Animal models have played a key role in providing an understanding of the mechanisms that govern the pathophysiology of intestinal diseases. To expand on the repertoire of organisms available to study enteric diseases, we report on the use of the Drosophila melanogaster model to identify a novel function of an effector protein secreted by Vibrio parahaemolyticus, which is an enteric pathogen found in contaminated seafood.
KEYWORDS: Drosophila melanogaster, FAK, Vibrio parahaemolyticus, VopA, antiapoptotic, genetic models
ABSTRACT
Animal models have played a key role in providing an understanding of the mechanisms that govern the pathophysiology of intestinal diseases. To expand on the repertoire of organisms available to study enteric diseases, we report on the use of the Drosophila melanogaster model to identify a novel function of an effector protein secreted by Vibrio parahaemolyticus, which is an enteric pathogen found in contaminated seafood. During pathogenesis, V. parahaemolyticus secretes effector proteins that usurp the host’s innate immune signaling pathways, thus allowing the bacterium to evade detection by the innate immune system. One secreted effector protein, VopA, has potent inhibitory effects on mitogen-activated protein kinase (MAPK) signaling pathways via the acetylation of critical residues within the catalytic loops of mitogen-activated protein kinase kinases (MAPKKs). Using the Drosophila model and cultured mammalian cells, we show that VopA also has potent modulating activity on focal adhesion complex (FAC) proteins, where VopA markedly reduced the levels of focal adhesion kinase (FAK) phosphorylation at Ser910, whereas the phosphorylation levels of FAK at Tyr397 and Tyr861 were markedly increased. Cultured cells expressing VopA were also impaired in their ability to migrate and repopulate areas subjected to a scratch wound. Consistently, expression of VopA in Drosophila midgut enterocytes disrupted the normal enterocyte arrangement. Finally, VopA inhibited apoptosis in both Drosophila tissues and mammalian cultured cells. Together, our data show that VopA can alter normal intestinal homeostatic processes to facilitate opportunities for V. parahaemolyticus to prolong infection within the host.
INTRODUCTION
Drosophila melanogaster is widely used as a genetically tractable animal model for discovering the mechanisms of diseases. Many fundamental physiological processes are conserved between Drosophila and mammals, with a homolog of about 75% of human disease-associated genes being present in the fly (1). In identifying the mechanisms whereby pathogenic bacteria elicit enteric diseases, the traditional methodology, mainly performed in rodent models, has employed the approach of infecting mice with either a wild-type or a mutated strain of pathogenic bacteria (2–4). Alternatively, the genetic tractability of Drosophila allows for a more reductionist approach to discovering the mechanism of bacterial pathogenesis. In the fly, the open reading frames of bacterial virulence factors can be inserted into the Drosophila chromosome, thereby creating a transgenic fly stably harboring the bacterial virulence factor. By specifically expressing the virulence factor in Drosophila tissue with determinate growth, such as the eye or wing, novel mechanisms of bacterial protein modulation of innate immunity have been revealed (5, 6). However, to date, few studies have taken the approach of ectopically expressing bacterial virulence factors in Drosophila intestinal epithelial cells. This is an overlooked opportunity because the Drosophila and human intestine share impressive transkingdom functional conservation, where the fly gut harbors many of the same cell types as the mammalian gut and common cell signaling pathways function in intestinal homeostasis and response to injury (7, 8). In addition, flies and mammals share conserved mechanisms of host cell and commensal microbe interactions (9, 10).
The gut pathogen Vibrio parahaemolyticus is a causal agent of gastroenteritis, typically following ingestion of contaminated seafood. Symptoms associated with disease include vomiting, abdominal cramping, and diarrhea, although illness is commonly acute and self-limiting (11, 12). V. parahaemolyticus harbors two type III secretion systems (T3SS), one on chromosome 1 (T3SS1) and the other on chromosome 2 (T3SS2) (13), with the genes on each of the secretion systems contributing to pathogenic infection (14). One secreted protein coded on T3SS2 is VopA (also known as VopP). Biochemical analysis of the VopA function revealed that it is a potent inhibitor of mitogen-activated protein kinase (MAPK) signaling (15, 16). However, the pathological outcome of VopA activity remains unclear. VopA is a member of the YopJ-like family of bacterial effector proteins, which includes YopJ from Yersinia enterocolitica (17), AvrA of Salmonella enterica serovar Typhimurium (2, 6, 18), and AopP of Aeromonas salmonicida (5, 19), all of which are known enteropathogens. VopA shares amino acid sequence similarity with YopJ at 45%, with AvrA at 48%, and with AopP at 51% over the whole length of the protein, with a highly conserved region of >90% amino acid similarity spanning a 20-amino-acid region that aligns with residues 175 to 195 of AvrA (Fig. 1A). This region harbors a conserved cysteine residue essential for the catalytic activity of each effector protein. VopA has been shown to have inhibitory effects against the extracellular signal-regulated kinase (ERK), p38, and Jun N-terminal protein kinase (JNK) (MAPK) pathways by a mechanism that involves the acetylation of the Ser, Thr, and Lys residues of the catalytic and activation loops of mitogen-activated protein kinase kinases (MAPKKs), residues which are normally phosphorylated during pathway activation (16). Acetyltransferase activity has also been shown in other YopJ-like proteins, including YopJ and AvrA (6, 20), although it is known that each family member has evolved a specificity of activity against particular members of the mammalian MAPKK and IκB kinase (IKK) superfamily (Fig. 1B) (21). Furthermore, the specificity of the YopJ-like protein against MAPK or NF-κB has the potential to influence cell fate during infection. For example, YopJ induces apoptosis and dampens cytokine production due to its broad inhibition of the MAPK and NF-κB pathways (22). AopP is proapoptotic due to it being a potent inhibitor of NF-κB signaling (5), whereas AvrA is antiapoptotic due to its inhibitory activity against the JNK pathway (2, 6). This property of AvrA has recently been exploited in the generation of nanoparticles harboring AvrA for the treatment of inflammatory bowel disease (23).
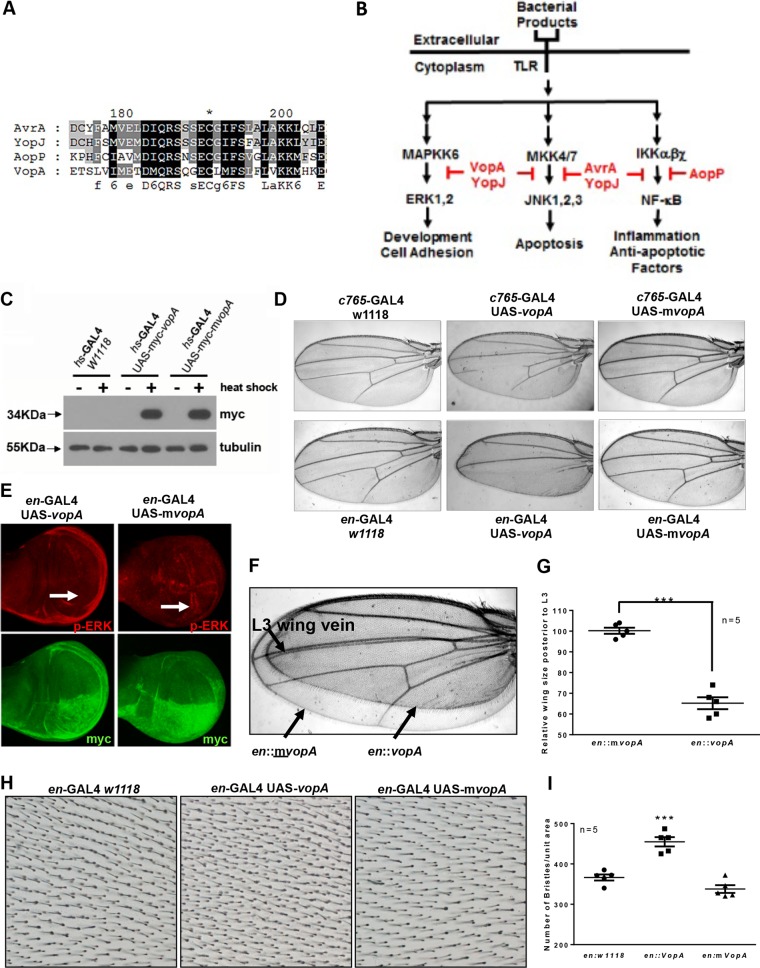
FIG 1.
