Orientia tsutsugamushi is an obligate intracellular bacterium that infects mononuclear and endothelial cells to cause the emerging global health threat scrub typhus. The ability of O. tsutsugamushi to survive in monocytes facilitates bacterial dissemination to endothelial cells, which can subsequently lead to several potentially fatal sequelae.
KEYWORDS: Orientia, Rickettsia, adaptive immunity, intracellular bacteria, major histocompatibility complex, obligate intracellular bacterium
ABSTRACT
Orientia tsutsugamushi is an obligate intracellular bacterium that infects mononuclear and endothelial cells to cause the emerging global health threat scrub typhus. The ability of O. tsutsugamushi to survive in monocytes facilitates bacterial dissemination to endothelial cells, which can subsequently lead to several potentially fatal sequelae. As a strict intracellular pathogen that lives in the cytoplasm of host cells, O. tsutsugamushi has evolved to counter adaptive immunity. How the pathogen does so and the outcome of this strategy in monocytes versus endothelial cells are poorly understood. This report demonstrates that O. tsutsugamushi reduces cellular levels of NOD-, LRR-, and CARD-containing 5 (NLRC5), a recently identified specific transactivator of major histocompatibility complex class I (MHC-I) component gene expression, to inhibit MHC-I biosynthesis. Importantly, the efficacy of this approach varies with the host cell type infected. In nonprofessional antigen-presenting HeLa and primary human aortic endothelial cells, the O. tsutsugamushi-mediated reduction of NLRC5 results in lowered MHC-I component transcription and, consequently, lower total and/or surface MHC-I levels throughout 72 h of infection. However, in infected THP-1 monocytes, which are professional antigen-presenting cells, the reductions in NLRC5 and MHC-I observed during the first 24 h reverse thereafter. O. tsutsugamushi is the first example of a microbe that targets NLRC5 to modulate the MHC-I pathway. The differential ability of O. tsutsugamushi to modulate this pathway in nonprofessional versus professional antigen-presenting cells could influence morbidity and mortality from scrub typhus.
INTRODUCTION
Major histocompatibility complex class I (MHC-I) molecules are essential for adaptive immunity. MHC-I complexes are constitutively expressed on nearly all nucleated cells. After being loaded with peptides derived from proteasome processing of intracellular antigens, they are directed to the cell surface, where they present the peptide antigens to the T cell receptors of CD8+ T cells (1, 2). NOD-, LRR-, and CARD-containing 5 (NLRC5)/class I transactivator (CITA), a recently identified specific transactivator of MHC-I genes, plays a prominent role in the adaptive immune response through the regulation of MHC-I expression (3). NLRC5-deficient mice poorly induce antigen-specific CD8+ T cell activation and are susceptible to infections that require CD8+ T cell responses (4–6). Class II transactivator (CIITA), a master regulator for MHC-II gene expression, can also contribute to the expression of MHC-I genes in antigen-presenting cells (APCs) in which both it and NLRC5 are expressed (2). As MHC-I is critical for adaptive immune responses to intracellular microbes, many of these pathogens have evolved mechanisms to disrupt various steps in the MHC-I biosynthetic pathway, including MHC-I retention in the endoplasmic reticulum, inhibition of peptide loading, increased MHC-I degradation, and modulation of MHC-I trafficking to the cell surface (7). However, no pathogen has been shown to alter the MHC-I pathway by specifically targeting NLRC5.
Orientia tsutsugamushi is a chigger-vectored obligate intracellular bacterium that causes scrub typhus (8, 9). More than 1 million new cases are diagnosed annually. The disease occurs primarily in the Asia-Pacific, but also in the Middle East, Africa, and South America, threatening one-third of the world’s population (9–11). Scrub typhus presents as an acute febrile illness accompanied by several nonspecific clinical manifestations and often a maculopapular rash. In the absence of appropriate antibiotic therapy at disease onset, severe symptoms can result and include lung injury, respiratory distress, renal failure, hepatitis, myocarditis, encephalitis, and systemic vascular collapse. The median mortality rate is 7% to 15% but can be as high as 70% (9, 12). At the chigger inoculation site, O. tsutsugamushi infects dendritic and mononuclear cells (13, 14). Peripheral blood mononuclear cells obtained from scrub typhus patients and experimentally infected monkeys and dogs harbor O. tsutsugamushi (14–16). The bacterium replicates in monocytes and monocyte-derived dendritic cells in vitro (16, 17), yet O. tsutsugamushi replication in monocytes is less efficient than that in other cell types, as it lags at between 24 h and 72 h and resumes thereafter (16). These observations support the premise that monocytes are sites of early O. tsutsugamushi replication and conduits that disseminate the bacterium to endothelial cells via the lymphatics (18). Endothelial cell damage due to infection and vasculitis lead to rash presentation and many of the serious sequelae (8). In BALB/c and C57BL/6 mice, CD8+ T cells and MHC-I are essential for protection against lethality following inoculation with a sublethal dose of O. tsutsugamushi (19, 20). Adaptive immunity in response to the bacterium is short-lived (11). Whether O. tsutsugamushi counters the adaptive immune response by modulating MHC-I is unknown.
Here, we demonstrate that O. tsutsugamushi decreases NLRC5 levels in MHC-II-negative HeLa and endothelial cells, which translates to reductions in total and/or surface MHC-I. In monocytes, which are professional APCs, the bacterium reduces NLRC5 and MHC-I total and surface levels early during infection, but these trends reverse thereafter. These data evidence the first example of a pathogen targeting NLRC5 to inhibit MHC-I expression and establish a link for how the differential ability of O. tsutsugamushi to modulate this pathway in nonprofessional APCs versus professional APCs could influence the progression of scrub typhus.
RESULTS
O. tsutsugamushi decreases MHC-I host cell surface levels by reducing the total amounts of HLA-ABC and β2M.
As a first step in determining if O. tsutsugamushi modulates the MHC-I pathway, the levels of MHC-I molecules on the surfaces of infected and uninfected HeLa cells were compared. HeLa cells were ideal for this purpose because they are models for studying O. tsutsugamushi-host cell interactions and constitutively express MHC-I (21–27). Also, because they are nonprofessional APCs, HeLa cells express NLRC5 but not CIITA and are therefore useful for analyzing MHC-I gene expression exclusively in the context of NLRC5 function. Indeed, for this reason, the use of HeLa cells contributed to the discovery of NLRC5’s role as a transcriptional regulator of MHC-I genes, and they continue to be utilized in this capacity (2, 3, 28–30). Classical MHC-I molecules consist of a heavy chain (HLA-A, -B, or -C) and β2-microglobulin (β2M) (25). Flow cytometric analyses using an HLA-ABC heavy-chain antibody revealed that MHC-I levels were decreased by 55.6% and 76.5% in O. tsutsugamushi-infected cells at 24 and 72 h, respectively (Fig. 1A and B). O. tsutsugamushi infection was confirmed via Western blot analysis using antibody against 56-kDa type-specific antigen (TSA56) (Fig. 1C and E), an outer membrane protein that the bacterium expresses throughout infection (31–33). The observed loss in MHC-I surface levels correlated with an overall reduction in MHC-I cellular levels, as confirmed by Western blotting (Fig. 1C to F). Whereas at 24 h HLA-ABC and β2M chain amounts were slightly lower in O. tsutsugamushi-infected cells than in uninfected cells, by 72 h they had significantly decreased by 74% and 65%, respectively. Gamma interferon (IFN-γ), a cytokine whose levels are elevated during O. tsutsugamushi infection of humans and mice, can stimulate expression of MHC-I components (1, 34–38). Even in the presence of IFN-γ, the bacterium pronouncedly reduced the cellular levels of HLA-ABC and β2M. Thus, O. tsutsugamushi decreases the total amounts of MHC-I heavy and β2M chains, which leads to a reduction in their presentation on the infected cell surface.
