zhao Z, Zhang X, Li X, Zhu M, Li P, Yang Z, Wang Y, Yan G, Shang H, Cao Y, Fan Q, Cui L. 2016. Naturally acquired antibody responses to Plasmodium vivax and Plasmodium falciparum merozoite surface protein 1 (MSP1) C-terminal 19 kDa domains in an area of unstable malaria transmission in Southeast Asia. PLoS One 11:e0151900. doi: 10.1371/journal.pone.0151900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodberry T, Minigo G, Piera KA, Hanley JC, de Silva HD, Salwati E, Kenangalem E, Tjitra E, Coppel RL, Price RN, Anstey NM, Plebanski M. 2008. Antibodies to Plasmodium falciparum and Plasmodium vivax merozoite surface protein 5 in Indonesia: species-specific and cross-reactive responses. J Infect Dis 198:134–142. doi: 10.1086/588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal GP, Vengesai A, Cao Y, Mduluza T, Kumar N. 2017. Antibodies elicited during natural infection in a predominantly Plasmodium falciparum transmission area cross-react with sexual stage-specific antigen in P. vivax. Acta Trop 170:105–111. doi: 10.1016/j.actatropica.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa JD, Zanchi FB, Rodrigues FL, Honda ER, Katsuragawa TH, Pereira DB, Taborda RL, Tada MS, Ferreira RDG, Pereira-da-Silva LH. 2013. Cross-reactive anti-PfCLAG9 antibodies in the sera of asymptomatic parasite carriers of Plasmodium vivax. Mem Inst Oswaldo Cruz 108:98–105. doi: 10.1590/S0074-02762013000100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braga EM, Fontes CJ, Krettli AU. 1998. Persistence of humoral response against sporozoite and blood-stage malaria antigens 7 years after a brief exposure to Plasmodium vivax. J Infect Dis 177:1132–1135. doi: 10.1086/517412. [DOI] [PubMed] [Google Scholar]
- 20.Fontes CJ, Bathurst I, Krettli AU. 1991. Plasmodium vivax sporozoite antibodies in individuals exposed during a single malaria outbreak in a non-endemic area. Am J Trop Med Hyg 44:28–33. doi: 10.4269/ajtmh.1991.44.28. [DOI] [PubMed] [Google Scholar]
- 21.Culleton R, Ndounga M, Zeyrek FY, Coban C, Casimiro PN, Takeo S, Tsuboi T, Yadava A, Carter R, Tanabe K. 2009. Evidence for the transmission of Plasmodium vivax in the Republic of the Congo, West Central Africa. J Infect Dis 200:1465–1469. doi: 10.1086/644510. [DOI] [PubMed] [Google Scholar]
- 22.Krause DR, Gatton ML, Frankland S, Eisen DP, Good MF, Tilley L, Cheng Q. 2007. Characterization of the antibody response against Plasmodium falciparum erythrocyte membrane protein 1 in human volunteers. Infect Immun 75:5967–5973. doi: 10.1128/IAI.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arévalo-Herrera M, Forero-Peña DA, Rubiano K, Gómez-Hincapie J, Martínez NL, Lopez-Perez M, Castellanos A, Céspedes N, Palacios R, Oñate JM, Herrera S. 2014. Plasmodium vivax sporozoite challenge in malaria-naive and semi-immune Colombian volunteers. PLoS One 9:e99754. doi: 10.1371/journal.pone.0099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obiero JM, Shekalaghe S, Hermsen CC, Mpina M, Bijker EM, Roestenberg M, Teelen K, Billingsley PF, Sim BK, James ER, Daubenberger CA, Hoffman SL, Abdulla S, Sauerwein RW, Scholzen A. 2015. Impact of malaria preexposure on antiparasite cellular and humoral immune responses after controlled human malaria infection. Infect Immun 83:2185–2196. doi: 10.1128/IAI.03069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher CR, Sutton HJ, Kaczmarski JA, McNamara HA, Clifton B, Mitchell J, Cai Y, Dups JN, D’Arcy NJ, Singh M, Chuah A, Peat TS, Jackson CJ, Cockburn IA. 2017. T-dependent B cell responses to Plasmodium induce antibodies that form a high-avidity multivalent complex with the circumsporozoite protein. PLoS Pathog 13:e1006469. doi: 10.1371/journal.ppat.1006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schofield L, Uadia P. 1990. Lack of Ir gene control in the immune response to malaria. I. A thymus-independent antibody response to the repetitive surface protein of sporozoites. J Immunol 144:2781–2788. [PubMed] [Google Scholar]