VopA inhibits ERK pathway signaling in developing Drosophila tissues. (A) Alignment of the amino acid sequences of VopA, AvrA, AopP, and YopJ over a highly conserved region present in each protein that aligns with residues 175 to 205 of the AvrA sequence. The asterisk denotes the conserved cysteine residue essential for the enzymatic activity of each of the aligned proteins. Black shading denotes residues similar in all four proteins, dark gray denotes residues similar in three of the aligned proteins, and light gray denotes residues similar in only two of the aligned proteins. (B) Recognition of bacterial products by TLRs results in the activation of innate immune pathways that function to eliminate the bacterial threat. At the same time, some pathogens secrete effector proteins, including VopA, AvrA, AopP, or YopJ, which usurp signaling pathways that transduce innate immune alarm messages from the cell surface to the nucleus. The blockade of innate immune signaling allows enteropathogens to persist undetected in the mucosa and evade host innate immunity. (C) Expression of the vopA and mvopA transgenes in flies of the indicated genotypes following 24 h of heat shock of third instar larvae. Each lane was loaded with 20 μg total protein extracted from larval whole bodies. Note the presence of a band following immunoblotting with myc antibody signifying expression of myc-VopA or myc-mVopA following heat shock. The blot is representative of blots from experiments done in triplicate. (D) Phenotypes of Drosophila adult wings expressing VopA or mVopA under c756-GAL4 (top) or engrailed-GAL4 (en-GAL4) (bottom). (E) Immunostain analysis of Drosophila third instar larvae wing disks expressing VopA or mVopA under en-GAL4 with antibodies against phosphorylated ERK and myc. Note the VopA-mediated loss of p-ERK activity in the posterior wing disk (white arrows). (F) Superimposition of adult Drosophila wings of phenotypes en-GAL4::vopA (smaller wing) and en-GAL4::mvopA, presented in panel D. The smaller-wing phenotype was observed at 100% penetrance in en-GAL4::vopA flies. (G) Quantification of the size of the adult wing posterior to the L3 wing vein from the phenotypes described in panel D. The average measured wing size of the control en::mvopA fly was taken as 100%, and the area for the en::vopA fly is presented as a value relative to that for the en::mvopA fly. Statistical analysis was calculated by Student's t test (n = 5). ***, P < 0.001. (H) Wing bristles within the posterior adult wings of the indicated phenotypes. (I) Quantification of the number of wing bristles within the selected area of the posterior adult wing in panel F. Statistical analysis was performed by one-way ANOVA (n = 5). ***, P < 0.001.
MAPKs have been shown to function in many cellular processes, including proliferation, oncogenesis, differentiation, inflammation, and stress responses. Recently, MAPK signaling has been reported to play a role in regulating cell migration, with both the ERK and JNK pathways being reported to function as mediators of extracellular signals that induce cell movement by interactions with proteins of the focal adhesion complex (FAC) (24, 25). Because VopA is coded within T3SS2 in V. parahaemolyticus and thus may be secreted by V. parahaemolyticus into cells of the epithelium, including enterocytes, we reasoned that VopA may have modulatory influences on gut epithelial cell migration and normal homeostasis in the intestine. Herein, we report on the outcome of VopA activity in enterocytes using a novel approach in Drosophila. Consistent with its known activity in cultured mammalian cells, VopA potently inhibited ERK pathway activity in Drosophila enterocytes, showing transkingdom conservation of VopA activity. Ectopic VopA expression in Drosophila intestinal epithelial cells resulted in increased organismal mortality, disrupted epithelial cell arrangement, and resulted in fewer apoptotic cells in the Drosophila midgut. Consistent with these observations, in cultured mammalian cells, VopA disrupted focal adhesion complex (FAC) dynamics by altering the phosphorylation states of critical regulatory residues within focal adhesion kinase (FAK), Paxillin, and Src. In addition, transfection of VopA significantly slowed the movement and migration of cultured enterocytes. Together, these results suggest that V. parahaemolyticus has evolved the VopA protein to have refined biochemical activity that inhibits the migration of enterocytes and the apoptotic response that enterocytes undergo when sensing an irreversible pathogenic threat, thus highlighting the delicate modification of host responses that some bacteria can engender.
RESULTS
VopA inhibitory activity is conserved in Drosophila.
A limitation of investigating V. parahaemolyticus pathogenicity is that an oral infection-based animal model of the human disease is still in development. This paucity was recently addressed by investigators using infant rabbit, porcine, and murine models, with some encouraging advancements in disease models being seen (14, 26, 27). Alternatively, our research group has successfully used the Drosophila animal model to study the function of bacterial effector proteins secreted by pathogens (5, 6). The utility of the Drosophila system is that it allows us to undertake initial discoveries that may then be confirmed in a well-developed and relevant mammalian model. To establish the feasibility of using Drosophila to study host/pathogen interactions in the midgut, we assessed whether the VopA inhibitory profile is conserved in the fly. We created transgenic Drosophila flies harboring either upstream activation sequence (UAS)-myc-vopA or the catalytically inactive UAS-myc- mvopA (C181A) mutant form. Because a functional ERK pathway is necessary for the normal development of the Drosophila wing, disruption of ERK pathway signaling results in markedly altered phenotypes (28–30). We first detected expression of the transgenes by crossing fly lines harboring UAS-myc-vopA and UAS-myc-mvopA to the heat shock (hs)-GAL4 driver line, which expresses the Saccharomyces cerevisiae yeast transactivator GAL4 in all cells upon incubation of Drosophila at 30°C (Fig. 1C). A tissue that requires a functional ERK pathway for development is the wing, where inhibition of ERK pathway signaling results in disruption of wing vein formation (29, 30). Consistently, VopA expression under c765-GAL4 or with engrailed-GAL4 (en-GAL4), which targets expression to the posterior compartment of the wing, resulted in the complete loss of wing vein formation from the posterior wing (Fig. 1D). Immunostaining of developing wing imaginal disks expressing VopA under en-GAL4 revealed a marked decrease in the levels of phosphorylated ERK in the posterior wing, whereas phosphorylated ERK was detected in the wing margin on the anterior portion of the same wing or in the entire wing margin tissue expressing a mutated and catalytically inactive version of VopA (mVopA) under en-GAL4 (Fig. 1E). Overlay of VopA- and mVopA-expressing wings revealed that VopA expression results in about a 30% decrease in wing size (Fig. 1F and G). However, enumeration of wing bristles within the posterior wing revealed about a 30% denser number of bristles per unit area of the VopA-expressing wing tissue (Fig. 1H and I). Furthermore, the arrangement of bristles in VopA-expressing wing tissue was noticeably altered, where bristles are not formed in a regular diagonal arrangement, as is evident in the wild type of mVopA-expressing wing tissue (Fig. 1H). Together, these data indicate that ectopic VopA activity in the Drosophila wing tissue results in ERK pathway inhibition and altered tissue phenotypes characteristic of the inhibition of tissue development. Furthermore, although VopA reduced the wing size by about 30%, the increased numbers of bristles per unit area of the same wing indicate that VopA-expressing cells are smaller but have no detectable differences in proliferation during development.
VopA disrupts epithelial homeostasis in the Drosophila intestine.
