Abstract
The gold standard assay for the characterization of stem/progenitor cells in terms of their self-renewal, tissue regeneration, and tumorigenesis is the in-vivo transplantation. To do so, researchers would need an enriched population of stem cells as they represent a small fraction of a given tissue. An enriched population of stem/progenitor cells would considerably increase the chance of engraftment and consequently decrease the number of recipient animals needed for in vivo transplantation. Mammosphere formation by mammary epithelial stem and progenitor cells have been widely adopted for enriching stem/progenitor cells. They also allow researchers to study the genetic and epigenetic properties, interaction with other types of cells, and process of differentiation and oncogenic transformation of stem/progenitor cells. However, generation of mammospheres involves many steps and requires certain skills. Here, we describe a detailed mammosphere assay protocol, including isolation and culture of human primary mammary epithelial stem/progenitor cells and their differentiation and passage in 3D organoid culture. We also describe a protocol for ex vivo culture of fresh human breast tissue used for mimicking clinical treatment. The methods are described in sufficient step-by-step detail from tissue handling to stem/progenitor cell-generated 3D organoid passage, which can be useful for the assessment of mammary stem/progenitor cell properties, functions, and neoplastic transformation.
Keywords: mammospheres, stem cells, progenitors, primary epithelial cells
INTRODUCTION
Mammary stem and progenitor cells from fresh breast tissues have been widely used for studying their self-renewal and lineage specific regeneration of mammary ductal structure as well as their role in mammary tumorigenesis. The mammosphere assay has been widely used in both culturing and maintaining mammary stem and progenitor cells. Although it is a relatively simple assay to understand, it can be difficult to master. Here, we describe a step-by-step detailed mammosphere assay protocol, including isolation, culture, and differentiation assay of mammary epithelial stem and progenitor cells. This protocol can be used to culture and maintain undifferentiated human mammary stem and progenitor cells, and evaluate the effect of agents on self-renewal and differentiation of mammary stem and progenitor cells.
Human mammary gland is mainly composed of fat and fibrous tissues in addition to mammary ducts. A mixture of collagenase and hyaluronidase is used to digest the tissue. Fat is removed after centrifugation at 4°C (see Basic Protocol 1). Breast tissue also contains blood cells and stroma cells in addition to epithelial cells. Flow cytometry sorting (see Basic Protocol 2) has proven to be an effective and rapid method for separation of epithelial cells from blood cells and stroma cells. Mammosphere formation is achieved in non-adherent culture conditions (see Basic Protocol 3). The mammospheres formed by basal or luminal stem/progenitor cells are further distinguished morphologically in 3D extracellular matrix culture, which allows us to study self renewal capacity of stem and progenitor cells in a serial passage assay (see Basic Protocol 4). This method is based on the combination of several steps: isolation, the mammosphere assay, differentiation assay (3D Organoid culture) and 3D organoid passage.
NOTE: The research with human tissue specimens should be conducted with the appropriate approvals by the Institutional Review Board and Biosafety Committee.
NOTE: All procedures are performed in a Class II biological hazard flow hood.
NOTE: All solutions and equipment coming into contact with tissue and cells must be sterile, and proper aseptic techniques should be used.
NOTE: All incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
STRATEGIC PLANNING
The time schedule for the entire procedure is shown in Table 1.
Table 1.
Strategic Planning
| Time | Event |
|---|---|
| Day1 | Tissue digestion |
| Day2 | 1. Isolation of mammary epithelial cells 2. Mammosphere formation assay |
| Day8 | Stem/progenitor cell differentiation with 3D organoid culture in extracellular matrix (Matrigel) |
| Day17 | 3D organoid passage |
BASIC PROTOCOL 1: Single mammary cells preparation from fresh human breast tissue
In this protocol, human breast tissue is digested using collagenase/hyaluronidase and followed by trypsin-EDTA and dispase treatment as detailed in previous methods (Dong et al., 2013; Dontu, Abdallah, et al., 2003).
Materials
Fresh human normal breast tissue adjacent to breast tumors from woman patients
Sterile Phosphate-buffered saline (PBS)
Sterile forceps, scissors, and scalpel
DMEM F12 (1:1), CAT#12400-024, GIBCO.
