Significance
Calcineurin inhibitors (CNIs) are potent immunosuppressants; hypertension and hyperkalemia are common adverse effects. Activation of the renal Na-Cl cotransporter (NCC) is implicated in this toxicity; however, the mechanism is unknown. CNIs’ renal effects mimic the hypertension and hyperkalemia resulting from mutations in WNK kinases or in KLHL3-CUL3 ubiquitin ligase. WNKs activate NCC and are degraded by ubiquitylation upon their binding to KLHL3. The binding of WNKs to KLHL3 is prevented by KLHL3 mutations or by PKC-mediated KLHL3 phosphorylation at serine 433. This work shows that calcineurin dephosphorylates KLHL3S433, promoting WNK4 degradation. Conversely, CNIs inhibit KLHL3S433 dephosphorylation, preventing WNK degradation. These findings implicate calcineurin in the normal regulation of KLHL3’s binding of WNK4 and identify a direct target causing CNI-induced pathology.
Keywords: pseudohypoaldosteronism type II, posttranslational modification, blood pressure, kidney
Abstract
Calcineurin is a calcium/calmodulin-regulated phosphatase known for its role in activation of T cells following engagement of the T cell receptor. Calcineurin inhibitors (CNIs) are widely used as immunosuppressive agents; common adverse effects of CNIs are hypertension and hyperkalemia. While previous studies have implicated activation of the Na-Cl cotransporter (NCC) in the renal distal convoluted tubule (DCT) in this toxicity, the molecular mechanism of this effect is unknown. The renal effects of CNIs mimic the hypertension and hyperkalemia that result from germ-line mutations in with-no-lysine (WNK) kinases and the Kelch-like 3 (KLHL3)–CUL3 ubiquitin ligase complex. WNK4 is an activator of NCC and is degraded by binding to KLHL3 followed by WNK4’s ubiquitylation and proteasomal degradation. This binding is prevented by phosphorylation of KLHL3 at serine 433 (KLHL3S433-P) via protein kinase C, resulting in increased WNK4 levels and increased NCC activity. Mechanisms mediating KLHL3S433-P dephosphorylation have heretofore been unknown. We now demonstrate that calcineurin expressed in DCT is a potent KLHL3S433-P phosphatase. In mammalian cells, the calcium ionophore ionomycin, a calcineurin activator, reduces KLHL3S433-P levels, and this effect is reversed by the calcineurin inhibitor tacrolimus and by siRNA-mediated knockdown of calcineurin. In vivo, tacrolimus increases levels of KLHL3S433-P, resulting in increased levels of WNK4, phosphorylated SPAK, and NCC. Moreover, tacrolimus attenuates KLHL3-mediated WNK4 ubiquitylation and degradation, while this effect is absent in KLHL3 with S433A substitution. Additionally, increased extracellular K+ induced calcineurin-dependent dephosphorylation of KLHL3S433-P. These findings demonstrate that KLHL3S433-P is a calcineurin substrate and implicate increased KLHL3 phosphorylation in tacrolimus-induced pathologies.
Calcineurin, a serine-threonine phosphatase, plays an important role in activation and proliferation of T cells (1, 2). Engagement of the T cell receptor results in increased cytoplasmic Ca2+ levels, which activate calcineurin. Activated calcineurin dephosphorylates nuclear factor of activated T cells (NFAT) transcription factors, enabling their nuclear translocation and IL-2 transcription, promoting T cell proliferation. NFAT also regulates the transcription of other cytokines including IL-4, IL-10, and IFN-γ, in concert with other transcription factors (2). Calcineurin inhibitors (CNIs), such as tacrolimus and cyclosporine A, are immunosuppressants commonly used to prevent rejection of transplanted organs. CNIs have a number of adverse effects, including hypertension along with hyperkalemia and variable distal renal tubular acidosis in a significant number of patients (3).
In searching for the cause of renal CNI toxicity, attention has been drawn to a pathway identified from studies of a Mendelian disease featuring the same cardinal features of hypertension and hyperkalemia with variable acidosis (pseudohypoaldosteronism type II, PHAII; also known as familial hyperkalemic hypertension, FHHt). Our group identified mutations in any of four different genes that can cause this trait; these include WNK1 and WNK4, which encode serine-threonine kinases, and two components of a ubiquitin ligase, Kelch-like 3 (KLHL3), and Cullin 3 (CUL3) (4, 5). These findings have led to elucidation of a previously unrecognized physiologic pathway that allows the kidney to modulate the balance between renal salt reabsorption and K+ excretion, allowing distinct physiological responses to volume depletion and hyperkalemia, states that both use the steroid hormone aldosterone as a signaling hormone. Characterization of the physiology of this system has shown that WNK kinases increase the activity of NCC (6–8) via a mechanism involving WNK phosphorylation of SPAK, which, in turn, phosphorylates and increases NCC activity (9–15). KLHL3 binds to WNK kinases, targeting them for ubiquitylation and proteasomal degradation (16–18). Several disease-causing mutations in WNK and KLHL3 occur at the sites of physical interaction between these two proteins and impair their binding to one another (17). Moreover, this binding is blocked in vivo by phosphorylation of KLHL3 at serine 433 (KLHL3S433-P), which is mediated by protein kinase C (PKC), acting downstream of angiotensin II (AngII) signaling (19). These findings implicated AngII signaling in promoting increased NCC activity and Na-Cl reabsorption without K+ loss. Inhibition of NCC with thiazide diuretics corrects the hypertension and hyperkalemia of PHAII (4, 5, 20).
