Abstract
Introduction:
Although the loss or reversal of brain laterality is one of the most consistent modalities in schizophrenia (SCZ) and bipolar disorder (BD), its molecular basis remains elusive. Our limited previous studies indicated that epigenetic modifications are key to the asymmetric transcriptomes of brain hemispheres.
Methods:
We used whole-genome expression microarrays to profile post-mortem brain samples from subjects with SCZ, psychotic BD [BD(+)] or non-psychotic BD [BD(−)], or matched controls (n=10/group, corresponding to different brain hemispheres) and performed whole-genome DNA methylation (DNAM) profiling of the same samples (n=3–4/group) to identify pathways associated with SCZ or BD(+) and genes/sites susceptible to epigenetic regulation. qRT-PCR and quantitative DNAM analysis were employed to validate findings in larger sample sets (n=35/group).
Results:
Gene Set Enrichment Analysis (GSEA) demonstrated that BMP signaling and astrocyte and cerebral cortex development are significantly (FDR q<0.25) coordinately upregulated in both SCZ and BD(+), and glutamate signaling and TGFβ signaling are significantly coordinately upregulated in SCZ. GSEA also indicated that collagens are downregulated in right versus left brain of controls, but not in SCZ or BD(+) patients, and Ingenuity Pathway Analysis predicted that TGFB2 is an upstream regulator of these genes (p=0.0012). While lateralized expression of TGFB2 in controls (p=0.017) is associated with a corresponding change in DNAM (p≤0.023), lateralized expression and DNAM of TGFB2 are absent in SCZ or BD.
Conclusion:
Loss or reversal of brain laterality in SCZ and BD corresponds to aberrant epigenetic regulation of TGFB2 and changes in TGFβ signaling, indicating potential avenues for disease prevention/treatment.
Keywords: Schizophrenia, Brain asymmetry, DNA methylation, TGFB2, NR2E1
INTRODUCTION
Schizophrenia (SCZ) and bipolar disorder (BD) are two interrelated life-long mental illnesses, each affecting 1% of the population worldwide. Despite several decades of extensive research, it has been challenging to define the key molecular pathways and corresponding etiologies of these diseases. During the last two decades, the use of advanced imaging techniques such as functional magnetic resonance imaging (fMRI), gamma synchrony and mapping of scalp electrical activity have helped to identify SCZ and BD endophenotypes, including atrophy of the cingulate gyrus and dorsolateral prefrontal cortex, mainly in the left brain hemisphere (Rockstroh et al., 1998; Walter et al., 2003; Weiss et al., 2004; Cullen et al., 2006; Prasad and Keshavan 2008; Venkatasubramanian et al., 2008a,b; Meda et al., 2008; Bangalore et al., 2009; Williams et al., 2009; Bleich-Cohen et al., 2012; Narayanaswamy et al., 2015; Mahon et al., 2015; Li et al., 2016; Mwansisya et al., 2017). In fact, the loss or reversal of brain asymmetry/laterality is one of the most consistent observations that have eluded an understanding in neuro-developmental diseases like SCZ and BD (Williams et al., 2009; Wang et al., 2014; Ho et al., 2017). It has been proposed that a compromised left brain’s logical dominance [or right brain in left-handed individuals] is linked to the lack of insight on wishes, fears and perceptual distortions, leading to delusional thinking and hallucination in SCZ (Crow 2000). Thus, “an individual’s own thoughts [originated from the right brain] are perceived as an external intruding voice” [by the left brain] (Angrilli et al, 2009).
For more than two decades, mutations in genes that are presumed to be involved in the establishment of brain laterality remained as the dominant hypothesis to explain the underlying mechanism of aberrant brain laterality in SCZ (Crow 1989 and 2000). However, more than a thousand linkage and genetic association studies, including the most recent genome-wide association studies (GWASs) involving thousands of cases and controls, have failed to identify a specific gene or group of genes with major effects responsible for the genesis of SCZ or BD (Purcell et al., 2009, Hamshere et al., 2013). On the other hand, recent research findings overwhelmingly support key roles for epigenetic modifications of genes in cell differentiation and the development of structural and functional diversity of different cells/tissues, which may include brain hemispheres. Epigenetic codes are inherited and referred to as epigenetic memory (Monk 1995; Bird 2002); however, environmental insults such as contaminants, infectious elements, malnutrition, or ecological and social conditions can change epigenetic memory, resulting in sporadic or inherited neurodevelopmental diseases (Abdolmaleky et al., 2008 and 2015a; Szyf 2009). Additionally, a role for environmental effects arising from choline, methionine and folic acid deficiency, the gut microbiome, inflammation and oxidative stress, which are associated with the failure of the functionality of methylation machinery and the corresponding loss of epigenetic memory, has been described in major mental diseases (reviewed in Abdolmaleky et al., 2015a; Alam et al., 2017). At least 30 gene-specific and more than 10 whole genome DNA methylation analyses have reported aberrant DNA methylation of more than 100 genes in SCZ and BD (Abdolmaleky et al., 2015a).
A number of studies have also provided evidence for epigenetic fine tuning of brain laterality affecting expression of monoaminergic genes such as DRD2 (Popendikyte et al., 1999), MB-COMT (Abdolmaleky et al., 2006), HTR2A and DTNBP1 in normal individuals, which is lost in SCZ and BD patients (Abdolmaleky et al., 2006, 2011 and 2015b). However, a comprehensive understanding of the identity of target genes and mechanisms underlying their dysregulation leading to partial loss or reversal of brain laterality remains unclear in SCZ and BD.
We performed gene expression profiling and DNA methylome analysis of the dorsolateral prefrontal cortex (Brodmann’s area 46), one of the well characterized dysfunctional brain regions in SCZ and BD (Stanley et al., 2007; Minzenberg et al., 2009; Ragland et al., 2009; Dell’Osso et al., 2015), in post-mortem brain samples from the left and right hemispheres of SCZ and BD patients and controls to identify pathways that are affected in SCZ and BD, as well as those that lose normal brain laterality in these disease states. Our results provide evidence for shared pathway alterations in SCZ and BD, as well as aberrant brain asymmetry/laterality in SCZ and BD. We also identified epigenetic modifications that correspond to gene expression alterations of specific genes. Several key genes identified in these analyses were subjected to verification in a larger set of post-mortem brain samples using qRT-PCR analysis. Additionally, iPSC derived neurons and astrocytes were also analyzed to identify cell type origins for the expression of significant genes.
METHODS
Samples
One hundred and five RNA and DNA samples extracted from the dissects of post-mortem brains (Brodmann’s area 46, dorsolateral prefrontal cortex) of patients with SCZ or BD or control subjects (n=35 per group) were obtained from the Stanley Medical Research Center (SMRC). DNA was extracted using a phenol-chloroform protocol and total RNA was isolated using TRIzol reagent (Invitrogen). An A260/A280 ratio greater than 1.7 was achieved with more than 95% of the DNA/RNA samples used in these studies. The samples used in the analysis were matched for sex, ethnicity, brain laterality, age and other demographics as described previously (Abdolmaleky et al., 2014). We also had access to other detailed information such as age of onset and duration of disease, psychotic feature, cause of death, length of stay in hospital, history/status of smoking and alcohol, antipsychotic, mood stabilizer or antidepressant use (and the type of drugs) at the time of death, brain weight and pH, and post-mortem and refrigeration intervals.
