Significance
Humans spend much of their waking life engaged in mind wandering. Underlying brain systems supporting this complex ability have been established in healthy individuals, yet it remains unclear how mind wandering is altered in neuropsychiatric populations. We reveal changes in the thought profiles elicited during periods of low cognitive demand in dementia, resulting in reduced mind wandering and an increased propensity toward stimulus-bound thought. These altered thought profiles were associated with structural and functional brain changes in the hippocampus, default, and frontoparietal networks, key regions implicated in internal mentation in healthy individuals. Our findings provide a unique clinical validation of current theoretical models of mind wandering and reveal a dimension of cognitive dysfunction that has received scant attention in dementia.
Keywords: mind wandering, default mode network, Alzheimer’s disease, behavioral variant frontotemporal dementia, hippocampus
Abstract
Mind wandering represents the human capacity for internally focused thought and relies upon the brain’s default network and its interactions with attentional networks. Studies have characterized mind wandering in healthy people, yet there is limited understanding of how this capacity is affected in clinical populations. This paper used a validated thought-sampling task to probe mind wandering capacity in two neurodegenerative disorders: behavioral variant frontotemporal dementia [(bvFTD); n = 35] and Alzheimer’s disease [(AD); n = 24], compared with older controls (n = 37). These patient groups were selected due to canonical structural and functional changes across sites of the default and frontoparietal networks and well-defined impairments in cognitive processes that support mind wandering. Relative to the controls, bvFTD patients displayed significantly reduced mind wandering capacity, offset by a significant increase in stimulus-bound thought. In contrast, AD patients demonstrated comparable levels of mind wandering to controls, in the context of a relatively subtle shift toward stimulus-/task-related forms of thought. In the patient groups, mind wandering was associated with gray matter integrity in the hippocampus/parahippocampus, striatum, insula, and orbitofrontal cortex. Resting-state functional connectivity revealed associations between mind wandering capacity and connectivity within and between regions of the frontoparietal and default networks with distinct patterns evident in patients vs. controls. These findings support a relationship between altered mind wandering capacity in neurodegenerative disorders and structural and functional integrity of the default and frontoparietal networks. This paper highlights a dimension of cognitive dysfunction not well documented in neurodegenerative disorders and validates current models of mind wandering in a clinical population.
Mind wandering is fundamental to the human experience, yet its alteration in clinical populations remains poorly understood. Dynamic interactions within and between large-scale brain networks govern the initiation and maintenance of mind wandering (1, 2). Of particular interest in this context are interactions between the default network and the frontoparietal control network (3–5). In a recently proposed framework, spontaneous and unconstrained internally oriented thought is generated by fluctuations in the medial temporal lobe system of the default network with weak influence from frontoparietal regions (2). More deliberative thought corresponds to reduced variability in the medial temporal system and increased coupling between the frontoparietal network and the default network core (2). The medial temporal lobe system therefore emerges as influential in the origin of spontaneous thoughts with frontoparietal control regions becoming increasingly important for subsequent elaboration and metacognitive processing (6).
Exploring mind wandering in clinical populations can provide unique information about its cognitive and neural substrates. Altered mind wandering is documented in many conditions and may constitute an important neurocognitive endophenotype across disorders. Perseverative mind wandering that is more frequent or salient with negative content has been reported in depressive rumination, neuroticism, and dysphoria (7, 8) and is suggested to reflect an overly constrained mode of function in the default network, leading to excessive stability of thoughts (2). In contrast, higher rates of unintentional spontaneous mind wandering are associated with increased obsessive-compulsive and attention-deficit/hyperactivity symptomatology in nonclinical samples (9, 10). Similarly, higher frequencies of mind wandering have been noted in schizophrenia, which correlate with the severity of positive symptoms (11). An unconstrained default network due to local hyperactivity or relaxed influence from frontoparietal regions may underpin excessive variation and incoherence of thoughts as seen in psychosis (2).
Previous studies in neuropsychiatric populations have tended to explore network alterations with respect to specific maladaptive expressions of mind wandering, for example, rumination in depression (12, 13). Notably, however, there exists a paucity of data directly relating brain network dysfunction to mind wandering capacity in clinical populations. As such, it remains unclear how pathological brain states impact the frequency and phenomenology of mind wandering.
The present paper addresses this by directly testing whether pathological changes in the default and frontoparietal networks are associated with alterations in mind wandering capacity in neurodegenerative disorders. Dementia syndromes afford a unique opportunity to study the impact of network level dysfunction on mind wandering, given well-established pathology primarily targeting, but not restricted to, nodes of the default and frontoparietal networks (14, 15). This approach is an extension to recent work confirming that focal lesions to the default network in the hippocampus and medial prefrontal cortex can impact the content of mind wandering or reduce its frequency (16, 17). Moreover, on the cognitive level, many of the component processes implicated in mind wandering are disrupted in neurodegenerative disorders, for example, autobiographical memory retrieval (18, 19), mental construction (20, 21), working memory, and shifting attention (22, 23).
Given these well-established neurocognitive changes, it follows that distinct alterations in the frequency and phenomenology of mind wandering should be present in dementia. A recent study reported reduced mind wandering in mild Alzheimer’s disease during concurrent performance of a sustained attention task (24). Reduced spontaneous mind wandering was also recently demonstrated in mild cognitive impairment during a simple vigilance task (25). In this paper, we empirically investigate how alterations in structural and functional brain network integrity across dementia syndromes relate to mind wandering.