Glutamine 200 mM, CAT# MT-25-005-CI, FISHER
Penicillin/Streptomycin 10,000 U/mL, CAT# MT-30-002-CI, FISHER
Collagenase/Hyaluronidase, CAT# 07912, STEM CELL TECHNOLOGIES
Epidermal growth factor (EGF), CAT# E9644, SIGMA
Cholera Toxin, CAT# C9903, SIGMA
Insulin, CAT# 91077C, SIGMA
Hydrocortisone, CAT #07925, STEM CELL TECHNOLOGIES
Bovine serum albumin (BSA), CAT# A7906, SIGMA
Fetal bovine serum (FBS), CAT# S11150, ATLANTA BIOLOGICS
Ammonium Chloride Solution, CAT# 07850, STEM CELL TECHNOLOGIES
Trypsin-EDTA (0.25%), CAT# 07901, STEM CELL TECHNOLOGIES.
Dispase in Hanks’ Balanced Salt Solution (5 U/mL), CAT# 7913, STEM CELL TECHNOLOGIES
DNase I Solution (1 mg/mL), CAT#07900, STEM CELL TECHNOLOGIES
15 mL and 50 mL sterile Polypropylene Conical Centrifuge Tubes, REF 352097 and 352098, FALCON
100 mm X 20 mm and 60 mm X 15 mm sterile Polypropylene culture dishes, REF 430167 and 430196, FALCON
Individually-wrapped sterile pipettes, REF 4488, COSTAR
Strainer, 40 μm, CAT# 352340, FALCON
Steps
Prepare breast tissue
-
1
Prior to dissection, prepare the digestion medium: DMEM/F12 (1:1) supplemented with 1 mg/mL BSA (needs to be sterile by filtration), glutamine at 2 mM, Penicillin/Streptomycin (100 U/mL/300 U and mL collagenase respectively), 100 U/mL hyaluronidase, 10 ng/mL epidermal growth factor (EGF), 100 ng/mL cholera toxin, 5 μg/mL insulin, 0.5 μg/mL hydrocortisone and 5% FBS.
-
2
Rinse 0.3 to 5 grams of breast tissue with cold PBS and transfer the tissue in a 60 mm culture dishes placed on ice. Rapidly mince tissue with scissor to obtain a pulp.
Note: This step must be performed on ice and should be done rapidly, otherwise the yield of epithelial cells will decreases.
-
3
Add the digestion medium in a weight (gram) to volume (mL) ratio of 2:3, transfer to a 15 mL or 50 mL polypropylene conical centrifuge tubes, and incubate the minced tissue for 18 hours at 37°C in a tissue culture incubator. Loose the cap of the tube.
Note: Tissue with less than 2 gram is digested in 3 mL digestion medium.
Remove fat and lyse the red cells
-
4
Vortex the tube gently and intermittently for 15 seconds, then stop the digestion by adding cold 1X PBS containing 2% FBS, mix gently by inverting the tube and then centrifuge at 700g for 5 min at 4°C.
-
5
Discard the supernatant containing adipose cells/tissue and resuspend the pellet into 2 mL of 1X PBS containing 2% FBS, then lyse the red cells by adding 8 mL of Ammonium Chloride Solution to the tissue. Incubate for 5 min on ice and then centrifuge at 700g for 5 min at 4°C.
Prepare single cells
-
6
Discard the supernatant and add 2 mL pre-warmed (at 37°C) 0.25% Trypsin-EDTA, then gently pipette up/down for 3 min with a 1 mL pipette.
Note: Cut the end of the pipette tip if the tissue is too viscous.
-
7
Stop Trypsin by adding 10 mL cold 1X PBS containing 2% FBS and centrifuge at 700g for 5 min at 4°C
-
8
Discard the supernatant and resuspend the pellet in 2 mL pre-warmed (at 37°C) 5 U/mL dispase containing DNase I 100 μg/mL, then gently pipetted up/down for 3 min. Centrifuge at 700g for 5 min at 4°C.
-
9
Stop Dispase by adding 10 mL cold 1X PBS containing 2% FBS and filtrate the digested tissue through a 40-μm strainers. Centrifuge at 700g for 5 min at 4°C. If the tissue is very fibrous, filtrate the digested tissue with a 100-μm strainer (FALCON, CAT# 352360).
-
10
Discard the supernatant and resuspend the pellet in 1X PBS (100–200 μL depending on the size of the pellet to obtain 1×107 cell/mL for flow cytometry analysis). Count the cells and evaluate their viability with 0.4% Trypan blue, which will dye dead cells. The dissociated cells are ready for flow cytometry analysis and sorting as described in Basic Protocol 2.
