Abstract
Introduction:
Chronic idiopathic constipation (CC) is highly prevalent worldwide. A subset of patients with CC have reduced fecal (and by inference, intra-colonic) bile acids (BA). Elobixibat, a locally-acting ileal bile acid transporter (IBAT) inhibitor, leads to increased BA delivery to the colon and represents a new class of treatment for CC. BAs accelerate colonic transit and increase colonic secretion. Therefore, IBAT inhibitors have potential to treat patients with CC.
Areas covered:
Rationale for IBAT inhibitor in therapeutics, and preclinical and clinical pharmacology of elobixibat: In vitro, elobixibat is a highly potent, selective IBAT inhibitor. In humans, elobixibat accelerated colonic transit. In phase 2A, 2B and 3 studies in CC, elobixibat was efficacious, well tolerated and safe. An open-label, phase 3 trial (52 weeks) confirmed the safety of elobixibat. Elobixibat reduces LDL cholesterol, increases serum GLP-1, and has potential in metabolic syndrome.
Expert commentary:
Uniquely among current treatments of CC, elobixibat stimulates both motor and secretory functions in the colon. These dual effects suggest that, when approved, elobixibat may be a first-line choice for constipation associated with colonic BA deficiency and a second-line treatment for all patients with CC and constipation-predominant irritable bowel syndrome. Further studies are required to confirm efficacy for relief of CC. Once approved, elobixibat will likely become a second-line choice for treatment of CC.
Keywords: bile acid, enterohepatic circulation, ileal bile acid transporter (IBAT), irritable bowel syndrome, pharmacodynamics, pharmacokinetics
1.0. Introduction
Increased fecal bile acids (BAs) and hepatic BA synthesis, which are markers of BA malabsorption or diarrhea, have been identified in patients with chronic diarrhea or diarrhea-predominant irritable bowel syndrome (IBS-D) [1,2]. In approximately 25-33% of patients with chronic functional diarrhea [3,4], excess BAs enter the colon and activate the various mechanisms listed in Table 1[5-29], resulting in diarrhea and abdominal discomfort. Shin et al. demonstrated that these biochemical changes translate to clinical findings. Participants with higher total fecal BAs had higher numbers of bowel movements, looser stool consistency, and faster colonic transit at 24 hours [1]. Other biomarkers in patients with IBS-D, in addition to elevated 48-hour total fecal BAs, are increased fasting serum 7-α-hydroxy-4-cholesten-3-one (7αC4) [2,30], decreased serum fibroblast growth factor 19 (FGF19) [31,32], and 75-selenium homocholic acid taurine (75SeHCAT) retention <15% [3].
Table 1.
Mechanisms of stimulation of colonic water and electrolyte movements and motility by BAs. Adapted with permission from reference 5.
| Mechanism | Mediators/Co-factors | Effects |
|---|---|---|
| Stimulation of intracellular mediators [6-11] | ↑cAMP, epidermal growth factor receptor, and mediators including exchange protein directly activated by cAMP and Ca ++ ions | CFTR - induced chloride secretion |
| ↑ intestinal permeability [12-15] | detergent or structure-activity properties of the BAs, TGR5 activation, ↓occludins | ↑ secretion, ↑ motility, ↓ transepithelial barrier |
| Aquaporin channels [16,17] | ↑ aquaporin channels 3 and 8 in rats | ↑ water secretion |
| Enteroendocrine mechanisms [18-21] | ↑ serotonin | ↑ fluid and mucus secretion; activation of enteric neurons to ↑ colonic motility |
| Neurocrine mediation [21-25] | Activate basal TGR5 and submucosal cholinergic neurons; TGR5 activation of cholinergic and nitrergic myenteric neurons | ↑ colonic motility, transit and secretion |
| Decreased sodium and water absorption [26] | ↓ sodium potassium ATPase β1 unit in colon and α1 unit in proximal colon | ↓ sodium and water reabsorption |
| ↑ Myoelectrical activity [27-29] | Mechanism(s) unclear | ↑ motility index |
| ↑ HAPC [23] | ↑ frequency of colonic propagating pressure wave sequences; mechanism(s) unclear | ↑ colonic mass movements |
ATP, adenosine triphosphatase; cAMP, cyclic adenosine monophosphate; CFTR, cystic fibrosis transmembrane conductance regulator
Conversely, there is now evidence that approximately 15% of patients with constipation predominant irritable bowel syndrome (IBS-C) has low 48-hour fecal BAs, and the proportion of lithocholic acid (LCA) (non-secretory BA) was increased and deoxycholic acid (DCA) (secretory BA) was decreased in IBS-C compared to controls [33]. Fasting serum 7αC4 was decreased (indicating decreased hepatic BA synthesis) and FGF19 was elevated (suggestive of increased negative feedback and, therefore, decreased hepatic BA synthesis) [33]. The associations of indices of BA synthesis or excretion with colonic functions were supported by 7αC4 being directly and FGF19 inversely correlated with colonic transit [33]. The reduction in total fecal bile acids and, particularly, decreased DCA in patients with functional constipation and IBS-C requires confirmation.
2.0. Body
2.1. The enterohepatic circulation
BAs are detergent molecules that aid in fat and fat soluble vitamin absorption. BAs are synthesized within the liver from cholesterol via the addition of hydroxyl groups to the cholesterol ring, changing the structure and function of the sterol. They are subdivided into two categories: primary and secondary BAs [34]. Primary BAs are those synthesized and conjugated with glycine or taurine in the liver. The rate limiting step in hepatic BA synthesis is the addition of the hydroxyl group in the carbon-7 position via 7αC4. As noted in Figure 1, both primary BAs have an α-hydroxyl group in the carbon-7 position of the cholesterol ring [35]. Secondary BAs are the metabolites resulting from deconjugation and 7α-dehydroxylation of the primary conjugated BAs by colonic bacteria [36].
Figure 1. Chemical structures and colonic functions of individual bile acids. Modified with permission from reference 35.
