Significance
The proteasome is required for the full virulence of Mycobacterium tuberculosis. However, the extent of its role as a regulator of bacterial physiology remains unclear. In this work, we demonstrate a function of the proteasome system in maintaining the expression of essential chaperonin genes. This activity by the proteasome is required for M. tuberculosis to use nitrate as a nitrogen source. Furthermore, we identified a specific growth condition that robustly decreases the abundance of pupylated proteins. This observation strongly suggests the presence of a yet-to-be-determined mechanism of control over the Pup-proteasome system in M. tuberculosis that is induced in nitrate.
Keywords: Mycobacterium, tuberculosis, proteasome, nitrate, chaperonins
Abstract
The human pathogen Mycobacterium tuberculosis encodes a proteasome that carries out regulated degradation of bacterial proteins. It has been proposed that the proteasome contributes to nitrogen metabolism in M. tuberculosis, although this hypothesis had not been tested. Upon assessing M. tuberculosis growth in several nitrogen sources, we found that a mutant strain lacking the Mycobacterium proteasomal activator Mpa was unable to use nitrate as a sole nitrogen source due to a specific failure in the pathway of nitrate reduction to ammonium. We found that the robust activity of the nitrite reductase complex NirBD depended on expression of the groEL/groES chaperonin genes, which are regulated by the repressor HrcA. We identified HrcA as a likely proteasome substrate, and propose that the degradation of HrcA is required for the full expression of chaperonin genes. Furthermore, our data suggest that degradation of HrcA, along with numerous other proteasome substrates, is enhanced during growth in nitrate to facilitate the derepression of the chaperonin genes. Importantly, growth in nitrate is an example of a specific condition that reduces the steady-state levels of numerous proteasome substrates in M. tuberculosis.
The pathogen Mycobacterium tuberculosis, which is the causative agent of the human disease tuberculosis, encodes a proteasome that is essential for its lethality in mice (1, 2). The central component of all proteasomes is a 28-subunit complex of four stacked rings known as the 20S core particle (20S CP). In M. tuberculosis, two identical outer rings, each composed of seven α-subunits (PrcA), serve as a gated entryway for protein substrates, and two identical inner rings, composed of a total of 14 β-subunits (PrcB), form the catalytic active sites of the protease (1, 3–5). While essential in eukaryotes and archaea, proteasomes are found only in a subset of bacteria primarily belonging to the Actinomycetales and Nitrospirales orders, and are not always essential for bacterial viability (6, 7).
In eukaryotes and bacteria, proteasomes carry out the regulated proteolysis of specific cellular substrates. Interest in the M. tuberculosis proteasome emerged after a screen for mutations that rendered this bacterial species sensitive to nitric oxide (NO), a host-derived molecule that is critical for controlling M. tuberculosis growth in mice (8), identified mutations in genes linked to prcBA. Over the years, it was determined that some proteasome substrates in M. tuberculosis are covalently modified with a small protein called prokaryotic ubiquitin-like protein (Pup) by a dedicated ligase, proteasome accessory factor A (PafA) (9–11). These pupylated proteins are recognized by a proteasomal activator, mycobacterial proteasome ATPase (Mpa) (also known as ARC), which uses ATP hydrolysis to power the unfolding and delivery of proteins into 20S CPs for degradation (1, 12). Pup can also be removed from substrates by an enzyme called deamidase of Pup (Dop) (13, 14), as well as by PafA (15). Collectively, Dop, PafA, Pup, Mpa, and 20S CPs constitute the core “Pup-proteasome system” (PPS). At least 60 M. tuberculosis proteins are currently known to be pupylation substrates (9, 16, 17), while studies performed in other Pup-bearing bacteria, including Mycobacterium smegmatis, have identified hundreds of additional potential targets of pupylation (18–21). Of note, many pupylated proteins in M. tuberculosis are not degraded under routine culture conditions for reasons that are unknown (16). This observation suggests pupylation may not immediately send proteins to the proteasome and could possibly serve a nondegradative regulatory role, as is observed in Corynebacteria (22).
In addition to being highly sensitive to NO in vitro, PPS mutants are highly attenuated for virulence in mouse infection models (2, 12, 23). The failure to degrade a single pupylated substrate, Log, is responsible for the NO hypersensitivity phenotype of a PPS (mpa) mutant. However, while genetic disruption of log completely restores NO resistance to an mpa strain in vitro, it does not fully rescue the virulence defect of this strain in mice (17). Therefore, there are likely to be other components of M. tuberculosis physiology whose regulation by the PPS is important for establishing lethal infections.
In addition to its central role in the posttranslational regulation of various cellular pathways, an essential function of the eukaryotic proteasome is to maintain nutrient homeostasis by recycling amino acids (24, 25). In light of this observation, there has been interest in the question of whether or not the proteasome has a similar function in bacteria. Studies in M. smegmatis suggest that pupylation is required to maintain nitrogen homeostasis. Deletion of pup renders M. smegmatis more sensitive to nitrogen starvation (26), during which several enzymes involved in nitrogen metabolism are pupylated (21). In M. tuberculosis, amino acids serve as the primary nitrogen donors for most anabolic processes (27, 28). Additionally, optimal M. tuberculosis growth, both in vitro and in vivo, requires the uptake of exogenous amino acids as a nitrogen source (29–32). It has therefore been hypothesized that the products of bulk proteolysis by the M. tuberculosis proteasome could be an important source of nitrogen under nutrient-limiting conditions. For this reason, we sought to determine whether the M. tuberculosis proteasome contributed to nitrogen metabolism. Contrary to what was proposed in M. smegmatis, we found that proteasomal degradation did not provide a survival advantage to M. tuberculosis during nitrogen starvation. However, we discovered that the proteasome was essential for the ability of M. tuberculosis to use nitrate as a nitrogen source. Through a genetic suppressor screen, we identified a putative PPS substrate whose inactivation rescued the ability of an M. tuberculosis PPS mutant to assimilate nitrogen from nitrate. Our data revealed an essential role for the PPS to facilitate the activity of nitrite reductase, possibly in two different ways, during growth in nitrate. Finally, we identified growth in nitrate broth alters the abundance of numerous pupylated proteins in M. tuberculosis.
Results
The M. tuberculosis Proteasome Does Not Provide a Survival Advantage During Nitrogen Starvation in Vitro.
It has been previously reported that an M. smegmatis mutant-lacking pup (also known as prcS in M. smegmatis) and prcBA cannot survive as well as a wild type (WT) strain during several weeks of nitrogen starvation; however, the phenotype of this mutant is almost fully complemented by pup alone, suggesting that proteasomal degradation itself may have a minor role in M. smegmatis nitrogen metabolism. Nonetheless, it was proposed that the proteasome supported bacterial survival during nitrogen starvation by recycling amino acids (26). We therefore sought to test whether or not proteasomal degradation contributed to M. tuberculosis survival during nitrogen starvation. We incubated WT, ∆mpa::hyg (“mpa”), and ∆prcBA::hyg (“prcBA”) strains (SI Appendix, Table S1) in Proskauer–Beck (PB) minimal medium lacking any nitrogen source and measured bacterial survival over time. In contrast to what is observed in M. smegmatis, we found that the WT strain had no survival advantage over the PPS mutant strains during 3 wk of nitrogen deprivation (Fig. 1A). Thus, amino acid recycling by the proteasome was not required for bacterial survival during complete nitrogen starvation. However, we cannot rule out a role for the proteasome in recycling amino acids under other conditions.
