Significance
Although half of the world’s population could face severe food crisis as a result of global warming by the end of this century, the effects of environmental temperature and host nutritional status in host defense to viral infection in vivo are less clear. Here, we demonstrated that exposure of mice to the high ambient temperature of 36 °C reduced their food intake and impaired adaptive immune responses to influenza virus infection. In addition, we found that administration of glucose or dietary short-chain fatty acids restored influenza virus-specific adaptive immune responses in high heat-exposed mice. Our results imply possible public health problems and concerns that outside temperature and host nutritional status may be critical determinants of viral pathogenesis or vaccine efficacy.
Keywords: global warming, vector-borne diseases, immunity to viral infection, autophagy, inflammasomes
Abstract
Although climate change may expand the geographical distribution of several vector-borne diseases, the effects of environmental temperature in host defense to viral infection in vivo are unknown. Here, we demonstrate that exposure of mice to the high ambient temperature of 36 °C impaired adaptive immune responses against infection with viral pathogens, influenza, Zika, and severe fever with thrombocytopenia syndrome phlebovirus. Following influenza virus infection, the high heat-exposed mice failed to stimulate inflammasome-dependent cytokine secretion and respiratory dendritic cell migration to lymph nodes. Although commensal microbiota composition remained intact, the high heat-exposed mice decreased their food intake and increased autophagy in lung tissue. Induction of autophagy in room temperature-exposed mice severely impaired virus-specific CD8 T cells and antibody responses following respiratory influenza virus infection. In addition, we found that administration of glucose or dietary short-chain fatty acids restored influenza virus-specific adaptive immune responses in high heat-exposed mice. These findings uncover an unexpected mechanism by which ambient temperature and nutritional status control virus-specific adaptive immune responses.
The innate immune system, the first line of defense against pathogens, utilizes pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (1, 2). The recognition of influenza virus plays a key role not only in limiting virus replication and inflammatory responses at early stages of infection, but also in initiating and orchestrating virus-specific adaptive immune responses (3, 4). Influenza virus is recognized by at least three PRRs. First, influenza genomic RNA is recognized by TLR-7 in late endosomes (5, 6). Second, the cytosolic sensor retinoic acid inducible gene I (RIG-I) directly interacts with the panhandle structure of the viral nucleocapsid and detects the uncapped 5′-triphosphate RNA of the viral genome (7–10). Third, the influenza virus M2 protein, a proton-selective ion channel, stimulates ion flux from the trans-Golgi network and activates the Nod-like receptor family, pyrin domain-containing 3 (NLRP3) (11). Upon activation, the NLRP3 is recruited to the mitochondria via mitochondrial antiviral signaling (MAVS) or mitofusin 2, which in turn recruits the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase-1 to form the multimolecular protein complex termed the NLRP3 inflammasome (12, 13). Formation of the NLRP3 inflammasome activates caspase-1, which cleaves the precursor forms of proinflammastory cytokines, such as IL-1β and IL-18, and stimulates their secretion (14).
After infection with influenza virus, inflammasome activation in the lung and IL-1R signaling in pulmonary dendritic cells (DCs) are essential for migration of antigen-captured DCs from the lung to the draining mediastinal lymph nodes (mLNs) to generate virus-specific adaptive immune responses (15, 16). We previously demonstrated that antibiotic-treated (Abx) mice fail to stimulate inflammasome-dependent cytokine release in the lung and mount protective CD8+ T cell responses following influenza virus infection (17). Intact microbiota in the gut may provide tonic IFN signals (18) leading to the expression of mRNA for pro–IL-1β and pro–IL-18 in the lung at steady state (15). Consistent with their beneficial effects on antiviral immunity, recent studies highlight the importance of microbiota-derived metabolites in protection against influenza virus infection (19, 20). Although several extrinsic factors such as diet or cold exposure may affect the gut microbiota composition (21, 22), it remains unclear whether ambient temperature critically regulates the generation of influenza virus-specific adaptive immune responses through the changes in the gut microbiota composition.
Here, we show that exposure of mice at the high ambient temperature of 36 °C severely impaired adaptive immune responses against influenza virus infection. The inability of heat-exposed mice to mount the virus-specific adaptive immune responses was not due to change of gut microbiota composition but was instead due to reduction of their food intake. Notably, we found that administration of glucose or short-chain fatty acids (SCFAs) restored the virus-specific adaptive immune responses in high heat-exposed mice. Our findings here have identified a previously unknown mechanism by which outside temperature and nutritional status control the virus-specific adaptive immune responses.
Results
Defective Immune Responses Against Influenza Virus Infection in High Heat-Exposed Mice.