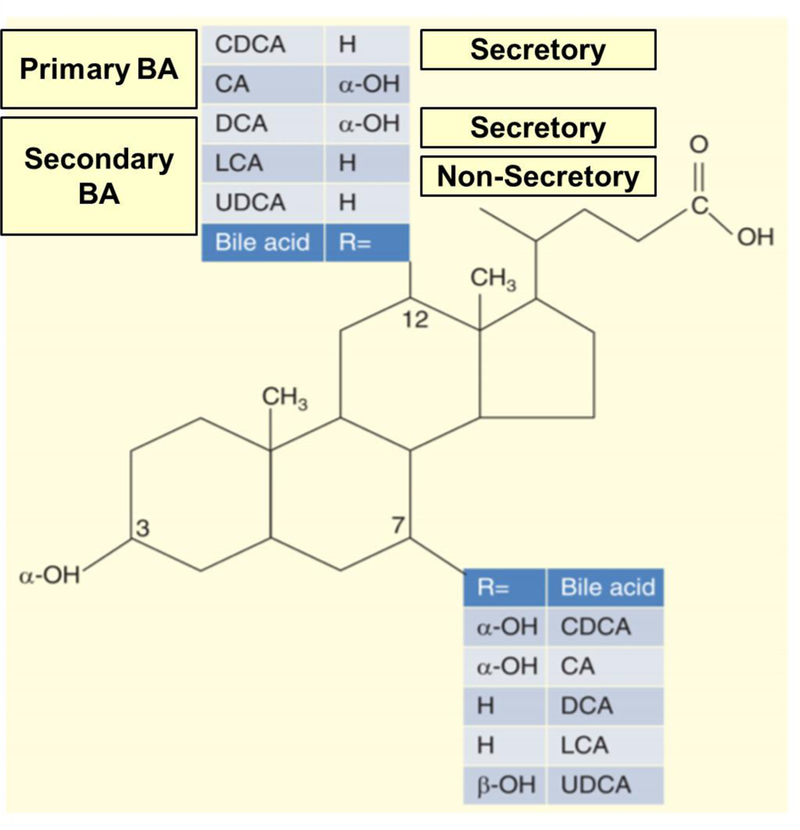
BA, bile acids; CDCA, chenodeoxycholic acid; CA, cholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid
After synthesis and storage in the gall bladder, BAs are released into the duodenum after fat enters the small intestine and cholecystokinin is released, resulting in contraction of the gall bladder. BAs emulsify fat and allow absorption within the small bowel [37]. After fat moieties are absorbed, the intraluminal free BAs are reabsorbed within the terminal ileum via the IBAT, with an efficiency of approximately 95% [38]. As depicted in Figure 2 [39], having entered the ileal enterocytes, BAs then activate the nuclear farnesoid X receptor (FXR), which increases the transcription of FGF19 [5]. FGF19 acts as an endocrine hormone, reaching the liver through the portal venous system. FGF19 binds to fibroblast growth factor receptor 4 (FGFR4) and klotho Β, and this leads to inhibition of hepatic CYP7A1 (rate-limiting enzymes for synthesis of 7αC4 and the primary BAs) [38,40].
Figure 2.
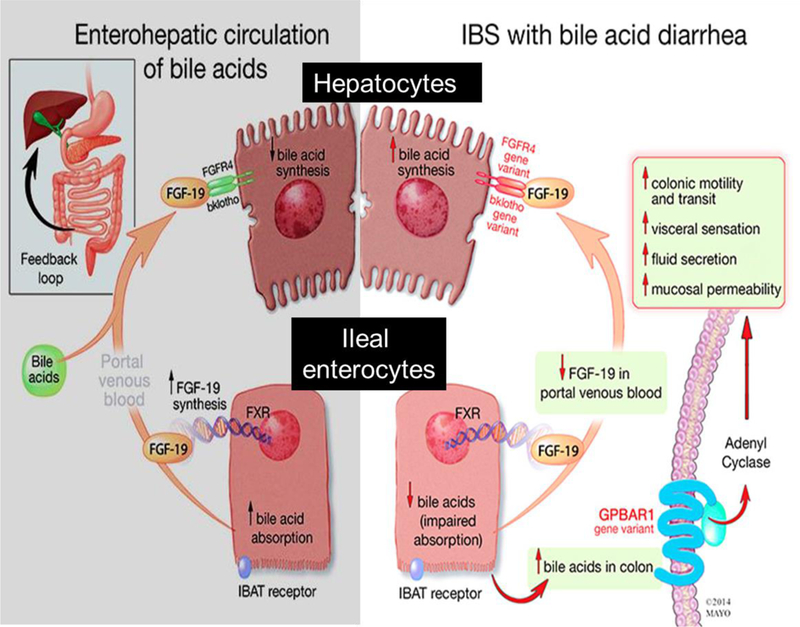
Enterohepatic circulation. The left panel shows the enterohepatic circulation in patients without bile acid malabsorption. The right panel demonstrates enterohepatic circulation in patients with bile acid malabsorption. Reproduced from reference [39] (no permission needed; it is a free PMC article). IBAT, ileal bile acid transporter; IBS, irritable bowel syndrome;
There are four major BAs in humans: chenodeoxycholic acid (CDCA), cholic acid (CA), deoxycholic acid (DCA), and lithocholic acid (LCA). As seen in Figure 1, these molecules differ structurally based on the locations of the hydroxyl groups, which alter the functions of the BAs [5,35]. CDCA (3α, 7α) and DCA (3α, 12α) have 2 α-hydroxyl groups which result in secretory properties compared to LCA which has non-secretory functions. In contrast, a fifth BA, ursodeoxycholic acid (3α, 7β), is a non-secretory BA in the colon [35].
3.0. Diagnostic testing for changes in bile acid synthesis or fecal bile acid excretion
There are presently two direct and two indirect methods of measuring excess or decreased BA synthesis or excretion. The two direct methods are the 75Selenium HomotauroCholic Acid Test (75SeHCAT) retention and 48-hour total fecal BA excretion. The 75SeHCAT utilizes radiolabeled BAs and monitors the percent retained over a period of seven days. Patients with excess BA loss have decreased retention (<15%) at seven days [3]. This test is available in Europe, but not in the United States. The alternative direct measurement of loss of BAs is the 48-hour total and individual fecal BA excretion; this requires ingestion of a 100-gram fat diet for four days with stool collection in the final two days [1,2,35]. Because of the dietary requirements and cumbersome stool collections required for measurement of fecal 48-hour bile excretion, patients may not be willing to complete this testing. Abnormal BA levels have been reported in patients with constipation; relative to healthy controls, there was reduced total fecal BAs and, specifically, DCA (which may result in reduced colonic secretion and motility) with increased LCA [33] and increased fasting plasma levels of taurine-conjugated DCA, CA, and LCA [41], which the authors attributed to greater BA reabsorption as a result of constipation.