The similarity of renal effects of CNIs to PHAII has led to exploration of the activity of the WNK kinase-NCC pathway with CNIs (21–23). Although CNIs affect many physiological systems that can modulate blood pressure (24–28), increased renal salt reabsorption resulting from aberrant Na-Cl cotransporter (NCC) activity appears to play a key role in tacrolimus-induced hypertension because thiazide diuretics, which selectively inhibit NCC, correct CNI-induced hypertension and hyperkalemia (21, 23, 29). Moreover, in animal models, tacrolimus has been shown to increase levels of WNK4, phosphorylated SPAK, and phosphorylated NCC (29–32). To date, however, the direct targets of the phosphatase calcineurin in DCT epithelia remain unknown.
The striking similarity of the mechanisms of renal toxicity of CNIs and PHAII strongly suggest that CNIs inhibit the dephosphorylation of a protein that results in NCC activation. We report herein that calcineurin normally dephosphorylates KLHL3S433-P and that this action is inhibited by CNIs, thereby increasing WNK levels, which, in turn, increases NCC levels and activity.
Results
Calcineurin Activation Decreases KLHL3S433-P in HEK and DCT Cells.
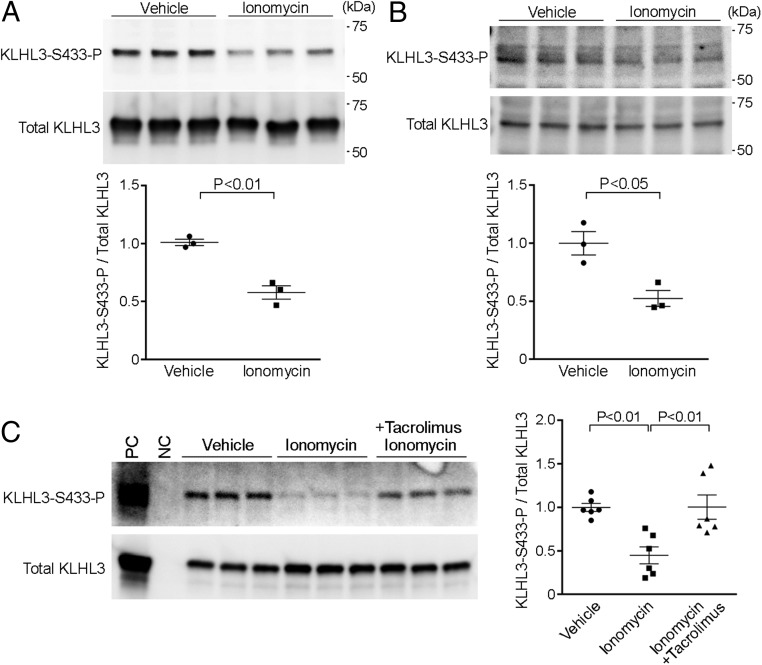
The findings that KLHL3 normally binds WNK kinases, targeting them for degradation, and that mutation or phosphorylation of KLHL3S433 prevents this binding, increasing WNK kinase levels and leading to increased NCC activity (16–19), suggested that calcineurin might normally dephosphorylate KLHL3S433-P, with CNIs preventing this activity. To address this possibility, we first tested the effects of ionomycin, a calcium ionophore that activates calcineurin, in mammalian cells (33). HEK cells expressing FLAG-tagged human KLHL3 (FLAG-KLHL3) were incubated with 3 μM ionomycin for 5 min, as described (33, 34). Cells were then lysed and analyzed by Western blot to detect KLHL3S433-P levels, using a well-characterized antibody that is highly specific for KLHL3S433-P (35). Ionomycin reduced KLHL3S433-P levels by a mean of 42% in independent replicates (Fig. 1A). To determine whether calcineurin affects endogenous KLHL3S433-P, we incubated mouse distal convoluted tubule (mDCT) cells (36) with 3 μM ionomycin. Similar to HEK cells, ionomycin decreased endogenous KLHL3S433-P levels by 47% in the DCT cells (Fig. 1B).
Fig. 1.
Calcineurin dephosphorylates KLHL3S433-P in HEK and DCT cells. (A) HEK cells expressing FLAG-KLHL3 were incubated with ionomycin for 5 min. Lysates of cells were analyzed by Western blotting using antibodies for KLHL3 phosphorylated at S433 (KLHL3S433-P) and for FLAG to detect total KLHL3. Blots show biological replicates. Dot-plot graphs show the results of quantification. Error bars in all graphs indicate SEM. (B) mDCT cells were incubated with ionomycin for 5 min. Changes in endogenous levels of KLHL3S433-P and total KLHL3 were analyzed by Western blotting. Dot-plot graphs show the results of quantification. (C) HEK cells expressing FLAG-KLHL3 was incubated with ionomycin in the presence or absence of tacrolimus, a calcineurin inhibitor. KLHL3S433-P and total KLHL3 levels were analyzed by Western blotting. Dot-plot graphs show the results of quantification. NC, negative control; PC, positive control (immunopurified KLHL3).
If the reduction in KLHL3S433-P in cells incubated with ionomycin is mediated by calcineurin, this effect should be blocked by calcineurin inhibition. Therefore, we incubated KLHL3-expressing HEK cells with ionomycin in the presence or absence of 100 nM tacrolimus, a potent calcineurin inhibitor (34, 37). Tacrolimus significantly attenuated the KLHL3S433-P dephosphorylation induced by ionomycin (Fig. 1C).
