Significance
Regulatory T cells (Tregs) inhibit autoimmune responses and are essential for immune homeostasis. By contrast, in settings of cancerous inflammation, Tregs represent a strong protumorigenic immune cell population via the incapacitation of various tumoricidal immune cells. By using multiple gene-deficient mouse systems, we show that IL-27 specifically induces ectonucleotidase CD39 expression on tumor-infiltrating Tregs in a STAT1-dependent manner. Protumorigenic activity of Tregs is significantly ameliorated by inhibiting CD39. Lack of IL-27Ra prevents CD39 upregulation on Tregs, which rescues cytotoxic CD8+ T cells from Treg-mediated inhibition in tumor tissue. Our findings unveil a IL-27–STAT1–CD39 axis as a crucial mechanism for the protumorigenic function of Tregs. Targeting this new molecular pathway may facilitate the development of antitumor therapeutic approaches.
Keywords: regulatory T cell, tumor immunity, CD39, IL-27, STAT1
Abstract
Expression of ectonucleotidase CD39 contributes to the suppressive activity of Foxp3+ regulatory T cells (Tregs) by hydrolyzing immunogenic ATP into AMP. The molecular mechanism that drives CD39 expression on Tregs remains elusive. We found that tumor-infiltrating Tregs (Ti-Tregs) failed to up-regulate CD39 in mice lacking EBI3 subunit of IL-27 or IL-27Ra. Mixed bone marrow chimera and in vitro studies showed that IL-27 signaling in Tregs directly drives CD39 expression on Ti-Tregs in a STAT1-dependent, but STAT3- and T-bet–independent, manner. Tregs stimulated with IL-27 showed enhanced suppressive activities against CD8+ T cell responses in vitro. Moreover, IL-27Ra–deficient Tregs and STAT1-deficient Tregs were less efficient than WT Tregs in suppressing antitumor immunity in vivo. CD39 inhibition significantly abolished IL-27–induced suppressive activities of Tregs. Thus, IL-27 signaling in Tregs critically contributes to protumorigenic properties of Tregs via up-regulation of CD39.
Tumors evade immune surveillance by multiple mechanisms, and one key is suppressive immune cells (1). Among them, regulatory T cells (Tregs) critically contribute to the generation of a protumorigenic environment (2). Proposed mechanisms for protumorigenic properties of Tregs include secretion of immunosuppressive cytokines such as TGF-β and IL-10, depletion of IL-2 via CD25, expression of coinhibitory molecules such as CTLA4 and PD-1, and hydrolysis of immunogenic ATP through CD39 (3). Compared with Tregs in the secondary lymphoid organs, Tregs infiltrated in tumor exhibit activated phenotypes and elevated expression of these immunosuppressive molecules. Infiltration of Tregs in tumors is associated with poor prognosis (4). Hence, Treg-associated immunosuppressive molecules serve as attractive targets in the development of cancer immunotherapy (2).
Among Treg-associated molecules, CD39 is an ectonucleotidase that hydrolyzes ATP and ADP into AMP. CD73 is another ectonucleotidase that converts AMP into adenosine (5). Whereas ATP exerts multiple immune-stimulatory functions, adenosine has strong immunosuppressive activities (6). Expression of CD39 is inversely correlated with overall survival and recurrence-free survival in patients with cancer (7, 8). Analysis of tumor-infiltrating immune cells revealed that Tregs express the highest levels of CD39 in patients with malignancy (9). Notably, a recent study with CD39-deficient mice demonstrated that CD39 on Tregs is required to suppress antitumor immunity in vivo (10). Thus, induction of CD39 on Tregs critically contributes to the establishment of a protumorigenic environment; however, little is known regarding the regulation of CD39 expression on Tregs. Elucidating the regulation of CD39 expression on Tregs is important not only for the understanding of the tumor microenvironment, but also for the development of novel therapeutic strategies against tumor. Here, we found that IL-27 signaling enhances CD39 expression on tumor-infiltrating Tregs (Ti-Tregs) in a STAT1-dependent manner. Moreover, IL-27Ra–deficient Tregs and STAT1-deficient Tregs appeared to be less protumorigenic than WT Tregs in vivo as a result of reduced expression of CD39.
Results
Ti-Tregs Exhibit a Distinct Phenotype.