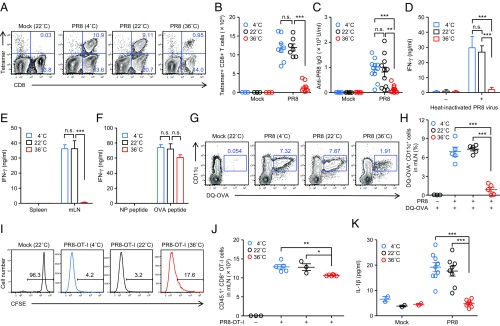
To assess the effects of ambient temperature in the induction of adaptive immunity to influenza virus infection, mice were kept at 4, 22, or 36 °C for 7 d before influenza virus infection. Cold or high-heat exposure of naïve mice was generally well tolerated (SI Appendix, Fig. S1A). Although cold-exposed naïve mice exhibited significant increase in their food intake compared with room temperature (RT)-exposed group (SI Appendix, Fig. S1B), high-heat exposure of naïve mice significantly reduced their food intake and body weight by 10% (SI Appendix, Fig. S1 B and C). Cold- or high heat-exposed mice were then infected i.n. with a sublethal dose (30 pfu) of A/PR8 influenza virus. After infection with influenza virus, cold- or high heat-exposed mice were kept at 4 or 36 °C, respectively, for the entire duration of the experiments. Remarkably, both frequency (Fig. 1A and SI Appendix, Fig. S2) and number (Fig. 1B) of influenza virus-specific CD8+ T cells in the lung as well as the virus-specific IgG antibody (Fig. 1C) and CD4+ T cell responses (Fig. 1D) were severely impaired in high heat-exposed mice. As a consequence, viral titer in the lung remained significantly elevated in the high heat-exposed mice at 7 d postinfection (p.i.) (SI Appendix, Fig. S3A). Further, freshly isolated mLN DCs from high heat-exposed mice infected with recombinant influenza A virus expressing the MHC-I OVA peptide SIINFEKL in the neuraminidase (NA) stalk of the A/PR8 backbone (PR8–OT-I virus), in the absence of exogenous peptide, induced no differentiation of OT-I CD8 TCR Tg T cells specific for the OVA epitope presented on H-2Db ex vivo (Fig. 1E). This defect was likely due to impairment of migration of antigen-captured lung DCs to the mLN in high heat-exposed mice, because the same mLN DCs were able to differentiate OT-I naïve CD8 T cells after exogenous OVA peptide addition (Fig. 1F). In addition, migration of antigen-captured lung DCs to the mLN was severely impaired in high heat-exposed mice (Fig. 1 G and H), in which MHC-II+ CD11c+ lung DCs were comparable to that seen in RT-exposed mice (SI Appendix, Fig. S4). These data suggest that migration of antigen-captured lung DCs to the mLN are impaired in high heat-exposed mice. Consequently, proliferation of adoptively transferred naïve OT-I CD8+ T cells was reduced in the mLNs of high heat-exposed mice at 5 d after infection with PR8–OT-I virus (Fig. 1 I and J).
Fig. 1.
High heat-exposed mice fail to induce adaptive immunity to influenza virus infection. Mice were kept at 4, 22, or 36 °C for 7 d before influenza virus infection (30 pfu per mouse) and throughout infection. (A) Ten days later, lymphocytes were isolated from the lung. Influenza virus-specific CD8+ T cells were then detected using the H-2Db influenza virus nucleoprotein (NP) tetramer. (B) Total numbers of influenza virus-specific CD8+ T cells in the lung are shown. (C) Serum was collected at 10 d postinfection. Influenza virus-specific serum IgG levels were measured by ELISA. (D) CD4+ T cells were isolated from spleen and restimulated with irradiated splenocytes in the presence or absence of inactivated influenza virus for 72 h, and IFN-γ production from CD4+ T cells was measured by ELISA. (E and F) Naïve OT-I CD8+ T cells (2 × 105 cells per well) were cocultured with CD11c+ DCs (1 × 105 cells per well) that were isolated from the mLNs of PR8–OT-I virus-infected animals with (F) or without (E) NP or OVA peptide. Splenic DCs (1 × 105 cells per well) from infected animals were used as a negative control. Seventy-two hours later, IFN-γ production was measured by ELISA. (G and H) Mice were inoculated intranasally with DQ-ovalbumin (DQ-OVA). Six hours later, mice were infected with 1,000 pfu of PR8 viruses. Eighteen hours after infection, mLNs were collected. Numbers adjacent to outlined areas indicate percent DQ-OVA+ CD11c+ DCs (G). Percentages of DQ-OVA+ CD11c+ DCs are shown (H). (I) Mice were injected with CFSE-labeled OT-I CD8+ CD45.1+ T cells on the day before infection with 1,000 pfu of PR8–OT-I viruses. Five days after influenza virus infection, mLNs were excised to assess T cell proliferation by CFSE dilution. (J) Total numbers of CD45.1+ cells in the mLNs are shown. (K) The BALF was collected from influenza virus-infected animals at 2 d postinfection. IL-1β levels in BALF were determined by ELISA. The data are representative of three independent experiments (A, G, and I) or are from three independent experiments (B, F, H, J–K; mean ± SEM). *P < 0.05, **P < 0.01, and ***P < 0.001; n.s., not significant (one-way ANOVA and Tukey’s test).