Among the isoforms of calcineurin, calcineurin A-α is responsible for the effects of calcineurin in the kidney (38). To further confirm that the effects of ionomycin on KLHL3S433-P are mediated by calcineurin, we introduced siRNA targeting calcineurin A-α into HEK cells. siRNA directed against calcineurin A-α, but not control siRNA, knocked down calcineurin A levels by 50% (Fig. 2A) and reversed the reduction in KLHL3S433-P levels induced by ionomycin (Fig. 2B).
Fig. 2.
Knockdown of calcineurin prevents the dephosphorylation of KLHL3S433-P by ionomycin in HEK cells. (A) siRNA directed against calcineurin A-α (CaNA-α) or control siRNA and FLAG-KLHL3 were introduced in HEK cells by transient transfection. Cells were then incubated with or without ionomycin for 5 min. Lysates were then analyzed for calcineurin A and tubulin levels by Western blotting. Blots show biological replicates. Dot-plot graphs show the results of quantification. (B) Indicated siRNAs were introduced in HEK cells by transient transfection. FLAG-KLHL3 was induced as described in Materials and Methods. Cells were then incubated with or without ionomycin for 5 min. Lysates were then analyzed for KLHL3S433-P and total KLHL3 levels by Western blotting. Dot-plot graphs show the results of quantification.
Calcineurin Directly Dephosphorylates KLHL3S433-P.
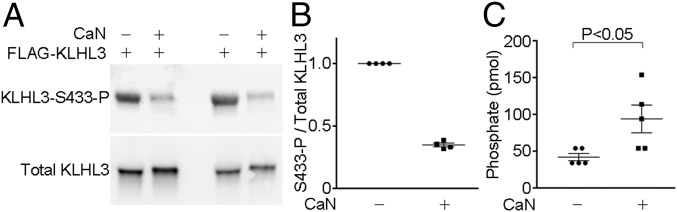
To test whether calcineurin directly dephosphorylates KLHL3S433-P, we performed an in vitro phosphatase assay. We purified FLAG-KLHL3 from HEK cells by immunoprecipitation using FLAG antibodies and incubated the protein with calcineurin (0.6 μM) in the presence of Ca2+ and calmodulin (39). Calcineurin reduced KLHL3S433-P levels by ∼70%, without effect on total KLHL3 levels (Fig. 3 A and B). To confirm these results, we incubated purified total KLHL3 with calcineurin, and the liberated amount of phosphate was quantitated by malachite green assay. The results demonstrated that free phosphate levels were significantly increased by calcineurin (Fig. 3C), confirming the dephosphorylation of KLHL3 by calcineurin.
Fig. 3.
Calcineurin dephosphorylates KLHL3 at S433 in vitro. (A and B) FLAG-tagged human KLHL3 was expressed in HEK cells, purified by IP, and incubated with or without calcineurin (CaN) in the presence of Ca2+ and calmodulin. The levels of KLHL3 phosphorylated at S433 (KLHL3S433-P) were detected by Western blotting with KLHL3S433-P antibody, and the levels of total KLHL3 were detected by FLAG antibody. Blots show biological replicates. Dot-plot graphs in B show the ratio of KLHL3S433-P to total KLHL3 levels in four independent experiments. (C) KLHL3 was expressed in HEK cells, purified by IP, and incubated with or without calcineurin in the presence of Ca2+ and calmodulin. Free phosphate levels were then quantified by malachite green phosphate assay.
Tacrolimus Increases KLHL3S433-P, Which Is Associated with Increased WNK and NCC Levels.
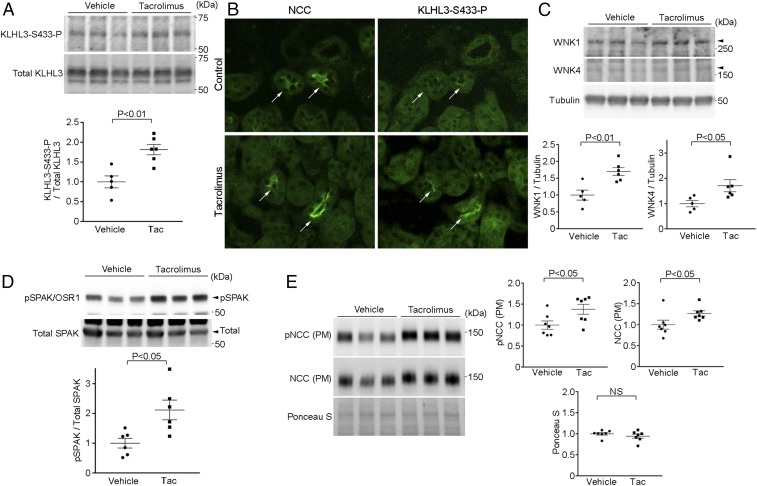
We next evaluated whether calcineurin regulates KLHL3S433-P levels in vivo. We administered the calcineurin inhibitor tacrolimus (1 mg/kg per body weight, s.c.) (29) or vehicle to C57BL/6J mice for 2 d, and analyzed the renal levels of KLHL3S433-P. We found that tacrolimus significantly increased KLHL3S433-P abundance in the kidney (Fig. 4A). Immunostaining revealed that this increase occurred in cells that also expressed NCC, indicating that these are DCT cells (Fig. 4B). As reported (29), the levels of the KLHL3 binding targets WNK1 and WNK4 were also increased by tacrolimus (Fig. 4C), consistent with loss of KLHL3 binding. Although NCC in the plasma membrane-enriched fraction was not altered after 2 d of tacrolimus treatment, after 14 d, we observed both a significant increase in NCC and phosphorylated NCC levels in the plasma membrane-enriched fraction, as well as a significant increase in phosphorylated SPAK (Fig. 4 D and E). Consistent with previous observations (29), mice receiving a high salt (8%) diet and tacrolimus showed a significant blood pressure elevation compared with control mice (SI Appendix, Fig. S1).