RNA expression microarray profiling
Gene expression profiling was performed using the AffymetrixGeneChip Human Genome U133 Plus 2.0 array with 1 µg of total RNA from each sample (n=10 per group: control, SCZ and BD). All RNA samples used for microarray analysis had RIN (RNA Integrity Number) values greater than 7 as measured by Agilent Bioanalyzer. CEL files were normalized to produce gene-level expression values using the implementation of the Robust Multiarray Average (RMA) in the affy R package (version 1.36.1) and an Entrez Gene-specific probeset mapping (version 14.0.0) from the Molecular and Behavioral Neuroscience Institute (Brainarray) at the University of Michigan (Dai et al 2005). Array quality was assessed by computing Relative Log Expression (RLE) and Normalized Unscaled Standard Error (NUSE) using the affyPLM R package (version 1.34.0). All samples had median RLE values less than 0.1 and median NUSE values less than 1.05. Principal Component Analysis (PCA) was performed using the prcomp R function with expression values that had been normalized across all samples to a mean of zero and a standard deviation of one. Moderated (empirical Bayesian) t tests were performed using the implementation in the limma R package (version 3.14.4), i.e., creating simple linear models with the lmFit function, followed by empirical Bayesian adjustment with the eBayes function. Samples from subjects with BD who were not in a psychotic state at the time of death were excluded; only subjects with psychotic BD (BD(+)) were included in the analysis. The expression of each gene was modeled twice: first as a linear function of disease state (SCZ vs control, BD(+) vs control) and laterality (right vs left) (the “reduced” model), and then as a linear function of disease state, laterality, and the interaction of the two (SCZ:laterality, BD(+):laterality) (the “full” model). The significance of each disease state versus control was assessed using the reduced model, and the significance of each interaction effect was assessed using the full model. Correction for multiple hypothesis testing was accomplished using the Benjamini-Hochberg false discovery rate (FDR). All microarray analyses were performed using the R environment for statistical computing (version 2.15.1).
Gene Set Enrichment Analysis (GSEA)
GSEA (version 2.2.1) was used to identify biological terms, pathways and processes that are coordinately up- or down-regulated with respect to given effects in the linear models described above. The Entrez Gene identifiers of the genes interrogated by the array were ranked according to the moderated t statistics of the SCZ or BD(+) coefficients in the “reduced” model, or for the SCZ:laterality or BD(+):laterality coefficients of the “full” model. Each ranked list was then used to perform pre-ranked GSEA analyses (default parameters with random seed 1234) using the Entrez Gene versions of the Hallmark, BioCarta, KEGG, Reactome, and Gene Ontology (GO) gene sets obtained from the Molecular Signatures Database (MSigDB) (version 6.0).
qRT-PCR analysis
Real-time qRT-PCR analysis was performed using SYBR Green and ABI 7900 Real-Time PCR System (384-well plate) in duplicate, using cDNA synthesized from the same RNA samples used for the microarray as well as additional samples which passed quality controls (35 samples per group). Quantification of the expression of candidate genes was performed using the ΔΔCT method normalized with the CT (cycle threshold) of β-actin and GAPDH as previously described (Abdolmaleky et al., 2008 and 2014).
Analysis of gene expression in iPSC-derived neurons and astrocytes
Four human iPSC lines of normal controls from the Simons Foundation were cultured in StemFlex medium (Thermo Fisher catalog #A3349401). Cells were induced to differentiate to NSC and then to either astrocytes or neuronal cells using standard protocols. In brief, iPSCs were cultured in StemFlex medium for 3 passages and were induced to differentiate to NSC using neuronal induction medium (Thermo Fisher, Catalog #A1647801). NSCs were then allowed to differentiate into neurons (using neurobasal medium and B-27 supplement) or astrocytes (using DMEM, 0.5% FBS and N2 supplement). After two weeks of differentiation, acquisition of neuronal or astrocyte phenotypes were confirmed with immunohistochemical staining with corresponding primary (e.g. MAP2 and synapsin) and secondary antibodies. Cells were cultured for another two weeks, then harvested. RNA was extracted using TRIzol (ZYMO Research, Direct-zol RNA MiniPrep, catalog #R2050) and used for cDNA synthesis (Invitrogen Life Technologies, catalog# 18090050). SYBR Green based qRT-PCR was used to perform gene expression analysis.
DNA methylation microarray profiling
To identify genes that are regulated by DNA methylation in the human brain, we assessed gene-specific DNA methylation status in representative post-mortem brain samples that had been evaluated for gene expression (four samples per group) using the Illumina Infinium Human Methylation 27 (27k) BeadChip array. Following bisulfite treatment of 1 µg of DNA using an EZ DNA Methylation kit (Zymo Research, Cat #D5001), 0.5 µg of bisulfite-modified DNA was processed for DNA methylation analysis. The 27k array is designed to measure methylation at approximately 27,000 CpG sites, primarily at gene promoters. When treated with bisulfite, non-methylated cytosines are converted to uracil while methylated cytosines remain unchanged. Two types of beads (U and M), which are only able to hybridize to either unmethylated or methylated sites, respectively, trigger single-base extension for detection. Extensions with green and red fluorescence represent methylated and unmethylated bases, respectively, and methylation status was computed from the ratio of green (methylated) fluorescent signal to the total fluorescent intensity (beta). Raw intensities in the green and red channels of the fluorescent signal were read and converted to methylation status by the methylumi R package, with beta values calculated using the formula beta = M/(M+U+100) (Du et al., 2010). Raw intensities were normalized using the normalizeMethyLumiSet function to correct dye bias and beta values were recalculated based on the corrected intensities.
Gene-specific DNA methylation analysis
The initial evaluation of CpG islands using DNA methylation microarray in representative samples enabled us to assess the overall methylation levels of potential target CpG islands for further analysis. Next, methylation specific PCR (MSP) and/or bisulfite sequencing analyses were used (Abdolmaleky et al., 2008; 2011; 2014) to confirm the presence of DNA methylation in a selected group of candidate CpGs. The bisulfite modified placental DNA and in vitro methylated DNA were used as negative and positive controls for methylation, respectively (Abdolmaleky et al., 2008 and 2014). Whenever MSP was not feasible due to highly GC-rich sequences, enzymatic digestion of methylated DNA coupled with qPCR was used to evaluate 5-mc (5-methyl cytosine) as well as 5-hmC (5-hydroxymethyl cytosine) content. 5-hmC is an intermediate formed during demethylation of methylated DNA, and has been reported to be abundant in human brain and to correspond to induction of gene expression (Song et al. 2011; Nestor et al., 2012), even before its conversion to unmethylated cytosine (Wu and Zhang 2011). In order to differentiate between 5-mC and 5-hmC, we used qPCR analysis of MspI and HpaII restricted DNA treated by T4 Phage β-glucosyltransferase (T4-BGT) with uridine diphosphoglucose (UDP-Glu) using the EpiMark 5-hmC and 5-mC Analysis Kit according to the instructions of the manufacturer (New England BioLabs, Cat# E3317S). For quantification, ΔΔCT method was used and normalized with the CT of uncut DNA.
RESULTS
Study demographics
The demographics of the subjects of this study are outlined in Table 1. Subjects with BD are divided into those with or without psychotic features, or those in whom the presence or absence of psychosis is unknown, denoted as BD(+), BD(−), and BD(unknown), respectively.
Table 1.
Demographics of samples in study. The sex and age of the subjects is shown, divided by disease state and brain laterality.
| Used in both qPCR and microarray analysis (n=105) | ||||||||||
| Disease state | control | SCZ | BD(−) | BD(+) | BD (unknown) | |||||
| Laterality | left | right | left | right | left | right | left | right | left | right |
| n | 16 | 19 | 17 | 18 | 8 | 4 | 11 | 10 | 1 | 1 |
| Sex (% male) | 88% | 63% | 71% | 78% | 63% | 75% | 27% | 50% | 0% | 100% |
| Age (mean ± sd) | 45.6 ± 6.6 | 43.1 ± 8.4 | 41.3 ± 8.1 | 43.8 ± 8.8 | 43.8 ± 11.0 | 45.2 ± 18.9 | 43.7 ± 9.8 | 48.3 ± 9.1 | 48 | 42 |
| Used in gene expression microarray analysis only (n=30) | ||||||||||
| Disease state | control | SCZ | BD(−) | BD(+) | BD (unknown) | |||||
| Laterality | left | right | left | right | left | right | left | right | left | right |
| n | 5 | 5 | 5 | 5 | 4 | - | 3 | 3 | - | - |
| Sex (% male) | 80% | 60% | 40% | 80% | 75% | - | 0% | 100% | - | - |
| Age (mean ± sd) | 45.0 ± 8.6 | 45.2 ± 10.3 | 43.2 ± 4.2 | 41.6 ± 6.9 | 37.8 ± 8.5 | - | 47.7 ± 16.2 | 41.3 ± 8.5 | - | - |
Principal Component Analysis (PCA)
Gene expression microarrays were used to profile gene expression in the left or right dorsolateral prefrontal cortex (Brodmann’s area 46) of post-mortem brain samples obtained from patients with SCZ or BD or from matched controls (n=10 per group). We used Principal Component Analysis (PCA) to cluster these 30 samples with regard to the expression of all ~19,000 genes interrogated by the microarray platform (Supplementary Figure 1). Although there was no clear division among samples with respect to disease state, brain hemisphere, or sex, there was also no indication of any outliers that separated them from the other samples used in the study.
