Abstract
Risk factors for adverse events after total knee arthroplasty (TKA) relating to malignancy have not been well studied. Thus, the purpose of this study was to conduct a retrospective case–control outcome and cost analysis after TKA in this population. Patients with a history of breast cancer (BrCa) were identified based on the International Classification of Disease 9th revision codes. An age- and sex-matched cohort was also identified of patients without a history of BrCa. Complications, length of stay, comorbidity burden, and reimbursements were tracked at 90 days. Each cohort comprised 92,557 patients. Length of stay was similar between cohorts (p = 0.627). Comorbidity status and incidence of pulmonary embolism (PE), lower extremity ultrasound, and chest computed tomography (CT) use were higher in patients with a history of BrCa (p< 0.05 for all). Control patients had a lower incidence of acute myocardial infarction (0.14 vs. 0.21%; p< 0.001). Surgical complications were similar. The 90-day reimbursements were greater in patients with a history of BrCa (US $13,990 vs. US$13,033 for controls; p = 0.021). Surgeons should be aware of the increased risk of PE after TKA in patients with a history of BrCa as well as increased 90-day costs, which warrant great attention.
Keywords: total knee arthroplasty, cancer, breast, outcomes, cost
Total knee arthroplasty (TKA) is one of the most common orthopaedic procedures performed in the United States.1–3 TKA has demonstrated significant benefits to patients by decreasing pain, improving function, and allowing return to work and sports.4 Adverse postoperative events, however, can have tremendous detrimental health effects.5 Recent research efforts have been aimed to evaluate risk factors for poor outcomes. A particular population of interest is patients with a previous history of malignancy.
Recent literature has highlighted the relationship between cancer and its effects on postoperative outcomes.6 Epidemiological research has demonstrated that the number of patients living with cancer is expected to increase in the coming years due to the improving efficacy of anticancer therapies and management options.7 This increased survivorship translates into an enlarging population of aging patients with a history of cancer who develop knee osteoarthritis.8 Deviations from normal physiology during and after the treatment for malignancy in this patient population are well-noted.9,10 These changes lead to an increased risk of thromboembolic events such as deep vein thrombosis (DVT), pulmonary embolism (PE), and changes in bone quality after radiation therapy and/or antineoplastic management,11–14 potentially putting these patients at increased risk of poor outcomes after major joint reconstructions.
Breast cancer is the most common form of cancer in the United States.15 The American Cancer Society estimated that in 2017, more than 250,000 new cases of breast cancer would develop.16 Furthermore, the mortality of this disease has been declining at a mean of 1.8% per year with a 5-year survival rate of 91.3%, highlighting the fact that most patients survive.15 With such a great survival rate, it is expected that these patients will suffer age-related diseases as they become older. Given the magnitude of patients currently living with a history of breast cancer, we aimed at studying this question in its relationship to TKA. Thus, the purpose of this study was to evaluate outcomes of patients with a previous history of breast cancer who underwent TKA. We hypothesized that these patients would have similar outcomes to age-matched controls, as tumor control is greatly successful.
Materials and Methods
This was a retrospective, matched case–control study using data extracted from a comprehensive Medicare database (PearlDiver Supercomputer, Warsaw, IN). This server holds the entire Medicare Standard Analytical Files and allows researchers to query the data through the International Classification of Disease (ICD) 9th revision codes as well as Current Procedural Terminology codes. It is a commercially and publicly available database that is compliant with the Health Insurance Portability and Accountability Act. The ICD codes used were similar to those used in previous literature.17–20 The query was performed to include patients who underwent TKA from 2005 to 2014. Following initial identification of patients who underwent TKA, patients with a previous history of breast cancer were identified. A random cohort of patients without a previous history of breast cancer who underwent TKA was also extracted to serve as the control. A 1:1 matching was then performed to ensure that the control group had the same age and sex distributions as the group of breast cancer patients to diminish the potential of confounding based on demographical variables. Of note, this type of coding allowed us to identify only patients with a history of the disease, and given the standard billing coding practices, patients on any medication for active breast cancer are not coded as having a history and therefore no confounding with patients with active disease occurs.
The outcomes of interest in this study were medical and surgical complications within the 90-day global period after surgery and length of stay (LOS).21 Costs, as reflected by reimbursements, were also tracked and analyzed based on the day of surgery (DOS) and 90-day episode of care reimbursements. The 90-day postoperative period was the period of interest evaluated as it represents the most common pay period used in the Comprehensive Care for Joint Reconstruction payment model. The Charlson Comorbidity Index (CCI) score was also extracted and compared for both groups.