FIG 1.
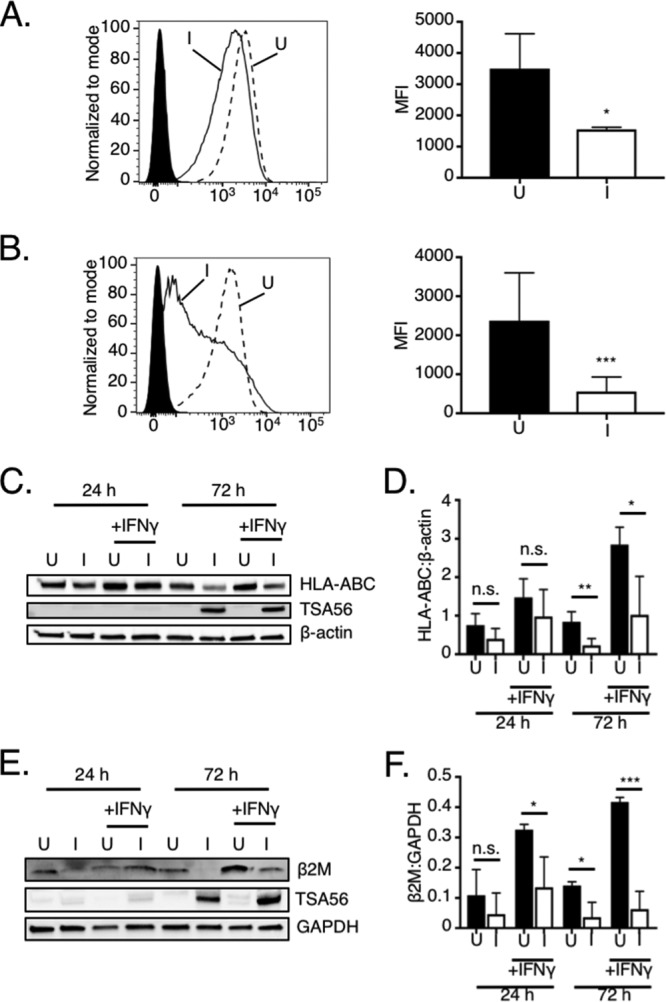
O. tsutsugamushi decreases MHC-I host cell surface levels by reducing the total amounts of HLA-ABC and β2M. (A and B) HeLa cells were incubated with O. tsutsugamushi (infected [I]) at an MOI of 10 or mock infected (uninfected [U]). At 24 or 72 h, the cells were assessed for MHC-I component surface levels using flow cytometry. (Left) Representative histograms of uninfected (hatched line) and infected (solid line) HeLa cells incubated with HLA-ABC antibody or uninfected cells incubated with the isotype control (filled histogram). (Right) The median fluorescence intensities (MFI) ± SD of HLA-ABC surface signals were calculated from at least four biological replicates at 24 h (A) and 72 h (B). (C to F) Whole-cell lysates of uninfected and infected HeLa cells that had been treated with IFN-γ or not were subjected to Western blot analyses. (C and E) Blots were probed with antibodies against HLA-ABC (C) or β2M (E). The blots were stripped and reprobed with TSA56 antibody (C and E), after which they were stripped and reprobed once more with β-actin (C) or GAPDH (E) antibody. (D and F) Mean normalized ratios ± SD of HLA-ABC/β-actin (D) and β2M/GAPDH (F) from three independent experiments were calculated using densitometry. Statistically significant values are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
O. tsutsugamushi inhibits the transcriptional expression of MHC-I components.
It was next determined if the O. tsutsugamushi-induced loss of MHC-I heavy and β2M chain cellular levels is linked to transcriptional repression. Total RNA isolated from infected and uninfected HeLa cells that had been treated with IFN-γ or not was analyzed by quantitative reverse transcription-PCR (qRT-PCR) using primers targeting B2M and HLA-A, the latter of which is first in the HLA locus (39, 40). At 24 h, HLA-A transcript levels were comparable between infected and uninfected cells whether or not they had been exposed to IFN-γ, while the levels of B2M were significantly decreased in untreated infected cells but equivalent in IFN-γ-treated cells (Fig. 2A and B). By 72 h, HLA-A and B2M mRNA levels had decreased by 70% and 81%, respectively. IFN-γ failed to even partially reverse the transcriptional inhibition at 72 h. These results correlate with the translational inhibition data and indicate that O. tsutsugamushi ultimately reduces MHC-I surface levels by antagonizing mRNA expression of both MHC-I heavy-chain and β2M components.
FIG 2.
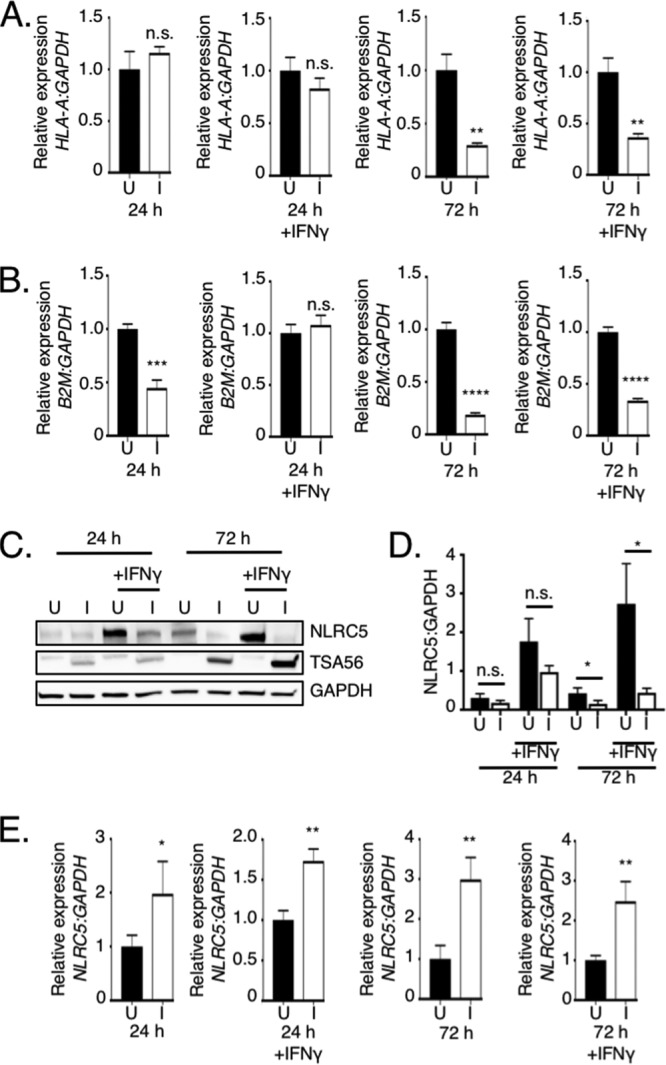
O. tsutsugamushi inhibits transcription of MHC-I components by reducing cellular levels of NLRC5. (A, B, and E) O. tsutsugamushi inhibits HLA-A and B2M mRNA but not NLRC5 mRNA expression. Total RNA isolated at 24 or 72 h from triplicate samples of uninfected (U) and infected (I) HeLa cells that had been treated with IFN-γ or not was subjected to qRT-PCR analysis. The 2−ΔΔCT method was used to determine the relative HLA-A (A), β2M (B), and NLRC5 (E) expression level normalized to that of GAPDH. (C and D) NLRC5 levels are decreased in O. tsutsugamushi-infected cells. (C) Whole-cell lysates recovered from uninfected and infected cells treated with IFN-γ or not were subjected to Western blot analyses using NLRC5 antibody. The blots were successively stripped and reprobed with TSA56 and GAPDH antibodies. (D) Mean normalized densitometric ratios ± SD of NLRC5/GAPDH from three separate experiments were calculated. Statistically significant values are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; n.s., not significant.