Identification of differentially regulated pathways in SCZ and BD(+)
A linear modeling approach was then used to model gene expression as a function of disease state (after correcting for brain laterality) and to perform a t test on the coefficient of the SCZ and BD(+) model terms to assign significance to each disease state. BD(−) subjects were excluded from analysis in order to reduce confounding variables and because BD(+) has previously been shown to be more similar to SCZ than BD(−) (Choi et al., 2008). The full set of results from this statistical analysis is provided in Additional File 1. Only one gene passed FDR correction at a reasonable threshold (q < 0.25) for the SCZ term, and none passed FDR correction for the BD(+) term, indicating that the experiment is not powered to discriminate true and false positives at the level of individual genes.
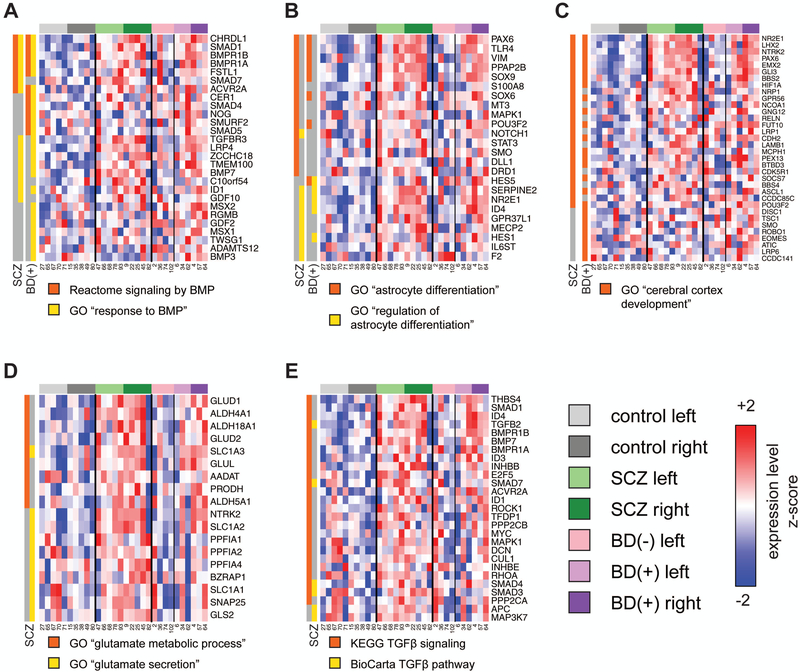
To improve statistical power, all genes interrogated by the array were ranked according to the t statistics computed from the SCZ or BD(+) terms of the model and Gene Set Enrichment Analysis (GSEA) was used to identify pathways that are coordinately regulated in either disease state with respect to controls. The full set of GSEA results are provided in Additional File 2. We found that gene sets related to BMP signaling, astrocyte differentiation, and cerebral cortex development are significantly (FDR q < 0.25) coordinately upregulated in both SCZ and BD(+); the leading edges of these gene sets are shown in Figure 1. These include the Reactome BMP signaling pathway (SCZ FDR q = 0.024, BD(+) FDR q = 0.014) and the Gene Ontology (GO) term “response to BMP” (SCZ FDR q = 0.18, BD(+) FDR q = 0.06), which includes the TGFβ receptor gene TGFBR3 and several Smad genes (Figure 1A); the GO terms “astrocyte differentiation” (SCZ FDR q = 0.016, BD(+) FDR q = 0.15), which includes the transcription factor SOX9, and “regulation of astrocyte differentiation” (SCZ FDR q = 0.18, BD(+) FDR q = 0.24), which includes the transcription factor NR2E1 (Figure 1B); and the GO term “cerebral cortex development” (SCZ FDR q = 0.13, BD(+) FDR q = 0.17), which includes the transcription factors PAX6, LHX2 and NR2E1 (Figure 1C).
Figure 1: Leading edges of gene sets in pathways coordinately upregulated by GSEA in SCZ and/or BD(+).
Linear modeling was performed to assign t statistics to each gene with respect to SCZ or BD(+), and GSEA was performed to identify Gene Ontology (GO) terms or BioCarta, KEGG or Reactome pathways with significant coordinate regulation in either or both disease states. Groups of significant gene sets representing several pathways or processes were selected, and the expression of the genes in any of the “leading edges” of each set (i.e., the genes that contributed the most to the significance of the result)is shown across all subjects (including non-psychotic BD, which was not included in the analysis). Rows and columns correspond to genes and samples, respectively. Expression values were scaled by z-normalizing expression values to a mean of zero and standard deviation of one across all samples in each row, with red and blue indicating z-scores ≥ 2 or ≤ −2, respectively. Genes are arranged in descending order from top to bottom by the magnitude of the t statistic used to rank genes for GSEA. Gene sets are grouped according to theme: (A) BMP signaling; (B) astrocyte differentiation; (C) glutamate metabolism and secretion; (D) cerebral cortex development; (E) TGFβ signaling. The membership of each gene in the leading edge of each gene set for each disease state (SCZ or BD(+)) in each analysis is denoted in a separate color (orange or yellow) for each gene set in each panel.
We also found that pathways related to glutamate signaling and TGFβ signaling showed significant coordinate upregulation in SCZ with weak or no association with BD(+). These include the GO terms “glutamate metabolic process” (SCZ p = 0.0045, FDR q = 0.065; BD(+) p = 0.044, FDR q = 0.33) and “glutamate secretion” (SCZ p = 0.016, FDR q = 0.16; BD(+) p = 0.63, FDR q = 0.92), indicating the involvement of glutamate/glucose transporters SLC1A2 and SLC1A3 (Figure 1D); and the KEGG (SCZ p < 0.001, FDR q = 0.086; BD p = 0.021, FDR q = 0.32) and BioCarta (SCZ p = 0.046, FDR q = 0.24; BD p = 0.82, FDR q = 0.98) highlighting the role of TGFβ pathways (Figure 1E). As the TGFβ ligand genes TGFB1 and TGFB3 were poorly expressed in the dorsolateral prefrontal cortex (called Present in only 10% or 13% of samples, respectively) and are not significantly associated with SCZ (p > 0.5), and TGFB2 was called Present in all samples and is strongly associated with both SCZ (p = 0.007) and BD(+) (p = 0.0097), TGFβ2 appears to be the ligand driving the TGFβ pathway GSEA results.
Loss of lateralized/asymmetric gene expression in the brain of SCZ and BD(+) patients
In the same manner, a linear modeling approach was then used to assign statistical significance to each gene with respect to the interaction of each disease state (SCZ or BD(+)) and brain laterality. The full set of results from this statistical analysis are provided in Additional File 1. As no genes passed FDR correction for these interaction coefficients, GSEA was used to identify pathways that are regulated in a disease-dependent manner with regard to brain laterality (full set of results provided in Additional File 2). Sets of genes annotated with Gene Ontology (GO) terms associated with collagen deposition are significantly associated with the interaction of either or both of SCZ and BD(+) with laterality (“complex of collagen trimers”: SCZ FDR q = 0.022, BD(+) FDR q = 0.058; “collagen trimer”: SCZ FDR q = 0.11), and show a pattern of coordinate downregulation in the right versus left brain of controls and upregulated or unchanged expression in the right versus left brain of SCZ or BD(+) patients (Figure 2A). These include the gene COL3A1 (SCZ:laterality p = 0.0052; BD(+):laterality p = 0.11; control: 1.5-fold lower in right brain; SCZ: 1.26-fold higher in right brain; BD(+): 1.01-fold lower in right brain).