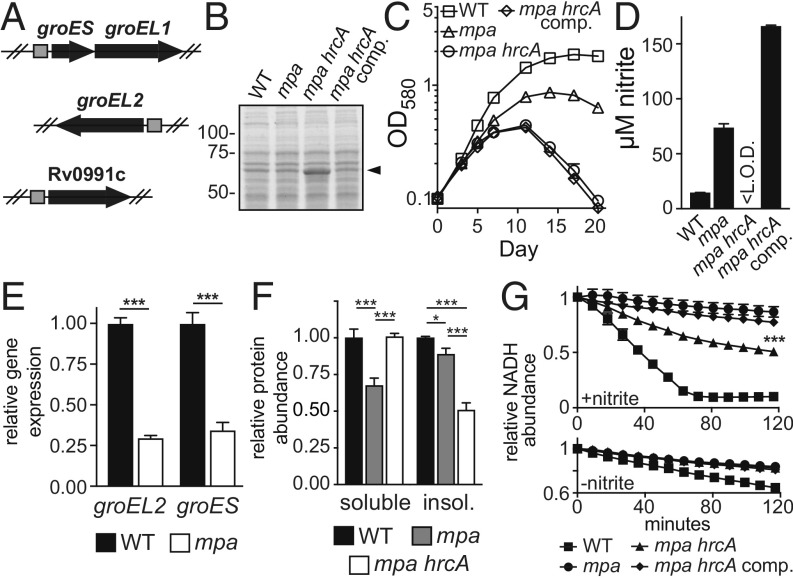
Fig. 1.
The M. tuberculosis Pup-proteasome system (PPS) is required for growth in nitrate. (A) The PPS does not promote survival of M. tuberculosis during complete nitrogen starvation. Survival of M. tuberculosis wild-type (WT), mpa (MHD149), and prcBA strains was measured by number of colony-forming units (CFU) per milliliter of culture at the indicated time points. At week 3, the fold change in CFU from input was determined to be statistically insignificant (one-way ANOVA, P > 0.05) for mpa and prcBA strains compared with the WT strain. Experiment represents data from six replicate cultures. (B) The PPS is not essential for growth of M. tuberculosis in ideal nitrogen sources. Growth of M. tuberculosis strains in Proskauer–Beck (PB) minimal media supplemented with single nitrogen sources asparagine (PB-Asn) or glutamate (PB-Glu) was measured by optical density at 580 nm (OD580). (C) An intact PPS is essential for M. tuberculosis growth when provided nitrate as the sole nitrogen source. M. tuberculosis strains were grown in PB supplemented with arginine (PB-Arg), nitrate (PB-nitrate), or ammonium (PB-ammonium). For each condition in B and C, the growth defect of the mpa mutant compared with the WT strain was statistically significant (one-way ANOVA, P < 0.01). (D) Complementation of the mpa mutant growth defect in PB-nitrate. (E) Pupylation and proteasomal degradation are required for M. tuberculosis nitrate utilization, as assessed by the growth of pafA (MHD2), mpa (MHD5), and prcBA strains in PB-nitrate. (F) Schematic of the M. tuberculosis enzymes that catalyze reduction of nitrate to ammonium (33, 34). (G) PPS mutants (as in E) secrete excess nitrite into culture supernatants during growth in PB-nitrate. Statistical significance was determined using one-way ANOVA; ***P < 0.001. Experiments in B–E and G each contain data from three replicate cultures.
M. tuberculosis Requires the PPS to Use Nitrate as a Nitrogen Source.
Following our observation that M. tuberculosis proteasome-defective strains did not have a survival disadvantage during nitrogen starvation, we next determined if the PPS was required for growth in a specific nitrogen source. M. tuberculosis can use both organic and inorganic sources of nitrogen, although asparagine and glutamate support growth most effectively in vitro (29). We compared the growth of WT and mpa strains in PB media supplemented with asparagine or glutamate and found that the mpa mutant had a minor growth defect compared with the WT strain (Fig. 1B); notably, a modest defect is also observed for an mpa mutant under routine culture conditions in rich broth (1). When the same strains were provided the suboptimal nitrogen sources arginine, ammonium, or nitrate, bacterial growth was predictably slower for both strains; remarkably, however, growth of the mpa mutant was almost completely abrogated in nitrate compared with the WT strain (Fig. 1C). A single copy of mpa integrated on the chromosome restored growth of the mpa strain in nitrate (Fig. 1D).
To determine if the inability of an mpa mutant to use nitrate was specifically related to a failure to degrade pupylated proteins, we assessed the growth of a pafA mutant (pafA::MycoMarT7) and the prcBA strain. Both mutants were attenuated for growth similarly to an mpa mutant in PB-nitrate, demonstrating that both pupylation by PafA and proteolysis by 20S CPs were required for using nitrate as a nitrogen source (Fig. 1E).
M. tuberculosis uses a highly conserved pathway for nitrogen assimilation from nitrate (Fig. 1F). Once imported into the cell, nitrate is reduced to nitrite by the NarGHIJ nitrate reductase complex (33). Nitrite is then reduced to ammonium by the nitrite reductase complex NirBD (34). Finally, ammonium is incorporated into glutamate and glutamine, which comprise the major intracellular nitrogen pool (27). Notably, M. tuberculosis secretes into its extracellular space any nitrite that cannot be immediately reduced to ammonium (34).
We hypothesized that the inability of PPS mutants to productively grow in PB-nitrate was caused by a failure of one or more reactions within nitrate catabolism. Upon growing M. tuberculosis in PB-nitrate, we discovered that supernatants of pafA, mpa, and prcBA mutant cultures contained 10- to 15-fold higher concentrations of nitrite than those of a WT strain (Fig. 1G). This result suggested that these mutants, while capable of importing and reducing nitrate, were unable to reduce nitrite to ammonium, causing the secretion of excess nitrite. Further supporting this model, an mpa mutant strain was capable of growing in PB-ammonium, which bypasses the requirement of nitrite reduction, nearly as well as the WT strain (Fig. 1C).
In M. tuberculosis, the nitrite reductase complex is encoded by the nirBD (Rv0252-Rv0253) operon. An M. tuberculosis nirBD mutant is unable to grow when nitrate is provided as the single nitrogen source, implicating NirBD as the only nitrite reductase in M. tuberculosis (34). Therefore, we hypothesized that degradation of one or more pupylated proteins is required for the in vivo activity of NirBD.
Suppressor Mutations in hrcA or nadD Restore Growth of an mpa Mutant in Nitrate.
Most of an mpa mutant culture ultimately dies upon extended incubation in PB-nitrate (Fig. 1A), an observation that provided a powerful phenotype to screen for suppressor mutations that might identify specific substrates of the PPS whose degradation is necessary for NirBD activity. We previously generated a transposon mutant library of an M. tuberculosis mpa strain, consisting of ∼72,000 unique double-mutant clones (17). To enrich for mutants with a suppressor phenotype, we incubated this library in PB-nitrate for 4–5 wk, until cultures were turbid. Surviving bacteria were further expanded in rich media and subjected to a second round of incubation in PB-nitrate. We isolated 16 clones from six independent pools and tested them individually for growth in PB-nitrate. Interestingly, while all of the suppressor mutants grew more productively than the parental mpa strain, no mutant grew as well as the WT strain (SI Appendix, Fig. S1A).