ALTERNATE PROTOCOL 1: Explant culture of fresh breast tissue before single cell isolation
Here, we also provide an alternate protocol for explant culture of fresh human breast tissue used for mimicking clinical drug treatment. The tissue was cut into small pieces and cultured/treated on sponge (Kogata & Howard, 2015; van Bogaert, 1976).
Materials
RPMI 1640 medium
FBS
Hydrocortisone, CAT #07925, STEM CELL TECHNOLOGIES
Insulin, CAT# 91077C, SIGMA
Absorbable Gelatin Sterile Sponges, CAT #59-9863, HARVARD APPARATUS
6-well cell culture plate, REF 3516, COSTAR
Sterile surgical scissors
Antibiotic/antimycotic, CAT# 15290-018, FISHER
Protocol steps
Prepare explant media (RPMI 1640 with 10% FBS + 1% antibiotic/antimycotic + 0.01 mg/mL hydrocortisone + 0.1 mg/mL insulin).
Gelatin sponges should be pre-soaked in 3 mL of explant media in 6 well plate for 30 mins.
Dice 0.3–0.5 grams of tumor tissue in to small pieces (approximately around 3×3×3 mm pieces) and washed with RPMI 1640 basic medium.
Placed pre-washed tissue pieces on gelatin sponge in the 6 well plate and add another 2 mL explant medium. A representative photo of the set-up is shown in Figure 1.
After 2 hours’ incubation at 37°C, treatments of any drug of choice can be done with additional 1mL explant media contains 6X concentration of drugs. Set up vehicle and drug treatments in triplicates or quadruplicates.
After culture for 48 hours at 37°C, tissue fragments can be processed for digestion as described in “BASIC PROTOCOL 1”.
Figure 1.
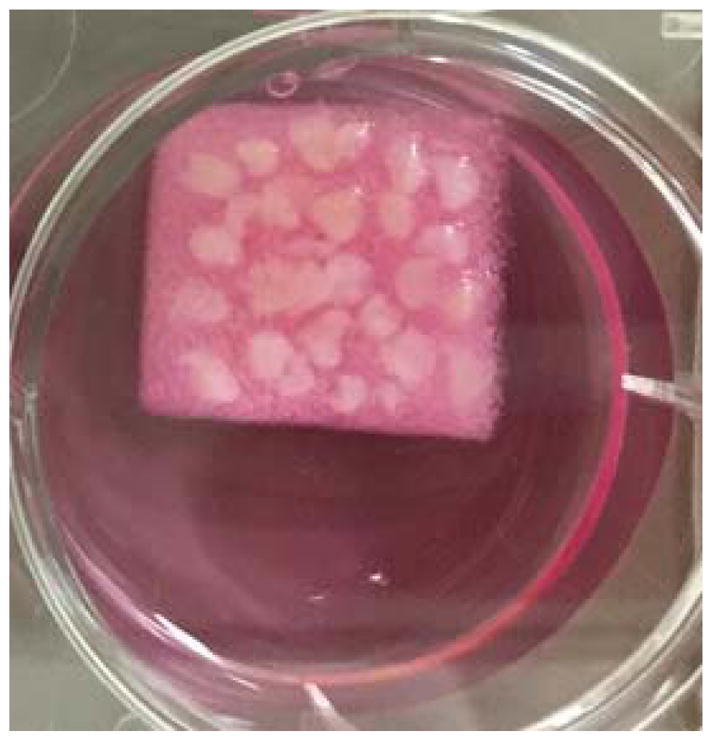
Ex vivo culture of fresh breast tissue. The tissue was cut into small pieces and cultured on the sponge in a well of a 6-well plate as shown in the figure.
BASIC PROTOCOL 2: Isolation of mammary epithelial stem/progenitor cells by flow cytometry sorting
In this protocol, single cells are labeled with several antibodies targeting different cell surface markers and sorted using flow cytometry as described by Stingl and co-workers (Stingl et al., 2006).
Materials
1 X Phosphate-buffered saline (1 X PBS), Sterile
Biotinylated Anti-CD31 antibody, CAT# 13-0319-82, eBioscience
Biotinylated Anti-CD235a, CAT#13-9987-80, eBioscience
Biotinylated Anti-CD45, CAT# 13-0459-82, eBioscience
Brilliant Violet 605™ Streptavidin, CAT# 405229, BioLegend, Inc.
