Abstract
About one third of cellular proteins in eukaryotic cells are localized to membrane-enclosed organelles in the endomembrane system. Trafficking of these membrane proteins (including soluble lumenal proteins) among the organelles is mediated by small sac-like vesicles. Vesicle-mediated membrane trafficking regulates a broad range of biological processes, many of which are still poorly understood at the molecular level. A powerful approach to dissect a vesicle-mediated membrane trafficking pathway is unbiased genome-wide genetic screening, which only recently became possible in mammalian cells with the isolation of haploid human cell lines and the development of CRISPR-Cas9 genome editing. Here, we describe a FACS-based method to select populations of live mutant cells based on the surface levels of endogenous proteins or engineered reporters. Collection of these mutant populations enables subsequent deep sequencing and bioinformatics analysis to identify genes that regulate the trafficking pathway. This method can be readily adapted to genetically dissect a broad range of mammalian membrane trafficking processes using haploid genetics or CRISPR-Cas9 screens.
Keywords: Membrane trafficking, vesicle transport, flow cytometry, genome-wide genetic screen, CRISPR-Cas9
INTRODUCTION
A defining feature of the eukaryotic cell is its intricate endomembrane system consisting of functionally specialized membrane-bound organelles, including the endoplasmic reticulum (ER), the Golgi apparatus, the endosome, the lysosome, and the plasma membrane (1,2). All organelle proteins in the endomembrane system are synthesized, folded and assembled in the ER before they are carried by vesicles to their destination organelles (3–5). Vesicle-mediated membrane trafficking was first genetically dissected in yeast, leading to the identification of membrane trafficking mediators conserved in all eukaryotes (6,7). Membrane trafficking is significantly more complex in mammalian cells with additional regulatory layers that adjust the speed and direction of cargo flow in response to intracellular and extracellular stimuli (1,8). However, few mammalian membrane trafficking pathways have been systematically dissected at the genome scale, largely due to a lack of robust methods to introduce loss-of-function mutations.
The advent of haploid genetics and the CRISPR-Cas9 genome editing system revolutionized mammalian cell genetics, enabling unbiased genome-wide genetic dissection of biological pathways (9–15). Pooled libraries of cultured mutant cells can be generated and selected based on specific cellular phenotypes in order to identify the genes underlying a biological pathway (10,13–19). The haploid genetics approach takes advantage of haploid mammalian cells such as HAP1 (derived from a human patient with myeloid leukemia) and haploid mouse embryonic stem cells (13,15,20–22). Since these haploid cells possess only one copy of each gene, mutagenesis of the gene (e.g., using retrovirus-delivered gene-traps) generates a complete knockout. Notably, haploid genetics allows for genome-wide screens not limited to annotated genes or specifically targeted mutations (15). Findings of haploid genetics generally apply to other cell types (14,15,20,23). In the CRISPR-Cas9 system, the Cas9 nuclease and guide RNAs introduce loss-of-function mutations into genes through non-homologous end joining (24). Unlike haploid genetics, which is restricted to available haploid cell lines, CRISPR-Cas9 screens can be performed in virtually any cell type including primary cells.
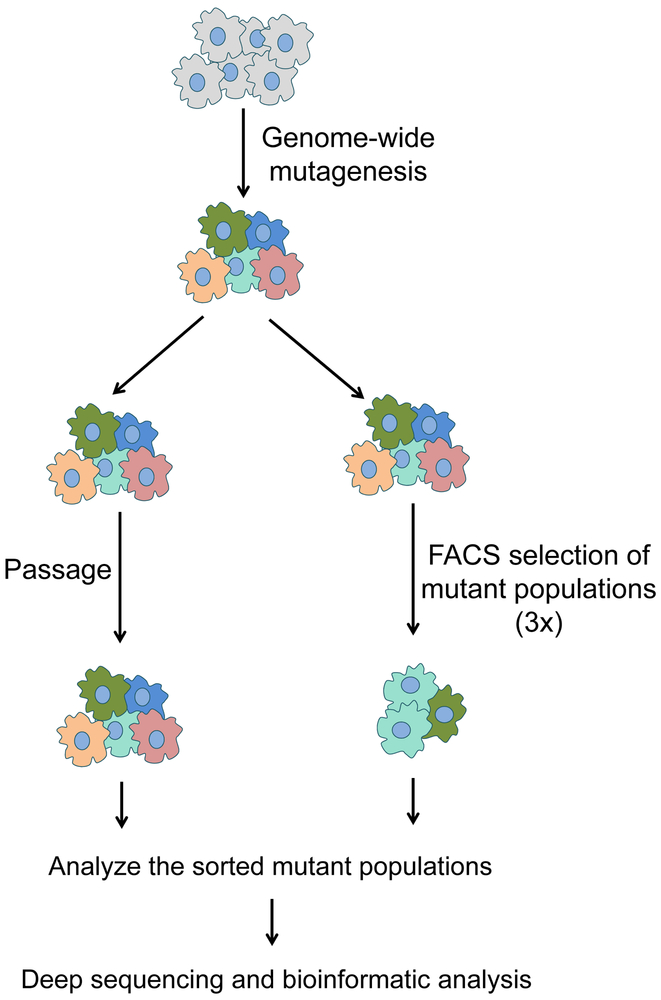
Previous haploid and CRISPR-Cas9 genetic screens were mainly based on straightforward cell viability or growth advantage assays (16,17,25–29), which cannot be directly used to dissect multifaceted membrane trafficking pathways. In this work, we describe a FACS-based method to dissect membrane trafficking in live cells by sorting mutant cells according to surface levels of endogenous proteins or engineered reporters (Fig. 1). This method can be adapted to genetically dissect a broad range of mammalian membrane trafficking pathways using haploid genetics or CRISPR screens.
Figure 1. Genetic screen workflow using FACS.
Note: all of these experimental procedures should be carried out under sterile conditions. Whenever possible, perform the experiments in a laminar flow cell culture hood. After FACS, return collected cells to sterile culture conditions as soon as possible. Basic Protocols 1–3 should be carried out on the same day (see Time Considerations in Critical Parameters).