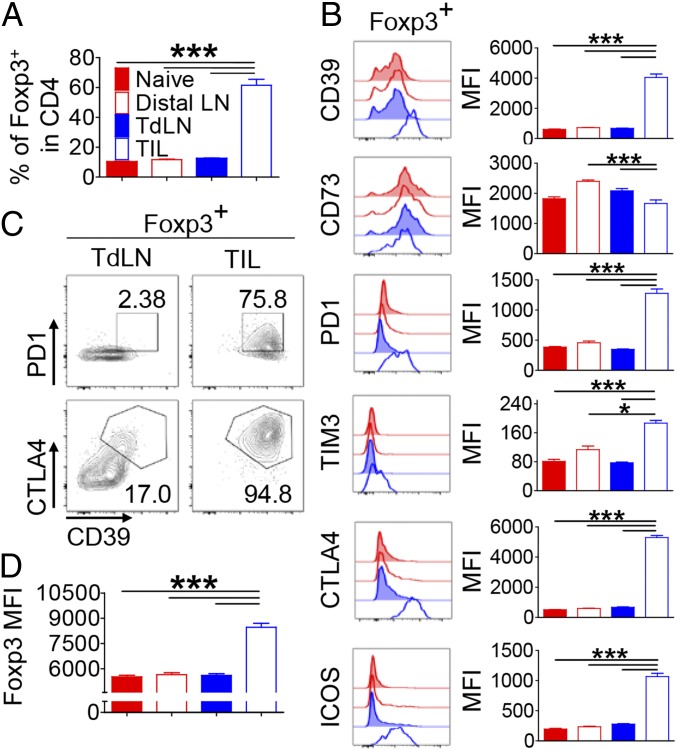
To determine the impact of tumor environment on the phenotypic changes of Tregs, we comparatively analyzed the expression of inhibitory molecules on Tregs obtained from lymph nodes (LNs) in naïve mice with those from the distal LNs, the tumor-draining LNs (TdLNs), and the tumor-infiltrating lymphocytes (TILs) in mice bearing s.c. B16F10 melanoma. The frequencies of Foxp3+ Tregs in the LNs of naïve and tumor-bearing mice were comparable (∼11% of CD4+ T cells). However, it was significantly increased in the TILs (∼61% of CD4+ T cells; Fig. 1A). Consistent with previous studies (9, 11), Ti-Tregs expressed increased levels of PD-1, CTLA4, ICOS, and, to a lesser extent, Tim-3 compared with those in the distal LNs and TdLNs (Fig. 1B). Ti-Tregs also expressed significantly elevated levels of CD39 compared with those in the LNs, whereas the expression of CD73 was slightly reduced in the former. Among those inhibitory molecules, the expression of CD39, CD73, and PD-1 on tumor-infiltrating Foxp3−CD4+ T cells was slightly increased vs. those shown on CD4+ T cells in the LNs, although the levels of expression were marginal compared with that of Ti-Tregs (SI Appendix, Fig. S1). As a result, a significant portion of Ti-Tregs coexpressed CD39, PD-1, and CTLA4, which was not observed on Tregs in the TdLNs (Fig. 1C). Moreover, the level of Foxp3 was also significantly higher in the Ti-Tregs than in Tregs in the TdLNs (Fig. 1D).
Fig. 1.
Tregs acquire a highly inhibitory phenotype in tumor tissue. Naïve and B16F10 s.c. tumor-bearing mice were analyzed at 15 d after inoculation for the phenotype of Tregs. CD4+Foxp3+ T cells in tumor-distal LN, TdLN, tumor tissue, and LN from naïve mice were analyzed by FACS. (A) The frequency of Foxp3+ cells in CD4+ T cells. (B) Histograms and geometric mean fluorescence intensity (MFI) of indicated proteins’ expression on Foxp3+ cells. (C) Coexpression of PD-1, CTLA-4, and CD39 on Foxp3+ cells from TdLN and tumor tissue. (D) Geometric MFI of Foxp3 in Tregs. The graphs show means ± SEM (*P < 0.05 and ***P < 0.001). Data are representative of two independent experiments (n = 6–7).
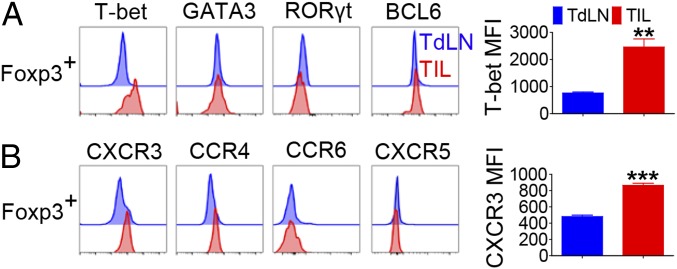
Foxp3+ Tregs can be subcategorized into multiple subsets based on the expression of transcription factors and chemokine receptors (12–15). Notably, we observed that most Ti-Tregs expressed T-bet but not GATA3, RORγt, or BCL6 (Fig. 2A). Such elevation of T-bet expression in Tregs was tumor site-specific, as few Tregs in the TdLN expressed T-bet. T-bet+ Tregs are known to coexpress CXCR3 (16). Consistent with this notion, Ti-Tregs predominantly expressed CXCR3 and, to a lesser extent, CCR4, but did not express CCR6 or CXCR5 (Fig. 2B). Selective accumulation of CXCR3+ T-bet+ Tregs in the tumor was also observed in mice bearing s.c. EL4 thymoma (SI Appendix, Fig. S2). Collectively, these results demonstrated that Ti-Tregs were phenotypically distinct from those in the LNs with profoundly elevated expressions of PD-1, CTLA4, and CD39 as well as T-bet and CXCR3 in solid tumor models in mice.
Fig. 2.
Ti-Tregs preferentially express T-bet and CXCR3. CD4+Foxp3+ T cells in TdLN and tumor tissue from B16F10 s.c. tumor-bearing mice were analyzed by FACS at 15 d after tumor inoculation. (A) The histograms and geometric mean fluorescence intensity (MFI) for indicated transcription factors and (B) chemokine receptors. The graphs show means ± SEM (**P < 0.01 and ***P < 0.001). Data are representative of two independent experiments (n = 3–4).
Role of IL-12 Family Cytokines on the Phenotypic Changes of Ti-Tregs.