To this end, we explored the mind wandering capacity in two dementia subtypes: AD and bvFTD. AD, characterized by prominent episodic memory deficits, is associated with pathological changes in the default and frontoparietal networks, particularly the hippocampus, medial temporal lobe subsystem, and posterior cingulate cortex, extending into the prefrontal and parietal regions with disease progression (15, 26, 27). In contrast, bvFTD is distinguished by behavioral dysfunction, including disinhibition, apathy, emotional blunting, stereotypical behaviors, and loss of insight. Early pathological changes in bvFTD target key regions of the salience and default networks, including the dorsomedial and ventromedial prefrontal cortices as well as widespread changes across the amygdalae, thalamus, and striatum with disease progression (15, 26, 28). We predicted that both of these groups would show an overall reduced propensity for mind wandering relative to controls. Given that environmentally dependent behaviors are characteristic of the bvFTD syndrome (29), we further predicted an increase in stimulus-bound forms of thought in bvFTD.
Quantifying the nature and content of mind wandering in clinical disorders is inherently challenging. Dominant experimental approaches require subjects to monitor or self-identify extraneous thoughts during an ongoing cognitive task. Such approaches rely on dual-tasking and metacognitive capacities that are diminished in dementia, limiting the extent to which reliable conclusions can be drawn from existing measures. To circumvent these methodological constraints, we developed a paradigm to measure mind wandering under conditions of low cognitive demand (30). The task quantifies mind wandering as thoughts unrelated to the immediate environment or to the task at hand, consistent with current theoretical frameworks in which mind wandering is operationalized as stimulus-independent task-unrelated thought (31). Thoughts are therefore classified along a continuum ranging from stimulus-bound to stimulus-/task-related, through to fully fledged instances of mind wandering (i.e., stimulus-independent task-unrelated thought).
The objectives of the current paper were twofold. First, we aimed to quantify the capacity for mind wandering in dementia syndromes during conditions of low cognitive demand. Second, we sought to characterize how disease-related alterations in (i) regional gray matter and (ii) seed-based functional connectivity in the default and frontoparietal networks relate to mind wandering performance. In doing so, we aimed to validate current frameworks of mind wandering in a clinical model by showing that the integrity of the default and frontoparietal networks is essential to support mind wandering capacity.
Results
Overall Mind Wandering Performance.
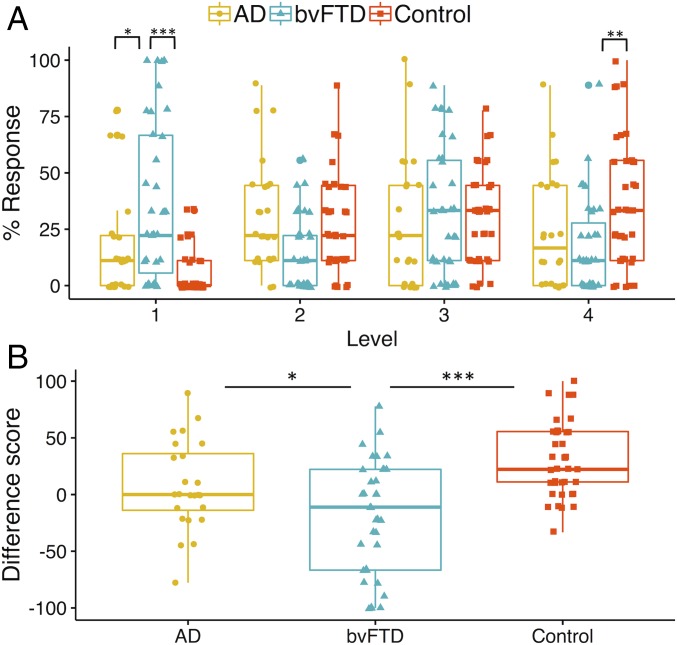
The task scoring system conceptualizes mind wandering along a continuum, ranging from Level 1 (stimulus-bound thought) to Level 4 (mind wandering). Fig. 1A displays the percentage of responses at each scoring level across the nine trials. The main finding was that bvFTD patients displayed significantly increased stimulus-bound responses (Level 1) in the context of significantly decreased mind wandering responses (Level 4) relative to the controls.
Fig. 1.
(A) Overall proportion of mind wandering scores across participant groups. Percentage responses across the mind wandering continuum. The asterisks show the main results of group differences at Level 1 and Level 4. Level 1 responses represent stimulus-bound thoughts; Level 4 responses denote fully fledged instances of mind wandering. (B) Average mind wandering index scores. Mind wandering index (i.e., percentage difference in Level 4 minus Level 1 responses). Higher scores reflect an increased propensity to engage in mind wandering as opposed to stimulus-bound thought with lower scores reflecting a tendency toward stimulus-bound thought. *P < 0.05; **P < 0.01; ***P < 0.001.