- 27.Noland GS, Hendel-Paterson B, Min XM, Moormann AM, Vulule JM, Narum DL, Lanar DE, Kazura JW, John CC. 2008. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun 76:5721–5728. doi: 10.1128/IAI.00591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. 2007. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J 6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longley RJ, White MT, Takashima E, Morita M, Kanoi BN, Li Wai Suen CSN, Betuela I, Kuehn A, Sripoorote P, Franca CT, Siba P, Robinson LJ, Lacerda M, Sattabongkot J, Tsuboi T, Mueller I. 2017. Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PLoS Negl Trop Dis 11:e0005888. doi: 10.1371/journal.pntd.0005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nussenzweig RS, Chen D. 1974. The antibody response to sporozoites of simian and human malaria parasites: its stage and species specificity and strain cross-reactivity. Bull World Health Organ 50:293–297. [PMC free article] [PubMed] [Google Scholar]
- 31.Barlow DJ, Edwards MS, Thornton JM. 1986. Continuous and discontinuous protein antigenic determinants. Nature 322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- 32.Doud MB, Koksal AC, Mi LZ, Song G, Lu C, Springer TA. 2012. Unexpected fold in the circumsporozoite protein target of malaria vaccines. Proc Natl Acad Sci U S A 109:7817–7822. doi: 10.1073/pnas.1205737109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haste Andersen P, Nielsen M, Lund O. 2006. Prediction of residues in discontinuous B-cell epitopes using protein 3D structures. Protein Sci 15:2558–2567. doi: 10.1110/ps.062405906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babon JJ, Morgan WD, Kelly G, Eccleston JF, Feeney J, Holder AA. 2007. Structural studies on Plasmodium vivax merozoite surface protein-1. Mol Biochem Parasitol 153:31–40. doi: 10.1016/j.molbiopara.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Morgan WD, Birdsall B, Frenkiel TA, Gradwell MG, Burghaus PA, Syed SE, Uthaipibull C, Holder AA, Feeney J. 1999. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J Mol Biol 289:113–122. doi: 10.1006/jmbi.1999.2753. [DOI] [PubMed] [Google Scholar]
- 36.Pizarro JC, Chitarra V, Verger D, Holm I, Petres S, Dartevelle S, Nato F, Longacre S, Bentley GA. 2003. Crystal structure of a Fab complex formed with PfMSP1-19, the C-terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: a malaria vaccine candidate. J Mol Biol 328:1091–1103. doi: 10.1016/S0022-2836(03)00376-0. [DOI] [PubMed] [Google Scholar]
- 37.Offeddu V, Thathy V, Marsh K, Matuschewski K. 2012. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol 42:535–548. doi: 10.1016/j.ijpara.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, Masika P, Mosha J, Bousema T, Shekalaghe S, Cook J, Corran P, Ghani A, Riley EM, Drakeley C. 2009. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One 4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Druilhe P, Pradier O, Marc JP, Miltgen F, Mazier D, Parent G. 1986. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect Immun 53:393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hope IA, Hall R, Simmons DL, Hyde JE, Scaife JG. 1984. Evidence for immunological cross-reaction between sporozoites and blood stages of a human malaria parasite. Nature 308:191–194. doi: 10.1038/308191a0. [DOI] [PubMed] [Google Scholar]
