Abstract
Study Objective:
Previous studies have identified shifts in gut microbiota associated with atypical antipsychotic (AAP) treatment, which may link AAPs to metabolic burden. Dietary prebiotics such as resistant starch may be beneficial in obesity and glucose regulation, but little is known mechanistically about its ability to modify gut microbiota in AAP-treated individuals. This investigation was undertaken to mechanistically delineate the effects of AAP treatment and resistant starch supplementation on gut microbiota in a psychiatric population.
Design:
Cross-sectional cohort study.
Setting:
The study was performed in an outpatient setting.
Patients:
Thirty-seven adults with a diagnosis of bipolar disorder or schizophrenia who were treated with an AAP (clozapine, olanzapine, risperidone, quetiapine, or ziprasidone [21 patients]) or lithium and/or lamotrigine (16 patients) for at least 6 months.
Intervention:
Patients in the AAP group received raw unmodified potato starch (resistant starch) daily for 14 days.
Measurements and Main Results:
Of the 37 patients, the mean ± SD age was 52.2 ± 12.5 years, and 57% were male. The primary outcome was gut microbiome DNA composition. Microbiome DNA obtained from stool samples from all patients was subject to 16S rRNA gene sequencing before and during resistant starch supplementation. Inter- and Intragroup microbial diversity measures were performed by permutational multivariate analysis of variance and Inverse Simpson Diversity Index, respectively. Differentially abundant organisms were detected by using linear discriminant analysis effect size. Although no significant difference in overall microbiota composition was detected at baseline between AAP users and nonusers, non-AAP users showed increased fractional representation of Alistipes. AAP-treated females exhibited decreased diversity compared with non–AAP-treated females. Although the microbiome of AAP-treated patients varied with resistant starch administration, an increased abundance of the Actinobacteria phylum was observed.
Conclusion:
These data suggest that AAP treatment is associated with measurable differences in gut microbiota, particularly in female AAP-treated patients in whom reduced species richness was observed. Additionally, variable microbiome responses to resistant starch supplementation were seen, with a significant increase in starch degraders.
Keywords: Atypical Antipsychotic, Schizophrenia, Bipolar Disorder, Resistant Starch, Microbiome
Individuals with a serious mental illness experience a greater risk of cardiovascular disease (CVD) and metabolic-related morbidity and mortality1. Although atypical antipsychotics (AAPs) are often the first-line medication for these patients, this class of medication independently contributes to increased CVD and metabolic risk. A recent meta-analysis of 48 studies revealed that the risk of metabolic syndrome ranges from 10.2–47.2% depending on the patient population and specific antipsychotic studied2. Although AAPs may vary in their capacity to contribute to metabolic syndrome and CVD, studies show that virtually all AAPs are associated with weight gain3.
In addition, research shows a sex disparity in CVD and metabolic consequences of AAP treatment4. A comparison of Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) participants with the Third National Health and Nutrition Examination Survey (NHANES III) demonstrated sex-specific elevations in CVD risk (50% more likely in females vs 34% more likely in males)4–6 and metabolic syndrome (137% more likely in females vs 85% more likely in men)7 in those treated with an AAP compared with matched healthy controls. Development of CVD and metabolic abnormalities in AAP-treated patients is undeniably multifactorial, but the mechanisms underlying the increased CVD and metabolic disease burden in patients treated with AAPs, especially among women, is poorly understood.
AAP-associated weight gain is often linked to increased caloric intake, which also contributes to CVD and metabolic abnormalities8,9. However, increased dietary intake was not shown in our previous work comparing diets in AAP-treated schizophrenia patients and healthy controls using the NHANES database10.
The human gut is host to a great abundance of microbial organisms, called the gut microbiome, that perform a number of beneficial functions critical to maintaining health11. Recent work has also increased our appreciation of how disruption of commensal microbes is associated with the development of metabolic-related disease pathologies such as obesity and glucose dysfunction12,13. The gut microbiome plays an important role in drug metabolism, which can potentially explain some of the variability among individuals regarding treatment efficacy and adverse events14–16. Inversely, a substantial amount of nonantibiotic drugs has the potential to affect the composition and function of gut microbial communities, which may also contribute to the wide variability in individual treatment response17. The relationship between gut microbial communities and the development of adverse treatment events is particularly interesting given the ease with which the microbiome can be altered by factors such as dietary interventions14.
Recent studies have examined the inverse correlation between diets consisting of resistant starch and the occurrence of obesity and diabetes mellitus in the general population18,19. Nondigestible plant fibers, such as resistant starches, create an optimal environment for gut anaerobic microbes20,21, and consumption of dietary fiber has been studied as a preventive measure for the accumulation of fat mass22, feelings of satiety23, and increasing glucose control24. Resistant starch degradation by specific microbiota results in production of short-chain fatty acids (SCFAs). Butyrate (a SCFA) in particular is associated with preservation of the gut epithelial barrier and improvements in mood25.