Fig. 4.
NCC activation in tacrolimus-treated mice is associated with the increase in KLHL3S433-P levels. (A) C57BL/6J mice received daily injection of tacrolimus (Tac) or vehicle for 2 d. Renal levels of KLHL3S433-P and total KLHL3 were measured by Western blotting. Blots show biological replicates. Dot-plot graphs show the results of quantitation. (B) Serial kidney sections stained for NCC and KLHL3S433-P antibodies in vehicle- and tacrolimus-injected mice. Arrows indicate DCT cells. The increase in KLHL3S433-P expression is mainly detected in the DCT cells. (C) Lysates from A were analyzed by Western blotting using antibodies against WNK1, WNK4, and tubulin. Blots show biological replicates. Dot-plot graphs show the results of quantitation. (D) C57BL/6J mice received a daily injection of tacrolimus (s.c.) or vehicle for 14 d. Renal levels of the active, phosphorylated SPAK (Lower) and total SPAK (Upper) were analyzed by Western blotting. Blots show biological replicates. Dot-plot graphs show the results of quantitation. (E) C57BL/6J mice received a daily injection of tacrolimus (s.c.) or vehicle for 14 d. Levels of total NCC and NCC phosphorylated at T53 (pNCC) in the plasma membrane enriched fraction (PM) were analyzed in the kidney. Ponceau S staining served as a loading control. Blots show biological replicates. Dot-plot graphs show the results of quantitation.
Tacrolimus Prevents Ubiquitylation of Wild-Type KLHL3 but Not Nonphosphorylatable KLHL3S433A.
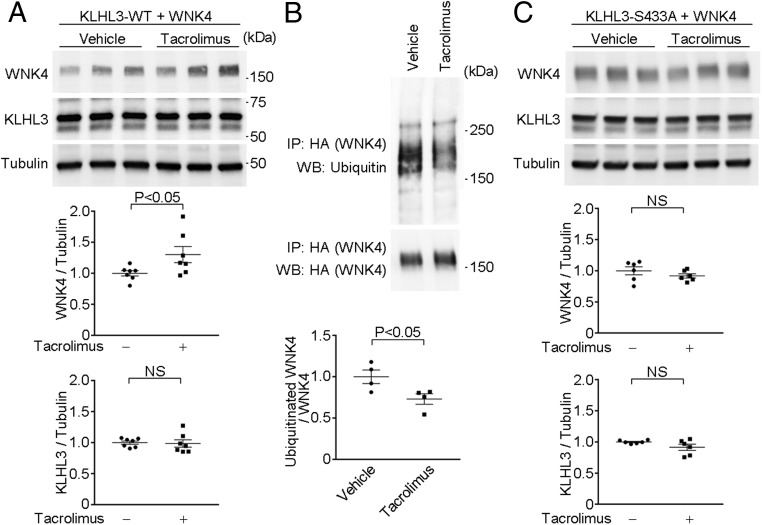
If tacrolimus produces WNK4 elevation by reducing its binding and ubiquitylation via KLHL3/CUL3, we expect levels of ubiquitylated WNK4 to be reduced (17). We transfected HEK cells with wild-type FLAG-KLHL3WT and WNK4-HA. We then incubated these cells with or without tacrolimus for 3 h, immunopurified WNK4, and analyzed the ubiquitylated WNK4 levels (17). Tacrolimus significantly increased WNK4 levels but reduced ubiquitylated WNK4 levels (Fig. 5 A and B). Moreover, these effects were abolished by the nonphosphorylatable alanine substitution KLHL3S433A (Fig. 5C), demonstrating that they were mediated by KLHL3S433 phosphorylation.
Fig. 5.
Calcineurin inhibitor tacrolimus abrogates WNK4 ubiquitylation, resulting in increased WNK4 levels. (A) Wild-type FLAG-KLHL3 and WNK4-HA plasmids were transfected in HEK cells, followed by incubation with tacrolimus or vehicle. Lysates were analyzed for WNK4, KLHL3, and tubulin levels by Western blotting. Blots show biological replicates. Dot-plot graphs show the results of quantitation of biological replicates normalized to the tubulin levels. (B) Wild-type FLAG-KLHL3 and WNK4-HA plasmids were transfected in HEK cells, followed by incubation with tacrolimus or vehicle. WNK4 was pulled down by HA-IP, and then Western blot analysis was performed using anti-ubiquitin antibody. Dot-plot graphs show the results of quantification. (C) FLAG-KLHL3 plasmid carrying the nonphosphorylatable S433A substitution and WNK4-HA plasmids were transfected in HEK cells, followed by incubation with tacrolimus or vehicle. Lysates were analyzed for WNK4, KLHL3, and tubulin levels by Western blotting. Blots show biological replicates. Dot-plot graphs show the results of quantitation normalized to the tubulin levels. NS, not significant.
Tacrolimus Inhibits K+-Induced Dephosphorylation of KLHL3S433-P.
