Significance
Attachment mechanisms of climbing animals provide inspiration for biomimetics, but many natural adaptations are still unexplored. Animals are known to grip by interlocking claws with rough surfaces, or engaging adhesive pads on smooth substrates. Here we report that insects can use a third, fundamentally different attachment mechanism on plant surfaces. When accelerating for jumps, froghoppers produce traction by piercing plant surfaces with sharp metal-enriched spines on their hind legs, deforming the cuticle plastically and leaving behind microscopic holes, like a biological nanoindenter. This mechanism depends on the substrate’s hardness, and requires special adaptations of the cuticle at the spine tips. Piercing may represent a widespread attachment strategy among plant-living insects, promising inspiration for novel robotic grippers and climbers.
Keywords: biomechanics, biomaterials, penetration, attachment, Auchenorrhyncha
Abstract
Attachment mechanisms used by climbing animals facilitate their interactions with complex 3D environments and have inspired novel types of synthetic adhesives. Here we investigate one of the most dynamic forms of attachment, used by jumping insects living on plants. Froghopper insects can perform explosive jumps with some of the highest accelerations known among animals. As many plant surfaces are smooth, we studied whether Philaenus spumarius froghoppers are able to take off from such substrates. When attempting to jump from smooth glass, the insects’ hind legs slipped, resulting in weak, uncontrolled jumps with a rapid forward spin. By contrast, on smooth ivy leaves and smooth epoxy surfaces, Philaenus froghoppers performed strong jumps without any slipping. We discovered that the insects produced traction during the acceleration phase by piercing these substrates with sharp spines of their tibia and tarsus. High-speed microscopy recordings of hind legs during the acceleration phase of jumps revealed that the spine tips indented and plastically deformed the substrate. On ivy leaves, the spines of jumping froghoppers perforated the cuticle and epidermal cell walls, and wounds could be visualized after the jumps by methylene blue staining and scanning electron microscopy. Improving attachment performance by indenting or piercing plant surfaces with sharp spines may represent a widespread but previously unrecognized strategy utilized by plant-living insects. This attachment mechanism may also provide inspiration for the design of robotic grippers.
Attachment devices used by climbing animals such as geckos, spiders, and insects have outstanding properties that make them excellent models for biomimetics. The adhesives they use for locomotion are rapidly controllable, reusable, and self-cleaning (1–6), and have therefore inspired new types of synthetic adhesives (7–10). However, many natural attachment systems are still unexplored.
Strong grip and highly dynamic surface attachment are particularly important for animals which jump to escape from predators or rapidly move through complex environments, and the action of jumping brings unique biomechanical challenges. Consequently, studying jumping animals may reveal novel solutions to biomechanical problems (11), and can also provide new insights into attachment mechanisms (12).
In this study, we show that jumping froghoppers produce traction on plant surfaces by piercing them with sharp spines on their hind legs. The use of claws and spines for attachment is widespread in animals, and has inspired the foot design for walking and climbing robots (13–15). Previous studies have focused on the interlocking of spines with rough surfaces (16–18). However, little is known about attachment by penetration of surfaces in robotic and natural systems, in terms of both the underlying mechanisms and the biological adaptations involved (but see refs. 15 and 19).
Most jumping insects live on plants, which can have smooth surfaces. Accelerating forward from such surfaces without slipping requires high friction forces. To allow forward jumps with a takeoff angle of <45° relative to the surface, the friction forces have to be larger than the normal load, implying that the friction coefficient between legs and the substrate must be very large (>1). How do jumping insects avoid slipping during takeoff?
Some of the fastest and most powerful jumps are performed by plant sap-sucking bugs of the order Hemiptera, which includes froghoppers, leafhoppers, and planthoppers. Philaenus spumarius froghoppers use a catapult mechanism to reach extreme accelerations of 550 g and takeoff velocities of up to 4.7 m⋅s−1 (20–22). In these jumps, the acceleration can last less than 1 ms. In a previous study, we showed that Aphrodes bicinctus/makarovi leafhoppers were able to jump from smooth glass substrates by briefly bringing some soft tarsal pads (platellae) on their hind legs into surface contact during the acceleration phase of the jump (12). In contrast, froghoppers such as P. spumarius lack soft platellae on their hind legs; they slipped when attempting to jump from glass, resulting in uncontrolled upward jumps with a rapid forward spin (12, 23). How, therefore, do froghoppers jump successfully from the plants on which they live? Smooth plant surfaces differ from glass in that they are more hydrophobic and softer (24, 25). In this study, we investigated how P. spumarius froghoppers are able to jump from smooth plant surfaces and hydrophobic polymer substrates, and the interaction between their hind feet and the substrate during the acceleration phase.
