Significance
Mutually exclusive expression of the var gene family contributes to antigenic variation and immune evasion in Plasmodium falciparum. Our work reveals that the entire var gene family is silenced on knockout of the PfRecQ1 DNA helicase. Our study shows that PfRecQ1 maintains var gene clonal expression, possibly by mediating the nuclear periphery localization of the var gene family and decreasing heterochromatic histone modifications at the var gene loci. This work reveals a path of var gene regulation through chromatin structure and identifies PfRecQ1 as a target for antimalarial drug development.
Keywords: DNA helicases, heterochromatin, virulence gene, mutually exclusive expression, Plasmodium falciparum
Abstract
The Plasmodium falciparum var gene family encodes ∼60 surface antigens by which parasites escape the host immune responses via clonal expression of var genes. However, the mechanism controlling this mutual exclusivity, associated with alterations in chromatin assembly, is not understood. Here, we determined how expression of the var gene family is regulated by two RecQ DNA helicase family members, PfRecQ1 and PfWRN, in P. falciparum. Through genetic manipulation, we found that the complete var repertoire was silenced on PfRecQ1 knockout, whereas their expression did not show noticeable changes when PfWRN was knocked out. More important, mutually exclusive expression of var genes could be rescued by complementation of PfRecQ1. In addition, knocking out either of these two helicase genes changed the perinuclear cluster distribution of subtelomeres and subtelomeric var genes. Whereas deletion of PfRecQ1 increased the heterochromatin mark trimethylated (H3K9me3) at the transcription start site (TSS) of the var gene upsC1, that deletion had no effect on the global distribution of H3K9me3 over gene bodies, including those for the var genes. ChIP-seq assay showed that PfRecQ1 was enriched globally at the TSSs of all genes, whereas PfWRN-enriched regions occurred at the gene bodies of the var gene family, but not of other genes or at TSSs of all genes. On PfRecQ1 deletion, the upsC1 var gene moved from the active perinuclear transcription region to a silenced region of the upsC type. These findings imply that PfRecQ1, but not PfWRN, is essential for maintaining the clonal expression of var genes.
Malaria is an infectious disease that presents a serious threat to human life and health (1). The World Health Organization reported that ∼3.2 billion people are currently at risk for malaria (2), and among the human malaria parasites, Plasmodium falciparum is the deadliest. The surface variant antigen P. falciparum Erythrocyte Membrane Protein 1 (PfEMP1), encoded by ∼60 var genes, mediates binding of parasitized erythrocytes to endothelial surface and contributes to the major pathogenesis in severe malaria. var genes are classified into three major types, upsA, upsB, and upsC, depending on their promoter structures. Their chromosomal locations vary: upsA and most of upsB var genes are at the subtelomeric regions, whereas all upsC and a few upsB var genes are at the central regions of the chromosomes (3). Through mutually exclusive expression of var genes, P. falciparum escapes the host immune responses and parasite killing (4–8). The underlying molecular mechanism of var gene regulation is incompletely understood, but it is known to involve epigenetic factors such as specific alterations in histone modifications and chromatin assembly (8, 9).
The RecQ protein family is a highly conserved group of DNA helicases, the members of which play important roles in DNA replication, transcription, repair, recombination, and telomere maintenance (10–12). Five family members have been identified in humans: RecQ1, RecQ2 (BLM), RecQ3 (WRN), RecQ4, and RecQ5 (11, 12). In human embryonic stem cells, WRN can associate with heterochromatin proteins SUV39H1, HP1α, and nuclear lamina-heterochromatin anchoring protein LAP2β, and WRN deficiency results in a global loss of trimethylated histone H3K9 (H3K9me3) associated with changes in heterochromatin architecture (13). P. falciparum contains only two members of the RecQ family: PfWRN, encoded by the PF3D7_1429900 gene, and PfRecQ1 (also named PfBLM), encoded by the PF3D7_0918600 gene (14). Both proteins possess an ATPase and a 3′–5′ direction-specific DNA helicase activity (15, 16). Recently, it has been reported that both PfRecQ1 and PfWRN may play roles in genome stability, gene expression patterns, and DNA replication dynamics (17). However, the molecular mechanisms of the two helicases in var gene regulation remain unclear.