A group (bvFTD, AD, control) by Level (1–4) repeated measures ANOVA revealed no main effect of group on the task [F(1.91, 89.00) = 1.13, P = 0.328]. A significant main effect of response level [F(2.87, 267.01) = 4.82, P < 0.01] was driven by a significant difference between percentage of responses at Level 3 vs. Level 1 (P < 0.001). No other significant differences across response levels were observed (P values > 0.07). The group by response level interaction was significant [F(5.74, 264.14) = 7.42, P < 0.00001] and followed by tests of simple effects. Responses differed significantly between the groups at Level 1 [simple effect, F(2, 93) = 14.51, P < 0.00001], Level 2 [simple effect, F(2,93) = 7.64, P < 0.001], and at Level 4 [simple effect, F(2, 93) = 5.81, P < 0.01]; the groups did not differ at Level 3 [simple effect, F(2, 93) = 0.590, P = 0.557]. Sidak-corrected pairwise comparisons confirmed that bvFTD patients provided significantly more stimulus-bound Level 1 responses than AD (P < 0.05) and controls (P < 0.00001) (AD vs. controls, P = 0.116). In contrast, bvFTD Level 4 responses were significantly reduced relative to controls (P < 0.01) indicating a significant reduction in mind wandering (AD vs. controls P = 0.142; AD vs. bvFTD, P = 0.670). BvFTD Level 2 responses were also significantly reduced compared with AD and controls (P values < 0.01) (AD vs. controls, P = 0.795). Finally, all groups displayed higher average scores on longer-duration trials, consistent with an increased propensity for mind wandering with increasing stimulus duration (SI Appendix, Fig. S1).
To explore group differences in the overall pattern of responses, we performed a linear trend analysis. This was to determine if observed responses across the levels were best described by a linear fit such that participants would have a progressively higher percentage of responses across Levels 1–4 consistent with the response profile predicted for healthy controls (30). In line with our predictions, controls’ data were well fit by a linear model [F(1, 146) = 44.65, P < 0.00001 with an R2 of 0.23]. In contrast, a significant linear trend was not observed in either the bvFTD [F(1,138) = 2.28, P = 0.13 with an R2 of 0.02] or the AD [F(1, 94) = 0.53, P = 0.47 with an R2 of 0.006] group, suggesting that the overall response profile in the two dementia groups differed from that of the controls.
Mind Wandering Index Score.
To compare the proportion of Level 1 (stimulus-bound) with Level 4 (mind wandering) responses, an index score was created by subtracting the percentage of Level 1 responses from the percentage of Level 4 responses. A larger positive index score reflects a tendency to engage in mind wandering as opposed to stimulus-bound thought, with negative scores reflecting the reverse profile. Fig. 1B shows the average mind wandering index score across participant groups. Significant group differences were observed on the mind wandering index [F(2, 93) = 13.57, P < 0.00001]. Sidak-corrected pairwise comparisons confirmed the bvFTD group scored significantly lower than both the AD group (P < 0.05) and the controls (P < 0.00001), whereas AD patients did not differ significantly from the controls (P = 0.110).
Gray Matter Correlates of Mind Wandering Performance.
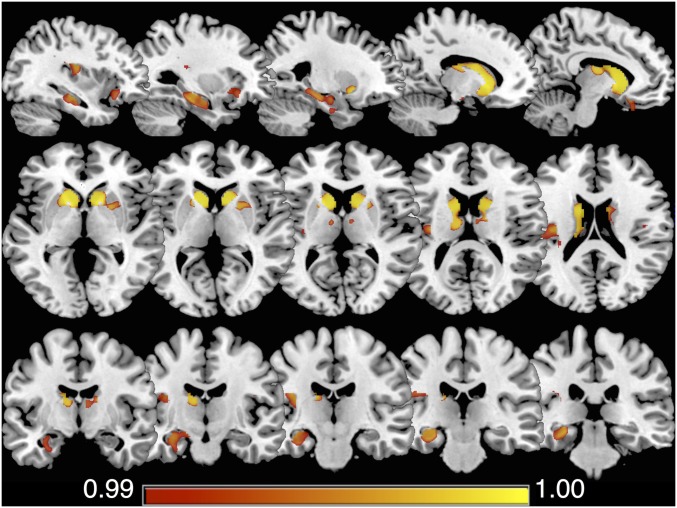
Voxel-based morphometry (VBM) was used to determine the relationship between the mind wandering index and regional gray matter intensity in the patient groups. Fig. 2 displays clusters in which a significant positive correlation emerged between gray matter intensity and the mind wandering index in both bvFTD and AD groups combined. Reduced gray matter intensity in these regions was associated with lower mind wandering index scores, reflecting the tendency toward stimulus-bound thought. Three main clusters were identified: (i) striatum (including caudate, putamen, and nucleus accumbens) and anterior–mid thalamus, extending to the left subcallosal, medial/lateral orbitofrontal, and anterior insular cortices; (ii) left hippocampus and parahippocampal gyrus; (iii) left posterior insular cortex (See SI Appendix, Table S2 for coordinates). These regions showed considerable overlap with areas of gray matter intensity reduction in the patient groups relative to the controls (SI Appendix, Fig. S3 and Table S4). To illustrate the resting-state networks that these regions overlapped with, the results are overlaid on the Yeo et al. (32) 17-network cortical parcellation scheme (SI Appendix, Fig. S4). Of note, the anterior insula cluster overlapped with the salience and default networks, the subcallosal/orbitofrontal cluster overlapped with the limbic network, and the parahippocampal cluster overlapped with the limbic and default networks.