Statistical Analysis
Data analysis was performed on SPSS Software Version 20 (SPSS Inc., Armonk, NY) using Student’s t-test for parametric data and Mann–Whitney U test for nonparametric data. The Kolmogorov–Smirnov test was used to assess normality. Chisquare analysis was also used for categorical variables. Odds ratios (OR) with the respective 95% confidence intervals (CIs) were reported. An α value of <0.05 was deemed as significant. For the CCI analysis, a weighted score was obtained from each cohort of patients.
Results
Our initial query identified a total of 2,369,594 TKAs from 2005 to 2014. After match-pairing, 92,557 patients were included in the cohort of patients with a history of breast cancer and in the cohort of patients without such a history. Table 1 demonstrates the demographic characteristics of the studied patients.
Table 1.
Demographical characteristics of both cohorts after matching
| 64 and under | 4,625 | 5% |
| 65–69 | 22,797 | 24.6% |
| 70–74 | 23,572 | 25.5% |
| 75–79 | 21,299 | 23% |
| 80–84 | 13,735 | 14.8% |
| 85 and over | 5,459 | 5.9% |
| Unknown | 1,070 | 1.2% |
| Total | 92,557 | |
| Female | 90,913 | 98.2% |
| Males | 574 | 0.6% |
| Unknown | 1,070 | 1.2% |
| Total | 92,557 |
Medical Complications
Patients with a previous history of breast cancer had a higher incidence of PE (0.65 vs. 0.56%; OR: 1.147; 95% CI: 1.019– 1.290) (Table 2). This was associated with a greater use of diagnostic ultrasound (0.22 vs. 0.17%; OR 1.274; 95% CI: 1.034–1.571) and chest computed tomography (CT) (1.67 vs. 1.36%; OR: 1.233; 95% CI: 1.144–1.329). Interestingly, patients without a history of breast cancer demonstrated significantly higher incidences of acute myocardial infarction (AMI), pneumonia, and intubation (p < 0.05 for all).
Table 2.
Medical complication rates and comparison
| Medical complications | ICD code | Breast cancer history (%) | No breast cancer history (%) | Odds ratio | 95% confidence interval | P-Value |
|---|---|---|---|---|---|---|
| Acute postoperative anemia | 285.1 | 2.60 | 2.50 | 1.04 | 0.982–1.102 | 0.184 |
| Pulmonary embolism | 415.1 | 0.65 | 0.56 | 1.147 | 1.019–1.290 | 0.023 |
| Acute myocardial infarction | 410 | 0.14 | 0.21 | 0.657 | 0.524–0.824 | <0.001 |
| Deep venous thrombosis | 453.4 | 1.24 | 1.20 | 1.027 | 0.945–1.1 16 | 0.525 |
| Pneumonia | 480–486 | 0.46 | 0.54 | 0.852 | 0.749–0.970 | 0.015 |
| Pulmonary insufficiency | 518.5 | 0.04 | 0.04 | 1.088 | 0.683–1.734 | 0.722 |
| Postoperative bleeding | 998.11 998.12 | 0.23 | 0.22 | 1.049 | 0.866–1.272 | 0.624 |
| Cardiac complication | 997.1 | 0.06 | 0.06 | 1.076 | 0.740–1.563 | 0.703 |
| Peripheral vascular complication | 997.2 | 0.07 | 0.07 | 1 | 0.709–1.410 | 1 |
| Urinary complication | 997.5 | 0.03 | 0.03 | 1.192 | 0.708–2 | 0.508 |
| Transfusion of blood | 99.X | 1.17 | 1.18 | 0.988 | 0.908–1.075 | 0.779 |
| Diagnostic ultrasound of peripheral vascular system | 88,77 | 0.22 | 0.17 | 1.274 | 1.034–1.571 | 0.023 |
| Chest computed tomography | 71,250, 71,260, 71,270, 71,275 | 1.67 | 1.36 | 1.233 | 1.144–1.329 | <0.001 |
Abbreviation: ICD, International Classification of Disease.
Note: Statistically significant differences are presented in bold.
Surgical Complications
The majority of reported surgical complications occurred at approximately the same rate in both groups (Table 3). Prosthetic joint dislocations were more common in patients with a history of breast cancer (OR: 1.522; 95% CI: 1.049– 2.208). The incidence of nonspecific osteomyelitis was greater in the control group, but the 90-day incidence of this complication was less than 1% in both groups.