Therefore, manipulating gut microbiota with prebiotics, such as resistant starch, may be an effective strategy to combat AAP-associated metabolic abnormalities. It was previously shown that resistant potato starch could increase fecal butyrate in healthy young adults26. However, little is known about the ability of prebiotics to modify gut microbiota in those taking AAPs or the tolerability of oral resistant starch in this population. Therefore, the aim of this study was to examine baseline microbiota differences between patients with a serious mental illness receiving an AAP and a psychiatric control population not treated with this class of medication. Additionally, for those receiving an AAP, we examined the effect of short-term resistant starch supplementation on gut microbiota composition and patient tolerability to taking resistant starch several times a day.
Methods
Study Design and Patient Population
This cross-sectional cohort study was approved by the University of Michigan Institutional Review Board and was not designated as a clinical trial, as the primary outcome was gut microbiome DNA composition. Patients were included in this study if they had a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnosis of Bipolar I, Bipolar II, or Bipolar not otherwise specified; Bipolar with Psychosis; Schizophrenia; or Schizoaffective Disorder; and were treated with an AAP—clozapine, olanzapine, risperidone, quetiapine, or ziprasidone— or lithium and/or lamotrigine for at least 6 months. Patients were excluded if they were unable to give informed consent. Other exclusion criteria were hospitalization or antimicrobial exposure within 6 months; receipt of systemic corticosteroids, immunosuppressant drugs, cytotoxic agents, or hormonal contraceptives; presence of any serious medical condition that would significantly affect weight changes; uncontrolled gastrointestinal disorders; chronic heavy alcohol consumption; or recent unstable dietary history. A complete list of inclusion and exclusion criteria can be found in Supplementary Table 1. Patients were split into groups according to AAP use (AAPs vs no AAPs), and detailed medical and medication histories were acquired at baseline after informed consent was obtained.
Resistant Starch Supplementation
After the baseline visit and assessments, patients receiving an AAP were asked to consume raw unmodified potato starch (Bob’s Red Mill, Milwaukie, OR) daily for 14 days. The packets consumed by patients contained approximately 50% resistant starch (type 2) by weight. This starch was gradually introduced into their normal diet as they were instructed to consume one packet (24 g) of Bob’s Red Mill Resistant Starch with a cold beverage for 3 days. On day 4, patients consumed one packet twice daily (48 g/day) and then continued taking this dosage for the remainder of the 14-day duration.
Patient Assessments
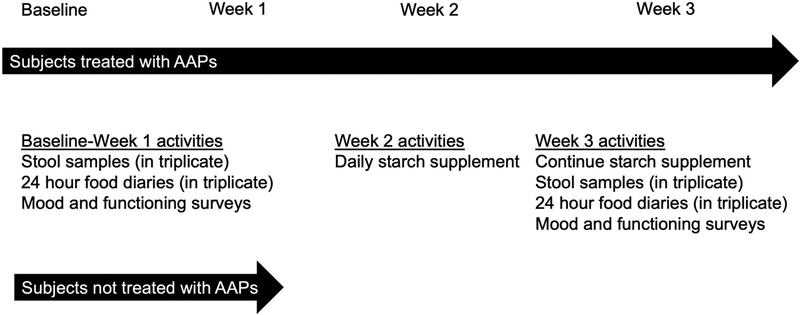
All patients underwent the following assessments at the baseline visit, with select assessments repeated after starch administration for patients receiving an AAP. A schematic of the study interventions is shown in Figure 1.
Figure 1.
Schematic of the study interventions.
Standard Study Assessments
Vital signs (height [cm], weight [kg], and blood pressure [mm Hg]) were obtained for each participant at baseline and after 14 days of resistant starch supplementation to assess tolerability. Current smoking status for each participant was also recorded as a yes or no variable.
Dietary Questionnaires
For the AAP-treated patients, 24-hour dietary recalls were administered at three independent time points during the baseline fecal collection (described below) and then repeated at three independent time points after at least 7 days of resistant starch administration. Data were analyzed using the Automated Self-Administered 24-Hour dietary assessment tool (National Cancer Institute, Bethesda, MD)27. Diet data were also converted to the Healthy Eating Index–2010 (HEI-2010) by the SAS code provided on the ASA24 website (https://epi.grants.cancer.gov/asa24/researcher/). The HEI-2010 measures diet quality and can be used to indicate adherence to dietary guidelines defined by the United States Department of Agriculture28. Mean nutritional variables for the three recalls at each fecal collection period were used for this analysis to account for daily variance in nutrient intake as significant changes in diet could potentially serve to confound microbiota composition.