Anti CD49f-APC, CAT# 17-0495-82, eBioscience
Anti EpCAM-FITC, CAT# 10109, STEM CELL TECHNOLOGIES
5 mL Polystyrene Round-Bottom Tube with Cell-Strainer Cap (12 X 75 mm style), REF 352235, FALCON
5 mL Polypropylene Round-Bottom Tube (12 X 75 mm style), REF 352063, FALCON
BD FACSAria cell sorter
Protocol steps
-
Setup the controls for the flow cytometry in polystyrene flow cytometry tube as shown in Table 2:
Tube “Blank”: 40,000 cells from Step 10 of Basic Protocol 1. Keep the tube on ice until sorting.
Tube “Single color control”: 40,000 cells from Step 10 of Basic Protocol 1 in each tube. Set up 3 tubes for the 3 markers labeled “Lin”, “CD49f” and “EpCAM”.
The cells to be sorted from Step 10 of Basic Protocol 1 are also transferred into a polystyrene flow cytometry tube ( labeled “Sample”). Adjust the cell concentration at 1×107 cell/mL in PBS.
Add biotinylated CD235a, CD31 and CD45 antibodies (volumes are indicated in Table 2) in the “Lin” control tube and in the “Sample” tube. Incubate for 15 min on ice.
Add 4 mL cold 1 X PBS to “Lin” and “Sample” tubes. Centrifuge the tubes at 700g for 5 min at 4°C. Discard the supernatant and resuspend the cells in 100–200 μL of 1X PBS.
Add the correct volume of Brilliant Violet 605™ Streptavidin to the “Lin” and “Sample” tubes (see Table 2). Add the correct volume of CD49f and EpCAM antibodies in the “Sample” tube and the corresponding “Control” tubes. Incubate on ice for 15 min. The tubes should be protected from light.
Add 4 mL cold 1 X PBS in each tube and centrifuge the tubes at 700g for 5 min at 4°C. Discard the supernatant, then add 400 μL of cold 1X PBS containing 2% FBS to each tube, keep those tubes on ice for sorting.
Prepare 3 collection tubes (5 mL Polypropylene Round-Bottom Tube) for each sample, add 0.5 mL of cold 1X PBS containing 2% FBS to each tube, keep those tubes on ice for collection of sorted cells.
-
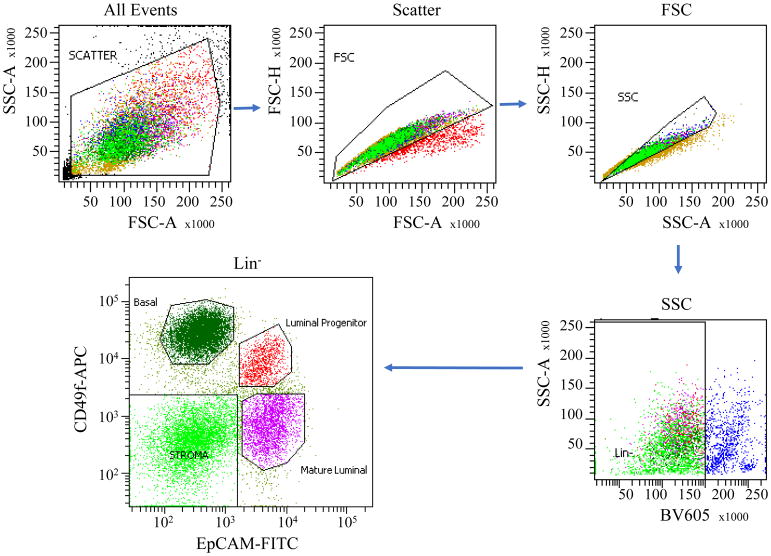
Sort basal mammary epithelial cells (Lin−/CD49fhigh/EpCAM−), luminal progenitor cells (Lin−/CD49flow/EpCAM+), and mature luminal cells (Lin−/CD49f−/EpCAM+) with a flow cytometry sorter such as BD FACSAria cell sorter. A representative flow cytometry setup and sorting data are shown in Figure 2.
Note: Keep the cells on ice to prevent cells from re-aggregating and for use in Basic Protocol 3.
Note: UltraComp eBeads (CAT# 01-2222-42, THERMOFISHER SCIENTIFIC) can be used instead of cells for the controls, particularly when working with limited number of cells after dispase treatment.
Table 2.