Table 3.
Surgical complication rates and comparison
| Surgical complications | ICD code | Breast cancer (%) | No cancer (%) | Odds ratio | 95% confidence interval | p-Value |
|---|---|---|---|---|---|---|
| Osteomyelitis | 730 | 0.02 | 0.04 | 0.424 | 0.227–0.793 | 0.006 |
| Mechanical complication of the orthopaedic device | 996.4 | 0.30 | 0.29 | 1.042 | 0.880–1.234 | 0.635 |
| Unspecified mechanical complication of internal orthopaedic device, implant, and graft | 996.40 | 0.03 | 0.04 | 0.889 | 0.552–1.431 | 0.628 |
| Mechanical loosening of the prosthetic joint | 996.41 | 0.02 | 0.02 | 0.636 | 0.326–1.244 | 0.182 |
| Dislocation of the prosthetic joint | 996.42 | 0.08 | 0.05 | 1.522 | 1.049–2.208 | 0.026 |
| Broken prosthetic joint implant | 996.43 | 0.01 | 0.0 | 0.706 | 0.337–1.478 | 0.353 |
| Periprosthetic fracture around the prosthetic joint | 996.44 | 0.06 | 0.05 | 1.17 | 0.793–1.728 | 0.428 |
| Other mechanical complication of prosthetic joint implant | 996.47 | 0.07 | 0.06 | 1.123 | 0.786–1.605 | 0.524 |
| Other mechanical complication of other internal orthopaedic device, implant, and graft | 996.49 | 0.03 | 0.03 | 1.167 | 0.676–2.013 | 0.579 |
| Infection of orthopaedic device | 996.66 | 0.32 | 0.32 | 1.01 | 0.860–1.186 | 0.902 |
Abbreviation: ICD, International Classification of Disease.
Note: Statistically significant differences are presented in bold.
Length-of-Stay Analysis
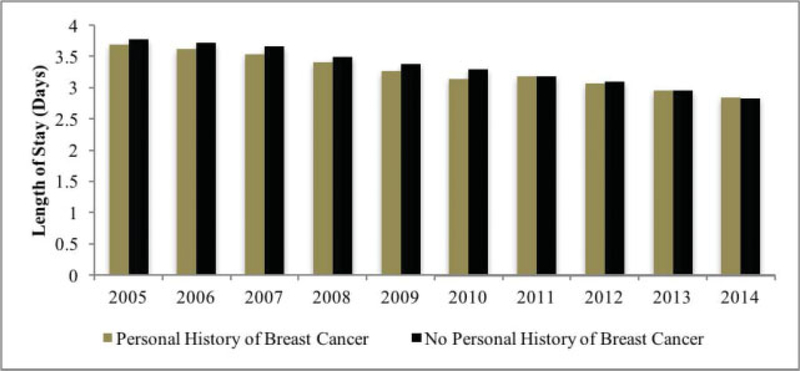
Patients with a personal history of breast cancer had a mean LOS of 3.269 ± 0.287 days, which was not statistically different compared with the controlgroup (3.375 0.325 days; p = 0.627) (Fig. 1).
Fig. 1.
Length of stay over time.
Charlson Comorbidity Analysis
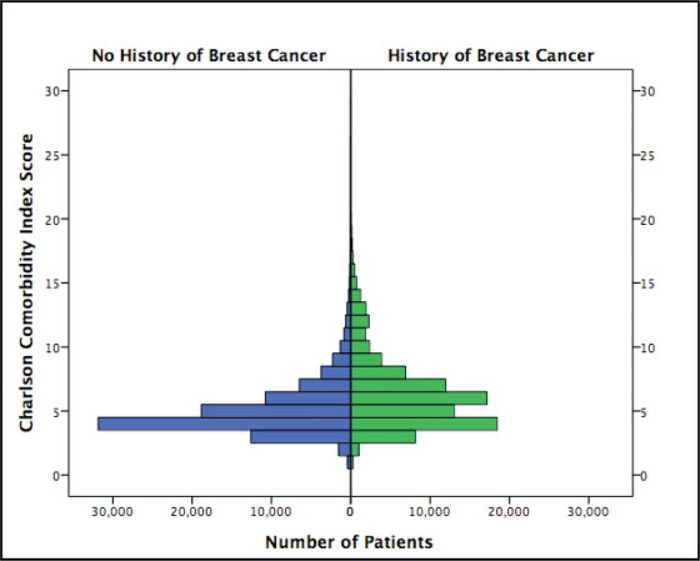
At the time of the operation, both cohorts were found to have nonnormally distributed CCI scores (p < 0.001 for both). The median CCI score was 6 ± 2.97 for patients with a history of breast cancer and 4 ± 2.14 for those without a breast cancer history (p < 0.001) (Fig. 2).
