Significance
Ovarian cancer is the leading cause of death among gynecological malignancies and has a poor prognosis characterized by resistance to chemotherapy. MCL1 has been found to play an essential role in chemoresistance and could be a promising therapeutic target. However, designing specific inhibitors targeting MCL1 remains challenging. Here, we found that deubiquitinating enzyme 3 (DUB3) stabilizes MCL1 via deubiquitination. We identified that O6-methylguanine-DNA methyltransferase (MGMT) is a key activator of DUB3 transcription and that the MGMT inhibitor PaTrin-2 effectively suppresses ovarian cancer cells with elevated MGMT-DUB3-MCL1 expression. We further showed that histone deacetylase (HDAC) inhibitors could significantly activate MGMT/DUB3 expression to sensitize ovarian cancer cells to PaTrin-2, providing an ideal therapeutic option involving the combined treatment of HDACis and PaTrin-2 in ovarian cancer.
Keywords: chemoresistance, DUB3, MCL1, MGMT, ovarian cancer
Abstract
Chemoresistance is a severe outcome among patients with ovarian cancer that leads to a poor prognosis. MCL1 is an antiapoptotic member of the BCL-2 family that has been found to play an essential role in advancing chemoresistance and could be a promising target for the treatment of ovarian cancer. Here, we found that deubiquitinating enzyme 3 (DUB3) interacts with and deubiquitinates MCL1 in the cytoplasm of ovarian cancer cells, which protects MCL1 from degradation. Furthermore, we identified that O6-methylguanine-DNA methyltransferase (MGMT) is a key activator of DUB3 transcription, and that the MGMT inhibitor PaTrin-2 effectively suppresses ovarian cancer cells with elevated MGMT-DUB3-MCL1 expression both in vitro and in vivo. Most interestingly, we found that histone deacetylase inhibitors (HDACis) could significantly activate MGMT/DUB3 expression; the combined administration of HDACis and PaTrin-2 led to the ideal therapeutic effect. Altogether, our results revealed the essential role of the MGMT-DUB3-MCL1 axis in the chemoresistance of ovarian cancer and identified that a combined treatment with HDACis and PaTrin-2 is an effective method for overcoming chemoresistance in ovarian cancer.
Ovarian cancer is the second most common and the most lethal gynecological malignancy worldwide, with a mortality rate exceeding 60%. According to one source of cancer statistics in China, ∼52,100 new cases and 22,500 deaths were related to ovarian cancer in 2015 (1). In the United States, there will be an estimated 22,240 new cases and 14,070 deaths in 2018 (2). Debulking surgery, followed by platinum/paclitaxel-based chemotherapy, is the standard treatment for patients with ovarian cancer. However, most patients relapse with chemoresistance after prolonged chemotherapy administration. The B-cell lymphoma 2 (BCL-2) family encodes more than 20 proteins, including proapoptotic and antiapoptotic proteins that regulate the intrinsic apoptosis pathway, which maintains the balance between cell survival and cell death (3). The up-regulation of the antiapoptotic BCL-2 family members has been recognized as essential for tumors to evade chemotherapy and become drug resistant (4). Because of its short half-life, MCL1 is distinct from the other BCL-2 family members, providing a tactic for cells to switch to either survival or apoptotic status in response to various stresses (5).
Ubiquitination, which is a reversible and significant posttranslational modification responsible for regulating the stability and activity of modified proteins, is involved in the regulation of nearly all biological process and is associated with tumorigenesis and development (6). In contrast, deubiquitinating enzymes (DUBs) can cleave ubiquitin from substrate proteins, edit ubiquitin chains, and process ubiquitin precursors to counterbalance the ubiquitination process (7). To date, six E3s (Mule, SCFβ-TRCP, SCFFBW7, APC/CCDC20, Trim17, and FBXO4) have been found to be involved in the ubiquitination process of MCL1 in proteasomal degradation (8, 9). In addition, USP9X and USP13 have been reported to deubiquitinate MCL1 for stabilization (10, 11). However, USP9X exhibits tissue-specific expression primarily in the brain and immune system (12), and sometimes functions as a tumor suppressor in certain biological processes and malignancies (13, 14). Similarly, USP13 acts as a tumor suppressor by stabilizing PTEN expression in breast cancer (15). Moreover, neither USP9X nor USP13 could completely cleave the ubiquitin chains on MCL1 in ubiquitination assays, suggesting that other DUBs essential for MCL1 deubiquitination likely exist.
In this study, we identified that DUB3 is a critical deubiquitinase that stabilizes MCL1. We further showed that the 40th lysine within the N terminus of MCL1 is mainly modified by DUB3. Moreover, we revealed that PaTrin-2 is an effective small-molecule inhibitor (SMI) that suppresses DUB3 at the transcriptional level by targeting the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT). The in vitro and in vivo studies showed that the up-regulation of the MGMT-DUB3-MCL1 signaling axis contributed to chemoresistance in ovarian cancer. In contrast, the depletion of DUB3 or administration of PaTrin-2 could significantly induce apoptosis in ovarian cancer cells via the down-regulation of MCL1. Interestingly, we observed that histone deacetylase (HDAC) inhibitors (HDACis) could transcriptionally activate MGMT expression, which sensitized the ovarian cancer cells to PaTrin-2. Our results provide a promising therapeutic approach involving the combined administration of HDACis and PaTrin-2 to overcome chemoresistance in ovarian cancer.
Results
Identification of DUB3 as a Deubiquitinase Stabilizing MCL1.