Results
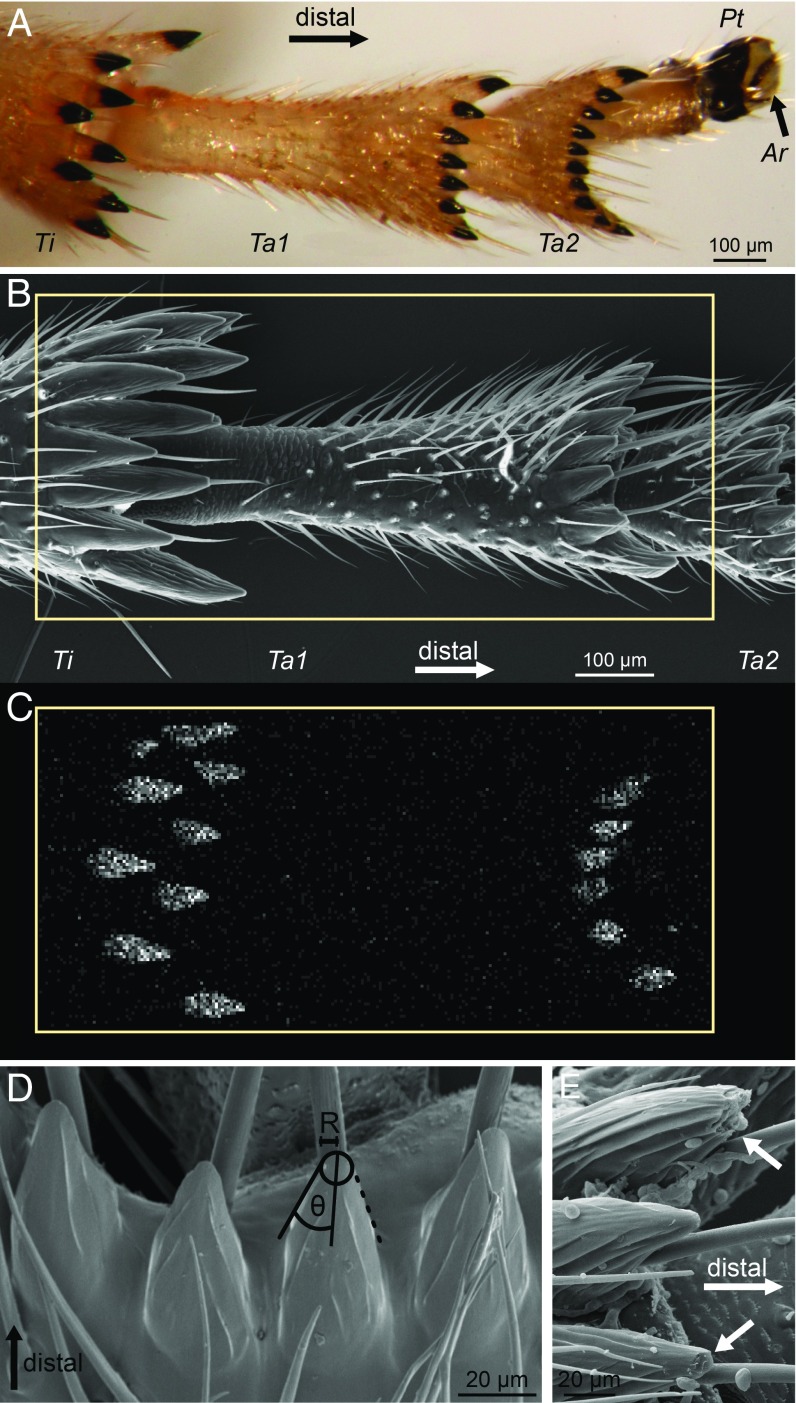
The feet of P. spumarius froghoppers consist of three tarsal segments (tarsomeres) and a pretarsus with a pair of claws and an arolium between the claws (Fig. 1). The hind legs (but not the two other leg pairs) are equipped with arc-shaped rows of distally oriented, strongly sclerotized spines, located ventrally on the distal margins of the tibia and first two tarsomeres. A single, long hair (“acutella”; ref. 26) protrudes from the dorsal side of each spine. The spines are approximately conical (half opening angle 18.5 ± 3.2°, 137 spines of 11 animals; Fig. 1D) and have sharp tips (tip radius of curvature 3.6 ± 1.0 µm, 115 spines without signs of wear of 11 animals). The tips of the spines are dark brown and more sclerotized than the lighter surrounding cuticle. Energy-dispersive X-ray spectroscopy (EDX) analysis revealed that zinc is incorporated in the tips of the spines; zinc could be detected in the distal 50 μm to 85 µm of each spine (Fig. 1C). One out of three froghoppers directly collected from the field and prepared for SEM had several spines with fractured tips, indicating that high stresses are acting on them under natural conditions. In animals that were not immediately killed after capture, more spines were fractured and fractures were larger (Fig. 1E).
Fig. 1.
Hind leg morphology of P. spumarius froghoppers. (A) Ventral view of distal tibia and tarsus. The dark brown color of the spines indicates strong sclerotization. (B) Scanning electron micrograph of hind leg (ventral view). (C) EDX scan of the same leg as in B, showing the location of zinc (Kα X-ray emission) in the tips of the spines. Rectangle in B shows the area sampled in C. (D) Conical spines on the distal end of the first tarsal segment. (E) Broken spine tips on the first tarsal segment (arrows, ventral view). Ar, arolium; Pt, pretarsus; R, tip radius; Ta1, tarsomere 1; Ta2, tarsomere 2; Ti, tibia.