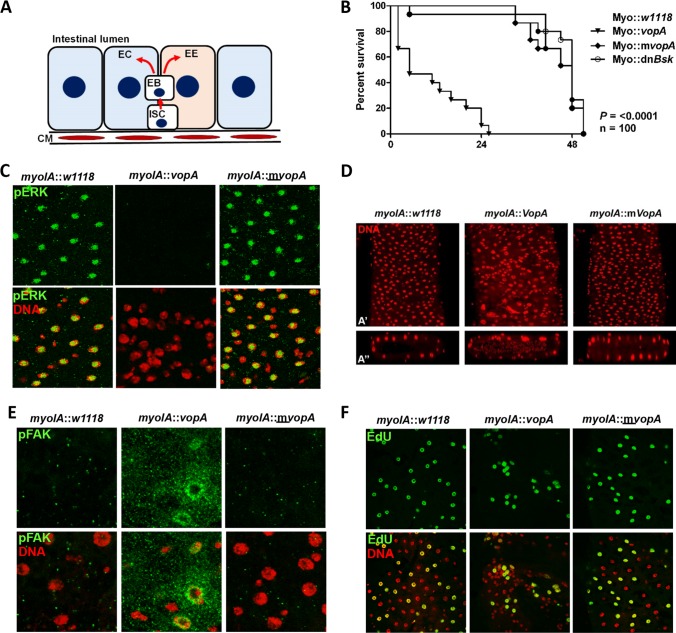
To model the influence of VopA in isolation in an in vivo model, we ectopically expressed VopA under the myoIA-GAL4 driver, which specifically expresses GAL4 in fly enterocytes but not in proliferating intestinal stem cells (ISCs) in the fly midgut (10). Many articles comparing the development of the fly and mammalian gut and detailing the fly’s relevance as a model for human intestinal physiology have been published (1, 31–33). Briefly, the Drosophila midgut is made up of an epithelial monolayer interspersed with hormone-producing enteroendocrine cells. Adult Drosophila midgut cells are continuously replenished by a population of pluripotent ISCs. The ISCs adjoin the intestinal basement membrane, their progenitors, and differentiated enterocytes (34, 35) (Fig. 2A). We first observed a significant loss of organismal viability following expression of VopA in the adult Drosophila midgut enterocytes, indicating that VopA may influence gut physiology and/or homeostasis (Fig. 2B). Importantly, inhibition of JNK pathway signaling by the constitutive expression of a dominant negative form of Basket (Bsk) did not influence survival, indicating that the loss of viability may be due to the inhibition of ERK pathway signaling (Fig. 2B). Because the decrease in survival is as a result of gut enterocyte-specific expression of VopA, we anticipated that the loss of viability may be as a result of an impairment to gut function and/or barrier integrity. Therefore, we investigated the effects of VopA expression of the gut tissue architecture and cell signaling pathways. Immunostain analysis of 7-day-old adult Drosophila distal midgut for phosphorylated ERK (p-ERK) revealed strong staining in the nucleus of enterocytes in control flies, whereas no p-ERK was detected in enterocytes expressing VopA (Fig. 2C, top). In addition, expression of VopA in enterocytes resulted in marked changes in cell arrangement in the distal midgut, with the loss of the regular enterocyte patterning compared to that in the controls being observed (Fig. 2C and D). Furthermore, Drosophila enterocytes in the distal midgut are normally arranged as a layer of single epithelial cells overlaying a basement membrane and circular muscle. A transverse section through the Drosophila gut by confocal z-stack analysis revealed that a disruption of the normal architecture was altered in VopA-expressing enterocytes (Fig. 2D, bottom). The movement of enterocytes in the gut is controlled, in part, by the activity of proteins that form the focal adhesion complex (FAC), including focal adhesion kinase (FAK) (24, 25). Immunostaining of the Drosophila midgut using an antibody against phospho-FAK Tyr397 revealed a marked increase in the fluorescent signal in VopA-expressing enterocytes (Fig. 2E). Finally, pulse analysis of 5-ethynyl-2′-deoxyuridine (EdU) incorporation into midgut cells revealed no marked difference in the total number of cells which had incorporated EdU over a 24-h period but did reveal differences in their distribution. Here, EdU incorporates into ISCs, which then mature and differentiate into enterocytes. Enterocytes organize in the fly midgut to distinct special patterning, as seen following expression in control, myoIA::w1118, or mutant myoIA::mVopA cells. However, enterocyte-specific expression of VopA results in EdU-positive cells being arranged in distinct clusters (Fig. 2F). Because VopA is not expressed in ISCs, the results point to VopA-mediated enterocyte disorganization occurring as a result of the inability of enterocytes to spatially organize, which may be as a result of VopA-mediated inhibition of ERK and FAC protein signaling. Together, we show that VopA alone can decrease p-ERK levels, increase FAK phosphorylation at Tyr397, and alter the normal enterocyte arrangement in the Drosophila intestine.
FIG 2.
VopA disrupts epithelial homeostasis in the Drosophila intestine. (A) Schematic depiction of a transverse section of an adult Drosophila midgut. ISC, intestinal stem cell; EC, enterocyte; EB, enteroblast; CM, circular muscle; EE, enteroendocrine cells. Note that the enteroblast in adult Drosophila flies is equivalent to transit-amplifying cells in mammals. ISCs proliferate to generate enteroblasts. The enteroblasts then differentiate to either enterocytes or EEs, which form the gut epithelium in adult Drosophila flies. (B) Life span of adult Drosophila expressing VopA, mVopA, or dominant negative Basket (dnBsk) under myoIA-GAL4. Temperature-sensitive GAL80 (tsGAL80) was also included in each genetic background. Drosophila flies were propagated at 18°C until they were 2-day-old adults, whereupon they were moved to 29°C. Percent survival was recorded at regular intervals. Data are for 100 flies per group. Data are representative of those from experiments undertaken in triplicate. (C) Immunostaining of 7-day-old adult Drosophila midguts of the indicated genotypes in panel B using an antibody against p-ERK. Images were captured by confocal microscopy at a ×40 magnification. (D) Staining of DNA within the distal midgut of Drosophila expressing VopA or mVopA under myoIA-GAL4. (A′) Enface image; (Aʺ) transverse section generated by z-stack analysis of images captured by confocal microscopy. (E) Immunostaining of 7-day-old adult Drosophila posterior midguts dissected from the indicated genotypes from panel B using an antibody against FAK phosphorylated at Tyr397 (p-FAKTyr397). Images captured by confocal microscopy at a ×100 magnification. (F) Detection of proliferating cells in the adult Drosophila posterior midgut by chase analysis. Seven-day-old adult Drosophila flies were fed EdU for 24 h, and cellular EdU incorporation was detected by confocal microscopy at a ×20 magnification. For the images presented in panels C to F, the VopA-mediated phenotype was detected with 100% penetrance, and the images presented are representative of 10 intestinal dissections (n = 10) per group.
VopA alters the phosphorylation state of FAC proteins.
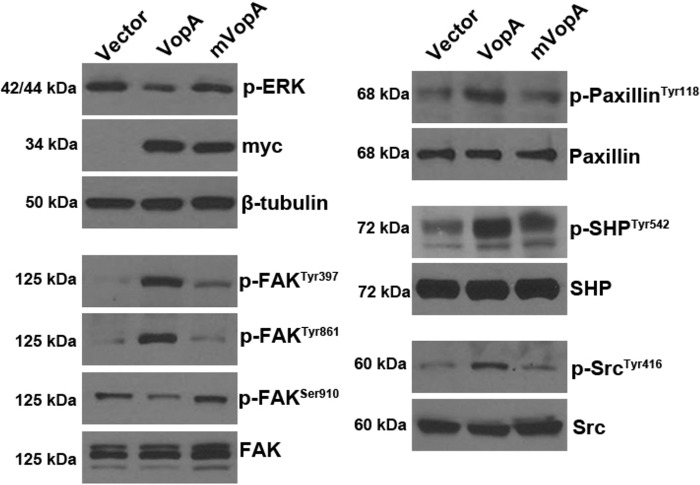
To establish if VopA alters the activity of focal adhesion complex (FAC) proteins in cultured mammalian cells, plasmids harboring either wild-type VopA or a mutated and catalytically inactive version of VopA (mVopA) with a cysteine-to-alanine transition at position 181 were transfected into HEK293 cultured cells. Consistent with previous established reports (15, 16), VopA activity resulted in reduced levels of phosphorylated ERK at steady state (Fig. 3). Strikingly, VopA markedly elevated the levels of phosphorylation of FAK at Tyr397 and Tyr861, whereas, in contrast, VopA mediated reduced levels of FAK phosphorylation at Ser910 (Fig. 3). In addition, VopA caused elevated levels of phosphorylation of Paxillin at Tyr118, SHP-2 phosphorylation at Tyr542, and Src phosphorylation at Tyr416 (Fig. 3). Together, these data show that VopA-mediated inhibition of ERK has marked downstream influences on the activity of proteins that make up the FAC and associated signaling pathways. Importantly, these changes were detected at steady state and not following serum starvation and pathway induction, as is commonly carried out to reveal effector protein activity on the cell.