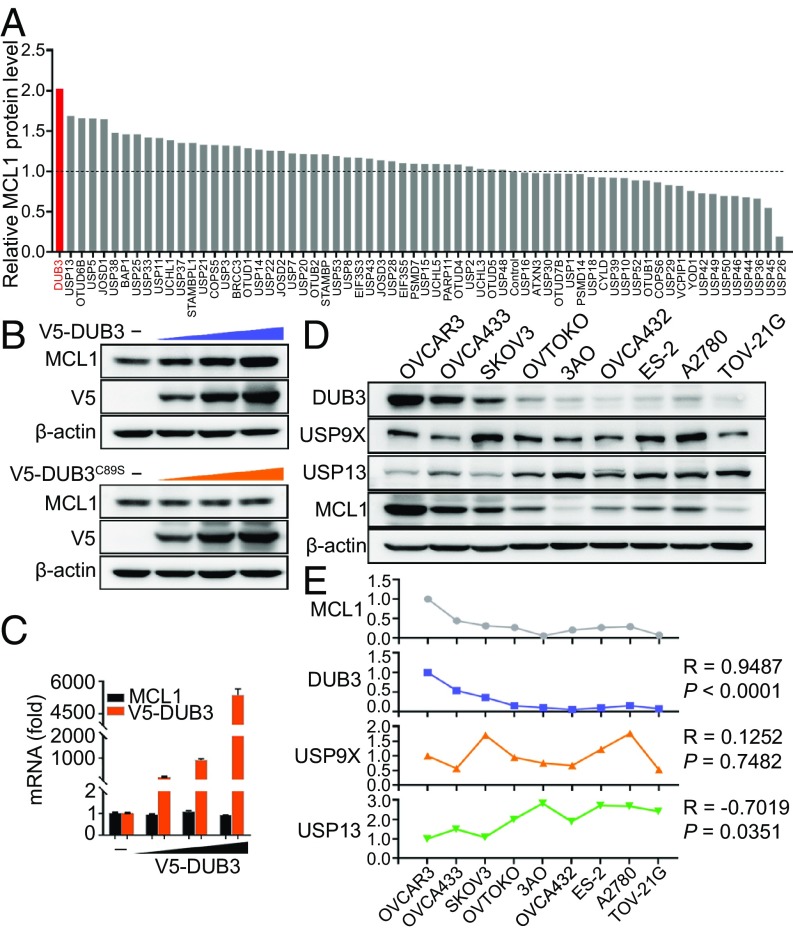
To systematically identify potential DUBs responsible for MCL1 stabilization, we performed an unbiased screening by individually transfecting 66 DUBs into 293T cells and detecting the endogenous MCL1 protein expression. Among the tested genes from the DUB plasmid library, DUB3 emerged as the DUB with the strongest up-regulation of MCL1 (Fig. 1A and SI Appendix, Fig. S1A). The ectopic expression of DUB3, but not its catalytically inactive C89S mutant (catalytic Cys89 is replaced by Ser, DUB3C89S), resulted in an elevation in the endogenous MCL1 protein but not mRNA expression level in a dose-dependent manner (Fig. 1 B and C). Consistently, the cycloheximide pulse-chase assay showed that DUB3 overexpression could significantly prolong the half-life of endogenous MCL1 (SI Appendix, Fig. S1 B and C). Although USP9X and USP13 also stabilize the MCL1 protein, their effects are slightly weaker than that of DUB3 (SI Appendix, Fig. S1 B and C). To further validate the stabilizing effect of the three DUBs on MCL1 in ovarian cancer, we detected the expression of MCL1, DUB3, USP9X, and USP13 in nine ovarian cancer cell lines (Fig. 1D). The correlation analysis revealed a strong positive correlation between DUB3 and MCL1 expression, whereas no obvious positive correlation between either USP13 or USP9X and MCL1 was observed (Fig. 1E). Furthermore, the stable overexpression of DUB3, but not DUB3C89S, in 3AO, A2780, or TOV-21G cells led to a conspicuous increase in the endogenous MCL1 protein levels without affecting the MCL1 mRNA expression (SI Appendix, Fig. S1 D–F). The depletion of DUB3 in the OVCAR3, OVCA433, or SKOV3 cells using short hairpin RNAs (shRNAs) resulted in the down-regulation of the MCL1 protein but had no effect on the MCL1 mRNA levels (SI Appendix, Fig. S1 G–I). The cycloheximide pulse-chase assay also showed accelerated MCL1 degradation in ovarian cancer cells after the DUB3 depletion (SI Appendix, Fig. S1 J–L).
Fig. 1.
DUB3 stabilizes MCL1. (A) Quantitative analysis of the MCL1 protein bands shown in SI Appendix, Fig. S1A. (B) Immunoblotting of MCL1 expression in 293T cells transfected with increasing amounts of DUB3 and DUB3C89S. (C) qRT-PCR detection of DUB3 and MCL1 expression in 293T cells transfected with increasing amounts of DUB3. (D and E) Immunoblotting and correlation analysis of the expression of DUB3, USP9X, USP13, and MCL1 in ovarian cancer cell lines. Pearson correlation coefficients are shown. The error bars indicate the mean ± SEM of three biological replicates.
DUB3 Interacts with and De-Polyubiquitinates MCL1.
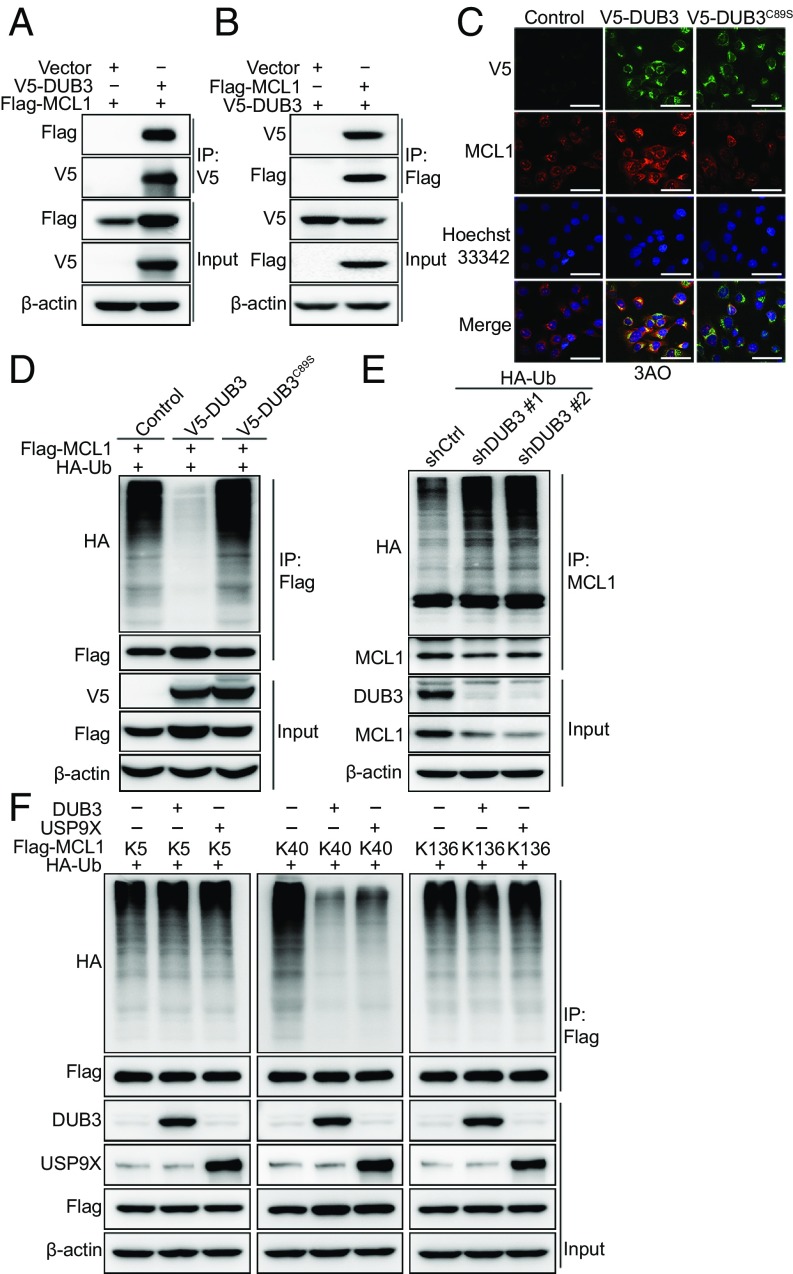
To investigate the DUB3-mediated regulation of MCL1, we first performed a coimmunoprecipitation (co-IP) analysis. V5-DUB3 and Flag-MCL1 were coexpressed in 293T cells, and after IP with anti-V5-tag pAb-agaroses, we detected MCL1, and vice versa (Fig. 2 A and B). To explore whether DUB3 selectively targets MCL1, immunoprecipitation was performed in Flag-DUB3-overexpressed 293T cells after both mass spectrometry and immunoblotting. The results demonstrated that only MCL1 was strongly associated with DUB3, whereas no other BCL-2 family member was detected (SI Appendix, Fig. S2 A and B and Table S1). Consistently, DUB3 overexpression specifically up-regulated the expression of MCL1, but not that of other BCL-2 family members in 3AO cells (SI Appendix, Fig. S2C). The interaction between endogenous MCL1 and DUB3 was further confirmed in the ovarian cancer cell lines by performing co-IP with an anti-MCL1 antibody (SI Appendix, Fig. S2D). To map the specific DUB3-binding region on MCL1, we constructed the following two DUB3 deletion mutants: N-terminal DUB3, which includes the catalytic domain, and C-terminal DUB3, which contains two hyaluronic acid-binding protein motifs (SI Appendix, Fig. S2E) (16). The co-IP analysis revealed that the N-terminal catalytic domain of DUB3, but not the C-terminal fragment, mediated the physical interaction with MCL1 and was responsible for the stabilization of MCL1 (SI Appendix, Fig. S2F). MCL1 contains two PEST (proline, glutamate, serine, and threonine) domains, four BH (BCL-2 homology) domains, and a carboxyl-terminal transmembrane domain (SI Appendix, Fig. S2E). The co-IP analysis indicated that only the N terminus of MCL1 could interact with DUB3 (SI Appendix, Fig. S2G). The immunofluorescence analysis further verified the interaction between DUB3 and MCL1 in the cytoplasm of ovarian cancer cells (Fig. 2C and SI Appendix, Fig. S2H).