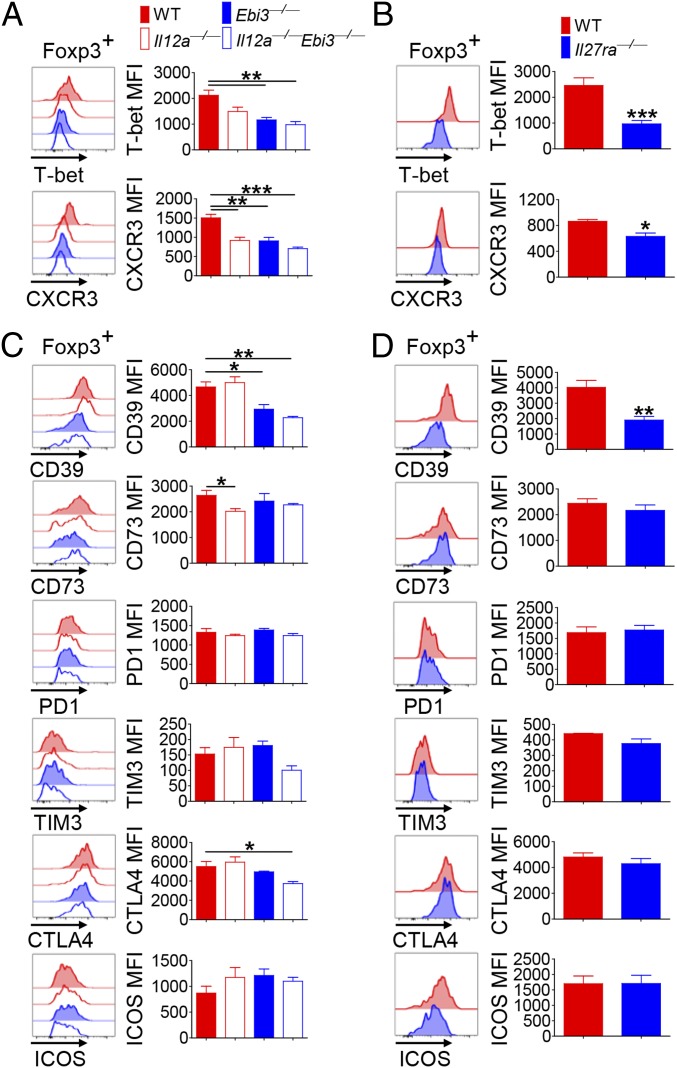
We next sought to determine the mechanism by which tumor environment induces the observed phenotypic changes in Ti-Tregs. To this end, we assessed the involvement of IL-12 family cytokines in this process because IL-27 has been shown to induce T-bet+ CXCR3+ Tregs in animal models of infection and inflammation (17). We employed Il12a−/− (lacking p35 subunit of IL-12 and IL-35), Ebi3−/− (lacking EBI3 subunit of IL-27 and IL-35), and Il12a−/−Ebi3−/− mice and compared the phenotypes of Ti-Tregs after s.c. inoculation with B16F10. Compared with that in WT mice, the level of T-bet expression in Ti-Tregs was significantly reduced in Ebi3−/− and Il12a−/−Ebi3−/− mice whereas such reduction was not statistically significant in Il12a−/− mice (Fig. 3A). On the contrary, CXCR3 expression on Ti-Tregs was significantly reduced in all gene-deficient mice compared with WT (Fig. 3A). These results prompted us to hypothesize that IL-27 but not IL-12 induces T-bet expression in Ti-Tregs, whereas both cytokines induce CXCR3 expression on Ti-Tregs. To further determine the role of IL-27, we analyzed Ti-Tregs from Il27ra−/− mice and found a significantly reduced expressions of T-bet and CXCR3 (Fig. 3B). The frequencies of Foxp3+CD4+ T as well as Foxp3−CD4+ T cells among TILs were comparable between WT and Il27ra−/− mice, but the absolute numbers of these cells were lower in Il27ra−/− mice because of a diminished number of total TILs (SI Appendix, Fig. S3 A and B). Diminished infiltration of Il27ra−/− T cells into tumor was further confirmed by using mixed bone marrow (BM) chimeric mice reconstituted with a 1:1 mixture of WT and Il27ra−/− BM cells (SI Appendix, Fig. S3C). Consistent with a previous study (18), the migration and cytokine secretion of natural killer (NK) and natural killer T (NKT) cells among TILs were lower in Il27ra−/− mice (SI Appendix, Fig. S4).
Fig. 3.
IL-27 induces CD39 expression on Tregs. WT, Il12a−/−, Ebi3−/−, Il12a−/−Ebi3−/− (A and C), or Il27ra−/− mice (B and D) were inoculated s.c. with B16F10 tumor cells. CD4+Foxp3+ T cells in tumor tissue from these mice were analyzed by FACS at 15 d after tumor inoculation. Histograms and geometric mean fluorescence intensity (MFI) for T-bet/CXCR3 (A and B) and indicated proteins (C and D). The graphs show means ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001). Data are representative of three independent experiments (n = 3–4).
Of note, we observed a significantly reduced level of CD39 on Ti-Tregs in Ebi3−/− mice and in Il12a−/−Ebi3−/− mice, but not in Il12a−/− mice, compared with WT (Fig. 3C). By contrast, the expression levels of CD73, PD-1, TIM3, and ICOS on Ti-Tregs were largely comparable among the groups (Fig. 3C). CTLA4 expression on Ti-Tregs was slightly lower in Il12a−/−Ebi3−/− mice than in WT. Consistently, Ti-Tregs from Il27ra−/− mice expressed a significantly reduced level of CD39, but they expressed comparable levels of CD73, PD1, TIM3, CTLA4, and ICOS in comparison with WT mice (Fig. 3D). The tumor growth was delayed in Ebi3−/− and, to a lesser extent, in Il27ra−/− mice in comparison with WT mice (SI Appendix, Fig. S5). These results together suggest that IL-27 was particularly required for the optimal up-regulation of CD39 on Ti-Tregs, whereas this cytokine played little role in the expression of the other Treg-specific cell surface molecules in vivo.
IL-27 Signal in Tregs Drives CD39 Expression via STAT1.
Our findings strongly suggest a critical contribution of IL-27 to CD39 expression on Ti-Tregs. Among tumor-infiltrating immune cells, CD11c− macrophages expressed higher levels of Il27 and Ebi3 transcripts than CD11c+ macrophages and T cells (SI Appendix, Fig. S6 A and B). As the tumor grew, the level of IL-27 and the frequencies of CD11c− macrophages and Tregs in tumor were also increased, whereas the level of CD39 expression on Ti-Tregs was maintained (SI Appendix, Fig. S6 C–G).