We next examined whether influenza virus replicates in the lung of high heat-exposed mice. We found that high heat-exposed mice sustained high virus burden in the lung until 7 d p.i. (SI Appendix, Fig. S3A). In addition, the extent of infection by the influenza virus in the lung of high heat-exposed mice was similar to that of RT-exposed mice (SI Appendix, Fig. S3B), suggesting that the inability of heat-exposed mice to mount the virus-specific adaptive immune responses was not due to viral replication in the lung tissue. Migration of antigen-captured lung DCs to the mLN and induction of influenza virus-specific CD8+ T cell responses require inflammasome activation and IL-1R signaling in pulmonary DCs (15, 16). This led us to consider the possibility that high heat-exposed mice fail to stimulate inflammasome-dependent cytokine secretion following influenza virus infection. To test this possibility, we measured the levels of secreted IL-1β in the bronchoalveolar lavage fluid (BALF) of cold-, RT-, or high heat-exposed mice infected with influenza virus. Notably, high heat-exposed mice impaired secretion of mature IL-1β in the BALF (Fig. 1K) as well as mRNA expression of pro–IL-1β in the lung tissues and secretion of IFN-α, IL-6, IL-12p40, and TNF-α (SI Appendix, Fig. S5) following influenza virus infection. These data indicated that immune responses in high heat-exposed mice are impaired, and critical pathways known to initiate adaptive immune responses including inflammasome activation and lung DC migration are severely compromised in mice maintained at high-heat ambient temperature.
Effects of High-Heat Exposure of Mice in the Induction of Adaptive Immunity to Vector-Borne Pathogens.
To examine whether the high-heat exposure of mice resulted in general immunodeficiency, we immunized cold-, RT-, and high heat-exposed mice with formalin-inactivated influenza virus vaccine and aluminum adjuvant. Unlike lung infection with influenza virus (Fig. 1), immunization with influenza virus vaccine and alum led to normal antibody (SI Appendix, Fig. S6 A and B) and T cell responses (SI Appendix, Fig. S6 C and D) in high heat-exposed mice. We next examined the effects of high-heat exposure of mice in the induction of adaptive immune response to vector-borne pathogens. To this end, we infected RT- or high heat-exposed mice i.p. with Zika virus (ZIKV) or severe fever with thrombocytopenia syndrome phlebovirus (SFTSV), a tick-borne human pathogenic virus. We observed normal IgG antibodies specific for ZIKV and SFTSV in high heat-exposed mice (SI Appendix, Fig. S7 A and B). In contrast, IFN-γ–producing CD4+ T cells were considerably reduced in high heat-exposed mice following i.p. infection with ZIKV or SFTSV (SI Appendix, Fig. S7 C–H). These data suggested that the effects of high-heat exposure of mice in the induction of adaptive immune responses are also impaired against other viral pathogens.
Commensal Bacteria Composition Remains Intact in High Heat-Exposed Mice.
Cold exposure of mice leads to change in the gut microbiota composition (21). In addition, intact commensal microbiota is required for adaptive immune responses to influenza virus infection (17, 18). These observations led us to consider the possibility that high-heat exposure of mice changes commensal bacteria composition, which could dampen adaptive immune responses to influenza virus infection. To test this possibility, we assessed the effects of high-heat exposure on bacterial load and composition in the cecum. Consistent with previous reports (17, 23), antibiotic treatment resulted in significant changes in the composition of commensal bacteria (SI Appendix, Fig. S8 A and B). Both frequency (SI Appendix, Fig. S8C) and number (SI Appendix, Fig. S8D) of influenza virus-specific CD8+ T cells in the lung were diminished in the Abx mice. Consequently, Abx mice enhanced susceptibility to both low (50 pfu) and high (200 pfu) doses of influenza virus infection (SI Appendix, Fig. S8 E and F). Further, rectal inoculation of LPS restored immune responses to influenza virus infection in Abx mice (SI Appendix, Fig. S8 G–I).
In contrast to Abx mice, high heat-exposed mice did not change in the amount of 16S rRNA (SI Appendix, Fig. S9A) and the composition of commensal bacteria (SI Appendix, Fig. S9 B and C) present in the cecum. Consistent with a previous report (21), profiling of the microbiota composition by 16S rRNA gene sequencing, followed by principal coordinates analysis (PCoA) revealed significantly different clustering of the microbiota in the cecum of cold-exposed mice that was distinct from that of RT-exposed mice (SI Appendix, Fig. S9D). In contrast, high-heat exposure of mice did not significantly change the bacterial clusters in the cecum (SI Appendix, Fig. S9D). In addition, rectal inoculation of LPS did not restore immune responses to influenza virus infection in high heat-exposed mice (SI Appendix, Fig. S9 E–G). These data indicated that rectal TLR stimulation is insufficient to restore immune responses in high heat-exposed mice and further suggested that commensal bacteria composition is unlikely to account for immune defects in high heat-exposed mice.