Fig. 2.
DUB3 interacts with and deubiquitinates MCL1. (A and B) Immunoblotting analysis of V5-DUB3 and Flag-MCL1 expression in a co-IP assay performed in 293T cells with anti-V5 (A) or anti-Flag (B) agarose beads, respectively. (C) Confocal microscopy detection of the colocalization of V5-DUB3 (green) and MCL1 (red) in 3AO cells. Nuclei were stained using Hoechst33342 (blue). (Scale bars, 60 μm.) (D) Immunoblotting to detect the ubiquitination of MCL1 in 293T cells cotransfected with Flag-MCL1, HA-Ubiquitin, and V5-DUB3 (wild-type or C89S). (E) Immunoblotting to detect the ubiquitination of MCL1 in OVCAR3 cells expressing HA-Ubiquitin along with nontargeting shRNA or shRNAs targeting DUB3. (F) Immunoblotting to detect the ubiquitination of MCL1 mutants (K5, K40, and K136 indicate that all lysines, except for one lysine at K5, K40, or K136, were mutated to arginines) in 293T cells cotransfected with Flag-MCL1 mutants, V5-DUB3 and HA-Ubiquitin. Representative results of three biological replicates are shown.
To explore whether DUB3 stabilizes MCL1 as a bona fide deubiquitinase, we coexpressed Flag-MCL1 with either DUB3 or DUB3C89S in 293T cells. After the pulldown of MCL1 from cells treated with MG132, we found that coexpression of DUB3 almost completely abolished MCL1 ubiquitination, whereas the cells expressing DUB3C89S did not exhibit this effect (Fig. 2D). Consistently, the depletion of endogenous DUB3 by two independent shRNAs in the OVCAR3 cells markedly promoted MCL1 polyubiquitination (Fig. 2E). To further determine the region of MCL1 that is modulated by DUB3, we performed a ubiquitination assay with the N-terminal, C-terminal, or full-length constructs of MCL1 with DUB3 and found that DUB3 mainly cleaved the polyubiquitin chains on the N terminus of MCL1, but exerted no effect on the C terminus of MCL1 (SI Appendix, Fig. S3A). In addition, USP9X and USP13 mainly cleaved the polyubiquitin chains on the N terminus and C terminus of MCL1, respectively (SI Appendix, Fig. S3 B and C). Of note, the deubiquitination ability of DUB3 and USP9X on the full-length MCL1 is much stronger than that of USP13 (SI Appendix, Fig. S3 A–C). In total, 13 lysines are located in the full-length MCL1 protein, including three lysines distributed on the N terminus numbered K5, K40, and K136. The K40 and K136 sites were predicated to be the dominant ubiquitination sites of MCL1 from UbPred (SI Appendix, Fig. S3 D and E) (17). To determine the detailed function of DUB3 and USP9X at these three lysine sites within the N terminus of MCL1, we first generated a null MCL1 with all 13 lysines replaced with arginines (MCL113R), and mutated these three arginine residues back to lysines, respectively. A ubiquitination assay indicated that K40 is the key site on MCL1 deubiquitinated by both DUB3 and USP9X (Fig. 2F). Subsequently, we determined the type of ubiquitin modification of MCL1K40 edited by DUB3 and USP9X. As shown in SI Appendix, Fig. S3F, the DUB3 and USP9X-mediated MCL1 deubiquitination was K48-specific, which is canonical for proteasomal degradation. These results illustrated that although DUB3 and USP9X both deubiquitinate the K40 site of MCL1 in 293T cells, the poor correlation between USP9X and MCL1 in ovarian cancer cell lines suggests that DUB3 is the dominant DUB stabilizing MCL1 in ovarian cancer.
DUB3-MCL1 Drives Chemoresistance in Ovarian Cancer via Inhibition of the Apoptotic Cascade.
