Significance
The RNA modification N6-methyladenosine (m6A) was the first physiological substrate of FTO to be discovered. Recently, cap N6,2′-O-dimethyladenosine (m6Am), internal m6Am, and N1-methyladenosine were also found to be physiological substrates of FTO. However, the catalytic mechanism through which FTO demethylates its multiple RNA substrates remains largely mysterious. Here we present the first structure of FTO bound to N6-methyldeoxyadenosine–modified ssDNA. We show that N6-methyladenine is the most favorable nucleobase substrate of FTO and that the sequence and the tertiary structure of RNA can affect the catalytic activity of FTO. Our findings provide a structural basis for understanding FTO’s catalytic mechanism for the demethylation of multiple RNA substrates and shed light on the mechanism through which FTO is involved in diseases or biological processes.
Keywords: RNA modification, RNA demethylase, FTO, enzyme catalysis, structure
Abstract
FTO demethylates internal N6-methyladenosine (m6A) and N6,2′-O-dimethyladenosine (m6Am; at the cap +1 position) in mRNA, m6A and m6Am in snRNA, and N1-methyladenosine (m1A) in tRNA in vivo, and in vitro evidence supports that it can also demethylate N6-methyldeoxyadenosine (6mA), 3-methylthymine (3mT), and 3-methyluracil (m3U). However, it remains unclear how FTO variously recognizes and catalyzes these diverse substrates. Here we demonstrate—in vitro and in vivo—that FTO has extensive demethylation enzymatic activity on both internal m6A and cap m6Am. Considering that 6mA, m6A, and m6Am all share the same nucleobase, we present a crystal structure of human FTO bound to 6mA-modified ssDNA, revealing the molecular basis of the catalytic demethylation of FTO toward multiple RNA substrates. We discovered that (i) N6-methyladenine is the most favorable nucleobase substrate of FTO, (ii) FTO displays the same demethylation activity toward internal m6A and m6Am in the same RNA sequence, suggesting that the substrate specificity of FTO primarily results from the interaction of residues in the catalytic pocket with the nucleobase (rather than the ribose ring), and (iii) the sequence and the tertiary structure of RNA can affect the catalytic activity of FTO. Our findings provide a structural basis for understanding the catalytic mechanism through which FTO demethylates its multiple substrates and pave the way forward for the structure-guided design of selective chemicals for functional studies and potential therapeutic applications.
The FTO gene was originally cloned in a study of a fused-toe mutant mouse and named Fatso (FTO); its function was unknown (1). It was renamed the fat mass and obesity-associated (FTO) gene after genome-wide associated studies linked it with human obesity (2, 3). A human obesity-related function was further substantiated by phenotypes observed in FTO knockout and overexpression mouse models (4, 5). Genetic variants in the FTO gene are also associated with cancers (6, 7), metabolic disorders (8, 9), and neurological diseases (10, 11). These intriguing phenotypes and genetic functions attracted tremendous research interest in the molecular mechanisms and physiological substrate(s) of FTO.
FTO was identified as a homolog of the Fe(II)/α-ketoglutarate acid (α-KG)–dependent AlkB family dioxygenases and was first reported to catalytically demethylate 3-methylthymine (3mT) in ssDNA and 3-methyluracil (m3U) in ssRNA (12, 13). The crystal structure of FTO provided valuable information about the composition and conformation of the enzyme catalytic pocket and activity (14). Later on, FTO was identified as the first RNA demethylase that catalyzes oxidative demethylation of N6-methyladenosine (m6A) on mRNA in vitro and in vivo (15, 16). This discovery stimulated extensive worldwide research efforts in recent years into dynamic m6A and other RNA modifications in biological regulation (17–25). FTO-mediated m6A demethylation has been found to regulate many biological processes, including preadipocyte differentiation (22), heat shock stress-induced cap-independent translation (23), UV-induced DNA damage (24), and acute myeloid leukemia (25). N6,2′-O-dimethyladenosine (m6Am)—a distinct form of m6A with a 2′-O-methylation at the ribose ring—is a substrate of FTO in vitro (26). It has long been known that m6Am marks exist predominantly at the +1 position following the N7-methylguanosine (m7G) cap at the 5′ terminus of mRNA molecules (henceforth termed cap m6Am). The m6A distribution along mRNA, as mapped by N6-methyladenosine sequencing, found a distinct peak immediately following the transcription start site (27), which in fact represents cap-associated m6Am, considering that the m6A antibody recognizes both m6A and m6Am. m6A individual-nucleotide-resolution cross-linking and immunoprecipitation identified certain mRNAs containing cap m6Am (28). Cap m6Am marks occur much less frequently than internal m6A marks; for instance, mRNA from H1-ESC cells had 33-fold more internal m6A than cap m6Am (29).
In 2017, Mauer et al. (30) proposed that FTO mediates cap m6Am demethylation and shows almost no demethylation activity on internal m6A in cells, a conclusion that diametrically opposes numerous previous findings (15, 21–25). A recent finding characterized the cap m6Am writer (CAPAM) and reported that CAPAM knockout cells grow well and show a similar growth rate than wild-type cells (31), which is different from the phenotypic features of FTO knockdown cells (22, 25) and indicates the cap m6Am is not the major substrate of FTO for its phenotypes and genetic functions. Recently published results systematically identified the in vivo substrates of FTO, including m6A and cap m6Am in mRNA, m6A and m6Am in snRNA, and m1A in tRNA, and thereby revealed that the subcellular localization of FTO affects its ability to perform different RNA modifications (32). However, the molecular mechanism for the enzymatic demethylation of FTO toward multiple RNA substrates remains unclear.