Figure 2: Collagen gene sets are coordinately regulated downstream of TGF-beta with respect to brain laterality in a disease-dependent manner.
(A) Leading edges of collagen-related gene sets with significant interaction between SCZ or BD(+) with laterality. Linear modeling was performed to assign t statistics to each gene with respect to the interaction of SCZ or BD(+) with laterality, and GSEA was performed to identify Gene Ontology (GO) terms or BioCarta, KEGG or Reactome pathways with coordinate regulation in either or both disease states with respect to laterality that differs significantly from that in controls. The expression of the genes in any of the “leading edges” of each set (i.e., the genes that contributed the most to the significance of the result) is shown across all subjects (including non-psychotic BD, which was not included in the analysis). Rows and columns correspond to genes and samples, respectively. Expression values were scaled by z-normalizing expression values to a mean of zero and standard deviation of one across all samples in each row, with red and blue indicating z-scores ≥ 2 or ≤ −2, respectively. Genes are arranged in descending order from top to bottom by the magnitude of the t statistic used to rank genes for GSEA. The membership of each gene in the leading edge of each gene set for each disease state (SCZ or BD(+)) in each analysis is denoted in a separate color (orange or yellow) for each gene set. (B) Mechanistic network of TGFB2-driven regulation of genes with significant SCZ:laterality interaction. Ingenuity Pathway Analysis was used to predict upstream regulators of the 265 genes with SCZ:laterality p< 0.05 and called Present in ≥ 3 samples. TGFB2 and SMAD3 were predicted to be activated, and SMAD7 was predicted to be inhibited, leading to coordinate downregulation of genes such as collagens in the right brain of controls only.
Prediction of TGFB2 as an upstream regulator of loss of brain laterality in SCZ
Ingenuity Pathway Analysis (IPA) was then used to identify upstream regulators of the loss of laterality of gene expression observed in SCZ relative to controls. In a Core Analysis performed using the 265 genes with SCZ:laterality p < 0.05 and called Present in at least 3 samples (i.e., the size of the smallest experimental group in the analysis), TGFB2 was predicted to be a likely upstream regulator of these changes in gene expression (p = 0.0012). A mechanistic network including TGFB2 and the genes that support this prediction is shown in Figure 2B.
Assessment of cell-type-specific TGFB2 expression
To determine which cell types contribute to increased TGFβ signaling in these samples, we used qRT-PCR to examine the expression of TGFB2 in human iPSC-derived astrocytes and neurons. This analysis indicates that the expression of TGFB2 is significantly higher in astrocytes than in neurons (p = 0.029) (Supplementary Figure 2), suggesting that astrocytes are the cell type responsible for this effect.
Validation of NR2E1 and TGFB2 expression
We then performed qRT-PCR analysis to validate the differential expression of NR2E1 and TGFB2 (primers listed in Table 2) using a larger set of post-mortem brain samples (n=35 per diagnosis). The expression of these genes by qPCR was highly concordant with the microarray data (NR2E1 Pearson r = 9.6 × 10−5; TGFB2 Pearson r =0.0041) (Supplementary Figure 3). The expression of NR2E1 was significantly increased in both SCZ (1.26-fold, p=0.038) and BD(+) (1.64-fold, p=0.016) (Figure 3A), whereas TGFB2 expression was significantly increased in SCZ (1.22-fold, p=0.026) but only trended in that direction in BD(+) (1.32-fold, p=0.11) (Figure 3B). Surprisingly, the expression of TGFB2 with respect to laterality (1.26-fold higher in the right brain of controls, p = 0.017) was the opposite of that predicted by IPA from the expression of collagens and other downstream genes (which were more highly expressed in the left brain of controls).
Table 2.
Primers for gene expression qRT-PCR analysis. The primers for NR2E1 and TGFB2 were obtained from the Harvard primer bank.
| Gene | Forward (5’−3’) | Reverse (5’−3’) |
|---|---|---|
| ACTB | CGAGCACAGAGCCTCGCCTTTGCC | TGTCGACGACGAGCGCGGCGATAT |
| NR2E1 | CAAACGGAGCATCCGAAGGAA | GTCCACGGAAGTAGAGGGC |
| TGFB2 | AGAGTGCCTGAACAACGGATT | CCATTCGCCTTCTGCTCTT |
Figure 3: Validation of NR2E1 and TGFB2 gene expression by qPCR.
Relative expression of (A) NR2E1 (n=100) and (B) TGFB2 (n=102), normalized to beta-actin, divided by disease state and brain laterality. The mean of each group is indicated by a heavy gray line. Filled circles indicate samples that were profiled by gene expression microarray.
Assessment of methylation at TGFB2 locus
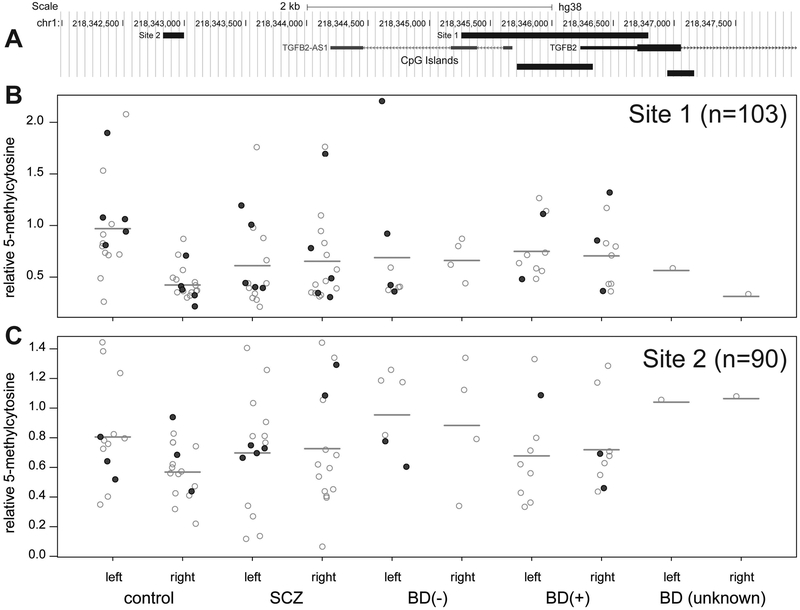
Enzymatic digestion of methylated DNA followed by qPCR was then used to measure methylation within two sites: 1) an amplicon encompassing a CpG island in the 5’ UTR of the TGFB2 gene locus, and 2) a 170-base amplicon approximately 3.4 kb upstream of the TGFB2 transcriptional start site (Figure 4A; primers listed in Table 3). As described in the Methods, we measured total methylation as well as 5-hmC at each site. Because we found that the 5-hmC:5-mC ratio varies substantially with respect to disease state (Supplementary Figure 4), we subtracted 5-hmC signal from total methylation signal in order to obtain a pure measurement of 5-mC at each site. The amount of 5-mC at site 1 (Figure 4B) was significantly lower in the right brain of controls than in the left brain of controls (1.59-fold, p = 6.8 × 10−3), and was unchanged with respect to brain laterality in SCZ (p = 0.76) or BD(+) (p = 0.77). A similar but less significant pattern was observed at site 2 (Figure 4C) (1.2-fold lower in right brain of controls, p = 0.023; SCZ right vs. left p = 0.84; BD(+) right vs. left p = 0.91). This is the inverse of the pattern of lateralized TGFB2 expression observed by qPCR, indicating that the laterality of this gene is regulated at least in part by DNA methylation. However, in contrast to the TGFB2 expression qPCR, there was no statistically significant change in 5-mC with regard to disease state at either site (SCZ vs. control p = 0.47 and 0.64 and BD(+) vs. control p = 0.92 and 0.11 for sites 1 and 2, respectively).
Figure 4: Measurement of TGFB2 DNA methylation by qPCR.