BASIC PROTOCOL 1
Labeling cells in suspension
Basic Protocol 1 describes experimental procedures to label surface molecules in live cells in suspension using fluorescent antibodies. The surface molecule can be either an endogenous protein or an engineered reporter expressing an epitope tag.
For adherent cells that cannot be labeled in suspension, see Alternate Protocol 1. For simultaneous labeling of multiple surface molecules, see Alternate Protocol 2 (for cells in suspension) or Alternate Protocol 3 (for adherent cells).
Materials:
Library of mutant cells
Fetal bovine serum (FBS)
Basic culture medium (e.g., MEM-a, DMEM, RPMI-1640, IMDM)
Phosphate-buffered saline (PBS, without Ca2+ or Mg2+)
Accutase cell detachment solution (Innovative Cell Technologies, #AT104)
Antibodies against surface proteins of interest
6-, 10-, or 15-cm cell culture dishes
15 and 50 mL polypropylene conical centrifuge tubes
Hemocytometer
Trypan blue
Inverted light microscope
Benchtop centrifuge with adaptors for 15 and 50 mL conical centrifuge tubes
Sterile cell strainer with 50 μm pore size (e.g., Sysmex CellTrics filters, #04–004-2327)
Sterile flow cytometry tubes (e.g., USA scientific #1450–0810)
- Plate the full library of mutant cells the day before sorting. To maintain the complexity of the library, plate ~108 cells in a haploid genetic screen and ~4 × 107 cells in a CRISPR-Cas9 screen using the GeCKO V2 library. Since library preparation protocols have been thoroughly discussed in previous publications (11,15,17,20,23,30–32), they are not covered here.
- When plating cells for the first sorting, divide the mutant cell library into two batches with equal numbers of cells, each at full complexity. One batch will be used for sorting while the other batch will be used as a passage control.
- When sorting cells based on the localization of an inducible surface protein such as the HA-GLUT4-GFP reporter (32), plate ~106 additional cells from the mutant library as an uninduced control. Process and label the uninduced control cells using the same procedure as the main population. This control population will be used to establish cell collection conditions during FACS in Basic Protocol 2.
- If using additional control cell lines or treatment conditions as suggested in Basic Protocol 2 and the Critical Parameters, plate ~106 cells of each control population.
On the day of sorting, prepare the cell labeling solutions including PBS, PBS + 1% FBS, and antibodies diluted in PBS at previously determined concentrations (see Critical Parameters). Keep these solutions on ice until use. Warm up cell culture materials including media, PBS and Accutase to 37 °C.
- Harvest the mutant cells plated the day before. For the first sorting, it is essential to collect at least the number of cells required to maintain library complexity. If significant cell loss is observed during labeling or sorting, prepare additional cells to compensate for the loss.
-
If working with adherent cells that can be labeled in suspension, dissociate the cells from culture dishes. Aspirate media from the dishes and wash twice with PBS. Add 1 mL Accutase solution to each 10-cm dish and incubate at 37 °C for 5–10 minutes or until all cells are dissociated. Add 5 mL complete media to each dish and gently pipette up and down until no visible clumps are observed. Transfer the cells to 15 or 50 mL centrifuge tubes.Unlike trypsin, Accutase dislodges cells without damaging surface proteins.
- For cells grown in suspension, pipette up and down gently until no visible clumps are observed. Transfer the cells to 15 or 50 mL centrifuge tubes.
- After transferring the cells, wash each 10-cm dish with 5 mL complete media and combine these residual cells with cells collected in a or b. Repeat this step if necessary until all the cells are collected.
-
Centrifuge the cells at 300 xg at 4 oC for 10 minutes.
Aspirate the media without disturbing the cell pellet.
Resuspend the cells in complete media at an estimated concentration of ~106 cells/mL.
Count the live cells using a hemocytometer in the presence of Trypan blue and record the total number of live cells.
-
Wash the cells twice with ice-cold PBS.
Before the final centrifugation step, remove ~106 cells as an unlabeled control sample. If a fluorophore-conjugated secondary antibody is used during the labeling protocol, remove an additional control sample of ~106 cells to label with this secondary antibody only (without the primary antibody). Resuspend the unlabeled control sample in 0.5 mL of PBS + 1% FBS and keep on ice until step 15. If using a secondary antibody control sample, resuspend the cells in 0.5 mL of PBS + 1% FBS and keep on ice until step 13.
After the final centrifugation, aspirate the supernatant as completely as possible without disturbing the cell pellet.
Resuspend the cell pellet in the diluted antibody solution prepared in Step 2 at a concentration of 1 – 2 × 107 live cells/mL. Gently pipette up and down to ensure no obvious cell clumps are observed.
-
Incubate the cells on ice for 1 hour. When fluorophore-conjugated antibodies are used, protect the sample from light.
Tap the tube every 10 minutes to ensure the cells remain in suspension and are labeled evenly with antibody.
Centrifuge the cells at 300 g at 4 oC for 10 minutes and wash three times with ice-cold PBS.
If the cells are labeled with fluorophore-conjugated primary antibodies in Steps 10–12, proceed directly to Step 14. If the cells are labeled with unconjugated primary antibodies in Steps 10–12, resuspend the cell pellet in diluted fluorophore-conjugated secondary antibodies. Incubate and wash the cells as described in Steps 10–12.
After the final centrifugation, resuspend the cell pellet at a concentration of 1 – 2 × 107 cells/mL in PBS + 1% FBS. Pipette up and down gently to disrupt cell clumps.
-
Add the cells to a sterile 50 μm cell strainer and filter into a sterile flow cytometry tube by gravity.
Each flow cytometry tube can collect 1 – 4 mL of cells.
-
Store the samples in capped flow cytometry tubes on ice protected from light until sorting (Basic Protocol 2).
Sort the cells within 1 to 2 hours of antibody labeling to preserve cell viability.
For each population to be collected during FACS, prepare one collection tube containing 1 mL of PBS + 1% FBS. When sorting large populations of cells, prepare one collection tube for every 107 cells to be sorted.