In this study, our in vitro and in vivo biochemical results conclusively establish that FTO demethylates both internal m6A and cap m6Am marks in mRNA. Given the considerable challenges of crystallizing FTO in a complex with nucleic acids, we rationally designed double mutations outside of FTO’s catalytic pocket and thus successfully obtained the structure of human FTO bound to N6-methyldeoxyadenosine–modified ssDNA (FTO-6mA). We investigated the recognition modes of multiple RNA substrates in FTO’s catalytic pocket and investigated which nucleobase is the most energetically favorable for binding with FTO; 6mA, m6A, and m6Am share the same recognition mode in FTO’s catalytic pocket, except for structural differences of the ribose ring. We explored whether the structural differences of the ribose ring may affect the demethylation activities of FTO when internal m6A and m6Am are positioned in the same RNA sequence and further investigated how FTO binds RNA and tested whether the sequence and the structure of RNA affect FTO’s activity. Our results demonstrate that N6-methyladenine is the favored nucleobase for FTO and find that FTO exhibits the same demethylation activity toward internal m6A and m6Am positioned in the same RNA sequence. Our work also shows that the sequence and the tertiary structure of RNA affect the demethylation activity of FTO.
Results
FTO Mediates Extensive Demethylation of Internal m6A and Cap m6Am in Vitro and in Vivo.
Mauer et al. (30) proposed that cap m6Am and not m6A is the cellular physiological substrate of FTO, which diametrically opposes most previous findings (15, 21–25). Seeking to resolve this apparent discrepancy and to further characterize the physiological substrate of FTO, we investigated the demethylation functions of FTO with biologically relevant substrates in vitro and in vivo. Here we used an mRNA digestion procedure which allowed us to simultaneously detect both internal m6A and cap m6Am marks and to measure the ratios of m6A to A (m6A/A) and m6Am to A (m6Am/A) using quantitative ultraperformance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS).
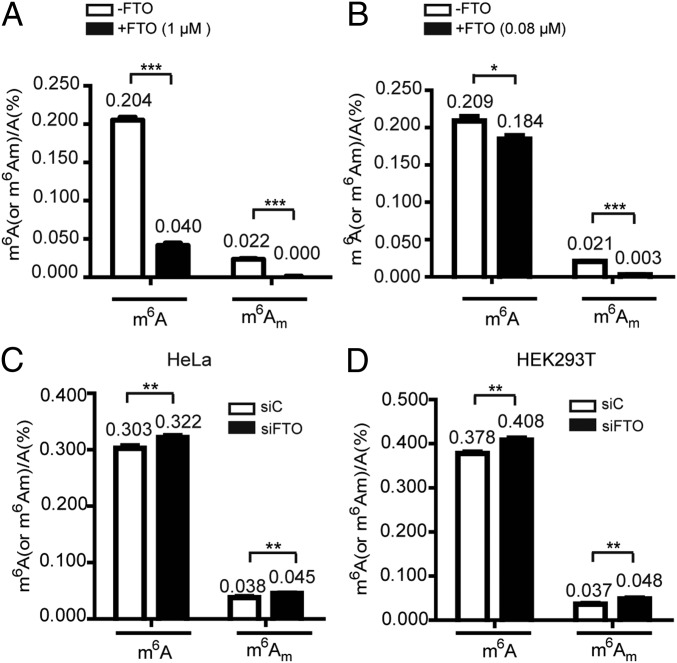
We first performed in vitro demethylation assays with recombinant FTO (SI Appendix, Fig. S1) and mRNA isolated from HeLa cells. This analysis showed that the total amount of m6A was ∼10-fold larger than the amount of cap m6Am in HeLa mRNAs, and FTO (1 μM in 50 μL) demethylated nearly all of the cap m6Am (>99%) and 80% of the internal m6A in 400 ng of mRNA (Fig. 1A and SI Appendix, Fig. S2A). We lowered the FTO concentration to achieve incomplete demethylation of cap m6Am to estimate the in vitro catalytic efficiency of FTO for m6A and for cap m6Am. We observed that 0.08 μM of FTO (50 μL) demethylated 86% of cap m6Am and 12% of internal m6A in 400 ng of isolated mRNA (Fig. 1B and SI Appendix, Fig. S2B). Note that the total amount of m6A was ∼10-fold larger than the amount of cap m6Am in mRNAs; the absolute number of m6A bases (0.245 per 1,000 A bases) reversed by FTO (0.08 μM in 50 μL) is ∼1.3-fold more than that of m6Am (0.178 per 1,000 A bases) (SI Appendix, Fig. S3A). We next examined the in vivo demethylation performance of FTO by performing siRNA knockdown assays in HeLa and HEK293T cells (SI Appendix, Fig. S4). Upon FTO knockdown in HeLa cells, FTO demethylates 0.185 m6A and 0.071 cap m6Am molecules per 1,000 A bases (Fig. 1C and SI Appendix, Fig. S3B). Consistently, a similar result was also observed in HEK293T cells (Fig. 1D and SI Appendix, Fig. S3C). Collectively, these results confirm that FTO can demethylate both internal m6A and cap m6Am in vitro and in vivo. During the revision of this paper, Wei et al. (32) reported that FTO can demethylate both m6A and cap m6Am in vitro and in cells, which is consistent with our results.
Fig. 1.