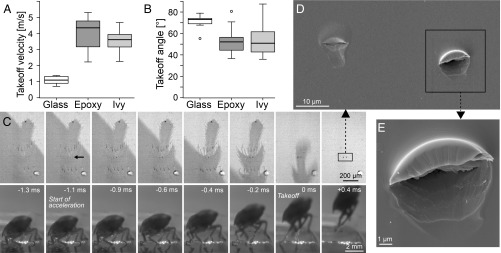
When P. spumarius froghoppers jumped from smooth glass surfaces, their hind legs always slipped, resulting in steep jumps with a rapid forward spin and a low takeoff velocity (Movie S1 and ref. 12). By contrast, P. spumarius froghoppers never slipped when jumping from smooth epoxy, resulting in fast jumps with a low takeoff angle (Movie S2). Takeoff velocity on epoxy ranged from 2.2 m⋅s−1 to 5.3 m⋅s−1 (mean: 3.9 ± 1.1 m⋅s−1; 11 jumps), much higher than for jumps from glass (1.1 ± 0.2 m⋅s−1; Welch’s t test: t10.98 = 8.46, P < 0.001; Fig. 2A; data for glass from ref. 12); takeoff angles ranged from 36.6° to 80.7° (mean: 53.2 ± 13.1°), significantly lower than for jumps from glass (71.3 ± 6.5°; t14.88 = 4.07, P = 0.001; Fig. 2B). The froghoppers avoided slipping on epoxy surfaces by plastically indenting the surface with the sharp zinc-enriched spines on their hind legs during the acceleration phase (Movie S3). Before the acceleration phase of the jump, the pretarsal arolium (in six out of seven jumps) and acutellae on the first and second tarsomere (in four out of seven jumps) contacted the surface. At the start of the acceleration (defined here as the first frame with a visible leg movement), four to seven spines (per leg) on the first and second tarsomere indented the epoxy substrate (seven jumps by five froghoppers; Fig. 2C). The spines plastically deformed the epoxy so that the indentation marks remained visible in the substrate after takeoff (Fig. 2 D and E).
Fig. 2.
Takeoff performance and foot-substrate interaction of P. spumarius while jumping. (A) Takeoff velocity and (B) takeoff angle for jumps from smooth glass, smooth epoxy, and ivy leaves. (C) Images of a P. spumarius jumping from epoxy in side view, captured at 4,700 frames per second (Bottom), and ventral view using coaxial illumination (Top). Before the jump, only acutellae and arolium were visible in surface contact. At the start of the acceleration phase, spines started to pierce into the surface, and indentations remained visible even after the insect’s takeoff (arrow marks first visible indentation). Takeoff was defined as the first frame in which the animal was airborne (time set to 0 ms), and start of acceleration was defined as the frame with the first visible hind leg movements. (D and E) Scanning electron micrographs of the plastic deformation of epoxy caused by the tarsal spines.
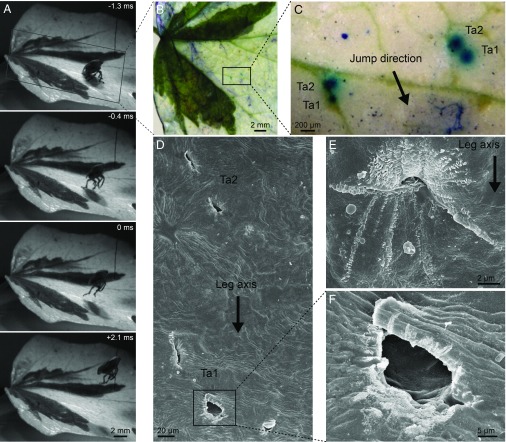
P. spumarius froghoppers were also able to jump from smooth plant surfaces (Movie S4). When jumping from ivy leaves, P. spumarius froghoppers never slipped, and reached takeoff velocities of 3.6 ± 0.6 m⋅s−1 in forward jumps, with takeoff angles ranging from 35.9° to 87.4° (mean: 53.6 ± 14.1°; two jumps each by 12 froghoppers), both results similar to epoxy but significantly different from glass (takeoff velocity: epoxy: t12.88 = 1.03, P = 0.32; glass: t31.93 = 17.40, P < 0.001; takeoff angle: epoxy: t20.91 = 0.08, P = 0.94; glass: t31.56 = 5.01, P < 0.001; Fig. 2 A and B). After the froghoppers had jumped from the ivy leaves, subsequent staining with methylene blue always revealed one or two blue spots at the position of the first two tarsal segments of the hind legs during the acceleration phase, indicating that the surface had been perforated by the spines (41 jumps by nine froghoppers from 10 leaves, Fig. 3 A–C). Some smaller blue spots were also visible in other areas of the leaf, but these were also present in leaves where no froghoppers had jumped (Fig. 3C). The tracks left in the leaves by the froghoppers were also visible by SEM (Fig. 3 D–F). Jumping froghoppers left between three and nine indents per leg, which were arranged in the same way as the froghopper spines in one or two transverse, curved rows. Both the spacing between spines in each row and the distance between rows matched the dimensions of the spines on the first two tarsal segments of the froghoppers’ hind legs as measured by SEM [spacing between spines on tarsomere 1, ivy tracks: 57.2 ± 14.6 µm (n = 4 tarsomeres), hind tarsi: 51.0 ± 8.4 µm (n = 8); spacing between spines on tarsomere 2: ivy tracks: 39.3 ± 9.2 µm (n = 4), hind tarsi: 38.2 ± 6.9 µm (n = 8); distance between tarsomere rows, ivy tracks: 217.6 ± 26.6 µm (n = 4), hind tarsi: 201.4 ± 10.7 µm (n = 12); see Fig. 3 C and D]. In 29 out of 34 indents from nine jumps, the spines appeared to have penetrated the outer cell wall of the epidermis (Fig. 3F).
Fig. 3.
Jumps of P. spumarius from ivy leaves, and tracks left in the leaf surface. (A) Image sequence of P. spumarius jumping from variegated ivy leaf. (B and C) Same leaf stained with methylene blue after the jump in A, showing blue marks at the position of both hind feet during the jump. (D–F) Scanning electron micrographs of damage to leaf tissue left by froghopper spines. Arrows point in the proximal direction of the leg, corresponding approximately to the jump direction; Ta1 and Ta2, indentations by spines on hind left tarsomeres 1 and 2.
Discussion
Insects employ a combination of different attachment mechanisms allowing them to live on plant surfaces. They use claws and spines to interlock with asperities on rough surfaces, and soft adhesive pads to cling to smooth substrates (27). Many insects also possess special “heel” pads on the tarsus that produce high friction when pressed against the substrate (12, 28–30). Our study shows that insects can use a fundamentally different mechanism to grip on smooth plant surfaces.