Here we report on the effect of selectively deleting the PfRecQ1 or PfWRN gene on epigenetic features of the var genes in P. falciparum. We find that transcription of the previously activated var gene was dramatically reduced in the PfRecQ1-knockout parasite strains (PfRecQ1∆), but not in the PfWRN-knockout strain (PfWRN∆). The phenotype of the clonal var expression could be successfully rescued by complementation of PfRecQ1. We show that knockout of either helicase gene resulted in more accumulated perinuclear distribution of subtelomeric var genes, whereas PfRecQ1∆ led to increase of H3K9me3 at the transcription start site (TSS) of the upsC1 var gene, concomitant with the inactivation of this var gene. ChIP-seq assay showed that PfRecQ1 was enriched globally at the TSSs of all genes. In contrast, PfWRN-enriched regions were seen at the gene bodies of the var gene family, but not of other genes or at TSSs of all genes. In addition, on PfRecQ1 deletion, upsC1 var gene moved from the active perinuclear transcription region to a silenced region of the upsC type. This study implies an essential role for PfRecQ1 in the maintenance of var expression in clonal P. falciparum strains, possibly through decreasing the amount of silence mark H3K9me3 at the TSS and keeping a perinuclear localization of the active var gene in the active expression site.
Results
PfRecQ1∆ Completely Abolishes Expression of the var Gene Family.
PfRecQ1 and PfWRN are two known PfRecQ helicase members. Knockout strains of these two genes were generated separately, using the CRISPR/Cas9 system (SI Appendix, Fig. S1A), and confirmed by PCR (SI Appendix, Fig. S1B) and DNA sequencing of the PCR products. Transcriptomic analysis of the knockout parasites at the ring stage by RNA sequencing revealed that the deletion of either PfRecQ1 or PfWRN altered the expression of many genes (SI Appendix, Fig. S1 C–H and Tables S1 and S2). Notably, the transcription of genes implicated in the DNA replication pathway dramatically increased (SI Appendix, Fig. S1 G and H), consistent with the roles of both PfRecQ gene members in DNA replication. With regard to down-regulated genes, there was no particular gene family except the var genes. PfRecQ1∆, in particular, markedly inhibited the dominantly expressed var gene (PF3D7_1240600, upsC1) in the 3D7C8 (WT) strain, leading to the overall silencing of the entire var gene family (SI Appendix, Fig. S1E). Such a drastic silencing effect was not observed in the PfWRN∆ strain (SI Appendix, Fig. S1F).
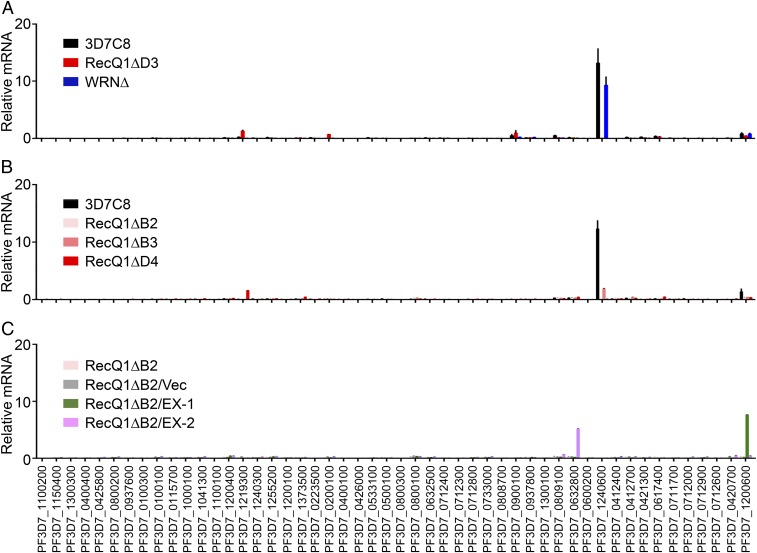
To confirm that the silencing of the var gene family was the direct result of PfRecQ1 knockout, RT-qPCR for each var gene was carried out on multiple clones from different transfection experiments. PfRecQ1∆ D4 and D3 are clones from the first transfection of 3D7C8, which dominantly expressed the upsC1 var gene PF3D7_1240600. B2 and B3 are clones from another transfection of 3D7C8, whereas D9 is a clone from transfection of 3D7G4, which dominantly expressed another upsC var gene, PF3D7_0711700. PfRecQ1∆ consistently silenced the entire var repertoire in all these strains (Fig. 1 A and B and SI Appendix, Fig. S2).
Fig. 1.
Expression status of var genes on knock-out of PfRecQ1 or PfWRN. Var genes expression level (A) in WT 3D7C8, PfRecQ1∆ (strain D3), and PfWRN∆ were detected by RT-qPCR with five repeats, and (B) in different strains of PfRecQ1∆ with at least two repeats. PfRecQ1∆D4 and PfRecQ1∆D3 were clones from first transfection into 3D7C8 (PF3D7_1240600, dominantly expressed); PfRecQ1∆B2 and PfRecQ1∆B3 were clones from second transfection into 3D7C8. 3D7C8 is the WT parasite strain. (C) In PfRecQ1∆B2, PfRecQ1∆B2/vec, PfRecQ1∆B2/EX-1, and PfRecQ1∆B2/EX-2. PfRecQ1∆B2/vec was PfRecQ1∆B2 transfected with PCC4-G418 vector, as control of complementation transfection. PfRecQ1∆B2/EX-1 and PfRecQ1∆B2/EX-2 were PfRecQ1∆B2 transfected with PCC4-TY1-FlagRecQ1-G418 plasmid to episomally express TY1-FlagPfRecQ1 to rescue the functions of PfRecQ1. PfRecQ1∆B2/EX-1 and PfRecQ1∆B2/EX-2 were results from twice-independent complementation transfections. Experiments were repeated three times. The y axis gives the relative abundance of var genes mRNA; var genes IDs are shown below the x axis. Arginyl-tRNA synthetase (PF3D7_0913900), a housekeeping gene, is used as internal control. Error bars represent SEM.