To identify the suppressor mutations, we cloned DNA containing the transposon insertion from each of the 16 isolates. We identified two strains with unique transposon insertions in the coding region of hrcA (Rv2374c). The remaining suppressor strains contained transposon insertions in different operons, with no obvious functional connections. We therefore suspected that these strains had additional, spontaneous mutations, possibly in hrcA. We PCR-amplified and sequenced the hrcA gene in the remaining mutants and discovered that five more strains had point mutations in hrcA. We next used whole-genome sequencing to identify mutations in the remaining nine suppressor mutants; seven strains had mutations in either the promoter or coding region of nadD (Rv2421c). Importantly, all of these suppressor mutations were the result of independent events (SI Appendix, Table S1).
Expression of Chaperonin Genes Promotes Growth in Nitrate.
Seven of the suppressor strains had mutations in hrcA, which encodes a transcriptional repressor that is conserved in many bacterial species as a regulator of molecular chaperones of the Hsp60 family (reviewed in ref. 35). In M. tuberculosis, HrcA directly represses four genes, including three chaperonin-encoding genes groES (Rv3418c), groEL1 (Rv3417c), and groEL2 (Rv0440); the fourth gene, Rv0991c, is uncharacterized (36) (Fig. 2A). Chaperonins, which are represented in all domains of life, facilitate the folding of protein substrates (37, 38). In bacteria, Hsp60-type chaperonins are composed of two stacked, heptameric rings of Hsp60 (GroEL) subunits, forming a chamber in which proteins fold; the chamber is capped by a heptamer of Hsp10 (GroES) subunits (reviewed in ref. 39). M. tuberculosis is unusual among prokaryotes by encoding two GroEL homologs, both of which form complexes that are likely capped by GroES subunits (40). It was previously shown that an M. tuberculosis strain with a deletion-disruption mutation in hrcA exhibits high expression of groEL1, groEL2, and groES genes, the products of which are detectable in cell lysates by Coomassie brilliant blue staining of SDS/PAGE gels (36). Using this method, we observed protein species corresponding to GroEL1 or GroEL2 that were more abundant in an mpa hrcA double mutant (Δmpa::hyg hrcA::MycoMarT7) (Fig. 2B) and in an hrcA single mutant (∆hrcA::hyg), as well as in the other six mpa hrcA suppressor mutants (SI Appendix, Fig. S1B). We transformed an mpa hrcA strain with an integrative plasmid encoding hrcA under the control of its native promoter, creating strain MHD1344 (SI Appendix, Table S1). Complementation of the hrcA mutation successfully restored GroEL to lower, WT levels (Fig. 2B), and reversed the suppressor phenotypes, as observed by failed growth in PB-nitrate (Fig. 2C) and excessive nitrite secretion (Fig. 2D). Collectively, these data support the hypotheses that the expression of one or more genes of the HrcA regulon is required for nitrite reduction, and that HrcA regulon expression is reduced in PPS mutants.
Fig. 2.
Disruption of hrcA increases chaperonin production and restores nitrite reductase activity to an mpa mutant. (A) The M. tuberculosis HrcA regulon, illustrating operons repressed by HrcA. Positions of HrcA consensus binding sites are shown as gray squares (36). (B) A transposon mutation in hrcA results in the overproduction of chaperonins. Lysates from WT (MHD1350), mpa (MHD1352), mpa hrcA (MHD1347), and mpa hrcA complemented (MHD1344) strains were separated by SDS/PAGE, and proteins were stained with Coomassie brilliant blue. Molecular weight markers are indicated at Left. Characteristic migration pattern of GroEL1 and GroEL2, which are similar in size, is indicated with an arrowhead. (C) Disruption of hrcA partially rescues the growth defect of an mpa mutant in PB-nitrate. (D) Disruption of hrcA returns bacterial nitrite secretion to WT levels in an mpa mutant during growth in PB-nitrate. <L.O.D., below the limit of detection. (E) The chaperonin genes are transcriptionally repressed in an mpa mutant (MHD149) compared with the WT parental strain. Statistical significance was determined using Welch’s t test. (F) The mpa mutant contains less soluble protein than WT or mpa hrcA (MHD1297) strains during growth in PB-nitrate. Additionally, the mpa hrcA strain contains less insoluble protein than WT or mpa strains. Statistical significance was determined using one-way ANOVA. (G) An mpa mutant is defective in nitrite reduction, which is partially rescued by hrcA disruption. Nitrite reductase activity was measured in normalized protein extracts from bacteria grown in PB-nitrate. Extracts were supplemented with NADH, NAD+, and nitrite (above), and nitrite reductase activity was assessed by measuring NADH oxidation over time (the nitrite reductase NirBD catalyzes electron transfer from NADH to nitrite to generate ammonium) (50). As a control, background NADH oxidation in the absence of nitrite was determined using the same extracts (below). The difference in nitrite reductase activity between mpa and mpa hrcA extracts was determined to be statistically significant as indicated (one-way ANOVA). Experiments in C–G each contain data from three replicate cultures. Statistical significance is indicated as follows: *P < 0.05; **P < 0.01; ***P < 0.001. For all panels, “comp” indicated complementation with hrcA.
To assess gene expression in a PPS mutant, we performed a global transcriptional analysis of WT and mpa strains grown in PB-nitrate by RNA sequencing (RNA-seq) (Experimental Procedures). Because an mpa mutant cannot productively grow in this media (Fig. 1C), we prepared RNA from cultures grown to early logarithmic phase [optical density at 580 nm (OD580) = 0.3]. RNA-seq demonstrated that groES and groEL2 were repressed in an mpa mutant compared with the parental WT strain (Fig. 2E). The remaining genes in the HrcA regulon, groEL1 and Rv0991c, were also significantly repressed in an mpa mutant, although by a factor of less than twofold (Dataset S1). This analysis suggested HrcA might be a PPS substrate.
To determine whether the repression of the chaperonin genes leads to changes in protein abundance, we measured global protein levels in WT and mpa strains grown in PB-nitrate using tandem mass tag (TMT)-based quantitative mass spectrometry (MS) (Experimental Procedures). Quantitative MS demonstrated that both GroEL2 and GroES were significantly less abundant in the mpa mutant compared with the WT strain; GroEL1 and Rv0991c levels were not significantly changed. Furthermore, this experiment confirmed that GroES, GroEL1, GroEL2, and Rv0991c were the four most abundant proteins in an mpa hrcA strain compared with the mpa parental mutant (Dataset S2).
groES and groEL2 are essential (41); thus, we were unable to disrupt these genes to test their requirement for growth in PB-nitrate. However, previous work identified a mutant with a transposon insertion in groEL1 (1), and additionally, we deleted and disrupted Rv0991c (∆Rv0991c::hyg) (SI Appendix, Table S1). Unlike a PPS mutant, the groEL1 and Rv0991c mutants grew well in PB-nitrate (SI Appendix, Fig. S2A). These data suggest that the GroES-GroEL2 (“GroESL2”) complex was needed for efficient nitrite reduction; however, we cannot exclude the possibility that other effects of hrcA disruption contributed to nitrate metabolism in an mpa hrcA strain.