Philaenus froghoppers were able to perform powerful jumps with takeoff angles as low as 36° from ivy leaves and smooth epoxy surfaces, but they slipped on glass (Fig. 2B). When accelerating for a jump, the sharp backward-pointing spines on the tibia and tarsus of their hind legs pierced the epoxy substrate and the ivy leaves, but not the glass surface.
Piercing involves plastic deformation or fracture of the substrate and depends mainly on the substrate’s material properties rather than its topography (roughness) or wettability.
To investigate the substrate properties required for this interaction, we estimated the forces acting on a single spine during the acceleration phase of a jump. The force in the direction of the jump can be calculated from the takeoff velocity , the acceleration time , and the froghopper’s mass m as (assuming constant acceleration). Assuming that both hind legs engaged the same number of spines and that all spines carried the same load, the four jumps where we simultaneously recorded both takeoff angle/velocity and the number of spines in contact produced forces of 4.2 mN to 7.9 mN per spine.
A minimum estimate of the pressure at the tip of the spine is obtained by assuming that the tip is loaded uniformly; this pressure (where R = 3.6 µm is the spine tip radius) ranges from 103.2 MPa to 194.0 MPa, significantly exceeding the compressive strength of epoxy (40 MPa) but not that of borosilicate glass (yield strength ∼264 MPa to 384 MPa; see ref. 31). [To discuss the material’s resistance to plastic deformation, we are using available literature values for compressive strength or yield strength, the latter being linearly related to the more commonly measured hardness: (32–34).]
This implies that the tip of the spine will plastically deform the substrate and sink in on epoxy but not on glass. Therefore, each froghopper spine acts like a conical nanoindenter that can determine the hardness of a material.
The stresses at the tips of froghopper spines also clearly exceed those needed to plastically deform and pierce natural plant surfaces. The strength of plant leaves measured by punch or tear tests ranged from 0.69 MPa to 11.2 MPa (35). More localized nanoindentation measurements of leaf surfaces yielded higher strengths (3 MPa to 127 MPa; refs. 36 and 37), but these values were obtained from dried specimens and likely overestimate the strength of hydrated epidermis. In plants, compressive strengths exceeding the pressures produced by froghopper spines have only been reported from nanoindentation studies on specialized silica cells in rice leaves and bamboo stems (as high as 900 MPa; refs. 37 and 38), suggesting that only exceptionally hard plant surfaces could cause any difficulties for froghoppers.
The estimated pressure may also come close to the yield strength of sclerotized insect cuticle (ca. 100 MPa to 500 MPa; refs. 39 and 40). As the yield strength of epoxy and plant tissue is lower, however, these substrates will yield first, and higher stresses may not be reached.
During attempted jumps from glass, however, the pressure at the spine tips may reach the level estimated above, and the contact pressure in the center of the spine tip, calculated using the Hertz theory, is even higher (2.6 GPa to 3.3 GPa; see SI Appendix, Eq. S6). These high contact pressures therefore suggest that, during a jump from glass, the tips of the spines should become plastically deformed or fractured.
However, the tips of the tibial and tarsal spines in Philaenus are adapted to minimize plastic deformation and fracture by the high zinc content of their tips (Fig. 1C). Sclerotized insect cuticle with incorporated metals such as zinc and manganese has been found to exhibit increased hardness, corresponding to yield strengths as high as 500 MPa (39, 41, 42). Moreover, when Philaenus froghoppers slip on glass, most of the energy of the jump is dissipated by the rapid slipping and kicking of the hind legs. The body’s kinetic energy is more than ninefold reduced (12), and the fourfold smaller takeoff velocity may result in a proportional reduction in the pressure at the center of the spines (ca. 0.6 GPa to 0.8 GPa). Thus, the tips of the spines may still escape plastic deformation when they slip on glass substrates. Nevertheless, jumps from rough and hard substrates such as rocks would probably cause deformation, wear, or fracture of the spine tips. As froghoppers spend most of their life on plants, they will only rarely perform jumps from such substrates under natural conditions.
Plowing Friction Model for Spines on Smooth Substrates.
What friction forces can froghopper spines achieve? When the spines sink into the substrate, their friction coefficient can be estimated using a simple theory proposed by Bowden and Tabor (ref. 43 and SI Appendix) that considers a rigid conical spine with half opening angle in contact with a smooth surface of a softer, purely plastic material (SI Appendix, Fig. S1)
| [1] |
where τ is the shear stress of the spine−substrate interface.
Estimating for the shear stress of cuticle on epoxy (44, 45), and , it can be seen that the interfacial shear term is negligible compared with the plowing term: . With such a high friction coefficient, froghoppers should be able to jump forward with takeoff angles as low as , consistent with the observation that Philaenus froghoppers never slipped on epoxy.
The above estimate of the friction coefficient is a simplification for several reasons. First, the model considers a perpendicularly oriented conical spine, whereas froghopper spines during the acceleration phase may be tilted by some angle. Second, the model considers a fully plastic substrate material (thereby potentially overestimating plowing friction; ref. 46) and ignores the shear resistance arising from material piling up ahead of the sliding cone (thereby potentially underestimating plowing friction). These factors are considered in more complex models of plowing friction (47) but are difficult to quantify, and their opposite effects on friction may approximately cancel out.