ALTERNATE PROTOCOL 1
Labeling cells attached to culture dishes
Alternate Protocol 1a describes the labeling of adherent cells that cannot be labeled in suspension. Use this alternative protocol if the cells are sensitive to prolonged incubation in suspension or are difficult to collect by centrifugation.
Materials:
Materials listed in Basic Protocol 1
Krebs-Ringer-HEPES-bicarbonate (KRH) buffer: see recipe in REAGENTS AND SOLUTIONS.
Rockers
-
The day before sorting, plate the full complexity library of mutant cells as described in Basic Protocol 1.
Plate ~106 additional cells for each control sample in a separate dish. Include at least an unlabeled control as described in Basic Protocol 1. When sorting cells based on the localization of an inducible surface protein, plate ~106 additional cells from the mutant library as an uninduced control.
On the day of sorting, prepare KRH buffer, KRH + 2% FBS, KRH + 5% FBS, PBS + 5% FBS, and antibodies diluted in KRH buffer + 2% FBS at the previously determined concentrations (see Critical Parameters). Keep these solutions on ice until use.
Aspirate media from the cultured cells and wash twice with 5 mL ice-cold KRH buffer per 10-cm dish to remove any residual media. Scale volume as needed based on dish surface area.
Add 5 mL ice-cold KRH buffer + 5% FBS (blocking solution) to each 10-cm dish. Incubate at 4 °C for 20 minutes without rocking.
Aspirate the blocking solution and add 2.5 mL of the diluted antibody solution prepared in Step 2 to each 10-cm dish. Incubate on a rocker for 1 hour in a 4 oC cold room. When fluorophore-conjugated antibodies are used, protect the sample from light.
Remove the antibody solution and wash three times using 5 mL of ice-cold KRH buffer + 5% FBS for each 10-cm dish. For each wash step, incubate on ice or in a 4 oC cold room for 5 minutes.
If the cells are labeled with fluorophore-conjugated primary antibodies in Steps 5–6, proceed directly to Step 8. If the cells are labeled with unconjugated primary antibodies in Steps 5–6, add 2.5mL of the diluted fluorophore-conjugated secondary antibodies to each 10-cm dish. Incubate and wash as described in Steps 5–6.
Wash the cells once with ice-cold PBS.
Add 1 mL Accutase to each 10-cm dish and incubate at 37 °C for 5 – 10 minutes or until all cells are dissociated.
Add PBS + 5% FBS to the cells such that the cell concentration is approximately 106 cells/mL. Transfer the cells to 15 or 50 mL centrifuge tubes.
Count the cells with a hemocytometer in the presence of Trypan blue.
-
Resuspend the cells in PBS. Centrifuge the cells at 300 g at 4 oC for 10 minutes. Aspirate the supernatant from the cell pellet. Repeat the PBS wash once. Resuspend the cell pellet to a concentration of 1 – 2 × 107 live cells/mL in PBS + 5% FBS. Pipette up and down gently to disrupt cell clumps.
If working with cells that cannot be collected by benchtop centrifugation, skip the wash step and proceed immediately to step 13.
Filter the cells through a 50 μm cell strainer into a sterile flow cytometry tube. Each tube can collect 1 – 4 mL of cells.
Store the capped flow cytometry tubes on ice until sorting (Basic Protocol 2). Protect the samples from light. Sort the cells within 1 to 2 hours of antibody labeling to preserve cell viability.
For each sample to be sorted, prepare at least one collection tube containing 1 mL of PBS + 1% FBS. When sorting large populations of cells, prepare one collection tube for every 107 cells to be sorted.
ALTERNATE PROTOCOL 2
Labeling cells in suspension using antibody mixtures
While Basic Protocol 1 describes the labeling of a single surface molecule with cells labeled in suspension, Alternative Protocol 2 describes simultaneous labeling of multiple surface molecules (endogenous proteins or engineered reporters) using fluorophore-conjugated antibodies. This protocol is identical to Basic Protocol 1 except that a mixture of fluorophore-conjugated primary antibodies are used to label the cells in Step 10 and that Step 13 (labeling with secondary antibodies) is omitted. In addition to an unlabeled control sample, prepare additional control samples in which only one of the fluorophore-conjugated antibodies is used to label the cells. These controls are essential for the analysis and compensation performed during FACS (see Basic Protocol 2 for discussion). Fluorophore-conjugated primary antibodies are used in this protocol to simplify the procedure and minimize background. The emission spectra of the fluorophores must be fully distinguishable during FACS (see Critical Parameters). Specific fluorophore-conjugated antibodies against surface proteins and epitope tags are readily available from commercial sources. If a fluorophore-labeled antibody is unavailable, primary antibodies can be conjugated to a range of fluorophores using commercial antibody labeling kits (e.g., Thermo/Molecular Probes antibody labeling kits). Determine optimal antibody concentrations for labeling, individually and in combination, before performing Alternate Protocol 1b (see Critical Parameters).
ALTERNATE PROTOCOL 3
Labeling attached cells using antibody mixtures
While Alternate Protocol 1 describes the labeling of a single surface molecule with cells attached to dishes, Alternative Protocol 3 describes the procedure for simultaneous labeling of multiple surface molecules (endogenous proteins or engineered reporters) using fluorophore-conjugated antibodies. The experimental procedure of this protocol is identical to Alternative Protocol 1, except that a mixture of fluorophore-conjugated antibodies is used to label the cells in Step 5 and Step 7 (labeling with secondary antibodies) is omitted. In addition to an unlabeled control sample, prepare additional control samples in which only one of the fluorophore-conjugated antibodies is used to label the cells. See Alternate Protocol 2 for additional discussion.
BASIC PROTOCOL 2
Collecting mutant cell populations using FACS
Basic Protocol 2 describes using FACS to collect mutant cell populations according to fluorescence intensity of the cells. It includes guidelines on setting analysis gates for live and single cells, and setting collection gates to sort for cells with a desired phenotype.
Materials:
FACS sorter (e.g., Beckman Coulter MoFlo XDP or BD Biosciences FACSAria)
Flow cytometry analysis software (e.g., FlowJo)
- Establish analysis gates for live and single cells using the unlabeled control cells prepared in Basic Protocol 1 or Alternate Protocol 1–3 (Fig. 2a).
