Significance
Epithelial-to-mesenchymal transition (EMT) is a process in which epithelial cells become elongated and spindle-like mesenchymal cells. EMT plays essential roles in organogenesis, tissue repair, tissue regeneration, organ fibrosis, and cancer progression. Therefore, studying EMT provides insight into how cells regulate cell plasticity that is linked to their changes in functions. ERK-MAP kinase is a critical coordinator of EMT in that many key EMT-inducing signals originated from growth factor receptor signaling and oncogene products, and metastasis drivers converge on ERK. Despite key roles of ERK in EMT induction, the molecular mechanisms by which ERK induces EMT are not well understood. Our study provides a conceptual advance and mechanistic insight into previously unexplored ERK signaling to promote EMT, cell migration/invasiveness, and survival.
Keywords: ERK2, epithelial-to-mesenchymal transition, EMT, FoxO, Dock10
Abstract
ERK is a key coordinator of the epithelial-to-mesenchymal transition (EMT) in that a variety of EMT-inducing factors activate signaling pathways that converge on ERK to regulate EMT transcription programs. However, the mechanisms by which ERK controls the EMT program are not well understood. Through an analysis of the global changes of gene expression mediated by ERK2, we identified the transcription factor FoxO1 as a potential mediator of ERK2-induced EMT, and thus we investigated the mechanism by which ERK2 regulates FoxO1. Additionally, our analysis revealed that ERK2 induced the expression of Dock10, a Rac1/Cdc42 GEF, during EMT. We demonstrate that the activation of the Rac1/JNK signaling axis downstream of Dock10 leads to an increase in FoxO1 expression and EMT. Taken together, our study uncovers mechanisms by which epithelial cells acquire less proliferative but more migratory mesenchymal properties and reveals potential therapeutic targets for cancers evolving into a metastatic disease state.
Changes of cell morphology induced by genetic, epigenetic, or environmental factors are often indicative of altered cell properties. Epithelial-to-mesenchymal transition (EMT) is a process in which cellular signaling programs in epithelial cells are reprogrammed and often promote morphological changes, specifically more elongated spindle-like mesenchymal cells with increased migratory and invasive properties, while exhibiting reduced rates of cell proliferation (1). Partial or complete EMT plays essential roles in normal physiology such as organogenesis, tissue repair, and tissue regeneration and in pathological situations such as organ fibrosis and cancer progression. In cancer, EMT is often associated with cancer invasiveness and aggressiveness, cancer cell survival, anticancer drug resistance, and poor prognosis (2, 3). In fact, complete or partial EMT has been observed at the leading edge of cancer by recently developed techniques such as intravital imaging, genetic lineage tracing, and capturing and characterizing circulating tumors (4, 5). Although the term “EMT” describes plasticity between epithelial and mesenchymal cells, processes resembling EMT also occur in nonepithelial cells (6–8). For example, during endothelial-to-mesenchymal transition, which is also observed in cancer progression, endothelial cells use signaling and transcriptional programs very similar to those involved in EMT (8). Many nonepithelial lineage cancer types such as melanoma also use EMT-like systems, which contribute to tumor progression and metastasis (6). Therefore, understanding EMT can help inform how both epithelial cells and nonepithelial cells regulate cell plasticity that is directly linked to their changes in functions in physiological and pathological processes. Ultimately, these mechanisms are very attractive therapeutic targets in cancer and associated diseases.
For complete or partial EMT to induce mesenchymal or quasi-mesenchymal cells, it is critical for epithelium-like cells to invoke mechanisms to reorganize their cytoskeleton, alter their signaling and gene expression programs, and rebalance their proliferation and migration (8). As part of this extensive reprogramming, mesenchymal cells acquire more migratory and invasive capabilities. Related to these properties, mesenchymal cells are better able to survive in adverse conditions such as hypoxia, cell detachment, and antitumor drug treatment (1, 3). The major physiological or pathological extracellular contributors of EMT are growth factors (i.e., FGF, IGF1, and EGF) and transforming growth factor-β (TGF-β), although environmental factors such as hypoxia, UV radiation, and extracellular inputs for WNT, Hedgehog, and Notch signaling can also contribute to EMT (1, 8). In addition to extracellular factors, genetic and/or epigenetic changes of intracellular molecules such as mutation of signaling proteins (i.e., Ras and Raf) and overexpression of receptor tyrosine kinases (i.e., EGFR1, ERRB2, and IGF1R) are also responsible for activation of EMT signaling. Often, these signaling molecules cooperate with each other to drive more efficient EMT programs. Although EMT can be induced by many EMT upstream molecules, the signaling changes induced by these molecules often converge on the main EMT regulators such as ERK and PI3K/Akt. ERK is one of the key mediators of Ras/Raf-, growth factors/growth factor receptor-, and TGF-β–mediated EMT, which play pivotal roles in cancer promotion and progression (8). In fact, sustained active ERK signaling is associated with cell transformation, metastasis, anticancer drug resistance, and poor prognosis in cancer in line with its roles in inducing EMT (9–11).
Despite the pathological roles of ERK and EMT in tumor progression, the molecular mechanisms by which ERK induces EMT are not well understood. Our previous studies showed isoform-specific functions of the ERK kinases; ERK2, but not ERK1, induces EMT and cancer stemness in breast epithelial cells, allowing them to gain more migratory and invasive properties while rendering them anoikis (cell-detachment–induced death)-resistant (12). We demonstrated that this EMT induction was through ERK2-mediated stabilization of Fra1 and subsequent up-regulation of Zeb (12). Subsequent reports also supported our findings on the isoform-specific functions of ERK2 in EMT, cell migration, and resistance to stress (13–16), as well as important roles for Fra1 and Zeb1 in mediating these processes (13, 16, 17).
Based on the physiological and pathological importance of EMT and our previous findings on ERK2’s major role in this process, we set out to further define the molecular mechanisms by which ERK2 regulates EMT and its associated properties such as increased cell migration/invasion and decreased cell proliferation. Through an analysis of global changes of mRNA levels induced by ERK2 and detailed biochemical and cellular analysis, we have found that FoxO1 is a major transcriptional mediator of ERK2-induced EMT, migration, and inhibition of cell proliferation. We also have found that ERK2-induced Dock10 is responsible for Rac1/JNK activation, which regulates FoxO1 and subsequent Zeb1 expression during EMT.
Results
ERK2 Regulates Global mRNA Changes During EMT.