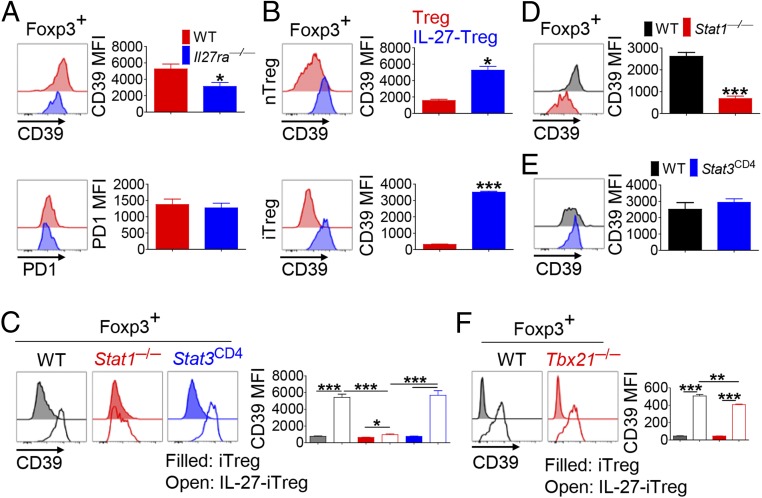
To determine whether IL-27 directly induced CD39 expression on Ti-Tregs, we generated WT:Il27ra−/− mixed BM chimera (SI Appendix, Fig. S7A) and found that CD39 expression in Il27ra−/− Tregs were slightly but significantly lower than WT Tregs in splenic, but not thymic, Tregs in tumor-free recipient mice (SI Appendix, Fig. S7B). In recipient mice bearing s.c. B16F10 tumor cells, Il27ra−/− Ti-Tregs showed significantly diminished CD39 expression compared with WT Ti-Tregs (Fig. 4A), indicating that IL-27 signaling in Tregs is critical for the up-regulation of CD39 in vivo. The level of PD-1 expression was comparable between WT and Il27ra−/− Tregs. To illustrate more precisely the cell-intrinsic function of IL-27 on Tregs, we stimulated natural Tregs (nTregs) isolated from Foxp3YFP-Cre reporter mice with anti-CD3 in the presence or absence of IL-27 (nTregs vs. IL-27-nTregs). IL-27 stimulation significantly elevated the expression of CD39 on the nTregs (Fig. 4B, Top). Moreover, addition of IL-27 significantly increased CD39 expression on Foxp3+ Tregs generated by stimulating naïve CD4+ T cells under induced Treg (iTreg)-skewing condition (iTregs vs. IL-27-iTregs; Fig. 4B, Bottom). Unlike IL-27, addition of IL-35, a cytokine that shares the EBI3 subunit with IL-27, had little effect on CD39 expression of iTregs (SI Appendix, Fig. S8).
Fig. 4.
T cell-intrinsic IL-27 signal regulates CD39 expression on Tregs via STAT1, not STAT3 and T-bet. CD4+Foxp3+ T cells in tumor tissue from WT:Il27ra−/− (A), WT:Stat1−/− (D), and WT:Stat3CD4 (E) mixed BM chimeric mice were analyzed at 15 d after tumor inoculation for CD39 expression by FACS. (B, Top) Purified nTregs from Foxp3YFP-Cre reporter mice were incubated in the presence or absence of IL-27 during T cell receptor stimulation. Naïve CD4+ T cells from WT (B, Bottom), Stat1−/−, Stat3CD4 (C), or Tbx21−/− mice (F) were differentiated into iTregs in the presence or absence of IL-27. CD39 expression on Foxp3+ cells was analyzed. Representative plots and graphs for geometric mean fluorescence intensity (MFI) are shown. The graphs show means ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001). Data for mixed BM chimera experiments are representative of two independent experiments (n = 3–4).
IL-27 induces STAT1 and STAT3 activation (19). To determine if these STATs are required to induce CD39 expression on Tregs, naïve CD4+ T cells from Stat1−/− mice or CD4-Cre × Stat3f/f (Stat3CD4) mice were differentiated into iTregs or IL-27-iTregs. We found that, even though IL-27 significantly increased CD39 expression on WT and Stat3−/− iTregs (approximately sevenfold), it only marginally increased CD39 expression on Stat1−/− iTregs (Fig. 4C). To determine the role of the STATs for CD39 expression on Tregs in vivo, we generated mixed BM chimera by transferring WT (CD45.1) and Stat1−/− or Stat3CD4 (CD45.2) BM cells into BM-ablated Tcrb−/− mice. We found that the level of CD39 expression on Stat1−/− Tregs was significantly lower than that of WT Tregs in the TILs of tumor-bearing recipients as well as in the spleens of tumor-free recipients (Fig. 4D and SI Appendix, Fig. S7B). By contrast, the level of CD39 was comparable between WT and Stat3−/− Tregs (Fig. 4E). These in vitro and in vivo studies convincingly demonstrate a critical contribution of STAT1, but not STAT3, to CD39 up-regulation on Tregs. There are five putative STAT1-binding sites with a dissimilarity of <6% in the conserved regions within the Entpd1 (encoding CD39) gene locus (SI Appendix, Fig. S9A, region 1–5). Interestingly, Foxp3 and Smad3 were also predicted to bind to region 1–4 (SI Appendix, Fig. S9B), raising a possibility that STAT1, Foxp3, and Smad3 orchestrate the expression of Entpd1 in Tregs upon IL-27 signal. In addition to IL-27, IFN-γ also signals through STAT1 and is produced by TILs. When tumor-bearing WT or Il27ra−/− mice were treated with anti–IFN-γ or isotype control, WT Ti-Tregs showed comparable expression level of CD39 irrelevant to anti–IFN-γ treatment. Anti–IFN-γ, however, slightly but significantly down-regulated CD39 expression on Il27ra−/− Ti-Tregs (SI Appendix, Fig. S10A). In vitro, addition of IFN-γ increased CD39 expression on Foxp3+ iTregs in a STAT1-dependent manner, but it was far less potent than IL-27 (SI Appendix, Fig. S10B). Thus, IFN-γ could induce CD39 expression on Ti-Tregs via STAT1, especially in the absence of IL-27.