- Perform initial analysis of ~5,000 unlabeled control cells. Plot forward scatter linear (FS-lin or FS height) on the y-axis and side scatter linear (SS-lin or SS height) on the x-axis. If needed, adjust the forward scatter gain and side scatter photomultiplier tube (PMT) voltage until the majority of detected cells appear in the center half or center two-thirds of the plot.
- Gate for live cells as indicated in Fig. 2a. Live cells form a distinct population in the central portion of the plot. Events with low values for both forward and side scatter are usually debris and should be excluded from further analysis. Cells with low values for forward scatter and a large range of values for side scatter are usually dead or damaged and likewise should be excluded from analysis.
- Of those live cells, plot forward scatter linear on the y-axis and forward scatter area on the x-axis. Gate for single cells as indicated in Fig 2a. Single cells, which form the majority of the population, have a linear relationship between the forward scatter linear value and the forward scatter area value. By contrast, cell aggregates have higher forward scatter area value for a given linear value than the majority of the population. These cell aggregates should be excluded from further analysis.
- Of the single cells, plot forward scatter linear on the y-axis and the fluorescent channel of interest on the x-axis. If sorting using multiple colors, establish a separate plot for each fluorescent channel. Use the log scale for all fluorescent channels analyzed during this protocol. Save a data file of the unlabeled control population for subsequent analysis.
- When establishing a new protocol file on the FACS instrument, adjust the photomultiplier tube (PMT) voltage for each fluorescent channel of interest such that all of the unlabeled control cells are detected in the first order of magnitude on the x-axis.
- If examining surface proteins by antibody labeling, use unlabeled control cells for this step.
- If using a genetically encoded fluorescent reporter, use control cells lacking the reporter for this step.
-
If multiple fluorophore-conjugated antibodies or fluorescent proteins are used as described in Alternate Protocol 2 or 3, perform initial analysis of ~5,000 cells for each single-color control population. Save a separate data file of each single-color control population for subsequent analysis.
If necessary, compensate for overlapping emission spectra, or fluorescence spillover, in order to accurately measure cellular phenotypes and establish sorting gates. Refer to the user manual for your FACS instrument and software for details on how to perform accurate compensation on that instrument.
-
If sorting based on inducible surface proteins or fluorescent reporters, perform an initial analysis of ~5,000 cells from the uninduced control population as described in Steps 1–3. Save a data file of this control for subsequent analysis.
This control population can also be used to tentatively establish a collection gate for mutant cells that do not respond to the induction. However, this tentative collection gate may need to be adjusted to meet the criteria described in Step 6.
- Perform initial analysis of ~5,000 cells from the population to be sorted. First confirm that the previously established gates for live cells and single cells also apply to this population. Of the single, live cells, plot forward scatter linear on the y-axis and the fluorescent channel of interest on the x-axis. Confirm that the labeled cells can be distinguished from the unlabeled control cells.
- If sorting based on multiple fluorophore-conjugated antibodies or fluorescent proteins, repeat this step for each fluorescent channel.
- If using multiple fluorophores to define cell subsets before sorting from a particular subset, perform that analysis before proceeding to step 6.
- Once the final subset of cells to use for sorting has been identified, define collection gates. If sorting based on a single fluorescent channel, plot forward scatter linear on the y-axis and the fluorescent channel of interest on the x-axis. This allows for a more accurate and detailed gating than plotting a histogram. If sorting based on two fluorescent channels as described in Alternative Protocol 2 or 3, plot one fluorescent channel on the x-axis and the other on the y-axis.
- For the first sorting from the full-complexity library, define a population of 1 to 5% of the total cells with the lowest fluorescence intensity, and a separate population of 1 to 5% of the total cells with the highest fluorescence intensity. Define similar gates if sorting based on inducible surface proteins or fluorescent reporters (see Critical Parameters).
- If mutant cells (e.g., deletion of a known regulator) corresponding to a desired phenotype are available, these cells can be used to define collection gates.
- If sorting based on two fluorescent channels, draw distinct collection gates rather than dividing the plot into quadrants in order to improve the purity of the collected populations.
- For subsequent rounds of sorting, use the same gates defined during the first round of sorting. Alternatively, if distinct populations with desired phenotypes emerge in subsequent rounds of sorting, adjust the gates to collect those populations. See Fig. 2b for example gates based on emerging populations.
- Save a data file of this initial analysis separately from the file for the complete population to be sorted. This file containing data from the small test population is often more convenient to analyze using flow cytometry software than the larger file containing data from the entire sorted population.
- Once the collection gates are established, sort the full population of library cells. Collect cells in flow cytometry tubes containing 1 mL PBS + 1% FBS.
- After collecting ~1 mL of cells, transfer the tube to ice and replace with a fresh collection tube.
- Once the sorting is complete, record the final number of cells isolated by each collection gate as reported by the FACS instrument in order to guide cell recovery described in Basic Protocol 3. Record the total number of live cells analyzed in order to ensure representation of the full library from the starting population.
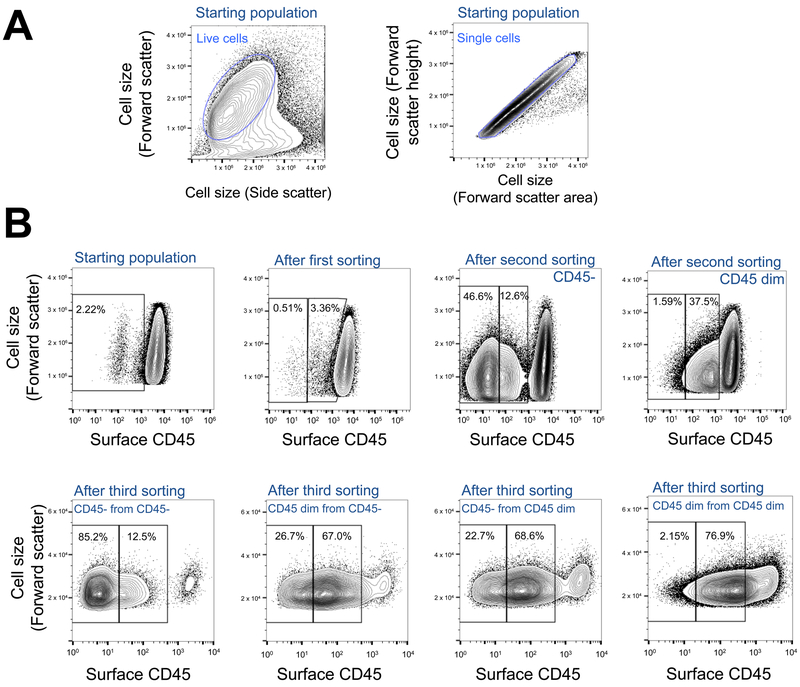
Figure 2. Sorting of mutant KBM-7 cells with decreased surface levels of CD45.