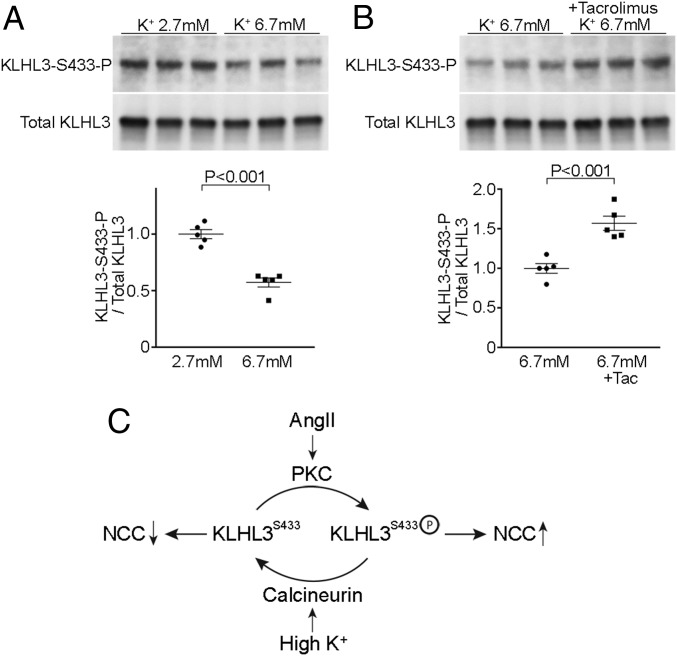
Prior research suggested that the elevation in plasma K+ level lowers WNK abundance, thereby reducing NCC activity and promoting distal nephron K+ secretion (35, 40). We hypothesized that the reduction of WNK4 with hyperkalemia might result from calcineurin-mediated dephosphorylation of KLHL3S433-P. To test this, HEK cells expressing KLHL3 were incubated in DMEM containing either 2.7 or 6.7 mM K+ for 1 h. We found that the increase in extracellular K+ levels resulted in significantly reduced KLHL3S433-P levels without altering total KLHL3 levels (Fig. 6A). If this effect is mediated by calcineurin, we expected that tacrolimus should prevent dephosphorylation. Therefore, cells were incubated with 6.7 mM K+ in the absence or presence of tacrolimus. We found that tacrolimus attenuated the dephosphorylation of KLHL3S433-P induced by high K+ (Fig. 6B). We therefore infer that calcineurin-mediated dephosphorylation of KLHL3S433-P mediates reduced levels of WNK4 seen with hyperkalemia.
Fig. 6.
Hyperkalemia dephosphorylates KLHL3S433-P through calcineurin. (A) HEK cells expressing FLAG-KLHL3 were incubated with DMEM containing either 2.7 or 6.7 mM K+ for 1 h. Lysates of cells were analyzed by Western blotting. Blots show biological replicates. Dot-plot graphs show the results of quantification. (B) HEK cells expressing FLAG-KLHL3 were incubated with DMEM containing 6.7 mM K+ in the presence or absence of tacrolimus (100 nM) for 1 h after preincubation with tacrolimus or vehicle for 10 min in DMEM containing 2.7 mM K+. Dot-plot graphs show the results of quantification. (C) Proposed mechanism that regulates KLHL3S433 phosphorylation levels. Calcineurin dephosphorylates KLHL3S433-P and activates KLHL3, resulting in reduced NCC activity by promoting WNK degradation. Conversely, PKC phosphorylates KLHL3S433, activating NCC via WNK/SPAK pathway.
Discussion
Previous studies in mice have shown that CNIs caused NCC activation and hypertension, consistent with studies in humans implicating NCC in the pathogenesis of CNI-induced hypertension (21–23). Although several lines of evidence indicate that these effects of CNIs are mediated in full or in part by the increased WNK abundance (29, 30), the direct target of calcineurin remained undetermined. We have previously shown that KLHL3 binds WNK4, resulting in WNK4 ubiquitylation and degradation (17). AngII signaling produces the phosphorylation of KLHL3 at Ser433, blocking WNK4 binding, thereby increasing WNK4 levels, and promoting the activation of NCC (19). We have demonstrated herein that calcineurin directly dephosphorylates KLHL3S433-P, and its inhibition by tacrolimus leads to an increase in WNK4 levels. The increase in WNK4 with tacrolimus treatment was sufficient to produce increased levels of phosphorylated SPAK and increased NCC in vivo, resulting in salt-sensitive hypertension.
Our findings are analogous to the observations in mutant KLHL3 knock-in mice, in which heterozygous KLHL3 mutation caused a modest increase in WNK levels (41). In these mice, the increase in WNK4 and in WNK1 was 1.4-fold and 1.8-fold, respectively, and these changes were sufficient to increase SPAK phosphorylation by more than threefold. These observations may be explained by the fact that KLHL3 targets both WNK4 and WNK1 isoforms for degradation; therefore, a KLHL3 mutation increases levels of both WNK4 and WNK1, acting synergistically to increase SPAK activity at a greater extent than would be seen with a WNK4 mutation alone. This inference is consistent with the observation that PHAII subjects with KLHL3 mutations have a markedly more severe phenotype than those carrying WNK4 or WNK1 mutations (5).