FTO demethylates both internal m6A and cap m6Am in vitro and in vivo. (A and B) UPLC-MS/MS quantification of internal m6A/A and cap m6Am/A ratios in mRNA treated with FTO protein in vitro. Here 400 ng of purified mRNA from HeLa cells were treated with 1 μM of FTO (A) or 0.08 μM of FTO (B) under standard demethylation conditions in 50 μL of reaction mixture for 1 h at 37 °C. (C and D) UPLC-MS/MS quantification of internal m6A/A and cap m6Am/A ratios in mRNA isolated from HeLa (C) and HEK293T (D) cells with or without FTO knockdown. Error bars indicate the mean ± SEM (n = 6, three biological replicates × two technical replicates), determined using an unpaired Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Rational Design of FTO Mutations Facilities Crystallization of FTO–Oligonucleotide Complex.
To elucidate how FTO recognizes and demethylates its physiological substrates, we decided to crystallize an FTO–oligonucleotide complex. However, we had a hard time obtaining crystals of an FTO–ssRNA complex for X-ray diffraction. This was not surprising, as crystallization of the AlkB family protein–nucleic acid complexes is known to be challenging due to the weak binding of these proteins with nucleic acids (33). Two strategies have been successfully used to overcome the difficulty: chemical bisulfide cross-linking and active-site mutation (34, 35). Here we chose to engineer FTO with site-directed mutagenesis to increase the binding ability of FTO to nucleic acids. The enzymatic activity of AlkB family proteins mainly depends on the recognition of a methylated nucleobase in the catalytic pocket (34). Considering that 6mA, m6A, and m6Am share the same nucleobase, we crystallized the complex of FTO bound to 6mA-modified ssDNA to characterize FTO’s catalytic mechanism for the demethylation of multiple RNA substrates.
We generated FTO variants with site-directed mutations; these were subsequently searched for variants that (i) exhibit increased binding affinity for 6mA-modified oligo but (ii) do not alter the enzyme’s demethylation activity. Superimposition of the apo FTO structure with a structure of an AlkB-1mA (N1-methyladenine) modified ssDNA complex led us to select five amino acids—inside and outside of the FTO catalytic pocket—for rational mutation (E234A, R96A, Y106F, Q86K, and Q306K) (SI Appendix, Fig. S5A). We then separately expressed and purified wild-type FTO (termed as FTOWT) and these FTO mutants from E. coli (SI Appendix, Fig. S1) and determined their binding affinities (equilibrium binding constants) with fluorescein-labeled 6mA-modified ssDNA using fluorescence anisotropy measurements (36). The Q86K and Q306K mutations increased the binding affinity of FTO to ssDNA by, respectively, ∼1.5-fold and ∼10-fold, while R96A and Y106F both decreased binding affinity by approximately twofold; the E234A mutation did not significantly affect binding affinity (SI Appendix, Figs. S5B and S6). We then generated a Q86K/Q306K double-mutation FTO variant (termed as FTOQ86K/Q306K) and found this variant had an ∼16-fold increase in binding affinity over FTOWT (Kd = 0.23 μM). We further confirmed that FTOQ86K/Q306K does not obviously alter the m6A demethylation activity (SI Appendix, Fig. S5C), which makes sense given that the Q86K/Q306K mutations are in the oligonucleotide binding motif of FTO, not in its catalytic pocket (SI Appendix, Fig. S5A).
The Structure of FTO Bound to 6mA-Modified ssDNA Reveals a Specific Substrate Binding and Catalytic Mechanism.
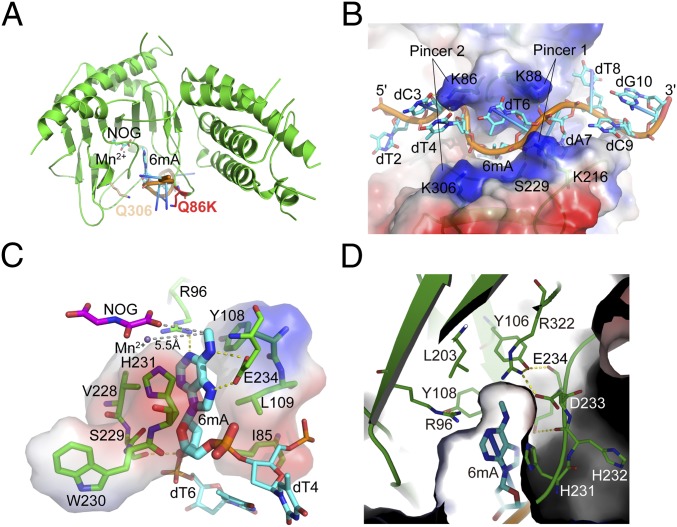
Our strategy of increasing the FTO substrate binding affinity facilitated the crystallization of FTOQ86K/Q306K bound to 6mA-modified 10-mer ssDNA (Fig. 2A). Needlelike crystals appeared within 1 wk. However, the diffraction of these crystals showed an obvious anisotropy property with two directions (b and c) diffracting to 3.0 and 3.1 Å but the other direction (a) diffracting to only 3.7 Å (SI Appendix, Fig. S7). We finally scaled the overall resolution to 3.3 Å, optimized the data, and solved the structure by molecular replacement using the published apo FTO structure [Protein Data Bank (PDB) ID code 3LFM] (14) (SI Appendix, Supplementary Text and Table S1). Notably, we found that most of the nucleotides (except the first one at the 5′ terminus) in the structure, especially 6mA, are well fitted into the electron density, although the resolution is low (SI Appendix, Fig. S8).
Fig. 2.