Fig. 2.
Charlson Comorbidity Index distribution.
Reimbursement Analysis
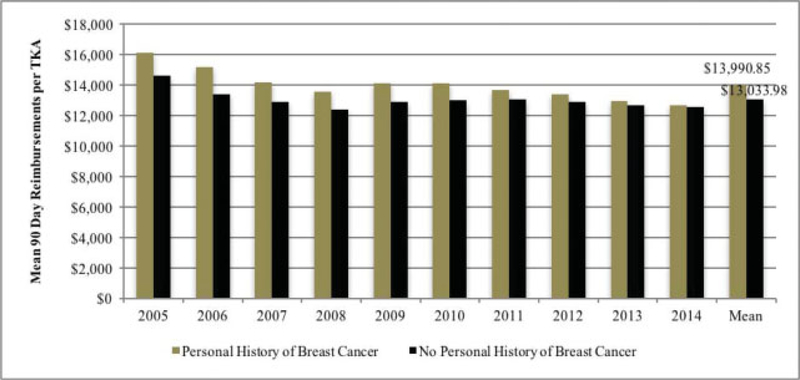
Mean DOS reimbursements were US$12,525 ± US$1,957 and US$11,633 ± US$1,446 for the breast cancer and control groups, respectively (p = 0.261). In contrast, 90-day reimbursements were significantly greater in patients with a history of breast cancer compared with controls, that is, US $13,990 ± US$1,020 versus US$13,033 ± US$625, respectively (p = 0.021) (Fig. 3).
Fig. 3.
Ninety-day reimbursements over time.
Discussion
The purpose of this study was to examine the effects of a personal history of breast cancer on the outcomes of TKA among Medicare beneficiaries. We conducted a retrospective case–control study with matching of demographics at a 1:1 ratio. This study demonstrated that patients with a history of breast cancer were more likely to sustain pulmonary emboli and prosthetic joint dislocations than age- and sex-matched controls. This supports our hypothesis that these patients are at a heightened risk of certain complications. The increased rate of PE in patients with a history of breast cancer resulted in greater use of vascular ultrasound and chest CT. Shahi et al22 previously evaluated the economic burden of inhospital venous thromboembolism (VTE) in the United States using the National Inpatient Sample and found that a VTE was associated with a significantly longer LOS and greater hospital charges when compared with patients who did not sustain a VTE. The authors found that the median charges increased significantly from US$38,791 to US$53,307, which is in line with our findings. Dasta et al,23 using a database of more than 45 million inpatient discharges within the United States, further demonstrated that the daily hospital costs of patients who sustain a DVT or PE were substantial, with means of US$1,594 (range: US$1,263–US$2,321) and US$1,735 (range: US$1,276–US$2,602). These data display the important association between VTE-related events and increased 90-day costs. Previous research efforts by van Erkel et al24 demonstrated that the cost of chest CT for the investigation of PE was on average US$143 dollars. These factors associated with the likelihood of greater medical attention are likely the culprit of increased 90-day reimbursements.
The increased risk of prosthetic joint dislocation in patients with a history of breast cancer is most likely multifactorial. Lebel and Lewallen25 evaluated all the TKA performed at their institution since 1970 and found only 58 cases of dislocations, with a described incidence of 0.93% for primary TKAs. The authors concluded that associated factors were a history of ligament laxity, extensor mechanism deficiency, and prosthetic joint infection (PJI). In our study, PJI rates were similar in both cohorts, leading us to believe that the dislocations were more likely due to other reasons. Jethanandani et al26 evaluated 14 patients who presented to their clinic with tibiofemoral dislocations.26 Of the 14, 10 (71%) had instability (either flexion instability or posterior/ medial cruciate ligament instability). High levels of estrogen have been related to increase in anterior cruciate ligament laxity, and thus a high level of this hormone may predispose TKA patients to lax joints that may change with antineoplastic therapy including estrogen decreasing agents,27,28 but focused studied on this topic are required to understand this difference.