Regulation of WNK abundance and activity plays a critical role in AngII- and K+-mediated control of NCC. AngII, via PKC, activates the SPAK/NCC cascade by increasing WNK4 levels and kinase activity (15, 19, 42, 43). AngII-induced NCC activation is completely lost in WNK4 knockout mice (15) and in SPAK knock-in mice carrying nonphosphorylatable, inactive form of SPAK (42). Similarly, K+ depletion increases WNK4 abundance and activity in the kidney, likely mediated by increased KLHL3S433-P (35, 40). This low K+-induced NCC activation is abolished by WNK knockdown (40). The current study indicates that the phosphatase calcineurin antagonizes PKC-mediated phosphorylation of KLHL3 at Ser433, thereby regulating WNK abundance. These data are consistent with a recent study showing that basophilic kinases including PKC are associated with the mammalian calcineurin substrate network (44). In addition, calcineurin is shown to modestly prefer sites with a basic residue at the −3 position (45, 46), which fits with Arg430 at the −3 position found in KLHL3.
Aldosterone is produced in two distinct physiological states, intravascular volume depletion and hyperkalemia. Previous studies suggested that NCC and pendrin are involved in mechanisms whereby the kidney differentially responds to aldosterone in these conditions (8, 13, 19, 35, 40, 47, 48). Our observation that high K+ dephosphorylates KLHL3S433-P through calcineurin provides further insight into these mechanisms (Fig. 6C). In volume depletion, AngII promotes phosphorylation of KLHL3S433 through PKC activation (19). This results in KLHL3 inactivation and WNK accumulation, activating NCC through WNK/SPAK pathway. In contrast, in hyperkalemia, calcineurin dephosphorylates KLHL3S433-P, promoting WNK degradation and inhibiting NCC activation. These mechanisms likely act in concert with the regulation of WNK kinase activity by phosphorylation (43), and also by intracellular Cl− (8, 40, 49, 50).
As described above, AngII-dependent effects on NCC require the integrity of WNK/SPAK pathway (15, 19, 42, 43). In K+-mediated modulation of NCC, however, several lines of evidence indicate that both WNK/SPAK-dependent and WNK/SPAK-independent pathways may be involved (8, 32, 40, 42, 51–53). The current study demonstrates the involvement of calcineurin in the former pathway. The precise role of calcineurin in the latter pathway needs further evaluation. The elevation in extracellular K+ levels can depolarize DCT cells. However, the mechanism whereby this change results in increased calcineurin activity in DCT cells remains to be elucidated. This will be an interesting avenue for further studies.
Materials and Methods
Cell Culture.
HEK cells were incubated in DMEM supplemented with 10% FBS (19). Transfection was carried out using nonliposomal polymer (Mirus Bio) (17, 19). The expression plasmids encoding FLAG-KLHL3 and WNK4-HA were prepared as described (17, 19). Cells were depleted of serum for 12 h before the experiment. Ionomycin was added at 3 μM in accordance with previous studies (33, 34). Tacrolimus was added at 100 nM 3 h before ionomycin treatment. The concentration was determined according to previous studies (34, 37). To evaluate the effect of extracellular K+ on KLHL3S433-P levels, potassium-free DMEM was prepared in the laboratory, and K+ concentrations were adjusted by adding potassium gluconate in accordance with previous studies (40). HEK cells expressing KLHL3 were incubated with DMEM containing 2.7 mM K+ for 12 h before the experiments. Cells were then incubated with DMEM containing 2.7 or 6.7 mM K+ for 1 h. To test the involvement of calcineurin, HEK cells expressing KLHL3 were incubated with DMEM containing 2.7 mM K+ for 12 h before the experiments. Before changing to DMEM containing 6.7 mM K+, cells were preincubated with 100 nM tacrolimus or vehicle for 10 min. Then, medium was changed to DMEM containing 6.7 mM K+ with tacrolimus or vehicle, and the cells were incubated for 1 h. mDCT cells (36) were kindly provided by Peter Friedman, University of Pittsburgh, Pittsburgh, and were incubated with DMEM/F12 supplemented with 5% FBS.
Western Blotting.
Western blotting was performed as described (47). Plasma membrane fraction was purified using plasma membrane isolation kit (Invent biotechnologies). Enrichment in plasma proteins was confirmed in the laboratory (54). Equal amounts of protein were mixed with Laemmli sample buffer, separated on polyacrylamide gel, and transferred to nitrocellulose membrane. The membrane was incubated with primary and peroxidase-conjugated secondary antibodies, followed by imaging using ECL reagents (Perkin-Elmer). Tubulin and Ponceau S staining were used to ensure equal loading and transfer of samples. For the detection of ubiquitylated WNK4, WNK4-HA was purified by HA- immunoprecipitation (IP) from the cell lysates, and the ubiquitylated WNK4 was detected by Western blotting using anti-ubiquitin antibody (47). Monoclonal mouse antibody against KLHL3S433-P was created in the laboratory as described (35) and was further characterized in this study. Rabbit polyclonal antibodies against NCC phosphorylated at T53 is kindly provided by Johannes Loffing, University of Zurich, Zurich. Other antibodies include anti-KLHL3 (19), anti-FLAG, anti-tubulin (all from Sigma), anti-WNK4 (created in the laboratory) (35), anti-phospho SPAK/OSR1, anti-NCC (all from Millipore), anti-total SPAK, anti-ubiquitin, anti-WNK1 (55), and anti-calcineurin A (Cell Signaling).
RNAi Studies.