High-Heat Exposure-Induced Autophagy Regulates Adaptive Immune Responses to Influenza Virus Infection.
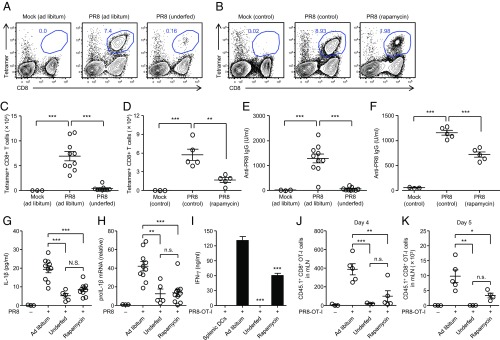
Next, we asked how high-heat exposure of mice suppresses the generation of adaptive immunity to influenza virus in the lung. High heat-exposed mice reduced their food intake and body weight by 10% (SI Appendix, Fig. S1). As a consequence, the level of light chain 3 (LC3)-II, a marker specific for autophagosome, was increased in the lung of high heat-exposed mice (SI Appendix, Fig. S10 A and B). In addition, flow cytometric analysis revealed that both CD45.2− epithelial cells and CD11c+ DCs significantly increased autophagy in the lung of high heat-exposed mice (SI Appendix, Fig. S10 C–E). Autophagy and mitophagy restrict inflammasome-dependent cytokine release by regulating the amounts of pro–IL-1β and damaged mitochondria, respectively (24, 25). These observations led us to consider the possibility that high-heat exposure of mice induces autophagy in the lung, which inhibits IL-1β secretion following influenza virus infection. To determine the importance of autophagy in the induction of adaptive immune responses against influenza virus infection, we induced autophagy in vivo by rapamycin treatment or nutrient starvation by established methods (26, 27) before influenza virus infection. The level of LC3-II was increased in the lung of mice starved for 24 h or injected i.v. with rapamycin (SI Appendix, Fig. S11), without affecting viral load in the lung at 3 d p.i. (SI Appendix, Fig. S12), the amount of 16S rRNA (SI Appendix, Fig. S13A), the gut microbiota composition (SI Appendix, Fig. S13 B and C), or their clusters (SI Appendix, Fig. S9D). Notably, induction of autophagy by nutrient starvation or treatment with rapamycin impaired frequency (Fig. 2 A and B) and total number (Fig. 2 C and D) of influenza virus-specific CD8+ T cells in the lung as well as the virus-specific IgG antibody (Fig. 2 E and F). Further, secretion of mature IL-1β in the BALF (Fig. 2G) as well as mRNA expression of pro–IL-1β (Fig. 2H) were impaired in the lung tissues of food-restricted or rapamycin-treated groups compared with ad libitum-fed mice. Consequently, migration of antigen-captured lung DCs to the mLN (Fig. 2I and SI Appendix, Fig. S14) as well as proliferation of OT-I naïve CD8 T cells in the mLNs at 4 and 5 d p.i. (Fig. 2 J and K) were reduced in food-restricted or rapamycin-treated groups. Collectively, these data indicated that induction of autophagy in the lung by high-heat exposure impaired adaptive immune responses to influenza virus infection.
Fig. 2.
Starvation-induced autophagy impaired influenza virus-specific adaptive immune responses. Mice were treated i.v. with rapamycin daily from day −2 to day 9 during infection or kept on food-restricted or ad libitum-fed condition for 7 d before influenza virus infection (30 pfu per mouse) and throughout infection. (A and B) Ten days later, lymphocytes were isolated from the lung. Influenza virus-specific CD8+ T cells were then detected using the H-2Db influenza virus NP tetramer. Numbers adjacent to outlined areas indicate percent tetramer-positive CD8+ T cells. (C and D) Total numbers of influenza virus-specific CD8+ T cells in the lung are shown. (E and F) Serum was collected at 10 d postinfection. Influenza virus-specific serum IgG levels were measured by ELISA. (G) The BALF was collected from influenza virus-infected animals at 2 d postinfection. IL-1β levels in BALF were determined by ELISA. (H) Total RNAs were extracted from the lung of the mice at 0 and 24 h postinfection. mRNA levels of pro–IL-1β were assessed by quantitative RT-PCR. GAPDH was used as an internal control. (I) Naïve OT-I CD8+ T cells (1 × 105 cells per well) were cocultured with CD11c+ DCs (1 × 105 cells per well) that were isolated from the mLNs of PR8–OT-I virus-infected animals. Splenic DCs (1 × 105 cells per well) from infected animals were used as a negative control. Seventy-two hours later, IFN-γ production was measured by ELISA. (J and K) Mice were injected with CFSE-labeled OT-I CD8+ CD45.1+ T cells on the day before infection with 1,000 pfu of PR8–OT-I viruses. Four (J) or 5 (K) d after influenza virus infection, mLNs were excised to assess T cell proliferation by CFSE dilution. Total numbers of CD8+ CD45.1+ OT-I cells in the mLNs are shown. The data are representative of two independent experiments (A and B) or from two independent experiments (C–K; mean ± SEM). *P < 0.05, **P < 0.01, and ***P < 0.001; n.s., not significant (one-way ANOVA and Tukey’s test).