Chaperonin Production Promotes NirBD Activity in M. tuberculosis.
To begin to understand the association between chaperonins and nitrite reduction, we first checked whether NirB or NirD abundance varied in WT, mpa, and mpa hrcA M. tuberculosis strains in our MS dataset (Dataset S2). While we observed a significant decrease in NirB abundance in an mpa mutant compared with the WT strain, this phenotype was not reversed upon disruption of hrcA; a similar trend was observed for NirD (SI Appendix, Fig. S2B). We thus concluded that changes in NirBD abundance alone could not explain the differences in nitrite reduction between the WT and mpa strains.
Bacterial chaperonins are required for folding many newly translated proteins, as well as for counteracting protein misfolding and aggregation under certain stress conditions (42–45). Consistent with this function of chaperonins, we always recovered less soluble protein from cell lysates of an mpa mutant than from the WT strain, a phenotype that was rescued by hrcA disruption (Fig. 2F). Based on these data, we hypothesized that chaperonins promote the solubility and activity of many M. tuberculosis proteins, including NirBD. To test this hypothesis, we measured NirBD activity in M. tuberculosis extracts. Bacterial extracts were supplemented with excess substrate (nitrite) and nicotinamide adenine dinucleotide (NAD), a cofactor that is required for NirBD activity (46). Compared with extracts made from the WT strain, nitrite reduction in mpa mutant extracts was at or below the limit of detection. Importantly, we observed a partial restoration of activity in mpa hrcA mutant extracts (Fig. 2G). This result suggested there was an intrinsic defect in NirBD activity in the mpa mutant that was restored by chaperonin overproduction. Notably, the incomplete rescue of nitrite reductase activity in an mpa hrcA strain might be explained by our observation that NirB levels were not restored by disruption of hrcA (SI Appendix, Fig. S2B).
HrcA Can Be Pupylated in Vitro.
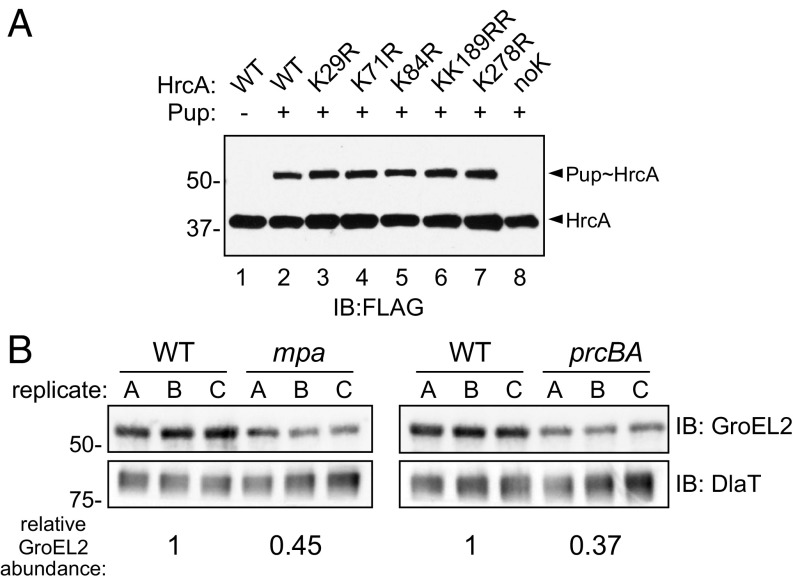
The observations that the HrcA regulon was repressed in an mpa mutant (Fig. 2E and Dataset S1) and that the disruption of hrcA rescued a growth defect of a PPS mutant (Fig. 2C) suggested that HrcA might be a proteasome substrate. To test this hypothesis, we first determined if HrcA could be pupylated in vitro. We purified M. tuberculosis HrcA with C-terminal FLAG and hexahistidine (His6) tandem-affinity tags (HrcATAP) from Escherichia coli. Following incubation of HrcATAP with purified His6-PupGlu and PafA-His6, which are sufficient to pupylate proteins, we observed the appearance of a higher–molecular-weight species corresponding to the expected size of His6-Pup∼HrcATAP (Fig. 3A, compare lanes 1 and 2). In M. tuberculosis, proteins are usually pupylated at a specific lysine (16); we thus attempted to identify a pupylation site on HrcA. We made several HrcATAP variants, each with lysine-to-arginine mutations in one or two of the six lysines in HrcA. Surprisingly, no single lysine was essential for the in vitro pupylation of HrcATAP (Fig. 3A, lanes 3 through 7). Meanwhile, substitution of all six lysines abolished HrcATAP pupylation (Fig. 3A, lane 8). Importantly, because the epitope tag on HrcATAP contained two nonnative lysines, this experiment demonstrated some substrate specificity for HrcA pupylation by PafA.
Fig. 3.
HrcA is a pupylated protein that is likely degraded by the M. tuberculosis proteasome. (A) Purified HrcA can be pupylated on any of its lysines by PafA. His6-PupGlu and PafA-His6 were coincubated with HrcATAP WT or lysine-to-arginine (K>R) variants, and both native and pupylated HrcA were detected by immunoblotting (IB) using an antibody that recognizes an affinity tag on HrcATAP (FLAG). Data are representative of three independent experiments. (B) GroEL2 abundance is low in both mpa (MHD149) and prcBA strains compared with the WT parental strain. Immunoblots for GroEL2 and dihydrolipoamide acyltransferase (DlaT) were performed on the same membrane using samples obtained from replicate PB-nitrate cultures. For each lane, GroEL2 was normalized to DlaT, a protein that is not regulated by the PPS. The difference in normalized GroEL2 abundance between strains is indicated at the Bottom; for comparison of WT and mpa strains, this difference had a P value of 0.07; the difference in GroEL2 abundance between WT and prcBA strains had a P value of <0.01.
We sought to test if the pupylation of HrcA leads to its degradation in vivo. However, we were unable to observe endogenous HrcA in M. tuberculosis under any condition. We were unsuccessful in generating antibodies to detect HrcA in M. tuberculosis lysates, and HrcA was barely detected by TMT-based quantitative MS (Dataset S2). We also tried to use an epitope-tagged HrcA allele, but the tag abolished its repressor function. Finally, we introduced an hrcA allele lacking all of its lysines into an hrcA-null mutant; however, this hrcA allele also completely lost its repressor activity.
Despite the technical limitations preventing us from observing pupylation or degradation of HrcA in vivo, our observation that mpa, pafA, or prcBA mutants could not grow in nitrate (Fig. 1E) suggested that optimal chaperonin expression requires PPS-dependent proteolysis. We therefore predicted that the HrcA regulon would be repressed similarly in the mpa and prcBA mutants. We compared the abundance of GroEL2 in lysates from WT, mpa, and prcBA strains grown in PB-nitrate. We observed a similarly low abundance of GroEL2 in both the mpa and prcBA strains compared with the WT strain (Fig. 3B). Collectively, the genetic evidence along with the pupylation assays suggests that the degradation of HrcA is necessary for maintaining chaperonin gene expression in M. tuberculosis grown in nitrate.
Gain-of-Function Mutations in nadD Rescue a Defect in NAD Availability in an mpa Mutant.