FIG 3.
VopA alters the phosphorylation state of proteins that make up the focal adhesion complex (FAC). The results of immunoblot analysis of lysates from cultured HEK293 cells transfected with plasmid vector pCMV (control) or pCMV harboring myc-vopA or a mutant and catalytically inactive species of vopA, myc-mvopA (C181A), for 24 h are shown. Cell lysate (0.5 μg) was loaded into each well, and protein abundance was assayed using antibodies against the indicated proteins. The data presented are representative of those from three independent experiments (n = 3). p-Paxillin, phosphorylated Paxillin.
VopA inhibits migration of cultured enterocytes.
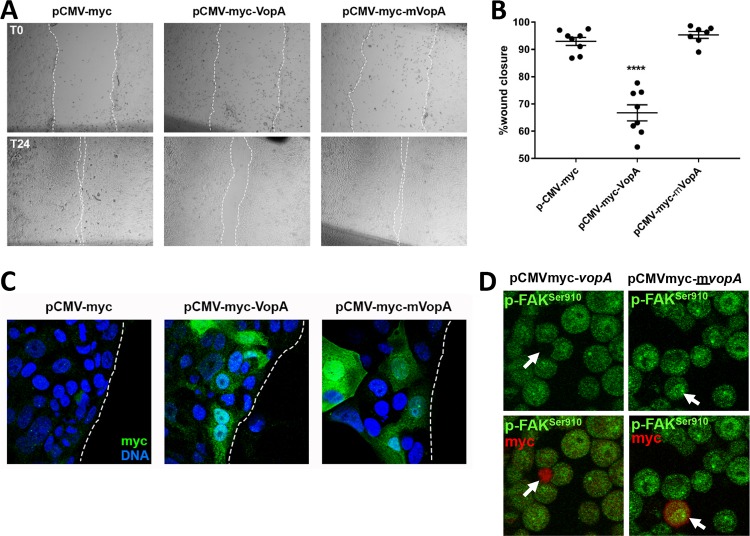
Focal adhesion complexes (FACs) are macromolecular protein assemblies that, through their dynamic assembly and disassembly, mediate enterocyte migration (36). FAC assembly and disassembly are controlled by the phosphorylation and protein complex constituents, including FAK. We thus reasoned that VopA may have modulatory influences on enterocyte migration. Indeed, cultured SKCO15 cells, which are cells of a human intestinal epithelial cell (IEC) line, transfected with plasmids harboring vopA had a significantly reduced capacity to repopulate an area of the confluent cell monolayer damaged by a needle-inflicted scratch wound compared to cells transfected with the mutant and catalytically inactive form, mvop (Fig. 4A to C). SKCO15 cells have a modest transfection efficiency, which we estimated to be about 30% in this experiment (Fig. 4C). Nevertheless, this transfection rate was sufficient to elicit a potent inhibitory influence on the closure of a scratch wound in the scratch wound assay. Consistent with the findings presented in Fig. 3, immunostain analysis of transfected HEK293 cells revealed decreased levels of phosphorylated FAK at Ser910 in VopA-expressing HeLa cells, showing that the activity of VopA is not specific to one cell type (Fig. 4D). Together, these data show that VopA activity has measurable influences on cell movement.
FIG 4.
VopA inhibits migration of cultured enterocytes. (A) Images of scratch wounds inflicted by a needle on confluent monolayers of SKCO15 cultured cells transfected with plasmid vector pCMV (control) or with pCMV harboring myc-vopA or a mutant and catalytically inactive species of vopA, myc-mvopA (C181A). Wounds were inflicted at 24 h posttransfection (time zero [T0]) (top). Repopulation of the wound area was assessed at 24 h after wound infliction (time 24 h [T24]) (bottom). (B) Quantification of the percent closure of wounds inflicted by a needle scratch shown in panel A. Data are represented as mean ± standard error of the mean (nonparametric unpaired t test; ****, P < 0.0001; n = 8). (C) Immunostain analysis of cells at the wound edge in the assays described in the legends to panels A and B using antibodies against myc to detect the transfection of myc-VopA, and myc-mVopA. (D) Immunostain analysis of cultured HeLa cells transfected with pCMV-myc-vopA or pCMV-myc-mvopA using an antibody against FAK phosphorylated at Ser910 and an antibody against the myc tag. White arrows point to transfected cells expressing VopA or mVopA.
VopA inhibits JNK pathway activity and apoptosis in Drosophila.
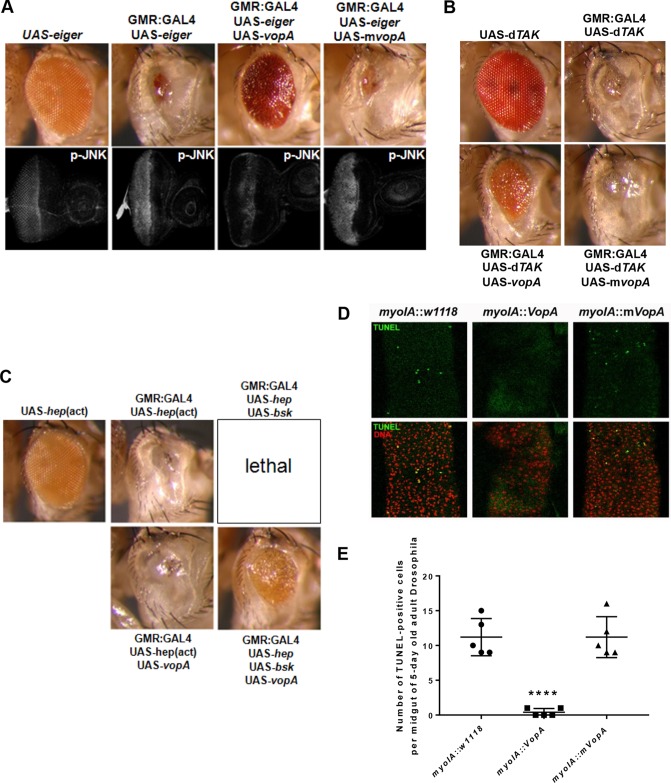
Our previous investigations showed that the S. enterica serovar Typhimurium-secreted protein AvrA dampens apoptosis by inhibiting JNK pathway signaling (2, 6). VopA has also been shown to be a potent inhibitor of JNK signaling in cultured mammalian cell systems (15). We thus investigated the extent to which VopA influences apoptosis in the fly. To this end, we induced JNK pathway activation and tissue apoptosis by constitutive expression of Eiger, the Drosophila ortholog of mammalian tumor necrosis factor alpha (TNF-α) (37). Eiger expression in Drosophila eye tissue caused an extreme-small-eye phenotype and elevated levels of phosphorylated JNK in eye imaginal disks of third instar larvae (Fig. 5A), findings which are consistent with past reports (6, 37). Coexpression of VopA with Eiger markedly rescued the small-eye phenotype (Fig. 5A) and inhibited the Eiger-induced phosphorylation of JNK in the developing eye, whereas mVopA expression exhibited no phenotypic change (Fig. 5A, bottom). A central regulator of JNK signaling is the mitogen-activated protein kinase kinase kinase (MAPKKK) family member Drosophila TAK-1 (dTAK-1). Constitutive expression of dTAK-1 is proapoptotic in Drosophila tissue. Similar to the findings obtained with Eiger, eye-specific expression of dTAK-1 also resulted in a strong small-eye phenotype (Fig. 5B). Coexpression of VopA with dTAK-1 markedly suppressed the small-eye phenotype, whereas coexpression of mVopA with dTAK-1 did not (Fig. 5B). These data indicate that VopA mediates its suppressive activity at the level of or downstream of dTAK-1. dTAK-1 is a MAPKKK that functions as a Jun N-terminal protein kinase kinase kinase (JNK-KK). Hemipterous (Hep) (mitogen-activated protein kinase kinase 4/7 [MKK4/7] in mammals) is the downstream MAPKK (Jun N-terminal protein kinase kinase [JNK-K]) in the JNK pathway. Expression of Hep or of Basket (Drosophila JNK) individually does not alter the eye phenotype. However, concurrent expression of Hep and Basket in the eye during development is lethal at the pupal stage. Importantly, expression of VopA with Hep and Basket in the eye resulted in a viable adult Drosophila fly with a markedly rescued eye phenotype (Fig. 5C). Furthermore, a constitutively active allele of Hemipterous [UAS-hep(act)] (with S326D and T330D amino acid replacements in the kinase activation loop) also mediated a rough-eye phenotype. This phenotype could not be reversed by VopA expression (Fig. 5C), indicating that VopA inhibits the JNK pathway at the level of Hep (MKK4/7). Similar to the natural loss of enterocytes at the tip of mammalian intestinal villi through anoikis, the Drosophila midgut cells undergo apoptosis (35). Consistent with these reports and as part of normal homeostasis in the Drosophila midgut, sporadic terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL)-positive cells were observed to be interdispersed between midgut enterocytes in control flies or flies with enterocyte-specific expression of mVopA (Fig. 5D and E). In contrast, few or no TUNEL-positive cells were detected in VopA-expressing enterocytes (Fig. 5D and 5E). Thus, consistent with the observation that VopA inhibits Eiger-induced apoptosis in Drosophila eye tissue, VopA expression in Drosophila also inhibits natural anoikis apoptosis in the Drosophila midgut.