To investigate the mechanistic link between ERK2 and EMT, we used distinct mammary epithelial cell models: MCF10A and NMuMG cells. These cells have been used widely to study EMT, and we have previously shown that ERK2 expression induces EMT in these and other cells (12). We first exploited well-established binding mechanisms between ERK and its substrates to study ERK downstream signaling in EMT. Many ERK substrates have well-defined ERK interaction motifs such as D (Docking) domain (e.g., RSK, ELK1) and/or DEF (Docking site of ERK, F-X-F) motifs (e.g., Fra1, Jun) (12, 18). In our previous report, we utilized an ERK mutant that is unable to bind D-domain–containing substrates (D319N) or a mutant that is unable to bind DEF-motif–containing substrates (Y261A) (12). We showed that only the ERK2 D-domain mutant (ERK2-D319N) that selectively binds to DEF-motif–containing substrates induces EMT through up-regulation of Fra1, a DEF-motif–containing ERK substrate (12). Wild-type ERK2 (ERK2-WT) also exerts similar effects through the same mechanisms although with slower kinetics (12). To gain a complete understanding of the ERK2-driven transcription programs that promote EMT, we assessed global changes of mRNA expression induced by ERK2. We first generated breast epithelial cells stably expressing ERK2-WT, ERK2-D319N, or ERK2-Y261A. As shown in SI Appendix, Fig. S1A, the ERK2-WT– or ERK2-D319N–expressing cells showed an elongated fibroblast-like morphology 6–8 d after selection. Conversely, vector control cells or cells expressing ERK2-Y261A that does not regulate DEF-motif targets displayed typical epithelial cell morphology. In line with these morphological changes, ERK2-WT or ERK2-D319N expression also resulted in a time-dependent decrease in E-cadherin, an epithelial marker, and an increase in fibronectin, a mesenchymal marker (Fig. 1A). In addition to these changes, one of the major characteristics of cells undergoing EMT is reduced proliferation rates (1), and it is known that sustained hyperactive ERK signaling suppresses cell proliferation (19). We found that ERK2-WT or ERK2-D319N–expressing cells clearly exhibited suppressed cell proliferation rates compared with ERK2-Y261A–expressing cells or control cells (Fig. 1B). Using the ERK2-D319N– or ERK2-Y261A–expressing cells at day 7, we extracted RNAs and used microarray chips (Affymetrix Human Transcriptome Array 2.0) detecting around 67,500 genes to look at global changes of mRNA levels during the EMT process induced by ERK2. Comparison of the up-regulated and down-regulated genes between the ERK2-D319N–expressing cells and the ERK2-Y261A–expressing cells revealed many candidate genes that might be involved in the induction of EMT and/or inhibition of cell proliferation (Fig. 1C). Through this analysis, we were able to identify a number of genes that were up-regulated (Fig. 1D) or down-regulated (Fig. 1E) specifically by ERK2-D319N. Analysis of differentially expressed genes revealed that many of the up-regulated gene products are involved in cell migration/invasion, extracellular matrix (ECM)-receptor interaction, and ECM degradation (Fig. 1F and SI Appendix, Fig. S1B). In contrast, genes involved in cell cycle progression were found to be down-regulated by ERK2-D319N (Fig. 1G and SI Appendix, Fig. S1C).
Fig. 1.
ERK2 induces global changes of mRNA expression during EMT. (A and B) MCF10A cells stably expressing vector control, WT ERK2, Y261A ERK2, or D319N ERK2 were grown for the indicated time. (A) Immunoblot analysis was performed, and (B) cell proliferation was measured by counting the cell numbers. Data are the means ± SD of three separate experiments. (C–G) MCF10A cells stably expressing vector control, Y261A ERK2, or D319N ERK2 were grown for 7 d. Total mRNAs were purified, and microarray analysis was performed. (C) Heat map was generated using the top 60 genes, the mRNA levels of which were differentially regulated by D319N ERK2. 1: D319N ERK; 2: Control; 3: Y261A ERK2. (D and E) Venn diagram of up-regulated (D) or down-regulated (E) mRNAs by D319N ERK2. (F and G) Functional analysis was performed using the genes, the mRNA levels of which were up-regulated (F) or down-regulated (G) by D319N ERK2.
FoxO1 Participates in EMT.
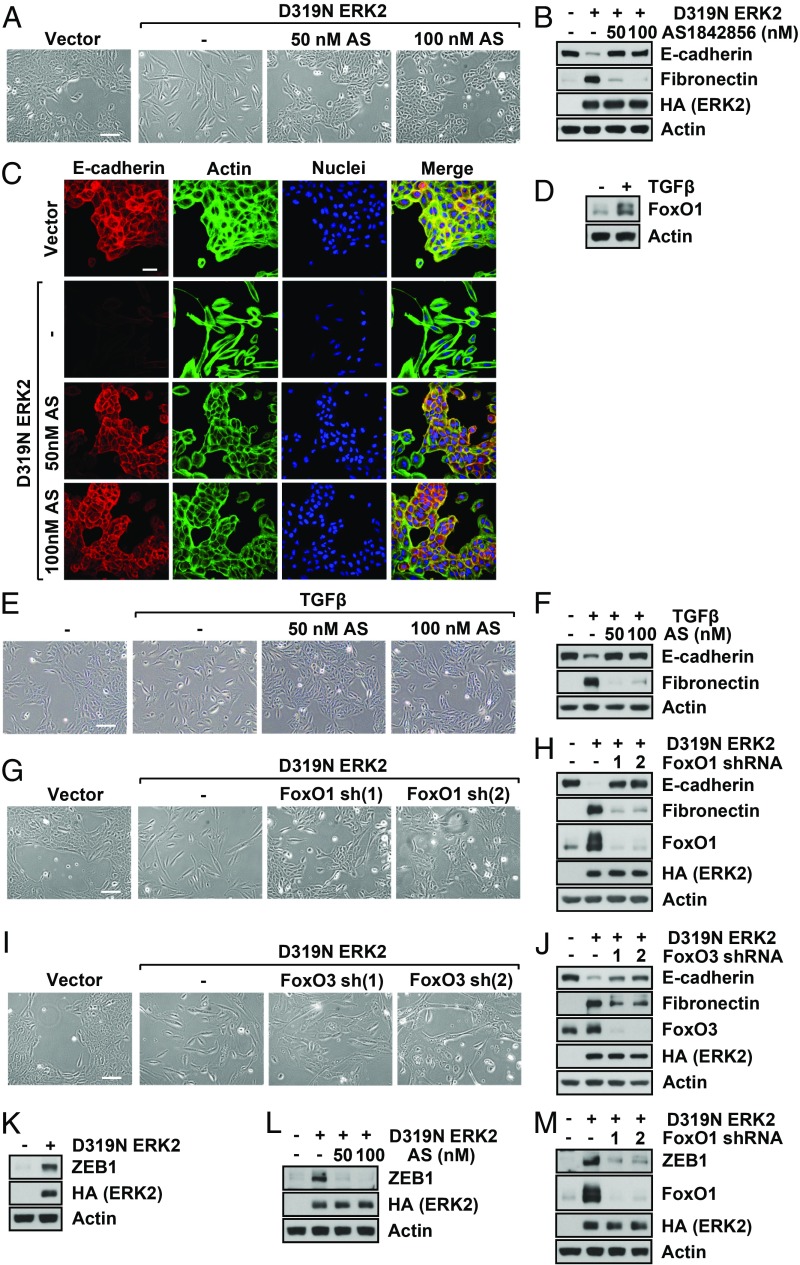
To identify major transcription programs that ERK2 utilizes to induce EMT, we first determined the genes, the mRNA levels of which were up-regulated or down-regulated at least twofold by ERK2. We then analyzed the ERK2-regulated genes using transcription factor prediction programs and identified several transcription factors as potential key mediators of ERK2-induced mRNA level changes. Most of the identified transcription factors (SI Appendix, Fig. S2A), such as TCF7L2 (WNT signaling), CTNNB1 (β-catenin, WNT signaling), GLI1 (Sonic hedgehog signaling), and Myc, are already known to be involved in EMT (8), which validates our datasets and approach to identifying novel EMT regulators. Among the candidate transcription factors, we were interested in FoxOs as they are well-known tumor suppressors that inhibit tumor growth/proliferation and migration/invasion (20). However, despite the seemingly contradictory roles of ERK and FoxOs, both hyperactive ERK2 and FoxOs are known to inhibit cell proliferation and provide resistance mechanisms in response to stress (19, 21). In addition, some reports show that FoxOs can contribute to metastasis, invasion, and wound healing in specific cancers or conditions (21–24). Therefore, we sought to explore FoxOs involvement in ERK2-induced EMT. First, we blocked FoxO1 activity in ERK2-expressing breast epithelial cells using a FoxO1 inhibitor, AS1842856, which preferentially inhibits FoxO1 over FoxO3 (25). Importantly, FoxO1 inhibition suppressed ERK2-induced EMT as evidenced by cell morphology and EMT markers (Fig. 2 A–C and SI Appendix, Fig. S2 B and C). We also found that inhibition of FoxO1 suppressed ERK2-induced EMT in NMuMG cells (SI Appendix, Fig. S2 D and E). ERK is an important mediator of TGF-β–induced EMT (8), and ERK and TGF-β cross-talk enhances their functions (26, 27). Therefore, we sought to determine if FoxO1 was involved in EMT induced by TGF-β. As shown in SI Appendix, Fig. S2 F and G, inhibition of ERK activity blocked TGF-β–induced EMT, which suggests that ERK activation is critical for TGF-β–mediated EMT. Based on these findings, we then asked whether FoxO1 was also necessary for TGF-β–induced EMT. As expected, TGF-β increased FoxO1 protein levels (Fig. 2D), and blocking FoxO1 also suppressed EMT induced by TGF-β (Fig. 2 E and F). Taken together, these results suggest a significant role of FoxO1 in EMT induced by ERK and TGF-β.