To further confirm the specific silencing effect of PfRecQ1∆ on the var gene family, phenotypic rescue experiments were performed, where the PCC4-TY1-FlagRecQ1-G418 plasmid was transfected into one PfRecQ1∆ strain to episomally express TY1-FlagPfRecQ1 (SI Appendix, Fig. S3). In two independent transfection experiments (named PfRecQ1∆B2/EX-1 and PfRecQ1∆B2/EX-2), we observed rescue of clonal var expression in the complementation strains (Fig. 1C). As expected, this phenotypic rescue was not observed in the control parasite PfRecQ1∆B2/Vec that carries the plasmid PCC4-G418 lacking PfRecQ1 (Fig. 1C). Interestingly, the var genes rescued in these two complementation experiments were different (Fig. 1C), with one being PF3D7_0632800 (a upsB type) and the other PF3D7_1200600 (a upsE type). These results demonstrated that PfRecQ1 was essential for maintaining expression of the active var genes in clonal parasites, and its effect was not specific to the upsC type. In contrast to PfRecQ1∆, PfWRN∆ did not affect var gene expression, and the dominantly expressed upsC1 var gene in WT 3D7C8 was still transcribed (Fig. 1A).
PfRecQ1∆ Alters Heterochromatin.
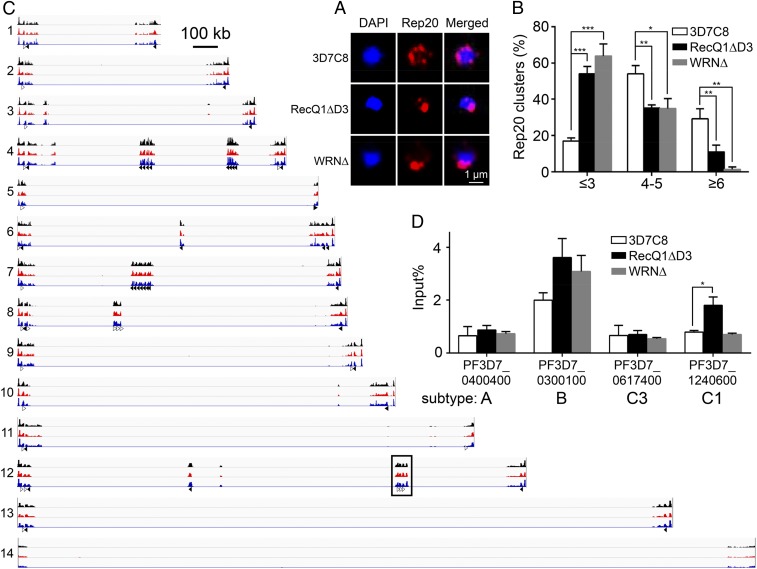
Subtelomeric regions of the P. falciparum chromosomes are clustered into several foci at the perinuclear space, where the var genes locate. The transcription status of var genes is related to their spatial distribution in the perinuclear area (18). To obtain a mechanistic understanding of the influence of PfRecQ proteins on var gene expression, we first studied the perinuclear distribution of the subtelomer var genes. For this analysis, we used DNA-FISH, targeting Rep20, a conserved repetitive sequence present at the subtelomeric regions of all P. falciparum chromosomes. Because Rep20 flanks subtelomeric var genes, its spatial distribution mimics that of subtelomeric var genes (19). Consistent with earlier observations (20), chromosome ends in WT parasites at the ring stage typically formed 4–7 Rep20 clusters. Deletion of either PfRecQ DNA helicase resulted in significant clumping of the chromosomal ends. Parasites containing 4–5 and ≥6 Rep20 foci greatly decreased, whereas the proportions of parasite nuclei containing 1–3 Rep20 foci increased from 17% in WT to 54% and 64% in PfRecQ1∆ and PfWRN∆, respectively (Fig. 2 A and B). This result suggests that each of the two helicases might, directly or indirectly, regulate the spatial distribution of subtelomeres and subtelomeric var genes, reminiscent of our hypothesis that PfRecQ1’s regulation on var genes is not specific to upsC type.