Why do froghopper spines slip on glass? The Hertzian estimate for the contact area of the spines (SI Appendix, Eq. S7) gives maximally 3.6 µm2 on glass. Assuming 45° jumps, producing the required forward thrust of 3.0 mN to 5.6 mN would involve shear stresses of 833 MPa to 1,555 MPa. These values exceed shear stresses measured for adhesive cuticle by at least two orders of magnitude (44, 45), confirming that the elastic increase in contact area alone is insufficient to produce the required friction forces. Only when stresses exceed the yield strength of the substrate can the spines plastically deform the substrate, thereby allowing high friction and jumps without slipping.
Wider Implications: Biology and Robotics.
All jumping insects living on plants face the challenge that they have to take off from surfaces which can be microscopically smooth (48, 49). To achieve large jump distances, takeoff angles of 45° or slightly less are optimal (50), but such jumps require high forces parallel to the ground. For takeoff angles of <45°, these shear forces have to exceed the normal force, which is only possible for friction coefficients greater than 1. However, friction coefficients for rigid, dry surfaces are usually less than 1 (51), indicating that insects have to develop special adaptations to solve this biomechanical problem.
We recently showed that leafhoppers (A. bicinctus/makarovi, Cicadellidae) possess several soft, pad-like structures (platellae) on their hind tarsi, which contact the surface briefly during the acceleration phase of the jump, thereby producing the high friction forces required for a jump (12). Platellae are absent in froghoppers, which explains why Aphrodes but not Philaenus were able to jump from smooth glass surfaces without slipping (12). On natural plant surfaces, however, Philaenus can jump successfully by piercing the surface with sharp spines.
Why have two lineages of the Hemiptera evolved such different solutions to the same problem? A key biomechanical difference between Philaenus froghoppers and Aphrodes leafhoppers is that Philaenus have hind legs 1.8 times shorter than Aphrodes, and that they accelerate with a 2.6 times higher force acting on the feet (21, 52).
Therefore, using soft, pad-like structures for jumping may not work for froghoppers, as producing higher friction forces over a shorter acceleration time with adhesive pads would require these to have much larger contact areas, and to attach and detach extremely rapidly, thereby exposing these soft structures to significant damage and wear.
For Aphrodes leafhoppers, on the other hand, using spines to pierce plant surfaces may not be feasible, as high forces and stresses are required to use this strategy efficiently. Moreover, Aphrodes possess very short spines at the same locations as those of Philaenus, and we did not detect any zinc in them, both factors making them even less suitable for piercing plant surfaces. The tibial spines of Aphrodes leafhoppers are also flexibly articulated with the tibia, whereas the spines are not hinged in Philaenus froghoppers (12). It is likely that the compliant linkage in Aphrodes will help distribute the load between different spines and thereby reduce peak stresses, which will be beneficial for gripping on rough surfaces (a principle recently explored in climbing robots; ref. 18). By contrast, the stiff, nonarticulated spines in Philaenus may serve to concentrate stresses on a small number of spines, helpful for penetrating plant tissue.
Aphrodes could theoretically compensate for their lower jumping forces by developing sharper spines (with a times smaller tip radius, assuming that they have to achieve similar spine stress levels as Philaenus). However, such sharp structures might be at a high risk of fracture or wear during other types of locomotion.
The importance of tip strength is highlighted by the fact that some spine tips in Philaenus were broken (Fig. 1E). The large number of spines on the tibia and the first two tarsomeres provide some redundancy so that slipping is still prevented if a few individual spines have become blunt or have broken off.
The spines of froghoppers may not only be adapted for high sharpness and strength, but also for preventing excessive penetration, to allow easy detachment. Sinking too deep into soft tissue may be avoided by the spines being relatively short and by the hairs protruding from their dorsal side (Fig. 1 A and B), which might act as penetration arresters.
Piercing of plant tissue by insects is common among plant sap-sucking insects and insects ovipositing in plant tissue. The mouthparts and ovipositors that pierce and cut into plant tissue also possess sharp tooth-like structures enriched with zinc and manganese, but the sensory, chemical, and biomechanical adaptations are much more complex, as they include mechanisms for continued cutting and targeted steering through plant tissue, prevention of buckling, egg transport and deposition, fluid injection and drinking, and inhibition of plant defenses (53–58).
Piercing of plant surfaces by sharp spines may represent a widespread attachment strategy but has been little documented. We are aware of only one report of a similar interaction in crawling caterpillars, where sharp claw-like crochets on the abdominal prolegs cut visible footprints into leaf tissue (59, 60), and it is still unclear under which biomechanical conditions these footprints are produced. Unlike the situation in hind legs during a jump, climbing insects can produce high forces against the substrate, independent of their body acceleration, by pulling together opposite legs (adduction), potentially allowing their claws to grip by piercing. Future work should explore the distribution of this attachment mechanism among plant-living insects, and what adaptations insects and plants have evolved for it.
Our findings may provide biological inspiration for robotic grippers. Insect-inspired spines have been used to enhance surface attachment in wall-climbing robots (14); moreover, the improved traction mediated by spines of jumping locusts and crickets has inspired new foot designs for jumping robots (15, 19). Such robots can navigate large obstacles and could be used for search and rescue missions in disaster areas (61, 62). Generally, gripping smooth and plastic materials is an engineering challenge with many potential applications. Needle grippers have been used for handling soft foodstuff such as meat and cakes (63), but could also be adapted for handling of plastic and cardboard packaging. Studying the detailed biomechanics of penetration-based grip in natural systems and the relevant adaptations in plants and insects may provide information for the design of new biomimetic grippers.
Materials and Methods
Animals.