Fig. 2.
FoxO1 is a critical mediator of ERK2-induced EMT. (A–C) MCF10A cells stably expressing vector or D319N ERK2 were grown for 7 d with or without FoxO1 inhibitor (AS1842856). Cell images were taken (A), or immunoblot analysis (B) or confocal microscopy (C) was performed. [Scale bar: 500 µm (A) and 50 µm (C).] (D–F) MCF10A cells were treated with TGF-β (5 ng/mL) every other day for 9 d with or without FoxO1 inhibitor (AS1842856). Immunoblot analysis was performed (D and F), or cell images were taken (E). (Scale bar: 500 µm.) (G–J) MCF10A cells stably expressing vector or D319N ERK2 were grown for 7 d with or without FoxO1 shRNAs (G and H) or FoxO3 shRNAs (I and J). Cell images were taken (G and I), or immunoblot analysis was performed (H and J). (Scale bar: 500 µm.) (K–M) MCF10A cells stably expressing vector or D319N ERK2 were grown for 7 d without (K) or with FoxO1 inhibitor (L) or FoxO1 shRNAs (M). Cells were lysed, and immunoblot analysis was performed.
Two major proteins in the FoxO family are FoxO1 and FoxO3. Although the DNA-binding domains (DBDs) of FoxO1 and FoxO3 are highly homologous, sequence conservation outside of the DBD is low (28). It is known that FoxO1 and FoxO3 have both overlapping and distinctive functions (29). We used shRNAs specific for FoxO1 over FoxO3 (SI Appendix, Fig. S2H) and found that FoxO1 knockdown inhibited ERK2-induced EMT (Fig. 2 G and H). We next knocked down FoxO3 to determine whether this protein was also involved in ERK2-induced EMT. As shown in Fig. 2 I and J, knockdown of FoxO3 suppressed EMT, but its effect was partial in comparison with FoxO1 inhibition. These results indicate that the nonconserved regions of these FoxO family members may contribute to overlapping as well as distinct functions.
The major transcription factors that promote EMT through repression of E-cadherin expression are Snail, Slug, Twist, and Zeb. In our previous report, we showed that Zeb1 was essential for ERK2-induced EMT and cell migration/invasion (12). In addition to EMT, the aberrant Zeb1 expression is linked to metastasis, therapy resistance, high tumor grade, high metabolic plasticity, and tumor recurrence (30). We investigated whether FoxO1 was involved in the regulation of Zeb1 expression. As shown in Fig. 2K and SI Appendix, Fig. S2C, ERK2 overexpression increased Zeb1 levels. Critically, inhibition of FoxO1 by inhibitor treatment dramatically reduced Zeb1 expression (Fig. 2L and SI Appendix, Fig. S2C), suggesting that FoxO1 mediates the up-regulation of Zeb1 by ERK2. Using FoxO1 knockdown, we also confirmed that FoxO1 is involved in Zeb1 expression (Fig. 2M).
FoxO1 Is Required for ERK2-Induced Cell Migration/Invasion and Decreased Cell Proliferation.
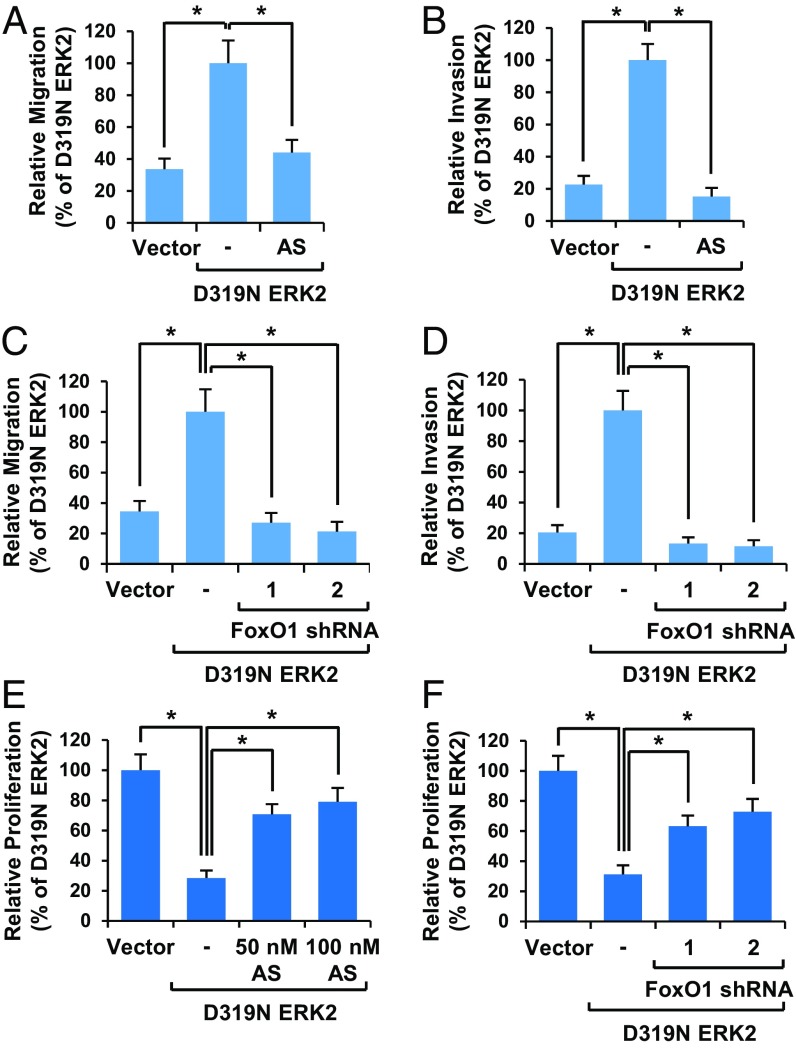
Because there is a strong positive correlation between EMT and cell migration/invasion (1, 3), we wondered if FoxO1 was involved in this shift to a more migratory phenotype. As expected, expression of ERK2 dramatically increased cell migration (Fig. 3A and SI Appendix, Fig. S3A) and invasion (Fig. 3B and SI Appendix, Fig. S3B), which was suppressed with FoxO1 inhibition (Fig. 3 A and B and SI Appendix, Fig. S3 A and B). Knockdown of FoxO1 also profoundly inhibited cell migration (Fig. 3C and SI Appendix, Fig. S3C) and invasion (Fig. 3D and SI Appendix, Fig. S3D) induced by ERK2. Although FoxO3 knockdown also suppressed ERK2-induced cell migration (SI Appendix, Fig. S3E) and invasion (SI Appendix, Fig. S3F), its effect was partial compared with that of FoxO1 knockdown (Fig. 3 C and D). While EMT leads to an increase in migration/invasion, these changes often negatively correlate with cell proliferation (1). As FoxO1 is a well-known suppressor of cell proliferation (21), we wondered if FoxO1 mediates ERK2-induced inhibition of cell proliferation during EMT. As shown in Fig. 3 E and F, ERK2 expression reduced the cell proliferation rate. However, inhibition of FoxO1 by inhibitor treatment (Fig. 3E) or knockdown (Fig. 3F) reversed the proliferation suppressive functions of ERK2. Together, these results indicate that ERK2 induces EMT and regulates its associated properties including increased cell migration/invasion and reduced cell proliferation mainly through FoxO1.