Starch Tolerability Assessment
For the AAP-treated patients, research staff administered the 36-Item Short-Form Health Survey (SF-36) to all patients at baseline and after 14 days of resistant starch supplementation29,30. The SF-36 includes measures of mental and physical health based on the self-report to the questions and was used to gain a baseline assessment of the patient’s perception of health status before and after 14 days of resistant starch administration. Patients were also interviewed on the last day of starch supplementation to assess the breadth and severity of gastrointestinal-related adverse effects. Each adverse effect was rated as either mild, moderate, or severe. These assessments were added to determine tolerability related to the resistant starch administration.
Fecal Sample Collection and Storage
After the initial study visit, each participant was trained to collect fecal samples at their residence. Patients were provided with waxed tissue paper (Epitope Diagnostics, San Diego, CA), which was used as a barrier between the sample and the toilet water. Fecal samples were collected using the OMNIgene-GUT kit OMR-200 (DNA Genotek, Ontario, Canada). Samples were frozen immediately on collection by the patient and were transported on dry ice by the study team and stored at −80°C. Samples were collected in triplicate at baseline and then on days 8–14 following resistant starch supplementation in patients treated with an AAP.
Fecal Sample Processing and Sequencing
Total DNA was isolated from 300 μL of the fecal sample using the PowerMag soil DNA isolation kit (Mo Bio, Carlsbad, CA), optimized for Eppendorf’s epMotion liquid handling robot (Hauppauge, NY). The DNA libraries were prepared by the Microbiome Core at the University of Michigan. The bacterial V4 16S rRNA region was amplified and sequenced with a barcoded primer set using 250 base pair, paired end kits on an Illuminia MiSeq platform31. Mothur software was used to curate sequences as described previously32,33. In brief, assembled contigs were filtered for chimeric sequences using UCHIME34 and aligned to a mothur-adapted SILVA bacterial reference database. A 97% cutoff was used to bin sequences into operational taxonomic units (OTUs). The number of sequences per sample was rarified to 2500 to prevent bias from uneven sampling. To identify species of interest in butyrate production, a representative binned sequence was identified and blasted against the National Center for Biotechnology Information (NCBI) database. Species designations indicate 100% precision to identity a single species in the database. A complete list of commands for data processing, statistical analysis, and data presentation are available at: https://github.com/StephanieAFlowers/Ellingrod_AAP.git
Statistical Analysis
Microbiome 16S sequencing and patient data were analyzed by using a combination of the mothur v.1.39.5 (University of Michigan, Ann Arbor, MI) and R v3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) software programs. Demographic and microbial differences between treatment groups were determined with the use of standard t tests, χ2 tests, and Wilcoxon tests. We determined normality for each outcome, and the corresponding nonparametric test was performed for outcomes with a skewed distribution. Permutational multivariate analysis of variance (PERMANOVA) using Bray-Curtis similarity was used to compare microbial communities between medication groups35. For longitudinal analysis, each individual was used as the blocking factor to account for repeated measures of individual microbiota composition. Permutational analysis of dispersions (PERMDISP) and PERMANOVA were all performed by using the vegan package in R36. Deferentially abundant OTUs between groups were identified by using linear discriminant analysis effect size (LEfSe) analysis that was calculated by using mothur software37. Mothur was also used to calculate alpha diversity, as measured using an Inverse Simpson Diversity Index estimate. Linear regressions comparing diversity estimates with medication and sex cohorts were adjusted for diagnosis and concomitant medications for hyperlipidemia.
Results
Patient Population
Thirty-seven patients were recruited for this investigation. The majority were Caucasian (81%), college educated, and obese (body mass index [BMI] >30 kg/m2), with a mean age of 52 years (Table 1). As previously stated, two general medication groups were formed (AAP vs non-AAP). Within these groups, significant differences were found with regard to represented diagnoses (p=0.02), with the AAP group showing higher Schizoaffective and Schizophrenia inclusion. No significant group differences were noted with regard to sex, BMI, and baseline nutrition parameters. Despite similar intergroup BMIs, those treated with AAPs experienced a higher frequency of concomitant treatment for hypertension (although not statistically significant; p=0.051) and hyperlipidemia (p=0.02). Patients not treated with AAPs were more likely to also take an antidepressant, although this observation was not statistically significant (p=0.051).