The two indirect methods for measurement of BA balance are serum fasting biomarkers (7αC4 and FGF19) that indirectly measure BA synthesis. 7αC4 directly correlates with BA synthesis, and FGF19 indirectly assesses BA synthesis [30,31]. A meta-analysis evaluating the frequency of positive tests suggestive of BA mechanisms in functional diarrhea or IBS-D demonstrated yields of 75SeHCAT of 0.308, total fecal BAs 0.255, 7αC4 0.171, and FGF19 0.248 [42]. However, it is important to note that none of the cohorts evaluated the diagnostic tests head-to-head.
4.0. Bile acids are physiological laxatives
Conjugated and non-conjugated BAs are physiologic laxatives that stimulate colonic motility [43] and secretion [44].
4.1. Effects of bile acids on colonic secretion and motility
When BAs enter the colon, there are a number of mechanisms activated that result in increased colonic contractility, increased colonic mucosal permeability, increased water and electrolyte secretion, and decreased water reabsorption. Table 1 summarizes the diverse mechanisms as summarized elsewhere [5].
BAs are known to stimulate colonic motility. Numerous studies have demonstrated increased colonic motility in both animals and humans after infusing DCA and CDCA, both of which are also secretory BAs (Table 1). BAs also activate TGR5 (takeda G-protein coupled receptor 5) receptors, induce high amplitude propagating contractions, and directly stimulate enteric neurons to increase serotonin secretion. All of these effects increase colonic motility.
4.2. Effects of bile acids on visceral sensitivity
Deoxycholic acid infusion into the colon resulted in persistent visceral hypersensitivity and referred pain in rats [45]. Another study evaluated esophageal pain with esophageal barostat balloon distension in 10 patients with functional heartburn and six healthy controls. There was a significant increase in pain when CDCA was infused via the nasogastric tube [46], indicating that the secretory BAs may play a role in visceral sensitivity.
4.3. Experimental effects of BAs on human colonic transit and bowel function
In a randomized, double-blind, placebo-controlled study of 20 healthy volunteers, delayed release formulation of sodium chenodeoxycholate (CDC) significantly accelerated colonic transit at 24 and 48 hours compared to placebo. It also significantly increased stool frequency, ease of passage and evacuation, and decreased stool consistency [47].
A randomized, controlled pharmacodynamics trial using the delayed release formulation of CDC in 36 females with IBS-C demonstrated a significant improvement in stool frequency, stool consistency and ease of passage compared to placebo [48]. In addition, CDC resulted in significant acceleration of overall colonic transit and ascending colon emptying (AC T½). There was a strong positive association between fasting serum 7αC4 and colonic transit (rs=0.749, p=0.003, placebo group) [48]. This trial further validated the role of BAs in the acceleration of colonic transit and the potential for exploring the role of modulating BA synthesis and enterohepatic circulation of BA pathways for the treatment of CC and IBS-C.
5.0. Proof of concept studies of IBAT inhibitor
5.1. Chemistry, preclinical pharmacology and pharmacokinetics of elobixibat
Elobixibat (formerly A3309) is an IBAT inhibitor that modulates the enterohepatic circulation of BAs. Elobixibat is a pure enantiomer of a synthetically modified 1,5-benzothiazepine, based on a seven-membered heterocyclic ring attached to a benzene ring (chemical formula C36H45N3O7S2) [49] (Figure 3). The expected half-life (T1/2) in humans is <4 hours. After oral administration, there is minimal systemic exposure, and systemically available drug is highly protein bound (>99.5%). After radiolabeling elobixibat and oral ingestion, there was limited tissue distribution and was found only in the gastrointestinal tract at 24 hours. The highest serum concentrations were found at 4 hours after individual dose or 2 hours after multiple doses over two weeks. There was no accumulation of elobixibat or associated metabolites within the plasma or urine.
Figure 3.
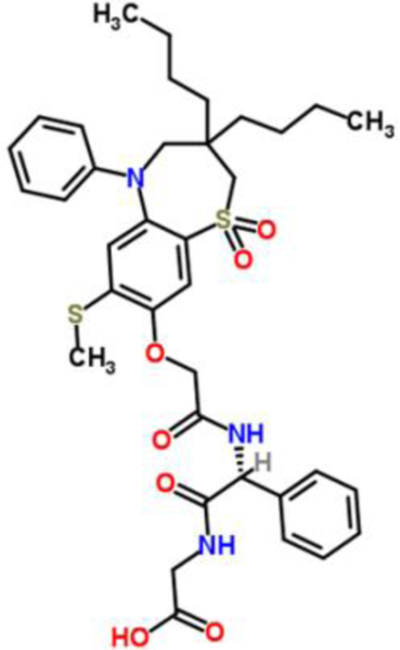
Two dimensional molecular structure of elobixibat
Elobixibat does inhibit CYP3A4 in vitro. However, the significance of this inhibition in drug-drug interactions is limited, secondary to the decreased oral bioavailability.
Elobixibat is highly selective for IBAT, with an affinity more than 400-fold higher than for human liver sodium-dependent BA transporter and more than 1000-fold higher than for neutral amino-acid transporter [49].
5.1.1. In vitro studies in HEK293 cells
Elobixibat was found to be highly potent and selective for IBAT in an in vitro study in transfected HEK293 cells, which express various BA transporters (IC50=0.53±0.17 nM for the human transporter). In contrast, IC50 for binding to the human liver (basolateral) sodium/BA co-transporter was 0.24±0.02 μM. Thus, IBAT inhibition by elobixibat was 400-fold higher than the inhibition of the basolateral co-transporter and more than 1000-fold higher than the neutral amino acid transporters in HEK293 cells. Elobixibat is, therefore, highly potent and selective for IBAT in humans based on in vitro studies [49]. It is proposed that elobixibat acts as a partial inhibitor of the re-uptake of BAs in the terminal ileum, since there are still some BAs absorbed in a dose-dependent manner after elobixibat treatment, as illustrated by the incomplete suppression of FGF19 production or stimulation of 7αC4 levels [50].
5.1.2. In vivo preclinical model (constipated dogs)
When studied in vivo in dogs with constipation induced by a meat diet, elobixibat increased feces weight in a dose-dependent manner compared to the 5-HT4 receptor agonist, tegaserod. This also correlated with a dose-dependent increase in serum 7αC4 concentration by 3- to 7-fold after 26 days of treatment. Given the duration of the biochemical effects, the data suggest that elobixibat has a lasting effect on increased BA synthesis in dogs with constipation, suggesting potential clinical utility [49].
5.2. Pharmacodynamics, safety and efficacy of elobixibat in humans
A summary of all the clinical trials discussed in the following section is available in Table 2 [50-54].