Fig. 2.
H3K9me3 modification and DNA-FISH of subtelomeric var genes on PfRecQ1∆ or PfWRN∆. (A) Rep20 DNA-FISH in WT 3D7C8, PfRecQ1∆D3, and PfWRN∆. Rep20 probe was labeled with Biotin and then incubated with streptavidin (Alexa Fluor 568 conjugate, Thermo Fisher, red); chromosomal DNA was stained with DAPI (blue), representing the nuclear area. Parasites synchronized at ring stage were used for this DNA-FISH. Resolution of A is 600 dpi. (B) Graphic representation of the statistical analysis of Rep20 DNA-FISH result. The experiment was repeated three times, each with a count of 100 nuclei at ring stage. Nuclei were classified as type 1 (1–3 foci/nucleus), type 2 (4–5 foci/nucleus), or type 3 (≥6 foci/nucleus). The percentage of each type per 100 nuclei was calculated. (Scale bars of all pictures of DNA-FISH, 1 µm.) Here all error bars were SEM, *P < 0.05; **P < 0.01; ***P < 0.001. (C) Integrative genomic view of ChIP-seq result of H3K9me3 at ring stage in P. falciparum. In parasites of WT-3D7C8 (black), RecQ1∆D3 (red), and WRN∆ (blue) at ring phase, H3K9me3 bound specially to sequences of telomeres and var genes. Var genes are categorized as being in the forward orientation (unfilled arrows) or reverse orientation (filled arrows). Telomere sequences were at the end of chromosomes, which were not labeled. ChIP was performed with the anti-H3K9me3 antibody twice, and the average was used for this IGV. Each read was normalized by the total number of uniquely mapped ChIP-seq reads. Values on the y axis were calculated by ChIP reads divided by input reads, representing the net signal of ChIP. Chromosomal numbers are shown to the left. Two var genes in the boxed region are amplified for a detailed view in SI Appendix, Fig. S4C. A scale bar representing 100 kilobases (kb) is shown to the right of chromosome 1. The data range on the y axis used for IGV here was 0–40. (D) ChIP-qPCR of H3K9me3. The primers at the TSS of four var genes were used for this assay. PF3D7_0400400 is a upsA type, also used as A1 var gene for paired DNA-FISH. PF3D7_0300100 is a upsB type var gene. PF3D7_0617400 is a upsC type, named as C3. PF3D7_1240600, is another upsC type, also used as C1 var genes for paired DNA-FISH. Var A1, B, C3 genes are silent in WT, PfRecQ1∆, and PfWRN∆; Var C1 is dominantly transcribed in WT or PfWRN∆, but silent in PfRecQ1∆. Primers for ChIP-qPCR are shown in SI Appendix, Table S3. The experiment was repeated five times. The P value of upsC1 between WT and PfRecQ1∆ was 0.013, whereas the others were >0.05 (no significant difference).
In humans, the WRN helicase is important for maintaining global distribution of H3K9me3 and heterochromatin architecture (13). In P. falciparum, H3K9me3 is known to repress var gene expression (21). Therefore, we next wanted to determine whether the two helicase genes in P. falciparum were also involved in maintaining chromatin structure. ChIP and sequencing (ChIP-seq) analysis showed that H3K9me3 at the ring stage is specifically enriched at telomeric sequences and var genes in the WT 3D7C8 strain (Fig. 2C). In the PfRecQ1∆ and PfWRN∆ strains, there were no changes of H3K9me3 distribution on gene bodies globally (SI Appendix, Fig. S4 A–C). Given that H3K9me3 alteration at the TSS is generally more critical for gene silencing (8, 22), we further evaluated H3K9me3 at TSS of var genes. ChIP-qPCR was used to circumvent the problem associated with the high sequence similarity in the untranslated regions of the var genes (8). The result revealed that the H3K9me3 level at the TSS of upsC1 var gene was markedly increased when this gene became silenced on PfRecQ1∆ (Fig. 2D), whereas we saw no significant changes of the H3K9me3 levels at the TSS of the other three selected var genes. In contrast, the H3K9me3 levels at the TSS of four representative var genes did not change in the PfWRN∆ line compared with those in the WT parasites.
PfRecQ1 and PfWRN Display Disparate Chromosomal Localizations.