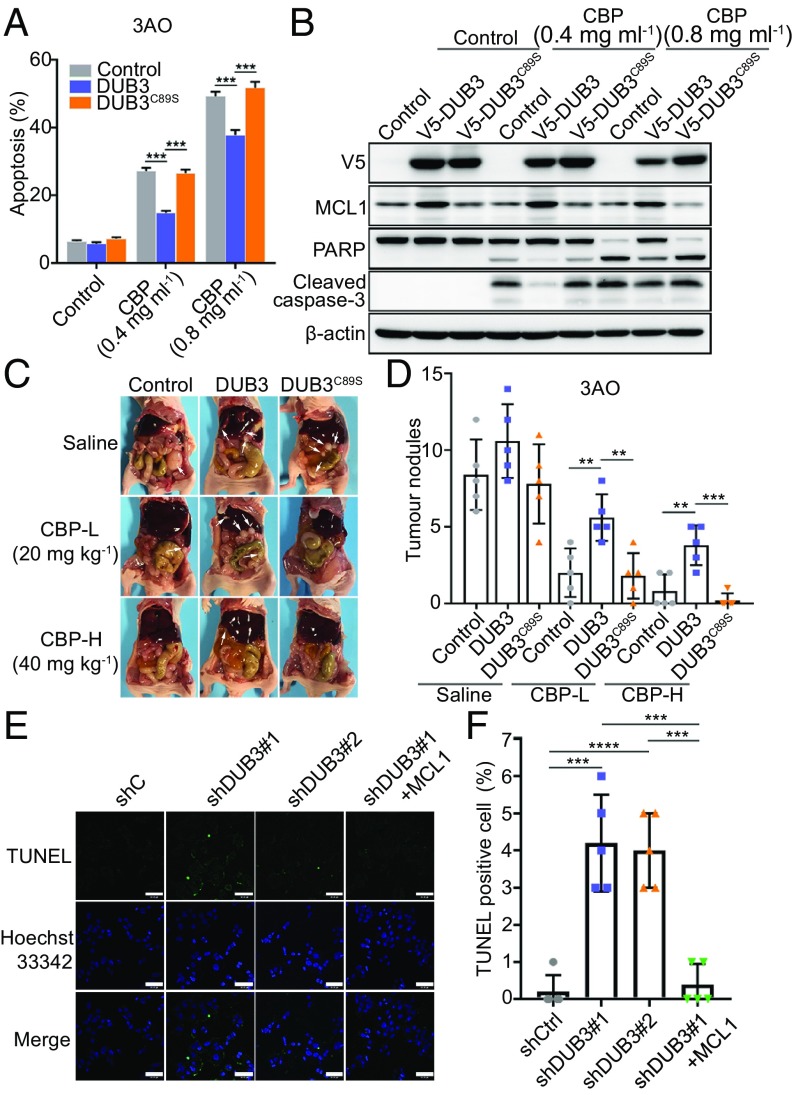
To determine the roles of DUB3 and MCL1 in the chemoresistance of ovarian cancer, we first detected the IC50 values of carboplatin (CBP) in nine ovarian cancer cell lines. The results showed that the three cell lines with high DUB3 expression exhibited more resistance to CBP than the cells with low DUB3 expression (SI Appendix, Fig. S4A), indicating that DUB3 might contribute to chemoresistance in ovarian cancer cells. Subsequently, we stably overexpressed DUB3 or DUB3C89S in 3AO and TOV-21G cells. The viability of both DUB3-overexpressing cell lines was conspicuously higher than that of the control cells after the treatment with different concentrations of CBP, but the viability of the DUB3C89S-overexpressing cells was the same as that of the control group after the CBP treatment (SI Appendix, Fig. S4B). We also detected the apoptosis rate of DUB3- or DUB3C89S-overexpressing ovarian cancer cells treated with CBP, using a flow cytometric analysis. As shown in Fig. 3A and SI Appendix, Fig. S4C, compared with the control cells, the ectopic expression of DUB3, but not DUB3C89S, in the 3AO cells reduced the apoptotic rate. Because MCL1 is a key modulator of the mitochondrial apoptotic pathway, we performed immunoblotting to detect apoptosis markers in both 3AO and TOV-21G cells. The expression level of both cleaved poly (ADP-ribose) polymerase (PARP) and cleaved caspase-3 in the DUB3-overexpressing cells was significantly decreased after the CBP treatment compared with that in either the vehicle- or DUB3C89S-overexpressing cells (Fig. 3B and SI Appendix, Fig. S4D). To further validate the antiapoptotic effect of DUB3 in vivo, we intraperitoneally injected luciferase-labeled 3AO cells expressing DUB3, DUB3C89S, or an empty vector into BALB/c nude mice and administered CBP after 6 d. At 12 and 18 d after the cell injection, the mice in the DUB3 group showed a much stronger luciferase signal after the CBP treatment (SI Appendix, Fig. S4 E and F). One month after the cell injection, the cells ectopically expressing DUB3 formed many more nodules than the cells in the other groups (Fig. 3 C and D). To further validate that the chemoresistance caused by DUB3 was dependent on MCL1, we depleted MCL1 in DUB3-overexpressing 3AO cells (SI Appendix, Fig. S4G). The depletion of MCL1 could absolutely abolish the chemoresistance caused by DUB3 both in vitro and in vivo (SI Appendix, Fig. S4 H–K). Furthermore, treatment with S63845, a MCL1 inhibitor, also rescued the chemoresistance caused by the DUB3 overexpression (SI Appendix, Fig. S4L).
Fig. 3.
DUB3 contributes to chemoresistance in ovarian cancer cells. (A) Quantitative analysis of the apoptosis rate of 3AO cells expressing an empty vector, DUB3 or DUB3C89S, and treated with CBP. (B) Immunoblotting of indicated proteins in 3AO cells expressing an empty vector, DUB3 or DUB3C89S and treated with CBP. (C and D) Representative images and quantitative analysis of metastatic nodules in the abdomens of mice injected with 3AO cells stably expressing an empty vector, DUB3 or DUB3C89S, and administered CBP (n = 5). (E and F) Representative images and quantitative analysis of TUNEL-stained apoptotic OVCA433 cells expressing nontargeting shRNA, MCL1-targeting shRNAs, or together with ectopic MCL1. Two-tailed Student’s t test; **P < 0.01, ***P < 0.001, and ***P < 0.001; error bars indicate the mean ± SD. In (A), (B), (E), and (F), representative results of three biological replicates are shown.