O. tsutsugamushi decreases NLRC5 cellular levels.
The genes encoding HLA-A and β2M are on separate chromosomes (41, 42), yet O. tsutsugamushi reduces the mRNA levels of both to similar degrees. In nonprofessional APCs, including HeLa cells, NLRC5 is the sole transactivator of MHC-I component gene expression (1, 43). We rationalized that the pathogen might transcriptionally repress HLA-A and B2M by targeting NLRC5. Accordingly, NLRC5 protein and mRNA levels were assessed in uninfected and O. tsutsugamushi-infected cells that had been treated with IFN-γ or not. We continued to use HeLa cells for these studies because MHC-I expression in these cells can be solely linked to NLRC5 and they express detectable basal levels of NLRC5 compared to other nonprofessional APC immortalized cell lines (44). Similar to the findings observed for HLA-ABC and β2M, NLRC5 levels were significantly reduced at 72 h but not at 24 h (Fig. 2C and D). As previously reported, IFN-γ stimulated higher NLRC5 expression (3, 45, 46). Nonetheless, O. tsutsugamushi still negatively affected NLRC5, as the cellular levels of the transactivator were reduced by 64% at 72 h. NLRC5 transcript levels were significantly elevated in all infected samples (Fig. 2E), indicating that the loss of NLRC5 protein was not attributable to a decrease in NLRC5 mRNA. Significant reductions in NLRC5, HLA-ABC, and β2M levels were not observed for HeLa cells that had been incubated with paraformaldehyde-fixed O. tsutsugamushi and stimulated with IFN-γ or for O. tsutsugamushi-infected HeLa cells that had been treated with tetracycline and IFN-γ stimulated (Fig. 3). Thus, in HeLa cells, O. tsutsugamushi promotes the reduction of NLRC5 in a bacterial protein synthesis-dependent manner to ultimately lessen the cellular levels of total and surface-localized MHC-I.
FIG 3.
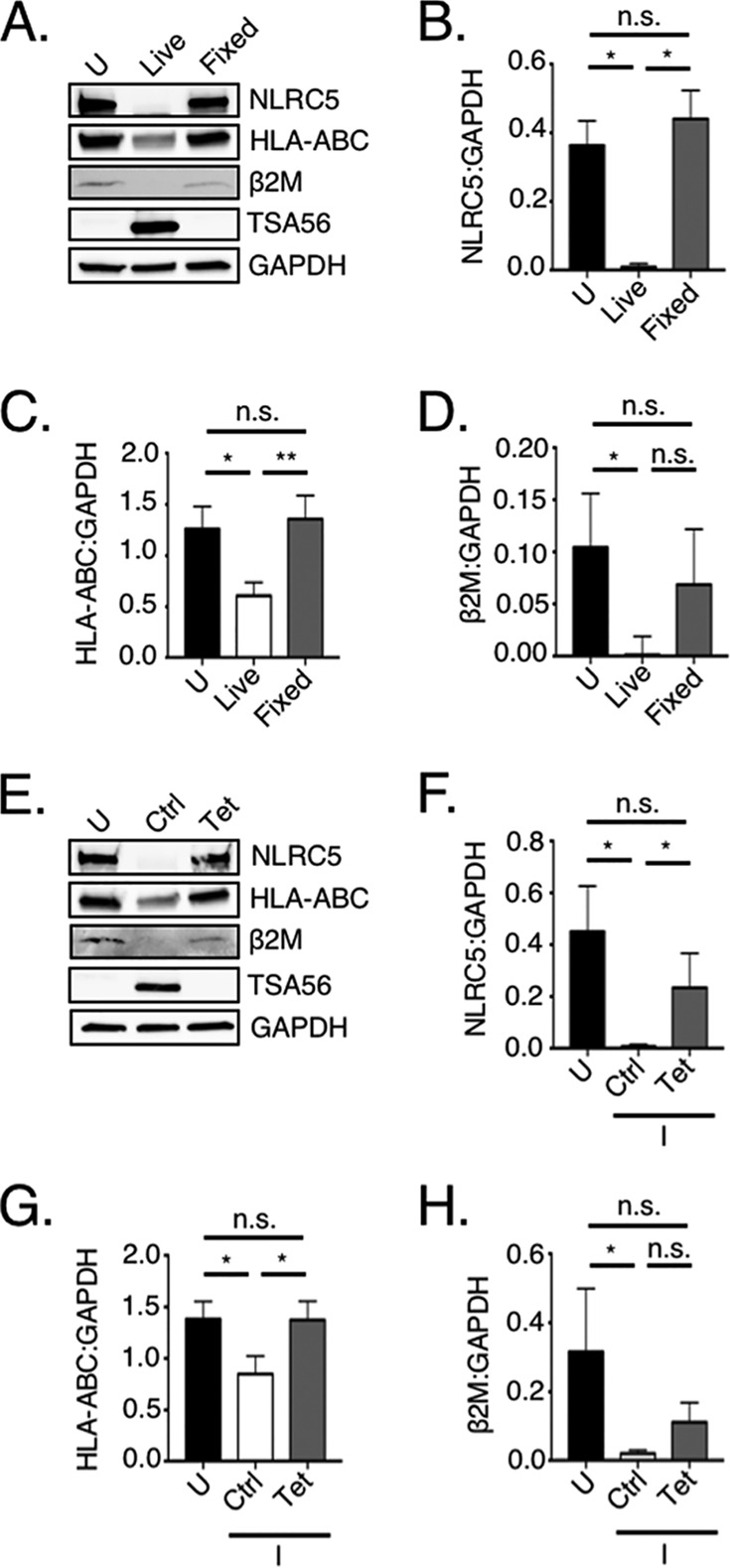
Protein synthesis by viable O. tsutsugamushi is essential for reduction of NLRC5, HLA-ABC, and β2M. HeLa cells were mock infected (uninfected [U]), infected with O. tsutsugamushi (Live), or incubated with paraformaldehyde-fixed O. tsutsugamushi (Fixed) at an MOI of 10 or infected (I) with O. tsutsugamushi followed by treatment with tetracycline (Tet) or vehicle control (Ctrl). After treatment with IFN-γ, whole-cell lysates were subjected to Western blot analyses using antibodies against NLRC5, HLA-ABC, β2M, and TSA56. The blots were stripped and reprobed with GAPDH antibody (A and E). The mean normalized ratios ± SD of NLRC5/GAPDH (B and F), HLA-ABC/GAPDH (C and G), and β2M/GAPDH (D and H) from three independent experiments were calculated using densitometry. Statistically significant values are indicated. *, P < 0.05; **, P < 0.01; n.s., not significant.