Pre-coating cells with specific antibody
| Antibody | Tube
|
||||
|---|---|---|---|---|---|
| Blank | Single color control
|
Sample | |||
| Lin | CD49f | EpCAM | |||
| Anti CD31 | - | 1 μL | - | - | 1 μL for 106 cells |
| Anti CD235a | - | 1 μL | - | - | 1 μL for 106 cells |
| Anti CD45 | - | 1 μL | - | - | 1 μL for 106 cells |
| Brilliant Violet 605Streptavidin | - | 0.5 μL | 0.5 μL | ||
| Anti CD49f-APC | - | - | 3 μL | - | 3 μL for 106 cells |
| Anti EpCAM-FITC | - | - | - | 3 μL | 110 μL for 106 cells |
Figure 2.
Flow cytometer sorting profile of input human mammary cell preparations showing the gates used to isolate fractions of stroma cells, mature luminal cells, luminal and basal cells containing progenitor/stem cells.
BASIC PROTOCOL 3: Mammosphere formation from sorted human mammary epithelial cells
Sphere formation is performed in ultralow attachment 96-well plates with complete MammoCult™ Human Medium. After 6 days, the individual sphere number is counted (Dontu, Abdallah, et al., 2003; Dontu & Wicha, 2005).
Materials
MammoCult™ Human Medium Kit, CAT# 05620, STEM CELL TECHNOLOGIES
Hydrocortisone, CAT #07925, STEM CELL TECHNOLOGIES
Heparin, CAT #07980, STEM CELL TECHNOLOGIES
Penicillin/Streptomycin 1000 U/mL
Ultralow attachment 96-well plates, REF 3474, Corning
Steps
-
Prepare complete MammoCult™ Human Medium for mammosphere culture according to Table 3.
Note: Complete MammoCult™ Human Medium must be used within one week when stored at 4°C.
Spin down the sorted cells from Step 8 of Basic Protocol 2 at 700g for 5 min at 4°C. Then remove as much liquid as possible using a pipette.
Resuspend the cell pellet using complete MammoCult™ Human Medium and plate cells on 96 well ultra-low attachment plates at 5,000–20,000 cells per well for basal cells and 2,000–10,000 cells per well for luminal cells respectively. A volume of 100 μL MammoCult™ Human Medium is used per well. Feed the cells every 3 days by adding another 50 μL medium each time.
-
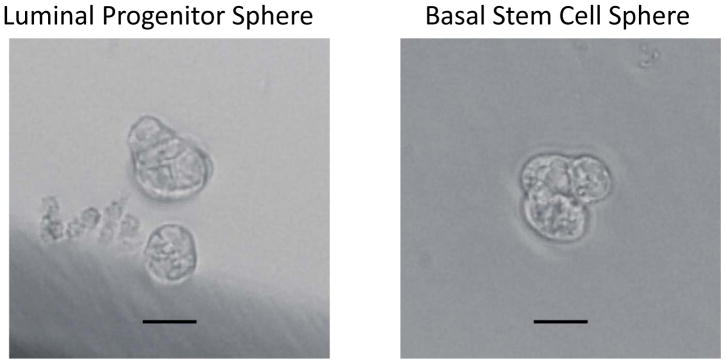
After 6 days of culture, count the number of spheres with diameter greater than 30 μm. Representative photos of spheres formed by basal stem cells and luminal progenitor cells are shown in Figure 3.
Note: A minority of cells in the sorted basal and luminal epithelial population will form spheres. They are considered stem/progenitor cells. Various studies have shown that the luminal cells and their spheres cannot regenerate mammary ducts, whereas the basal cells and their spheres can when transplanted in humanized cleared mammary fat pads of immune-deficient mice (Lloyd-Lewis, Harris, Watson, & Davis, 2017). Therefore, we call the sphere-forming luminal cells as Luminal Progenitor Cells and the sphere-forming basal cells as Basal Stem Cells in Figure 3.
Table 3.
Complete MammoCult™ Human Medium for mammosphere
| Item | Volume |
|---|---|
| MammoCult™ Basal Medium (Human, CAT #05621) | 9 mL |
| MammoCult™ Proliferation Supplement (Human, CAT #05622) | 1 mL |
| Heparin, 0.2 % | 20 μL |
| Hydrocortisone, 2.5 mg/mL | 1.9 μL |
| Penicillin/Streptomycin | 100 μL |
Figure 3.
Mammospheres formed by luminal progenitor cells and basal stem cells after 6 days of cultivation. Scale bar represents 25 μm.