Crystal structure of FTO bound to 6mA-modified ssDNA. (A) The Q86K and Q306K double-mutation sites of FTO in the structure. (B) Overall structure of FTO-6mA. The electrostatic surface of FTO and sticks of ssDNA were generated by PyMOL. The color range from red (negative) to blue (positive) represents the surface electrostatic potentials of −73.5 to +73.5 e/kT. ssDNA is colored in cyan. (C) Detailed interactions in the catalytic pocket of FTO to accommodate 6mA. The gray dashes represent the distance between the N6-methyl group with NOG and Mn2+. The electrostatic surface of the residues involved in hydrophobic interactions with 6mA is shown. (D) The hydrophobic cave around the N6-methyl group of 6mA. Residues involved in the interactions are shown and labeled.
The asymmetric crystallographic unit contains four FTO–ssDNA complexes, in which every two FTO molecules stack two ssDNA strands under the same 5′ to 3′ direction (SI Appendix, Fig. S9). Within the complex, two pairs of positively charged residues from two critical loops near the oligonucleotides binding area of FTO contribute most of the hydrophilic interactions with the oligonucleotide. They hold the oligonucleotide like two pairs of pincers and bend it into an M shape (Fig. 2B). The first pincer (pincer 1) consists of two lysine residues: K88 and K216. K88 is located on a short loop (residues 86–88) between β2 and β3, while K216 is within a long loop (residues 210–223, henceforth called the FTO unique loop) between β7 and β8; FTO is the only human AlkB family member that contains this type of loop (SI Appendix, Fig. S10). K88 and K216 stabilize the ssDNA through hydrogen bonds (H bonds) between their side chains and the phosphates of, respectively, A7 and T6 of the ssDNA molecule; these bonds effectively twist the strand ∼45° as a result of steric hindrances with the side chains.
The second pincer (pincer 2) consists of two mutated lysine residues: K86 and K306 (glutamines in FTOWT) (Fig. 2B). K86 (Q86 in FTOWT) is located on the short loop between β2 and β3 next to K88, and its side chain forms strong hydrophilic interactions with the O2 atom of the pyrimidine rings of C3 and T4 in the ssDNA; given this interaction, it is likely that residues at this position contribute strongly to substrate sequence recognition and stabilization. In contrast, K306 (Q306 in FTOWT) is located on β13 and has a hydrophilic interaction with the phosphate backbone of 6mA. These side chain–base interactions significantly increase the binding affinity of FTOQ86K/Q306K to ssDNA compared with FTOWT, which is consistent with the observations from the fluorescence anisotropy measurements (SI Appendix, Figs. S5B and S6). Moreover, whereas the nucleic acid binding tunnel of pincer 1 is narrow, the distance between the two residues (K86 and K306) of pincer 2 is significant longer (11.2 Å), generating a flat and large space next to pincer 2 that potentially accommodates tertiary structured RNAs like stem loops as substrates (SI Appendix, Fig. S11A). Additionally, the 5′ and 3′ ends of the 10-mer ssDNA have few interactions with FTO (SI Appendix, Supplementary Text and Fig. S12).
Inside the catalytic pocket, the purine ring of 6mA is stacked between Y108, L109, V228, and H231, and the deoxyribose ring is stacked between I85, V228, S229, W230, and H231 through hydrophobic interactions (Fig. 2C). The N1 atom on the 6mA purine ring interacts with R96 via a H bond, while the N6 and N7 atoms form H bonds with E234, thereby locking the base in place. Note that the N6-methyl group is stabilized in a hydrophobic pocket formed by the side chains of R96, Y106, Y108, L203, and R322 (Fig. 2D) and is orientated to Fe(II) and α-KG for oxidation (Fig. 2C). These residues form a stable H bond network with each other, making the pocket stable and robust. These structural insights help explain the aforementioned biochemical results that the R96A and Y106F mutations significantly reduced the binding affinity: each mutation would disrupt the H bond network and reduce the stability of the hydrophobic pocket used for holding the N6-methyl group of 6mA (SI Appendix, Figs. S5B and S6).
Structural-based sequence alignment among AlkB family members shows that most of the residues involved in hydrophobic interactions with substrate bases are conserved (such as Y108, L109, and H231 in FTO), suggesting that these residues strongly contribute to base stabilization across the entire protein family (Fig. 2 C and D and SI Appendix, Fig. S10). However, given the extensive variation among the family members of the residues involved in hydrophilic interactions with substrate bases, it seems clear that the R96 and E234 residues of FTO are responsible for specific substrate base recognition (SI Appendix, Fig. S10). In addition, cofactors Mn2+ [which occupies the Fe(II)-binding site but does not support catalysis] and α-KG analog N-oxalylglycine (NOG) molecules are stabilized in the FTO catalytic pocket predominantly through H bonds (N205, D233, Y295, H307, R316, S318, and R322) and coordinate bonds (H231, D233, and H307) (SI Appendix, Figs. S10 and S11B), indicating a highly conserved catalytic mechanism among the AlkB family members.
FTO Exhibits a Preference for the Nucleobase N6-Methyladenine over Its Other Reported Substrates.
In vivo and in vitro evidence has established that FTO demethylates multiple methylated modifications (12, 13, 15, 30, 32), yet it is not known how FTO recognizes multiple substrates in the catalytic pocket or for which substrates FTO exhibits the highest affinity. Thus, we further structurally elucidate the catalytic mechanisms using the computational superimposition strategy.