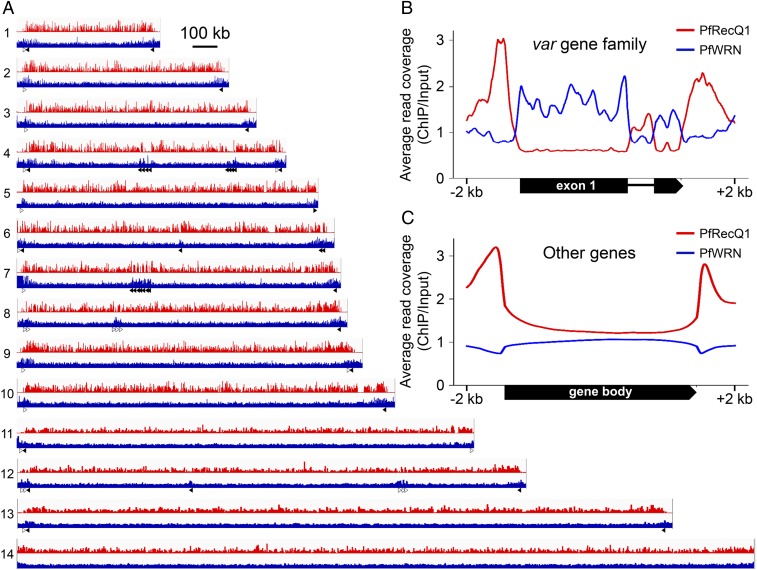
To determine whether the observed differential global effects of the helicase gene knockouts on var genes were a result of their distinct associations with chromosomal regions, we established parasite lines with PfRecQ1 and PfWRN tagged at their N termini with 3×HA-Flag and GFP, respectively, in 3D7 parasites (named HA-FlagRecQ1 and GFPWRN), using the CRISPR/Cas9 system (Methods and SI Appendix, Fig. S5A). Tagging of these two genes was confirmed by PCR (SI Appendix, Fig. S5B) and sequencing on the PCR products, as well as by Western blotting to detect the tagged proteins (SI Appendix, Fig. S5C). ChIP-seq analysis of the HA-FlagRecQ1 strain showed genomewide enrichment of PfRecQ1 at the TSS sequences of all genes, including the var genes, suggesting the involvement of PfRecQ1 in transcription. In contrast, PfWRN was not associated with the TSS of P. falciparum genes, but instead was enriched only at the gene bodies of the var gene family (Fig. 3).
Fig. 3.
Localization of PfRecQ1 and PfWRN proteins to P. falciparum chromatins. (A) Integrative genomic view of ChIP-seq result of HA-FlagRecQ1 (red) and GFPWRN (blue). Anti-Flag and anti-GFP were used for ChIP of PfRecQ1 and PfWRN, respectively. y axis values were calculated using each ChIP signal as the numerator and the respective signal input as the denominator. Sixty var genes distributed along P. falciparum chromosomes 1–13 are indicated by forward orientation (unfilled) and reverse orientation (filled) arrows. The data range of values on the y axis used for RecQ1 and WRN was 0–15 and 0–3, respectively. (B and C) Average reads coverage profile of HA-FlagRecQ1 (red line) and GFPWRN (blue line) at gene bodies of (B) var gene family and (C) other genes (nonvar genes). The curves were normalized per million mapped reads. y axis values were calculated as each ChIP signal divided by its respective input signals.
The Active var Gene Is Relocated to a Silent Perinuclear Site on PfRecQ1∆.
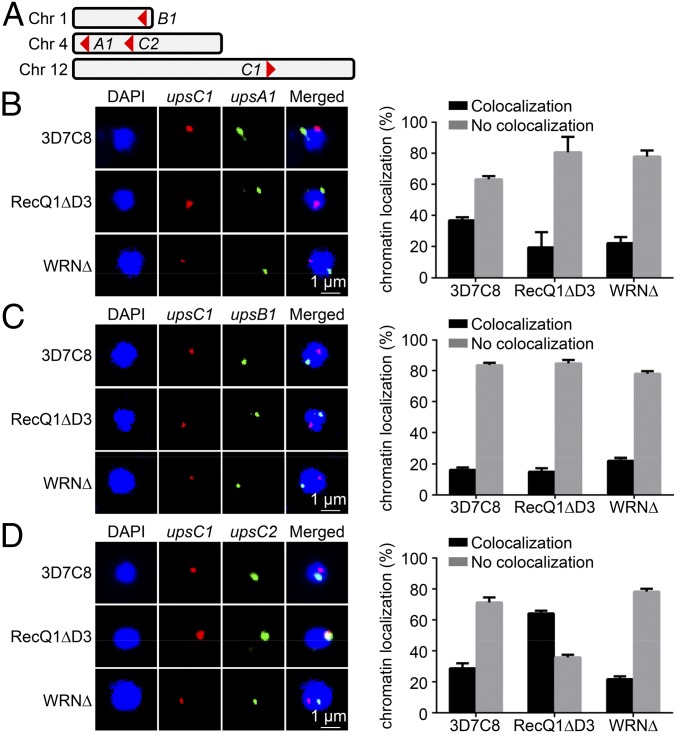
In light of the spatial clumping of chromosomal ends in the knockout strains, we further aimed to investigate whether the relative positions of different var gene types were also changed. For this purpose, we selected different var gene types for paired DNA-FISH analysis, including upsC1, the predominantly expressed var gene in WT 3D7 but silenced in PfRecQ1∆, PF3D7_0400400 (upsA1), PF3D7_0115700 (upsB1), and PF3D7_0412400 (upsC2) (Fig. 4A). The result showed that upsC1 was predominantly not colocalized with those of upsA1 or upsB1 in both WT 3D7 and PfRecQ1∆, suggesting the spatial distribution of upsC1 relative to the upsA or upsB type was unaffected by deletion of PfRecQ1 (Fig. 4 B and C). Interestingly, upsC1 became predominantly colocalized with upsC2 in PfRecQ1∆ (Fig. 4D). As expected, the perinuclear distribution of upsC1 relative to other var genes was not changed in PfWRN∆; upsC1 was largely separated from the other three var genes tested in both WT 3D7 and PfWRN∆ (Fig. 4 B–D), consistent with its remaining dominant expression after PfWRN was knocked out. These results indicated that PfRecQ1 might regulate heterochromatin at TSS of upsC1, leading to its relocation from an active transcription region to a silenced region of the upsC type.