(A) Analysis of KBM-7 cells using a Beckman MoFlo XDP. In order to accurately collect cells during FACS, live cells were identified based on Forward Scatter Linear and Side Scatter Linear. Of those live cells, single cells (singlets) were identified based on Forward Scatter Linear and Forward Scatter Area. This gating procedure is further described in Step 1 of Basic Protocol 2. (B) Mutant KBM-7 cells collected during a haploid genetic screen according to surface levels of CD45, a transmembrane protein involved in hematopoietic cell signaling (36). Mutant KBM-7 cells were labeled with anti-CD45 antibodies (BioLegend #304002) and anti-mouse IgG-APC antibodies (eBioscience #17-4015-82) as described in Basic Protocol 1. Cells were gated for live and single cells as shown in A. Cells were then sorted for decreased CD45 surface labeling using a Beckman MoFlo XDP as described in Basic Protocol 2. During the first round of FACS, a single collection gate was used to isolate CD45- cells (background level of surface CD45). During the second round of FACS, two separate collection gates were used to isolate CD45- and CD45 dim cells (decreased surface level of CD45) according to their fluorescence intensity. During the third round of FACS, each of those separate populations were further sorted into CD45- and CD45 dim populations. After the third round of FACS, surface levels of CD45 were measured by flow cytometry using a Beckman CyAn analyzer as described in Basic Protocol 4.
BASIC PROTOCOL 3
Growing the sorted cells
Basic Protocol 3 describes experimental procedures to culture sorted cells to maximize cell recovery and survival.
Materials:
FBS
Basic culture medium (e.g., MEM-a, DMEM, RPMI-1640, IMDM)
PBS
Accutase cell detachment solution
6-, 10-, or 15-cm culture dishes
15 and 50 mL polypropylene conical centrifuge tubes
Hemocytometer
Trypan blue
Dimethyl sulfoxide (DMSO)
-
Add complete media to the collected cells in flow cytometry tubes to adjust the volume to 4 mL. Transfer the cells to 15 mL conical tubes. Wash each flow cytometry tube twice with 2 mL complete media in order to collect all cells.
Alternatively, if the cells are fragile and tend to undergo apoptosis after extensive handling, transfer the collected cells directly to culture dishes without Steps 2 – 3.
Centrifuge the cells at 300 xg at room temperature for 10 minutes.
-
Resuspend the cells in complete media at 105 to 106 cells/mL based on the number of collected cells recorded by the FACS instrument. Count live cells using a hemocytometer in the presence of Trypan blue.
The actual cell number recovered at this step is likely lower than the number of collected cells reported by the FACS instrument.
-
Plate the cells at a density appropriate for the cell type.
To prevent bacterial contamination, use antibiotics such as penicillin and streptomycin while culturing of the sorted cells, even if antibiotics are not included in routine culturing of the cells.
- Passage the cells until they expand 10- to 20-fold.
- This procedure ensures sufficient library complexity of the sorted population.
- The cell expansion time depends upon cell type and is usually complete within one week for established cell lines such as HAP1, HeLa and HCT116 cells.
- Maintain the passage control population (without sorting) at full complexity during this expansion procedure.
- After expansion, a portion of the cells can be further sorted to improve the signal-to-noise ratio whereas the rest of the cells can be frozen.
- Plate four to five times the number of cells collected by FACS and repeat Basic Protocol 1 or Alternate Protocol 1, 2, or 3 to label cells for another round of sorting.
- Perform Basic Protocol 4 with 105 to 106 cells to examine the enrichment of the mutant cells compared to the passage control.
- Suspend the remaining cells in a freezing medium containing 10% DMSO and 90% complete media at 1 – 3 × 107 cells/mL. Freeze the cells in a - 70 to −80 °C freezer overnight before transferring the cells to liquid nitrogen for long-term storage.
BASIC PROTOCOL 4
Phenotypic analysis of the sorted cells
Basic Protocol 4 describes flow cytometry analysis of sorted cells based on the gates set in Basic Protocol 2. Perform this protocol after each round of FACS and expansion of the sorted cells. Use the outcome of this analysis to determine if a sorted population should be subjected to additional rounds of sorting or harvested for deep sequencing. The enrichment of mutant populations depends on the repetition and stringency of sorting. Stronger phenotypic enrichment usually results in a lower false positive rate but may result in a higher false negative rate. On the other hand, less stringent phenotypic enrichment can identify more genes that regulate the pathway under study, but may result in a higher false positive rate. One way to balance these considerations is to sequence all mutant cell populations that exhibit significant enrichment.
Materials:
FBS
Basic culture medium (e.g. MEM-a, DMEM, RPMI-1640, IMDM)
PBS
Accutase cell detachment solution
Antibodies against the surface proteins of interest
DMSO
6-, 10-, or 15-cm culture dishes
15 and 50 mL polypropylene conical centrifuge tubes
Hemocytometer
Trypan blue
Flow cytometer (e.g., Beckman Coulter CyAn ADP)
Flow cytometry analysis software (e.g., FlowJo)
After expansion of sorted cells described in Basic Protocol 3, label 1 – 5 × 105 cells as described in Basic Protocol 1 or Alternate Protocol 1–3. Include the same control samples used during these initial protocols.