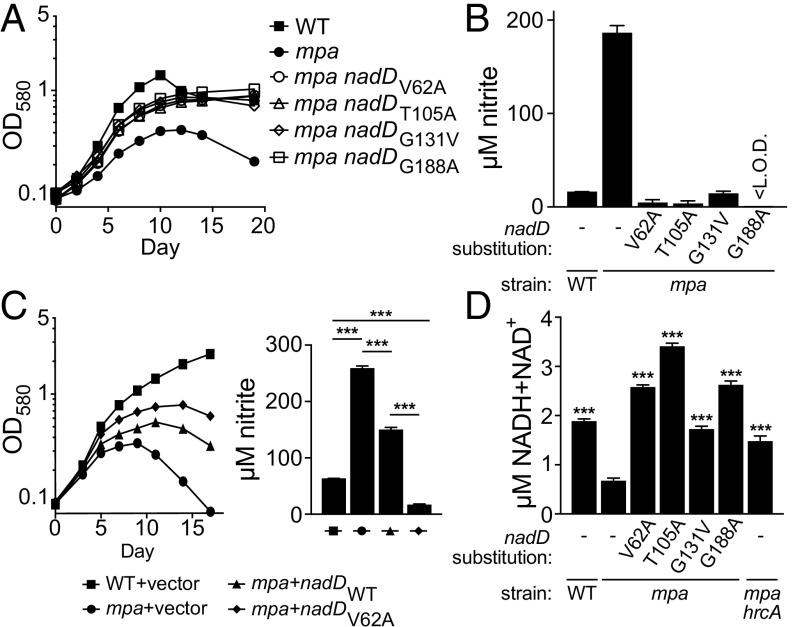
We identified four different point mutations in nicotinate mononucleotide adenylyltransferase (nadD) (SI Appendix, Table S1) that each rescued the growth of an mpa mutant in PB-nitrate (Fig. 4A). NadD catalyzes a committed step in the biosynthesis of NAD, a molecule that serves as an electron carrier in a wide variety of essential redox reactions (47). Through ATP hydrolysis, NadD transfers adenosine monophosphate to nicotinic acid mononucleotide (NaMN), generating nicotinic acid adenine dinucleotide (NaAD), a direct precursor to NAD (48). In M. tuberculosis, NadD is constitutively required for the production of NAD (49).
Fig. 4.
Point mutations in nadD restore nitrate growth to an mpa mutant and increase NAD abundance in bacteria. (A) Amino acid substitutions in NadD partially rescue growth of an mpa mutant in PB-nitrate. Strains WT, MHD149, MHD1294, MHD1300, MHD1301, and MHD1311 are represented. Note that strains MHD1294, MHD1300, MHD1301, and MHD1311 each have transposon insertions in unrelated genes (see SI Appendix, Table S1 for full genotypes). (B) nadD mutations restore nitrite secretion by the mpa strain to WT levels during growth in PB-nitrate. (C) Ectopic expression of WT nadD or nadDV62A partially rescues growth of an mpa mutant in PB-nitrate (Left) and lowers nitrite secretion by the mpa mutant (Right); statistical significance was determined using one-way ANOVA. Strains MHD1350, MHD1352, MHD1440, and MHD1456 are represented. (D) An mpa mutant contains less NAD than a WT strain, a defect that is rescued both by mutations in nadD and by disruption of hrcA (MHD1297). Total NAD [oxidized (NADH) and reduced (NAD+) forms] was quantified in lysates of bacteria grown in PB-nitrate; statistical significance is indicated by comparison with the mpa single mutant (one-way ANOVA). Experiments in A–D each contain data from three replicate cultures. **P < 0.01; ***P < 0.001.
The four mutations we identified in nadD resulted in the amino acid substitutions V62A, T105I, G131V, and G188A (V, valine; A, alanine; T, threonine; I, isoleucine; G, glycine); two additional strains, recovered from independent mutant pools, also encoded a NadDV62A allele (SI Appendix, Table S1). Consistent with their ability to grow in PB-nitrate, all four mpa nadD suppressor strains secreted low nitrite levels comparable to the parental WT strain, demonstrating that NirBD activity was restored in these strains (Fig. 4B).
Because NadD is essential for the growth of M. tuberculosis (49), we predicted that these nadD suppressor mutations resulted in a gain of function. To test this hypothesis, we transformed a single copy of either WT nadD or nadDV62A into an mpa strain and assessed growth of these transformants in PB-nitrate. As expected, ectopic expression of nadDV62A partially rescued the growth of the mpa parental strain, while ectopic expression of WT nadD had an intermediate phenotype. Likewise, ectopic expression of nadDV62A had a dominant effect to reduce nitrite secretion, even in the presence of the endogenous, WT nadD (Fig. 4C). We also measured the levels of total oxidized and reduced NAD (NAD+ and NADH, respectively) in M. tuberculosis lysates from our strains. Interestingly, we observed a nearly threefold reduction in NAD abundance in an mpa mutant relative to the parental WT strain. Importantly, all four nadD mutations restored NAD abundance in an mpa mutant to levels equal to or greater than that of the WT strain (Fig. 4D). Thus, nadD gain-of-function mutations rescued a defect in NAD availability in the mpa strain, an effect that was sufficient to restore NirBD activity in this mutant.
NAD depletion could affect many redox-associated enzymes in M. tuberculosis; however, there exists a direct link between NAD and nitrate catabolism. NirBD catalyzes electron transfer from NADH to nitrite, producing ammonium and NAD+ (50). This reaction also requires the presence of NAD+ itself (46). Accordingly, the reduced levels of NAD in the mpa mutant could also contribute to this strain’s defect in NirBD activity.
We sought to understand the molecular basis by which amino acid substitutions in NadD result in increased production of NAD in vivo. We produced WT and variant (V62A, T105I, G131V, and G188A) NadD in E. coli, and purified these proteins to homogeneity. We first measured NadD protein stability using a thermal shift assay (Experimental Procedures). Remarkably, three of the four NadD variants (T105I, G131V, and G188A) displayed a more than 13 °C increase in melting temperature compared with WT NadD (Table 1 and SI Appendix, Fig. S3A). We next measured the rate of ATP hydrolysis by WT and variant NadD upon incubation with NaMN. While NadD G131V and G188A hydrolyzed ATP at a higher rate, the remaining two NadD variants had less activity than the WT enzyme (Table 1).
Table 1.
Analysis of NadD variants
| Protein sample | Tm (SD),* °C | Activity (SD), nmol⋅min−1⋅mg−1 |
| NadD WT | 54.4 (1.06) | 1.09 (0.01) |
| NadD V62A | 48.7 (0.94) | 0.48 (0.015) |
| NadD T105I | 68.0 (1.21) | 0.11 (0.05) |
| NadD G131V | 68.9 (0.82) | 1.95 (0.005) |
| NadD G188A | 67.7 (0.78) | 2.34 (0.07) |
Tm, melting temperature.
We mapped the amino acid substitutions on the NadD crystal structure. T105I and G131V are located in the core of the NadD monomer (SI Appendix, Fig. S3B). This region is characterized by hydrophobic interactions between a central β-sheet and several α-helices (51); accordingly, such hydrophobic amino acid substitutions may stabilize NadD by increasing core packing, which might explain their increased thermal stability. In contrast, substitutions V62A and G188A lie at a subunit-to-subunit interface in the NadD tetramer (SI Appendix, Fig. S3B). NadD forms both dimers and tetramers in vitro (51); while the state of NadD assembly in vivo is unknown, it is possible that substitutions at the surface of NadD monomers influence the oligomeric state of NadD to affect its catalytic activity (either positively or negatively) in M. tuberculosis. While we cannot yet explain why two of the NadD mutant alleles show slower activity in vitro, our genetic data suggest NadD activity is higher in vivo for all four mutants.