Fig. 3.
ERK2 regulates cell migration/invasion and proliferation through FoxO1. Where applicable, data are the means ± SD of three separate experiments. Results were statistically significant (*P < 0.01) as assessed by t test. (A–F) MCF10A cells stably expressing vector control or D319N ERK2 were grown for 7 d with or without FoxO1 inhibitor (AS1842856) (A, B, and E) or FoxO1 shRNAs (C, D, and F). Cell migration (A and C) or invasion (B and D) assay was performed. (E and F) Cell proliferation rate was measured by counting cell numbers.
FoxO1 Requires ERK2 to Induce EMT.
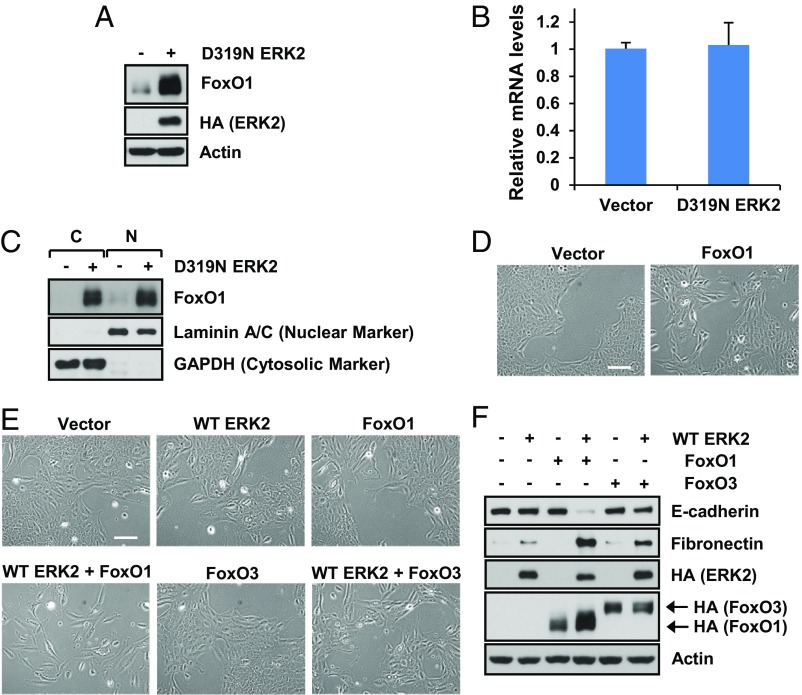
Intrigued by the findings that FoxO1 is a major mediator of ERK2-generated EMT and changes of cell migration/invasion and proliferation rate, we measured FoxO1 protein and mRNA levels to determine if ERK2 regulates FoxO1 expression. Interestingly, we found that ERK2 increased FoxO1 protein levels (Figs. 2 H and M and 4A and SI Appendix, Fig. S4A) without affecting FoxO1 mRNA levels (Fig. 4B). Because FoxO1 is a transcription factor in which cytoplasmic and nuclear distribution are known to be regulated, we determined the FoxO1 levels in the nucleus. As shown in Fig. 4C and SI Appendix, Fig. S4B, ERK2 increased nuclear as well as cytoplasmic FoxO1 protein levels. Compared with FoxO1, FoxO3 protein levels were slightly increased by ERK2 (Fig. 2J). Because ERK2-induced EMT requires FoxO1 (Fig. 2), we wondered whether FoxO1 alone is sufficient to induce EMT. To test this, we expressed FoxO1 in cells for more than 10 d, but FoxO1 alone did not induce significant morphological changes (Fig. 4D). Thus, we expressed ERK2 and FoxO1 together and found that FoxO1 profoundly accelerated EMT in the presence of ERK2-WT even 5 d after selection when ERK2-WT expression alone did not induce the complete EMT phenotype (Fig. 4 E and F). ERK2 and FoxO3 coexpression also promoted EMT, but at a slower rate compared with ERK2/FoxO1 expression (Fig. 4 E and F) as expected based on the partial phenotype observed in Fig. 2 I and J. These results indicate that ERK2 requires FoxO1 as a major EMT-inducing factor, but that FoxO1 expression itself is not sufficient to induce EMT.
Fig. 4.
FoxO1 requires ERK2 to induce EMT. (A–C) MCF10A cells stably expressing vector control or D319N ERK2 were grown for 7 d. (A) Immunoblot analysis or (B) RT-qPCR was performed to determine FoxO1 mRNA levels. Data are the means ± SEM of four separate experiments. (C) Cells were lysed and fractionated, and immunoblot analysis was performed. (D) MCF10A cells stably expressing vector control or FoxO1 were grown for 10 d, and cell images were taken. (Scale bar: 500 µm.) (E and F) MCF10A cells stably expressing vector control, WT ERK2, FoxO1, WT ERK2+FoxO1, FoxO3, or WT ERK2+FoxO3 were grown for 5 d. (E) Cell images were taken, or (F) immunoblot analysis was performed. (Scale bar: 500 µm.)
ERK2-Induced FoxO1 Up-Regulation Is Mediated by JNK.
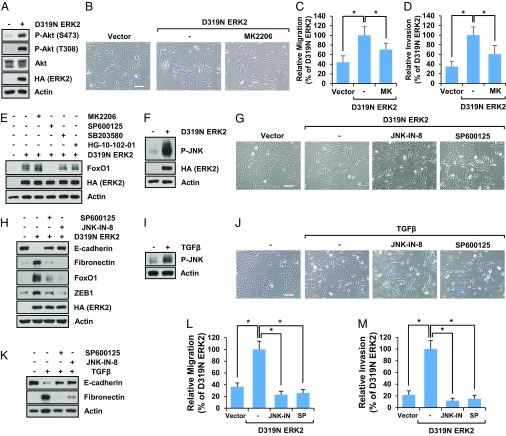
FoxO1 protein levels are regulated by posttranslational modifications (21). Akt is a well-known FoxO1 kinase that phosphorylates several sites at FoxO1-Thr-24, Ser-256, and Ser-319. Although we found that ERK2 increases Akt phosphorylation (Fig. 5A), inhibition of Akt did not block ERK2-induced EMT (Fig. 5B). Akt is a well-known regulator of cell migration and invasion. Since ERK2 induced Akt activation, we wondered whether activated Akt contributed to the cell migration and invasion associated with EMT. As shown in Fig. 5 C and D and SI Appendix, Fig. S5 A and B, inhibition of Akt suppressed ERK2-induced cell migration and invasion, which suggests that Akt is one of the mediators of ERK2-induced cell motility. However, it was not clear if Akt was a major regulator of FoxO1 during EMT because Akt is known to induce FoxO1 degradation, which is in contrast to our observations that ERK2 positively regulates FoxO1 abundance and that inhibition of Akt does not block ERK2-induced cell plasticity.
Fig. 5.