Analyze the cells using a flow cytometer. A FACS instrument is not required for this analysis step. Use the same gates established in Basic Protocol 2 to identify live cells, single cells, and cells with the phenotype of interest. Save the same control and sample files described in Basic Protocol 2.
- After collecting the flow cytometry data, quantify changes in the sorted population compared to the passage control population.
- Plot the flow cytometry data as described in Step 6 of Basic Protocol 2. Use the collection gates established in Basic Protocol 2 to determine the proportion of the sorted population with the phenotype of interest.
- On the same plot as above, use a contour or zebra visualization to determine whether a distinct population of mutant cells has emerged within those sorting gates, and whether that population is absent in the passage control population.
- Plot the histogram of each relevant fluorescent channel in order to determine whether the median fluorescence intensity of the sorted population has shifted away from the passage control population.
If a distinct population with a desired phenotype (e.g., increased or decreased surface level of a molecule) is not observed, perform additional rounds of labeling and sorting until the mutant population is significantly enriched (e.g., more than five-fold enrichment compared to the passage control).
- Once a mutant population has been enriched to the desired level, expand the final sorted cell population 10- to 20-fold. Harvest the cells and proceed to subsequent steps of the genetic screen including genomic DNA isolation, PCR, deep sequencing, and bioinformatics analysis to identify significant hits (17,20,23,32,33).
- Harvest the passage control cells for genomic DNA isolation, PCR, and deep sequencing.
- Freeze the remaining sorted and passage control cells as described in Basic Protocol 3.
REAGENTS AND SOLUTIONS
KRH buffer:
121 mM NaCl, 4.9 mM KCl, 1.2 mM MgSO4, 0.33 mM CaCl2, and 12 mM HEPES. Adjust pH to 7.4. Used Milli-Q water for buffer preparation. Sterilize the buffer by filtering through a 0.22 µm bottle top filter (e.g., Corning #431097). Store at room temperature.
COMMENTARY
Critical Parameters
Choice of cell type:
This FACS-based method is designed for proliferating cells that can be readily expanded and sorted for multiple rounds. However, the method can be adapted to screen post-mitotic cells (e.g., adipocytes and neurons) or cells with limited lifespan in culture (e.g., primary fibroblasts and macrophages). In this case, perform one round of the screen in triplicate with three independent library generation, antibody labeling, and FACS procedures. This strategy relies on additional sequencing and statistical analysis rather than additional rounds of sorting to achieve the desired signal-to-noise ratio. The triplicate sorting strategy can also be used to select mutant cells based on intracellular antigen labeling, in which the cells are fixed and permeabilized prior to antibody labeling. Finally, it is often possible to recapitulate a biological process in a proliferating cell line even if the process is not found in the substitute cell type. The hits identified in the substitute cell type should be individually validated in the physiologically relevant cell type.
Assay development:
The success of a genetic screen largely depends on identifying a cellular phenotype and a robust assay to monitor that phenotype. For a FACS-based screen, a cellular phenotype needs to be translated into a fluorescent signal. For endogenous proteins or engineered reporters localized to the cell surface, fluorescent labeling of these molecules without cell permeabilization directly reflects the biosynthetic and trafficking pathways controlling the surface level of the molecules. Before generating a mutant cell library, optimize the antibody labeling protocol to identify a condition that distinguishes between labeled and unlabeled cells. If treating the cells to induce surface localization of these molecules, optimize the treatment protocol to identify a condition that clearly distinguishes treated and untreated cells. Optimizing the labeling and treatment conditions makes it possible to accurately identify and collect cells with altered phenotypes during the screen.
If a membrane trafficking pathway does not directly cause changes in the surface level of a molecule, a different strategy must be devised to monitor pathway function using flow cytometry. For example, activation of the membrane-anchored transcription factor ATF6 by ER stress requires its translocation from the ER to the Golgi to be proteolytically processed to release its cytoplasmic active domain, which then migrates to the nucleus to induce target gene expression (34,35). This ER-to-Golgi trafficking event can be monitored indirectly by a reporter gene (e.g., GFP) driven by a promoter containing ATF6-binding sites. Other trafficking pathways that cause gene expression changes can be monitored using a similar strategy. When first creating a reporter cell line, reporter gene expression often varies within the population. Thus, it is advantageous to isolate individual clones with homogenous expression of the reporter gene and, if treating the cells to alter membrane trafficking and reporter gene expression, with robust changes of the reporter signal.
In order to identify both positive and negative regulators of a membrane trafficking pathway, collect at least two populations during FACS: a population with decreased fluorescence compared to the majority of the cells, and a population with increased fluorescence. If treating cells to induce surface localization or activate a trafficking pathway, collect cells that are activated in the absence of treatment in order to identify negative regulators of the pathway, and collect cells that are not activated in the presence of treatment to identify positive regulators of the pathway.
Choices of fluorophore:
Genetic dissection of a multifaceted membrane trafficking pathway often requires simultaneous detection of several fluorescent signals with minimal overlap between the excitation and emission spectra of the fluorophores. The choice of fluorophores depends in part upon the FACS instrument used to sort cells during the screen, as different instruments have different combinations of excitation lasers and emission filters. Three fluorophores can be successfully combined in antibody-based labeling: Brilliant Violet BV421 (Excitation maximum 407 nm/Emission maximum 421 nm), Alexa Fluor 488 (Excitation maximum 490/Emission maximum 525), and Allophycocyanin (APC) (Excitation maximum 650 nm/Emission maximum 660 nm). If using a genetically encoded fluorescent protein to define cellular phenotypes, GFP (Excitation maximum 488 nm/Emission maximum 510 nm) can be used together with Brilliant Violet BV421- and/or APC-conjugated antibodies with minimal interference.