We found that the low NAD levels in an mpa mutant were also restored by disruption of hrcA (Fig. 4D). It is possible that either the HrcA regulon is needed to support NAD synthesis, or that in the absence of Mpa function, one or more NAD-consuming enzymes deplete the cellular stores of this cofactor.
Nitrogen Sources Affect Steady-State Pupylome Levels.
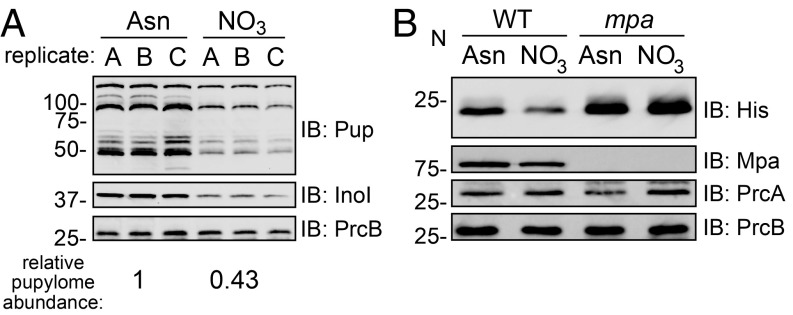
Our results up to now suggest that the PPS degrades HrcA to allow for the expression of chaperonin genes in bacteria growing in nitrate. However, we did not know whether these observations reflected the specific degradation of HrcA, or a mass degradation of substrates by the proteasome. To address this question, we grew M. tuberculosis in PB-Asn, which permits robust growth of an mpa mutant (Fig. 1B), or in PB-nitrate and quantified the abundance of pupylated proteins in bacterial lysates detectable by immunoblotting. We observed a nearly twofold decrease in pupylome abundance in bacteria grown in PB-nitrate compared with PB-Asn. We also observed a decrease in the abundance of (unpupylated) inositol-3-phosphate synthase (Ino1), a model PPS substrate (16), but not of PrcB (Fig. 5A). This result suggested that there was an increase in the degradation of pupylated proteins, rather than a decrease in pupylation, during growth in PB-nitrate. To further test this point, we used a reporter protein, Pup-Zur-His6, to specifically observe the degradation of a “prepupylated” protein in M. tuberculosis. Zinc uptake regulator (Zur) (Rv2359) is an M. tuberculosis protein that lacks lysines and therefore cannot be pupylated in vivo. Pup is translationally fused to Zur through a linear amide, rather than an isopeptide bond, and cannot be depupylated; thus, the abundance of this reporter specifically assesses proteolysis by the Mpa-proteasome (52). In WT M. tuberculosis, we observed a decrease in Pup-Zur-His6 abundance in bacteria grown in PB-nitrate compared with bacteria cultured in PB-Asn. Meanwhile, in an mpa mutant, there was no difference in Pup-Zur-His6 levels upon growth in either medium, supporting a model whereby the Mpa-proteasome degrades pupylated proteins during growth in nitrate (Fig. 5B).
Fig. 5.
Abundance of pupylated proteins depends on the nitrogen source. (A) M. tuberculosis contains a lower abundance of pupylated proteins, and of a model PPS substrate, when grown in PB-nitrate compared with PB-Asn. Pupylated proteins were detected by immunoblot (IB) using a monoclonal antibody that recognizes M. tuberculosis Pup. The same immunoblot membranes were used to detect inositol-3-phosphate synthase (Ino1) and PrcB. The relative pupylome abundance between growth conditions (Bottom) was normalized by PrcB abundance and was statistically significant (Welch’s t test, P < 0.05). (B) Pup-Zur-His6 levels are reduced in WT M. tuberculosis grown in PB-nitrate compared with PB-Asn. The normalized Pup-Zur-His6 intensity in the second lane relative to the first lane is 0.62. PrcB and Mpa were detected on the same membrane, while PrcA was detected using a membrane separately prepared with the same lysates.
Previous work has shown that total nitrogen starvation is associated with a decrease in pupylated proteins in M. smegmatis. This phenomenon was attributed to a greater abundance of 20S CPs upon nitrogen starvation, suggesting that M. smegmatis regulates the production of the degradation machinery in response to nitrogen availability (26). However, we found that while the abundance of the pupylome, Ino1, and Pup-Zur-His6 decreased during growth in PB-nitrate, the levels of Mpa, PrcA, and PrcB remained unchanged (Fig. 5B). Therefore, we propose that the regulation of proteasomal degradation in M. tuberculosis during growth in nitrate does not require significantly altering levels of the known proteolytic components.
Discussion
In this work, we established that M. tuberculosis requires an intact PPS to assimilate nitrogen from nitrate. Specifically, the ability of M. tuberculosis to reduce nitrite depended on the expression of the Hsp60 chaperonin genes, including groES and groEL2. We found that HrcA, a repressor of the groES and groEL1/2 genes, is most likely pupylated and degraded by the proteasome to allow for the production of the GroESL2 complex during growth in nitrate. Additionally, we found that NAD levels were reduced in the absence of a functional PPS, which could also contribute to the observed defect in nitrite reduction in PPS mutants. Last, we showed that the abundance of PPS substrates changed depending on the nitrogen source provided to M. tuberculosis.
While mouse models of M. tuberculosis infection have demonstrated a requirement for bacterial uptake of asparagine and aspartate as nitrogen sources (30, 31), the importance of nitrate as a nutrient during infection is less clear. An M. tuberculosis narG mutant, which is unable to reduce nitrate, is fully virulent in mice (53). However, unlike in humans, M. tuberculosis lesions in most inbred mouse lines are not hypoxic (53, 54); because nitrate import and reduction occur most abundantly under anaerobic conditions (55–57), these infection models may not accurately reflect nitrate utilization during a human infection. Notably, abundant nitrate reduction by M. tuberculosis occurs in primary human macrophages, where the bacteria experience hypoxia (58, 59).
Our results suggest that M. tuberculosis NirBD activity requires derepression of the HrcA regulon and support a model by which HrcA is degraded in a PPS-dependent manner (Fig. 6). Studies of HrcA from other bacterial species have shown that this repressor acts as a thermosensor: An increase in temperature induces the dissociation of HrcA from DNA, presumably allowing for the expression of factors necessary to respond to heat-induced protein misfolding (60, 61). We observed that the PPS alleviates HrcA repression in the absence of heat shock, suggesting that there are other ways of inducing the expression of the hsp60 protein quality control genes in M. tuberculosis. Importantly, it is unknown how many M. tuberculosis proteins depend on GroESL2 for folding. The identification of other GroESL2 substrates could potentially uncover additional pathways whose function depends on PPS-mediated control of chaperonin gene expression. Importantly, these pathways may at least partially explain how defects in the PPS lead to highly attenuated bacteria in animals.