IL-27 and STAT1 induce T-bet expression in Tregs (17). Il27ra−/− Ti-Tregs showed reduced levels of T-bet (Fig. 3B). Therefore, we determined whether T-bet is also necessary for IL-27–induced CD39 up-regulation on Tregs. We found that IL-27 up-regulated CD39 on Tbx21−/− Tregs as efficiently as on WT Tregs (Fig. 4F), indicating little role of T-bet in IL-27–induced CD39 expression on Tregs. Together, these results indicated that IL-27 induced CD39 expression on Ti-Tregs in a STAT1-dependent but STAT3- and T-bet–independent, manner.
IL-27–Stimulated Tregs Exhibit an Enhanced ATP-Hydrolyzing Activity and Inhibit Cytokine Production by CD8+ T Cell in Vitro.
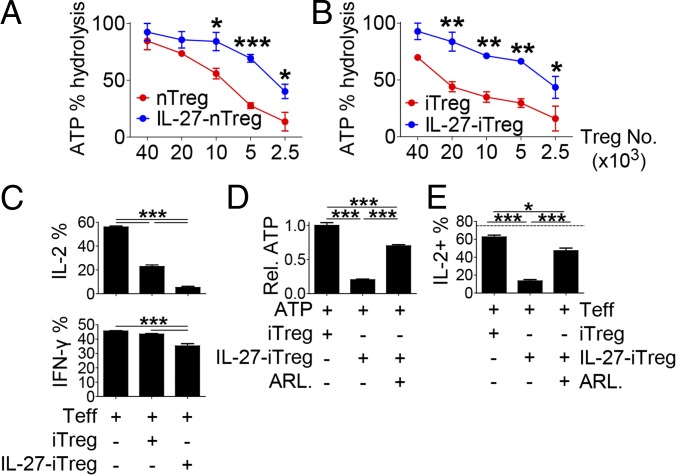
As we observed that IL-27 up-regulated CD39 on Tregs, we next asked if IL-27 impacts ATP hydrolytic capacity of Tregs. CD4+ T cells from Foxp3YFP-Cre reporter mice were stimulated with anti-CD3 in the presence or absence of IL-27. YFP+ Tregs were sorted and cocultured with ATP for 6 h, and the remaining ATP in the supernatant was measured (Fig. 5A). We observed that IL-27–stimulated Tregs were far more efficient in hydrolyzing ATP than IL-27–unstimulated Tregs. The number of Tregs required to hydrolyze 50% of ATP was ∼2.4 times lower in IL-27-nTregs than in nTregs (required cell numbers for nTregs vs. IL-27-nTregs, 9,000 vs. 3,750). Similarly, iTregs showed an enhanced ATP-hydrolyzing activity when generated in the presence of IL-27 (cell numbers required to hydrolyze 50% of ATP for iTregs vs. IL-27-iTregs, 22,000 vs. 2,900; Fig. 5B).
Fig. 5.
IL-27–stimulated CD39high Tregs mediate suppression via enhanced enzymatic activation. Foxp3+ cells from IL-27–stimulated and unstimulated Tregs (A, nTregs; B, iTregs) were incubated with exogenous ATP. The remaining ATP was measured in the supernatants, and percent ATP hydrolysis against control is shown. (C) The effect of iTregs or IL-27-iTregs on cytokine-producing CD8+ T cells. (D and E) The effect of CD39 blockade by ARL67156 on (D) ATP hydrolysis and (E) IL-2 production in CD8+ T cells. The representative graphs and plots are shown. The graphs show means ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001). Data are representative of two independent experiments.
To determine whether IL-27 signaling also regulates the immunosuppressive activity of Tregs, we stimulated naïve CD8+ T cells with anti-CD3/CD28 in the presence of iTregs or IL-27-iTregs. IL-27-iTregs and iTregs similarly suppressed the proliferation of CD8+ T cells (SI Appendix, Fig. S11A). By contrast, IL-27-iTregs were far more potent in suppressing IL-2 and, to a lesser extent, IFN-γ expression in CD8+ T cells compared with iTregs (Fig. 5C). The augmented ATP hydrolysis activity of IL-27-iTregs was significantly attenuated by the CD39 inhibitor ARL67156, although it was still higher than that of iTregs (Fig. 5D). Importantly, the frequency of IL-2–producing CD8+ T cells cocultured with IL-27-iTregs was significantly reversed by ARL67156, although it was still lower than that cocultured with iTregs (Fig. 5E). Furthermore, higher amounts of adenosine were detected in the supernatant cocultured with IL-27-iTregs than that with iTregs, which was significantly attenuated by ARL67156 (SI Appendix, Fig. S11B). Similarly, treatment with an adenosine A2A blocker, ZM241385, significantly diminished the suppression of IL-2 production in CD8+ T cell by IL-27-iTregs (SI Appendix, Fig. S11C). Collectively, these results indicate that IL-27 stimulation enhances the ATP hydrolyzing capacity and the suppression of IL-2 production from CD8+ T cells by Tregs in a CD39-dependent manner.
IL-27 Signal Is Crucial for Protumorigenic Activity of Tregs via Induction of CD39 in Vivo.