Number of cells to be sorted:
To maintain sufficient coverage of the mutant library for accurate statistical analysis, it is critical to determine the numbers of cells to be sorted and sequenced. The first round of cell preparation and sorting should be performed with a full complexity genome-wide library as described in Basic Protocol 1. For CRISPR-Cas9 screens using the GeCKO V2 library (30), this requires 4 – 5 × 107 cells, or ~300 cells per guide RNA (30,32). In screens using other CRISPR libraries, the number of cells needs to be adjusted based on the number of guide RNAs in the library. For haploid genetic screens, a full complexity population requires at least the number of cells originally infected during library preparation, usually ~108 cells (20,23). The passage control population should be maintained and sequenced at full complexity. Since the complexity of sorted populations decreases, lower numbers of cells are needed for subsequent rounds of sorting and deep sequencing: 1 – 5 × 106 cells in CRISPR-Cas9 screens and 1 – 2 × 107 cells in haploid genetic screens.
TROUBLESHOOTING
- Fluorescence profiles of cells in the large-scale FACS selection procedure differs from those in small-scale flow cytometry experiments.
-
Possible cause: incorrect photomultiplier tube (PMT) voltage settings used on one of the instruments.Solution: adjust the PMT voltage of each fluorescent channel such that all the unlabeled control cells are detected in the first order of magnitude on the x-axis.
-
Possible cause: different cell concentrations are used during antibody labeling between the experiments.Solution: run small-scale optimization experiments using the same cell concentration as in the large-scale FACS selection to ensure consistent antibody labeling.
-
- Poor survival or expansion of sorted cells.
- Possible cause: Too few cells collected by FACS.
- Solution: repeat the first sorting procedure using a larger cell population.
- Solution: if possible, broaden the collection gates to increase the number of collected cells.
- Possible cause: The cells are sensitive to the antibody labeling and/or sorting procedures.
- Solution: sort the cells once and immediately process for DNA isolation and deep sequencing without further expansion. Perform triplicate preparation and sorting of the full complexity library to achieve the desired signal-to-noise ratio (see Critical Parameters).
- Solution: if possible, perform the screen in a more robust cell type in which the pathway is conserved (see Critical Parameters). Then validate the hits in the original cell type.
- Possible cause: (when applicable) treatment used to induce surface localization of the labeled molecules causes growth arrest or cell death.
- Solution: sort the cells only once using the triplicate sorting strategy (see Critical Parameters).
- Solution: identify an improved treatment condition that preserves cell viability while achieving the desired induction.
- Insufficient enrichment of a mutant cell population after two or three rounds of sorting.
- Possible cause: the collection gates used in FACS are too broad and include cells lacking the desired phenotype.
- Solution: repeat sorting using more stringent gates.
- Solution: collect multiple mutant populations with various degrees of fluorescence perturbation (Fig. 2b). After expanding the cells as described in Basic Protocol 3, follow Basic Protocol 4 to determine which collection gates result in the desired enrichment of mutant cells. Use those gates for subsequent FACS procedures.
- Possible cause: the intensity of fluorescence is not fully distinguishable between the sorted and control populations.
- Solution: optimize antibody labeling conditions and/or test different antibodies to establish a labeling protocol that yields a labeling pattern clearly distinct from control samples in flow cytometry measurements.
- Solution: if an inducing agent is used to alter surface molecule localization, optimize the treatment conditions to minimize overlap between induced and uninduced cells in flow cytometry analysis.
Anticipated Results
While haploid genetics and CRISPR screens differ significantly in library preparation, deep sequencing, and bioinformatics analysis, this FACS-based selection method can be used in both types of screens. This method is designed to isolate one or more populations of mutant cells according to the surface levels of endogenous proteins or engineered reporters. These mutant populations correlate with either upregulated or downregulated surface levels of the molecules. Subsequent expansion of these mutant populations allow for identification of both positive and negative regulators of the trafficking pathway. It is often possible to perform multiple screens in parallel by monitoring surface levels of several molecules, either independently or simultaneously. Such comparative genetic screens offer a powerful way to identify common and specialized regulators of parallel trafficking pathways (20).
To distinguish true hits from false positives, it is necessary to perform secondary screens to validate the hits from the genome-wide screens. Pooled secondary screens can be first carried out using custom CRISPR libraries built using the top-ranking hits (e.g., top 1,000 genes) from the genome-wide screens, followed by individual gene validation in the same cell type as well as in other relevant cell types. This FACS-based method can be used to perform the pooled secondary screens as well. Subsets of the identified hits likely directly regulate the trafficking pathway, while other hits may indirectly regulate the pathway by controlling the expression or localization of direct regulators. Detailed mechanistic studies are required to reveal the precise molecular functions of new factors identified in the screens.
Time Considerations
The entire protocol described in this method takes one to six weeks to complete depending on the number of sorting procedures and the growth rate of the cells. It takes four to six hours to prepare and label a full library of mutant cells as described in Basic Protocol 1, Alternate Protocol 1, 2, or 3. It takes two to six hours to sort a full library of mutant cells (Basic Protocol 2) depending on cell number, volume of cell suspension solution, and the speed of the FACS instrument. The Beckman Coulter MoFlo XDP usually sorts 1 – 2 × 107 cells per hour. After each sorting, it usually takes four days to two weeks to expand the sorted cells (Basic Protocol 3) depending on the number of cells collected and the growth rate of the cells. Phenotypic analysis of sorted cells (Basic Protocol 4) takes four to six hours to accomplish, including 30 – 60 minutes to collect and analyze flow cytometry data.
SIGNIFICANCE STATEMENT.
Vesicle-mediated cargo transport is the molecular basis of a broad range of fundamental physiological processes, most of which remain poorly understood. The recent isolation of haploid human cell lines and the development of CRISPR-Cas9 genome editing enables unbiased genome-wide genetic dissection of vesicle-mediated membrane trafficking in mammalian cells. Here, we describe a fluorescence-activated cell sorting (FACS)-based method to select populations of mutant cells in genome-wide genetic screens. Translating a membrane trafficking pathway into a fluorescent signal makes it possible to collect cell populations with altered pathway function using FACS. Comparing the loss-of-function mutations enriched in the sorted populations to those found in the unsorted control population identifies regulatory factors of a membrane trafficking pathway.