Fig. 2.
The regions of gray matter intensity that covaried with the mind wandering index in bvFTD and AD patients combined. Significant clusters were identified in the striatum (including caudate, putamen, and nucleus accumbens) and the anterior–mid thalamus, extending to the left subcallosal, medial/lateral orbitofrontal and anterior insular cortices; the left hippocampus, parahippocampal gyrus, and the left posterior insular cortex. Results are familywise error (FWE) corrected at P < 0.01; significant clusters identified using threshold free cluster enhancement.
Seed Region Connectivity and Mind Wandering Performance.
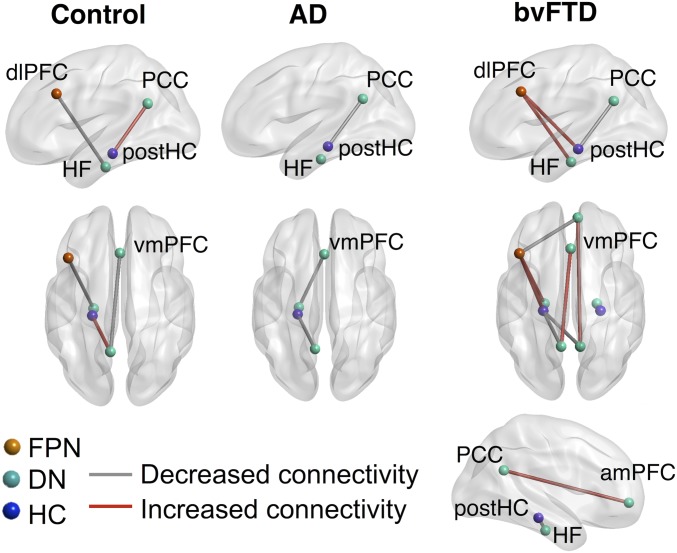
The relationship between seed region connectivity and the mind wandering index score was examined using seeds placed within the default, frontoparietal networks, and the hippocampus. Connections that significantly correlated with the mind wandering index within each group are shown in Fig. 3. In the controls, the tendency to mind wander (as opposed to stimulus-bound thought) was positively associated with left PCC–left posterior hippocampus connectivity (r = 0.14, q < 0.05) and negatively associated with left dlPFC–left hippocampal formation connectivity (r = −0.23, q < 0.05) and left PCC–vmPFC (r = −0.30, q < 0.05) connectivity.
Fig. 3.
Seed regions where connectivity was associated with a tendency to mind wander within the participant groups. Connections show where the mind wandering index was significantly correlated with connectivity such that a tendency to mind wander on the task (as opposed to stimulus-bound thought) was either positively (red lines) or negatively (gray lines) associated with connectivity. The color of regions of interest (ROIs) correspond to the networks the regions are taken from: DN, default network; FPN, frontoparietal network; HC, hippocampus. The results are false discovery rate (FDR) corrected at q < 0.05. amPFC, anteromedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; HF, hippocampal formation; PCC, posterior cingulate cortex; postHC, posterior hippocampus; vmPFC, ventromedial prefrontal cortex. Left view = left side of brain; Right view = right side of brain.
The AD group showed the opposite pattern for left PCC–left posterior hippocampus connectivity as this was negatively associated with a tendency to mind wander (r = −0.56, q < 0.05; this was significantly different from the controls’ connectivity between the same regions: Z = −2.09, P < 0.05). The tendency to mind wander in the AD group was also negatively associated with left hippocampal formation–vmPFC connectivity (r = −0.62, q < 0.05). Similar to the AD group, bvFTD patients showed the opposite (negative) relationship to the controls for left PCC–left posterior hippocampus connectivity (r = −0.44, q < 0.05; which differed significantly from the controls’ connectivity between the same regions: Z = −1.91, P < 0.05 but not from ADs’ connectivity between those regions: Z = −0.43, P = 0.33). The bvFTD group also showed the opposite relationship from the controls for left dlPFC–left hippocampal formation connectivity and left PCC–vmPFC connectivity, which were both positively associated with a tendency to mind wander (dlPFC–HF: r = 0.48, q < 0.05; PCC–vmPFC: r = 0.58, q < 0.05) and both of which were significantly different from the controls (dlPFC–HF: Z = 2.30, P < 0.05; PCC–vmPFC: Z = 2.97, P < 0.01). bvFTD showed additional significant positive associations with mind wandering, between left dlPFC–left hippocampal formation connectivity (r = 0.48, q < 0.05) and right PCC–right amPFC connectivity (r = 0.44, q < 0.05) as well as significant negative associations with left dlPFC–right amPFC connectivity (r = −0.47, q < 0.05), right PCC–left posterior hippocampal connectivity (r = −0.51, q < 0.05), and right posterior hippocampus–right hippocampal formation connectivity (r = −0.46, q < 0.05).
Discussion
This paper offers insights into mind wandering capacity and its associated neural substrates across dementia syndromes. Our results point to shifts in the thought profiles elicited during periods of low cognitive demand in dementia, which, in turn, are associated with structural integrity and resting-state functional connectivity in the default and frontoparietal networks. These findings corroborate current theoretical frameworks emphasizing the role of the medial temporal lobe and interactions between the default and the frontoparietal networks in supporting stimulus-independent task-unrelated thought (2).