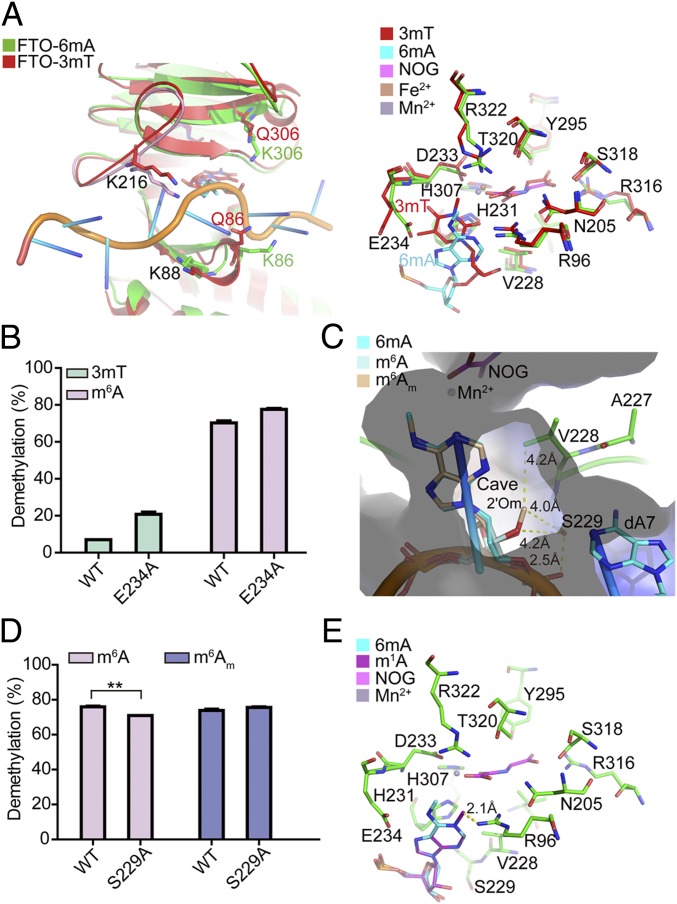
The superposition of the FTO-3mT nucleoside structure (PDB ID code 3LFM) (14) into the FTO-6mA structure indicates high similarity (rmsd = 0.615); most of the key residues inside the catalytic pocket adopt a similar conformation, except E234 (Fig. 3A). The side chain of E234 forms H bonds with the N6 and N7 atoms of the 6mA purine ring for base stabilization; however, the amide nitrogen of E234 in the FTO-3mT complex forms only a weak H bond with the O4 atom of 3mT. Moreover, the side chain of E234 is pushed toward the outside of the catalytic pocket (∼70°) by the 3-methyl group of 3mT during the ∼45° counterclockwise rotation of 3mT, causing an unfavorable and unstable conformation of 3mT in the catalytic pocket, which obviously weakens the catalytic activity of FTO on 3mT (13, 14). As expected, the E234A FTO mutant variant showed a threefold increase in enzymatic activity toward 3mT. In contrast, this variant had only a 10% increase in the m6A demethylation activity (Fig. 3B). Recall that this mutation did not interfere with oligonucleotide substrate binding (SI Appendix, Figs. S5B and S6), suggesting that E234 functions in nucleobase selection and recognition inside the catalytic pocket.
Fig. 3.
Structural basis for substrate preference of FTO in the catalytic pocket. (A) Superposition of FTO-3mT nucleoside structure into the FTO-6mA structure. (B) Enzymatic activity comparison of WT and E234A mutation of FTO in catalyzing 3mT and m6A for 1 h at 37 °C. m6A-modified Oligo2 (10 μM) was incubated with 0.5 μM of WT or E234A mutation of FTO in 50 μL of reaction mixture (pH 7.0), while 3mT-modified Oligo1 (10 μM) was incubated with 10 μM of WT and E234A mutation of FTO in 50 μL of reaction mixture (pH 6.5). (C) Superposition of m6A and m6Am nucleosides into the FTO-6mA structure. The electrostatic surface is shown. (D) Enzymatic activity comparison of 1 μM of WT and S229A mutation of FTO in catalyzing m6A- and m6Am-modified Oligo3 (10 μM) for 15 min at 37 °C. (E) Superposition of the m1A nucleoside into the FTO-6mA structure. Error bars indicate the mean ± SEM (n = 6, three biological replicates × two technical replicates), determined using an unpaired Student’s t test. **P < 0.01.
We next investigated the catalytic mechanism through which FTO recognizes m6A and m6Am in the catalytic pocket by examining the superposition of these two nucleosides into the FTO-6mA structure (Fig. 3C). As three confirmed FTO substrates share the same nucleobase (N6-methyladenine), any difference in recognition could be assumed to result from some influence of differences at the 2′ position of the ribose ring of these substrates (Fig. 3C). The 2′ position of the deoxyribose ring of 6mA points toward a small cave composed of residues V228 and S229 and nucleotide A7, and the side chain of S229 forms a H bond with the oxygen atom on the phosphate of T6, holding the oligonucleotide in place for catalysis (Fig. 2C).
The superposition of the m6A nucleoside into the FTO-6mA structure shows that the additional hydroxyl group (2′OH) of m6A on the 2′ position of the ribose ring further points toward the same cave. Although the distance between 2′OH and the side chain of S229 in the structure is likely too far to enable formation of a H bond (4.2 Å), it is possible that the insertion of the 2′OH induces an ∼15° rotation of the S229 side chain, which could potentiate the formation of a weak H bond for further stabilization of the m6A nucleoside (Fig. 3C and SI Appendix, Fig. S13). In contrast, the methoxy group on the 2′ position of the ribose ring of m6Am could be reasonably expected to insert further into this small cave. Thus, either the spatial configuration or the hydrophobic properties of the cave apparently accommodate and stabilize the 2′-OMe of m6Am; however, m6Am might lose the potential hydrophilic interaction between the 2′OH and S229 due to the methylation of the hydroxyl group, causing lower activity in catalyzing m6Am compared with m6A.