To further investigate the effect of DUB3 ablation on ovarian cancer cell apoptosis, we knocked down DUB3 and rescued MCL1 expression in OVCAR3 and OVCA433 cells (SI Appendix, Fig. S5A). DUB3 depletion severely reduced the viability of both cell lines, whereas the ectopic expression of MCL1 could completely rescue this effect (SI Appendix, Fig. S5 B–E). In consist, the TUNEL assay showed a significant increase in apoptosis in the OVCA433 cells with the DUB3 depletion, which could also be rescued by the ectopic expression of MCL1 (Fig. 3 E and F). Furthermore, both the cell viability assay and Annexin V/FITC double staining revealed that the DUB3 depletion could sensitize ovarian cancer cells to platinum treatment (SI Appendix, Fig. S5 F–H).
MGMT Transcriptionally Activates DUB3.
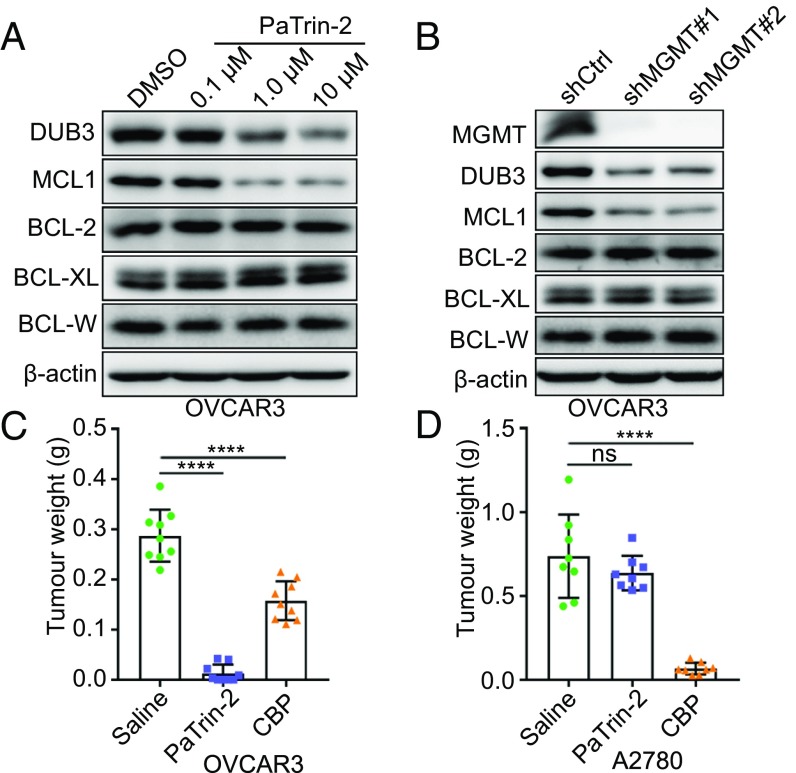
To determine whether targeting DUB3 could be an alternative choice for indirectly mitigating MCL1 activity, we screened several SMIs and detected the expression level of DUB3. Both the results from qRT-PCR screening and further immunoblotting validation revealed that PaTrin-2 was the most effective SMI in repressing DUB3 expression (SI Appendix, Fig. S6 A and B). PaTrin-2 treatment reduced the mRNA and protein levels of DUB3 in a dose-dependent manner, whereas only the protein expression level of MCL1 was decreased in response to treatment (Fig. 4A and SI Appendix, Fig. S6 C and D). PaTrin-2 is a potent and minimally toxic MGMT inhibitor that acts as a pseudosubstrate of MGMT (18). MGMT has been shown to be highly expressed in various cancer types and contributes to chemoresistance and treatment failure (19, 20). To verify that the PaTrin-2-induced suppression of DUB3 was mediated by the inhibition of MGMT, we knocked down MGMT in OVCAR3 and OVCA433 cells and found that the MGMT depletion significantly reduced the protein levels of both DUB3 and MCL1, but only the mRNA expression of DUB3 (Fig. 4B and SI Appendix, Fig. S6 E and F). The ubiquitin assay showed that MCL1 ubiquitination was increased after the PaTrin-2 treatment (SI Appendix, Fig. S6G), further confirming that PaTrin-2 promotes the ubiquitination and degradation of MCL1 by suppressing DUB3 transcription. To investigate the potential therapeutic effect of PaTrin-2 on chemoresistance in ovarian cancer, we administered PaTrin-2 to OVCAR3 and OVCA433 cells expressing high levels of MGMT-DUB3-MCL1 and A2780 and ES-2 cells expressing low levels of MGMT-DUB3-MCL1. The PaTrin-2 treatment significantly inhibited proliferation of ovarian cancer cells expressing high levels of MGMT-DUB3-MCL1, but had no effect on cell proliferation of ovarian cancer cells expressing low levels of MGMT-DUB3-MCL1 (Fig. 4 C and D and SI Appendix, Fig. S7 A–H).
Fig. 4.
PaTrin-2 transcriptionally suppresses DUB3 expression by targeting MGMT. (A) Immunoblotting of the protein levels of DUB3 and BCL-2 family members in OVCAR3 cells treated with PaTrin-2. (B) Immunoblotting of the protein levels of MGMT, DUB3, and BCL-2 family members in OVCAR3 cells expressing nontargeting or MGMT-targeting shRNAs. (C and D) Tumor weight of xenografts of OVCAR3 and A2780 cells treated with saline, PaTrin-2, or CBP. (n = 9). (A–C) Error bars indicate the mean ± SEM of three biological replicates. The error bars indicate the mean ± SD. Two-tailed Student’s t test; ****P < 0.0001; ns, no significant difference.
To further confirm that the suppressive effect of PaTrin-2 was dependent on enzymatic MGMT expression, we ectopically expressed MGMT in ES-2 and A2780 cells. The overexpression of MGMT, but not its dead mutant MGMTC145A, increased the protein expression of both DUB3 and MCL1 (SI Appendix, Fig. S7I), whereas only the mRNA expression level of DUB3, but not of MCL1, was increased (SI Appendix, Fig. S7J). The administration of PaTrin-2 promoted the chemosensitivity of the ES-2 and A2780 cells that ectopically expressed MGMT, but not that of the control cells with low MGMT expression (SI Appendix, Fig. S7 K and L). Supporting the positive regulation of the MGMT-DUB3-MCL1 axis, the expression level of MGMT in the ovarian cancer cell lines was correlated with the DUB3 mRNA level and DUB3/MCL1 protein expression level (SI Appendix, Fig. S7 M–O).
HDACis Elevate MGMT Expression to Sensitize PaTrin-2.