Table 2. Summary of the clinical trials in chronic constipation.
[<3 spontaneous bowel movements (SBM)/week + lumpy and or hard stools, straining and sensation of incomplete evacuation during defecation; pain not a predominant symptom]
| Author, year | Dose (mg/day) | Simren et al. 2011 [50] | Wong et al. 2011 [51] | Chey et al. 2011 [52] | Nakajima et al. 2018 [53] | Nakajima et al. 2018 - 2wk RCT [54] | Nakajima et al. 2018 - 52wk open label [54] |
| N (F/M) | 30 (23/7) | 36 (36/0) | 190 (170/20) | 163 (143/20) | 132 (109/23) | 340 (283/57) | |
| Study duration, wk | 2 | 2 | 8 | 2 | 2 | 52 | |
| Location of Study | Sweden | USA | Japan | ||||
| Primary Outcome | Safety | Colon transit | SBM | SBM at wk1 | SBM at wk1 | Safety, SBM and satisfaction over 52 wks | |
| BSFS (1-7 scale) | 3 | ↑ | |||||
| 5 | ↑ 1 | ↑ 1.3 | |||||
| 10 | ↑ | ↑ 1 | ↑ 1.7 | ↑ 1.8 | |||
| 15 | ↑ 1.3 | ↑ 1 | ↑ 2.0 | ||||
| 20 | ↑ 1.8 | ||||||
| Stool (SBM) #/ day | 3 | ↑ | |||||
| 5 | ↑ 2.5 | ↑ 1.7 | ↑ 3.3* | ||||
| 10 | ↑ | ↑ 4.0 | ↑ 4.1 | ↑ 6.4 | ↑ 3.3* | ||
| 15 | ↑ 0.5 | ↑ 5.4 | ↑ 3.8 | ↑ 3.3* | |||
| 20 | ↑ 1.17 | ||||||
| AE: Abdo pain | 3 | ||||||
| 5 | 10% | 23% | 24%* | ||||
| 10 | 11% | 26% | 19% | 24%* | |||
| 15 | 36% | 27% | 12% | 24%* | |||
| 20 | 50% | ||||||
| AE: Diarrhea | 3 | ||||||
| 5 | 8% | 9% | 15%* | ||||
| 10 | 6% | 5% | 13% | 15%* | |||
| 15 | 9% | 13% | 7% | 15%* | |||
| 20 | 36% | ||||||
Refers to the average response over 5 mg, 10 mg, and 15 mg. There were equal proportions of patients with each dose listed.
5.2.1. Phase 1b trial
In a phase 1b, randomized, double-blind, placebo-controlled trial conducted in 30 patients with chronic idiopathic constipation (CC), five escalating doses (range: 0.1, 0.3, 1, 3, and 10 mg⁄day) of elobixibat were studied in comparison to placebo over a 14-day period [50]. The study showed that, when administered orally, elobixibat was minimally absorbed and was present in picomolar concentrations in plasma. This supports the mechanism of action of elobixibat as a local inhibitor of IBAT [50]. Of note, the portion of absorbed elobixibat was mostly protein bound when in plasma, and the half-life was <4 hours. In the same cohort of patients with CC, elobixibat induced up to a 3-fold increase in BA synthesis as measured by serum 7αC4 and reduced the plasma FGF19 [50]. While the phase 1b trial was mainly focused on safety and tolerability of elobixibat, the efficacy of the drug was noted by changes in colonic transit time as measured by radiopaque markers. Compared to placebo, elobixibat, 10 mg, was effective in reducing colonic transit time, thereby demonstrating acceleration of colonic transit. Interestingly, elobixibat decreased plasma levels of total cholesterol and low-density lipoprotein (LDL) cholesterol in a dose-dependent fashion [50], as increased BA synthesis in the hepatocytes, and directs sterols to the BA synthesis pathway and away from cholesterol synthesis. In this trial, elobixibat was found to be safe and tolerable across the dose levels tested with no serious adverse events or discontinuations. However, this phase 1b trial [50] of 14 days’ duration did not represent evidence of efficacy for chronic constipation and, therefore, longer term studies were required, as discussed in the phase 2b (USA) trial [51] and the Japanese phase 3, open-label, 52-week trial [54].
5.2.2. Phase 2a study
Elobixibat was studied in a phase 2a, double-blind, placebo-controlled, parallel-group study in 36 females with CC randomized to three groups (placebo, 15 mg or 20 mg elobixibat) for 14 consecutive days [51]. The primary endpoint for efficacy was colonic transit at 24 hours using scintigraphy [geometric center (GC) at 24 hours]. Secondary endpoints included other measurements of small bowel (colonic filling at 6 hours) and colonic transit (GC at 48 hours and ascending colon T1/2), and symptoms-based (Bowel Pattern Diary) and biochemical [serum 7αC4, triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol, and LDL cholesterol] endpoints. Compared to placebo, elobixibat resulted in accelerated colonic transit, looser stool consistency, and reduced straining with defecation [51].
In general, elobixibat was well tolerated in this phase 2a trial, with only 1 of 13 patients in the placebo group and 2 of12 patients in the 20 mg elobixibat group withdrawing from the trial due to diarrhea. There were no reported serious adverse events. The most common adverse event was mild/moderate abdominal cramps/pain that occurred in 36% of the 15 mg elobixibat group and 50% of the 20 mg elobixibat group (respectively, p=0.039 and p=0.003 compared to placebo). It was noted that abdominal cramps/pain tended to precede the diarrhea and were relieved by bowel movements [51].
5.2.3. Phase 2b U.S. study
In a phase 2b, multicenter, randomized, double-blind, placebo-controlled, parallel-group trial conducted at 45 sites across the United States, 190 patients with CC (90% female) were randomized into four groups (placebo, 5, 10, or 15 mg/day elobixibat) over 8 weeks [52]. In this trial, the primary endpoint was change in the number of spontaneous bowel movements (SBMs) during week 1 compared to baseline. The secondary endpoints were similar to the phase 2a trial [weekly evaluation of SBMs and complete spontaneous bowel movements (CSBMs), daily stool consistency and straining, abdominal discomfort, serum 7αC4, total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides] except for the absence of measurements of colonic transit [52].