Fig. 4.
Var gene DNA redistribution correlates with expression status on RecQ1∆. (A) Localizations of four picked var genes for paired DNA-FISH in chromosomes. (B) Representative image of paired DNA-FISH of upsA1 (PF3D7_0400400, green) with upsC1 (PF3D7_1240600, red). In 3D7C8 and RecQ1∆, upsA1 was labeled with fluorescein, whereas upsC1 was labeled with biotin. In WRN∆, upsA1 was labeled with biotin, whereas upsC1 was labeled with fluorescein. (C) Representative image of paired DNA-FISH of upsB1 (PF3D7_ 0115700, green) with upsC1 (red). In all strains, upsB1 was labeled with biotin, whereas upsC1 was labeled with fluorescein. (D) Representative image of paired DNA-FISH of upsC2 (PF3D7_0412400, green) with upsC1 (red). In 3D7C8 and RecQ1∆, upsC2 was labeled with fluorescein and upsC1 was labeled with biotin. In WRN∆, upsC2 was labeled with biotin and upsC1 was labeled with fluorescein. Parasites synchronized at ring stage were used for this DNA-FISH. Biotin labeling was visualized by incubating with streptavidin-Alexa Fluor 568. DAPI staining (blue) was used to locate nuclear area. Graphs at right are statistical analyses of paired DNA-FISH results. Experiments were repeated three times, each with a count of ≥50 nuclei. The percentage of colocalization or no colocalization were calculated. Error bars were SEM. Scale bars of all pictures of DNA-FISH were 1 µm. In DNA-FISH of upsC1 and upsC2, the P value of RecQ1∆ to 3D7C8 was 0.006. Resolution of B, C, and D is 600 dpi.
Discussion
RecQ family members play vital roles in many aspects of DNA metabolism. The deadliest malarial parasite, P. falciparum, possesses just two PfRecQ family DNA helicases, PfRecQ1 and PfWRN. In this work, we show that PfRecQ1 participates in the regulation of var gene expression; deletion of PfRecQ1 abolishes the monoallelic expression pattern of the var genes and results in complete silencing of var gene family, which could also be rescued by complementation of PfRecQ1. This role is restricted to PfRecQ1, as PfWRNΔ does not affect var expression, although both of these DNA helicase members are associated with parasite chromosomes.
The involvement of PfRecQ1 in var gene regulation appears not be restricted to a particular var gene. Regardless of which individual var genes were active in the parasite strains tested, PfRecQ1Δ resulted in their repression. Complementation studies confirmed the role of PfRecQ1 in the maintenance of var gene expression, as episomally expressed PfRecQ1 restored the monoallelic expression pattern of var genes. Interestingly, the two complementation strains expressed two different var genes of the upsB and upsE types, respectively, instead of the original upsC1 gene, further implying that PfRecQ1’s role applies to the whole var gene family.