Our most striking behavioral finding was a bias toward stimulus-bound thought in bvFTD. Relative to controls, the bvFTD group displayed significantly more instances of stimulus-bound thought (Level 1) in the context of significantly reduced mind wandering (Level 4). This pattern was reflected by a lower negative mind wandering index in bvFTD relative to the other groups. By contrast, healthy older controls displayed a larger positive mind wandering index, reflecting preferential engagement in perceptually decoupled thought. Our findings suggest that bvFTD patients experience marked difficulties in disengaging from the immediate environment, leading to a predominantly stimulus-bound style of thought.
In contrast, no clear differences emerged between the AD group and the controls across any of the response levels or the mind wandering index. However, the distribution of responses across the four levels in AD suggested subtle alterations in their overall response profiles. Controls’ responses showed a positive linear trend across the four levels, consistent with a greater proportion of responses at higher levels on the task, replicating previous findings (30). This trend was not observed in AD, with an equivalent frequency of thoughts at each response level suggesting a shift from the normative profile. We tentatively suggest that, in the early stages of AD, thought content gradually shifts toward intermediate and increasingly stimulus-related forms of thought, leading to a comparable distribution of responses across task levels. Importantly, the disproportionate impairment in mind wandering in bvFTD relative to AD cannot be attributed to greater disease severity in the bvFTD group. The patient groups were matched for overall disease duration with characteristic profiles of greater behavioral impairment in bvFTD and greater overall cognitive impairment in AD (cf. SI Appendix, Table S1 results of the cognitive and behavioral screening assessments). Rather, our results suggest that, under conditions of low cognitive demand, AD patients can achieve a form of mind wandering despite their underlying widespread structural and functional brain changes.
Research investigating mind wandering in dementia is scarce, however, a recent study revealed increased on-task thoughts during the performance of an ongoing task [i.e., the sustained attention to response task (SART)] in early AD relative to the controls (24). This finding differs from the current results where we did not see an obvious reduction in mind wandering in AD. It is important to note, however, that the paradigms used in the two studies differ considerably in the cognitive demands imposed. Performance of an ongoing task is inherently more cognitively demanding than the thought sampling paradigm used in the current paper. For patients with AD, the increased attentional demands of the SART invariably leave less cognitive resources available, reducing the likelihood of engaging in mind wandering (24). In contrast, the very low cognitive demands of our task render it more conducive to mind wandering in the AD group. These differences across studies emphasize that dual-tasking requirements may be a crucial determinant of ongoing thought patterns in cognitively impaired groups. We note with interest that, when cognitive demands are lessened, AD patients appear capable of engaging in task-independent forms of thought. Whether the content, phenomenology, and intentional vs. unintentional nature of mind wandering in AD differs from that of the controls remains an important question for future study. Consistent with this possibility, individuals with selective bilateral hippocampal damage have been shown to mind wander as frequently as the controls, albeit with distinct differences in terms of content (17).
Substantial evidence has shown that component processes supporting mind wandering, particularly, those involving memory-based constructive simulation, are compromised in dementia. AD and bvFTD patients display comparable episodic memory dysfunction (33, 34). Both groups also display marked impairments in future-oriented thinking, including prospective memory (35, 36) and constructive simulation of future episodes (20, 37, 38). Accordingly, similar reductions in mind wandering might have been predicted. Our results, however, suggest that mind wandering capacity is more vulnerable in bvFTD, consistent with other features of the syndrome that point to a predisposition for stimulus-bound thought. The stimulus-bound thought style we observed in bvFTD resonates with reports of environmentally dependent behavior in their everyday life, including preoccupation with objects in the immediate environment and the inability to disengage from such external stimuli (39, 40). We suggest that the current findings capture a core feature of the broader bvFTD behavioral phenotype, not previously reported, namely, a change in spontaneous thought style. Naturalistic paradigms assessing mind wandering over extended time periods (e.g., ref. 17) would be an important extension to the current paper and would enable us to determine how task demands and contextual factors influence stimulus-bound thought in bvFTD.
Our neuroimaging findings underscore the role of key regions of the default and frontoparietal networks in supporting internally generated thought, corroborating previous reports in healthy individuals (3–5). The resting-state results showed that, in controls, the tendency to mind wander (as opposed to stimulus-bound thought) was associated with stronger left PCC–left posterior hippocampal connectivity and weaker left PCC–vmPFC and left dlPFC–left hippocampal formation connectivity. This suggests that relative engagement of a posterior memory system and disengagement of frontal systems may be important for mind wandering on our task in healthy individuals.
In the patient groups, we observed the opposite association between left PCC–left posterior hippocampal connectivity as mind wandering was associated with weaker connectivity between these regions. The bvFTD group showed additional opposite associations from the controls with stronger left PCC–vmPFC and left dlPFC–left hippocampal formation connectivity associated with mind wandering. This suggests that, for bvFTD, mind wandering in the context of our task may rely more on frontal systems. In general, bvFTD showed more widespread associations between mind wandering and connectivity, relative to both controls and AD. This pattern may reflect a compensatory reorganization of networks involved in mind wandering, or it may reflect dedifferentiation, that is, a loss of specialization in the regions or systems supporting a given function. Both of these mechanisms can occur in aging and neurodegeneration in response to local atrophy or structural change (41, 42), and future work is needed to disambiguate between these processes in the context of mind wandering in bvFTD. Our results suggest that, in bvFTD, and to a lesser extent in AD, perceptually decoupled thought may be supported by a different functional architecture or by different psychological characteristics compared to controls, which, in turn, may contribute to the observed behavioral differences.