To determine whether the structural difference in the ribose ring of m6A and m6Am affects the enzymatic activity of FTO, we performed demethylation assays with a purified protein (either FTOWT or FTOS229A) and a synthetic 15-mer RNA (Oligo3) containing either m6A or m6Am as the substrate (SI Appendix, Fig. S14). We found that FTOWT has the same demethylation activity for internal m6A and m6Am in the same RNA sequence; the S229A mutation slightly decreases the m6A demethylation activity of FTO (Fig. 3D). These results suggest that the substrate specificity of FTO primarily results from the interaction of residues in the catalytic pocket with the nucleobase N6-methyladenine rather than the ribose ring; further, they support that S229 does likely form a weak H bond with 2′OH of m6A.
In addition to m6A and m6Am, m1A has also been reported as a substrate of FTO (32). The superposition of the m1A nucleoside into the FTO-6mA structure suggests that the methyl group of m1A would undergo significant clashes with R96 (Fig. 3E). Further, m1A would have to rotate to facilitate catalysis, but steric hindrance with E234 on the opposite side of the catalytic pocket would likely interfere with such movement. Superposition of the FTO-6mA structure into the structure of AlkB–dsDNA with 1mA (AlkB-1mA; PDB ID code 3BI3) (34) confirmed this hypothesis. The structure showed that in the catalytic pocket of AlkB, the 1mA base rotates counterclockwise toward K134 (the corresponding residue of E234 in FTO) to avoid clashing with the side chain of M61 (the corresponding residue of R96 in FTO); meanwhile, the side chain of K134 flips ∼45° outward, generating enough space for 1mA base rotation (SI Appendix, Fig. S15). These observations revealed that the purine ring of m1A loses H bonds with both E234 and R96 and the positively charged N1 is averse to the holding N1-methyl group in the hydrophobic cave, explaining FTO’s significantly lower enzymatic activity reported for m1A/1mA compared with m6A or 3mT (12, 32). Collectively, we demonstrated that N6-methyladenine is the most favorable nucleobase substrate of FTO (SI Appendix, Fig. S16).
Consider our findings that FTO displays the same demethylation activity toward internal m6A and m6Am positioned in the same RNA sequence (Fig. 3D) but that FTO has been shown to exhibit a preference for cap m6Am over internal m6A in ssRNA (30). We found, upon superposition of an cap m6Am cap m6A substrate into the FTO-6mA structure, that the m7G cap can be accommodated in the large space next to pincer 2 (as expected) and that the m7G nucleobase is in close contact with residue K86 from pincer 2 (Q86 in FTOWT; SI Appendix, Fig. S17). We further examined whether residue Q86 would bind and recognize m7G, and found that the mutation of Q86A or Q86L in FTO does not affect the cap m6Am demethylation activity in isolated mRNA in vitro (SI Appendix, Fig. S18). Thus, whether FTO provides a special residue to recognize the m7G cap will remain a mystery until the complex structure of FTO bound to cap m6Am-modified RNA is solved. Moreover, the structure suggests that the large space next to pincer 2 can also accommodate other m6A-modified RNAs with tertiary structures like stem loops. Our enzymatic activity assays showed that the sequence and the structure of RNAs indeed affect the demethylation activity of FTO (SI Appendix, Figs. S19 and S20). FTO exhibits twofold higher demethylation activity for m6A positioned in a large stem loop compared with m6A in a linear ssRNA (SI Appendix, Fig. S20).
Comparison of the Complex Structure of FTO Bound to 6mA-Modified ssDNA with Other AlkB Family Proteins.
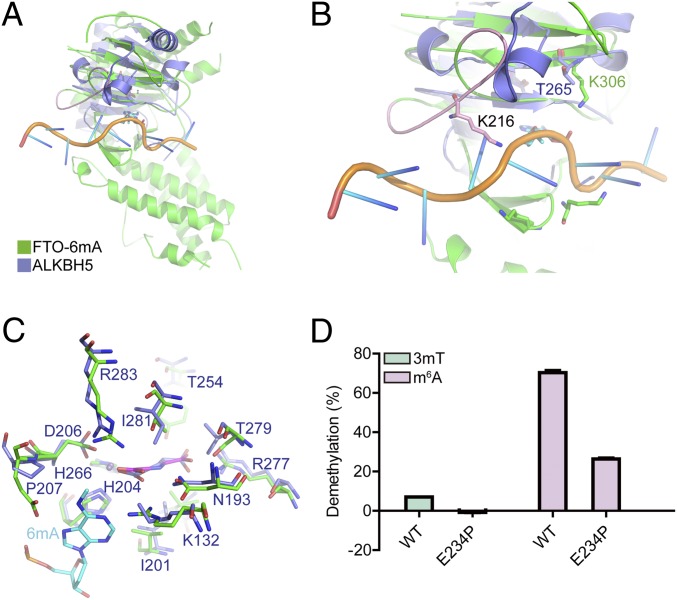
Multiple sequence alignment shows that AlkB family members share highly conserved active residues for catalysis, especially around the α-KG and Fe(II) binding sites (SI Appendix, Fig. S10). However, the structural conformation of the nucleic acid binding motifs varies a lot, for example, the unique loop (residues 210–223) and the short loop between β2 and β3 (residues 85–88) in FTO, where the two pincers are located. Our structure showed that the FTO unique loop is used for recognition of the sequence and structure of the RNA substrate (Figs. 2B and 4A); it interacts with the base of the RNA substrate through the key residue K216 and sterically prevents the binding of dsRNA and dsDNA (SI Appendix, Fig. S21). Notably, other AlkB family members (AlkB and ALKBH2) lose the corresponding motif of the FTO unique loop, explaining their capacity to take dsDNA as substrates for catalysis (SI Appendix, Fig. S21), while ALKBH5 and ALKBH8 prefer ssRNA due to the steric hindrance of a corresponding motif at this position. Specifically, ALKBH5 replaces the FTO unique loop with a short α-helix that prevents binding with dsRNA (Fig. 4B). Additionally, the other loop between β2 and β3 in FTO, where the two key residues K86 (Q86 in FTOWT) and K88 are located, is significantly shorter than the corresponding loop in the other AlkB family members, further contributing to the unique nucleic acid substrate capacity of FTO.