DNA encoding FLAG-KLHL3 was introduced by using TransIT-X2 reagent. After 24 h, the medium was changed and indicated siRNAs were introduced to HEK cells by using TransIT-X2 reagent, and incubated for another 24 h. We obtained siRNA against PPP3CA (encoding calcineurin A-α) and control siRNA from Dharmacon (On-Target plus). The specificity of the siRNA is validated by the vendor and is further confirmed in this study.
Immunoprecipitation and in Vitro Phosphatase Assay.
FLAG-KLHL3 was expressed in HEK cells and purified by immunoprecipitation. The purified KLHL3 (which contains KLHL3S433-P) was incubated with recombinant human calcineurin (Enzo; 0.6 μM) in a buffer containing 50 mM Hepes, 1 mg/mL BSA, 1 mM DTT, 1 mM CaCl2, and 0.5 μM calmodulin at 37 °C for 8 h, and KLHL3S433-P levels were analyzed by Western blotting. The liberated phosphate levels in the reaction mix were quantitated by malachite green assay using commercially available kit (Abcam). The concentrations of calcineurin and calmodulin are in accordance with previous studies showing NFAT dephosphorylation by calcineurin (39).
Animal Studies.
Animal procedures were approved by the Teikyo University Ethics Committee for Animal Experiments (no. 14-018). Male C57BL/6J mice (Tokyo Laboratory Animals) were fed ad libitum and housed under a 12-h light cycle. Animals received s.c. injection of tacrolimus (1 mg/kg per d) or vehicle for 2 d (n = 5 for control and n = 6 for tacrolimus group) and for 14 d (n = 7 for control and n = 7 for tacrolimus group) under anesthesia. The dose of tacrolimus was in accordance with the previous study (29). In some experiments, mice received a high-salt (8%) diet (n = 6 for control and n = 6 for tacrolimus group), in accordance with previous studies (29). Systolic blood pressure was measured using volumetric pressure recording (CODA; Kent Scientific), as described (54).
Immunostaining.
Immunofluorescence study was performed as described (19, 47). We used polyclonal rabbit anti-KLHL3S433-P antibodies for immunostaining (19). NCC and KLHL3S433-P were stained in the adjacent sections because both antibodies were made from rabbits.
Statistical Analysis.
The data are summarized as mean ± SEM. Unpaired t test was used for comparisons between two groups. For multiple comparisons, statistical analysis was performed by ANOVA followed by Tukey post hoc tests. A P value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Peter Friedman and Dr. Tatsuo Shimosawa for providing mDCT cells and Dr. Johannes Loffing for providing phosphorylated NCC antibodies. This work was supported by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research 15H04837 (to S.S.) and 17K16097 (to K.I.); the Suzuki Memorial Foundation (S.S.); the Takeda Science Foundation (S.S.), and NIH Grant P01DK17433 (to R.P.L.).
Footnotes
Conflict of interest statement: R.P.L. is a nonexecutive director of Roche and its subsidiary Genentech.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817281116/-/DCSupplemental.
References
- 1.Rusnak F, Mertz P. Calcineurin: Form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- 2.Fric J, et al. NFAT control of innate immunity. Blood. 2012;120:1380–1389. doi: 10.1182/blood-2012-02-404475. [DOI] [PubMed] [Google Scholar]
- 3.Morales JM, Domínguez-Gil B. Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J Am Soc Nephrol. 2006;17(12 Suppl 3):S296–S303. doi: 10.1681/ASN.2006080930. [DOI] [PubMed] [Google Scholar]
- 4.Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 5.Boyden LM, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinehart J, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chávez-Canales M, et al. WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension. 2014;64:1047–1053. doi: 10.1161/HYPERTENSIONAHA.114.04036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazúa-Valenti S, et al. The effect of WNK4 on the Na+-Cl- cotransporter is modulated by intracellular chloride. J Am Soc Nephrol. 2015;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalioti MD, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 11.Yang SS, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Richardson C, et al. Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 13.San-Cristobal P, et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SS, et al. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castañeda-Bueno M, et al. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109:7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohta A, et al. The CUL3-KLHL3 E3 ligase complex mutated in Gordon’s hypertension syndrome interacts with and ubiquitylates WNK isoforms: Disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013;451:111–122. doi: 10.1042/BJ20121903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci USA. 2013;110:7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakabayashi M, et al. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013;3:858–868. doi: 10.1016/j.celrep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S, et al. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci USA. 2014;111:15556–15561. doi: 10.1073/pnas.1418342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon RD, Hodsman GP. The syndrome of hypertension and hyperkalaemia without renal failure: Long term correction by thiazide diuretic. Scott Med J. 1986;31:43–44. doi: 10.1177/003693308603100114. [DOI] [PubMed] [Google Scholar]
- 21.Rojas-Vega L, et al. Increased phosphorylation of the renal Na+-Cl- cotransporter in male kidney transplant recipient patients with hypertension: A prospective cohort. Am J Physiol Renal Physiol. 2015;309:F836–F842. doi: 10.1152/ajprenal.00326.2015. [DOI] [PubMed] [Google Scholar]