In both patient groups, a tendency toward stimulus-bound thought at the expense of mind wandering (i.e., lower mind wandering index) was associated with decreased gray matter intensity in several regions. Most notable in the context of existing literature were associations between the mind wandering index and gray matter integrity in the left hippocampus and parahippocampus. Convergent measures show that activity in the hippocampus and parahippocampus (6), entorhinal cortex (43), and temporal cortex (44) precedes spontaneous free recall of episodic memories. This accords with rodent work implicating hippocampal sharp wave ripple events (SWRs) in the replay (and preplay) of previously learned or future behavioral sequences (45, 46) and in monkeys where hippocampal SWRs precede increased activation of the default network (47). Together, these findings link spontaneous activation in the hippocampus and surrounding regions with both recall and prospection, which may then engage the default network more broadly to support the elaboration of memories and simulations. A critical role for the hippocampus in mind wandering was also recently confirmed as individuals with selective bilateral hippocampal damage exhibited reduced diversity in their mind wandering content (17).
Significant associations were also observed between the mind wandering index score and gray matter integrity in areas overlapping with large-scale networks relevant for mind wandering. Gray matter clusters in the subcallosal/orbitofrontal and parahippocampal cortices overlapped with the limbic and default networks. Furthermore, a cluster in the anterior insula overlapped with the salience network. The salience network is proposed to mediate dynamic shifts between default and executive control networks (48) facilitating transitions between external and internal focus, which may be relevant for disengaging from external stimuli to engage in internally focused thought. Whereas the structural correlates of task-independent thought have received less attention relative to the functional correlates, cortical thickness in regions within and adjacent to the default and frontoparietal networks has been shown to covary with mind wandering performance in healthy individuals (49). The implication is that structural integrity in regions within and adjacent to these networks helps to promote their integration (49).
Finally, we found an association between the striatal gray matter loss and a reduced mind wandering index. The basal ganglia represent a network hub vulnerable to degeneration across neurodegenerative disorders (50), contributing to an array of cognitive and neuropsychiatric features (51). We speculate that the involvement of the basal ganglia in supporting large-scale network communication (52) may explain its association with mind wandering. This is consistent with known functional connectivity between the striatum and the large-scale cortical networks, including the default and frontoparietal (53) and the convergence of these functional networks in distinct zones of the striatum (54). Striatal degeneration may impair the dynamic integration of information from disparate brain networks, which is necessary to support abstract forms of cognition, including mind wandering (1, 55).
To summarize, this study empirically measures mind wandering under conditions of low cognitive demand in two dementia syndromes and correlates performance with structural and functional imaging. Our results show a change in the thought patterns of individuals with bvFTD and, to a much lesser extent, those with AD. The tendency to engage in mind wandering vs. stimulus-bound thought was associated with regional gray matter integrity and functional connectivity in the default and frontoparietal networks. Future work is needed to identify the trait level and phenomenological characteristics of altered mind wandering in dementia. Given the ubiquity of mind wandering in everyday life, we also stress the importance of further understanding of how the loss of this fundamental human capacity impacts well being and sense of self in individuals living with dementia.
Methods and Materials
Case Selection.
The study included 35 individuals meeting diagnostic criteria for bvFTD, 24 individuals with a clinically probable diagnosis of AD, and 37 healthy controls. See SI Appendix, Table S1. South Eastern Sydney Local Area Health and University of New South Wales ethics committees approved the study, and all participants provided informed consent. Data supporting this study are unavailable as ethics did not cover open data sharing, however, stimulus materials for the task are available from the authors upon request.
Mind Wandering Experimental Task.
Participants viewed static two-dimensional colored geometric shapes presented individually on a computer screen. Immediately following the presentation of each stimulus, participants were prompted to report any thoughts they had during the time that the stimulus was presented on screen (i.e., any thoughts that occurred within that trial). The task was composed of nine trials, each presenting a commonplace shape (e.g., blue square, yellow circle) for varying durations (Short: ≤20 s, Medium: 30–60 s, Long: ≥90 s). The scoring procedure for this task has been described previously (30). Briefly, responses are coded along a continuum ranging from stimulus-bound thinking directly related to the stimulus at hand (Level 1) to fully fledged instances of stimulus-/task-unrelated mind wandering (Level 4). Intermediary levels 2 and 3 capture the transition from stimulus-related to increasingly stimulus-independent responses. The final score awarded for each trial was the highest level achieved on that trial, ranging from one to four. Total percentages of each level across the task were calculated as well as the mind wandering index comparing the extent of Level 1 vs. Level 4 responses. The mind wandering index was used as the covariate of interest in the neuroimaging rather than the mind wandering percentage (Level 4) as many patients scored 0 for this, leading to reduced variance in the sample. See SI Appendix for the scoring protocol and representative responses.
VBM Analysis of Mind Wandering Performance.