Contrary to our hypothesis, we demonstrated that patients with a history of breast cancer presented with lower rates of AMI and pneumonia compared with controls. These findings may correlate with a likelihood of greater postoperative multispecialty monitoring of patients with a history of breast cancer and/or differences in other confounding variables such as obesity or obstructive sleep apnea.29 Furthermore, it is possible that some patients who have a history of breast cancer and had adverse events during treatment or may not have been candidates for a TKA, thus self-selecting patients who later received the surgery. In addition, many treatment regimens for breast cancer include docetaxel, cyclophosphamide, doxorubicin, and 5-fluorouracil.21,30 Some of these treatments, most notably doxorubicin, can cause cardiac abnormalities including heart failure, leading to these patients receiving cardiac ultrasound more often and to an increased likelihood of protective self-selection from a cardiac health standpoint and the risk of receiving a TKA.31 Patient-specific factors are mostly related to these adverse events, which may be more predictive of outcome instead of a personal history of breast cancer. A retrospective study of the Danish national registries identified that AMI rates are increased in patients who undergo TKA, especially for the first 2 weeks after surgery.32 The authors described that age played a strong effect modification and that patients younger than 60 years did not have any difference in AMI rates based on receiving a TKA or not. This later finding demonstrates how multiple confounders play a role in such adverse events, which should increase awareness for their occurrence and draw greater research efforts.
LOS did not differ between the two groups. This finding could be expected as we did not examine patients with a current breast cancer diagnosis and, as such, would expect similar postoperative protocols for both groups. This comparable LOS may have also helped diminish the possible differences in 90-day costs as a wider variation in LOS would have had a greater effect on overall costs.33 Nonetheless, patient-specific factors usually play a major role in deciding the optimal time to send a patient home, and thus a previous history of breast cancer would not have an impact on this measure.
Our analysis of the CCI aids in demonstrating that patients who have a history of breast cancer may also be at increased risk of complication even during remission. The significantly higher comorbidity status in patients with a history of breast cancer may aid surgeons in risk-stratifying these patients as comorbidities other than cancer may play a confounding role.
Limitations
This study is subject to similar limitations to those seen in all investigations using large databases. First, the information extracted from the database directly relies on the quality of the information put into the database. Thus, coding errors by physicians would lead to incorrect data and analysis. Second, several characteristics that may affect outcomes are not captured in this type of investigation, for example, hospital volume, operative time, and anesthetic technique. Moreover, the use of a large database of Medicare beneficiaries introduces selection bias because it precludes the inclusion of patients with private insurers. Additionally, the retrospective nature of this study introduced an inherent limitation because postoperative protocols could not be standardized. However, the use of a large sample size from this database provides the necessary number of patients to investigate postoperative complications associated with less commonly researched conditions in arthroplasty, such as breast cancer. An additional limitation of our study is the different comorbidity status between cohorts. Nonetheless, this demonstrates that patients with a history of breast cancer have a greater overall CCI, thus further explaining the cost differences in the 90-day global period. Despite these limitations, the large sample sizes afforded by the database allows for the determination of cost impact on a relevant population, which is of high interest in the currently evolving health care system.
Conclusion
Surgeons should be aware of the increased risk of PE after TKA in patients with a personal history of breast cancer. It appears that this complication and the increased use of diagnostic studies have an effect on increasing 90-day costs after TKA.