- 22.Moes AD, Hesselink DA, van den Meiracker AH, Zietse R, Hoorn EJ. Chlorthalidone versus amlodipine for hypertension in kidney transplant recipients treated with tacrolimus: A randomized crossover trial. Am J Kidney Dis. 2017;69:796–804. doi: 10.1053/j.ajkd.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Tutakhel OAZ, et al. NaCl cotransporter abundance in urinary vesicles is increased by calcineurin inhibitors and predicts thiazide sensitivity. PLoS One. 2017;12:e0176220. doi: 10.1371/journal.pone.0176220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciresi DL, Lloyd MA, Sandberg SM, Heublein DM, Edwards BS. The sodium retaining effects of cyclosporine. Kidney Int. 1992;41:1599–1605. doi: 10.1038/ki.1992.231. [DOI] [PubMed] [Google Scholar]
- 25.Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993;91:2144–2149. doi: 10.1172/JCI116440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marumo T, et al. Cyclosporin A inhibits nitric oxide synthase induction in vascular smooth muscle cells. Hypertension. 1995;25:764–768. doi: 10.1161/01.hyp.25.4.764. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, et al. Cyclosporine A-induced hypertension involves synapsin in renal sensory nerve endings. Proc Natl Acad Sci USA. 2000;97:9765–9770. doi: 10.1073/pnas.170160397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiyama A, et al. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension. 2003;42:754–760. doi: 10.1161/01.HYP.0000085195.38870.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoorn EJ, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011;17:1304–1309. doi: 10.1038/nm.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melnikov S, Mayan H, Uchida S, Holtzman EJ, Farfel Z. Cyclosporine metabolic side effects: Association with the WNK4 system. Eur J Clin Invest. 2011;41:1113–1120. doi: 10.1111/j.1365-2362.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 31.Lazelle RA, et al. Renal deletion of 12 kDa FK506-binding protein attenuates tacrolimus-induced hypertension. J Am Soc Nephrol. 2016;27:1456–1464. doi: 10.1681/ASN.2015040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoda W, et al. Calcineurin inhibitors block sodium-chloride cotransporter dephosphorylation in response to high potassium intake. Kidney Int. 2017;91:402–411. doi: 10.1016/j.kint.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Shaw KT, et al. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci USA. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 35.Ishizawa K, et al. Potassium depletion stimulates Na-Cl cotransporter via phosphorylation and inactivation of the ubiquitin ligase Kelch-like 3. Biochem Biophys Res Commun. 2016;480:745–751. doi: 10.1016/j.bbrc.2016.10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magyar CE, White KE, Rojas R, Apodaca G, Friedman PA. Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. Am J Physiol Renal Physiol. 2002;283:F29–F40. doi: 10.1152/ajprenal.00252.2000. [DOI] [PubMed] [Google Scholar]
- 37.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 38.Gooch JL, Roberts BR, Cobbs SL, Tumlin JA. Loss of the alpha-isoform of calcineurin is sufficient to induce nephrotoxicity and altered expression of transforming growth factor-beta. Transplantation. 2007;83:439–447. doi: 10.1097/01.tp.0000251423.78124.51. [DOI] [PubMed] [Google Scholar]
- 39.Aramburu J, et al. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 40.Terker AS, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Susa K, et al. Impaired degradation of WNK1 and WNK4 kinases causes PHAII in mutant KLHL3 knock-in mice. Hum Mol Genet. 2014;23:5052–5060. doi: 10.1093/hmg/ddu217. [DOI] [PubMed] [Google Scholar]
- 42.Castañeda-Bueno M, et al. Modulation of NCC activity by low and high K(+) intake: Insights into the signaling pathways involved. Am J Physiol Renal Physiol. 2014;306:F1507–F1519. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castañeda-Bueno M, et al. Phosphorylation by PKC and PKA regulate the kinase activity and downstream signaling of WNK4. Proc Natl Acad Sci USA. 2017;114:E879–E886. doi: 10.1073/pnas.1620315114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman A, et al. The calcineurin signaling network evolves via conserved kinase-phosphatase modules that transcend substrate identity. Mol Cell. 2014;55:422–435. doi: 10.1016/j.molcel.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donella-Deana A, Krinks MH, Ruzzene M, Klee C, Pinna LA. Dephosphorylation of phosphopeptides by calcineurin (protein phosphatase 2B) Eur J Biochem. 1994;219:109–117. doi: 10.1111/j.1432-1033.1994.tb19920.x. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Wilmanns M, Thornton J, Köhn M. Elucidating human phosphatase-substrate networks. Sci Signal. 2013;6:rs10. doi: 10.1126/scisignal.2003203. [DOI] [PubMed] [Google Scholar]
- 47.Shibata S, et al. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660–671. doi: 10.1016/j.cmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibata S, et al. ULK1 phosphorylates and regulates mineralocorticoid receptor. Cell Rep. 2018;24:569–576. doi: 10.1016/j.celrep.2018.06.072. [DOI] [PubMed] [Google Scholar]
- 49.Piala AT, et al. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang MX, et al. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018;93:893–902. doi: 10.1016/j.kint.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, et al. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1) Proc Natl Acad Sci USA. 2014;111:11864–11869. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wade JB, et al. SPAK-mediated NCC regulation in response to low-K+ diet. Am J Physiol Renal Physiol. 2015;308:F923–F931. doi: 10.1152/ajprenal.00388.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penton D, et al. Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl- -dependent and independent mechanisms. J Physiol. 2016;594:6319–6331. doi: 10.1113/JP272504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu N, et al. Hypokalemia and pendrin induction by aldosterone. Hypertension. 2017;69:855–862. doi: 10.1161/HYPERTENSIONAHA.116.08519. [DOI] [PubMed] [Google Scholar]
- 55.Gallolu Kankanamalage S, et al. Multistep regulation of autophagy by WNK1. Proc Natl Acad Sci USA. 2016;113:14342–14347. doi: 10.1073/pnas.1617649113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.