To elucidate the mechanisms by which PfRecQ family proteins regulate gene expression, we determined the association of PfRecQ proteins with chromosomal regions and their potential effects on chromatin structures. Both PfRecQ proteins were shown to regulate the spatial distribution of chromosomal ends; their deletion led to enhanced clustering of the subtelomeric regions containing var genes in perinuclear areas. Active and silent var genes are known to occupy distinct perinuclear sites, and var gene activation requires relocation to the active perinuclear site (18). Moreover, an active var from a telomere region could move to the silent sites enriched for telomeric vars when it is silenced. Consistently, our results showed that silencing of the active upsC1 var gene on PfRecQ1Δ is coupled with the redistribution of this gene from a transcriptionally active site to a silent site. This silent site colocalized with the silent upsC2 gene, but differed from the sites that the silent upsA and upsB genes occupied. This relocation could be directly related to the loss of PfRecQ1 protein on the loci, or indirectly to the absence of other active factors. Fine global mapping of the two helicases revealed that PfRecQ1 and PfWRN were associated with different chromosomal regions. Whereas PfRecQ1 had a global association with the TSS of all genes (including var genes), PfWRN had a more restricted role, enriched only in the gene bodies of var genes. This is consistent with the var gene silencing on deletion of PfRecQ1, but not PfWRN∆. The silencing of var genes in PfRecQ1∆ was also associated with the enrichment at its TSS of H3K9me3, a mark of heterochromatin.
On the basis of these findings, we speculate that PfRecQ1 functions in the maintenance of clonal var expression. With its DNA helicase activity, PfRecQ1 may regulate the DNA conformation of var genes and be essential for keeping related genes within such active expression regions at the nuclear periphery, providing var gene access to transcription machinery in this active region to maintain its active transcription. Thus, it may be that if PfRecQ1 protein is deficient, the var gene DNA reverts to an inactive conformation and is relocated to silenced region at nuclear periphery. In such conformation, the originally active var gene cannot escape silencing because of the heterochromatin environment.
PfEMP1 is known to mediate cyto-adherence of infected red blood cells to host receptors, which is essential for the survival of the parasite (1). With regard to the global silencing of all var genes in PfRecQ1∆ parasites, PfRecQ1 could serve as a target for therapeutic development against malaria. Furthermore, the finding that PfRecQ1 is involved in var gene expression identifies another layer of the complex regulatory mechanisms of antigenic switching in malaria parasites.
Materials and Methods
Parasite Culture and Transfection.
P. falciparum was cultured and synchronized following standard protocols (23). Ring-stage parasites were synchronized with Percoll-sorbitol and transfected with 100 μg plasmid DNA by electroporation (24). Transfected parasites were subjected to selection with the BSD and WR99210 drugs, which respectively targeted blasticidin S deaminase and human dihydrofolate reductase, to select parasites carrying both pL6CS and pUF1-BSD-Cas9. After resistant parasites emerged, genomic DNA was extracted for PCR validation, and parasites were cloned by limiting dilution.
The knockout and tagging of the PfRecQ1 and PfWRN genes were carried out separately, using the CRISPR/Cas9 system (SI Appendix, Figs. S1A and S5A), and was confirmed by PCR. For gene knockout, the entire 1,042-bp functional domain (210 bp away from ATG to 1,252 bp) of PfRecQ1 or 1,828 bp functional domain (167 bp away from ATG to 1,995 bp) of PfWRN was replaced by 5′-UTR-hdhfr-3′-UTR. Individual PCR was performed with genomic DNA as templates, and primer pairs P1/P2 or P3/P4 (SI Appendix, Fig. S1A). The knockout was confirmed by sequencing of the PCR products. For tagging, the coding sequences of HA-Flag or GFP were, respectively, fused to the N terminus of PfRecQ1 or PfWRN. Tagged parasites HA-FlagRecQ1 and GFPWRN were confirmed by PCR with genomic DNA and primer pairs P5/P6 and P7/P8, respectively (SI Appendix, Fig. S5 A and B), by sequencing of the PCR products, and by Western blotting (SI Appendix, Fig. S5C). All primers used are shown in SI Appendix, Table S3.
Complementation Assay.
The PCC4-TY1-FlagRecQ1-G418 and PCC4-G418 plasmids were transfected into PfRecQ1∆B2 strain to express TY1-FlagPfRecQ and be used as control, respectively. Two independent transfection experiments were performed for PCC4-TY1-FlagRecQ1-G418, named RecQ1∆B2/EX-1 and RecQ1∆B2/EX-2. Transfected parasites were subjected to G418 (neomycin) selection, which targets aminoglycoside phosphotransferase, to select parasites carrying the episomes of PCC4-TY1-FlagRecQ1-G418 or PCC4-G418. When resistant parasites emerged, ring-stage parasites were collected, and episomal expression of TY1-FlagPfRecQ1 was checked by RT-qPCRs of PfRecQ1 with primers P9/P10 (SI Appendix, Fig. S3 and Table S3) and confirmed by Western blotting with anti-TY1 antibody (Genscript).
Detailed Methods.
RNA-seq and RT-qPCR, Western blot analysis, DNA-FISH, ChIP, next-generation sequencing, and data analysis are described in detail in SI Appendix, Supplementary Materials and Methods. All the raw data of next-generation sequencing in this manuscript have been deposited in NCBI’s Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) and can be accessed through accession no. PRJNA506122 (25).