A total of 57 adult P. spumarius (Linnaeus, 1758) froghoppers were collected in and around Cambridge (United Kingdom) between late May and November (body mass: 12.0 ± 2.6 mg; data given as mean ± SD unless stated otherwise). P. spumarius can be found on diverse plant species but were mostly collected from thistle (Cirsium arvense) and, occasionally, ivy (Hedera helix). Ivy leaves possess a smooth cuticle membrane (64, 65) with an elastic modulus of ∼0.3 GPa (64). To produce epoxy substrates for microscopy, glass coverslips were coated with low-viscosity epoxy [PX672H/NC; Robnor Resins; elastic modulus ∼ 1.8 GPa (66); compressive strength: 40 MPa, from technical data sheet].
Morphology.
Hind legs of P. spumarius were investigated using light microscopy (Leica MZ 16; Leica Microsystems GmbH) and SEM (see SI Appendix, SI Materials and Methods). The presence of metals in tibial and tarsal spines was studied using EDX (see SI Appendix, SI Materials and Methods).
High-Speed Recordings of Jumps.
Jumps were recorded with two synchronized Phantom V7.1 high-speed cameras (Vision Research) at 4,700 frames per second. Froghoppers jumped voluntarily or were gently stimulated to jump with a single human hair. To film jumps from transparent glass or epoxy substrates [glass coverslips coated with low-viscosity epoxy PX672H/NC; Robnor Resins; elastic modulus ∼ 1.8 GPa (66); compressive strength: 40 MPa, from technical data sheet], one camera recorded a side view, while the other was attached to a Leica DMIRE2 inverted microscope (Leica Microsystems GmbH) to record the surface contact and movements of hind feet from below with high magnification and epi-illumination (5× lens; field of view: 3.6 mm × 2.7 mm). To film jumps from ivy leaves, the cameras were both oriented horizontally at an angle of 90° to each other to record side views of the jumps.
Study of Tracks Left on Leaf Surfaces.
After froghoppers had jumped from ivy, the leaves were stained with 0.1% methylene blue to reveal possible foot marks and imaged using SEM (SI Appendix, SI Materials and Methods).
Supplementary Material
Acknowledgments
We acknowledge the Engineering and Physical Sciences Research Council Engineering Instrument Pool for multiple loans of the Phantom high-speed camera system. We thank Jeremy Skepper for help with electron microscopy and sample preparation, and John Williams for comments on a draft of the manuscript. This study was supported by scholarships from the Gates Cambridge Trust, the Balfour Fund, and the Cambridge Philosophical Society (H.H.G.), a United Kingdom Biotechnology and Biological Research Council PhD Studentship, Grant BB/J014540/1 (to J.G.P.), and United Kingdom Biotechnology and Biological Sciences Research Council Grant BB/I008667/1 (to W.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814183116/-/DCSupplemental.
References
- 1.Autumn K. Gecko adhesion: Structure, function, and applications. MRS Bull. 2007;32:473–478. [Google Scholar]
- 2.Peattie AM. Functional demands of dynamic biological adhesion: An integrative approach. J Comp Physiol B. 2009;179:231–239. doi: 10.1007/s00360-008-0310-8. [DOI] [PubMed] [Google Scholar]
- 3.Federle W. Why are so many adhesive pads hairy? J Exp Biol. 2006;209:2611–2621. doi: 10.1242/jeb.02323. [DOI] [PubMed] [Google Scholar]
- 4.Autumn K, Dittmore A, Santos D, Spenko M, Cutkosky M. Frictional adhesion: A new angle on gecko attachment. J Exp Biol. 2006;209:3569–3579. doi: 10.1242/jeb.02486. [DOI] [PubMed] [Google Scholar]
- 5.Labonte D, Federle W. Biomechanics of shear-sensitive adhesion in climbing animals: Peeling, pre-tension and sliding-induced changes in interface strength. J R Soc Interface. 2016;13:20160373. doi: 10.1098/rsif.2016.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen WR, Autumn K. Evidence for self-cleaning in gecko setae. Proc Natl Acad Sci USA. 2005;102:385–389. doi: 10.1073/pnas.0408304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogue R. Biomimetic adhesives: A review of recent developments. Assem Autom. 2008;28:282–288. [Google Scholar]
- 8.Hancock MJ, Sekeroglu K, Demirel MC. Bioinspired directional surfaces for adhesion, wetting, and transport. Adv Funct Mater. 2012;22:2223–2234. doi: 10.1002/adfm.201103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue L, Steinhart M, Gorb SN. Biological and bioinspired micro- and nanostructured adhesives. In: Taubert A, Mano JF, Rodríguez-Cabello JC, editors. Biomaterials Surface Science. Wiley-VCH; Weinheim, Germany: 2013. pp. 409–439. [Google Scholar]
- 10.Kamperman M, Kroner E, del Campo A, McMeeking RM, Arzt E. Functional adhesive surfaces with “gecko” effect: The concept of contact splitting. Adv Eng Mater. 2010;12:335–348. [Google Scholar]
- 11.Burrows M, Sutton G. Interacting gears synchronize propulsive leg movements in a jumping insect. Science. 2013;341:1254–1256. doi: 10.1126/science.1240284. [DOI] [PubMed] [Google Scholar]
- 12.Clemente CJ, et al. Jumping without slipping: Leafhoppers (Hemiptera: Cicadellidae) possess special tarsal structures for jumping from smooth surfaces. J R Soc Interface. 2017;14:20170022. doi: 10.1098/rsif.2017.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spagna JC, Goldman DI, Lin PC, Koditschek DE, Full RJ. Distributed mechanical feedback in arthropods and robots simplifies control of rapid running on challenging terrain. Bioinspir Biomim. 2007;2:9–18. doi: 10.1088/1748-3182/2/1/002. [DOI] [PubMed] [Google Scholar]
- 14.Provancher WR, Clark JE, Geisler B, Cutkosky MR. 2005. Towards penetration-based clawed climbing. Climbing and Walking Robots, Proceedings of the 7th International Conference CLAWAR 2004, eds Armada M, González de Santos P (Springer, Berlin), pp 961–970.