O. tsutsugamushi modulates NLRC5 and MHC-I levels during infection of endothelial cells.
To determine if the O. tsutsugamushi-induced NLRC5 reduction recapitulates in a nonprofessional APC host cell type similar to that which the bacterium infects during scrub typhus, uninfected and infected primary human aortic endothelial cells (HAECs) were examined. Unlike O. tsutsugamushi-infected HeLa cells, infected endothelial cells exhibited NLRC5 levels that were significantly elevated by 5.3-fold at 24 h (Fig. 4A and B). This result correlated with increases in total and surface HLA-ABC levels of 3.4- and 1.7-fold, respectively (Fig. 4A to E). However, by 72 h O. tsutsugamushi had quelled this response, reducing NLRC5 by 55% and surface-detectable HLA-ABC by 43%, even though total HLA-ABC levels remained elevated (Fig. 4A to C, F, and G).
FIG 4.
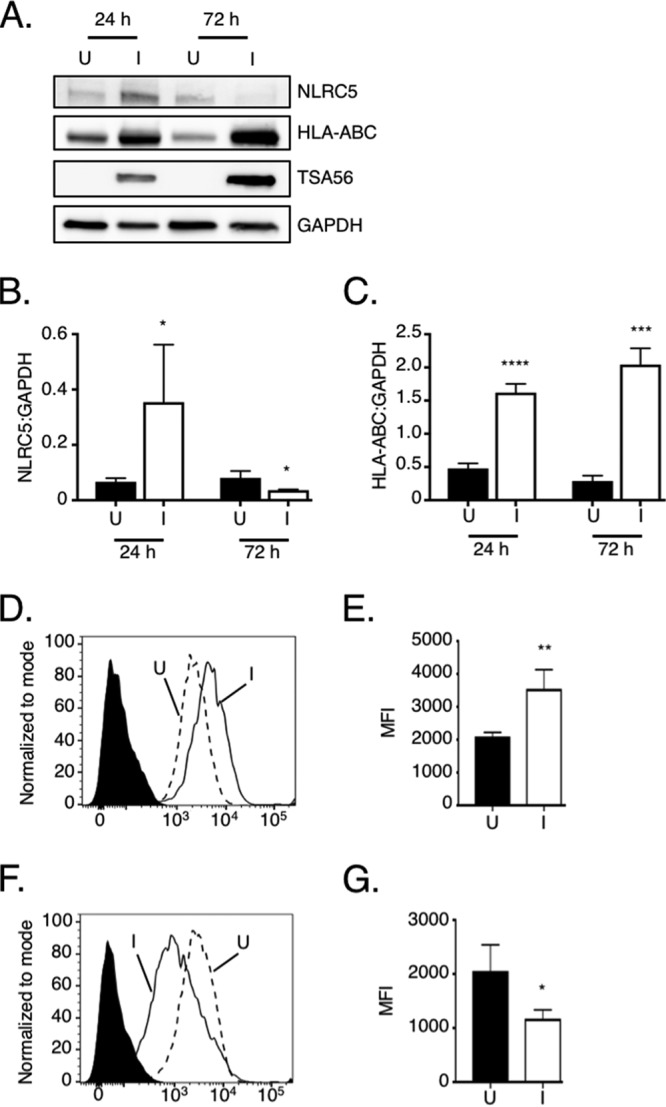
NLRC5 and MHC-I levels in O. tsutsugamushi-infected endothelial cells. HAECs were incubated with O. tsutsugamushi (infected [I]) at an MOI of 10 or mock infected (uninfected [U]). At 24 or 72 h, the cells were assessed for MHC-I component total and surface levels via Western blotting and flow cytometry, respectively. (A to C) Whole-cell lysates of uninfected and infected HAECs were subjected to Western blot analyses. (A) The Western blot was probed with NLRC5 antibody, after which it was successively stripped and reprobed with antibodies against HLA-ABC, TSA56, and GAPDH. (B and C) Mean normalized ratios ± SD of NLRC5/GAPDH (B) and HLA-ABC/GAPDH (C) from three separate experiments were calculated using densitometry. (D to G) The cells were assessed for MHC-I component surface levels using flow cytometry. (D and F) Representative histograms of uninfected (hatched line) and infected (solid line) HAECs incubated with HLA-ABC antibody or uninfected cells incubated with the isotype control (filled histogram) at 24 h (D) and 72 h (F) postinfection. (E and G) The median fluorescence intensities ± SD of HLA-ABC cell surface signals were calculated from at least four biological replicates at 24 h (E) and 72 h (G) postinfection. Statistically significant values are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
O. tsutsugamushi reduces NLRC5 and MHC-I levels in THP-1 monocytic cells during early infection, but these phenomena reverse thereafter.
Monocytes are infected by O. tsutsugamushi in vivo and are hypothesized to contribute to disseminating the bacterium (13–15, 18). Therefore, it was next examined if O. tsutsugamushi reduces NLRC5 levels and MHC-I expression in THP-1 monocytic cells. NLRC5 and HLA-ABC total and surface levels were significantly reduced at 24 h postinfection (Fig. 5A to E), yet by 72 h NLRC5 levels had partially rebounded and both total and surface HLA-ABC levels had significantly increased (Fig. 5A, C, F, and G). Concomitant with this observation and in agreement with the report that O. tsutsugamushi replication is stalled during the first 72 h postinfection of monocytes (16), the TSA56 band intensity was comparable between 24 and 72 h (Fig. 5A), suggesting that bacterial protein synthesis, replication, and/or overall fitness was compromised.
FIG 5.