FIG 5.
VopA inhibits JNK/Bsk phosphorylation resulting from constitutive Eiger or dTAK expression. (A) Adult Drosophila eye phenotypes resulting from constitutive Eiger expression or from constitutive coexpression of Eiger and VopA, with the genotypes indicated (top), and immunostain analysis of larval eye disks from the indicated phenotypes using antibodies against phosphorylated JNK (bottom). (B) Phenotypes of adult Drosophila eyes resulting from constitutive expression of dTAK-1 and from constitutive coexpression of dTAK-1 and VopA or mVopA, with the genotypes indicated. (C) Phenotypes of adult Drosophila eyes resulting from constitutive expression of a constitutively active form of Hemipterous [hep(act)] with VopA, from constitutive coexpression of Hemipterous (hep) and Basket (bsk), and from constitutive coexpression of Hemipterous, Basket, and VopA. (D) TUNEL analysis for the detection of apoptotic cells in the posterior midgut of flies of the indicated phenotypes. (E) Numeration of TUNEL-positive cells in panel D. ****, P < 0.0001 (n = 5). For panels A to C, representative images were observed in all eclosed adult flies of the indicated phenotype, indicating that the inhibitory effect of VopA on Eiger-induced JNK signaling in the eye occurred with 100% penetrance.
VopA inhibits apoptosis in cultured mammalian cells.
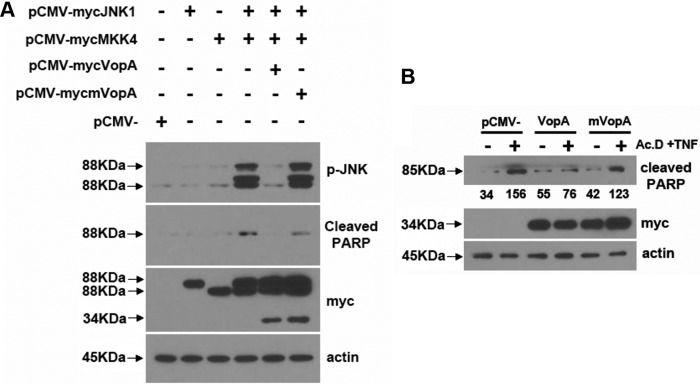
To corroborate the antiapoptotic activity detected in flies in mammalian cells, we activated the JNK pathway in human cultured cells with plasmids harboring JNK1 and MKK4, which together induce JNK phosphorylation and the ensuing cleavage of the apoptotic marker poly(ADP-ribose) polymerase (PARP). Cotransfection of plasmids harboring vopA totally abolished both MKK4-mediated JNK phosphorylation and PARP cleavage (Fig. 6A), thus indicating that VopA inhibits JNK activation and apoptosis at the level of MKK4 and/or JNK. In addition, cultured HeLa cells transfected with vopA had markedly less detectable cleaved PARP, which is a marker of apoptosis following the induction of cytotoxicity by the addition of actinomycin D and TNF-α for 24 h into the culture medium (Fig. 6B). These data indicate that the VopA protein, in isolation, not only can suppress the stress signaling JNK pathway but also can actively reduce markers of apoptosis in mammalian cells.
FIG 6.
VopA protects mammalian cultured cells from apoptosis. (A) Immunoblot analysis of lysates from cultured HEK293 cells transfected with the indicated plasmids, using antibodies against phosphorylated JNK or myc. (B) Immunoblot analysis of lysates from cultured HeLa cells transfected with the indicated plasmid before stimulation with actinomycin D (Ac.D) and TNF-α for 24 h, using an antibody against cleaved PARP or myc. The data presented are representative results from three independent experiments (n = 3).
DISCUSSION
Here, we show the utility of the Drosophila model to discover potential phenotypes elicited by the VopA effector protein in the intestinal epithelia. We show that the activity of VopA at inhibiting ERK MAPK signaling is conserved across kingdoms, i.e., between the fly and cultured mammalian cells. Examination of the phenotype elicited by VopA expression in Drosophila enterocytes, together with the known cross talk between ERK signaling and the FAC, pointed toward examining the effect of VopA on proteins involved in cell movement. To this end, we show that VopA has a modulating effect on FAC proteins, which was confirmed by immunoblot analysis of cultured mammalian cells transfected with VopA and functionally in a scratch wound assay measuring cell migration. The Drosophila model also revealed that VopA can elicit potent antiapoptotic activity in Drosophila tissue, which was also corroborated in cultured mammalian cells.
To date, VopA-related studies have focused on identifying the inhibitory spectrum and biochemical mechanisms of VopA, with less attention being given to the pathological outcome of VopA activity within cells. This in part is due to the lack of a satisfactory system to model V. parahaemolyticus infection, although advances were recently made to establish a mammalian model of disease (14, 26). The approach taken in many models is to infect subjects with the wild type or mutant variants of pathogens. The strength of using the Drosophila animal system is that a single protein can be ectopically expressed in target model tissue in the context of a whole organism (38). This reductionist approach has already been utilized to identify the pathogenic outcome of other pathogen-secreted proteins on host signaling networks (5, 6, 39–41). We show that VopA expression in the Drosophila developing wing leads to altered tissue phenotypes, including the loss of wing vein formation and reduced cell growth, consistent with the known function of ERK signaling in wing development (30). However, the Drosophila wing is a tissue of determinate size and is thus not a satisfactory model for tissues that undergo constant regeneration and homeostasis, as occur in the metazoan gut. The recent availability of a midgut enterocyte-specific myoIA-GAL4 driver fly enabled us to directly express a secreted protein from an enteric pathogen within the gut enterocytes of Drosophila. Furthermore, Drosophila is a suitable model organism due to the physiological similarities and developmental similarities between the fly midgut and the mammalian intestine (31).