- 15.Woodward MA, Sitti M. Morphological intelligence counters foot slipping in the desert locust and dynamic robots. Proc Natl Acad Sci USA. 2018;115:E8358–E8367. doi: 10.1073/pnas.1804239115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai Z, Gorb SN, Schwarz U. Roughness-dependent friction force of the tarsal claw system in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae) J Exp Biol. 2002;205:2479–2488. doi: 10.1242/jeb.205.16.2479. [DOI] [PubMed] [Google Scholar]
- 17.Pattrick JG, Labonte D, Federle W. Scaling of claw sharpness: Mechanical constraints reduce attachment performance in larger insects. J Exp Biol. 2018;221:jeb188391. doi: 10.1242/jeb.188391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asbeck AT, Kim S, Cutkosky MR, Provancher WR, Lanzetta M. Scaling hard vertical surfaces with compliant microspine arrays. Int J Robot Res. 2006;25:1165–1179. [Google Scholar]
- 19.Lee JS. 2018. Increased millirobot traction in running and jumping through leg spines. PhD dissertation (Univ of California, Berkeley)
- 20.Burrows M. Biomechanics: Froghopper insects leap to new heights. Nature. 2003;424:509. doi: 10.1038/424509a. [DOI] [PubMed] [Google Scholar]
- 21.Burrows M. Jumping performance of froghopper insects. J Exp Biol. 2006;209:4607–4621. doi: 10.1242/jeb.02539. [DOI] [PubMed] [Google Scholar]
- 22.Burrows M. Morphology and action of the hind leg joints controlling jumping in froghopper insects. J Exp Biol. 2006;209:4622–4637. doi: 10.1242/jeb.02554. [DOI] [PubMed] [Google Scholar]
- 23.Sutton GP, Burrows M. The mechanics of azimuth control in jumping by froghopper insects. J Exp Biol. 2010;213:1406–1416. doi: 10.1242/jeb.036921. [DOI] [PubMed] [Google Scholar]
- 24.Barthlott W, et al. Classification and terminology of plant epicuticular waxes. Bot J Linn Soc. 1998;126:237–260. [Google Scholar]
- 25.Lucas PW, Turner IM, Dominy NJ, Yamashita N. Mechanical defenses to herbivory. Ann Bot. 2000;86:913–920. [Google Scholar]
- 26.Emeljanov AF. Structure and evolution of the tarsus in the Dictyopharidae (Homoptera) Entomol Rev. 1982;61:44–59. [Google Scholar]
- 27.Gorb S. Attachment Devices of Insect Cuticle. Kluwer Acad; Dordrecht, The Netherlands: 2001. [Google Scholar]
- 28.Clemente CJ, Federle W. Pushing versus pulling: Division of labour between tarsal attachment pads in cockroaches. Proc Biol Sci. 2008;275:1329–1336. doi: 10.1098/rspb.2007.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endlein T, Federle W. On heels and toes: How ants climb with adhesive pads and tarsal friction hair arrays. PLoS One. 2015;10:e0141269. doi: 10.1371/journal.pone.0141269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labonte D, Federle W. Functionally different pads on the same foot allow control of attachment: Stick insects have load-sensitive “heel” pads for friction and shear-sensitive “toe” pads for adhesion. PLoS One. 2013;8:e81943. doi: 10.1371/journal.pone.0081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashby MF, Shercliff H, Cebon D. Materials: Engineering, Science, Processing and Design. Butterworth-Heinemann; Oxford: 2013. [Google Scholar]
- 32.Dugdale DS. Vickers hardness and compressive strength. J Mech Phys Solids. 1958;6:85–91. [Google Scholar]
- 33.Gilman JJ. Relationship between impact yield stress and indentation hardness. J Appl Phys. 1975;46:1435–1436. [Google Scholar]
- 34.Zhang P, Li SX, Zhang ZF. General relationship between strength and hardness. Mater Sci Eng A. 2011;529:62–73. [Google Scholar]
- 35.Read J, Sanson GD. Characterizing sclerophylly: The mechanical properties of a diverse range of leaf types. New Phytol. 2003;160:81–99. doi: 10.1046/j.1469-8137.2003.00855.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Ren L, Liu Y, Han Z, Yang Y. Mechanical characteristics of typical plant leaves. J Bionics Eng. 2010;7:294–300. [Google Scholar]
- 37.Sato K, et al. Effects of nanostructured biosilica on rice plant mechanics. RSC Advances. 2017;7:13065–13071. [Google Scholar]
- 38.Yu Z, Jiang Z, Zhang X, Yu Y. Mechanical properties of silica cells in bamboo measured using in situ imaging nanoindentation. Wood Fiber Sci. 2016;48:1–6. [Google Scholar]
- 39.Cribb BW, et al. Hardness in arthropod exoskeletons in the absence of transition metals. Acta Biomater. 2010;6:3152–3156. doi: 10.1016/j.actbio.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Wegst UGK, Ashby MF. The mechanical efficiency of natural materials. Philos Mag. 2004;84:2167–2186. [Google Scholar]
- 41.Edwards AJ, Fawke JD, McClements JG, Smith SA, Wyeth P. Correlation of zinc distribution and enhanced hardness in the mandibular cuticle of the leaf-cutting ant Atta sexdens rubropilosa. Cell Biol Int. 1993;17:697–698. [Google Scholar]