Signals Coming from ad Libitum-Fed Mice Restore Influenza Virus-Specific Adaptive Immune Responses in Underfed Mice.
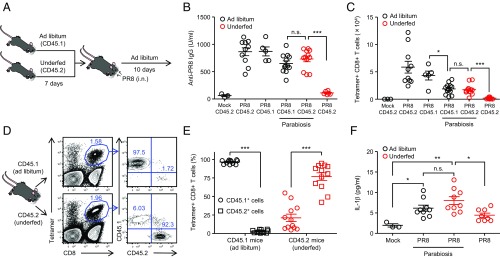
Next we investigated whether immune defects in underfed mice could be rescued by signals coming from ad libitum-fed mice. To this end, we surgically joined CD45.1+ ad libitum-fed mice with CD45.2+ underfed mice at the time of influenza virus challenge (Fig. 3A). After the challenge, the parabionts were kept under ad libitum self-feeding condition (Fig. 3A). Self-feeding condition after influenza virus infection was insufficient to restore influenza virus-specific CD8+ T cell responses of underfed mice (SI Appendix, Fig. S15). Strikingly, CD45.2+ underfed parabiotic mice surgically joined CD45.1+ ad libitum-fed mice restored influenza virus-specific IgG antibody (Fig. 3B) and CD8+ T cell responses in the lung (Fig. 3C). In addition, most of the virus-specific CD8+ T cells in the lung of underfed parabiotic mice were found to be of host-derived CD45.2+ cells (Fig. 3 D and E). Further, underfed parabiotic mice secreted IL-1β into the alveolar space to the levels of ad libitum-fed parabiotic mice in response to influenza virus infection (Fig. 3F). These data indicated that signals coming from ad libitum-fed parabiotic mice within 10 d restored immune responses to influenza virus infection in underfed parabiotic mice through systemic circulation.
Fig. 3.
Signals from ad libitum-fed mice restore immune defects in underfed mice. (A) Mice were kept on food-restricted or ad libitum-fed condition for 7 d before influenza virus infection (30 pfu per mouse). Pairs of age-matched underfed C57BL/6 (CD45.2) and ad libitum B6-Ly5.1 (CD45.1) mice were surgically joined at the time of infection and kept on ad libitum-fed condition for 10 d. (B) Serum was collected at 10 d postinfection. Influenza virus-specific serum IgG levels were measured by ELISA. (C) Ten days later, lymphocytes were isolated from the lung. Influenza virus-specific CD8+ T cells were then detected using the H-2Db influenza virus NP tetramer. Total numbers of influenza virus-specific CD8+ T cells in the lung are shown. (D) Ten days later, the presence of influenza virus-specific host and donor CD8+ T cells in the lung of parabiotic mice was analyzed by flow cytometry. (E) Host-derived and partner-derived tetramer-positive CD8+ T cells (n = 12 pairs) in lung tissue are shown. (F) The BALF was collected from influenza virus-infected animals at 2 d postinfection. IL-1β levels in BALF were determined by ELISA. The data are representative of two independent experiments (D) or from two independent experiments (B, C, E, and F; mean ± SEM). *P < 0.05, **P < 0.01, and ***P < 0.001; n.s., not significant (one-way ANOVA and Tukey’s test).
Glucose and SCFAs Restore Influenza Virus-Specific Adaptive Immune Responses in High Heat-Exposed Mice.
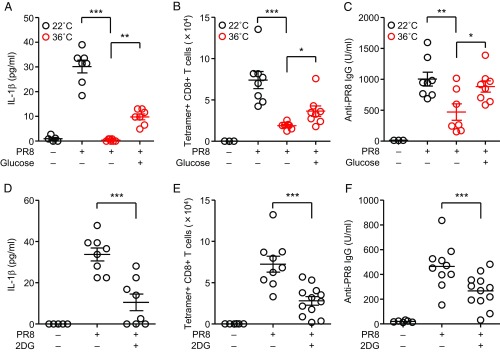
Thus far, our data indicated that reduced feeding behavior impaired the virus-specific CD8 T cells and antibody responses in underfed or high heat-exposed mice. A recent study has demonstrated that gavage of glucose protects mice from lethal influenza virus infection (28). In addition, Abx mice fail to mount protective CD8+ T cell responses following influenza virus infection (17) (SI Appendix, Fig. S8). These results led us to focus on the role of glucose or microbiota-derived SCFAs in the induction of adaptive immune responses to influenza virus infection. We hypothesized that glucose utilization or diet-derived SCFAs may be required for induction of adaptive immunity induced by influenza virus infection. To address these possibilities, high heat-exposed mice were given 1 M glucose in drinking water for the entire duration of the experiments. Then, we injected high heat-exposed mice with glucose i.v. daily from day −1 to day 3 during infection. Strikingly, we found that i.v. injection with glucose significantly enhanced secretion of mature IL-1β in the BALF, influenza virus-specific CD8 T cells, and antibody responses in high heat-exposed mice (Fig. 4 A–C). Injection of high heat-exposed naïve mice with glucose i.v. did not enhance secretion of mature IL-1β in the BALF (SI Appendix, Fig. S16). In addition, both secretion of mature IL-1β in the BALF and the virus-specific adaptive immune responses were significantly reduced in RT-exposed mice by blockade of glucose utilization with 2-deoxy-d-glucose (2DG) (Fig. 4 D–F), suggesting that glucose utilization is critical to mount adaptive immune responses following respiratory influenza virus infection.