The VopA protein is secreted by V. parahaemolyticus, which is predominantly an extracellular pathogen, as determined by strong supporting data from an infant rabbit model of infection (26), although other reports suggest that it may also actively invade nonphagocytic cells (42). A critical step in its pathogenesis is the initial binding to the host cell, where adhesion factors on the V. parahaemolyticus surface contact with host enterocytes to facilitate secretion of effector proteins from the bacteria to the host cell. These adhesion factors include the constitutively expressed bacterial protein multivalent adhesion molecule 7 (MAM7), which is conserved in many Gram-negative pathogens and which functions in establishing the immediate contact of V. parahaemolyticus with host cells, before the upregulation of other adhesion proteins and the subsequent secretion of virulence factors during pathogenesis (43, 44). At the same time, host cells express surface pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), that function as sentinels that detect microbe-associated molecular patterns (MAMPs). TLR-mediated sensing of specific MAMPs triggers MyD88- and TAK-1-dependent innate immune NF-κB and JNK pathway activation, which induces the secretion of proinflammatory cytokines or induces apoptosis, depending on the strength and duration of the MAMP signal (45). Therefore, strategies evolved by bacteria to subvert innate immunity will enhance their chances of prolonged survival within the intestine and opportunities for pathogenic activity.
Secreted effector proteins are one strategy evolved by pathogens to dampen the innate immune response, and these effector proteins are known to inhibit the activation of a number of cellular signaling pathways (46). As mentioned, members of the YopJ-like family of proteins have differential inhibitory effects on substrates at the MAPKK level (Fig. 1B). Moreover, MAPK pathways are known to cross talk with many other signaling pathways, including the FAK pathway (47). We confirmed the secondary effects of ERK pathway blockade on Src-FAK signaling both in cultured cells and in the Drosophila midgut and thus show the effects of an effector protein on factors that influence cell movement. This is significant because during intestinal homeostasis enterocytes of the intestine rapidly migrate from the bottom of the crypt to the villus tip, with a transit time of about 5 days (48). Once at the villus tip, enterocytes undergo anoikis, where cells detach from the surrounding extracellular matrix (ECM) and are eliminated into the fecal stream. By extension, any bacterium that is attached to the enterocyte or that has invaded the enterocyte is also eliminated. We speculate that inhibition of enterocyte migration would allow V. parahaemolyticus more time to remain attached to the enterocyte, thus increasing the chances of establishing a niche within the host. Similar to AvrA, which is secreted by S. enterica serovar Typhimurium (2, 5), VopA also has antiapoptotic activity both in cultured cells and in Drosophila enterocytes. Our data show that the VopA-mediated inhibition of apoptosis blocks the apoptotic fail-safe mechanism signaled through the JNK pathway that cells may undergo when they encounter an irreversible threat. Therefore, similar to slowing cell migration, inhibition of enterocyte apoptosis also increases the chances of V. parahaemolyticus establishing a niche in the host. Collectively, our data show the ability of VopA to influence specific MAPKK-mediated signaling, apoptosis, and cellular migration in a eukaryotic in vivo system.
As well as VopA, the V. parahaemolyticus type III secretion system on chromosome 2 (T3SS2) is known to secrete other effector proteins that are essential for colonization and pathogenicity. Another V. parahaemolyticus effector protein is VopZ, which was reported to inhibit the signaling of both the JNK and NF-κB pathways via its inhibitory activity on TAK-1 (49). The TAK-1 protein is a MAPKKK and is the upstream kinase that phosphorylates the JNK pathway intermediates MKK4/7, also known as Hemipterous in Drosophila. Both VopA and VopZ therefore exhibit activity that inhibits functionally related elements involved in the activation of JNK signaling. Interestingly, whereas the closest homologues to VopA, namely AvrA, YopJ, and AopP, all have reported inhibitory activity on NF-κB at the level of IKKβ (5, 6, 20), VopA, in contrast, apparently does not inhibit NF-κB pathway signaling (Fig. 1B). Therefore, V. parahaemolyticus may have necessarily evolved the VopZ effector protein to target NF-κB. Indeed, many of the V. parahaemolyticus-secreted effector proteins likely function synergistically during pathogenesis, independently targeting key cell signaling pathway intermediates to subvert the innate immune response and avoid detection and elimination by the host.
The utility of the Drosophila system is to make initial discoveries that may then be tested in a relevant mammalian model. Thus, a limitation of the current study is that the activity of VopA was not corroborated in a mammalian in vivo infection model, which needs to be performed. Nevertheless, by elucidating these VopA-mediated phenotypes in the fly and cultured cells, investigators now have a scientific premise on which to assay for them in a mammalian model. In addition, in light of the report suggesting that V. parahaemolyticus actively invades nonphagocytic cells (42), it is probable that invasion of enterocytes lining the mammalian intestine villus by V. parahaemolyticus does not occur in each enterocyte. Rather, invasion likely occurs only in a small number of cells, making such an analysis challenging and invasion difficult to detect in a mammalian in vivo infection model. Finally, the information generated in this study may be valuable, in light of the recent report of a study in which investigators exploited the inhibitory activity of AvrA on innate immunity in nanoparticles to treat inflammatory bowel disease (23). The nanoparticle study performed using AvrA revealed that expression of this class of protein at steady state in cells can by sufficient to dampen gut inflammation. Identification of enteric or other diseases that can be treated by modulating cell movement and apoptosis with VopA may point to a function for VopA in therapeutics.
MATERIALS AND METHODS
Plasmids and constructs.
Plasmids harboring the vopA or mvopA coding sequence were a gift from Kim Orth. The vopA and mvopA coding sequences were cloned into pCMV-myc, creating pCMV-myc-vopA and pCMV-myc-mvopA, respectively. The DNA amplicon myc-vopA or myc-mvopA was cloned into pP[UAST] (a gift from Kevin Moses), creating pP[UAST]-myc-vopA or pP[UAST]-myc-mvopA, respectively. The creation of pCMV-mycJNK1 and pCMV-mycMKK4 was described previously (6).
Drosophila lines.
The vectors pP[UAST]-myc-vopA and pP[UAST]-myc-mvopA were microinjected into w1118 embryos, creating fly lines harboring UAS-myc-vopA and UAS-myc-mvopA, respectively. Other fly stocks used included UAS-dTAK (50), UAS-eiger (37), UAS-hep(act), UAS-hep, and UAS-Bsk, all of which were obtained from the Bloomington Drosophila Stock Center. Driver line glass multiple reporter (GMR)-GAL4 (which specifically expresses GAL4 in omatidial cells during development) was a gift from Kevin Moses, c765-GAL4 (which expresses GAL4 in the wing pouch during development) was a gift from Daniel Marenda, and engrailed-GAL4 (which expresses GAL4 in the posterior compartment of the wing) was obtained from the Bloomington Drosophila Stock Center. myoIA-GAL4 was a gift from Shigeo Takashima (51).
Antibodies and reagents for Drosophila immunostaining and immunohistochemistry.
For immunostaining procedures, third instar larvae imaginal eye or wing disks were dissected in phosphate-buffered saline and fixed in 4% paraformaldehyde for 20 min. The tissues were washed 3 times for 10 min each time in 0.1% Triton X-100 and then placed in blocking solution (1% goat serum in 0.1% Triton X-100) for 30 min. The tissues were incubated in primary antibody overnight at 4°C with gentle rocking, before being washed 3 times for 10 min each time in 0.1% Triton X-100 and incubated in the secondary antibody for 1 h at ambient temperature. The antibodies used for Drosophila tissue immunostaining included anti-phospho-p44/42 MAPK (catalog number 4370), anti-phospho-FAK (Tyr397) (catalog number 3283), and β-tubulin (9F3) rabbit monoclonal antibody (catalog number 2128) (all from Cell Signaling Technology, Danvers, MA) and monoclonal anti-β-actin (clone AC-74; catalog number A2228; Sigma-Aldrich). DNA was stained with SYTO24 and TO-PRO-3 (Life Technologies, Grand Island, NY). Secondary antibody incubations were done using goat anti-rabbit immunoglobulin-Cy5 or goat anti-mouse immunoglobulin-fluorescein isothiocyanate (Jackson ImmunoReserach, West Grove, PA).