Fig. 4.
Comparison of FTO and ALKBH5 in catalyzing m6A. (A) Superposition of the ALKBH5 structure into the FTO-6mA structure. (B) The unique loop (pink) of FTO induces substrate selectivity variation between FTO and ALKBH5. (C) Superposition of the catalytic pocket of ALKBH5 with the FTO-6mA structure. (D) Enzymatic activity comparison of WT and the E234P mutation of FTO in catalyzing 3mT and m6A for 1 h at 37 °C. The reaction condition is the same as in Fig. 3D. Error bars indicate the mean ± SEM (n = 6, three biological replicates × two technical replicates), determined using an unpaired Student’s t test.
Considering that in vitro biochemistry assays have shown that FTO exhibits higher enzyme kinetics efficiency with m6A demethylation than does ALKBH5 (37, 38), we finally investigated the molecular mechanism for this kinetic difference. Sequence alignment and structural analysis suggest that E234 in human FTO is not conserved among human AlkB family members (SI Appendix, Fig. S10). The corresponding residue P207 in human ALKBH5 abolishes the hydrophilic interaction with N6 and N7 atoms of the 6mA purine ring (Fig. 4C). The mutation of E234P in FTO results in a 62% decrease in m6A demethylation activity (Fig. 4D), confirming the functional contribution of E234 in substrate recognition and, importantly, explaining the significant lower m6A demethylation activity (in vitro) reported for ALKBH5 compared with FTO (37, 38).
Discussion
To date, extensive efforts have been dedicated to identifying the physiological substrate(s) of FTO. It catalyzes the demethylation of m6A and cap m6Am in mRNA, m6A and m6Am in snRNA, and m1A in tRNA (15, 30, 32). However, many questions remain unanswered, including how FTO recognizes such multiple-modification substrates, whether FTO displays a substrate preference, why FTO exhibits a preference for cap m6Am over internal m6A in ssRNA, and why FTO has m1A demethylation activity in tRNA or loop-structured RNA but no activity for linear ssRNA and ssDNA. Here we presented the structure of FTO bound to 6mA-modified ssDNA, which enabled us to investigate these mechanisms at the molecular level. The main conclusions from the biochemical assays and structural analysis described above include the following: (i) FTO prefers the methylated nucleobase N6-methyladenine rather than 3mT and m1A in the catalytic pocket. Residues R96 and E234 of FTO specifically interact with the purine ring of N6-methyladenine, and a hydrophobic cave holds the N6-methyl group for demethylation. (ii) The demethylation activity of FTO is the same for internal m6A and m6Am within the same RNA sequence, suggesting the binding interaction between the residues in FTO’s catalytic pocket and the nucleobase N6-methyladenine (rather than the structural differences of the ribose ring) plays the predominant role in mediating the enzymatic activity of FTO. (iii) The sequence and the tertiary structure of RNA can affect the enzymatic activity of FTO, which helps explain the activity preference of FTO for cap m6Am over internal m6A in ssRNA and for m1A in tRNA or loop-structured RNA over m1A in linear ssRNA.
Here we demonstrated that the activity preference of FTO for cap m6Am over internal m6A in ssRNA is because of the sequence and structure of RNA but not the differences of the ribose ring between m6Am and m6A. The FTO-6mA structure showed that two pincers of FTO hold and bend the oligonucleotides for substrate demethylation. The feature of the cap structure (m7Gppp) including the positively charged m7G, the negatively charged triphosphate, and the 5′ terminus might increase the binding affinity with FTO and bend RNA easily for substrate demethylation. The subcellular localization of the FTO protein was found to affect its ability to perform different RNA modifications (32). Our FTO-6mA structure revealed that FTO does not accept dsRNA substrates but can accommodate ssRNA, RNA with tertiary structures like large stem loops, and cap structures. Therefore, the binding of FTO for various RNAs can further help define its targeted RNA modifications at specific RNAs. The m6A reader domain YTH recognizes the N6-methyl group through a hydrophobic cave (39); similarly, we also found FTO uses a hydrophobic cave for holding the N6-methyl group for demethylation.