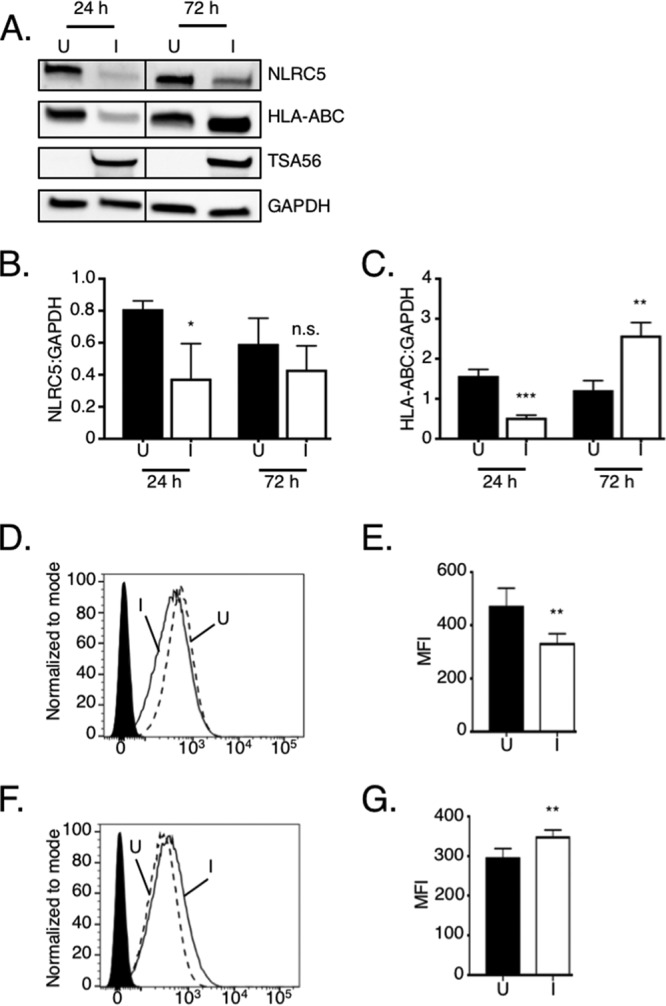
The initial reductions in MHC-I component and NLRC5 levels observed in O. tsutsugamushi-infected monocytic cells at 24 h are reversed by 72 h. (A to C) O. tsutsugamushi reduces NRLC5 and HLA-ABC total protein levels in THP-1 cells at 24 h. (A) Whole-cell lysates of mock-infected (uninfected [U]) and infected (I) THP-1 cells obtained at 24 and 72 h postinfection were subjected to Western blot analyses using NRLC5 antibody. The blot was successively stripped and reprobed with HLA-ABC, TSA56, and GAPDH antibodies. Vertical lines separating the samples obtained at 24 h and 72 h indicate where irrelevant lanes of the blot were cropped. (B and C) Mean normalized ratios ± SD of NLRC5/GAPDH (B) and HLA-ABC/GAPDH (C) from three separate experiments were calculated using densitometry. (D and F) Representative histograms of uninfected (hatched line) and infected (solid line) THP-1 monocytes incubated with HLA-ABC antibody at 24 h (D) and 72 h (F) postinfection or uninfected cells incubated with the isotype control (filled histogram). (E and G) Median fluorescence intensities ± SD of HLA-ABC cell surface signals for infected cells at 24 h (E) and 72 h (G) postinfection were calculated from at least four biological replicates. Statistically significant values are indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
DISCUSSION
As a strict intracellular pathogen that resides in the cytoplasm of professional and nonprofessional APCs, O. tsutsugamushi has evolved to counter adaptive immunity using poorly defined mechanisms. NLRC5, which is induced by IFN-γ, associates with the SXXY promoter as part of an enhanceosome to activate MHC-I component gene expression (3, 30, 43). This study demonstrates that O. tsutsugamushi utilizes the novel strategy of reducing host cell NLRC5 levels to modulate the MHC-I pathway. Strikingly, however, the efficacy of this approach varies with the host cell type that the bacterium has infected. In HeLa cells, O. tsutsugamushi pronouncedly reduces NLRC5 levels and, consequently, total and surface levels of MHC-I even in the presence of IFN-γ. The degree of the reduction in NLRC5 and MHC-I levels increases from 24 to 72 h, the timing of which corresponds to when intracellular bacterial population growth transitions from lag to log phase (24). Therefore, NLRC5 reduction could be bacterial load dependent or due to the temporal expression of a bacterial effector(s) that orchestrates the phenomenon.
Compared to HeLa cells, HAECs are more capable of responding to O. tsutsugamushi infection, as evidenced by robust increases in NLRC5, total HLA-ABC, and cell surface HLA-ABC protein levels at 24 h. Total MHC-I levels remain elevated at 72 h, even though the bacterium has pronouncedly reduced NLRC5 levels by this time, likely due to the initial excess of MHC-I that had been produced. Nonetheless, MHC-I surface levels are significantly lowered by 72 h. What could account for this discrepancy? O. tsutsugamushi blocks the secretory pathway using its effector, Ank9, and potentially other endoplasmic reticulum (ER)/Golgi apparatus-tropic effectors (21, 47). Thus, even though the response of primary endothelial cells to O. tsutsugamushi partially offsets the infection-induced NLRC5 deficiency, bacterial modulation of the secretory pathway would retain MHC-I components in the ER, rendering these host cells unable to replenish surface MHC-I complexes. O. tsutsugamushi therefore potentially uses a two-pronged approach—depletion of NLRC5 and secretory pathway inhibition—to reduce the MHC-I antigen presentation capability of host cells.
In THP-1 monocytic cells, yet a different scenario plays out. At 24 h, O. tsutsugamushi has reduced NLRC5, total HLA-ABC, and cell surface HLA-ABC levels, but by 72 h NLRC5 levels partially recover and total and surface MHC-I levels escalate. Conspicuously, TSA56 levels exhibit no to little increase from 24 to 72 h of infection in THP-1 cells, which agrees with a report that O. tsutsugamushi growth in monocytic cells stalls during this time period (16) and which is in contrast to the pronounced TSA56 level increase that occurs during infection in HeLa cells and HAECs. This observation suggests that bacterial replication, fitness, or, at the very least, protein synthesis is compromised during the first 72 h of infection of THP-1 cells, any of which could contribute to the bacterium’s inability to sustain NLRC5 reduction and inhibit the secretory pathway, especially given that these mechanisms likely require the energy-costly synthesis of bacterial effectors. The induction of MHC-I in infected THP-1 monocytes at 72 h is considerable, given that NLRC5 levels have been only partially restored at this time. NLRC5-independent activation of MHC-I component gene expression by CIITA, which is exclusively present in APCs (2), may contribute to the observed boost in MHC-I levels.