Elobixibat resulted in a significantly greater increase in the number of SBMs during week 1 from baseline compared to placebo [mean increase (95% confidence interval): 1.7 (0.7, 2.8) for placebo, 2.5 (1.5, 3.5), 4.0 (2.9, 5.0), and 5.4 (4.4, 6.4) for 5 mg, 10 mg (p<0.002), and 15 mg (p<0.001) elobixibat, respectively]. Elobixibat also resulted in increased stool frequency that was maintained over the 8-week treatment period. Additionally, patients in the elobixibat treatment arms reported decreased straining and bloating compared to the placebo group (p<0.05) [52].
Similar to previous trials, individuals with CC treated with elobixibat had increased 7αC4 and reduced LDL cholesterol. Similar to the phase 2a trial, elobixibat was well tolerated, with the most common adverse events being abdominal pain and diarrhea (more commonly in the 15 mg group). A total of 29 patients discontinued the trial (n=6 in each of placebo, 5mg and 10mg elobixibat groups; n=11 in the 15 mg elobixibat group). The majority of the discontinuations in the elobixibat treatment groups were related to adverse events, whereas, in the placebo group, the main reason for discontinuation was due to lack of efficacy [52].
5.2.4. Phase 2b Japan trial
In order to determine the optimal dosing of elobixibat for treatment of CC in Japanese patients, Nakajima et al. conducted a randomized, placebo-controlled trial in 163 patients randomized to four groups (placebo, 5, 10 or 15 mg/day elobixibat, orally) for 2 weeks [53]. The primary endpoint was the change from baseline in frequency of SBMs at end of the first week of treatment. Individuals treated with 10 mg or 15 mg elobixibat had a significant change from baseline in the frequency of SBMs [mean ± standard deviation, 5.7±4.2 (p=0.0005) and 5.6±3.5 (p=0.0001) times per week, respectively] compared to the placebo group (2.6±2.9 times per week). In addition, a subgroup analysis demonstrated elobixibat to be equally efficacious in patients with CC with or without IBS. The authors concluded that 10 mg elobixibat is the clinically optimal dose for treatment of CC in a Japanese cohort [53].
Similar to the previous trials, elobixibat was well tolerated, with the most common adverse events being mild abdominal pain and diarrhea in the treatment groups, and with no serious adverse events. Only 9/163 patients in the elobixibat groups discontinued the trial due to adverse events, and 1/40 in the placebo group discontinued due to lack of efficacy [53].
5.2.5. Short-term safety and efficacy, long-term safety and quality of life in Japanese phase 3 trials
Nakajima et al. conducted two phase 3 trials in patients with CC to determine the safety and efficacy of short-term treatment with elobixibat (Trial 1), and the safety, satisfaction and quality of life (QOL) with long-term treatment with elobixibat (Trial 2) [54].
5.2.5.1. Trial 1 (short-term treatment)
Trial 1 was a randomized, double-blind, placebo-controlled study in 133 patients with CC conducted at 16 clinics in Japan comparing 10 mg/day elobixibat to placebo for two weeks. The primary outcome was change from baseline during week 1 in the frequency of SBMs. During week 1, the change in SBM frequency/week was significantly increased in the elobixibat group [n=69, 6.4 (5.3, 7.6), least squares mean (95% CIs)] compared with placebo [n=63, 1.7 (1.3, 2.2), p<0.0001]. Elobixibat was well tolerated in this two-week trial, with only 4/133 patients withdrawing due to adverse events [abdominal pain (18.8%) and diarrhea (13.0%)] [54].
5.2.5.2. Trial 2 (long-term, open-label treatment)
Trial 2 was an open-label study in 340 patients with CC conducted at 34 clinics or hospitals in Japan over a period of one year. Participants were allowed to titrate the dose of elobixibat (5-15 mg/day) after 1 week of 10mg/day, based on the effectiveness of the drug and adverse events. The primary outcome of Trial 2 was to assess the safety of long-term use of elobixibat. Secondary outcomes focused on evaluation of efficacy relative to baseline, including bowel function assessment and health-related quality of life (QOL) and satisfaction scores.
Elobixibat was found to be safe, with no reported serious adverse events or deaths over the one-year period. Similarly, the most common adverse events in the elobixibat treatment group were abdominal pain (24.1%) and diarrhea (14.7%). Only 18/340 patients discontinued treatment due to adverse events. Approximately 25% of patients titrated the dose up to 15 mg/day and 25% of patients titrated the dose down to 5 mg/day within one month after their initial dose. Ultimately, there was approximately 33% of patients in each dose group of 5 mg, 10 mg or 15 mg [54].
Additionally, patients reported consistent improvements from baseline in numbers of SBMs and CSBMs, stool consistency, and QOL. The effects of elobixibat were sustained throughout the one-year treatment period.
6.0. Metabolic effects of elobixibat
Previous clinical trials of elobixibat in patients with constipation thus far have analyzed its effects on total cholesterol, LDL and HDL cholesterol as a secondary biochemical endpoint [50-54]. Interestingly, in all these trials, except for the short duration pharmacodynamics study of Wong et al. (phase 2a trial) [51], there was a significant reduction in total cholesterol and LDL cholesterol in a dose-dependent manner, with no effect on HDL cholesterol. This is consistent with results showing reduced ileal BA absorption in the ileum, reduced FXR stimulation and production of FGF19 leading to reduced negative feedback of hepatocyte BA synthesis, and, therefore, shunting of steroid synthesis in the hepatocyte from cholesterol and LDL cholesterol to increased BA synthesis [50-54].
A more specific assessment of the effects of elobixibat on lipid metabolism was conducted in a single-center, randomized, parallel-group, double-blind, placebo-controlled trial in 36 dyslipidemic patients treated for 28 consecutive days. In this trial, the primary endpoint was reduction in LDL cholesterol. At a dose of 5 mg, elobixibat reduced LDL cholesterol by 7.4% and decreased the LDL/HDL ratio by 18% [55]. The effect on LDL cholesterol alone with reduction in the LDL/HDL ratio is of particular importance for prevention of cardiovascular disease (CVD) [56]. Additionally, it is worth noting that constipation is reported to be a risk factor for CVD disease, as demonstrated in a study of post-menopausal women with severe constipation who had an approximate 25% increased risk of CVD attributable to the constipation [57]. Furthermore, patients with CVD had decreased fecal BA excretion in comparison to those without CVD [58]. These observations suggest that elobixibat may be potentially beneficial in women with constipation who also have risk factors for CVD [55].
Finally, elobixibat plays a role in stimulating the synthesis and secretion of glucagon-like peptide-1 (GLP-1), presumably due to the increase in colonic BAs, which would stimulate the TGR5 receptors on the intestinal L cells, that is, enteroendocrine cells that synthesize and secrete GLP-1 [55]. Additionally, the release of GLP-1 in response to IBAT inhibition by elobixibat can provide an explanation for the observation of delayed gastric emptying in contrast to the acceleration of colonic transit in response to elobixibat [51].