FoxO1 up-regulation by ERK2 is mediated by JNK. (A) MCF10A cells stably expressing vector control or D319N ERK2 were grown for 7 d. Cells were lysed, and immunoblot analysis was performed. (B) MCF10A cells expressing vector control or D319N ERK2 were grown for 7 d in the presence or absence of Akt inhibitor (MK2206, 5 µM), and cell images were taken. (Scale bar: 500 µm.) (C and D) Migration (C) or invasion (D) assay was performed using MCF10A cells expressing vector control or D319N ERK2 in the presence or absence of Akt inhibitor (MK2206, 5 µM). Data are the means ± SD of three separate experiments. Results were statistically significant (*P < 0.01) as assessed by t test. (E and F) MCF10A cells expressing vector control or D319N ERK2 were treated with or without inhibitors of Akt (MK2206), JNK (SP600125), p38 (SB203580), or LRRK2 (HG-10-102-01), and immunoblot analysis was performed. (G and H) MCF10A cells expressing vector control or D319N ERK2 were grown for 7 d in the presence or absence of JNK inhibitors (JNK-IN-8 or SP600125). Cell images were taken (G), or immunoblot analysis was performed (H). (Scale bar: 500 µm.) (I–K) MCF10A cells were treated with TGF-β (5 ng/mL) every other day for 9 d with or without JNK inhibitor. Immunoblot analysis was performed (I and K), or cell images were taken (J). (Scale bar: 500 µm.) (L and M) Migration (L) or invasion (M) assay was performed using MCF10A cells expressing vector control or D319N ERK2 in the presence or absence of JNK inhibitors (JNK-IN-8 or SP600125). Data are the means ± SD of three separate experiments. Results were statistically significant (*P < 0.01) as assessed by t test.
In contrast to our observation that ERK2 suppresses cell proliferation through up-regulation of FoxO1 levels (Fig. 3 E and F), a previous report showed that ERK could directly phosphorylate FoxO3, which facilitates its degradation and promotes cell growth/proliferation (31). Because direct ERK phosphorylation sites on FoxO3 are not completely conserved in FoxO1, and ERK functions are opposite in our system (inhibition of cell proliferation) compared with the system used by Yang et al. (31) (promotion of cell proliferation), we suspected that ERK2 exerted positive regulatory functions over FoxO1 during EMT. Given that FoxO1 mRNA levels did not change whereas protein levels were dramatically induced by ERK2, and since FoxO1 protein levels can be regulated by direct modifiers such as JNK, p38, and LRKK2 (21), we determined if inhibiting these kinases affected ERK2-induced FoxO1 protein levels. Among these candidate kinases, we found that inhibition of JNK dramatically suppressed ERK2-mediated FoxO1 up-regulation (Fig. 5E). Furthermore, we found that ERK2 induced JNK phosphorylation (Fig. 5F and SI Appendix, Fig. S5C) and that JNK inhibition suppressed ERK2-induced EMT as evidenced by cell morphology and EMT markers (Fig. 5 G and H and SI Appendix, Fig. S5 D–F) in MCF10A cells. We observed the involvement of JNK in ERK2-induced EMT in other epithelial cells as well (SI Appendix, Fig. S5 G–I). It has been shown that TGF-β regulates cell motility through JNK (32). We found that TGF-β increased JNK phosphorylation (Fig. 5I) and that JNK inhibition suppressed TGF-β–induced EMT (Fig. 5 J and K). Inhibition of JNK also profoundly suppressed ERK2-induced cell migration (Fig. 5L and SI Appendix, Fig. S5J) and invasion (Fig. 5M and SI Appendix, Fig. S5K). These results suggest that JNK activation by ERK2 and TGF-β is responsible for the increase in FoxO1 levels, EMT induction, and increased cell migration and invasion.
ERK2 Regulates JNK and EMT Through the Rac1 Pathway.
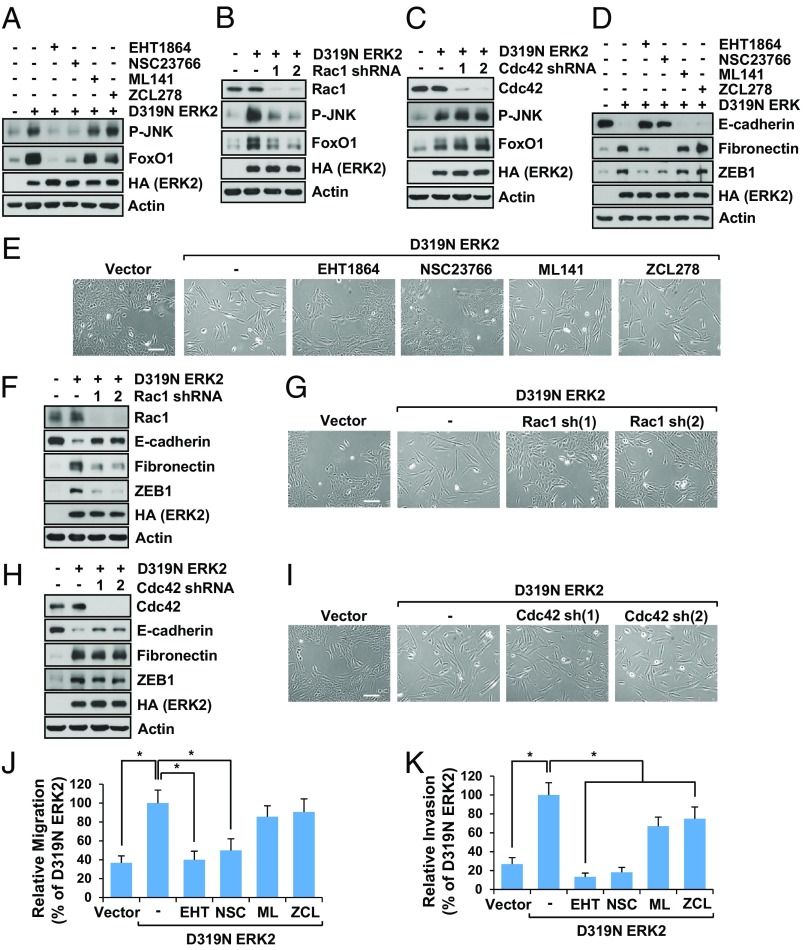
The major activators of JNK are Rac1 and Cdc42 (33). To determine whether ERK2-mediated JNK activation was dependent on Rac1 and/or Cdc42, we first suppressed the activities of Rac1 and Cdc42 with pharmacological inhibitors and examined JNK phosphorylation. Interestingly, inhibition of Rac1, but not Cdc42, blocked ERK2-induced JNK phosphorylation and FoxO1 up-regulation (Fig. 6A and SI Appendix, Fig. S6A). In line with this, knockdown of Rac1 (Fig. 6B), but not Cdc42 (Fig. 6C), prevented JNK phosphorylation and up-regulation of FoxO1 induced by ERK2. Intrigued by these findings, we examined if Rac1 inhibition suppressed ERK2-induced EMT. As shown in Fig. 6 D and E and SI Appendix, Fig. S6 B–D, inhibition of Rac1, but not of Cdc42, suppressed ERK2-mediated EMT as evidenced by EMT markers including Zeb1 expression (Fig. 6D and SI Appendix, Fig. S6B) and cell morphology (Fig. 6E and SI Appendix, Fig. S6 C and D). Rac1 inhibition also blocked ERK2-induced EMT in NMuMG cells (SI Appendix, Fig. S6 E and F). This finding was also confirmed by knockdown of Rac1 (Fig. 6 F and G) and Cdc42 (Fig. 6 H and I). As expected, inhibition of Rac1 dramatically reduced cell migration (Fig. 6J and SI Appendix, Fig. S6G) and invasion (Fig. 6K and SI Appendix, Fig. S6H) induced by ERK2. Cell migration induced by ERK2 was not affected by Cdc42 inhibition (Fig. 6J and SI Appendix, Fig. S6G). However, Cdc42 inhibition did suppress ERK2-induced cell invasion, although it was not as dramatic as Rac1 inhibition (Fig. 6K and SI Appendix, Fig. S6H). These results suggest that Rac1, but not Cdc42, is required for ERK2-induced JNK phosphorylation, FoxO1 up-regulation, and EMT.
Fig. 6.