BASIC PROTOCOL 4: 3D extracellular matrix (Matrigel) single sphere differentiation and passage
The mammospheres from Step 4 of Basic Protocol 3 can be manually picked using a microscope and SterileHamilton® syringe. This allows for accurate plating the number of spheres in Matrigel. After 9 days of culture in the presence of serum, the spheres will grow into hollow or solid 3D organoids, respectively. (Gjorevski et al., 2016; Timmins, Harding, Smart, Brown, & Nielsen, 2005).
Materials
EpiCult™-B Human Medium Kit, CAT# 05601, STEM CELL TECHNOLOGIES
Hydrocortisone, CAT #07925, STEM CELL TECHNOLOGIES
Fetal Bovine Serum
Glutamine 200 mM
Penicillin/Streptomycin 10000 U/mL
Dispase in Hanks’ Balanced Salt Solution (5 U/mL), CAT# 7913, STEM CELL TECHNOLOGIES
60 mm X 15 mm Polypropylene culture dish, REF 430196, FALCON
SterileHamilton® syringe, Model 705 RN SYR (Small Removable NDL, 22s ga, 2 in, point style 2, Specifications: 50 μL), CAT# 80530, HAMILTON
BD Matrigel™ Basement Membrane Matrix, 5 mL vial, CAT #356234, BD Biosciences
Ultralow attachment 24-well plates, REF 3473, CORNING
Steps
-
Prepare complete EpiCult™-B Human Medium for 3D-organoid culture according to Table 4.
Note: Complete EpiCult™-B Human Medium must be used within one week when stored at 4 °C.
Transfer all spheres from the same tissue sample into a 60-mm dish.
-
Using a microscope and under sterile condition, manually pick a total of 30 spheres using a blunt end Hamilton® syringe and transfer them in a 1.5 mL tube containing 25 μL cold EpiCult™-B Human Medium.
Note: The volume of medium containing spheres in the syringe should not exceed 5 μL.
-
Gently mix the spheres with 25 μL Matrigel and plate the sphere-Matrigel mixture as a droplet onto pre-warmed (in a 37°C incubator) 24-well ultra-low attachment plate for sphere differentiation assay.
Note: Gently mix the spheres with Matrigel to avoid bubbles before plating.
Let the droplet solidify inside a 37°C incubator for 15 minutes, then cover with 500 μL complete EpiCult™ Human Medium, and incubate at 37°C for 9 days.
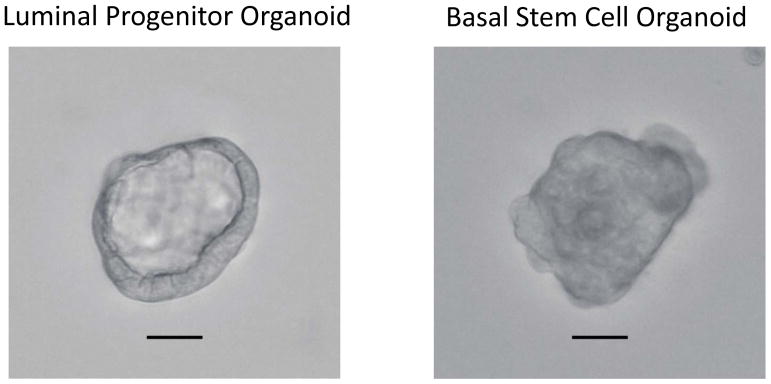
Count the number of 3D-organoids with a diameter greater than 100 μm. In general, luminal progenitor cell spheres give rise to both solid and hollow organoids, while basal cells give rise to only solid organoids. Representative photos of a luminal progenitor sphere-formed hollow 3D organoid and a basal stem cell sphere-formed solid 3D organoid are shown in Figure 4.
Discard most of the liquid medium in the well with a pipette and transfer the Matrigel with 3D-organoids to a 1.5 mL centrifuge tube. Pipette up and down with a 200 μL pipette to break the Matrigel and then centrifuge at 700g for 5 min.
Discard the supernatant and add 300 μL pre-warmed (at 37°C) 5 U/mL dispase to digest/dissolve Matrigel. Incubate at 37°C for 15 min, every 5 min gently pipette up and down to resuspend the organoids.
Stop dispase by adding 1 mL 1X PBS containing 2% FBS and centrifuge at 700g for 5 min.
Discard the supernatant and resuspend the organoid pellet in 2 mL pre-warmed (at 37°C) 0.25% Trypsin-EDTA, then gently pipetted up/down for 3 min to break organoids into single cells.