Fig. 4.
Glucose utilization is required to mount adaptive immunity against influenza virus infection. (A–C) Mice were kept at 22 °C or 36 °C for 7 d before influenza virus infection and throughout infection. High heat-exposed mice were treated with glucose i.v. daily from day −1 to day 3 during infection. (A) The BALF was collected from influenza virus-infected animals at 2 d postinfection. IL-1β levels in BALF were determined by ELISA. (B) Ten days later, lymphocytes were isolated from the lung. Influenza virus-specific CD8+ T cells were then detected using the H-2Db influenza virus NP tetramer. Total numbers of influenza virus-specific CD8+ T cells in the lung are shown. (C) Serum was collected at 10 d postinfection. Influenza virus-specific serum IgG levels were measured by ELISA. (D–F) Mice were treated i.p. with 2DG daily from day −1 to day 1 (D) or 9 (E–F) during infection. (D) The BALF was collected from influenza virus-infected animals at 2 d postinfection. IL-1β levels in BALF were determined by ELISA. (E) Ten days later, lymphocytes were isolated from the lung. Influenza virus-specific CD8+ T cells were then detected using the H-2Db influenza virus NP tetramer. Total numbers of influenza virus-specific CD8+ T cells in the lung are shown. (F) Serum was collected at 10 d postinfection. Influenza virus-specific serum IgG levels were measured by ELISA. The data are from three (A–C) or two (D–F) independent experiments (mean ± SEM). Statistical analysis was performed by two-tailed Student’s t test (A–C) or one-way ANOVA and Tukey’s test (D–F). *P < 0.05, **P < 0.01, and ***P < 0.001.
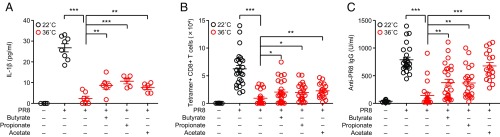
Finally, we examined whether SCFAs, such as butyrate, propionate, and acetate, can restore immune responses to influenza virus in high heat-exposed mice. A previous report indicates that i.v. injection with butyrate significantly enhances CD8 T cell response against influenza virus (20). Indeed, injection with butyrate enhanced secretion of mature IL-1β in the BALF (Fig. 5A), influenza virus-specific CD8 T cells (Fig. 5B), and antibody responses (Fig. 5C) in high heat-exposed mice. Remarkably, secretion of mature IL-1β in the BALF (Fig. 5A), influenza virus-specific CD8 T cells (Fig. 5B), and antibody responses (Fig. 5C) were partially restored in high heat-exposed mice by i.v. injection of propionate or acetate after influenza virus infection. These data collectively indicated that induction of autophagy by high-heat exposure or nutrient starvation impaired virus-specific CD8 T cells and antibody responses following respiratory influenza virus infection. Under such circumstances, both glucose and SCFAs might be important for efficient priming of inflammasome-dependent cytokine release and adaptive immune responses after infection with influenza virus.
Fig. 5.