Rac1 is involved in ERK2-induced EMT and JNK activation. (A) MCF10A cells stably expressing vector control or ERK2 were grown for 7 d in the presence or absence of Rac inhibitor (EHT1864 or NSC23766) or Cdc42 inhibitor (ML141 or ZCL278). Immunoblot analysis was performed. (B and C) MCF10A cells stably expressing vector control or D319N ERK2 were grown for 7 d with control, Rac1 shRNAs (B), or Cdc42 shRNA (C). Immunoblot analysis was performed. (D and E) MCF10A cells stably expressing vector control or ERK2 were grown for 7 d in the presence or absence of Rac inhibitor (EHT1864 or NSC23766) or Cdc42 inhibitor (ML141 or ZCL278). (D) Immunoblot analysis was performed, or (E) cell images were taken. (Scale bar: 500 µm.) (F–I) MCF10A cells stably expressing vector control or D319N ERK2 were grown for 7 d with control, Rac1 shRNAs (F and G), or Cdc42 shRNA (H and I). Immunoblot analysis was performed (F and H), or cell images were taken (G and I). (Scale bar: 500 µm.) (J and K) Migration (J) or invasion (K) assay was performed using MCF10A cells stably expressing vector control or ERK2 in the presence or absence of Rac inhibitor (EHT1864 or NSC23766) or Cdc42 inhibitor (ML141 or ZCL278). Data are the means ± SD of three separate experiments. Results were statistically significant (*P < 0.01) as assessed by t test.
Dock10 Regulates ERK2-Dependent Rac1 Activation.
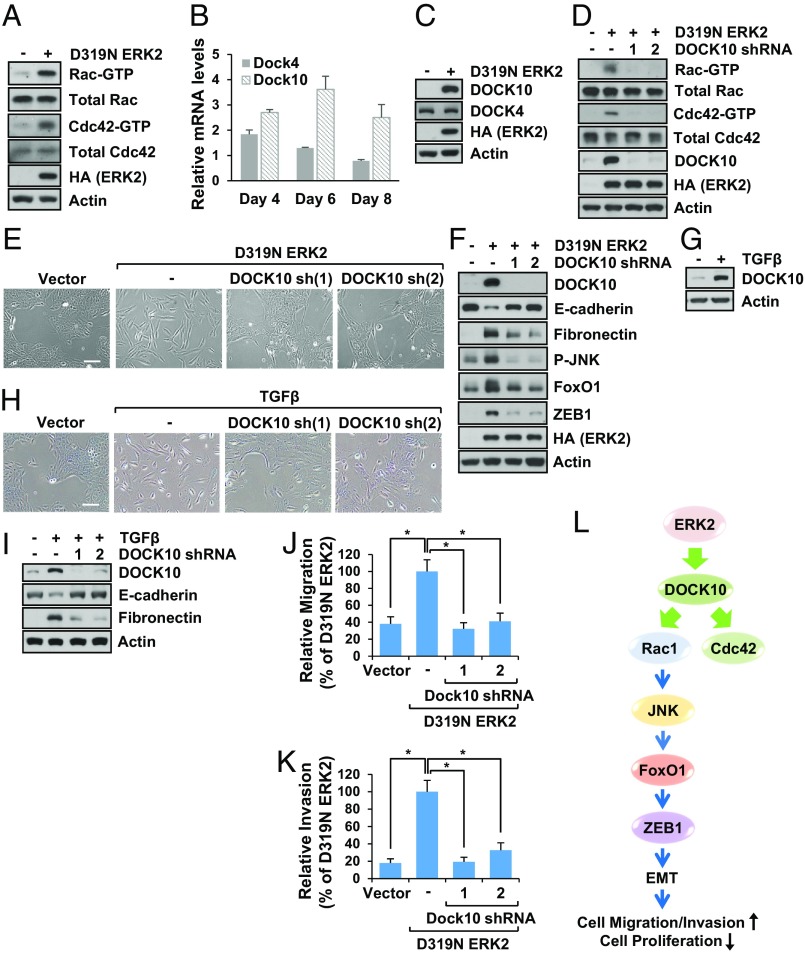
We next asked how Rac1 activity was regulated by ERK2. As Rho family GTPases, Rac1 and Cdc42 activities are regulated positively or negatively by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), respectively. Thus, we utilized Rac1 and Cdc42 activity assays and determined that ERK2 activated both Rac1 and Cdc42 (Fig. 7A and SI Appendix, Fig. S7A). Since ERK2 activated Rac and Cdc42, we investigated the molecular basis for this. Many Rho family GEFs and GAPs have been identified, which suggests that particular cellular processes induced by Rho-family GTPases are controlled by specific regulators under different conditions (34, 35). Analysis of our microarray data indicated that Dock4 and Dock10, GEFs for Rac and Cdc42, were increased by ERK2 (SI Appendix, Fig. S7B). However, validation by qPCR indicated that mRNA levels of Dock10, but not Dock4, were increased by ERK2 (Fig. 7B). Thus, we examined protein levels of Dock4 and Dock10. As shown in Fig. 7C and SI Appendix, Fig. S7C, Dock10, but not Dock4, levels were increased by ERK2. This was also confirmed in NMuMG cells (SI Appendix, Fig. S7D). To investigate whether increased Dock10 by ERK2 was responsible for Rac1 and Cdc42 activation, we knocked down Dock10 in ERK2-expressing cells and performed Rac1 and Cdc42 activity assays. As shown in Fig. 7D, Dock10 knockdown inhibited Rac1 and Cdc42 activation. To determine if ERK2-induced EMT is through increased Dock10 expression, we knocked down Dock10 and examined cell morphology and EMT markers. As shown in Fig. 7 E and F and SI Appendix, Fig. S7E, knockdown of Dock10 inhibited changes of morphology and EMT markers induced by ERK2. In addition, Dock10 knockdown inhibited JNK phosphorylation and up-regulation FoxO1 and Zeb1 levels by ERK2 (Fig. 7F). We then wondered whether Dock10 was also important for TGF-β–induced EMT. As expected, TGF-β increased Dock10 protein levels (Fig. 7G), and knockdown of Dock10 inhibited TGF-β–induced EMT (Fig. 7 H and I). We also determined the effects of Dock10 on cell migration and invasion. As shown in Fig. 7 J and K and SI Appendix, Fig. S7 F and G, Dock10 knockdown profoundly inhibited cell migration and invasion induced by ERK2. Taken together, these data suggest that ERK2 activates Rac1 and Cdc42 through Dock10 up-regulation (Fig. 7L), that the Dock10/Rac1/JNK signaling axis is the main mediator of ERK2-induced FoxO1 up-regulation, and that FoxO1 is a major contributor to EMT (Fig. 7L).
Fig. 7.
ERK2-induced Dock10 expression activates Rac1-mediated EMT. (A) Rac1 and Cdc42 activity assays were performed using vector control- or ERK2-expressing MCF10A cells that were grown for 7 d. (B) MCF10A cells stably expressing vector control or D319N ERK2 were grown for the indicated time, and RT-qPCR was performed. Fold changes are relative to control samples and normalized by changes in actin value. Data are the means ± SD of three separate experiments. (C) MCF10A cells stably expressing vector control or D319N ERK2 were grown for 7 d, and immunoblot analysis was performed. (D–F) MCF10A cells stably expressing control or D319N ERK2 were grown for 7 d in the presence or absence of Dock10 shRNAs. (D) Rac1 or Cdc42 activity assay was performed. Cell images were taken (E), or immunoblot analysis was performed (F). (Scale bar: 500 µm.) (G–I) MCF10A cells were treated with TGF-β (5 ng/mL) every other day for 9 d in the presence or absence of Dock10 shRNAs. Immunoblot analysis was performed (G and I), or cell images were taken (H). (Scale bar: 500 µm.) (J and K) Migration (J) or invasion (K) assay was performed using MCF10A cells expressing vector control or D319N ERK2 in the presence or absence of Dock10 shRNAs. Data are the means ± SD of three separate experiments. Results were statistically significant (*P < 0.01) as assessed by t test. (L) Schematic diagram showing mechanisms by which ERK2 regulates EMT, cell migration/invasion, and proliferation.