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Patients
| Characteristic | Atypical Antipsychotic–Treated Group (n=21) | Lithium- or Lamotrigine-Treated Group (n=16) | p Value |
|---|---|---|---|
| Sex | |||
| Male | 12 (57) | 9 (56) | 0.96 |
| Female | 9 (43) | 7 (44) | |
| Race | |||
| Caucasian | 16 (76) | 14 (88) | 0.35 |
| Age (yrs) | 54 ± 10 | 50 ± 15 | 0.31 |
| Education (yrs) | 15 ± 2.2 | 17 ± 3.4 | 0.08 |
| Diagnosis | |||
| Bipolar I | 9 (43) | 10 (63) | 0.02 |
| Bipolar II | 3 (14) | 6 (37) | |
| Schizoaffective Disorder | 5 (24) | 0 (0) | |
| Schizophrenia | 4 (19) | 0 (0) | |
| No. of Psychiatric Hospitalizations |
6 ± 8.1 | 3 ± 3.5 | 0.15 |
| Anthropometric Measurements | |||
| Body Mass Index (kg/m2) | 30.6 | 31.1 | 0.7 |
| Waist:Hip ratio | 0.98 | 1 | 0.25 |
| Metabolic syndrome | 8 (38) | 3 (19) | 0.2 |
| Activity Scorea | 4891 | 9774 | 0.28 |
| Smoker | 5 (24) | 5 (31) | 0.89 |
| Concomitant Medications | |||
| Antidepressants | 9 (43) | 12 (75) | 0.051 |
| Benzodiazepines | 5 (24) | 4 (25) | 0.9 |
| Medication for Hypertension | 12 (57) | 4 (25) | 0.051 |
| Medication for Diabetes Mellitus | 2 (10) | 0 (0) | 0.2 |
| Medication for Hyperlipidemia | 10 (48) | 2 (13) | 0.02 |
| Nutrition | |||
| Total (Kcal/day) | 2183 | 2181 | 0.99 |
| Protein (g/day) | 89 | 85 | 0.77 |
| Fat (g/day) | 92 | 83 | 0.41 |
| Carbohydrates (g/day) | 254 | 254 | 0.99 |
| Fiber (g/day) | 20 | 18 | 0.6 |
| HEI-2010c | 56 | 50 | 0.26 |
Data are no. (%) of patients, mean ± SD values, or mean values.
Activity Score = Global Physical Activity Questionnaire.
Atypical antipsychotics = clozapine, olanzapine, risperidone, quetiapine, ziprasidone.
Healthy Eating Index–2010
Gut Bacterial Communities between Medication Groups
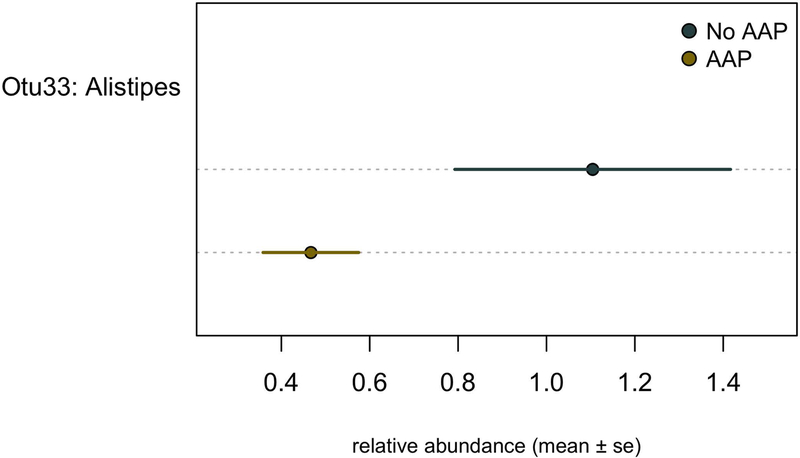
The average microbial community differences from three separate fecal samples collected over a period of a week were not statistically significant between medication groups, as calculated by using PERMANOVA (data not shown). Our LEfSe analysis revealed one differentially represented OTU between groups that was among the top 40 most abundant taxonomic units (Figure 2; linear discriminant analysis [LDA] log score = 3.4; p=0.03). OTU33 classified to Alistipes and was preferentially increased in patients not treated with AAPs.
Figure 2.
Differentially abundant members of gut microbiota in atypical antipsychotic (AAP)-treated with patients (blue) and non–AAP-treated patients (yellow). The mean relative abundance ± standard error (se) of differentially abundant operational taxonomic units (Otus) was identified by linear discriminant analysis effect size analysis.
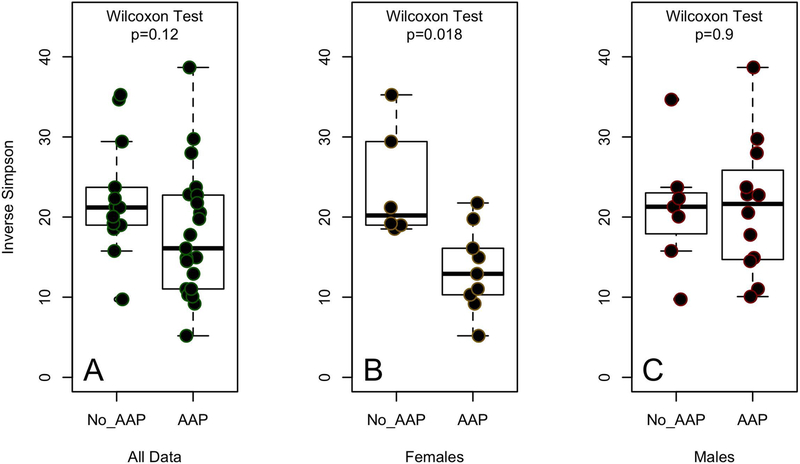
Additionally, there were no significant differences in microbial community diversity as measured by the Inverse Simpson Diversity Index between medication groups (Figure 3A; p=0.12). However, we previously observed a sex-specific difference in community species diversity in AAP-treated patients33. Therefore, when stratified by sex, APP-treatment was associated with decreased diversity in females (Figure 3B; p=0.018). No significant difference in diversity was found between medication groups of male patients (Figure 3C; p=0.9). Differences in microbiota diversity between the female medication cohorts remained significant after adjusting for diagnosis and treatment for hyperlipidemia (p=0.04, R2=0.40).