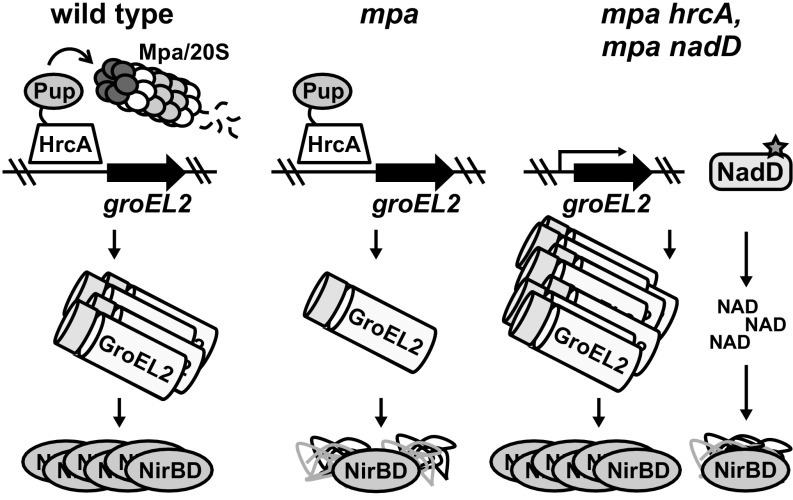
Fig. 6.
Model of PPS control over M. tuberculosis nitrate metabolism. (Left) HrcA, which represses the M. tuberculosis chaperonin genes including groEL2, is likely pupylated and degraded by the Mpa/20S CP proteasome to allow for the full expression of the chaperonins that promote the folding or assembly of the nitrite reductase NirBD. (Middle) Failure of proteasomal degradation in M. tuberculosis leads to the repression of the chaperonin genes, preventing the formation of functional NirBD. (Right) Disruption of hrcA restores NirBD activity in an mpa mutant through the full derepression the chaperonin genes, while gain-of-function mutations in nadD increase the abundance of NAD to promote NirBD catalysis.
According to the most well-characterized model of chaperonin activity in E. coli, misfolded or unfolded proteins become encapsulated within a GroES-GroEL chamber, a hydrophobic space in which substrates fold (37, 38). NirD has a mass of 12.5 kDa, a size that is within the range of most E. coli GroEL substrates (62); in contrast, the 90-kDa NirB subunit is too large to be fully encapsulated. Nonetheless, a mechanism of chaperonin-mediated folding of large proteins without encapsulation has been described (63, 64), and several high–molecular-weight E. coli proteins have been identified as in vivo GroEL substrates (62). Thus, it is possible that NirB and/or NirD are endogenous substrates of GroESL2 in M. tuberculosis.
While a failure of M. tuberculosis to maintain chaperonin levels is associated with a loss of NirBD function, we have also shown that an mpa mutant grown in nitrate lacks WT levels of NAD, which is required for NirBD activity. Proteomic analysis of WT and mpa strains did not identify alterations in the abundance of any enzymes within the NAD biosynthetic pathway that could explain the failure of an mpa mutant to maintain WT NAD levels (Dataset S2). It is possible that the NAD pool is exhausted by the accumulation of one or more PPS substrates that consume NAD. However, it is telling that, in addition to compensatory mutations in nadD, hrcA disruption is sufficient to restore NAD levels in an mpa mutant. Taken together, these observations suggest that GroESL2 may also promote the folding or assembly of NadD or other enzymes in the NAD synthetic pathway. In turn, the gain-of-function mutations in nadD that were selected for in our suppressor screen may allow NadD to remain functional despite reduced chaperonin levels. Importantly, NadD is thought to be essential in most bacteria, and is of interest as a drug target in a potentially diverse set of pathogens, including M. tuberculosis (49, 65). Thus, the NadD variants that we describe here highlight the importance of NadD activity under stress conditions and strengthen the potential of NadD as an underappreciated drug target.
In a study using M. smegmatis, Gur and colleagues (26) found that the pupylome is less abundant during nitrogen starvation, an observation that is similar to what we observed with M. tuberculosis grown in nitrate broth. It was proposed that altered levels of components of the PPS are responsible for this phenotype in M. smegmatis. In contrast, we did not observe conspicuous changes in proteasome component abundance in M. tuberculosis despite a dramatic change in substrate abundance, suggesting that there are differences in the regulation of proteasomal activity between these bacterial species. Instead of altering PPS component levels, it is conceivable that there are posttranslational modifications on the proteasome itself that alter its activity. For example, M. tuberculosis kinases PknA and PknB can phosphorylate PrcA and PrcB (66); although there is no indication that this activity occurs in a physiological setting, phosphorylation could potentially affect the activity of 20S CPs. Additionally, there may be factors that modulate the association of Mpa with the 20S CP, since attempts to observe a robust Mpa-20S CP interaction in vitro have been unsuccessful (67).
In addition to identifying a role of the M. tuberculosis PPS in regulating chaperonin and NAD levels during growth in nitrate, we found a specific condition during which proteasomal degradation appears to be stimulated. Because the Hsp60 system is undoubtedly required for the function of numerous proteins, it seems likely that other environmental cues could activate proteasomal degradation to induce hsp60 regulon expression. Thus, the molecular mechanisms by which PPS function might be altered, as well as other growth conditions that promote proteolysis, warrant further investigation.
Experimental Procedures
Bacterial Strains, Plasmids, Primers, and Culture Conditions.
Bacterial strains, plasmids, and primers used in this study are listed in SI Appendix, Table S1. Chemicals used for making all buffers and bacterial media were purchased from Thermo Fisher, unless otherwise indicated. M. tuberculosis was grown in “7H9” (BD Difco Middlebrook 7H9 broth with 0.2% glycerol and supplemented with 0.5% BSA, 0.2% dextrose, 0.085% sodium chloride, and 0.05% Tween 80). For culturing M. tuberculosis in single nitrogen sources, a base of PB minimal medium (68) with no nitrogen source (“PB-base”) was prepared with 0.5% potassium phosphate monobasic, 0.06% magnesium sulfate heptahydrate, 1.5% glycerol, 0.25% magnesium citrate dibasic anhydrous, and 0.05% Tween 80. The following nitrogen sources were added to a final concentration of 10 mM: asparagine (PB-Asn), glutamate (PB-Glu), arginine (PB-Arg), sodium nitrate (PB-nitrate), or ammonium chloride (PB-ammonium); pH was adjusted to 6.4 after nitrogen addition. PB broths were autoclaved or filtered before use. M. tuberculosis was incubated at 37 °C for all experiments.
For solid media, M. tuberculosis was grown on “7H11” agar (BD Difco Middlebrook 7H11) containing 0.5% glycerol and supplemented with 10% final volume of BBL Middlebrook OADC Enrichment. For selection of M. tuberculosis, the following antibiotics were used as needed: kanamycin (50 µg/mL), hygromycin (50 µg/mL), and gentamicin (15 µg/mL).
E. coli was cultured in BD Difco Luria–Bertani (LB) broth or on LB agar. Media were supplemented with the following antibiotics as needed: kanamycin (100 µg/mL), hygromycin (150 µg/mL), and gentamicin (15 µg/mL).
For all experiments in which M. tuberculosis was cultured in PB broth, bacteria were first grown in 7H9 to an OD580 of 0.5–0.8, washed three times in PBS-T [PBS (Corning) with 0.05% Tween 80], and resuspended in the appropriate PB broth. For growth curves, bacteria were harvested by centrifugation at 500 × g for 5 min to remove large clumps of bacteria before dilution into fresh broth.