These results emphasize the value of using multiple host cell types, including those reflective of what O. tsutsugamushi infects in vivo. Together with the following points, this report provides insight that the differential outcome between monocytic and endothelial cell infection influences scrub typhus progression. First, fatal/severe scrub typhus is associated with inadequate early control of bacterial growth, higher bacterial loads, and endothelial cell colonization (11, 48, 49). Second, the disease can become chronic, lasting for months in patients and experimentally infected animals; and it has been suggested that impaired T cell effector responses are causally linked to persistent infections (49, 50). Third, MHC-I and CD8+ T cells are essential for preventing fatal/severe infections (19, 20). Fourth, the degree to which O. tsutsugamushi downregulates MHC-I is comparable to the reduction levels observed for virus-infected cells that are attenuated for recognition by CD8+ T cells (51–53). Thus, monocytes, which are among the first cells infected at the chigger feeding site (13), play an important role in early scrub typhus because they partially restrict O. tsutsugamushi growth and overcome the NLRC5 reduction to retain the ability to present MHC-I complexes on their surfaces and contribute to the adaptive immune response. However, because monocytes are also key for disseminating O. tsutsugamushi (18), once organisms have egressed from infected monocytes to colonize endothelial cells, their fitness is no longer compromised. Moreover, the bacteria now reside in a host cell type that activates MHC-I expression exclusively using NLRC5 (2), which enables them to effectively lower NLRC5 levels and impair MHC-I complex delivery to/antigen presentation at the endothelial cell surface. This would allow the bacteria to replicate intracellularly relatively undetected, resulting in the high bacterial burdens observed in some organs during late infection. The differential ability of O. tsutsugamushi to modulate NLRC5 and MHC-I cell surface levels in these two host cell types is expected to contribute to immune system dysregulation and the severe sequelae associated with the disseminated form of scrub typhus.
Overall, we present the first example of a pathogen that reduces host cell MHC-I levels by specifically targeting NLRC5. We also provide direct evidence that the host cell type and the ability to respond to infection influence the tug-of-war between O. tsutsugamushi and adaptive immunity, which, in turn, has implications for disease outcome.
MATERIALS AND METHODS
Cultivation of cell lines and O. tsutsugamushi infections.
Uninfected and O. tsutsugamushi-infected HeLa cells were propagated as previously described (27). THP-1 cells (TIB-202; American Type Culture Collection [ATCC], Manassas, VA) were maintained in RPMI 1640 with l-glutamine (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gemini Bio-Products, Sacramento, CA, USA) at 37°C in a humidified incubator with 5% CO2. Primary human aortic endothelial cells (PCS-100-011; ATCC) were cultured in vascular cell basal medium (ATCC) supplemented with an endothelial cell growth kit-vascular endothelial growth factor (ATCC) at 37°C in a humidified incubator with 5% CO2. Host cell-free O. tsutsugamushi organisms were obtained for experimental use from highly infected HeLa cells and incubated with naive HeLa cells to initiate synchronous infections as described previously (27). In some experiments, O. tsutsugamushi organisms were treated with 2% paraformaldehyde for 30 min prior to incubation with host cells, or 10 μg ml−1 of oxytetracycline hydrochloride (Sigma-Aldrich) in 70% ethanol or the vehicle control was added at 4, 24, and 48 postinfection. In some cases, HeLa cells were treated with 20 ng ml−1 human IFN-γ (PeproTech, Rock Hill, NJ) at 2 h postinfection or postaddition of fixed bacteria. THP-1 cells were incubated with O. tsutsugamushi for 2 h with gentle shaking every 15 to 30 min. The inoculum was removed by centrifugation of the sample at 250 × g for 5 min, after which the supernatant was decanted. The pellet of infected THP-1 cells was resuspended in medium to a density of 1 × 106 ml−1. Host cell-free O. tsutsugamushi bacteria were incubated with HAECs for 2 h, at which point the inoculum was removed and replaced with fresh medium. All infected samples were processed and analyzed in parallel with mock-infected controls, which were prepared as previously described (27), to verify that any observed effect was not due to host cellular debris.
Immunofluorescence microscopy.
All infections were performed to achieve a multiplicity of infection (MOI) of 10, which was verified for infected HeLa cells or HAECs using immunofluorescence microscopy as previously described (27). To confirm the MOI for THP-1 cells, aliquots of 250,000 infected cells were removed from each sample per experiment and pipetted onto glass coverslips in 24-well plates. The plates were spun at 750 × g for 5 min. Following gentle removal of the supernatant, the coverslip was washed with phosphate-buffered saline, fixed with ice-cold methanol, and examined by immunofluorescence microscopy as described previously (27). Immunofluorescent images of the MOI coverslips and synchronously infected THP-1 cells were acquired by spinning disc confocal microscopy using a BX51 microscope (Olympus, Center Valley, PA) affixed with an Olympus disk spinning unit and an ORCA-R2 charge-coupled-device camera (Hamamatsu, Japan) or a Zeiss LSM 700 laser-scanning confocal microscope, the latter of which was located in the Virginia Commonwealth University School of Medicine Microscopy Core Facility.
Flow cytometry.
Cells were incubated with Fc block (Miltenyi Biotec, Bergusch Gladbach, Germany), followed by labeling with mouse anti-human HLA-ABC W6/32 (Invitrogen, Carlsbad, CA) or the isotype control (BioLegend, San Diego, CA) and allophycocyanin anti-mouse immunoglobulin secondary antibody (BioLegend). Cells were fixed with fixation buffer (BioLegend), and flow cytometry was performed as previously described (54). Data were captured on a BD LSRFortessa II flow cytometer (BD Biosciences, Franklin Lakes, NJ) and analyzed with FlowJo (version 10.4.2) software (BD Biosciences).
Western blotting.
SDS-PAGE was performed as described previously, except that the gels used to generate Western blots to be probed with β2M antibody were 4% to 20% mini-Protean TGX gradient gels (Bio-Rad) to achieve better resolution (47). Western blot analyses were performed as described previously (27) using mouse anti-HLA-ABC heavy chain (Abcam, Cambridge, UK) at 1:1,000, rabbit anti-β2M (Life Technologies, Carlsbad, CA) at 1:1,000, rat anti-NLRC5 (EMD Millipore, Burlington, MA) at 1:1,000, TSA56 antiserum at 1:1,000 (21), mouse anti-GAPDH (anti-glyceraldehyde-3-phosphate dehydrogenase; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:2,500, mouse anti-β-actin (Santa Cruz) at 1:2,500, horseradish peroxidase (HRP)-conjugated horse anti-mouse IgG (Cell Signaling Technology, Danvers, MA) at 1:10,000, HRP-conjugated horse anti-rabbit IgG (Cell Signaling Technology) at 1:10,000, and HRP-conjugated horse anti-rat IgG (Cell Signaling Technology) at 1:10,000.
RNA isolation and qRT-PCR.
RNA isolation and qRT-PCR were performed as previously described (22) using primers NLRC5 forward (fwd), NLRC5 reverse (rev), HLA-A fwd, HLA-A rev, B2M fwd, and B2M rev (3). Expression of each gene of interest was normalized using GAPDH gene-specific primers 5′-ACATCATCCCTGCCTCTACTGG-3′ and 5′-TCCGACGCCTGCTTCACC-3′ and the 2−ΔΔCT threshold cycle (CT) method (55).
Statistical analyses.
Statistical analyses were performed using the Prism (version 5.0) software package (GraphPad, San Diego, CA). One-way analysis of variance (ANOVA) with Tukey's post hoc test was used to test for a significant difference among groups. The Student t test was used to test for a significant difference among pairs. Statistical significance was set at P values of <0.05.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI123346 and AI128152 (to J.A.C.). Services in support of the research project were generated by the VCU Massey Cancer Center Flow Cytometry Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center support grant P30 CA016059.