7.0. Conclusion
Elobixibat is a novel pharmacologic treatment for chronic constipation with promising results. Elobixibat was approved on January 19, 2018 by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) on the basis of these results.
8.0. Expert commentary
Chronic constipation is a highly prevalent condition worldwide, affecting approximately 27.2% of people in North America [59] and 28.4% of Japanese adults [60]. To date, pharmacologic advancements for treating CC have targeted increasing colonic motility via 5-HT4 receptor agonists and increasing colonic secretion with both osmotic laxatives and intestinal secretagogues [e.g. lubiprostone (activation of chloride channels) and linaclotide and plecanatide (guanylate cyclase C agonists)] [61]. Elobixibat provides a novel mechanism of action targeting the IBAT and increasing colonic bile acid, which active both increased colonic motility and secretion via the mechanisms listed above [44,50,51].
The multiple clinical trials evaluating elobixibat have demonstrated a consistent improvement in stool frequency and consistency, clinically meaningful endpoints, across various populations diagnosed with CC. Moving forward, it will be interesting to evaluate elobixibat’s efficacy in patients with IBS-C. The main concern is that abdominal pain may increase in this patient population. However, improvement of the constipation may improve the baseline abdominal pain that is associated with long-standing constipation. Additionally, further evaluation is needed to determine elobixibat efficacy in patients with normal versus low total fecal bile acids. In a hypothesis generating paper, 15% of patients with IBS-C were found to have reduced total fecal BAs, which correlated with physiological endpoints of retarded colonic transit [33]. The current trials of elobixibat in CC did not select participants with low BA synthesis. Despite that, elobixibat was efficacious in the treatment of CC. Prior to U.S. Food and Drug Administration approval, further investigation is required with 12-week, phase 3, randomized, controlled trials to confirm the efficacy for relief of CC and IBS-C. Once approved, we anticipate that elobixibat will become a second-line therapy for patients with CC. Although the cost of this medication will need to be considered to appraise its cost-benefit ratio, it is worth noting that emergency room visits for constipation are steadily rising with an associated cost of $1.6 million in 2011 [62]. After elobixibat is approved, it will be necessary to conduct studies to determine whether the efficacy leads to saving on health care dollars in both emergency room visits and hospitalizations associated with CC.
There are no genetic variations that would suggest clinically significant ethnic or racial differences in response to elobixibat among these populations. Indeed, the phase 2a and 2b studies conducted in the U.S., the phase 1 study conducted in Scandinavia, and the phase 2b and phase 3 studies conducted in Japan provide very similar results. This is more clearly apparent in Table 2 (49-53).
The previous clinical trials have not evaluated the pharmacogenetics of elobixibat in association with allelic variants in IBAT and other BA receptors. Rao et al. studied the role of genetic variations in proteins involved in the FGF19-mediated feedback control of BA synthesis in response to CDC, which had an influence on ascending colon emptying [48]. Thus, future trials should also focus on the effects of such genetic variations (e.g., FGFR4 or klotho ß) and the response to elobixibat. This would allow for individualizing therapy in those variants with a better response.
Interestingly, there was a similar rise in GLP-1 with both elobixibat and dipeptidyl peptidase IV inhibitors (DPP4 inhibitors) such as vildagliptin [55,63]. Based on a meta-analysis of DPP4 inhibitors, there was an overall decrease in HbA1c of 0.77% (95% CI 0.72 - 0.82%), and future studies will need to assess whether elobixibat enhances glycemic control [64]. The positive effect of elobixibat on GLP-1 secretion may benefit patients with type 2 diabetes mellitus or pre-diabetes, but this would require additional clinical trials.
In conclusion, although it is still early to generalize, we anticipate that, once elobixibat is approved, it will be widely available for the treatment of CC and IBS-C. More data are still required prior to making elobixibat available for prescribers, such as a phase 3 trial for CC over 12 months. In the coming years, elobixibat is likely going to be approved and available in the U.S. since it is, ultimately, a promising novel drug with a favorable profile for the treatment of CC and IBS-C. Alternative indications might be possible in the next five years, given the potential benefits in treating metabolic diseases such as hyperlipidemia and type 2 diabetes mellitus. It is conceivable that the metabolic effects of elobixibat, probably mediated by effects of BAs on enteroendocrine cells, will have potential benefits in patients with CC and type 2 diabetes.
Key Issues.
The enterohepatic circulation is an efficient method of recycling bile acids (BAs) and involves several key molecules: the ileal bile acid transporter (IBAT), farnesoid X receptor, the hormone fibroblast growth factor 19 (FGF19), and 7-α-hydroxy-4-cholesten-3-one (7αC4), the intermediary step in conversion of cholesterol to BAs. Free BAs are efficiently reabsorbed (~95%) in the terminal ileum via the IBAT.
Conjugated and non-conjugated BAs are physiologic laxatives that stimulate colonic motility and secretion.
Elobixibat is a highly potent and selective IBAT partial inhibitor that modulates the enterohepatic circulation of BAs, resulting in increased BAs in the colon and compensatory increase in hepatic BA synthesis from cholesterol.
The use of elobixibat in individuals with chronic constipation (CC) results in faster colonic transit, increased spontaneous bowel movements (SBMs) and complete SBMs. Elobixibat was found to be safe and well tolerated across the dose levels tested in phase 2B and 3 trials, with no serious adverse events or discontinuations.
Metabolic effects of elobixibat include reduction of serum LDL cholesterol, reflecting diversion of sterols to BA synthesis.
Elobixibat increases circulating levels of glucagon like peptide-1 (GLP-1), presumably reflecting BA stimulation of enteroendocrine cells.