Immunoblot and immunostain analysis.
For immunoblot analysis, human embryonic kidney (HEK293) cells grown in tissue culture to 50% confluence in 24-well plates were transfected with 0.5 μg of plasmid pCMV-myc, pCMV-myc-vopA, or pCMV-myc-mvopA, using 0.75 μl of the Lipofectamine 2000 reagent per well. Control wells were transfected with a plasmid expressing green fluorescent protein in order to quantify the transfection efficiency. At 24 h posttransfection, the transfected cells were harvested in 100 μl Laemmli sample buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue, 0.125 M Tris HCl; pH, approximately 6.8) per well. The amount of protein was quantified using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific Pierce) before 0.5 μg of protein was loaded per well. The presence of specific proteins transferred to immunoblots was detected using the following antibodies: from Cell Signaling Technology (Danvers, MA), anti-phospho-p44/42 MAPK (catalog number 4370), anti-phospho-FAK (Tyr397) (catalog number 3283), anti-FAK (catalog number 3285), anti-phospho-Src (Tyr416) (catalog number 2101), anti-Src (catalog number 2109), anti phospho-Paxillin (Tyr118) (catalog number 2541), anti-Paxillin (catalog number 2542s), anti-phospho-SHP (Tyr542) (catalog number 3751s), and anti-SHP-2 (catalog number 3752); from BD Biosciences, anti-human c-Myc (catalog number 631206); and from Sigma-Aldrich, anti-phospho-FAK (pTyr861) (catalog number F9176) and monoclonal anti-β-actin (clone AC-74; catalog number A2228). Immunoreactive species were detected using anti-rabbit immunoglobulin-horseradish peroxidase (HRP) or anti-mouse immunoglobulin-HRP, followed by visualization with an ECL chemiluminescence detection reagent (GE Healthcare Biosciences, Piscataway, NJ). Experiments were conducted in triplicate, and the average density of the band was quantified using ImageJ software. For immunofluorescence analysis, HeLa cells were grown on coverslips submerged in cell culture dishes to 70% confluence, whereupon they were transfected with 0.5 μg of plasmid pCMV-myc, pCMV-myc-vopA, or pCMV-myc-mvopA. At 24 h posttransfection, the coverslips were recovered from the dishes and the cells were fixed in 4% paraformaldehyde. The levels of phosphorylated FAK at serine 910 within the cells were detected using antibody anti-FAK Ser910 (44-596G; Bio-source Inc.) and anti-human c-Myc (catalog number 631206; BD Biosciences).
Detection of markers of apoptosis and cytotoxicity in cultured mammalian cells.
HEK293 cells grown in tissue culture to 50% confluence in 12-well plates were transfected with combinations of 0.5 μg each of plasmid pCMV-myc, pCMV-myc-vopA, pCMV-myc-mvopA, pCMV-mycJNK1, or pCMV-mycMKK4, using 2.5 μl of the Lipofectamine 2000 reagent per well. The total amount of DNA transfected per well was normalized by the addition of pCMV-myc, to make a total of 1.5 μg DNA in the transfection mix. At 24 h posttransfection, the transfected cells were harvested in 200 μl Laemmli sample buffer per well. The amount of protein was quantified using a BCA protein assay kit (Thermo Scientific Pierce) before 0.5 μg of protein was loaded per well. The presence of specific proteins transferred to immunoblots was detected using the following antibodies: antibodies against cleaved PARP (Asp214) (human specific; catalog number 9541), phospho-SAPK/JNK (Thr183/Tyr185) (catalog number 9251), and anti-human c-Myc (catalog number BD631206) (all from BD Biosciences). In addition, to measure the effect of VopA on cytotoxicity, HeLa cells were grown in 12-well dishes to 70% confluence, whereupon they were transfected with 0.5 μg of plasmid pCMV-myc, pCMV-myc-vopA, or pCMV-myc-mvopA. At 24 h posttransfection, actinomycin D (final concentration, 1 μg/ml) and TNF-α (final concentration, 200 ng/ml) were included for a further 24 h. Cells were then harvested in 200 μl Laemmli sample buffer per well, protein was quantified using a BCA protein assay kit (Thermo Scientific Pierce), and 0.5 μg of protein was loaded per well. The presence of specific proteins transferred to immunoblots was detected using antibodies against cleaved PARP (Asp214) antibody (human specific; catalog number 9541; Cell Signaling Technologies) and anti-human c-Myc (catalog number BD631206; BD Biosciences).
Cell migration assay.
For the cell migration assay, human SKCO15 intestinal epithelial cells at 85 to 90% confluence in 24-well plates were transfected with 0.5 μg of plasmid pCMV-myc, pCMV-myc-vopA, or pCMV-myc-mvopA, using 0.75 μl of the Lipofectamine 2000 reagent per well. At 48 h posttransfection, the monolayer of epithelial cells was scratched with a pipette tip connected to a vacuum line, and the initial width of the scratch wound was photographed by microscopy, with a marked line being used as the reference point. On the following morning (elapsed time, approximately 18 h), the scratch wound was imaged again at the reference line and the covered area (the initial area subtracted from the final area) was determined using ImageJ software. The covered area was averaged across 10 replicates per transfected plasmid and was represented as the percent scratch wound closure.
EdU incorporation assay.
The EdU incorporation assay was done as described by Luo et al. (52). Briefly, pellets of dried yeast were made into a paste with water containing 1 mg/ml EdU. Aliquots of the paste were put into vials containing fly food. After 24 h, Drosophila intestines were dissected and EdU incorporation into chromosomal DNA was detected using Click-iT EdU cell proliferation assays (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol.
ACKNOWLEDGMENTS
R.M.J. is supported in part by NIH grant R01DK098391. J.D.M. is funded by the Crohn’s and Colitis Foundation of America (CCFA). B.S.R. is funded by training grant T32DK108735-03.