- 42.Schofield RMS, Nesson MH, Richardson KA. Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften. 2002;89:579–583. doi: 10.1007/s00114-002-0381-4. [DOI] [PubMed] [Google Scholar]
- 43.Bowden FP, Tabor D. The Friction and Lubrication of Solids. Oxford Univ Press; Oxford: 1950. [Google Scholar]
- 44.Federle W, Baumgartner W, Hölldobler B. Biomechanics of ant adhesive pads: Frictional forces are rate- and temperature-dependent. J Exp Biol. 2004;207:67–74. doi: 10.1242/jeb.00716. [DOI] [PubMed] [Google Scholar]
- 45.Drechsler P, Federle W. Biomechanics of smooth adhesive pads in insects: Influence of tarsal secretion on attachment performance. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:1213–1222. doi: 10.1007/s00359-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 46.Lafaye S, Gauthier C, Schirrer R. The ploughing friction: Analytical model with elastic recovery for a conical tip with a blunted spherical extremity. Tribol Lett. 2006;21:95–99. [Google Scholar]
- 47.Mishra M, Egberts P, Bennewitz R, Szlufarska I. Friction model for single-asperity elastic-plastic contacts. Phys Rev B. 2012;86:045452. [Google Scholar]
- 48.Barthlott W, Mail M, Bhushan B, Koch K. Plant surfaces: Structures and functions for biomimetic innovations. Nano-Micro Lett. 2017;9:23. doi: 10.1007/s40820-016-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riederer M, Müller C. Biology of the Plant Cuticle. Blackwell; Oxford: 2006. [Google Scholar]
- 50.Vogel S. Living in a physical world II. The bio-ballistics of small projectiles. J Biosci. 2005;30:167–175. doi: 10.1007/BF02703696. [DOI] [PubMed] [Google Scholar]
- 51.Rabinowicz E. Friction and Wear of Materials. Wiley; New York: 1995. [Google Scholar]
- 52.Burrows M. Kinematics of jumping in leafhopper insects (Hemiptera, Auchenorrhyncha, Cicadellidae) J Exp Biol. 2007;210:3579–3589. doi: 10.1242/jeb.009092. [DOI] [PubMed] [Google Scholar]
- 53.Kundanati L, Gundiah N. Biomechanics of substrate boring by fig wasps. J Exp Biol. 2014;217:1946–1954. doi: 10.1242/jeb.098228. [DOI] [PubMed] [Google Scholar]
- 54.Cerkvenik U, van de Straat B, Gussekloo SWS, van Leeuwen JL. Mechanisms of ovipositor insertion and steering of a parasitic wasp. Proc Natl Acad Sci USA. 2017;114:E7822–E7831. doi: 10.1073/pnas.1706162114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leopold RA, Freeman TP, Buckner JS, Nelson DR. Mouthpart morphology and stylet penetration of host plants by the glassy-winged sharpshooter, Homalodisca coagulata, (Homoptera: Cicadellidae) Arthropod Struct Dev. 2003;32:189–199. doi: 10.1016/S1467-8039(03)00047-1. [DOI] [PubMed] [Google Scholar]
- 56.Polidori C, García AJ, Nieves-Aldrey JL. Breaking up the wall: Metal-enrichment in ovipositors, but not in mandibles, co-varies with substrate hardness in gall-wasps and their associates. PLoS One. 2013;8:e70529. doi: 10.1371/journal.pone.0070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollard DG. Plant penetration by feeding aphids (Hemiptera, Aphidoidea): A review. Bull Entomol Res. 1973;62:631–714. [Google Scholar]
- 58.Quicke DLJ, Wyeth P, Fawke JD, Basibuyuk HH, Vincent JFV. Manganese and zinc in the ovipositors and mandibles of hymenopterous insects. Zool J Linn Soc. 1998;124:387–396. [Google Scholar]
- 59.Bown AW, Hall DE, MacGregor KB. Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol. 2002;129:1430–1434. doi: 10.1104/pp.006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall D, MacGregor K, Nijsse J, Bown A. Footsteps from insect larvae damage leaf surfaces and initiate rapid responses. Eur J Plant Pathol. 2004;110:441–447. [Google Scholar]
- 61.Lee JS, Plecnik M, Yang J-h, Fearing RS. 2018 IEEE International Conference on Robotics and Automation. Inst Electr Electron Eng; New York: 2018. Self-engaging spined gripper with dynamic penetration and release for steep jumps; pp. 1–8. [Google Scholar]
- 62.Kovač M, Schlegel M, Zufferey J-C, Floreano D. Steerable miniature jumping robot. Auton Robots. 2010;28:295–306. [Google Scholar]
- 63.Lien TK. Gripper technologies for food industry robots. In: Caldwell DG, editor. Robotics and Automation in the Food Industry. Woodhead; Cambridge, UK: 2013. pp. 143–170. [Google Scholar]
- 64.Wiedemann P, Neinhuis C. Biomechanics of isolated plant cuticles. Bot Acta. 1998;111:28–34. [Google Scholar]
- 65.Ensikat HJ, Boese M, Mader W, Barthlott W, Koch K. Crystallinity of plant epicuticular waxes: Electron and X-ray diffraction studies. Chem Phys Lipids. 2006;144:45–59. doi: 10.1016/j.chemphyslip.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Robinson A, Steiner U, Federle W. Insect adhesion on rough surfaces: Analysis of adhesive contact of smooth and hairy pads on transparent microstructured substrates. J R Soc Interface. 2014;11:20140499. doi: 10.1098/rsif.2014.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.