ACKNOWLEDGEMENTS
We thank Yan Ouyang and Yuming Han for technical assistance. This work was supported by National Institutes of Health (NIH) grants DK095367 (J.S.) and GM126960 (J.S.), an NIH T32 training grant (B.M., L.C., and E.D.), an American Heart Associate (AHA) Predoctoral Fellowship (D.G.), and a University of Colorado Seed Grant (J.S.).
Literature Cited
- 1.Bonifacino JS, and Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116, 153–166 [DOI] [PubMed] [Google Scholar]
- 2.Wickner W, and Schekman R (2008) Membrane fusion. Nat Struct Mol Biol 15, 658–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schekman R, and Novick P (2004) 23 genes, 23 years later. Cell 116, S13–15, 11 p following S19 [DOI] [PubMed] [Google Scholar]
- 4.Sudhof TC, and Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins Science (New York, N.Y.) 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wandinger-Ness A, and Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harbor perspectives in biology 6, a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novick P, and Schekman R (1979) Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 76, 1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novick P, Field C, and Schekman R (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 [DOI] [PubMed] [Google Scholar]
- 8.Bryant NJ, Govers R, and James DE (2002) Regulated transport of the glucose transporter GLUT4. Nature reviews. Molecular cell biology 3, 267–277 [DOI] [PubMed] [Google Scholar]
- 9.Doudna JA, and Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9 Science (New York, N.Y.) 346, 1258096. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, and Weissman JS (2014) Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 159, 647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, and Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems Science (New York, N.Y.) 339, 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, and Church GM (2013) RNA-guided human genome engineering via Cas9 Science (New York, N.Y.) 339, 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeb M, and Wutz A (2011) Derivation of haploid embryonic stem cells from mouse embryos. Nature 479, 131–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, and Brummelkamp TR (2011) Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477, 340–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carette JE, Guimaraes CP, Varadarajan M, Park AS, Wuethrich I, Godarova A, Kotecki M, Cochran BH, Spooner E, Ploegh HL, and Brummelkamp TR (2009) Haploid genetic screens in human cells identify host factors used by pathogens Science (New York, N.Y.) 326, 1231–1235 [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Wei JJ, Sabatini DM, and Lander ES (2014) Genetic screens in human cells using the CRISPR-Cas9 system Science (New York, N.Y.) 343, 80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, and Zhang F (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells Science (New York, N.Y.) 343, 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, and Yusa K (2014) Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol 32, 267–273 [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Zhu S, Cai C, Yuan P, Li C, Huang Y, and Wei W (2014) High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature 509, 487–491 [DOI] [PubMed] [Google Scholar]
- 20.Davis EM, Kim J, Menasche BL, Sheppard J, Liu X, Tan AC, and Shen J (2015) Comparative Haploid Genetic Screens Reveal Divergent Pathways in the Biogenesis and Trafficking of Glycophosphatidylinositol-Anchored Proteins. Cell Rep 11, 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Shi L, Wang BA, Liang D, Zhong C, Liu W, Nie Y, Liu J, Zhao J, Gao X, Li D, Xu GL, and Li J (2012) Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell 149, 605–617 [DOI] [PubMed] [Google Scholar]
- 22.Li W, Shuai L, Wan H, Dong M, Wang M, Sang L, Feng C, Luo GZ, Li T, Li X, Wang L, Zheng QY, Sheng C, Wu HJ, Liu Z, Liu L, Wang XJ, Zhao XY, and Zhou Q (2012) Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature 490, 407–411 [DOI] [PubMed] [Google Scholar]
- 23.Carette JE, Guimaraes CP, Wuethrich I, Blomen VA, Varadarajan M, Sun C, Bell G, Yuan B, Muellner MK, Nijman SM, Ploegh HL, and Brummelkamp TR (2011) Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nature biotechnology 29, 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shalem O, Sanjana NE, and Zhang F (2015) High-throughput functional genomics using CRISPR-Cas9. Nat Rev Genet 16, 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown KR, MacLeod G, Mis M, Zimmermann M, Fradet-Turcotte A, Sun S, Mero P, Dirks P, Sidhu S, Roth FP, Rissland OS, Durocher D, Angers S, and Moffat J (2015) High-Resolution CRISPR Screens Reveal Fitness Genes and Genotype-Specific Cancer Liabilities. Cell 163, 1515–1526 [DOI] [PubMed] [Google Scholar]
- 26.Sidik SM, Huet D, Ganesan SM, Huynh MH, Wang T, Nasamu AS, Thiru P, Saeij JP, Carruthers VB, Niles JC, and Lourido S (2016) A Genome-wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes. Cell 166, 1423–1435 e1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marceau CD, Puschnik AS, Majzoub K, Ooi YS, Brewer SM, Fuchs G, Swaminathan K, Mata MA, Elias JE, Sarnow P, and Carette JE (2016) Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, Zuiani A, Zhang P, Fernandez E, Zhang Q, Dowd KA, Pierson TC, Cherry S, and Diamond MS (2016) A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, and Sabatini DM (2015) Identification and characterization of essential genes in the human genome Science (New York, N.Y.) 350, 1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanjana NE, Shalem O, and Zhang F (2014) Improved vectors and genome-wide libraries for CRISPR screening. Nature methods 11, 783–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nature protocols 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulbranson DR, Davis EM, Demmitt BA, Ouyang Y, Ye Y, Yu H, and Shen J (2017) RABIF/MSS4 is a Rab-stabilizing holdase chaperone required for GLUT4 exocytosis. Proc Natl Acad Sci U S A 114(39), E8224–E8233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, Irizarry RA, Liu JS, Brown M, and Liu XS (2014) MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome biology 15, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen J, and Prywes R (2005) ER stress signaling by regulated proteolysis of ATF6. Methods 35, 382–389 [DOI] [PubMed] [Google Scholar]
- 35.Shen J, Chen X, Hendershot L, and Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Developmental cell 3, 99–111 [DOI] [PubMed] [Google Scholar]
- 36.Penninger JM, Irie-Sasaki J, Sasaki T, and Oliveira-dos-Santos AJ (2001) CD45: new jobs for an old acquaintance. Nature Immunology 2, 389–396 [DOI] [PubMed] [Google Scholar]