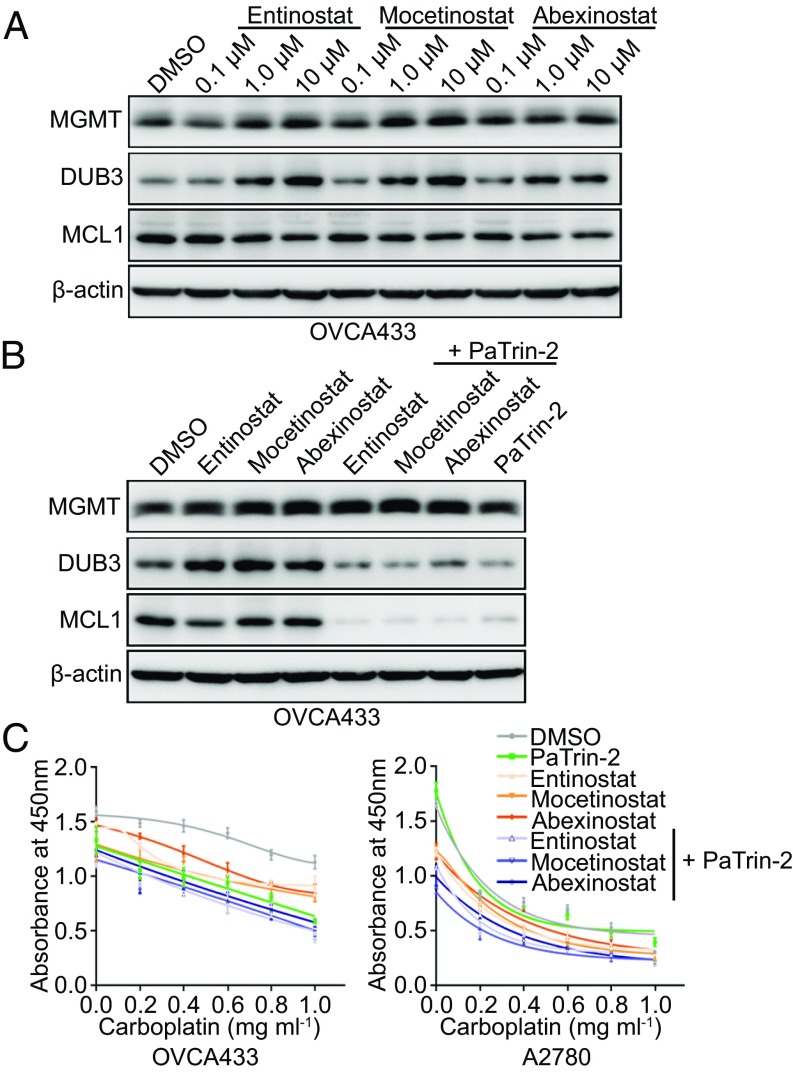
In our previous SMI screening experiment, several inhibitors were found to increase the expression of DUB3, among which the most obvious were some HDACis (SI Appendix, Fig. S6 A and B). Because of the specificity of the MGMT regulation of DUB3 expression, we speculated whether HDACis activate DUB3 via MGMT activation. Strikingly, when we treated the ovarian cancer cells with Entinostat, Mocetinostat, or Abexinostat, the mRNA expression levels of MGMT and DUB3 increased in a dose-dependent manner, whereas the MCL1 mRNA expression remained unchanged (SI Appendix, Fig. S8 A–C). The anti-tumor function of HDACis is mostly exerted via the induction of apoptosis, which activates the apoptosis cascade, including decreases in MCL1 (21, 22) and BCL-2 (23). However, the immunoblotting analysis showed that the administration of HDACis to OVCA433 cells only slightly decreased the MCL1 expression levels (Fig. 5A). We speculated that the MCL1 protein level might be stabilized by the increased MGMT-DUB3 expression triggered by HDACis. Consistent with our speculation, the combined administration of HDACis and PaTrin-2 significantly reduced MCL1 protein expression (Fig. 5B). To determine whether the combined administration of HDACis and PaTrin-2 could be an effective therapeutic method for ameliorating chemoresistance in ovarian cancer, we treated both OVCA433 and A2780 cells with HDACis, PaTrin-2, or a combination of these two drugs, and measured the cell viability. Although the HDACis showed some inhibitory effects on the viability of ovarian cancer cells, the combination of HDACis and PaTrin-2 significantly reduced the cell viability and induced apoptosis in the ovarian cancer cells (Fig. 5C and SI Appendix, Fig. S8 D and E). To further validate this therapeutic effect, we established the following four primary ovarian cancer cell lines: OVP1, OVP2, OVP3, and OVM1 (SI Appendix, Fig. S8F). Consistent with our earlier results, the combined administration of Mocetinostat and PaTrin-2 led to a highly effective therapeutic response (SI Appendix, Fig. S8G). Taken together, our results suggest that the combined use of HDACis and PaTrin-2 might be a promising approach for the treatment of ovarian cancer.
Fig. 5.
HDACis sensitize ovarian cancer cells to PaTrin-2 via the activation of the MGMT-DUB3-MCL1 axis. (A) Immunoblotting of the indicated proteins in OVCA433 cells treated with Entinostat, Mocetinostat, or Abexinostat. (B) Immunoblotting of the indicated proteins in OVCA433 cells treated with DMSO, Entinostat, Mocetinostat, or Abexinostat either alone or together with PaTrin-2. (C) Cell viability detection at an absorbance of 450 nm in OVCA433 and A2780 cells treated with DMSO, Entinostat, Mocetinostat, or Abexinostat either alone or together with PaTrin-2 after the administration of CBP. The error bars indicate the mean ± SD; representative results of three biological replicates are shown.
High MGMT-DUB3-MCL1 Expression Is Correlated with Chemoresistance in Human Ovarian Cancer Samples.