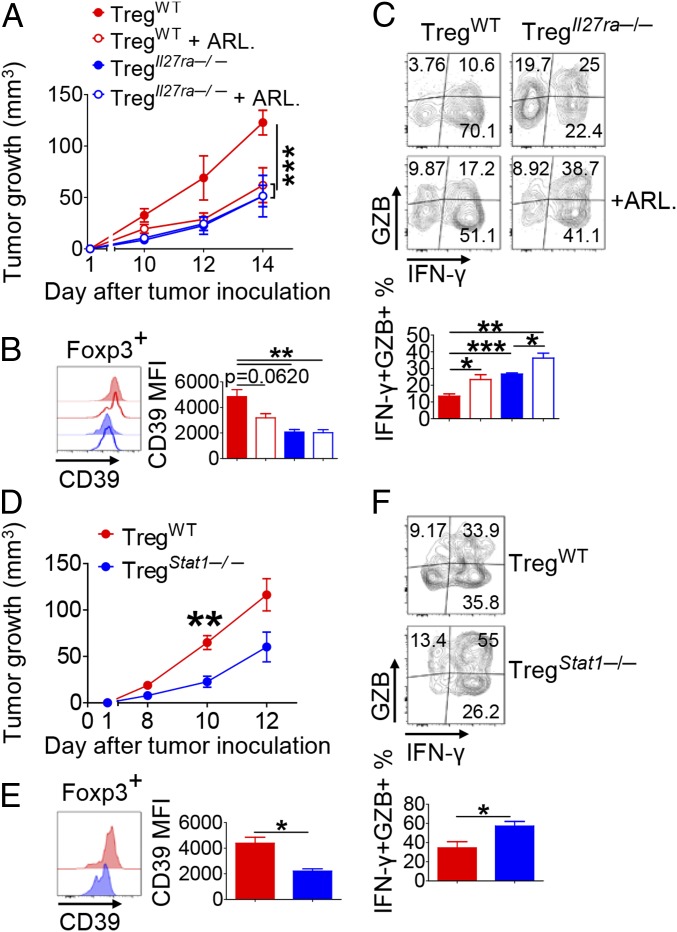
We next addressed whether IL-27 signaling in Tregs plays a role in protumorigenic activity in vivo. We generated Tregs by stimulating naïve CD4+ T cells from Foxp3YFP-Cre or Il27ra−/− × Foxp3YFP-Cre mice under iTreg-skewing condition. The sorted YFP+ iTregs (YFP+ > 95%, CD45.2+) were mixed with naïve CD4+ T and CD8+ T cells (CD45.1+) and transferred into Rag1−/− mice before the recipients were inoculated s.c. with B16F10. We observed a significant delay in tumor growth in the recipients of Il27ra−/− Tregs compared with those of WT Tregs (Fig. 6A). Consistent with the data from tumor-bearing Il27ra−/− or Ebi3−/− mice (Fig. 3), the level of CD39 on Il27ra−/− Ti-Tregs was significantly lower than that on WT Ti-Tregs (Fig. 6B). Moreover, the frequency of IFN-γ+ granzyme B (GZB)+ cells among CD8+ T cells was remarkably higher in the recipients of Il27ra−/− Tregs than in the recipients of WT Tregs (Fig. 6C). To determine whether CD39 contributes to the observed difference in tumor growth, we additionally treated the recipients with ARL67156. ARL67156 reduced the expression of CD39 on WT Ti-Tregs. By contrast, such treatment had little effect on the expression of CD39 on Il27ra−/− Ti-Tregs (Fig. 6B). Similarly, whereas ARL67156 significantly delayed tumor growth in the WT Treg recipients, it did not affect tumor growth in the Il27ra−/− Treg recipients (Fig. 6A). As a result, the difference in tumor growth between WT Treg recipients and Il27ra−/− Treg recipients disappeared with ARL67156 treatment. Furthermore, ARL67156 treatment significantly augmented the frequency of IFN-γ+ GZB+ cells among CD8+ T cells in WT Treg recipients and in Il27ra−/− Treg recipients (Fig. 6C). To directly assess the role of IL-27 signaling in Tregs on tumor growth and to rule out potential differences in their infiltration into the tumor, we employed a tumor/Treg s.c. cotransfer model (20, 21). We injected s.c. the mixture of B16F10 cells and YFP+ Tregs isolated from WT Foxp3YFP-Cre or Il27ra−/− × Foxp3YFP-Cre mice into Rag1−/− mice. The recipients were injected i.v. with naïve CD4+ T cells and CD8+ T cells. Tumor growth was significantly delayed in the recipients of Il27ra−/− Tregs, associated with a significant increase in the frequencies of IFN-γ+ GZB+ CD8+ T cells and IFN-γ+ NK cells (SI Appendix, Fig. S12 A–C). We next determined whether lack of IL-27 signaling or CD39 inhibitor administration impacts the survival or proliferation of Tregs in tumor. Although the total number of TILs was decreased in Il27ra−/− Treg recipients compared with WT recipient mice, the numbers of Tregs among TILs as well as the frequencies of Annexin V+ apoptotic and Ki67+ proliferative cells among Tregs appeared to be comparable between the two groups (SI Appendix, Fig. S12 D–G). Similarly, ARL67156 treatment played an insignificant role in the frequency of Annexin V+ cells or Ki67+ cells among Tregs in tumor (SI Appendix, Fig. S12 D–G).
Fig. 6.
IL-27 signal promotes protumorigenic activity of Tregs via CD39 in vivo. WT and Il27ra−/− Tregs (A–C) or WT and Stat1−/− Tregs (D–F) (CD45.2) were transferred into Rag1−/− mice in combination with naïve CD4+ and CD8+ T cells (CD45.1) and then inoculated s.c. with B16F10 tumor cells. Some mice were treated i.p. with ARL67156. The tumor growth (A and D), the geometric mean fluorescence intensity (MFI) of CD39 expression on Ti-Tregs (B and E), and the frequency of indicated cytokine-producing CD8+ T cells (C and F) were examined. The mice were analyzed at 15 d after tumor inoculation. Representative graphs and plots are shown. The graphs show means ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001). Data are representative of two independent experiments (n = 3–4).