(A) Location of two qPCR amplicons (site 1 and site 2) in the TGFB2 locus. (B, C) Measurement of 5-methylcytosine (5-mC) at each site (site 1: n=103 samples total; site 2: n=90 samples total), divided by disease state and brain laterality. The mean of each group is indicated by a heavy gray line. Filled circles indicate samples that were profiled by gene expression microarray.
Table 3.
Primers for methylation qRT-PCR analysis.
| Amplicon | Forward (5’−3’) | Reverse (5’−3’) |
|---|---|---|
| TGFB2 site 1 | CGTGGTTCAGAGAGAACTTATAAATCTCC | TCTGTCTTTCTCTTGTCAGGAGC |
| TGFB2 site 2 | CCTTTTACCATGAAGACTGTAGAGAC | TCAAATGATCAGTTCTTTGAAGACCT |
Genome-wide assessment of DNA methylation
Illumina 27k DNA methylation microarrays were also used to profile 3–4 of the same samples from each disease group (control, SCZ, and BD). The annotation for each probe and beta values (fraction of methylated CpG residues) are provided in Additional File 3.
To identify genes that might be regulated by methylation, we matched the Illumina 27k data with the HG-U133 Plus 2.0 data using the annotated Entrez Gene ID (generating a set of 25,972 CpG-gene pairs, representing 13,601 unique genes) and performed Spearman correlation tests for each pair. The complete set of these results are provided in Additional File 4. We identified the CCND1 locus as of particular interest, as it was interrogated by a large number of probes, its expression was significantly downregulated in SCZ (p = 0.00044) and BD(+) (p = 0.044), and its methylation was generally anti-correlated with its expression (11/18 probes with Spearman rho < 0). Methylation was increased to a greater extent in BD (up to 39%) than in SCZ (up to 14%) in probes interrogating a CpG island extending from upstream of CCND1 through its second intron; however, methylation was generally increased in both SCZ and BD (up to 17%) in and around a CpG island contained in its third intron.
DISCUSSION
Schizophrenia (SCZ) and bipolar disorder (BD) are two severe mental disorders that share some common clinical phenotypes and hundreds of SNPs and epigenetic alterations underlying their disease pathology. Although numerous studies have attempted to define molecular pathways corresponding to the pathology to devise effective therapeutics, the etiology of their pathogenesis remains elusive. At present, the treatment of these diseases relies predominantly on antipsychotic drugs and mood stabilizers that were serendipitously found to improve some of the disease symptoms. However, these drugs have little effect on cognitive dysfunction to improve the functional capabilities of patients, particularly those with SCZ. While one of the overlooked phenotypes in SCZ and to some extent in BD is the loss of brain laterality, its molecular basis has remained elusive and its elucidation may provide leads for implementing novel therapeutic approaches. Our limited previous studies provided evidence for epigenetic modifications as the key to asymmetric transcription of genes in the brain and corresponding functional diversity of brain hemispheres (Abdolmaleky et al., 2006, 2011 and 2014). For the first time to our knowledge, here we report the examination of gene expression profiles of post-mortem brain samples in the left versus right brain to identify differentially activated molecular pathways, and to correlate altered gene expression to differential DNA methylation patterns in order to decipher the molecular basis and underlying mechanisms for the loss or reversal of brain laterality in these interlinked diseases.
Based on our data, TGFB2 and NR2E1 appear to be key drivers for dysregulation of many genes in SCZ and BD. These findings are consistent with a previous meta-analysis which concluded that TGFβ is a disease state marker in SCZ, as its expression increased in acutely relapsed patients and those exhibiting first-episode psychosis, and returned to normal levels following antipsychotic treatment (Miller et al., 2011). TGFB2 is downregulated in the retina of NR2E1 knockout mice (Zhang et al 2006) and there are 20 binding sites for NR2E1 (AAGGTCA/TGACCTT) in the body of the TGFB2 gene, explaining the direct correlation between NR2E1 and TGFB2 expression. NR2E1 (TLX) is an orphan nuclear receptor (Benod et al., 2014) expressed exclusively in the brain (Yu et al., 1994) and has been shown to either act as a transcriptional repressor (when bound to the p21, PTEN, GFAP or S100b promoters) or an activator (if it binds to other specific proteins) (Benod et al., 2014). During the development and adulthood of vertebrates, NR2E1 is expressed in the forebrain, specifically in the neurogenic regions as well as in the retina, where it regulates multiple pathways involved in DNA replication, cell cycle and cell adhesion via repression or activation of target genes. NR2E1 is also “a master regulator of neural stem cell maintenance and neurogenesis” (Islam and Zhang, 2014), preventing senescence (O’Loghlen et al., 2015), mitigating the impacts of IL-1β on hippocampal neurogenesis (Ó’Léime et al., 2018) and involved in aggressive behavior (Abrahams et al., 2005). Furthermore, genetic mutations in NR2E1 have been linked to BD pathogenesis (Kumar et al., 2008).
A recent study combining serial analysis of gene expression (SAGE) and transcription factor binding site prediction identified 64 novel candidate target genes of NR2E1 involved in neocortical development (Schmouth et al., 2015). These include LHX2, which is involved in “post-mitotic regulation of sensory area patterning in the mammalian neocortex” (Zembrzycki et al., 2015) and its cofactor SOX9. It is also known that coordination and cooperation between NR2E1 and PAX6 is required for brain patterning during development (Stenman et al., 2003). Our gene expression microarray analysis indicated that these three genes are upregulated in SCZ and in BD, and contribute to the coordinate upregulation of gene sets related to cerebral cortex development or astrocyte differentiation in the corresponding disease states. NR2E1 also controls cyclin D expression via PTEN by binding to the PTEN promoter to suppress its activity during retinogenesis (Zhang et al., 2006). Interestingly, the expression of CCND1 was decreased in SCZ and BD, and its DNA methylation was increased in SCZ and BD, indicating that DNA hypermethylation may also partly explain this effect.
Some other genes known to be linked to psychiatric diseases also exhibited dysregulation in patients with SCZ and BD in our expression profiling studies. For example, the genes NOTCH2, which is a key player in neurodevelopment and SCZ pathogenesis (Sundararajan, Manzardo and Butler 2018), and SELENBP1, which exhibits increased expression in the frontal cortex and blood cells in SCZ (Glatt et al., 2005), are also up-regulated in SCZ and BD as evident from our microarray analysis. The expression of the glutamate/glucose transporter SLC1A2 (Koeglsperger et al., 2013), which is linked to lithium response in BD, and is associated with the AMPA2 glutamate receptor network in human brain, was also highly upregulated in SCZ, consistent with findings related to prefrontal cortex of SCZ patients (Matute et al., 2005). In GWAS studies SLC1A2 has an effect size of 0.3 in disease pathogenesis with an allelic frequency of 0.4 (Higgins, Allyn-Feuer & Athey, 2015). Interestingly, it has been shown that in mouse astrocytes, TGFβ induces the expression of SLC1A2 as well as the glutamate/glucose transporter SLC1A3 and an increase in astroglial glutamate uptake (Koeglsperger et al., 2013). In addition to SLC1A2, SLC1A3 also exhibited a trend for increased expression in SCZ and BD in our analysis, suggesting that TGFβ-mediated astrocyte dysfunction may be involved in the pathogenesis of these major mental diseases. Our examination of iPSC-derived astrocytes and neurons also found that astrocytes express TGFB2 more than in neurons supporting this notion.
According to our array data, several collagen gene products downstream of TGFβ signaling emerged as likely mediators for the establishment of brain lateralization in normal controls that is lost in SCZ and BD. Among them, COL3A1 is known to regulate cortical development and is the major ligand for GPR56 in the developing brain. Its binding to GPR56 inhibits neuronal migration and activates the RhoA pathway by coupling GPR56 to GNA13 and possibly to GNA12. It also appears that the expression of TGFB2, which appears to encode the active TGFβ isoform in the dorsolateral prefrontal cortex, is partially regulated by methylation. However, it is unclear why the transcription of TGFB2 is discordant with that of the collagen products with respect to laterality in control subjects. As it has been previously reported that the transcription and translation of TGFβ are regulated by distinct pathways (Xiao et al., 2008), it may be that translational and post-translational regulation of TGFβ signaling explains this discrepancy. This will needs to be examined in further studies.