SCFAs restored influenza virus-specific adaptive immune responses in high heat-exposed mice. (A–C) Mice were kept at 22 °C or 36 °C for 7 d before influenza virus infection and throughout infection. High heat-exposed mice were given butyrate (200 mM), propionate (200 mM), or acetate (200 mM) in drinking water for 7 d before influenza virus infection and throughout infection and treated with each SCFA i.v. daily from day −2 to day 7 during infection. (A) The BALF was collected from influenza virus-infected animals at 2 d postinfection. IL-1β levels in BALF were determined by ELISA. (B) Ten days later, lymphocytes were isolated from the lung. Influenza virus-specific CD8+ T cells were then detected using the H-2Db influenza virus NP tetramer. Total numbers of influenza virus-specific CD8+ T cells in the lung are shown. (C) Serum was collected at 10 d postinfection. Influenza virus-specific serum IgG levels were measured by ELISA. The data are pooled from four independent experiments (A and B; mean ± SEM). Statistical analysis was performed by two-tailed Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
Warm temperature restricts viral replication through type I IFN-dependent and -independent mechanisms in vitro (29–31). In addition, both humidity and temperature affect the frequency of influenza virus transmission among guinea pigs (32). In contrast, the effects of high-heat ambient temperature in host defense to viral infection in vivo are largely unknown. Here, we provide evidence that high-heat exposure of mice severely impaired adaptive immune responses following respiratory influenza virus infection. In addition, viral clearance was severely delayed in high heat-exposed mice at later stages of infection, highlighting the importance of outside temperature in the induction of adaptive immune responses to influenza virus infection. Although high heat-exposed mice also impaired CD4+ T cell responses against ZIKV and SFTSV infection, their antibody responses against ZIKV or SFTSV infection, or vaccination with inactivated influenza virus and aluminum adjuvant were intact, suggesting that the reduction of adaptive immune responses is not caused by a general immune deficiency in these mice. Interestingly, cold-exposed naïve mice significantly increased their food intake without affecting the virus-specific adaptive immune responses or their body weight, probably due to cold-induced increases in energy expenditure (33), compared with RT-exposed mice. In contrast, high-heat exposure of naïve mice significantly reduced their food intake and body weight by 10%. Although several possible mechanisms could explain how higher temperature dampens the generation of adaptive immune responses to influenza virus infection, our present study indicated that high-heat exposure of mice reduced their food intake, resulting in enhanced levels of autophagy in the lung. As a result of autophagy induction, inflammasome-dependent cytokine secretion in the lung and migration of antigen-captured lung DCs to the mLN were severely impaired in high heat-exposed mice following influenza virus infection (SI Appendix, Fig. S17). Since underfed or rapamycin-treated mice kept at 22 °C severely impaired the virus-specific adaptive immune responses, we believe that reduced feeding activity as a result of high-heat exposure is responsible for impaired adaptive immune responses in high heat-exposed mice.
We previously demonstrated that induction of influenza virus-specific CD8+ T cell responses requires the inflammasome-dependent cytokine secretion and downstream IL-1R signaling in respiratory DCs (15, 16). In the present study, we demonstrated that the high heat-exposed mice failed to stimulate inflammasome-dependent cytokine secretion after infection with influenza virus. Although the high heat-exposed mice did not change commensal microbiota composition, which supply priming signals necessary for IL-1β and IL-18 secretion (17), they increased the levels of autophagy in the lung due to reduction of food intake. Since autophagy and mitophagy restrict inflammasome-dependent cytokine release by regulating the amounts of pro–IL-1β and damaged mitochondria, respectively (24, 25), it is possible that elevated levels of autophagy in high heat-exposed mice suppress IL-1β secretion in the lung following influenza virus infection (SI Appendix, Fig. S17). Indeed, we found that high heat-exposed mice impaired secretion of mature IL-1β in the BALF as well as mRNA expression of pro–IL-1β in the lung tissues following influenza virus infection. Although recent studies have demonstrated an essential role for autophagy in antigen presentation by DCs or maintenance of memory B cell responses after infection with herpes simplex virus or influenza virus (27, 34, 35), our data indicated that induction of autophagy by high-heat exposure, nutrient starvation, or rapamycin treatment suppressed influenza virus-specific adaptive immune responses due to impairment of pro–IL-1β mRNA expression and inflammasome-dependent IL-1β secretion in the lung of infected mice, and any immunostimulatory role of autophagy was undermined by its role in regulating IL-1β secretion.
A recent study has demonstrated that gavage of glucose protects mice from lethal influenza virus infection, whereas inhibition of glucose utilization by 2DG exacerbated influenza virus-induced mortality (28). Although glucose utilization is required to mitigate endoplasmic reticulum (ER) stress response and CHOP, (an ER stress-induced transcription factor)-dependent tissue dysfunction in the brain of influenza virus-infected mice (28), it remains unclear whether glucose utilization regulates the generation of influenza virus-specific adaptive immune responses. In the present study, we found that glucose utilization is critical to mount adaptive immune responses following respiratory influenza virus infection. Although influenza virus-induced glycolysis in plasmacytoid DCs is essential for robust expression of IFN-α (36), our results suggested that glucose utilization also regulates adaptive immune responses during influenza virus infection. Recently, Trompette et al. (20) have demonstrated that dietary fiber increased influenza virus-specific CD8+ T cell responses when mice were born and raised on a low-fiber diet supplemented with either cellulose (control) or inulin (high-fiber diet). They found that SCFAs increased glycolysis and respiration of naïve splenic CD8+ T cells after a short period of in vitro stimulation. In addition to the importance of butyrate in the induction of adaptive immune responses to influenza virus infection, our results indicated that all SCFAs tested, including butyrate, propionate, and acetate, restored influenza virus-specific CD8+ T cells and antibody responses in high heat-exposed mice. In the present study, we found that ad libitum self-feeding condition after infection with influenza virus was insufficient to restore immune responses in underfed mice. In addition, ad libitum-fed parabiotic mice reduced the virus-specific CD8+ T cell responses compared with the ad libitum-fed control group, probably due to difficulty in food intake after parabiotic surgery, suggesting that host nutritional status before and after influenza virus infection might be important to maximize the virus-specific adaptive immune responses.