REFERENCES
- 1.Jongsma MLM, Guarda G, Spaapen RM. 7 December 2017. The regulatory network behind MHC class I expression. Mol Immunol doi: 10.1016/j.molimm.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi KS, van den Elsen PJ. 2012. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol 12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- 3.Meissner TB, Li A, Biswas A, Lee KH, Liu YJ, Bayir E, Iliopoulos D, van den Elsen PJ, Kobayashi KS. 2010. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci U S A 107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas A, Meissner TB, Kawai T, Kobayashi KS. 2012. Cutting edge: impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J Immunol 189:516–520. doi: 10.4049/jimmunol.1200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staehli F, Ludigs K, Heinz LX, Seguin-Estevez Q, Ferrero I, Braun M, Schroder K, Rebsamen M, Tardivel A, Mattmann C, MacDonald HR, Romero P, Reith W, Guarda G, Tschopp J. 2012. NLRC5 deficiency selectively impairs MHC class I-dependent lymphocyte killing by cytotoxic T cells. J Immunol 188:3820–3828. doi: 10.4049/jimmunol.1102671. [DOI] [PubMed] [Google Scholar]
- 6.Yao Y, Wang Y, Chen F, Huang Y, Zhu S, Leng Q, Wang H, Shi Y, Qian Y. 2012. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res 22:836–847. doi: 10.1038/cr.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuren AB, Costa AI, Wiertz EJ. 2016. Recent advances in viral evasion of the MHC class I processing pathway. Curr Opin Immunol 40:43–50. doi: 10.1016/j.coi.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Diaz FE, Abarca K, Kalergis AM. 2018. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection. Clin Microbiol Rev 31:e00076-17. doi: 10.1128/CMR.00076-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. 2017. A review of the global epidemiology of scrub typhus. PLoS Negl Trop Dis 11:e0006062. doi: 10.1371/journal.pntd.0006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocher C, Jiang J, Morrison AC, Castillo R, Leguia M, Loyola S, Ampuero JS, Cespedes M, Halsey ES, Bausch DG, Richards AL. 2017. Serologic evidence of scrub typhus in the Peruvian Amazon. Emerg Infect Dis 23:1389–1391. doi: 10.3201/eid2308.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soong L. 2018. Dysregulated Th1 immune and vascular responses in scrub typhus pathogenesis. J Immunol 200:1233–1240. doi: 10.4049/jimmunol.1701219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor AJ, Paris DH, Newton PN. 2015. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 9:e0003971. doi: 10.1371/journal.pntd.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, Ferguson DP, Blacksell SD, Newton PN, Day NP, Turner GD. 2012. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis 6:e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ro HJ, Lee H, Park EC, Lee CS, Il Kim S, Jun S. 2018. Ultrastructural visualization of Orientia tsutsugamushi in biopsied eschars and monocytes from scrub typhus patients in South Korea. Sci Rep 8:17373. doi: 10.1038/s41598-018-35775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirai A, Sankaran V, Gan E, Huxsoll DL. 1978. Early detection of Rickettsia tsutsugamushi in peripheral monocyte cultures derived from experimentally infected monkeys and dogs. Southeast Asian J Trop Med Public Health 9:11–14. [PubMed] [Google Scholar]
- 16.Tantibhedhyangkul W, Prachason T, Waywa D, El Filali A, Ghigo E, Thongnoppakhun W, Raoult D, Suputtamongkol Y, Capo C, Limwongse C, Mege JL. 2011. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus. PLoS Negl Trop Dis 5:e1028. doi: 10.1371/journal.pntd.0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu H, Park SM, Cheon IS, Park MY, Shim BS, Gil BC, Jeung WH, Hwang KJ, Song KD, Hong KJ, Song M, Jeong HJ, Han SH, Yun CH. 2013. Orientia tsutsugamushi infection induces CD4+ T cell activation via human dendritic cell activity. J Microbiol Biotechnol 23:1159–1166. doi: 10.4014/jmb.1303.03019. [DOI] [PubMed] [Google Scholar]
- 18.Paris DH, Richards AL, Day NP. 2015. Orientia, p 2057–2079. In Tang Y, Liu D, Schwartzman J, Sussman M, Poxton I (ed), Molecular medical microbiology, vol 3 Academic Press, New York, NY. [Google Scholar]
- 19.Hauptmann M, Kolbaum J, Lilla S, Wozniak D, Gharaibeh M, Fleischer B, Keller CA. 2016. Protective and pathogenic roles of CD8+ T lymphocytes in murine Orientia tsutsugamushi infection. PLoS Negl Trop Dis 10:e0004991. doi: 10.1371/journal.pntd.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu G, Mendell NL, Liang Y, Shelite TR, Goez-Rivillas Y, Soong L, Bouyer DH, Walker DH. 2017. CD8+ T cells provide immune protection against murine disseminated endotheliotropic Orientia tsutsugamushi infection. PLoS Negl Trop Dis 11:e0005763. doi: 10.1371/journal.pntd.0005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyer AR, Rodino KG, VieBrock L, Green RS, Tegels BK, Oliver LD Jr, Marconi RT, Carlyon JA. 2017. Orientia tsutsugamushi Ank9 is a multifunctional effector that utilizes a novel GRIP-like Golgi localization domain for Golgi-to-endoplasmic reticulum trafficking and interacts with host COPB2. Cell Microbiol 19:12727. doi: 10.1111/cmi.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodino KG, VieBrock L, Evans SM, Ge H, Richards AL, Carlyon JA. 2018. Orientia tsutsugamushi modulates endoplasmic reticulum-associated degradation to benefit its growth. Infect Immun 86:e00596-17. doi: 10.1128/IAI.00596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko Y, Cho NH, Cho BA, Kim IS, Choi MS. 2011. Involvement of Ca(2+) signaling in intracellular invasion of non-phagocytic host cells by Orientia tsutsugamushi. Microb Pathog 50:326–330. doi: 10.1016/j.micpath.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Giengkam S, Blakes A, Utsahajit P, Chaemchuen S, Atwal S, Blacksell SD, Paris DH, Day NP, Salje J. 2015. Improved quantification, propagation, purification and storage of the obligate intracellular human pathogen Orientia tsutsugamushi. PLoS Negl Trop Dis 9:e0004009. doi: 10.1371/journal.pntd.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. 2002. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A 99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villard J, Muhlethaler-Mottet A, Bontron S, Mach B, Reith W. 1999. CIITA-induced occupation of MHC class II promoters is independent of the cooperative stabilization of the promoter-bound multi-protein complexes. Int Immunol 11:461–469. doi: 10.1093/intimm/11.3.461. [DOI] [PubMed] [Google Scholar]