In summary, our results indicate that key genes of the TGFβ signaling pathway are upregulated in SCZ and BD, and the expression of collagen genes is associated with left/right brain asymmetry in controls but not in SCZ and BD.
CONCLUSIONS
Evidence from developmental biology research indicates that several TGFβ superfamily signaling proteins (e.g., lefty-1, lefty-2, nodal, ACVR2B, Smad2 and Smad5) are involved in left-right axis determination (Meno et al., 1996 and 1998; Nomura and Li 1998; Hamada et al., 2002; Chang et al., 2000; Smith et al., 2011). Despite the limited availability of post-mortem brain samples, our observations are consistent with and extend the previous findings of developmental biologists (Vonica et al., 2011) in supporting that the brain laterality could also be regulated by TGFβ superfamily signaling pathways. Furthermore, the association between the loss or reversal of brain laterality in SCZ and BD due to aberrant epigenetic regulation of the key affected genes suggests potential avenues for disease prevention at onset and/or during progression. For example, TGFB2 itself, as well as collagens or other genes downstream of TGFB2 exhibiting aberrant lateralized expression might be appropriate targets for designing novel therapeutics for the SCZ and BD patients. It is noteworthy that the currently available drugs affect both brain hemispheres and can only render partial improvement of symptoms and most of the patients remain dysfunctional throughout their life. Therefore, elucidating the hemisphere-specific molecular basis of SCZ and BD pathogenesis as described in this report is a good beginning for the quest to design novel therapeutic strategies (such as unilateral transcranial magnetic stimulation, or ECT) for these major psychiatric disorders with implications to other mental diseases (e.g. obsessive compulsive disorder).
Supplementary Material
Additional File 1: Summary of linear modeling analyses. Columns A-D contain annotation for each gene, including the Brainarray (MBNI) probeset ID, symbol and description, and a link to the Entrez Gene record. Column E contains the percentage of samples in which each gene was called Present. Columns F-Q contain the t statistics, nominal p values, and FDR q values for the moderated t tests performed on each coefficient of the linear models. Columns R-V contain signed linear fold changes computed for each disease state versus control and for right versus left brain within each disease state (e.g., +2 = 2-fold higher in SCZ than in control; −2 = 2-fold lower in SCZ than in control). Columns W-AZ contain log2 (expression) across all samples, laid over a colored representation (heatmap), scaled so that red and blue indicate expression values ≥ 2 standard deviations above and below, respectively, the row-wise mean (white) computed across all samples. Expression values that are called Absent or Present are indicated in italicized or normal type, respectively. The rows are sorted in descending order by SCZ t statistic.
Additional File 2: Summary of Gene Set Enrichment Analysis (GSEA) results. Column A indicates the category of the gene set. Column B indicates the gene set name provided by MSigDB, represented as a link to the MSigDB “card”. Column C indicates the number of genes in the gene set that overlap with the genes interrogated by the microarray. Columns D-O indicate the NES (Normalized Enrichment Score), nominal p value, and FDR q value for each gene set and each ranked list. Positive and negative NES values indicate that the t statistics in each ranked list are skewed towards positive or negative values, respectively. Nominal p values equal to 0 indicate p< 0.001 (as computed from 1000 permutations). FDR q values less than 0.25 are indicated in bold italics.
Additional File 3: Summary of Illumina 27k measurements. Columns A-AF contain annotation obtained from Gene Expression Omnibus Platform record GPL8490. Columns AG-AQ contain the beta values computed for each probe in each sample as described in Methods.
Additional File 4: Correlation analysis performed between Illumina 27k (DNA methylation) and HG-U133 Plus 2.0 (RNA expression) analyses. Columns A-J contain annotation obtained from Gene Expression Omnibus Platform record GPL8490. Column K indicates the total number of 27k probes per gene. Columns L-M indicate the Spearman rho and p values computed for each gene between the DNA methylation and RNA expression values. Column N indicates the number of samples in which each gene was called Present by gene expression microarray. Columns O and P contain the p values computed for each gene for SCZ and BD(+), respectively, in the gene expression microarray analysis. Columns Q-AA and AB-AL contain the methylation beta values and log2 (expression) values, respectively. Each set of values is laid over a colored representation (heatmap), scaled so that red and blue indicate expression values ≥ 2 standard deviations above and below, respectively, the row-wise mean (white) computed across all samples within each platform. Expression values that are called Absent or Present are indicated in italicized or normal type, respectively. The rows are sorted in ascending order by Spearman rho value.
Supplementary Figure 1: Principal Component Analysis (PCA). PCA was performed using all genes interrogated by the array, with expression values z-normalized to a mean of zero and standard deviation of one across all samples. Light and dark colors indicate samples obtained from the left and right hemisphere, respectively, and squares and circles indicate samples obtained from females and males, respectively.
Supplementary Figure 2: Cell-type specific expression of TGFB2. Expression of TGFB2 was measured by qRT-PCR in neurons and astrocytes (n=4 per group) and normalized to beta-actin.
Supplementary Figure 3: Expression values are highly correlated between qPCR and microarray platforms. The relative expression of NR2E1 and TGFB2 is plotted against the anti-logged (linear-scale) expression values obtained from the HG-U133 Plus 2.0 microarray platform.
Supplementary Figure 4: Relative content of 5-hydroxymethylcytosine (5-hmC) across experimental groups. Total methylation and 5-hmC were measured at each TGFB2 site by qPCR as described in Methods, and the fraction of total signal attributable to 5-hmC is shown.
ACKNOWLEDGMENTS
Post-mortem DNA and RNA samples were generously provided by The Stanley Brain Collection, courtesy of Drs. Michael B. Knable, E. Fuller Torrey, Maree J. Webster and Robert H. Yolken. S.T. was supported in part by NIH (CA165707), CTSI Boston University (NIH CTSA, UL1-TR00157) and the NARSAD independent investigator Award. A.C.G. was supported by the Boston University Clinical and Translational Science Institute (NIH NCATS award 1UL1TR001430).