Stop Trypsin by adding 1 mL 1X PBS containing 2% FBS, centrifuge at 700g for 5 min at 4°C.
-
Discard the supernatant and resuspend gently the single cells with a small volume (<50 μL) of EpiCult™-B Human Medium to count cells. Mix 1,000–5,000 cells with 30 μL Matrigel and plate the cell-gel droplet onto pre-warmed 24 well ultra-low attachment plate (Corning 3473) for 3D-organoids generation.
Note: Gently mix the single cells with Matrigel to avoid bubbles.
Let the sphere-gel droplet solidify inside a 37°C incubator for 15 minutes, covered with 500 μL complete EpiCult™ Human Medium, and incubate at 37°C for 9 days.
Count the number of 3D organoids with a diameter greater than 100 μm.
Step7 through 14 can be repeated as many times as needed according to the purpose of the experiment. However, the 3D organoid regeneration efficiency will gradually decrease after each passage, likely due to the exhaustion of the stem/progenitor cells.
Table 4.
Complete EpiCult™-B Human Medium for 3D-Organoid culture
| Item | Volume |
|---|---|
| EpiCult™ Basal Medium (Human, CAT #05602) | 100 mL |
| EpiCult™ Proliferation Supplement (Human, CAT #05603) | 1 mL |
| L-Glutamine 100X | 1 mL |
| FBS | 5 mL |
| Hydrocortisone, 2.5 mg/mL | 20 μL |
| Pennicillin/Streptomycin | 100 μL |
Figure 4.
3D organoids formed by luminal progenitor and basal stem cell spheres after 7 days of cultivation. Scale bar represents 50 μm
COMMENTARY
Background Information
Spheres assay was first established by Reynold et al. in 1992 to investigate the neural stem cells and was later modified for use in other tissues including both normal and malignant tissues (Reynolds & Weiss, 1992). As Shaw and co-workers (Shaw et al., 2012) reviewed before, the mammosphere assay has also been used to quantify both stem cell activity and self-renewal in both normal mammary tissue and breast cancer tissue for the investigation of breast cancer initiation and development (Dong et al., 2013; Dontu, Abdallah, et al., 2003; Eirew et al., 2008; Liu, Deng, Lehal, Kim, & Zacksenhaus, 2007; Rota, Lazzarino, Ziegler, LeRoith, & Wood, 2012; Shackleton et al., 2006; Sleeman, Kendrick, Ashworth, Isacke, & Smalley, 2006; Stingl, Eaves, Zandieh, & Emerman, 2001; Stingl et al., 2006). However, the way mammosphere assay is performed varies among laboratories, and thus the interpretation of the data is not always consistent. It is Dontu and co-workers who first demonstrated that non-adherent mammospheres are enriched in cells with functional characteristics of stem/progenitor cells in 2003 (Dontu, Abdallah, et al., 2003; Dontu & Wicha, 2005). They tested several culture conditions and determined that serum-free medium containing EGF and/or bFGF at saturating concentrations (10–20 ng/mL), supplemented with insulin, hydrocortisone, and B27, promoted optimal mammosphere formation. Recently the limiting dilution analysis (LDA) was used on primary and secondary (or passaged) mammospheres to quantify the sphere formation efficiency (SFE) (Deome, Faulkin, Bern, & Blair, 1959; Dontu, Al-Hajj, Abdallah, Clarke, & Wicha, 2003; Lindeman, Visvader, Smalley, & Eaves, 2008; Rota et al., 2012; Ziegler et al., 2012). In our present protocol, we generated mammospheres using commercial MammoCult™ Human Medium Kit (STEMCELL Technologies Inc. Vancouver, BC, Canada) to quantify the sphere formation efficiency (SFE) with different plating densities of the sorted cells.
Critical Parameters and Troubleshooting
We have successfully enriched and cultured human mammary epithelial stem and progenitor cells from same-day surgical tissues from a local hospital and from overnight-shipped tissues in a basic cell culture medium on ice from Cooperative Human Tissue Network (CHTN).
If the tissue is very fibrous, it is recommended to digest the tissue using a shaker at 37°C to maximize the yield in Basic Protocol 1.
If the cell pellet is still red (Basic Protocol 1) after lysing the red blood cells, ammonium chloride solution can be used a second time to get rid of remaining red blood cells.
If spheres appear aggregated, resuspend the spheres by pipetting up and down to separate them for accurate counting of the number of spheres.