- 41.Cochrane AH, Maracic M. 1991. Blood stage-induced Plasmodium brasilianum infection in the squirrel monkey induces antibodies which react with the circumsporozoite protein. Infect Immun 59:1180–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett JW, Yadava A, Tosh D, Sattabongkot J, Komisar J, Ware LA, McCarthy WF, Cowden JJ, Regules J, Spring MD, Paolino K, Hartzell JD, Cummings JF, Richie TL, Lumsden J, Kamau E, Murphy J, Lee C, Parekh F, Birkett A, Cohen J, Ballou WR, Polhemus ME, Vanloubbeeck YF, Vekemans J, Ockenhouse CF. 2016. Phase 1/2a trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Dis 10:e0004423. doi: 10.1371/journal.pntd.0004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spring M, Polhemus M, Ockenhouse C. 2014. Controlled human malaria infection. J Infect Dis 209(Suppl 2):S40–S45. doi: 10.1093/infdis/jiu063. [DOI] [PubMed] [Google Scholar]
- 44.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, Davidson SA, Dutta S, Griffith ME, Magee CD, Wojnarski M, Livezey JR, Kress AT, Waterman PE, Jongert E, Wille-Reece U, Volkmuth W, Emerling D, Robinson WH, Lievens M, Morelle D, Lee CK, Yassin-Rajkumar B, Weltzin R, Cohen J, Paris RM, Waters NC, Birkett AJ, Kaslow DC, Ballou WR, Ockenhouse CF, Vekemans J. 13 June 2016. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria infection and immunogenicity study. J Infect Dis doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 45.Ockenhouse CF, Regules J, Tosh D, Cowden J, Kathcart A, Cummings J, Paolino K, Moon J, Komisar J, Kamau E, Oliver T, Chhoeu A, Murphy J, Lyke K, Laurens M, Birkett A, Lee C, Weltzin R, Wille-Reece U, Sedegah M, Hendriks J, Versteege I, Pau MG, Sadoff J, Vanloubbeeck Y, Lievens M, Heerwegh D, Moris P, Guerra Mendoza Y, Jongert E, Cohen J, Voss G, Ballou WR, Vekemans J. 2015. Ad35.CS.01-RTS,S/AS01 heterologous prime boost vaccine efficacy against sporozoite challenge in healthy malaria-naive adults. PLoS One 10:e0131571. doi: 10.1371/journal.pone.0131571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell BA, Wood JF, Bansal R, Ragab H, Cargo J III, Washington MA, Wood CL, Ware LA, Ockenhouse CF, Yadava A. 2009. Process development for the production of an E. coli produced clinical grade recombinant malaria vaccine for Plasmodium vivax. Vaccine 27:1448–1453. doi: 10.1016/j.vaccine.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 47.Yadava A, Sattabongkot J, Washington MA, Ware LA, Majam V, Zheng H, Kumar S, Ockenhouse CF. 2007. A novel chimeric Plasmodium vivax circumsporozoite protein induces biologically functional antibodies that recognize both VK210 and VK247 sporozoites. Infect Immun 75:1177–1185. doi: 10.1128/IAI.01667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwenk R, DeBot M, Porter M, Nikki J, Rein L, Spaccapelo R, Crisanti A, Wightman PD, Ockenhouse CF, Dutta S. 2014. IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice. PLoS One 9:e111020. doi: 10.1371/journal.pone.0111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dutta S, Ware LA, Barbosa A, Ockenhouse CF, Lanar DE. 2001. Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli. Infect Immun 69:5464–5470. doi: 10.1128/IAI.69.9.5464-5470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Angov E, Aufiero BM, Turgeon AM, Van Handenhove M, Ockenhouse CF, Kester KE, Walsh DS, McBride JS, Dubois MC, Cohen J, Haynes JD, Eckels KH, Heppner DG, Ballou WR, Diggs CL, Lyon JA. 2003. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol Biochem Parasitol 128:195–204. doi: 10.1016/S0166-6851(03)00077-X. [DOI] [PubMed] [Google Scholar]
- 51.Saha S, Raghava GP. 2006. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 65:40–48. doi: 10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- 52.Saha S, Bhasin M, Raghava GP. 2005. Bcipep: a database of B-cell epitopes. BMC Genomics 6:79. doi: 10.1186/1471-2164-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponomarenko J, Bui HH, Li W, Fusseder N, Bourne PE, Sette A, Peters B. 2008. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics 9:514. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. 2014. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. 2009. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc 4:1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.