Acknowledgements
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding
M. Camilleri is funded by grant RO1-DK115950 from National Institutes of Health.
Footnotes
Declaration of interest
M. Camilleri performs consulting with EA Pharma with fee to his employer, Mayo Clinic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A, Girtman A, Zinsmeister AR: Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol (2013) 11(10):1270–1275 e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR: Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol (2012) 10(9):1009–1015 e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ: Systematic review: The prevalence of idiopathic bile acid malabsorption as diagnosed by sehcat scanning in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics (2009) 30(7):707–717. [DOI] [PubMed] [Google Scholar]
- 4.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhea in functional bowel disorder with diarrhea: a systematic review and meta-analysis. Gut (2016) 65(12):1951–1959. [DOI] [PubMed] [Google Scholar]
- 5.Vijayvargiya P, Camilleri M: Update on bile acid malabsorption: Finally ready for prime time? Current Gastroenterology Reports (2018) 20(3):10. [DOI] [PubMed] [Google Scholar]
- 6.Conley DR, Coyne MJ, Bonorris GG, Chung A, Schoenfield LJ: Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am J Dig Dis (1976) 21(6):453–458. [DOI] [PubMed] [Google Scholar]
- 7.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC: Chenodeoxycholic acid stimulates cl(−) secretion via camp signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in t84 cells. Am J Physiol Cell Physiol (2013) 305(4):C447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingue JC, Ao M, Sarathy J, Rao MC: Chenodeoxycholic acid requires activation of egfr, epac, and ca2+ to stimulate cftr-dependent cl- secretion in human colonic t84 cells. Am J Physiol Cell Physiol (2016) 311(5):C777–C792. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S et al. : A g protein-coupled receptor responsive to bile acids. J Biol Chem (2003) 278(11):9435–9440. [DOI] [PubMed] [Google Scholar]
- 10.Borg JY J; Wu Q; Lajczak N; Keely S; Fenton RA; Moeller H: Regulated expression of the na+/k+-atpase pump in colonic epithelium by bile acids. FASEB Journal (2017) 31(Suppl. 1):856. [Google Scholar]
- 11.Suhail M: Na+, k+-atpase: Ubiquitous multifunctional transmembrane protein and its relevance to various pathophysiological conditions. J Clin Med Res (2010) 2(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S: The bile acid receptor gpbar-1 (tgr5) modulates integrity of intestinal barrier and immune response to experimental colitis. PloS one (2011) 6(10):e25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF: Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. The Journal of laboratory and clinical medicine (1979) 94(5):661–674. [PubMed] [Google Scholar]
- 14.Jayashree S, Jo DS, Mei A, Nabihah K, Sydney F, Hafsa S, Tanushree N, C. RM: The yin and yang of bile acid action on tight junctions in a model colonic epithelium. Physiological Reports (2017) 5(10):e13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keely SJ, Scharl MM, Bertelsen LS, Hagey LR, Barrett KE, Hofmann AF: Bile acid-induced secretion in polarized monolayers of t84 colonic epithelial cells: Structure-activity relationships. American Journal of Physiology-Gastrointestinal and Liver Physiology (2007) 292(1):G290–G297. [DOI] [PubMed] [Google Scholar]
- 16.Yde J, Borg J, Fenton RA, Moeller HB: Altered expression of aquaporin water channels in a rat model of chronic diarrhea due to bile acid malabsorption. The FASEB Journal (2017) 31(1_supplement):703.714–703.714. [Google Scholar]
- 17.Ma T, Verkman AS: Aquaporin water channels in gastrointestinal physiology. J Physiol (1999) 517 ( Pt 2)(317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardhan PK, Rahman ASM, Islam S, Rahman M, Gyr K: Effects of tropisetron, a 5-hydroxytryptamine type 3 receptor blocker, on intestinal secretion induced by cholera toxin or deoxycholic acid in rabbits in vivo. Journal of International Medical Research (1993) 21(6):323–333. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Murphy R, Chadwick VS: Pharmacological inhibition of chenodeoxycholate-induced fluid and mucus secretion and mucosal injury in the rabbit colon. Digestive diseases and sciences (1982) 27(10):865–869. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman TW, Binder HJ: Serotonin-induced alteration of colonic electrolyte transport in the rat. Gastroenterology (1984) 86(2):310–317. [PubMed] [Google Scholar]
- 21.Duboc H, Tolstanova G, Yuan PQ, Wu V, Kaji I, Biraud M, Akiba Y, Kaunitz J, Million M, Tache Y, Larauche M: Reduction of epithelial secretion in male rat distal colonic mucosa by bile acid receptor tgr5 agonist, int-777: Role of submucosal neurons. Neurogastroenterol Motil (2016) 28(11):1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward JB, Mroz MS, Keely SJ: The bile acid receptor, tgr5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil (2013) 25(8):708–711. [DOI] [PubMed] [Google Scholar]
- 23.Bunnett NW: Neuro-humoral signalling by bile acids and the tgr5 receptor in the gastrointestinal tract. J Physiol (2014) 592(14):2943–2950.**This paper identifies that bile acids target the TGR5 receptors which activate colonic motility and directly on the enteric neurons.
- 24.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU: The receptor tgr5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology (2013) 144(1):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU: Expression and function of the bile acid receptor gpbar1 (tgr5) in the murine enteric nervous system. Neurogastroenterol Motil (2010) 22(7):814–825, e227-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekhjian HS, Phillips SF, Hofmann AF: Colonic absorption of unconjugated bile acids: Perfusion studies in man. Digestive diseases and sciences (1979) 24(7):545–550. [DOI] [PubMed] [Google Scholar]
- 27.Kirwan WO, Smith AN, Mitchell WD, Falconer JD, Eastwood MA: Bile acids and colonic motility in the rabbit and the human. Gut (1975) 16(11):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor I, Basu P, Hammond P, Darby C, Flynn M: Effect of bile acid perfusion on colonic motor function in patients with the irritable colon syndrome. Gut (1980) 21(10):843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flynn M, Hammond P, Darby C, Taylor I: Effects of bile acids on human colonic motor function in vitro. Digestion (1982) 23(3):211–216. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R: Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphac4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil (2009) 21(7):734–e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston IM, Nolan JD, Pattni SS, Appleby RN, Zhang JH, Kennie SL, Madhan GK, Jameie-Oskooei S, Pathmasrirengam S, Lin J, Hong A et al. : Characterizing factors associated with differences in fgf19 blood levels and synthesis in patients with primary bile acid diarrhea. The American journal of gastroenterology (2016) 111(3):423–432. [DOI] [PubMed] [Google Scholar]
- 32.Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW: A new mechanism for bile acid diarrhea: Defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol (2009) 7(11):1189–1194. [DOI] [PubMed] [Google Scholar]
- 33.Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M: Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol (2018) 16(4):522–527.**Proof of concept study that detailed ~15% of patients with constipation predominant irritable bowel syndromehave low total 48 hour fecal bile acid and increased non-secretory individual bile acids, providing a potential underlying mechanism for clinical symptoms.