In summary, our study demonstrated the effects of ambient temperature in host defense to viral infection in vivo. Our data indicated that a heat wave-level temperature and host nutritional status critically regulate the generation of virus-specific CD4+ and CD8+ T cells and antibody responses following respiratory influenza virus infection. Because half of the world’s population could face a severe food crisis as a result of global warming by the end of this century (37), our results imply possible public health problems and concerns that outside temperature and host nutritional status may be critical determinants of live attenuated influenza vaccine efficacy in tropical or developing countries. Finally, clinical management of emerging infectious diseases such as influenza, Zika, and Ebola in tropical or developing countries may require nutritional supplementation in addition to antiviral therapy.
Methods
Mice.
Age- and sex-matched C57BL/6 (WT) and B6-Ly5.1 (congenic CD45.1 mice on B6 background) mice were purchased from The Jackson Laboratory and Sankyo Laboratory Service, respectively. Cold (4 °C, 80% relative humidity), RT (22 °C, 53% relative humidity), or high-heat (36 °C, 25% relative humidity) exposures were started 7 d before infection and continued for the entire duration of the experiments. These mice were allowed free access to food and drinking water and kept on a 12 h light/dark cycle. All animal experiments were performed in accordance with The University of Tokyo’s Regulations for Animal Care and Use, which were approved by the Animal Experiment Committee of the Institute of Medical Science, The University of Tokyo (approval number H17–12).
Quantification and Statistical Analysis.
Statistical significance was tested by one-way ANOVA followed by Tukey’s test or unpaired t tests with PRISM software (version 5; GraphPad software). Data are presented as mean ± SEM. Statistical details can be found directly in the figure legends. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank A. Iwasaki (Yale University) for helpful discussions and comments on the manuscript, Y. Kawaoka (University of Wisconsin and The University of Tokyo) for providing the plasmids for reverse genetics, and T. Taniguchi (The University of Tokyo) for OT-I mice. This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (25713018, 16H05193) and the Research Program on Emerging and Re-emerging Infectious Diseases, of the Japan Agency for Medical Research and Development. M.M. is a research fellow of the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815029116/-/DCSupplemental.
References
- 1.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 6.Lund JM, et al. Recognition of single-stranded RNA viruses by toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornung V, et al. 5′-triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 8.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 9.Rehwinkel J, et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Weber M, et al. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe. 2013;13:336–346. doi: 10.1016/j.chom.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichinohe T, Yamazaki T, Koshiba T, Yanagi Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci USA. 2013;110:17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, Fox D, Man SM. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018;430:3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat Immunol. 2013;14:246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichinohe T, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abt MC, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steed AL, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trompette A, et al. Dietary fiber confers protection against Flu by shaping Ly6c- patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity. 2018;48:992–1005.e8. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Chevalier C, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Ussar S, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng M, et al. Microbiota modulate tumoral immune surveillance in lung through a γδT17 immune cell-dependent mechanism. Cancer Res. 2014;74:4030–4041. doi: 10.1158/0008-5472.CAN-13-2462. [DOI] [PubMed] [Google Scholar]
- 24.Zhong Z, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris J, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J Biol Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M, et al. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat Med. 2014;20:503–510. doi: 10.1038/nm.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang A, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166:1512–1525.e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foxman EF, et al. Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci USA. 2015;112:827–832. doi: 10.1073/pnas.1411030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foxman EF, Storer JA, Vanaja K, Levchenko A, Iwasaki A. Two interferon-independent double-stranded RNA-induced host defense strategies suppress the common cold virus at warm temperature. Proc Natl Acad Sci USA. 2016;113:8496–8501. doi: 10.1073/pnas.1601942113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonarkart C, Suptawiwat O, Sakorn K, Puthavathana P, Auewarakul P. Exposure to cold impairs interferon-induced antiviral defense. Arch Virol. 2017;162:2231–2237. doi: 10.1007/s00705-017-3334-0. [DOI] [PubMed] [Google Scholar]
- 32.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravussin Y, Xiao C, Gavrilova O, Reitman ML. Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PLoS One. 2014;9:e85876. doi: 10.1371/journal.pone.0085876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HK, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhl M, et al. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+ T cells. Cell Death Differ. 2009;16:991–1005. doi: 10.1038/cdd.2009.8. [DOI] [PubMed] [Google Scholar]
- 36.Bajwa G, et al. Cutting edge: Critical role of glycolysis in human plasmacytoid dendritic cell antiviral responses. J Immunol. 2016;196:2004–2009. doi: 10.4049/jimmunol.1501557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.