Discussion
Our findings reveal links in ERK2-driven cancer progression whereby ERK2 utilizes a Dock10/FoxO1 signaling axis to promote EMT, cell migration, and cell invasion at the expense of cell proliferation. Considering the physiological and clinical importance of ERK signaling and EMT in development, tissue repair, and progression of diseases such as cancer, our studies not only uncover previously undescribed connections between ERK2 and EMT but also identify additional potential therapeutic options for the treatment of aggressive cancers.
Our findings suggest that FoxO1, a well-known tumor suppressor due to its roles as an inhibitor of tumor growth/motility and an inducer of tumor death, has a previously unappreciated function of promoting EMT and cell migration/invasion in breast epithelial cells when it is regulated by sustained active ERK2. Tumor development and progression are multistep processes that are driven by gain-of-function of tumor promoters (oncogenes) and loss-of-function of tumor suppressors. Based on this paradigm, the current basis of cancer therapeutics is to inhibit tumor promoters and/or activate tumor suppressors, although most current targeted cancer therapies rely on targeting tumor promoters due to druggable properties of many oncogenes. Because FoxOs are tumor suppressors, their activation has been regarded as one of the promising strategies in cancer therapeutics (20). However, several cancer regulators do not fit into one of these two simplified categories and can indeed function as both tumor promoters and tumor suppressors depending on various conditions and cellular context (36–39). In these cases, targeting these molecules may not be an effective cancer therapy without a greater understanding of how they work. In contrast to the current general view that FoxOs function as tumor suppressors, our studies reveal that in our system ERK2-activated FoxO1 increases migratory and invasive potential (tumor promotion) by inducing EMT, while also inhibiting tumor proliferation (tumor suppression), suggesting dual functions for FoxO1. Thus, as shown, suppression of FoxO1 with its inhibitor or upon RNAi-mediated knockdown dramatically decreases migration/invasion of cells when ERK2 activity is sustained while increasing cell proliferation and inducing a mesenchymal-to-epithelium–like transition (MET), the reversal of EMT. Supporting our results, recent evidence shows the positive functions of FoxO1 in cancer cell migration/invasion and metastasis in specific cancers/conditions (21–24). FoxO1 function can also confer resistance to stress and certain drugs (40). Thus, understanding when, depending on cellular context or cancer stage, it is beneficial to target FoxO1 will be critical for therapeutic efficacy.
When highly expressed and/or highly active, the major EMT contributors such as TGF-β and EMT transcription factors (i.e., Snail, Zeb1) increase cell migration/invasion at the expense of cell proliferation (41). ERKs have been known as key positive regulators of cell proliferation by promoting cell cycle progression and mRNA translation. Therefore, ERKs have been one of the top candidates for anticancer therapies. Indeed, pharmacological inhibitors of Raf/MEK, direct upstream regulators of ERKs, and direct ERK inhibitors are used in clinics or clinical trials to treat cancer patients. However, depending on the duration and magnitude of ERK activation, ERK can promote very different cell fates (18). In some systems, it is known that a sustained strong ERK activation, which is observed in many cancer types because of hyperactive Ras and/or Raf, actually suppresses cell cycle progression, while a sustained mild activation of ERK or highly active ERK for short durations induces cell cycle progression and proliferation (19). In our model systems, we have found that inhibition of ERK in epithelial cells reduces cell proliferation without changing cell plasticity, indicating that ERK activity in these cells is necessary for cell proliferation. However, we show here that a sustained ERK2 activation in epithelial cells inhibits cell proliferation through up-regulation of FoxO1 while promoting conversion to mesenchymal cells with increased cell migration/invasion. All these properties of ERK in mesenchymal cells may underlie the reported resistance of metastatic cancer cells to chemotherapeutics (42). Our findings also have important implications regarding the dose of inhibitors used. For example, when ERK2-driven mesenchymal cells in our systems were treated with pharmacological ERK inhibitors in a concentration-dependent manner, we observed that mild inhibition of ERK induced MET transition, thereby rendering cells less migratory and invasive. This treatment converted less proliferative mesenchymal-like cells to more proliferative epithelium-like cells. However, when ERK activity was potently blocked in the mesenchymal cells, a MET-like conversion was induced, and cell migration/invasion and proliferation were profoundly inhibited as this treatment blocked even minimal ERK activity required for epithelial cell proliferation. These observations underscore the difficulties associated with targeted therapy in this case as finding the dose most effective at blocking migration/invasion, and proliferation may also promote general patient toxicities whereas intermediate doses may actually promote MET and stimulate reinitiation of cell proliferation of quiescent micrometastases at distant sites.
Rho GTPases such as Rac1 and Cdc42 are regulated by two classes of exchange factors: classical Dbl-related factors and atypical Dock family factors. Due to their relatively recent discovery, little is known about the functions and regulation of Dock family exchange factors (43). Among several Dock family factors, Dock10 was recently identified as an essential factor for cancer invasion and metastasis, and its expression is correlated with poor cancer patient outcome (44). As a GEF for Rac1 and Cdc42, Dock10’s functions are mediated by Rac1 and/or Cdc42. Indeed, we have found that ERK2 activates Rac1 and Cdc42 through Dock10. However, only Rac1, but not Cdc42, is necessary for ERK2-induced JNK/FoxO1 signaling and EMT. Although our data suggest that Cdc42 is not a major contributor to ERK2-induced EMT, we did observe that the inhibition of Cdc42 partially decreased cell invasion. This suggests that the ERK2/Dock10 axis may use both EMT-dependent and -independent mechanisms for regulation of cell invasion. Considering Dock10’s critical role in cancer cell invasion, metastasis, and poor prognosis (44), it will be important to investigate regulatory mechanisms and functions of Dock10 in the context of cancers having a highly active Ras/Raf/MEK/ERK signaling axis.
Based on our finding that ERK2 up-regulates Dock10 at the mRNA level, it is likely that ERK2 exerts its regulation of Dock10 through the regulation of transcription factors. However, ERK2 may also directly regulate Dock10 levels via phosphorylation of Dock10. ERK phosphorylates serine or threonine residues followed by a proline such as PX(S*/T*)P or simply (S*/T*)P. Analysis of posttranslational modifications of Dock10 using databases such as PhosphositePlus (Cell Signaling Technology) shows that serine (S292 and S877) or threonine (T471 and T1125) are phosphorylated and reside within this motif. In addition to the proline-based phosphorylation motif, ERK substrates typically have ERK-binding sites such as D domain or DEF motif. Analysis of amino acid sequence reveals several potential ERK DEF motifs (F-X-F) in Dock10. Thus, it is possible that Dock10 could be a direct substrate of ERK and could regulate its stability and/or activity. It will be interesting to investigate which transcription factors mediate ERK2’s function to induce Dock10 expression and to test if direct phosphorylation of Dock10 by ERK2 may also be important for Rac1-mediated cell migration/invasion.
Activation of ERK is closely linked to tumorigenesis, metastasis, anticancer drug resistance, and poor prognosis (9–11, 45–47). Among these, tumor metastasis requires signal reprogramming at various stages of this process including during complete or partial EMT, tumor migration/invasion, survival, MET, and tumor regrowth. Our study identifies an unexplored link between ERK2, Dock10, and FoxO1 as a key signaling axis that incorporates ERK2 signaling into the promotion of cancer progression by inducing EMT and increasing invasiveness and stress resistance. Although EMT and tumor invasion play important roles in tumor metastasis, they do not always correlate with metastatic potential (48–50). However, studies from our laboratory and others suggest the possibility that cancer cells possess the potential to regulate all of the steps of metastasis through the stage-specific fine-tuning of ERK activity, thereby promoting successful metastasis. Considering the active investigations of ERK pathway inhibitors for the treatment of cancer, our studies aimed at understanding ERK-mediated cell plasticity and its associated changes of cell behavior will provide valuable clues for more efficient and less resistant therapies.