By using a similar Treg transfer model as that shown in Fig. 6A, we examined the role of STAT1 in Tregs. Similar to that shown in the Il27ra−/− Treg recipient mice, Stat1−/− Treg recipients showed a significant delay in tumor growth associated with a remarkable down-regulation of CD39 on Ti-Tregs in comparison with WT Treg recipients (Fig. 6 D and E). Moreover, the frequency of IFN-γ+ GZB+ cells among CD8+ T cells was significantly higher in Stat1−/− Treg recipients than in WT Treg recipients (Fig. 6F). Moreover, we observed a significantly delayed tumor growth in Stat1−/− Treg recipients compared with WT Treg recipients in an s.c. cotransfer of tumor and Treg model (SI Appendix, Fig. S12H). The absolute numbers of Tregs among TILs as well as the frequencies of Annexin V+ apoptotic and Ki67+ proliferative cells among Tregs were comparable between the two groups (SI Appendix, Fig. S12 H–K). Taken together, these data indicate that IL-27–STAT1 signal in Tregs was crucial for the generation of protumorigenic environment through up-regulation of CD39 on Ti-Tregs, which significantly incapacitated antitumor activity of tumor-infiltrating CD8+ T cells in vivo (SI Appendix, Fig. S13).
Discussion
Despite the evident protumorigenic role of CD39 on Tregs, how CD39 expression is regulated in Tregs has been unclear. By using in vivo animal models of B16F10 tumor growth, we demonstrate that cell-intrinsic IL-27 signaling is crucial for the up-regulation of CD39 on Ti-Tregs based on the findings that Ti-Tregs in Ebi3−/− mice and Il27ra−/− mice exhibited reduced CD39 expression, and that IL-27 stimulation significantly increased CD39 expression on Tregs via STAT1. IL-27–stimulated Tregs were more efficient in hydrolyzing ATP and were more potent in suppressing IL-2 production by CD8+ T cells than IL-27–unstimulated Tregs, which was reversed by a CD39 inhibitor in vitro. Importantly, Il27ra−/− Tregs as well as Stat1−/− Tregs were less efficient in supporting tumor growth than WT Tregs as a result of reduced expression of CD39 in vivo. Our findings unveil an immunosuppressive mechanism by which IL-27 potentiates protumorigenic activities of Tregs via up-regulating CD39 in tumor environment.
IL-27 was initially reported to be a Th1-inducing cytokine because of its ability to induce the expression of T-bet and IFN-γ in T cells (22). Subsequent studies have revealed that IL-27 is an inhibitory cytokine, as Il27ra−/− and Ebi3−/− mice appeared to be more susceptible in animal models of infection and autoimmune diseases (23). IL-27 is known to induce a specialized subset of Tregs coexpressing T-bet and CXCR3 in animal models of infection (16, 17). Moreover, IL-27 signaling can trigger LAG3 expression on Tregs (24). The present study convincingly demonstrates that IL-27 is crucial for protumorigenic function of Tregs by inducing CD39 via STAT1. Although STAT3 has been shown to be required for CD39 expression on dendritic cells and Th17 and Tr1 cells (25–27), our in vitro and in vivo studies showed little role for STAT3 in inducing CD39 on Tregs. IFN-γ is known to signal through STAT1 to mediate antitumor immune responses. This cytokine also induces suppressive molecules such as PD-L1, CTLA-4, and IDO (28, 29). Our IFN-γ neutralization study revealed a marginal role of IFN-γ on CD39 expression in WT Ti-Tregs in vivo, indicating that IL-27 is likely superior to IFN-γ in inducing CD39 on Ti-Tregs. Hence, we propose that IL-27 and IFN-γ play nonredundant roles in promoting a protumorigenic environment by inducing distinct suppressive molecules. Whereas IL-27 stimulation induced the expression of CXCR3 and T-bet in Tregs, the expression of CD39 by IL-27 occurred independently of T-bet. T-bet+ CXCR3+ Tregs are known to be specialized for suppressing type 1 immunity in vivo (16, 17). Hence, IL-27 may have at least two distinct roles in Treg-mediated suppression of antitumor immunity: (i) by inducing the migration of Tregs into the sites where Th1 cells and cytotoxic T lymphocytes exist via CXCR3 and (ii) by enhancing ATP hydrolyzing activity via inducing CD39. The tumor/Treg mixture cotransfer study, which can rule out possible differences in Treg migration into the tumor, showed that Il27ra−/− Tregs were far less efficient in suppressing antitumor immunity than WT Tregs. Although a previous study proposed a potential role of IL-27 signal on the survival of Tregs in a murine model of colitis (30), our results demonstrate that IL-27 signal and STAT1 have minimal roles in the survival and proliferation of Tregs in tumor. Thus, we propose that up-regulation of CD39, rather than CXCR3, on Tregs is crucial for the IL-27–induced protumorigenic activity of Tregs. In this context, our observation that the CD39 inhibitor significantly diminished the suppressive activity of IL-27–stimulated Tregs strongly suggests that CD39 on Tregs acts as a metabolic checkpoint that controls antitumor immunity. IL-27 appeared to minimally affect the expression of coinhibitory molecules, including PD-1 and CTLA4, on Ti-Tregs in the present study. Thus, the IL-27–CD39 axis likely plays a nonredundant role with the other immune checkpoint molecules in suppressing antitumor immunity. Hence, targeting CD39, such as with a CD39 blocking antibody (31), or IL-27 signaling would be synergistic with immune checkpoint blockers such as anti-CTLA4 and anti–PD-1.