REFERENCES
- Abdolmaleky HM, Cheng KH, Faraone SV, Wilcox M, Glatt SJ, Gao F, Smith CL, Shafa R, Aeali B, Carnevale J, Pan H, Papageorgis P, Ponte JF, Sivaraman V, Tsuang MT, Thiagalingam S. 2006. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 15(21):3132–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolmaleky HM, Smith CL, Zhou RJ, Thiagalingam S 2008. Epigenetic Alterations of Dopaminergic System in Major Psychiatric Disorders (in “Pharmacogenomics in Drug Discovery and Development” Editor: Yan Q, Humana Press; ). Methods Mol Biol.448:187–212. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Yaqubi S, Papageorgis P, Lambert AW, Ozturk S, Sivaraman V, Thiagalingam S. 2011. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr Res. 129(2–3):183–190. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Nohesara S, Ghadirivasfi M, Lambert AW, Ahmadkhaniha H, Ozturk S, Wong CK, Shafa R, Mostafavi A, Thiagalingam S. 2014. DNA hypermethylation of serotonin transporter gene promoter in drug naïve patients with schizophrenia. Schizophr Res. 152(2–3):373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolmaleky HM, Zhou JR and Thiagalingam S. 2015a. An update on the epigenetics of psychotic diseases and autism. Epigenomics, Future Medicine, 7(3):427–449. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky HM, Pajouhanfar S, Faghankhani M, Joghataei MT, Mostafavi A, Thiagalingam S. 2015b. Antipsychotic drugs attenuate aberrant DNA methylation of DTNBP1 (dysbindin) promoter in saliva and post-mortem brain of patients with schizophrenia and Psychotic bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 168(8):687–696. [DOI] [PubMed] [Google Scholar]
- Alam R, Abdolmaleky HM, Zhou JR. 2017. Microbiome, inflammation, epigenetic alterations, and mental diseases.Am J Med Genet B Neuropsychiatr Genet. 174(6):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams BS, Kwok MC, Trinh E, Budaghzadeh S, Hossain SM, Simpson EM. 2005. Pathological aggression in “fierce” mice corrected by human nuclear receptor 2E1. J Neurosci. 25(27):6263–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benod C, Villagomez R, Filgueira CS, Hwang PK, Leonard PG, Poncet-Montange G, Rajagopalan S, Fletterick RJ, Gustafsson JÅ, Webb P. 2014. The human orphan nuclear receptor tailless (TLX, NR2E1) is druggable. PLoS One. 9(6):e99440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A, Spironelli C, Elbert T, Crow TJ, Marano G, Stegagno L. 2009. Schizophrenia as failure of left hemispheric dominance for the phonological component of language. PLoS One. 4(2):e4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore SS, Goradia DD, Nutche J, Diwadkar VA, Prasad KM, Keshavan MS. 2009. Untreated illness duration correlates with gray matter loss in first-episode psychoses. Neuroreport. 20(7):729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich-Cohen M, Sharon H, Weizman R, Poyurovsky M, Faragian S, Hendler T. 2012. Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr Res. 134(2–3):131–136 [DOI] [PubMed] [Google Scholar]
- Bird A DNA methylation patterns and epigenetic memory.2002. Genes Dev. 16:6–21. [DOI] [PubMed] [Google Scholar]
- Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. 2000. Smad5 is essential for left-right asymmetry in mice. Dev Biol. 219(1):71–78. [DOI] [PubMed] [Google Scholar]
- Choi KH, Elashoff M, Higgs BW, Song J, Kim S, Sabunciyan S, Diglisic S, Yolken RH, Knable MB, Torrey EF, Webster MJ. 2008. Putative psychosis genes in the prefrontal cortex: combined analysis of gene expression microarrays. BMC Psychiatry. 8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. 1989. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 46(12):1145–1150. [DOI] [PubMed] [Google Scholar]
- Crow TJ. 2000. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 31(2–3):118–129. [DOI] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. 2006. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry. 188:26–31. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Cinnante C, Di Giorgio A, Cremaschi L, Palazzo MC, Cristoffanini M, Fazio L, Dobrea C, Avignone S, Triulzi F, Bertolino A, Altamura AC. 2015. Altered prefrontal cortex activity during working memory task in Bipolar Disorder: A functional Magnetic Resonance Imaging study in euthymic bipolar I and II patients. J Affect Disord. 184:116–122 [DOI] [PubMed] [Google Scholar]
- Du P, Zhang X, Huang CC, Jafari N, Kibbe WA, Hou L, Lin SM. 2010. Comparison of Beta-value and M-value methods for quantifying methylation levelsby microarray analysis. BMC Bioinformatics.11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Everall IP, Kremen WS, Corbeil J, Sásik R, Khanlou N, Han M, Liew CC, Tsuang MT. 2005. Comparative gene expression analysis of blood and brain provides concurrent validation of SELENBP1 up-regulation in schizophrenia. Proc Natl Acad Sci U S A. 102(43):15533–15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Meno C, Watanabe D, Saijoh Y. 2002. Establishment of vertebrate left-right asymmetry. Nat Rev Genet. 3(2):103–113. [DOI] [PubMed] [Google Scholar]
- Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D, Jones I, Forty L, Jones L, Gordon-Smith K, Riley B, O’Neill FA, Kendler KS, Sklar P, Purcell S, Kranz J; Schizophrenia Psychiatric Genome-wide Association Study Consortium; Wellcome Trust Case Control Consortium+; Wellcome Trust Case Control Consortium 2, Morris D, Gill M, Holmans P, Craddock N, Corvin A, Owen MJ, O’Donovan MC. 2013. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry. 18(6):708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Allyn-Feuer A, Athey BD. 2015. Epigenomic mapping and effect sizes of noncoding variants associated with psychotropic drug response. Pharmacogenomics. 16(14):1565–1583. [DOI] [PubMed] [Google Scholar]
- Ho NF, Li Z, Ji F, Wang M, Kuswanto CN, Sum MY, Tng HY, Sitoh YY, Sim K, Zhou J. 2017. Hemispheric lateralization abnormalities of the white matter microstructure in patients with schizophrenia and bipolar disorder. J Psychiatry Neurosci. 42(4):242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Zhang CL. 2014. TLX: A master regulator for neural stem cell maintenance and neurogenesis. Biochim Biophys Acta. 1849(2):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeglsperger T, Li S, Brenneis C, Saulnier JL, Mayo L, Carrier Y, Selkoe DJ, Weiner HL 2013. Impaired glutamate recycling and GluN2B-mediated neuronal calcium overload in mice lacking TGF-β1 in the CNS. Glia. 61(6):985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, McGhee KA, Leach S, Bonaguro R, Maclean A, Aguirre-Hernandez R, Abrahams BS, Coccaro EF, Hodgins S, Turecki G, Condon A, Muir WJ, Brooks-Wilson AR, Blackwood DH, Simpson EM. 2008. Initial association of NR2E1 with bipolar disorder and identification of candidate mutations in bipolar disorder, schizophrenia, and aggression through resequencing. Am J Med Genet B Neuropsychiatr Genet. 147B(6):880–889. [DOI] [PubMed] [Google Scholar]
- Li X, Thermenos HW, Wu Z, Momura Y, Wu K, Keshavan M, Seidman L, DeLisi LE. 2016. Abnormal interactions of verbal- and spatial-memory networks in young people at familial high-risk for schizophrenia. Schizophr Res. 176(2–3):100–105. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Lee DS, Trinh H, Tward D, Miller MI, Younes L, Barta PE, Ratnanather JT. 2015. Morphometry of the amygdala in schizophrenia and psychotic bipolar disorder. Schizophr Res. 164(1–3):199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. 2005. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 49(3):451–5. [DOI] [PubMed] [Google Scholar]
- Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, Cascella N, Keshavan M, Kates W, Buchanan R, Sharma T, Pearlson GD. 2008. A large scale (N=400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res. 101(1–3):95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno C, Saijoh Y, Fujii H, Ikeda M, Yokoyama T, Yokoyama M, Toyoda Y, Hamada H. 1996. Left–right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature, 381(6578):151–155 [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. 1998. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell 94(3):287–297. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 70(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. 2009. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 66(8):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M 1995. Epigenetic programming of differential gene expression in development and evolution. Dev Genet 17:188–197. [DOI] [PubMed] [Google Scholar]
- Mwansisya TE, Hu A, Li Y, Chen X, Wu G, Huang X, Lv D, Li Z, Liu C, Xue Z, Feng J, Liu Z. 2017. Task and resting-state fMRI studies in first-episode schizophrenia: A systematic review. Schizophr Res. 189:9–18. [DOI] [PubMed] [Google Scholar]
- Narayanaswamy JC, Kalmady SV, Venkatasubramanian G, Gangadhar BN. 2015. Clinical correlates of superior temporal gyrus volume abnormalities in antipsychotic-naïve schizophrenia. J Neuropsychiatry Clin Neurosci. 27(2):e128–133 [DOI] [PubMed] [Google Scholar]
- Nestor CE, Ottaviano R, Reddington J, Sproul D, Reinhardt D, Dunican D, Katz E, Dixon JM, Harrison DJ, Meehan RR. 2012. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome Res. 22(3):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Li E. 1998. Smad2 role in mesoderm formation, left–right patterning and craniofacial development. Nature 393(6687):786–790. [DOI] [PubMed] [Google Scholar]
- O’Loghlen A, Martin N, Krusche B, Pemberton H, Alonso MM, Chandler H, Brookes S, Parrinello S, Peters G, Gil J. 2015. The nuclear receptor NR2E1/TLX controls senescence. Oncogene. 34(31):4069–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó’Léime CS, Kozareva DA, Hoban AE, Long-Smith CM, Cryan JF, Nolan YM. 2018. TLX is an intrinsic regulator of the negative effects of IL-1β on proliferating hippocampal neural progenitor cells. FASEB J. 32(2):613–624. [DOI] [PubMed] [Google Scholar]