Materials and Methods
Cells and Reagents.
MCF-10A and NMuMG mammary epithelial cells were obtained from the American Type Culture Collection and were cultured as previously described (12, 51, 52). The 293TD cells and lentiviral packaging and envelope plasmids were a generous gift from Andrew L. Kung (Dana-Farber Cancer Institute, Boston) and David Baltimore (California Institute of Technology, Pasadena, CA). TGF-β, anti-FoxO1, anti-FoxO3, anti–phospho-Akt, anti-Akt, anti–phospho-JNK, anti-HA tag, anti–phospho-ERK1/2, and anti-ERK1/2 antibodies were purchased from Cell Signaling Technology. Biocoat Matrigel invasion chamber, anti–E-cadherin, and anti-fibronectin antibodies were from BD Biosciences. Anti-Dock4 and anti-Dock10 antibodies were purchased from Bethyl Laboratories. Anti-Zeb1 and anti-HA tag antibodies were from Santa Cruz Biotechnology. Anti-actin antibodies and ML141 were obtained from Sigma. AS1842856, SB203580, ZCL278, and HG-10–102-01 were from EMD Millipore. Rac1 and Cdc42 activation assay kits were obtained from Cytoskeleton. JNK-IN-8, EHT1864, NSC23766, MK-2206, U0126, and SP600125 were purchased from Selleckchem.
Microarray.
Vector control, D319N ERK2, and Y261A ERK2 expression was induced by doxycycline in Tet-on MCF10A cell systems. Cells were grown for 7 d, and RNAs were purified from three individual replicates of each condition using an RNA isolation kit (Qiagen). Partners Healthcare Personalized Medicine performed a microarray using GeneChip Human Transcriptome Array 2.0 (Affymetrix). Data were analyzed using Expression Console (Applied Biosystems) and Transcriptome Analysis Console (Applied Biosystems). The microarray data were deposited in the Gene Expression Omnibus (GEO) database with accession no. GSE124947 (53).
Supplementary Material
Acknowledgments
We thank Drs. Andrew L. Kung and David Baltimore for generously providing reagents. This work was supported by a start-up fund from University of Illinois College of Medicine (to S.-O.Y.); a William E. McElroy Research grant (to S.-O.Y.); NIH Grants GM51405, HL121266, and CA46595 (to J.B.); and Canadian Institutes for Health Research Grants PJT-152995 and MOP-142374 (to P.P.R.). P.P.R. is a senior scholar of the Fonds de la Recherche du Québec-Santé.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE124947).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811923116/-/DCSupplemental.
References
- 1.Nieto MA. Epithelial plasticity: A common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 2.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Z, et al. In vivo visualization and characterization of epithelial-mesenchymal transition in breast tumors. Cancer Res. 2016;76:2094–2104. doi: 10.1158/0008-5472.CAN-15-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caramel J, et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell. 2013;24:466–480. doi: 10.1016/j.ccr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Heerboth S, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartholomeusz C, et al. High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist. 2012;17:766–774. doi: 10.1634/theoncologist.2011-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller H, et al. Potential prognostic value of mitogen-activated protein kinase activity for disease-free survival of primary breast cancer patients. Int J Cancer. 2000;89:384–388. doi: 10.1002/1097-0215(20000720)89:4<384::aid-ijc11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Whyte J, Bergin O, Bianchi A, McNally S, Martin F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 2009;11:209. doi: 10.1186/bcr2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38:114–127. doi: 10.1016/j.molcel.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong X, et al. Cancer drug addiction is relayed by an ERK2-dependent phenotype switch. Nature. 2017;550:270–274. doi: 10.1038/nature24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, et al. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J Clin Invest. 2014;124:2172–2187. doi: 10.1172/JCI71103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radtke S, et al. ERK2 but not ERK1 mediates HGF-induced motility in non-small cell lung carcinoma cell lines. J Cell Sci. 2013;126:2381–2391. doi: 10.1242/jcs.115832. [DOI] [PubMed] [Google Scholar]
- 16.Gu X, et al. Integration of mTOR and estrogen-ERK2 signaling in lymphangioleiomyomatosis pathogenesis. Proc Natl Acad Sci USA. 2013;110:14960–14965. doi: 10.1073/pnas.1309110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam WL, et al. Protein kinase C α is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24:347–364. doi: 10.1016/j.ccr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy LO, Blenis J. MAPK signal specificity: The right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 20.Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Eijkelenboom A, Burgering BM. FOXOs: Signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 22.Tenbaum SP, et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 23.Storz P, Döppler H, Copland JA, Simpson KJ, Toker A. FOXO3a promotes tumor cell invasion through the induction of matrix metalloproteinases. Mol Cell Biol. 2009;29:4906–4917. doi: 10.1128/MCB.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponugoti B, et al. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J Cell Biol. 2013;203:327–343. doi: 10.1083/jcb.201305074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagashima T, et al. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: Improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacol. 2010;78:961–970. doi: 10.1124/mol.110.065714. [DOI] [PubMed] [Google Scholar]
- 26.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 27.Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: The TGFβ and MAPK pathways in cancer progression. Cell Biosci. 2011;1:42. doi: 10.1186/2045-3701-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, et al. Geminin facilitates FoxO3 deacetylation to promote breast cancer cell metastasis. J Clin Invest. 2017;127:2159–2175. doi: 10.1172/JCI90077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Sun Y, Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang JY, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates transforming growth factor beta (TGFbeta)-stimulated fibronectin synthesis through TGFbeta-activated kinase 1 and activation of the JNK pathway. J Biol Chem. 2005;280:25920–25927. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases: Regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 34.Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 35.Fort P, Blangy A. The evolutionary landscape of Dbl-like RhoGEF families: Adapting eukaryotic cells to environmental signals. Genome Biol Evol. 2017;9:1471–1486. doi: 10.1093/gbe/evx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: It’s NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin S, et al. Glycogen synthase kinase-3β positively regulates protein synthesis and cell proliferation through the regulation of translation initiation factor 4E-binding protein 1. Oncogene. 2014;33:1690–1699. doi: 10.1038/onc.2013.113. [DOI] [PubMed] [Google Scholar]
- 38.Perkins ND. NF-kappaB: Tumor promoter or suppressor? Trends Cell Biol. 2004;14:64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Meulmeester E, Ten Dijke P. The dynamic roles of TGF-β in cancer. J Pathol. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- 40.Hornsveld M, Dansen TB, Derksen PW, Burgering BMT. Re-evaluating the role of FOXOs in cancer. Semin Cancer Biol. 2018;50:90–100. doi: 10.1016/j.semcancer.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, et al. 14-3-3ζ turns TGF-β’s function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell. 2015;27:177–192. doi: 10.1016/j.ccell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 43.Gadea G, Blangy A. Dock-family exchange factors in cell migration and disease. Eur J Cell Biol. 2014;93:466–477. doi: 10.1016/j.ejcb.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Westcott JM, et al. An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J Clin Invest. 2015;125:1927–1943. doi: 10.1172/JCI77767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oberst MD, et al. TDAG51 is an ERK signaling target that opposes ERK-mediated HME16C mammary epithelial cell transformation. BMC Cancer. 2008;8:189. doi: 10.1186/1471-2407-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoenerhoff MJ, et al. BMI1 cooperates with H-RAS to induce an aggressive breast cancer phenotype with brain metastases. Oncogene. 2009;28:3022–3032. doi: 10.1038/onc.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 48.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu W, et al. Comparison of three different methods for the detection of circulating tumor cells in mice with lung metastasis. Oncol Rep. 2017;37:3219–3226. doi: 10.3892/or.2017.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 52.Xie L, et al. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin S, Yoon S, Blenis J. 2019 ERK2-dependent gene expression. Gene Expression Omnibus. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE124947. Deposited January 11, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.