The role of IL-27 on tumor immunity has been controversial. A previous study showed that the growth of B16F10 tumor was exacerbated in Ebi3−/− mice compared with WT mice (32). By contrast, delayed tumor growth in Ebi3−/− mice was also reported in B16F10 metastatic and orthotopic pancreatic tumor models (33, 34). Similar controversy arises from studies with Il27ra−/− mice. One study showed exacerbated B16F10 tumor growth in Il27ra−/− mice (35), whereas another recent study showed delayed B16F10 tumor growth in Il27ra−/− mice associated with less exhausted tumor-infiltrating T cells (36). This discrepancy might result from the quantity of IL-27 produced and the type of responsive cells. For instance, IL-27 seemed to exert proinflammatory functions when used as an adjuvant (37), whereas it seemed to exert immunosuppressive function in steady state as shown in mice lacking IL-27 or its receptor (38). Our study provides an additional explanation for such a discrepancy in regard to the role of IL-27; IL-27 potentiates protumorigenic activities of Tregs, but also promotes antitumor activities of CD8+ T cells and NK cells.
In summary, our findings unveil the IL-27→STAT1→CD39 axis as a mechanism by which tumor environment induces protumorigenic activity of Ti-Tregs. Blockade of IL-27 signal and CD39 in Tregs would be an attractive strategy to overcome immunosuppressive environment in tumor-bearing hosts.
Materials and Methods
C57BL/6 mice were purchased from Orient Bio. Il12a−/−, Ebi3−/−, Il27ra−/−, Foxp3YFP-Cre, B6.SJL, Rag1−/− and Tcrb−/− mice were purchased from the Jackson Laboratory. Double-KO mice (Il12a−/−Ebi3−/−) were obtained by crossing Il12a−/− and Ebi3−/− mice. Foxp3YFP-Cre mice were crossed with Il27ra−/− or Stat1−/− mice to obtain Foxp3 reporter-KO mice (Il27ra−/− × Foxp3YFP-Cre and Stat1−/− × Foxp3YFP-Cre). STAT3flox/floxCD4-Cre mice were provided by Chen Dong (Tsinghua University, Beijing, China) and Shizuo Akira (Osaka University, Osaka, Japan). Tbx21−/− and Stat1−/− mice were provided by Eun Sook Hwang (Ewha Womans University, Seoul, Korea) and Hun Sik Kim (Asan Medical Center, Seoul, Korea), respectively. Mice aged 6–12 wk were used. All mice were maintained in a specific pathogen-free facility at Seoul National University. All experiments were performed according to a protocol approved by the institutional animal care and use committees of Seoul National University (SNU-150316-1-3). Additional information is provided in SI Appendix, Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Kyu-Won Kim and Sung-Jin Bae (Seoul National University) for their supports in flow cytometric analysis, Drs. Shizuo Akira (Osaka University) and Seung-Yong Sung (Seoul National University) for Stat3fl/fl mice, the entire laboratory of Y.C. for suggestions and discussion, and Ms. Da-Sol Kuen (Seoul National University) for proofreading the manuscript. This work is supported by National Research Foundation of Korea Grants 2017R1A2B3007392 (to Y.C.) and 0430-20150023 (to Y.-J.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810254116/-/DCSupplemental.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Ohara M, et al. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol Immunother. 2009;58:441–447. doi: 10.1007/s00262-008-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 7.Cai XY, et al. Overexpression of CD39 and high tumoral CD39+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. Int J Clin Exp Pathol. 2015;8:14757–14764. [PMC free article] [PubMed] [Google Scholar]
- 8.Cai XY, et al. Overexpression of CD39 in hepatocellular carcinoma is an independent indicator of poor outcome after radical resection. Medicine (Baltimore) 2016;95:e4989. doi: 10.1097/MD.0000000000004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plitas G, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Simone M, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BS, et al. Generation of RORγt+ antigen-specific T regulatory 17 cells from Foxp3+ precursors in autoimmunity. Cell Reports. 2017;21:195–207. doi: 10.1016/j.celrep.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall AO, et al. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J, et al. Critical role of dendritic cell-derived IL-27 in antitumor immunity through regulating the recruitment and activation of NK and NKT cells. J Immunol. 2013;191:500–508. doi: 10.4049/jimmunol.1300328. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 20.Koristka S, et al. Retargeting of human regulatory T cells by single-chain bispecific antibodies. J Immunol. 2012;188:1551–1558. doi: 10.4049/jimmunol.1101760. [DOI] [PubMed] [Google Scholar]
- 21.Maj T, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida H, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 23.Chung Y, et al. Epstein Barr virus-induced 3 (EBI3) together with IL-12 negatively regulates T helper 17-mediated immunity to Listeria monocytogenes infection. PLoS Pathog. 2013;9:e1003628. doi: 10.1371/journal.ppat.1003628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do J, et al. Treg-specific IL-27Rα deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc Natl Acad Sci USA. 2017;114:10190–10195. doi: 10.1073/pnas.1703100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascanfroni ID, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascanfroni ID, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmin F, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Zaidi MR, et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayers M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol. 2013;190:1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastid J, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res. 2015;3:254–265. doi: 10.1158/2326-6066.CIR-14-0018. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, et al. Epstein-Barr virus-induced gene 3-deficiency leads to impaired antitumor T-cell responses and accelerated tumor growth. OncoImmunology. 2015;4:e989137. doi: 10.4161/2162402X.2014.989137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer KA, et al. Immunosurveillance of lung melanoma metastasis in EBI-3-deficient mice mediated by CD8+ T cells. J Immunol. 2008;181:6148–6157. doi: 10.4049/jimmunol.181.9.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirlekar B, Michaud D, Searcy R, Greene K, Pylayeva-Gupta Y. IL35 hinders endogenous antitumor T-cell immunity and responsiveness to immunotherapy in pancreatic cancer. Cancer Immunol Res. 2018;6:1014–1024. doi: 10.1158/2326-6066.CIR-17-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinozaki Y, et al. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer. 2009;124:1372–1378. doi: 10.1002/ijc.24107. [DOI] [PubMed] [Google Scholar]
- 36.Zhu C, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salcedo R, et al. Immunologic and therapeutic synergy of IL-27 and IL-2: Enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol. 2009;182:4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.