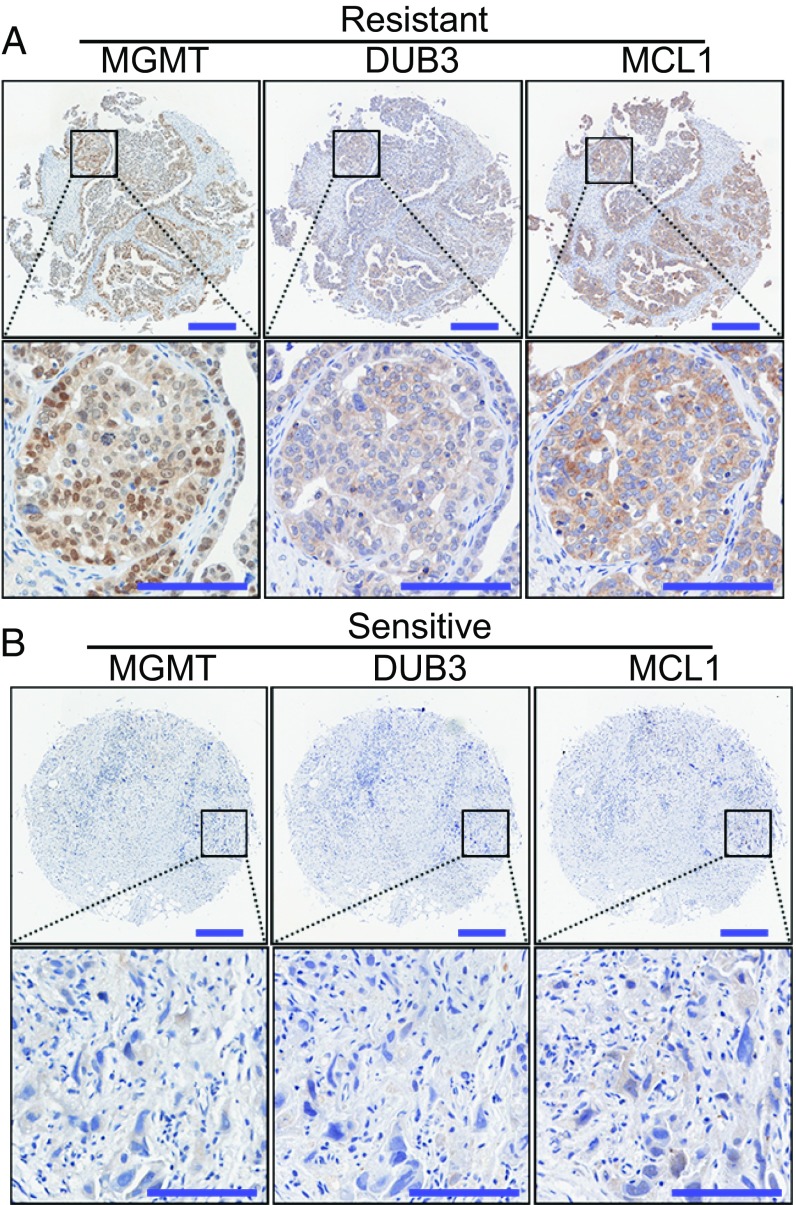
To further confirm the correlation between the MGMT-DUB3-MCL1 axis and chemoresistance in ovarian cancer, immunohistochemistry (IHC) was employed to stain an array of 150 human ovarian cancer specimens for the detection of MGMT, DUB3, and MCL1 expression. Consistent with their expression in the cell lines, the expression levels of MGMT, DUB3, and MCL1 were also positively correlated with each other in the 150 samples (SI Appendix, Fig. S9 A–C). Moreover, the MGMT, DUB3, and MCL1 expression levels were highly up-regulated in the chemoresistant samples compared with the levels in the chemosensitive samples (Fig. 6), further supporting our conclusion that the high expression of the MGMT-DUB3-MCL1 axis contributes to chemoresistance in ovarian cancer. Further analysis showed that the elevated expression of DUB3 or MCL1 was also correlated with pathological factors, such as the residual tumor size and CA125 levels in patients with ovarian cancer (SI Appendix, Fig. S9D). Moreover, the high levels of DUB3 and MCL1 were correlated with poor overall survival and progression-free survival (SI Appendix, Fig. S9 E and F). However, the expression level of MGMT was not obviously correlated with any other variables in this ovarian cancer cohort (SI Appendix, Fig. S9 D and G), which may be a result of the heterogeneity of MGMT expression in cancer. Collectively, these results further support our conclusion that high MGMT-DUB3-MCL1 expression contributes to chemoresistance in ovarian cancer.
Fig. 6.
MGMT-DUB3-MCL1 expression is correlated with chemoresistance in ovarian cancer. (A and B) Representative images of IHC staining of MGMT, DUB3, and MCL1 in platinum-resistant or platinum-sensitive ovarian cancer samples. [Scale bars, 250 µm (original), 100 µm (zoomed).]
Discussion
Ovarian cancer is the leading cause of death among gynecological malignancies, with a 5-y survival rate less than 50% in women diagnosed with late-stage ovarian cancer (24). In recent years, MCL1 has been considered an essential oncogene in the development of chemoresistance in ovarian cancer (25). S63845, the only MCL1-specific inhibitor, has shown an ideal effect in the treatment of blood tumors, but its effect on solid tumors is still restricted (26). DUB3 is a powerful oncogene that plays important roles in several cancer types, which is exemplified by its ability to stabilize Cdc25A and Snail1 to promote oncogenic transformation and metastasis in breast cancer (16, 27). To our knowledge, the functions of DUB3 have been investigated mainly in the nucleus, but its functions in the cytoplasm remain ill-defined. Furthermore, the role of DUB3 in chemoresistance is still poorly understood. Here, we found that the DUB3-mediated stabilization of MCL1 depends on the enzymatic activity of DUB3 as the ectopic expression of DUB3, but not its nonenzymatic mutant, led to a near-total abolishment of MCL1 ubiquitination. Further investigation showed that PaTrin-2 administration could effectively suppress the growth of ovarian cancer cells with amplified MGMT-DUB3-MCL1 expression. Most interestingly, the discovery that HDACis are strong activators of the MGMT/DUB3 axis provides a promising therapeutic approach involving a combination of HDACis and PaTrin-2.
MCL1 and BCL-2 are two pivotal members of the BCL-2 antiapoptotic family and play a critical role in chemoresistance by promoting cancer cell survival. Because of the antiapoptotic function of both MCL1 and BCL-2, the inhibition of either BCL-2 or MCL1 alone is insufficient to turn off the antiapoptotic pathway under some circumstances, whereas the combined targeting of both MCL1 and BCL-2 could alleviate this problem (11, 28). A previous study implied that the combined administration of PaTrin-2 and a BCL-2 inhibitor could sensitize ovarian cancer cells to temozolomide (29), but the underlying mechanism remains unknown. Our study uncovered that the synthetic effect of PaTrin-2 and HDAC inhibitors might be a result of the combined inhibition of both MCL1 and BCL-2. Very recently, another study showed that targeting DUB3 rescued resistance to a BET inhibitor in prostate cancer cells, and that the NCOR2-HDAC10 complex could transcriptionally activate DUB3 expression (30). In our study, these three HDACis mainly targeted the class I HDACs (HDAC1, HDAC2, and HDAC3) (31), illustrating that different HDAC family members might activate DUB3 via various mechanisms.
In conclusion, our study revealed that the high expression of the MGMT-DUB3-MCL1 axis plays an important role in chemoresistance and provides therapeutic targets for ovarian cancer. On the basis of these results, we categorized the ovarian cancer cases into three groups, as follows: (i) ovarian cancer cells expressing high levels of MGMT-DUB3-MCL1 that are resistant to traditional chemotherapeutic drugs but sensitive to PaTrin-2; (ii) ovarian cancer cells expressing low levels of MGMT-DUB3-MCL1, representing cases that are initially sensitive to standard chemotherapy; and (iii) ovarian cancer cells that express low levels of MGMT-DUB3-MCL1 but are still resistant to chemotherapies, which might be a result of the existence of other antiapoptotic proteins such as BCL-2. Under this condition, a combined regimen of HDACis and PaTrin-2 might achieve an effective therapeutic outcome (SI Appendix, Fig. S10).