Structural scans were available for 31 bvFTD, 23 AD, and 32 controls. To identify regions where gray matter intensity covaried with mind wandering performance, a general linear model was conducted in the patient groups combined (i.e., excluding controls) using the mind wandering index score as a covariate in the design matrix. A priori the ROI were specified as all cortical and subcortical regions within the Harvard-Oxford cortical and subcortical atlases; the cerebellum and brainstem were not included. We tested for interaction effects, and having confirmed that they were not significant, the main effect of the mind wandering index on gray matter intensity was reported, i.e., regions where a significant positive relationship with the mind wandering index in both groups combined was identified. Results are FWE corrected at P < 0.01 and clusters identified using threshold free cluster enhancement (See SI Appendix for details, preprocessing procedures, and group-level comparisons).
Seed Region Connectivity and Mind Wandering Performance.
A subset of 24 bvFTD, 17 AD, and 23 controls underwent task-free resting-state imaging (three bvFTD and three AD were removed from the analysis due to excessive motion; see SI Appendix for details). Thirteen seeds were placed in the default, the frontoparietal networks, and the hippocampus to determine the relationship between seed region connectivity and the mind wandering index. Within each of the three groups, participants’ mind wandering index scores were correlated with the connectivity between each ROI for the 13 seed ROI. Correlations that survived FDR correction at q < 0.05 are reported. To compare the strength of shared correlations across groups, Fisher’s r to z was calculated, and a one-tailed comparison at P < 0.05 (i.e., Z ≥ ± 1.645) was reported. (See SI Appendix for details, preprocessing procedures, and group-level comparisons.)
Supplementary Material
Acknowledgments
We thank Nadene Dermody and Jody Kamminga for assistance with data collection and scoring. C.O. was supported by a National Health and Medical Research Council (NHMRC) Neil Hamilton Fairley Fellowship Grant GNT1091310 and by the Wellcome Trust (Grant 200181/Z/15/Z). J.M.S. was supported by a NHMRC CJ Martin Fellowship Grant GNT1072403. J.R.A.-H. was supported by the University of Arizona and a grant from the John Templeton Foundation, “Prospective Psychology Stage 2: A Research Competition” to Martin Seligman. M.I. was supported by an Australian Research Council (ARC) Future Fellowship (Grant FT160100096) and an ARC Discovery Project (Grant DP180101548). This work was supported by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the NHMRC (Grant APP1037746) and the ARC Centre of Excellence in Cognition and its Disorders (Grant CE11000102). The opinions expressed in this paper are those of the author(s) and do not necessarily reflect the views of the John Templeton Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.W.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818523116/-/DCSupplemental.
References
- 1.Kucyi A. Just a thought: How mind-wandering is represented in dynamic brain connectivity. Neuroimage. 2018;180:505–514. doi: 10.1016/j.neuroimage.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: A dynamic framework. Nat Rev Neurosci. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 3.Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 5.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellamil M, et al. Dynamics of neural recruitment surrounding the spontaneous arising of thoughts in experienced mindfulness practitioners. Neuroimage. 2016;136:186–196. doi: 10.1016/j.neuroimage.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Smallwood J, O’Connor RC, Sudbery MV, Obonsawin M. Mind-wandering and dysphoria. Cogn Emotion. 2007;21:816–842. [Google Scholar]
- 8.Perkins AM, Arnone D, Smallwood J, Mobbs D. Thinking too much: Self-generated thought as the engine of neuroticism. Trends Cogn Sci. 2015;19:492–498. doi: 10.1016/j.tics.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Seli P, Smallwood J, Cheyne JA, Smilek D. On the relation of mind wandering and ADHD symptomatology. Psychon Bull Rev. 2015;22:629–636. doi: 10.3758/s13423-014-0793-0. [DOI] [PubMed] [Google Scholar]
- 10.Seli P, Risko EF, Purdon C, Smilek D. Intrusive thoughts: Linking spontaneous mind wandering and OCD symptomatology. Psychol Res. 2017;81:392–398. doi: 10.1007/s00426-016-0756-3. [DOI] [PubMed] [Google Scholar]
- 11.Shin D-J, et al. Away from home: The brain of the wandering mind as a model for schizophrenia. Schizophr Res. 2015;165:83–89. doi: 10.1016/j.schres.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Berman MG, et al. Does resting-state connectivity reflect depressive rumination? A tale of two analyses. Neuroimage. 2014;103:267–279. doi: 10.1016/j.neuroimage.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JP, et al. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irish M, Piguet O, Hodges JR. Self-projection and the default network in frontotemporal dementia. Nat Rev Neurol. 2012;8:152–161. doi: 10.1038/nrneurol.2012.11. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertossi E, Ciaramelli E. Ventromedial prefrontal damage reduces mind-wandering and biases its temporal focus. Soc Cogn Affect Neurosci. 2016;11:1783–1791. doi: 10.1093/scan/nsw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormick C, Rosenthal CR, Miller TD, Maguire EA. Mind-wandering in people with hippocampal damage. J Neurosci. 2018;38:2745–2754. doi: 10.1523/JNEUROSCI.1812-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irish M, et al. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia. 2011;49:2694–2702. doi: 10.1016/j.neuropsychologia.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Piolino P, et al. Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain. 2003;126:2203–2219. doi: 10.1093/brain/awg222. [DOI] [PubMed] [Google Scholar]