- 27.Evans SM, Rodino KG, Adcox HE, Carlyon JA. 2018. Orientia tsutsugamushi uses two Ank effectors to modulate NF-kappaB p65 nuclear transport and inhibit NF-kappaB transcriptional activation. PLoS Pathog 14:e1007023. doi: 10.1371/journal.ppat.1007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamkanfi M, Kanneganti TD. 2012. Regulation of immune pathways by the NOD-like receptor NLRC5. Immunobiology 217:13–16. doi: 10.1016/j.imbio.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neerincx A, Castro W, Guarda G, Kufer TA. 2013. NLRC5, at the heart of antigen presentation. Front Immunol 4:397. doi: 10.3389/fimmu.2013.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neerincx A, Rodriguez GM, Steimle V, Kufer TA. 2012. NLRC5 controls basal MHC class I gene expression in an MHC enhanceosome-dependent manner. J Immunol 188:4940–4950. doi: 10.4049/jimmunol.1103136. [DOI] [PubMed] [Google Scholar]
- 31.Ching WM, Wang H, Eamsila C, Kelly DJ, Dasch GA. 1998. Expression and refolding of truncated recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin Diagn Lab Immunol 5:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi S, Jeong HJ, Ju YR, Gill B, Hwang KJ, Lee J. 2014. Protective immunity of 56-kDa type-specific antigen of Orientia tsutsugamushi causing scrub typhus. J Microbiol Biotechnol 24:1728–1735. doi: 10.4014/jmb.1407.07048. [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Cho NH, Kim SY, Bang SY, Chu H, Choi MS, Kim IS. 2008. Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. J Infect Dis 198:250–257. doi: 10.1086/589284. [DOI] [PubMed] [Google Scholar]
- 34.Soong L, Wang H, Shelite TR, Liang Y, Mendell NL, Sun J, Gong B, Valbuena GA, Bouyer DH, Walker DH. 2014. Strong type 1, but impaired type 2, immune responses contribute to Orientia tsutsugamushi-induced pathology in mice. PLoS Negl Trop Dis 8:e3191. doi: 10.1371/journal.pntd.0003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizvi M, Sultan A, Chowdhry M, Azam M, Khan F, Shukla I, Khan HM. 2018. Prevalence of scrub typhus in pyrexia of unknown origin and assessment of interleukin-8, tumor necrosis factor-alpha, and interferon-gamma levels in scrub typhus-positive patients. Indian J Pathol Microbiol 61:76–80. doi: 10.4103/IJPM.IJPM_644_16. [DOI] [PubMed] [Google Scholar]
- 36.Kang SJ, Jin HM, Cho YN, Kim SE, Kim UJ, Park KH, Jang HC, Jung SI, Kee SJ, Park YW. 2017. Increased level and interferon-gamma production of circulating natural killer cells in patients with scrub typhus. PLoS Negl Trop Dis 11:e0005815. doi: 10.1371/journal.pntd.0005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramme S, An Le V, Khoa ND, Trin Le V, Tannich E, Rybniker J, Fleischer B, Drosten C, Panning M. 2009. Orientia tsutsugamushi bacteremia and cytokine levels in Vietnamese scrub typhus patients. J Clin Microbiol 47:586–589. doi: 10.1128/JCM.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki H, Takada N, Nakamura T, Ueda T. 1997. Increased levels of macrophage colony-stimulating factor, gamma interferon, and tumor necrosis factor alpha in sera of patients with Orientia tsutsugamushi infection. J Clin Microbiol 35:3320–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choo SY. 2007. The HLA system: genetics, immunology, clinical testing, and clinical implications. Yonsei Med J 48:11–23. doi: 10.3349/ymj.2007.48.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiina T, Hosomichi K, Inoko H, Kulski JK. 2009. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet 54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 41.Bjorkman PJ, Parham P. 1990. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem 59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- 42.Vitiello A, Potter TA, Sherman LA. 1990. The role of beta 2-microglobulin in peptide binding by class I molecules. Science 250:1423–1426. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]
- 43.Ludigs K, Seguin-Estevez Q, Lemeille S, Ferrero I, Rota G, Chelbi S, Mattmann C, MacDonald HR, Reith W, Guarda G. 2015. NLRC5 exclusively transactivates MHC class I and related genes through a distinctive SXY module. PLoS Genet 11:e1005088. doi: 10.1371/journal.pgen.1005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neerincx A, Lautz K, Menning M, Kremmer E, Zigrino P, Hosel M, Buning H, Schwarzenbacher R, Kufer TA. 2010. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J Biol Chem 285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benko S, Magalhaes JG, Philpott DJ, Girardin SE. 2010. NLRC5 limits the activation of inflammatory pathways. J Immunol 185:1681–1691. doi: 10.4049/jimmunol.0903900. [DOI] [PubMed] [Google Scholar]
- 46.Kuenzel S, Till A, Winkler M, Hasler R, Lipinski S, Jung S, Grotzinger J, Fickenscher H, Schreiber S, Rosenstiel P. 2010. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J Immunol 184:1990–2000. doi: 10.4049/jimmunol.0900557. [DOI] [PubMed] [Google Scholar]
- 47.VieBrock L, Evans SM, Beyer AR, Larson CL, Beare PA, Ge H, Singh S, Rodino KG, Heinzen RA, Richards AL, Carlyon JA. 2014. Orientia tsutsugamushi ankyrin repeat-containing protein family members are type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum. Front Cell Infect Microbiol 4:186. doi: 10.3389/fcimb.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonthayanon P, Chierakul W, Wuthiekanun V, Phimda K, Pukrittayakamee S, Day NP, Peacock SJ. 2009. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol 47:430–434. doi: 10.1128/JCM.01927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valbuena G, Walker DH. 2012. Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol 2:170. doi: 10.3389/fcimb.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 51.Quinn LL, Williams LR, White C, Forrest C, Zuo J, Rowe M. 2016. The missing link in Epstein-Barr virus immune evasion: the BDLF3 gene induces ubiquitination and downregulation of major histocompatibility complex class I (MHC-I) and MHC-II. J Virol 90:356–367. doi: 10.1128/JVI.02183-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemmermann NA, Fink A, Podlech J, Ebert S, Wilhelmi V, Bohm V, Holtappels R, Reddehase MJ. 2012. Murine cytomegalovirus immune evasion proteins operative in the MHC class I pathway of antigen processing and presentation: state of knowledge, revisions, and questions. Med Microbiol Immunol 201:497–512. doi: 10.1007/s00430-012-0257-y. [DOI] [PubMed] [Google Scholar]
- 53.Lemmermann NA, Bohm V, Holtappels R, Reddehase MJ. 2011. In vivo impact of cytomegalovirus evasion of CD8 T-cell immunity: facts and thoughts based on murine models. Virus Res 157:161–174. doi: 10.1016/j.virusres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 54.Martin RK, Damle SR, Valentine YA, Zellner MP, James BN, Lownik JC, Luker AJ, Davis EH, DeMeules MM, Khandjian LM, Finkelman FD, Urban JF Jr, Conrad DH. 2018. B1 cell IgE impedes mast cell-mediated enhancement of parasite expulsion through B2 IgE blockade. Cell Rep 22:1824–1834. doi: 10.1016/j.celrep.2018.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]