References
- 1.Jain NB, Higgins LD, Ozumba D, et al. Trends in epidemiology of knee arthroplasty in the United States, 1990–2000. Arthritis Rheum 2005;52(12):3928–3933 [DOI] [PubMed] [Google Scholar]
- 2.Laucis NC, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am 2016;98(09): 707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrotra C, Remington PL, Naimi TS, Washington W, Miller R. Trends in total knee replacement surgeries and implications for public health, 1990–2000. Public Health Rep 2005;120(03): 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res 2016;28(01):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373(17): 1597–1606 [DOI] [PubMed] [Google Scholar]
- 6.Karam JA, Huang RC, Abraham JA, Parvizi J. Total joint arthroplasty in cancer patients. J Arthroplasty 2015;30(05):758–761 [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(01):7–30 [DOI] [PubMed] [Google Scholar]
- 8.Millstone DB, Perruccio AV, Badley EM, Rampersaud YR. Factors associated with adverse events in inpatient elective spine, knee, and hip orthopaedic surgery. J Bone Joint Surg Am 2017;99(16): 1365–1372 [DOI] [PubMed] [Google Scholar]
- 9.Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiol Res Pract 2011;2011:394740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caine GJ, Stonelake PS, Lip GYH, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002;4(06):465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res 2010;126 (02):113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron AC, Touyz RM, Lang NN. Vascular complications of cancer chemotherapy. Can J Cardiol 2016;32(07):852–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol 2003;41(03):208–211 [DOI] [PubMed] [Google Scholar]
- 14.Groenen KH, Pouw MH, Hannink G, et al. The effect of radiotherapy, and radiotherapy combined with bisphosphonates or RANK ligand inhibitors on bone quality in bone metastases. A systematic review. Radiother Oncol 2016;119(02):194–201 [DOI] [PubMed] [Google Scholar]
- 15.Howlader NNA, Krapcho M, Miller D, et al. , eds. SEER Cancer Statistics Review, 1975–2014. Bethesda, MD: National Cancer Institute; Available at: https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(01):7–30 [DOI] [PubMed] [Google Scholar]
- 17.Heindel P, Tuchman A, Hsieh PC, et al. Reoperation rates after single-level lumbar discectomy. Spine 2017;42(08):E496–E501 [DOI] [PubMed] [Google Scholar]
- 18.Roche MW, Law TY, Triplet JJ, Hubbard ZS, Kurowicki J, Rosas S. Effect of hypoglycemia on the incidence of revision in total knee arthroplasty. J Arthroplasty 2017;32(02):499–502 [DOI] [PubMed] [Google Scholar]
- 19.Rosas S, Sabeh KG, Buller LT, Law TY, Roche MW, Hernandez VH. Medical comorbidities impact the episode-of-care reimbursements of total hip arthroplasty. J Arthroplasty 2017;32(07): 2082–2087 [DOI] [PubMed] [Google Scholar]
- 20.Sabeh KG, Rosas S, Buller LT, Roche MW, Hernandez VH. The impact of discharge disposition on episode-of-care reimbursement after primary total hip arthroplasty. J Arthroplasty 2017;32 (10):2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 2017;28(01):16–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahi A, Chen AF, Tan TL, Maltenfort MG, Kucukdurmaz F, Parvizi J. The incidence and economic burden of in-hospital venous thromboembolism in the United States. J Arthroplasty 2017;32(04): 1063–1066 [DOI] [PubMed] [Google Scholar]
- 23.Dasta JF, Pilon D, Mody SH, et al. Daily hospitalization costs in patients with deep vein thrombosis or pulmonary embolism treated with anticoagulant therapy. Thromb Res 2015;135(02): 303–310 [DOI] [PubMed] [Google Scholar]
- 24.van Erkel AR, van den Hout WB, Pattynama PM. International differences in health care costs in Europe and the United States: do these affect the cost-effectiveness of diagnostic strategies for pulmonary embolism? Eur Radiol 1999;9(09):1926–1931 [DOI] [PubMed] [Google Scholar]
- 25.Lebel B, Lewallen D. Total knee arthroplasty dislocation: incidence, etiology, and management. J Bone Joint Sur Br 2010;92-B (Supp_II):323 [Google Scholar]
- 26.Jethanandani RG, Maloney WJ, Huddleston JI III, Goodman SB, Amanatullah DF. Tibiofemoral dislocation after total knee arthroplasty. J Arthroplasty 2016;31(10):2282–2285 [DOI] [PubMed] [Google Scholar]
- 27.Choksi P, Williams M, Clark PM, Van Poznak C. Skeletal manifestations of treatment of breast cancer. Curr Osteoporos Rep 2013;11 (04):319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen M, Kjaer M. Sex hormones and tendon. Adv Exp Med Biol 2016;920:139–149 [DOI] [PubMed] [Google Scholar]
- 29.Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012;116(04): 788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senkus E, Kyriakides S, Ohno S, et al. ; ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26(Suppl 5): v8–v30 [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology 2010;115(02):155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalmohamed A, Vestergaard P, Klop C, et al. Timing of acute myocardial infarction in patients undergoing total hip or knee replacement: a nationwide cohort study. Arch Intern Med 2012; 172(16):1229–1235 [DOI] [PubMed] [Google Scholar]
- 33.Molloy IB, Martin BI, Moschetti WE, Jevsevar DS. Effects of the length of stay on the cost of total knee and total hip arthroplasty from 2002 to 2013. J Bone Joint Surg Am 2017;99(05):402–407 [DOI] [PMC free article] [PubMed] [Google Scholar]