Materials and Methods
Paraffin-embedded in situ tumor tissue blocks were collected from 150 patients with ovarian cancer, as previously described (32). Informed consent was obtained from all patients, and tissue samples were de-identified before use. IHC experiments were performed as previously described (33). All slides were scanned using an Aperio scanning system (Aperio). IHC staining was quantified as an H-score, as follows: H-score = ∑(pi × i), where pi represents the percentage of positively stained cells (0–100%) and i represents the staining intensity (0, negative; 1, weak; 2, moderate; 3, strong). The scoring was evaluated by two independent observers. Additional materials and methods are available in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Zhigang Zhang (Shanghai Jiao Tong University) for providing the OVCA433 and OVTOKO cell lines and Zhe Liu (Tianjin Medical University) for kindly providing the OVCA432 cells. We appreciate Beijing Qinglian Biotech Co., Ltd. for the mass spectrometric analysis. This work was supported by National Science Foundation of China Grant 81420108025, National Key R&D Program of China Grant 2016YFC1302100, and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences Grant 2016-I2M-1-001.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814742116/-/DCSupplemental.
References
- 1.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 4.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 5.Inuzuka H, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18:69–88. doi: 10.1038/nrc.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komander D, Clague MJ, Urbé S. Breaking the chains: Structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 8.Mojsa B, Lassot I, Desagher S. Mcl-1 ubiquitination: Unique regulation of an essential survival protein. Cells. 2014;3:418–437. doi: 10.3390/cells3020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng C, Yang F, Wang J. FBXO4 inhibits lung cancer cell survival by targeting Mcl-1 for degradation. Cancer Gene Ther. 2017;24:342–347. doi: 10.1038/cgt.2017.24. [DOI] [PubMed] [Google Scholar]
- 10.Schwickart M, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, et al. Deubiquitinase USP13 dictates MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat Commun. 2018;9:215. doi: 10.1038/s41467-017-02693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik E, et al. Regulation of proximal T cell receptor signaling and tolerance induction by deubiquitinase Usp9X. J Exp Med. 2014;211:1947–1955. doi: 10.1084/jem.20140860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan OM, et al. The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J Clin Invest. 2018;128:1326–1337. doi: 10.1172/JCI97325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toloczko A, et al. Deubiquitinating enzyme USP9X suppresses tumor growth via LATS kinase and core components of the hippo pathway. Cancer Res. 2017;77:4921–4933. doi: 10.1158/0008-5472.CAN-16-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, et al. Deubiquitylation and stabilization of PTEN by USP13. Nat Cell Biol. 2013;15:1486–1494. doi: 10.1038/ncb2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat Commun. 2017;8:14228. doi: 10.1038/ncomms14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radivojac P, et al. Identification, analysis, and prediction of protein ubiquitination sites. Proteins. 2010;78:365–380. doi: 10.1002/prot.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turriziani M, et al. O6-(4-bromothenyl)guanine (PaTrin-2), a novel inhibitor of O6-alkylguanine DNA alkyl-transferase, increases the inhibitory activity of temozolomide against human acute leukaemia cells in vitro. Pharmacol Res. 2006;53:317–323. doi: 10.1016/j.phrs.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Yu Z, et al. Inhibition of NF-κB results in anti-glioma activity and reduces temozolomide-induced chemoresistance by down-regulating MGMT gene expression. Cancer Lett. 2018;428:77–89. doi: 10.1016/j.canlet.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, et al. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat Commun. 2018;9:2949. doi: 10.1038/s41467-018-05373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song KH, et al. HDAC1 upregulation by NANOG promotes multidrug resistance and a stem-like phenotype in immune edited tumor cells. Cancer Res. 2017;77:5039–5053. doi: 10.1158/0008-5472.CAN-17-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Adorno AM, et al. Histone deacetylase inhibitor enhances the efficacy of MEK inhibitor through NOXA-mediated MCL1 degradation in triple-negative and inflammatory breast cancer. Clin Cancer Res. 2017;23:4780–4792. doi: 10.1158/1078-0432.CCR-16-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar JS, Suman S, Chandna S. Radioresistant Sf9 insect cells readily undergo an intrinsic mode of apoptosis in response to histone deacetylase (HDAC) inhibition. Mol Cell Biochem. 2018;444:207–218. doi: 10.1007/s11010-017-3245-0. [DOI] [PubMed] [Google Scholar]
- 24.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: New opportunities for translation. Nat Rev Cancer. 2009;9:415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, et al. Geraniin suppresses ovarian cancer growth through inhibition of NF-κB activation and downregulation of Mcl-1 expression. J Biochem Mol Toxicol. 2017;31 doi: 10.1002/jbt.21929. [DOI] [PubMed] [Google Scholar]
- 26.Kotschy A, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 27.Pereg Y, et al. Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat Cell Biol. 2010;12:400–406. doi: 10.1038/ncb2041. [DOI] [PubMed] [Google Scholar]
- 28.Arai S, et al. Tyrosine kinase inhibitors increase MCL1 degradation and in combination with BCLXL/BCL2 inhibitors drive prostate cancer apoptosis. Clin Cancer Res. 2018;24:5458–5470. doi: 10.1158/1078-0432.CCR-18-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barvaux VA, et al. Sensitization of a human ovarian cancer cell line to temozolomide by simultaneous attenuation of the Bcl-2 antiapoptotic protein and DNA repair by O6-alkylguanine-DNA alkyltransferase. Mol Cancer Ther. 2004;3:1215–1220. [PubMed] [Google Scholar]
- 30.Jin X, et al. DUB3 promotes BET inhibitor resistance and cancer progression by deubiquitinating BRD4. Mol Cell. 2018;71:592–605.e4. doi: 10.1016/j.molcel.2018.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu T, Li Y, Wu X, Li B, Liu Z. Down-regulation of HECTD3 by HER2 inhibition makes serous ovarian cancer cells sensitive to platinum treatment. Cancer Lett. 2017;411:65–73. doi: 10.1016/j.canlet.2017.09.048. [DOI] [PubMed] [Google Scholar]
- 33.Luo Q, et al. ARID1A ablation leads to multiple drug resistance in ovarian cancer via transcriptional activation of MRP2. Cancer Lett. 2018;427:9–17. doi: 10.1016/j.canlet.2018.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.