Both FTO and ALKBH5 mediate m6A demethylation in mRNA (15, 37); however, they lead to completely different phenotypes: FTO-deficient mice have lean body mass and growth retardation, while ALKBH5-deficient male mice have impaired fertility (4, 5, 37). Apparently, FTO and ALKBH5 must take different RNAs as targets for demethylation, thereby leading to different phenotypes. Our structural analysis showed that FTO and ALKBH5 contain different structural conformations of the nucleic acid binding motifs, further confirming that they bind distinct RNA targets at molecular level.
Collectively, our biochemical and cellular results confirm that FTO demethylates both m6A and cap m6Am in mRNA, thus providing a biochemical foundation for studying the mechanisms through which FTO is involved in biological processes and in human diseases. Moreover, our FTO-6mA structure provides a structural basis for understanding the mechanism of FTO-mediated m6A, m6Am, and m1A demethylation and will support the structure-guided design of selective inhibitors and/or activators for functional studies and potential therapeutic applications.
Materials and Methods
Experimental procedures for cloning, expression, and purification of wild-type and mutation FTO, knockdown of FTO, mRNA isolation, FTO demethylation activity assays, synthesis of the m6Am standard nucleoside and phosphoramidite, measurement of mRNA internal m6A and cap m6Am levels using UPLC-MS/MS, oligonucleotide synthesis and purification, fluorescence anisotropy assay, measurement of 3mT, m6A, and m6Am levels in oligonucleotides using HPLC, crystallization, data collection and structure determination, and statistical analysis are described in SI Appendix, Supplementary Materials and Methods.
Supplementary Material
Acknowledgments
We thank the staff from beam line BL19U1 at the Shanghai Synchrotron Radiation Facility for assistance with crystal diffraction data collection. This work was supported by the National Basic Research Program of China (Grant 2017YFA0505201) and the National Natural Science Foundation of China (Grants 21722802, 21820102008, 21432002, and 21572133).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.G.F. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID code 5ZMD).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820574116/-/DCSupplemental.
References
- 1.Peters T, Ausmeier K, Rüther U. Cloning of Fatso (Fto), a novel gene deleted by the Fused toes (Ft) mouse mutation. Mamm Genome. 1999;10:983–986. doi: 10.1007/s003359901144. [DOI] [PubMed] [Google Scholar]
- 2.Dina C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 3.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer J, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 5.Church C, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42:1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaklamani V, et al. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet. 2011;12:52. doi: 10.1186/1471-2350-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández-Caballero ME, Sierra-Ramírez JA. Single nucleotide polymorphisms of the FTO gene and cancer risk: An overview. Mol Biol Rep. 2015;42:699–704. doi: 10.1007/s11033-014-3817-y. [DOI] [PubMed] [Google Scholar]
- 8.Fawcett KA, Barroso I. The genetics of obesity: FTO leads the way. Trends Genet. 2010;26:266–274. doi: 10.1016/j.tig.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, et al. Association of metabolites with obesity and type 2 diabetes based on FTO genotype. PLoS One. 2016;11:e0156612. doi: 10.1371/journal.pone.0156612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller L, et al. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: A prospective cohort study. J Alzheimers Dis. 2011;23:461–469. doi: 10.3233/JAD-2010-101068. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Shen H, Liu H, Guo G. Mixture SNPs effect on phenotype in genome-wide association studies. BMC Genomics. 2015;16:3. doi: 10.1186/1471-2164-16-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerken T, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia G, et al. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008;582:3313–3319. doi: 10.1016/j.febslet.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Z, et al. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464:1205–1209. doi: 10.1038/nature08921. [DOI] [PubMed] [Google Scholar]
- 15.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fustin JM, et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Geula S, et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 19.Haussmann IU, et al. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–304. doi: 10.1038/nature20577. [DOI] [PubMed] [Google Scholar]
- 20.Li HB, et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–342. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess ME, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042–1048. doi: 10.1038/nn.3449. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, et al. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Y, et al. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543:573–576. doi: 10.1038/nature21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y. 2012. Dynamic regulation of RNA modifications by AlkB family dioxygenases. PhD thesis (Univ of Chicago, Chicago)
- 27.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 28.Linder B, et al. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molinie B, et al. m(6)A-LAIC-seq reveals the census and complexity of the m(6)A epitranscriptome. Nat Methods. 2016;13:692–698. doi: 10.1038/nmeth.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauer J, et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akichika S, et al. Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science. 2018;363:eaav0080. doi: 10.1126/science.aav0080. [DOI] [PubMed] [Google Scholar]
- 32.Wei J, et al. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71:973–985.e5. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishina Y, Chen LX, He C. Preparation and characterization of the native iron(II)-containing DNA repair AlkB protein directly from Escherichia coli. J Am Chem Soc. 2004;126:16930–16936. doi: 10.1021/ja045066z. [DOI] [PubMed] [Google Scholar]
- 34.Yang CG, et al. Crystal structures of DNA/RNA repair enzymes AlkB and ABH2 bound to dsDNA. Nature. 2008;452:961–965. doi: 10.1038/nature06889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holland PJ, Hollis T. Structural and mutational analysis of Escherichia coli AlkB provides insight into substrate specificity and DNA damage searching. PLoS One. 2010;5:e8680. doi: 10.1371/journal.pone.0008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen B, McMurray C. Rapid method for measuring DNA binding to protein using fluorescence anisotropy. Protoc Exch. 2009 doi: 10.1038/nprot.2009.80. [DOI] [Google Scholar]
- 37.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou S, et al. N(6)-methyladenosine: A conformational marker that regulates the substrate specificity of human demethylases FTO and ALKBH5. Sci Rep. 2016;6:25677. doi: 10.1038/srep25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.