Figure 3.
Fecal microbiota diversity as measured by the Inverse Simpson diversity index in atypical antipsychotic (AAP)-treated and non–AAP-treated patients with bipolar disease: (A) comparison of diversity between AAP- and non–AAP-treated patients of both sexes (p=0.12, Wilcoxon test); (B) comparison of diversity between AAP- and non–AAP-treated female patients (p=0.018, Wilcoxon test); and (C) comparison of diversity between AAP- and non–AAP-treated male patients (p=0.9, Wilcoxon).
Resistant Potato Starch Supplementation and Clinical Measures
Nineteen of the 21 AAP-treated patients completed the 14-day administration of titrated dietary starch (Table 2). Two patients voluntarily dropped out of the study prior to starch supplementation. Otherwise, the starch was generally well tolerated, with moderate flatulence being the most common adverse effect reported by patients. Dietary habits and weight did not significantly change during the course of the intervention and, therefore, were not included as confounders in the final analysis. The tolerability analysis from baseline generally showed no change in mood but did measure improvement in the emotional well-being domain of the SF-36 (p=0.03).
Table 2.
Tolerability Measures and Dietary Changes before and during Potato Starch Administration for 14 Days (n=19)a
| Before Starch Administration | During Starch Administration | p Value | |
|---|---|---|---|
| Weight (kg) | 91.8 | 91.9 | 0.9 |
| SF-36 scores | |||
| Physical Functioning | 845 | 829 | 0.46 |
| Physical Health | 316 | 337 | 0.30 |
| Emotional Problems | 195 | 226 | 0.27 |
| Energy/Fatigue | 217 | 248 | 0.07 |
| Emotional Well-Being | 327 | 376 | 0.03 |
| Social Functioning | 159 | 172 | 0.38 |
| Pain | 161 | 176 | 0.12 |
| General Health | 286 | 312 | 0.16 |
| Nutrition | |||
| Total (Kcal/day)) | 2245 | 2035 | 0.32 |
| Protein (g/day) | 91 | 79 | 0.37 |
| Fat (g/day) | 97 | 84 | 0.27 |
| Carbohydrates (g/day) | 257 | 249 | 0.7 |
| Fiber (g/day) | 19 | 19 | 0.9 |
| HEI-2010 | 54 | 54.9 | 0.71 |
| Phylum Relative abundance | |||
| Actinobacteria | 1.27 ± 1.1 | 4.42 ± 5.9 | 0.03 |
| Bacteroidetes | 46.3 ± 16.9 | 43.5 ± 4.4 | 0.44 |
| Firmicutes | 44.6 ± 15 | 44.3 ± 14.9 | 0.92 |
| Proteobacteria | 4.9 ± 2.8 | 4.5 ± 3.9 | 0.50 |
Data are mean or mean ± SD values.
SF-36 = 36-Item Short Form Health Survey; HEI-2010 = Healthy Eating Index–2010.
19 of the 21 atypical antipsychotic–treated patients completed the 14-day administration of titrated dietary starch.