- Popendikyte V, Laurinavicius A, Paterson AD, Macciardi F, Kennedy JL, Petronis A. 1999. DNA methylation at the putative promoter region of the human dopamine D2 receptor gene. Neuroreport. 10(6):1249–1255. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Keshavan MS. 2008. Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct “extended endophenotypes”? Schizophr Bull. 34(4):774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. 2009. International Schizophrenia Consortium, Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 460(7256):748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. 2009. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 166(8):863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh B, Clementz BA, Pantev C, Blumenfeld LD, Sterr A, Elbert T. 1998. Failure of dominant left-hemispheric activation to right-ear stimulation in schizophrenia. Neuroreport. 9(17):3819–3822. [DOI] [PubMed] [Google Scholar]
- Schmouth JF, Arenillas D, Corso-Díaz X, Xie YY, Bohacec S, Banks KG, Bonaguro RJ, Wong SH, Jones SJ, Marra MA, Simpson EM, Wasserman WW. 2015. Combined serial analysis of gene expression and transcription factor binding site prediction identifies novel-candidate-target genes of Nr2e1 in neocortex development. BMC Genomics. 16:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, Noël E, Thurlings I, Rehmann H, Chocron S, Bakkers J. 2011. Bmp and nodal independently regulate lefty1 expression to maintain unilateral nodal activity during left-right axis specification in zebrafish. PLoS Genet. 7(9): e1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C. 2011. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 29(1):68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JA, Vemulapalli M, Nutche J, Montrose DM, Sweeney JA, Pettegrew JW, MacMaster FP, Keshavan MS. 2007. Reduced N-acetyl-aspartate levels in schizophrenia patients with a younger onset age: a single-voxel 1H spectroscopy study. Schizophr Res. 93(1–3):23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Yu RT, Evans RM, Campbell K. 2003. Tlx and Pax6 co-operate genetically to establish the pallio-subpallial boundary in the embryonic mouse telencephalon. Development. 130(6):1113–11122. [DOI] [PubMed] [Google Scholar]
- Sundararajan T, Manzardo AM, Butler MG. 2018. Functional analysis of schizophrenia genes using GeneAnalytics program and integrated databases. Gene. 30;641:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M The early life environment and the epigenome. 2009. Biochim Biophys Acta. 1790(9):878–885. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. 2008a. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naïve schizophrenia. Acta Psychiatr Scand. 117(6):420–431. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. 2008b. Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Res. 164(3):215–222. [DOI] [PubMed] [Google Scholar]
- Vonica A, Rosa A, Arduini BL, Brivanlou AH. 2011. APOBEC2, a selective inhibitor of TGFβ signaling, regulates left-right axis specification during early embryogenesis. Dev Biol. 350(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Wunderlich AP, Blankenhorn M, Schäfer S, Tomczak R, Spitzer M, Grön G. 2003. No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophr Res. 61(2–3):175–184. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jia Y, Feng Y, Zhong S, Xie Y, Wang W, Guan Y, Zhu D, Huang L. 2014. Overlapping auditory M100 and M200 abnormalities in schizophrenia and bipolar disorder: a MEG study. Schizophr Res 160(1–3):201–207. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. 2004. Brain activation patterns during a verbal fluency test-a functional MRI study in healthy volunteers and patients with schizophrenia. Schizophr Res. 70(2–3):287–291. [DOI] [PubMed] [Google Scholar]
- Williams LM, Whitford TJ, Gordon E, Gomes L, Brown KJ, Harris AW. 2009. Neural synchrony in patients with a first episode of schizophrenia: tracking relations with grey matter and symptom profile. J Psychiatry Neurosci. 34(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Y. 2011. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation.Genes Dev. 25(23):2436–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YQ, Freire-de-Lima CG, Schiemann WP, Bratton DL, Vandivier RW Henson PM. 2008. Transcriptional and translational regulation of TGF-β production in response to apoptotic cells. J Immunol. 181: 3575–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RT, McKeown M, Evans RM, Umesono K. 1994. Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature. 370: 375–379 [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Perez-Garcia CG, Wang CF, Chou SJ, O’Leary DD. 2015. Postmitotic regulation of sensory area patterning in the mammalian neocortex by Lhx2. Proc Natl Acad Sci U S A. 112(21):6736–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, Yu RT, Gage FH, Evans RM. 2006. Nuclear receptor TLX prevents retinal dystrophy and recruits the corepress oratrophin 1. Genes Dev. 20(10):1308–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Summary of linear modeling analyses. Columns A-D contain annotation for each gene, including the Brainarray (MBNI) probeset ID, symbol and description, and a link to the Entrez Gene record. Column E contains the percentage of samples in which each gene was called Present. Columns F-Q contain the t statistics, nominal p values, and FDR q values for the moderated t tests performed on each coefficient of the linear models. Columns R-V contain signed linear fold changes computed for each disease state versus control and for right versus left brain within each disease state (e.g., +2 = 2-fold higher in SCZ than in control; −2 = 2-fold lower in SCZ than in control). Columns W-AZ contain log2 (expression) across all samples, laid over a colored representation (heatmap), scaled so that red and blue indicate expression values ≥ 2 standard deviations above and below, respectively, the row-wise mean (white) computed across all samples. Expression values that are called Absent or Present are indicated in italicized or normal type, respectively. The rows are sorted in descending order by SCZ t statistic.
Additional File 2: Summary of Gene Set Enrichment Analysis (GSEA) results. Column A indicates the category of the gene set. Column B indicates the gene set name provided by MSigDB, represented as a link to the MSigDB “card”. Column C indicates the number of genes in the gene set that overlap with the genes interrogated by the microarray. Columns D-O indicate the NES (Normalized Enrichment Score), nominal p value, and FDR q value for each gene set and each ranked list. Positive and negative NES values indicate that the t statistics in each ranked list are skewed towards positive or negative values, respectively. Nominal p values equal to 0 indicate p< 0.001 (as computed from 1000 permutations). FDR q values less than 0.25 are indicated in bold italics.
Additional File 3: Summary of Illumina 27k measurements. Columns A-AF contain annotation obtained from Gene Expression Omnibus Platform record GPL8490. Columns AG-AQ contain the beta values computed for each probe in each sample as described in Methods.
Additional File 4: Correlation analysis performed between Illumina 27k (DNA methylation) and HG-U133 Plus 2.0 (RNA expression) analyses. Columns A-J contain annotation obtained from Gene Expression Omnibus Platform record GPL8490. Column K indicates the total number of 27k probes per gene. Columns L-M indicate the Spearman rho and p values computed for each gene between the DNA methylation and RNA expression values. Column N indicates the number of samples in which each gene was called Present by gene expression microarray. Columns O and P contain the p values computed for each gene for SCZ and BD(+), respectively, in the gene expression microarray analysis. Columns Q-AA and AB-AL contain the methylation beta values and log2 (expression) values, respectively. Each set of values is laid over a colored representation (heatmap), scaled so that red and blue indicate expression values ≥ 2 standard deviations above and below, respectively, the row-wise mean (white) computed across all samples within each platform. Expression values that are called Absent or Present are indicated in italicized or normal type, respectively. The rows are sorted in ascending order by Spearman rho value.
Supplementary Figure 1: Principal Component Analysis (PCA). PCA was performed using all genes interrogated by the array, with expression values z-normalized to a mean of zero and standard deviation of one across all samples. Light and dark colors indicate samples obtained from the left and right hemisphere, respectively, and squares and circles indicate samples obtained from females and males, respectively.
Supplementary Figure 2: Cell-type specific expression of TGFB2. Expression of TGFB2 was measured by qRT-PCR in neurons and astrocytes (n=4 per group) and normalized to beta-actin.
Supplementary Figure 3: Expression values are highly correlated between qPCR and microarray platforms. The relative expression of NR2E1 and TGFB2 is plotted against the anti-logged (linear-scale) expression values obtained from the HG-U133 Plus 2.0 microarray platform.
Supplementary Figure 4: Relative content of 5-hydroxymethylcytosine (5-hmC) across experimental groups. Total methylation and 5-hmC were measured at each TGFB2 site by qPCR as described in Methods, and the fraction of total signal attributable to 5-hmC is shown.