Understanding Results
The total yield of single mammary cells depends on the tissue quality. Usually, there are more epithelial ducts in the tissue near the nipple. The single cells should be ≥95% viable. Sphere formation efficiency (SFE) from Basal cells is about 5 per 1,000 cells. SFE from luminal cells is about 20 per 1,000 cells. Mature luminal cells cannot form any spheres.
Time Considerations
Preparation of mammary single cells (see Basic Protocol 1) usually takes 2 hr, depending on the size of tissue. Isolation of various populations of mammary epithelial cells by Flow Cytometry sorting (see Basic Protocol 2) requires an additional 1.5–2 hr including the antibody staining step. Mammosphere formation performed in the non-adherent culture condition (see Basic Protocol 3) takes 6 days. 3D organoid culture (see Basic Protocol 4) usually takes 9 days.
Significance Statement.
Successful maintenance of primary human mammary epithelial stem/progenitor cells allows research of their properties, functions, and transformation process. The protocols are provided for the isolation and culture of human mammary epithelial stem/progenitor cells as mammospheres, and their differentiation and passage in 3-dimentional (3D) organoid culture. We also provide a protocol for ex vivo culture and drug treatment of fresh human breast tissue for mimicking clinical treatment. The methods are presented in sufficient step-by-step detail from tissue handling to 3D organoid passage.
Acknowledgments
This work was supported in part by the grant from National Institutes of Health R01CA192564 to L-Z.S. We thank anonymous patients for donating their samples for our research. We also thank Dr. Ismail Jatoi and Dr. Alia Nazarullah in our School of Medicine, as well as Cooperative Human Tissue Network (CHTN) for the collection of human breast tissue samples for our study.
LITERATURE CITED
- Deome KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19(5):515–520. [PubMed] [Google Scholar]
- Dong Q, Wang D, Bandyopadhyay A, Gao H, Gorena KM, Hildreth K, … Sun LZ. Mammospheres from murine mammary stem cell-enriched basal cells: clonal characteristics and repopulating potential. Stem Cell Res. 2013;10(3):396–404. doi: 10.1016/j.scr.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10(1):75–86. doi: 10.1007/s10911-005-2542-5. [DOI] [PubMed] [Google Scholar]
- Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, Eaves CJ. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14(12):1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez-Moran P, … Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- Kogata N, Howard BA. A protocol for studying embryonic mammary progenitor cells during mouse mammary primordial development in explant culture. Methods Mol Biol. 2015;1293:51–62. doi: 10.1007/978-1-4939-2519-3_2. [DOI] [PubMed] [Google Scholar]
- Lindeman GJ, Visvader JE, Smalley MJ, Eaves CJ. The future of mammary stem cell biology: the power of in vivo transplants. Breast Cancer Res. 2008;10(3):402. doi: 10.1186/bcr1986. author reply 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res. 2007;67(18):8671–8681. doi: 10.1158/0008-5472.CAN-07-1486. [DOI] [PubMed] [Google Scholar]
- Lloyd-Lewis B, Harris OB, Watson CJ, Davis FM. Mammary Stem Cells: Premise, Properties, and Perspectives. Trends Cell Biol. 2017;27(8):556–567. doi: 10.1016/j.tcb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rota LM, Lazzarino DA, Ziegler AN, LeRoith D, Wood TL. Determining mammosphere-forming potential: application of the limiting dilution analysis. J Mammary Gland Biol Neoplasia. 2012;17(2):119–123. doi: 10.1007/s10911-012-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, … Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shaw FL, Harrison H, Spence K, Ablett MP, Simoes BM, Farnie G, Clarke RB. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia. 2012;17(2):111–117. doi: 10.1007/s10911-012-9255-3. [DOI] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8(1):R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Zandieh I, Emerman JT. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res Treat. 2001;67(2):93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, … Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Timmins NE, Harding FJ, Smart C, Brown MA, Nielsen LK. Method for the generation and cultivation of functional three-dimensional mammary constructs without exogenous extracellular matrix. Cell Tissue Res. 2005;320(1):207–210. doi: 10.1007/s00441-004-1064-6. [DOI] [PubMed] [Google Scholar]
- van Bogaert LJ. Glucose uptake by normal human breast tissue in organ culture. Influence of insulin and other additives. Cell Tissue Res. 1976;171(4):535–541. doi: 10.1007/BF00220244. [DOI] [PubMed] [Google Scholar]
- Ziegler AN, Schneider JS, Qin M, Tyler WA, Pintar JE, Fraidenraich D, … Levison SW. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30(6):1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]