- 34.Chiang JYL: Bile acid metabolism and signaling. Comprehensive Physiology (2013) 3(3):1191–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vijayvargiya P, Camilleri M, Shin A, Saenger A: Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol (2013) 11(10):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridlon JM, Kang DJ, Hylemon PB: Bile salt biotransformations by human intestinal bacteria. Journal of lipid research (2006) 47(2):241–259. [DOI] [PubMed] [Google Scholar]
- 37.Koop I: Role of bile acids in the control of pancreatic secretion and cck release. European journal of clinical investigation (1990) 20 Suppl 1(S51–57. [DOI] [PubMed] [Google Scholar]
- 38.Camilleri M: Advances in understanding of bile acid diarrhea. Expert review of gastroenterology & hepatology (2014) 8(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camilleri M: Physiological underpinnings of irritable bowel syndrome: Neurohormonal mechanisms. J Physiol (2014) 592(14):2967–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T, Chiang JY: Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev (2014) 66(4):948–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge Z, Duan Z, Yang H, Zhang S, Zhang S, Wang L, Yang D, Sun X, Zhang Z, Su L, Zhu H, Zhou D, Liu B, Shi H, Yu J, Yang H, Chang Q, Zhang N, Wu D, Chen JDZ: Home-based transcutaneous neuromodulation improved constipation via modulating gastrointestinal hormones and bile acids. Evid Based Complement Alternat Med (2018) April 29;2018:2086163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD: Systematic review with meta-analysis: The prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Alimentary pharmacology & therapeutics (2015) 42(1):3–11. [DOI] [PubMed] [Google Scholar]
- 43.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ: The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol (2002) 282(3):G443–449. [DOI] [PubMed] [Google Scholar]
- 44.Mekjian HS, Phillips SF, Hofmann AF: Colonic secretion of water and electrolytes induced by bile acids: Perfusion studies in man. J Clin Invest (1971) 50(8):1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y: A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology (2008) 135(6):2075–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiqui A, Rodriguez-Stanley S, Zubaidi S, Miner PB, Jr.: Esophageal visceral sensitivity to bile salts in patients with functional heartburn and in healthy control subjects. Digestive diseases and sciences (2005) 50(1):81–85. [DOI] [PubMed] [Google Scholar]
- 47.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR: Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol (2010) 8(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R, Zinsmeister AR: Chenodeoxycholate in females with irritable bowel syndrome-constipation: A pharmacodynamic and pharmacogenetic analysis. Gastroenterology (2010) 139(5):1549–1558, 1558.e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillberg P- G, Dahlström M, Starke I, Östlund-Lindqvist A- M: The ibat inhibition by a3309 - a potential mechanism for the treatment of constipation. Gastroenterology (2010) 138(5):S–224. [Google Scholar]
- 50.Simren M, Bajor A, Gillberg PG, Rudling M, Abrahamsson H: Randomised clinical trial: The ileal bile acid transporter inhibitor a3309 vs. Placebo in patients with chronic idiopathic constipation--a double-blind study. Alimentary pharmacology & therapeutics (2011) 34(1):41–50. [DOI] [PubMed] [Google Scholar]
- 51.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR: Effects of a3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. The American journal of gastroenterology (2011) 106(12):2154–2164. [DOI] [PubMed] [Google Scholar]
- 52.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H: A randomized placebo-controlled phase iib trial of a3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. The American journal of gastroenterology (2011) 106(10):1803–1812.**Proof of concept Phase 2b trial on the effect of elobixibat on chronic idiopathic constipation
- 53.Nakajima A, Seki M, Taniguchi S: Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in japanese patients with chronic constipation: A phase ii, multicenter, double-blind, placebo-controlled randomized clinical trial. Journal of gastroenterology (2018) 53(4):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakajima AM S; Taniguchi S; Ohta A; Gillberg P; Mattsson J; Camilleri M : Short-term, randomized trial of efficacy and open-label, long-term safety of ileal bile acid transporter inhibitor, elobixibat, in chronic constipation. In: Lancet Gastroenterology and Hepatology (2018).**Phase III Japanese trial showing the short-term and long-term safety of elobixibat in chronic idiopathic constipation.
- 55.Rudling M, Camilleri M, Graffner H, Holst JJ, Rikner L: Specific inhibition of bile acid transport alters plasma lipids and glp-1. BMC Cardiovasc Disord (2015) 15(75.**This paper reported that elobixibat increased down stream bile acid effects by increasing GLP1 and decreased serum LDL, likely associated with the increased hepatic bile acid synthesis as a result of increased bile acid loss into the colon.
- 56.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR et al. : Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA (2007) 297(5):499–508. [DOI] [PubMed] [Google Scholar]
- 57.Salmoirago-Blotcher E, Crawford S, Jackson E, Ockene J, Ockene I: Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med (2011) 124(8):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charach G, Grosskopf I, Rabinovich A, Shochat M, Weintraub M, Rabinovich P: The association of bile acid excretion and atherosclerotic coronary artery disease. Therapeutic advances in gastroenterology (2011) 4(2):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higgins PD, Johanson JF: Epidemiology of constipation in north america: A systematic review. The American journal of gastroenterology (2004) 99(4):750–759. [DOI] [PubMed] [Google Scholar]
- 60.Tamura A, Tomita T, Oshima T, Toyoshima F, Yamasaki T, Okugawa T, Kondo T, Kono T, Tozawa K, Ikehara H, Ohda Y et al. : Prevalence and self-recognition of chronic constipation: Results of an internet survey. J Neurogastroenterol Motil (2016) 22(4):677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson AD, Camilleri M, Chirapongsathorn S, Vijayvargiya P, Valentin N, Shin A, Erwin PJ, Wang Z, Murad MH: Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: A systematic review and network meta-analysis. Gut (2017) 66(9):1611–1622. [DOI] [PubMed] [Google Scholar]
- 62.Sommers T, Corban C, Sengupta N, Jones M, Cheng V, Bollom A, Nurko S, Kelley J, Lembo A: Emergency department burden of constipation in the united states from 2006 to 2011. The American journal of gastroenterology (2015) 110(4):572–579. [DOI] [PubMed] [Google Scholar]
- 63.Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A: Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. The Journal of clinical endocrinology and metabolism (2004) 89(5):2078–2084. [DOI] [PubMed] [Google Scholar]
- 64.Esposito K, Chiodini P, Maiorino MI, Capuano A, Cozzolino D, Petrizzo M, Bellastella G, Giugliano D: A nomogram to estimate the hba1c response to different dpp-4 inhibitors in type 2 diabetes: A systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open (2015) 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]