Starch-Induced Changes in Gut Microbiota of Atypical Antipsychotic Users
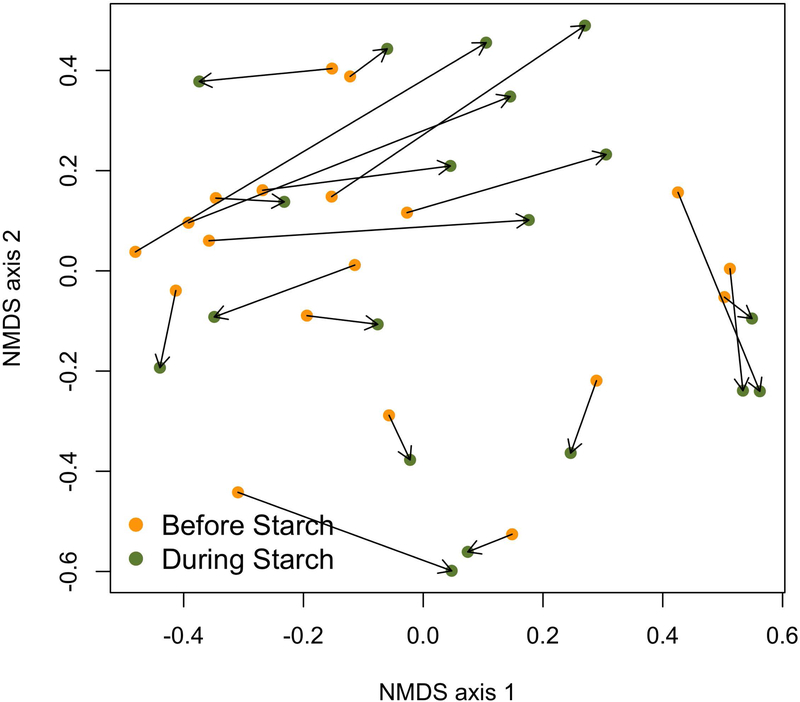
When assessing the impact of resistant starch on overall microbiota changes, we found that in the overall community-wide analysis, calculated by PERMANOVA and using each individual as a blocking factor to account for intraindividual variability (Figure 4; p=0.001), there were changes in overall microbiota composition in response to resistant starch administration. However, a PERMDISP analysis revealed substantial variance in the dispersion of communities (p=0.04). Specifically, the relative abundance of OTUs of the Actinobacteria phylum increased with resistant starch administration (p=0.03; Table 2), whereas the OTUs within the Firmicutes, Bacteriodetes, and Proteobacteria phyla remained stable during resistant starch administration. Resistant starch supplementation also resulted in a decrease in the fractional representation of OTU1 (LDA value = 4.48, p=0.023), which classified as Bacteroides, and OTU11 (LDA value = 4.16, p=0.032), which classified as Parabacteroides as detected by LEfSe analysis. Alpha diversity as measured by Inverse Simpson Diversity Index was not significant (p>0.05).
Figure 4.
Gut community structure in atypical antipsychotic (AAP)-treated patients before and during 14 days of resistant starch supplementation. Non-metric multidimensional scaling (NMDS) was used to plot the Bray and Curtis measured dissimilarity index.
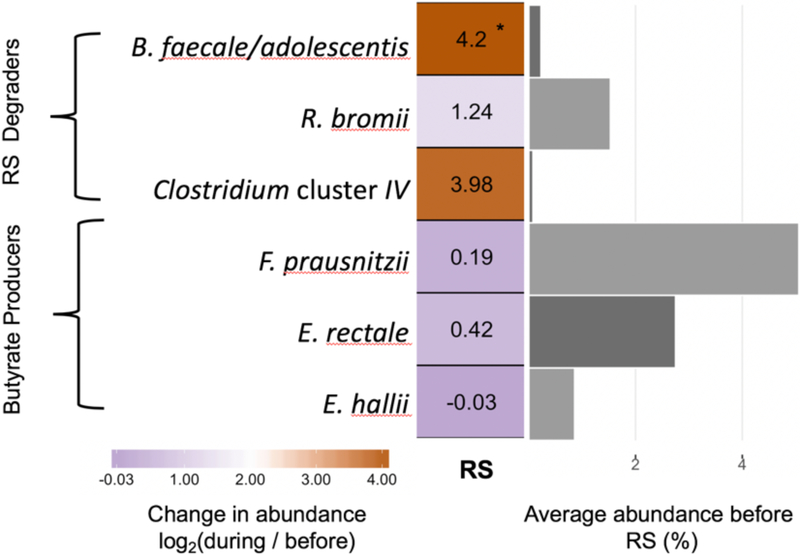
Several species of bifidobacteria and Ruminococcus bromii are known as resistant starch degraders and were expected to increase in response to the resistant starch supplements38,39. Specifically, we saw a 4.2-fold increase in the OTU corresponding to resistant starch–degrading species Bifidobacterium faecale and B. adolescentis (p<0.003, Wilcoxon Rank Sum Test), but no statistically significant change to any of the other bifidobacteria was detected for the group (Figure 5). The OTU corresponding to Ruminococcus bromii increased in a subset of individuals, but this was not significant when considering the group as whole (p=0.09). In addition to these organisms, an OTU classified as Clostridium cluster IV (OTU37), which was only initially detected in 3 of the 19 patients, demonstrated an average 3.98-fold increase and exceeded >5% relative abundance in these individuals during resistant starch supplementation. Among the most abundant butyrate-producing species detected in our population—Faecalibacterium prausnitzii, Eubacterium rectale, and E. hallii—there were no significant increases in our population (Figure 5).
Figure 5.
Heatmap showing the fold change in relative abundance of species of interest in response to resistant starch (RS) supplementation. To the right, the bar plot shows the average relative abundance of each species prior to fiber supplementation. *p<0.05, paired Wilcoxon test.