REFERENCES
- 1.Apidianakis Y, Rahme LG. 2011. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech 4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H, Jones RM, Neish AS. 2012. The Salmonella effector AvrA mediates bacterial intracellular survival during infection in vivo. Cell Microbiol 14:28–39. doi: 10.1111/j.1462-5822.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulte M, Hensel M. 2016. Models of intestinal infection by Salmonella enterica: introduction of a new neonate mouse model. F1000Res 5:F1000 Faculty Rev-1498. doi: 10.12688/f1000research.8468.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt W-D. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RM, Luo L, Moberg KH. 2012. Aeromonas salmonicida-secreted protein AopP is a potent inducer of apoptosis in a mammalian and a Drosophila model. Cell Microbiol 14:274–285. doi: 10.1111/j.1462-5822.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RM, Wu H, Wentworth C, Luo L, Collier-Hyams L, Neish AS. 2008. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe 3:233–244. doi: 10.1016/j.chom.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Naszai M, Carroll LR, Cordero JB. 2015. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut. Insect Biochem Mol Biol 67:9–14. doi: 10.1016/j.ibmb.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, Patel PH, Kohlmaier A, Pavlovic B, Zhang C, Edgar BA. 2017. Intestinal stem cell pool regulation in Drosophila. Stem Cell Rep 8:1479–1487. doi: 10.1016/j.stemcr.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones RM, Desai C, Darby TM, Luo L, Wolfarth AA, Scharer CD, Ardita CS, Reedy AR, Keebaugh ES, Neish AS. 2015. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep 12:1217–1225. doi: 10.1016/j.celrep.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RM, Luo L, Ardita CS, Richardson AN, Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, Lambeth JD, Denning PW, Neish AS. 2013. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J 32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair GB, Ramamurthy T, Bhattacharya SK, Dutta B, Takeda Y, Sack DA. 2007. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin Microbiol Rev 20:39–48. doi: 10.1128/CMR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotoh K, Kodama T, Hiyoshi H, Izutsu K, Park KS, Dryselius R, Akeda Y, Honda T, Iida T. 2010. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS One 5:e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino K, Oshima K, Kurokawa K, Yokoyama K, Uda T, Tagomori K, Iijima Y, Najima M, Nakano M, Yamashita A, Kubota Y, Kimura S, Yasunaga T, Honda T, Shinagawa H, Hattori M, Iida T. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V cholerae. Lancet 361:743–749. doi: 10.1016/S0140-6736(03)12659-1. [DOI] [PubMed] [Google Scholar]
- 14.Pineyro P, Zhou X, Orfe LH, Friel PJ, Lahmers K, Call DR. 2010. Development of two animal models to study the function of Vibrio parahaemolyticus type III secretion systems. Infect Immun 78:4551–4559. doi: 10.1128/IAI.00461-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trosky JE, Mukherjee S, Burdette DL, Roberts M, McCarter L, Siegel RM, Orth K. 2004. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus. J Biol Chem 279:51953–51957. doi: 10.1074/jbc.M407001200. [DOI] [PubMed] [Google Scholar]
- 16.Trosky JE, Li Y, Mukherjee S, Keitany G, Ball H, Orth K. 2007. VopA inhibits ATP binding by acetylating the catalytic loop of MAPK kinases. J Biol Chem 282:34299–34305. doi: 10.1074/jbc.M706970200. [DOI] [PubMed] [Google Scholar]
- 17.Orth K, Palmer LE, Bao ZQ, Stewart S, Rudolph AE, Bliska JB, Dixon JE. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920–1923. [DOI] [PubMed] [Google Scholar]
- 18.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. 2002. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol 169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 19.Fehr D, Casanova C, Liverman A, Blazkova H, Orth K, Dobbelaere D, Frey J, Burr SE. 2006. AopP, a type III effector protein of Aeromonas salmonicida, inhibits the NF-kappaB signalling pathway. Microbiology 152:2809–2818. doi: 10.1099/mic.0.28889-0. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 21.Jones RM, Neish AS. 2011. Recognition of bacterial pathogens and mucosal immunity. Cell Microbiol 13:670–676. doi: 10.1111/j.1462-5822.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 22.Orth K, Xu Z, Mudgett MB, Bao ZQ, Palmer LE, Bliska JB, Mangel WF, Staskawicz B, Dixon JE. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290:1594–1597. [DOI] [PubMed] [Google Scholar]
- 23.Herrera Estrada L, Wu H, Ling K, Zhang G, Sumagin R, Parkos CA, Jones RM, Champion JA, Neish AS. 2017. Bioengineering bacterially derived immunomodulants: a therapeutic approach to inflammatory bowel disease. ACS Nano 11:9650–9662. doi: 10.1021/acsnano.7b03239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu M, Iyengar R, Koshman YE, Kim T, Russell B, Martin JL, Heroux AL, Robia SL, Samarel AM. 2011. Serine-910 phosphorylation of focal adhesion kinase is critical for sarcomere reorganization in cardiomyocyte hypertrophy. Cardiovasc Res 92:409–419. doi: 10.1093/cvr/cvr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Jacobson K, Schaller MD. 2004. A role for JNK-paxillin signaling in cell migration. Cell Cycle 3:4–6. [PubMed] [Google Scholar]
- 26.Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, Davis BM, Bronson RT, Waldor MK. 2012. Inflammation and disintegration of intestinal villi in an experimental model for Vibrio parahaemolyticus-induced diarrhea. PLoS Pathog 8:e1002593. doi: 10.1371/journal.ppat.1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang RZ, Zhong YF, Gu XS, Yuan J, Saeed AF, Wang SH. 2015. The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front Microbiol 6:437. doi: 10.3389/fmicb.2015.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. 1998. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development 125:3875–3885. [DOI] [PubMed] [Google Scholar]
- 29.Vrailas-Mortimer AD, Majumdar N, Middleton G, Cooke EM, Marenda DR. 2007. Delta and Egfr expression are regulated by Importin-7/Moleskin in Drosophila wing development. Dev Biol 308:534–546. doi: 10.1016/j.ydbio.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marenda DR, Vrailas AD, Rodrigues AB, Cook S, Powers MA, Lorenzen JA, Perkins LA, Moses K. 2006. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development 133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Edgar BA. 2012. Intestinal stem cell function in Drosophila and mice. Curr Opin Genet Dev doi: 10.1016/j.gde.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karpowicz P, Perrimon N. 2010. All for one, and one for all: the clonality of the intestinal stem cell niche. F1000 Biol Rep 2:73. doi: 10.3410/B2-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P, Hou SX. 2010. Regulation of intestinal stem cells in mammals and Drosophila. J Cell Physiol 222:33–37. doi: 10.1002/jcp.21928. [DOI] [PubMed] [Google Scholar]
- 34.Micchelli CA, Perrimon N. 2006. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 35.Ohlstein B, Spradling A. 2006. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 36.Wehrle-Haller B. 2012. Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol 24:569–581. doi: 10.1016/j.ceb.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Moreno E, Yan M, Basler K. 2002. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol 12:1263–1268. doi: 10.1016/S0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 38.Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415. [DOI] [PubMed] [Google Scholar]
- 39.Guichard A, Park JM, Cruz-Moreno B, Karin M, Bier E. 2006. Anthrax lethal factor and edema factor act on conserved targets in Drosophila. Proc Natl Acad Sci U S A 103:3244–3249. doi: 10.1073/pnas.0510748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia J, Wang Y, Zhou L, Jin S. 2006. Expression of Pseudomonas aeruginosa toxin ExoS effectively induces apoptosis in host cells. Infect Immun 74:6557–6570. doi: 10.1128/IAI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leulier F, Marchal C, Miletich I, Limbourg-Bouchon B, Benarous R, Lemaitre B. 2003. Directed expression of the HIV-1 accessory protein Vpu in Drosophila fat-body cells inhibits Toll-dependent immune responses. EMBO Rep 4:976–981. doi: 10.1038/sj.embor.embor936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Krachler AM, Broberg CA, Li Y, Mirzaei H, Gilpin CJ, Orth K. 2012. Type III effector VopC mediates invasion for Vibrio species. Cell Rep 1:453–460. doi: 10.1016/j.celrep.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Orth K. 2013. Virulence determinants for Vibrio parahaemolyticus infection. Curr Opin Microbiol 16:70–77. doi: 10.1016/j.mib.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Krachler AM, Orth K. 2011. Functional characterization of the interaction between bacterial adhesin multivalent adhesion molecule 7 (MAM7) protein and its host cell ligands. J Biol Chem 286:38939–38947. doi: 10.1074/jbc.M111.291377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Front Immunol 5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galan JE. 2009. Common themes in the design and function of bacterial effectors. Cell Host Microbe 5:571–579. doi: 10.1016/j.chom.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano RL. 1997. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol 136:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Gewurz BE, Ritchie JM, Takasaki K, Greenfeld H, Kieff E, Davis BM, Waldor MK. 2013. A Vibrio parahaemolyticus T3SS effector mediates pathogenesis by independently enabling intestinal colonization and inhibiting TAK1 activation. Cell Rep 3:1690–1702. doi: 10.1016/j.celrep.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takatsu Y, Nakamura M, Stapleton M, Danos MC, Matsumoto K, O'Connor MB, Shibuya H, Ueno N. 2000. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol Cell Biol 20:3015–3026. doi: 10.1128/MCB.20.9.3015-3026.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. 2008. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 52.Luo L, Reedy AR, Jones RM. 2016. Detecting reactive oxygen species generation and stem cell proliferation in the Drosophila intestine. Methods Mol Biol 1422:103–113. doi: 10.1007/978-1-4939-3603-8_10. [DOI] [PubMed] [Google Scholar]