Supplementary Material
Acknowledgments
We thank Jose-Juan Lopez-Rubio of University Montpellier for kindly providing the pUF1-Cas9 and pL6-CS plasmids. We also thank Jiping Han for providing the technical support of parasite transfection. This research was supported by National Key R&D Program of China Grant 2018YFA0507300; National Science and Technology Major Project Grant 2018ZX10101004003001; National Natural Science Foundation of China Grants 31571345, 31771455, and 81772218; Chinese Academy of Sciences (CAS) Grant GJHZ1703; CAS President's International Fellowship Initiative Grant 153831WGZJTPYJY20170005; and National Institute of Allergy and Infectious Diseases of the National Institutes of Health Grants R01AI116466 and U19AI089672.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: All the raw data of next-generation sequencing reported in this paper have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), https://www.ncbi.nlm.nih.gov/sra (accession no. PRJNA506122).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811766116/-/DCSupplemental.
References
- 1.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Technical Strategy for Malaria 2016-2030. WHO; Geneva: 2015. [Google Scholar]
- 3.Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol. 2008;62:445–470. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- 4.Dzikowski R, Deitsch KW. Genetics of antigenic variation in Plasmodium falciparum. Curr Genet. 2009;55:103–110. doi: 10.1007/s00294-009-0233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherf A, et al. Antigenic variation in malaria: In situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 7.Yin S, Cheng X, Zhang X, Jiang L. Epigenetic regulations in immune evasion of the deadliest malaria parasite Plasmodium falciparum. Sci Found China. 2014;1:030. [Google Scholar]
- 8.Jiang L, et al. PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature. 2013;499:223–227. doi: 10.1038/nature12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ay F, et al. Three-dimensional modeling of the P. falciparum genome during the erythrocytic cycle reveals a strong connection between genome architecture and gene expression. Genome Res. 2014;24:974–988. doi: 10.1101/gr.169417.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudlow BA, Kennedy BK, Monnat RJ., Jr Werner and Hutchinson-Gilford progeria syndromes: Mechanistic basis of human progeroid diseases. Nat Rev Mol Cell Biol. 2007;8:394–404. doi: 10.1038/nrm2161. [DOI] [PubMed] [Google Scholar]
- 11.Khakhar RR, Cobb JA, Bjergbaek L, Hickson ID, Gasser SM. RecQ helicases: Multiple roles in genome maintenance. Trends Cell Biol. 2003;13:493–501. doi: 10.1016/s0962-8924(03)00171-5. [DOI] [PubMed] [Google Scholar]
- 12.Chu WK, Hickson ID. RecQ helicases: Multifunctional genome caretakers. Nat Rev Cancer. 2009;9:644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science. 2015;348:1160–1163. doi: 10.1126/science.aaa1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuteja R. Genome wide identification of Plasmodium falciparum helicases: A comparison with human host. Cell Cycle. 2010;9:104–120. doi: 10.4161/cc.9.1.10241. [DOI] [PubMed] [Google Scholar]
- 15.Rahman F, Tarique M, Tuteja R. Plasmodium falciparum Bloom homologue, a nucleocytoplasmic protein, translocates in 3′ to 5′ direction and is essential for parasite growth. Biochim Biophys Acta. 2016;1864:594–608. doi: 10.1016/j.bbapap.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Rahman F, Tarique M, Ahmad M, Tuteja R. Plasmodium falciparum Werner homologue is a nuclear protein and its biochemical activities reside in the N-terminal region. Protoplasma. 2016;253:45–60. doi: 10.1007/s00709-015-0785-6. [DOI] [PubMed] [Google Scholar]
- 17.Claessens A, et al. RecQ helicases in the malaria parasite Plasmodium falciparum affect genome stability, gene expression patterns and DNA replication dynamics. PLoS Genet. 2018;14:e1007490. doi: 10.1371/journal.pgen.1007490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ralph SA, Scheidig-Benatar C, Scherf A. Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci USA. 2005;102:5414–5419. doi: 10.1073/pnas.0408883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueiredo LM, Pirrit LA, Scherf A. Genomic organisation and chromatin structure of Plasmodium falciparum chromosome ends. Mol Biochem Parasitol. 2000;106:169–174. doi: 10.1016/s0166-6851(99)00199-1. [DOI] [PubMed] [Google Scholar]
- 20.Freitas-Junior LH, et al. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature. 2000;407:1018–1022. doi: 10.1038/35039531. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Rubio JJ, et al. 5′ flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol Microbiol. 2007;66:1296–1305. doi: 10.1111/j.1365-2958.2007.06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 24.Ghorbal M, et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, et al. 2018 DNA helicase RecQ1 regulates expression of virulence genes in Plasmodium falciparum via heterochromatin alteration. Available at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA506122/. Deposited November 29, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.