Discussion
In this investigation, we identified that non–AAP-treated patients with a serious mental illness exhibited a fractional increased abundance of OTU33, classified to Alistipes. Additionally, we observed that AAP-treated female patients exhibited less microbial diversity than those not treated with AAPs. After 14 days of resistant starch administration, an increase in organisms in the Actinobacteria phylum was detected. Resistant starch was fairly well tolerated during the 14-day administration.
Increased dietary intake and appetite have often been cited as playing a defining role in weight gain among AAP-treated patients8,40. Differences in the amount or quality of diet did not significantly differ between our medication groups; therefore, relating metabolic complications to dietary intake in AAP-treated patients fails to fully understand this relationship. The addition of an AAP to pharmacotherapy may also indicate increased seriousness of disease in bipolar disorder, which may independently affect gut microbial structure41. To account for this potential variable to the best of our abilities, we matched our medication cohorts for previous hospitalizations and other metabolic comorbidities.
As our current study identified that OTU33, Alistipes, was preferentially increased in patients not treated with AAPs, it is important to note that the Alistipes bacteria are within the Bacteroidetes phylum and are inversely associated with obesity and an animal-rich diet13,42. Alistipes have also shown to be decreased in patients with inflammatory bowel disorders42,43. Interestingly, our previous investigation in an independent bipolar cohort also showed Alistipes to drive AAP-associated separation in gut microbial communities33. Maier and colleagues have recently screened over 1,000 marketed drugs against the top 40 representative gut bacterial strains and observed that 24% of the human target drugs inhibit the growth of at least one strain in vitro17. As part of this investigation, the overall medication class of antipsychotics was noted by the authors as exhibiting significant antimicrobial activity. Interestingly, despite the structural dissimilarity of the antipsychotic class of medications, these drugs as a class showed more similar patterns of growth inhibition against specific commensal microbes than was expected from their chemical dissimilarity. Collectively, these results suggest that antipsychotic-associated inhibition of commensal bacteria might be important for the effectiveness or potentially adverse effects associated with these medications. Our cohort exhibited significant differences in diagnosis and concomitant use of medication for hypertension, which is a weakness to this study.
Our previous work has also documented significant decreased species diversity in the female AAP-treated cohort33, an observation replicated in this independent population. No significant differences were observed in alpha diversity between the male medication cohorts in either study. Gut species diversity is decreased in many disease states such as obesity and diabetes44; therefore, increased species diversity is often associated with gut health. A study investigating the effects of risperidone on microbiome structure exclusively in males documented an increase in species diversity45. These results highlight the importance in considering sex as a variable in future studies investigating interactions between gut microbiota and AAPs.
There is a strong interest in optimizing gut microbiota through dietary manipulation in an effort to improve human health. This, however, was not the purpose of our mechanistic study. As there is little information on the success of implementing an intervention of resistant starch supplementation on an outpatient basis in those with a serious mental illness, the willingness of our patients to adhere to resistant starch for 14 days was unknown. Like previous work investigating prebiotic supplementation in human populations26,46, we observed a variable resistant starch response in microbial communities. On a population level, only increases in B. faecale and B.adolescentis were significant in response to resistant starch. However, when we considered the individual responses to resistant starch, we observed that a subset of the cohort exhibited increases in R. bromii, and three individuals showed fractional increases in an OTU from Clostridium cluster IV. It is not known how our observed variation in resistant starch response translates into functional differences in SCFA production. In the future, quantifying fecal SCFAs and other microbial-derived metabolites will give us critical insight into how AAP-specific changes in microbiome composition affect microbiome output and, ultimately, host phenotype.
Conclusion
These data support our hypothesis that AAP treatment results in measurable differences in gut microbiota composition in a well-characterized clinical population. Additionally, we were also able to demonstrate that daily resistant starch supplementation for 14 days was tolerable by this patient population and showed measurable effects on their gut microbiome, including increased organisms associated with starch degradation and SCFA production. These changes can provide mechanistic insights into the effect of AAP use and the potential impact of resistant starch.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01MH082784) and the Michigan Institute for Clinical and Health Research (UL1TR002240). The Heinz C. Prechter Bipolar Research Program and the Richard Tam Foundation also supported this research.
Footnotes
Availability of Data and Materials
Sequences and metadata can be found through the NCBI short Read Archive under accession number PRJNA509882. Other data files can be found at the Git hub repository of Stephanie Flowers: https://github.com/StephanieAFlowers/Ellingrod_AAP.git
Conflict of interest statement
The authors declare no competing interests.