- 20.Irish M, et al. Scene construction impairments in Alzheimer’s disease–A unique role for the posterior cingulate cortex. Cortex. 2015;73:10–23. doi: 10.1016/j.cortex.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Duval C, et al. What happens to personal identity when semantic knowledge degrades? A study of the self and autobiographical memory in semantic dementia. Neuropsychologia. 2012;50:254–265. doi: 10.1016/j.neuropsychologia.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Stopford CL, Thompson JC, Neary D, Richardson AMT, Snowden JS. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex. 2012;48:429–446. doi: 10.1016/j.cortex.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Possin KL, et al. Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology. 2013;80:2180–2185. doi: 10.1212/WNL.0b013e318296e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gyurkovics M, Balota DA, Jackson JD. Mind-wandering in healthy aging and early stage Alzheimer’s disease. Neuropsychology. 2018;32:89–101. doi: 10.1037/neu0000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niedźwieńska A, Kvavilashvili L. Reduced mind-wandering in mild cognitive impairment: Testing the spontaneous retrieval deficit hypothesis. Neuropsychology. 2018;32:711–723. doi: 10.1037/neu0000457. [DOI] [PubMed] [Google Scholar]
- 26.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 28.Broe M, et al. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60:1005–1011. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- 29.Snowden JS, et al. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Callaghan C, Shine JM, Lewis SJG, Andrews-Hanna JR, Irish M. Shaped by our thoughts–A new task to assess spontaneous cognition and its associated neural correlates in the default network. Brain Cogn. 2015;93:1–10. doi: 10.1016/j.bandc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Smallwood J, Schooler JW. The science of mind wandering: Empirically navigating the stream of consciousness. Annu Rev Psychol. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- 32.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74:472–479. doi: 10.1212/WNL.0b013e3181cef85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irish M, Piguet O, Hodges JR, Hornberger M. Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer’s disease. Hum Brain Mapp. 2014;35:1422–1435. doi: 10.1002/hbm.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamminga J, O’Callaghan C, Hodges JR, Irish M. Differential prospective memory profiles in frontotemporal dementia syndromes. J Alzheimers Dis. 2014;38:669–679. doi: 10.3233/JAD-131118. [DOI] [PubMed] [Google Scholar]
- 36.Dermody N, Hornberger M, Piguet O, Hodges JR, Irish M. Prospective memory impairments in Alzheimer’s disease and behavioral variant frontotemporal dementia: Clinical and neural correlates. J Alzheimers Dis. 2016;50:425–441. doi: 10.3233/JAD-150871. [DOI] [PubMed] [Google Scholar]
- 37.Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. 2009;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irish M, Hodges JR, Piguet O. Episodic future thinking is impaired in the behavioural variant of frontotemporal dementia. Cortex. 2013;49:2377–2388. doi: 10.1016/j.cortex.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Lanata SC, Miller BL. The behavioural variant frontotemporal dementia (bvFTD) syndrome in psychiatry. J Neurol Neurosurg Psychiatry. 2016;87:501–511. doi: 10.1136/jnnp-2015-310697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh A, Dutt A, Bhargava P, Snowden J. Environmental dependency behaviours in frontotemporal dementia: Have we been underrating them? J Neurol. 2013;260:861–868. doi: 10.1007/s00415-012-6722-0. [DOI] [PubMed] [Google Scholar]
- 41.Kalpouzos G, Persson J, Nyberg L. Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging. 2012;33:623.e1–623.e13. doi: 10.1016/j.neurobiolaging.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Maillet D, Rajah MN. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Res Rev. 2013;12:479–489. doi: 10.1016/j.arr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Gelbard-Sagiv H, Mukamel R, Harel M, Malach R, Fried I. Internally generated reactivation of single neurons in human hippocampus during free recall. Science. 2008;322:96–101. doi: 10.1126/science.1164685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke JF, et al. Theta and high-frequency activity mark spontaneous recall of episodic memories. J Neurosci. 2014;34:11355–11365. doi: 10.1523/JNEUROSCI.2654-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diba K, Buzsáki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan R, et al. Hippocampal sharp-wave ripples influence selective activation of the default mode network. Curr Biol. 2016;26:686–691. doi: 10.1016/j.cub.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golchert J, et al. Individual variation in intentionality in the mind-wandering state is reflected in the integration of the default-mode, fronto-parietal, and limbic networks. Neuroimage. 2017;146:226–235. doi: 10.1016/j.neuroimage.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Crossley NA, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Callaghan C, Bertoux M, Hornberger M. Beyond and below the cortex: The contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J Neurol Neurosurg Psychiatry. 2014;85:371–378. doi: 10.1136/jnnp-2012-304558. [DOI] [PubMed] [Google Scholar]
- 52.Bell PT, Shine JM. Subcortical contributions to large-scale network communication. Neurosci Biobehav Rev. 2016;71:313–322. doi: 10.1016/j.neubiorev.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 53.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zabelina DL, Andrews-Hanna JR. Dynamic network interactions supporting internally-oriented cognition. Curr Opin Neurobiol. 2016;40:86–93. doi: 10.1016/j.conb.2016.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.