References
- 1.Colton CW & Manderscheid RW Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev. Chronic. Dis 3, A42 (2006). [PMC free article] [PubMed] [Google Scholar]
- 2.Vancampfort D et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 14, 339–347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bak M, Fransen A, Janssen J, van Os J & Drukker M Almost All Antipsychotics Result in Weight Gain: A Meta-Analysis. PLoS ONE 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEvoy JP et al. Prevalence of the metabolic syndrome in patients with schizophrenia: Baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr. Res 80, 19–32 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Daumit GL et al. Antipsychotic effects on estimated 10 year coronary heart disease risk in the CATIE Schizophrenia Study. Schizophr. Res 105, 175–187 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goff DC et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr. Res 80, 45–53 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Meyer JM et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: Prospective data from phase 1. Schizophr. Res 101, 273–286 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RC, Rachakonda S, Dwivedi S & Davis JM Olanzapine and risperidone effects on appetite and ghrelin in chronic schizophrenic patients. Psychiatry Res. 199, 159–163 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Teff KL et al. Antipsychotic-Induced Insulin Resistance and Postprandial Hormonal Dysregulation Independent of Weight Gain or Psychiatric Disease. Diabetes 62, 3232–3240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bly MJ et al. Metabolic Syndrome In Bipolar Disorder and Schizophrenia: Dietary and Lifestyle Factors Compared to the General Population. Bipolar Disord. 16, 277–288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flowers SA & Ellingrod VL The Microbiome in Mental Health: Potential Contribution of Gut Microbiota in Disease and Pharmacotherapy Management. Pharmacotherapy 35, 910–916 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Gu Y et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun 8, 1785 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke SF et al. Targeting the Microbiota to Address Diet-Induced Obesity: A Time Dependent Challenge. PLOS ONE 8, e65790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmody RN & Turnbaugh PJ Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J. Clin. Invest 124, 4173–4181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Björkholm B et al. Intestinal Microbiota Regulate Xenobiotic Metabolism in the Liver. PLoS ONE 4, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enright EF, Griffin BT, Gahan CGM & Joyce SA Microbiome-mediated bile acid modification: Role in intestinal drug absorption and metabolism. Pharmacol. Res 133, 170–186 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Maier L et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston KL, Thomas EL, Bell JD, Frost GS & Robertson MD Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet. Med 27, 391–397 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Higgins JA et al. Resistant starch consumption promotes lipid oxidation. Nutr. Metab 1, 8 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremaroli V & Bäckhed F Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Martínez I, Kim J, Duffy PR, Schlegel VL & Walter J Resistant Starches Types 2 and 4 Have Differential Effects on the Composition of the Fecal Microbiota in Human Subjects. PLOS ONE 5, e15046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbara Grube, Pee-Win Chong, Kai-Zhia Lau & Hans-Dieter Orzechowski. A natural fiber complex reduces body weight in the overweight and obese: A double-blind, randomized, placebo-controlled study. Obesity 21, 58–64 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark MJ & Slavin JL The effect of fiber on satiety and food intake: a systematic review. J. Am. Coll. Nutr 32, 200–211 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Silva FM et al. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr. Rev 71, 790–801 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Sun J et al. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci. Lett 618, 159–166 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Venkataraman A et al. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 4, 33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subar AF et al. The Automated Self-Administered 24-Hour Dietary Recall (ASA24): A Resource for Researchers, Clinicians and Educators from the National Cancer Institute. J. Acad. Nutr. Diet 112, 1134–1137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guenther PM et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J. Nutr 144, 399–407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellingrod VL et al. Dietary, Lifestyle and Pharmacogenetic Factors Associated with Arteriole Endothelial Dependent Vasodilatation In Schizophrenia Patients Treated with Atypical Antipsychotics (AAPs). Schizophr. Res 130, 20–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE & Sherbourne CD The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483 (1992). [PubMed] [Google Scholar]
- 31.Kozich JJ, Westcott SL, Baxter NT, Highlander SK & Schloss PD Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol AEM.01043–13 (2013). doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schloss PD et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol 75, 7537–7541 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flowers SA, Evans SJ, Ward KM, McInnis MG & Ellingrod VL Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy 37, 261–267 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Edgar RC, Haas BJ, Clemente JC, Quince C & Knight R UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beals EW Bray-Curtis Ordination: An Effective Strategy for Analysis of Multivariate Ecological Data. Adv. Ecol. Res 14, 1–55 (1984). [Google Scholar]
- 36.Anderson Marti J Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online (2014). doi: 10.1002/9781118445112.stat07841 [DOI] [Google Scholar]
- 37.Segata N et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ze X, Duncan SH, Louis P & Flint HJ Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 6, 1535–1543 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flint HJ, Scott KP, Duncan SH, Louis P & Forano E Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3, 289–306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroeze WK et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 28, 519–526 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Evans SJ et al. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res 87, 23–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdam FJ et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obes. Silver Spring Md 21, E607–615 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Saulnier DM et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 141, 1782–1791 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK & Knight R Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahr SM et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl. Psychiatry 5, e652 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maier TV et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 8, e01343–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.