A detailed description of plasmid construction is provided in SI Appendix, Supplementary Experimental Procedures.
Protein Purification, Antibody Production, and Immunoblotting.
Purification of PafA-His6 and His6-PupGlu was described previously (9, 15). HrcA was made with a C-terminal affinity tag consisting of FLAG and His6 epitopes separated by a 5-aa linker (“HrcATAP”). M. smegmatis PrcB was made with a C-terminal His6 (smPrcB-His6). HrcATAP, smPrcB-His6, and PrcA-His6 were produced in E. coli strain ER2566 and purified by affinity chromatography using Ni-NTA agarose (Qiagen) according to the manufacturer’s instructions (PrcA and PrcB were purified under urea denaturing conditions). To make rabbit polyclonal immune serum, ∼200 µg PrcA-His6 or smPrcB was used to immunize rabbits (Covance). Purification of recombinant NadD is described in SI Appendix, Supplementary Experimental Procedures. Antibodies to M. tuberculosis DlaT were a gift from R. Bryk and C. Nathan, Weill Medical College of Cornell University, New York.
Separation of proteins in in vitro assays and in M. tuberculosis lysates was performed using 10% SDS/PAGE gels, with the exception of the experiment shown in Fig. 5B, which used a 15% SDS/PAGE gel. Bio-Safe Coomassie Stain (Bio-Rad) was used to stain gels. For immunoblots, proteins were transferred from SDS/PAGE gels to nitrocellulose membranes (GE Amersham) and analyzed by immunoblotting as indicated. Detailed immunoblotting procedures are found in SI Appendix, Supplementary Experimental Procedures.
To quantify GroEL2 abundance in Fig. 3B, we used ImageJ (https://imagej.nih.gov) to measure the pixel density of GroEL2 and DlaT signals in immunoblot images. To normalize each lane, the GroEL2 density was divided by the DlaT density. Quantification of pupylome and Pup-Zur-His6 abundances in Fig. 5 was performed in the same manner, using the PrcB signal to normalize the pupylome or His signal for each lane. For the fractionation experiment shown in Fig. 2F, total protein content in soluble and insoluble lysate fractions was determined by separating samples on SDS/PAGE gels, staining gels with Coomassie brilliant blue, and using ImageJ to measure pixel density in scanned images.
Preparation of M. tuberculosis Extracts.
To generate protein extracts for gel separation and immunoblotting, M. tuberculosis cultures were grown to an OD580 of 0.3. Equal amounts of bacteria were harvested by centrifugation, resuspended in lysis buffer (50 mM Tris, 150 mM sodium chloride, and 1 mM EDTA, pH 8.0), and transferred to a tube containing 250 µL of 0.1-mm zirconia beads (BioSpec Products). Bacteria were lysed using a mechanical bead-beater (BioSpec Products). Whole lysates were mixed with 4× SDS sample buffer (250 mM Tris, pH 6.8, 2% SDS, 20% 2-mercaptoethanol, 40% glycerol, 1% bromophenol blue) to a 1× final concentration, and samples were boiled for 5 min. For preparing lysates from M. tuberculosis grown in 7H9, which contains BSA, an additional wash step with PBS-T was done before resuspension of bacteria in lysis buffer.
For the fractionation experiment shown in Fig. 2F, bacteria were lysed as described above; whole lysate was centrifuged at 16,000 × g for 5 min to pellet insoluble material. Supernatants were mixed with 4× SDS sample buffer, and pellets were resuspended in 250 µL of fresh lysis buffer and mixed with 4× SDS sample buffer; each sample was boiled for 5 min.
Sequencing of Suppressor Mutants.
M. tuberculosis chromosomal DNA was purified as described previously (69). Transposon insertion sites were cloned from M. tuberculosis genomic DNA, transformed into S17-λpir, and sequenced as previously described (1). For strains MHD149, MHD1294, MHD1300, MHD1301, MHD1302, MHD1304, MHD1306, MHD1307, MHD1308, and MHD1311, whole-genome sequencing was done by the Genome Technology Center at New York University Langone Health using an Illumina Hi-Seq platform. Reads were mapped to the H37Rv reference genome (National Center for Biotechnology Information) using BWA (bio-bwa.sourceforge.net/) and SAMtools (samtools.sourceforge.net/). Identification of nucleotide mutations was performed using HaplotypeCaller (Broad Institute).
Quantification of Nitrite Reductase Activity.
All experiments were performed using cultures growing in PB-nitrate to an OD580 of 0.3. The concentration of nitrite in M. tuberculosis culture supernatants was measured using the Griess assay (70) by mixing supernatant 1:1 with Griess reagent [2.5% phosphoric acid, 0.5% sulfanilamide, 0.05% N-(1-napthyl)-ethylenediamine], incubating for 10 min at 25 °C, and measuring absorbance at 550 nm (A550). A set of sodium nitrite solutions was used to make a standard curve for A550 measurements.
Direct measurement of nitrite reductase activity in M. tuberculosis extracts was performed as described previously, using NADH oxidation as a measure of NirBD activity (46). To eliminate background oxidation by NADH dehydrogenase, a membrane-associated complex, bacterial lysates were filtered and subjected to ultracentrifugation at 150,000 × g for 2 h to remove insoluble material. Extracts were then normalized by total protein content after measuring the protein concentration using the Bio-Rad Protein Assay. NADH was measured in the reactions by A340. Background NADH oxidation was determined in the same extracts by omitting sodium nitrite from the reaction.
Transcriptional Analysis.
To analyze gene expression, RNA was purified as previously described (71) from M. tuberculosis cultures grown in PB-nitrate to an OD580 of 0.3. Library preparation, sequencing by Illumina HiSeq, and analysis were performed by GENEWIZ. Sequence reads were mapped to the H37Rv genome using Bowtie2 (bowtie-bio.sourceforge.net/bowtie2/index.shtml). Unique gene hit counts were calculated using Subread (subread.sourceforge.net/), and differential gene expression analysis was performed using DeSeq2 (https://bioconductor.org/packages/release/bioc/html/DESeq2.html). To compare gene expression between strains, the Wald test was used to generate P values and log2 fold changes. Genes with an adjusted P value of <0.05 and absolute log2 fold change of >1 were called as differentially expressed genes for each comparison. Global gene expression analyses in WT and MHD149 strains are provided in Dataset S1. Raw sequencing data files are available in a PATRIC public workspace (https://patricbrc.org/workspace/public/shb360@patricbrc.org/shb2018).
Mass Spectrometry.
For analysis of protein content in M. tuberculosis strains, bacteria were grown in PB-nitrate to an OD580 of 0.3. Equal amounts of bacteria were harvested by centrifugation, resuspended in freshly prepared denaturing lysis buffer (100 mM Tris, 1 mM EDTA, 8 M urea, pH 8.0), and lysed by bead beating. Whole lysates were centrifuged at 16,000 × g for 5 min to pellet the urea-insoluble material. Supernatants were centrifuged through a 0.22-µm Spin-X cellulose-acetate filter (Corning) and stored at −80 °C. Detailed methods for TMT-based quantitative MS are in SI Appendix, Supplementary Experimental Procedures. A comparison of global protein abundances between WT, MHD149, and MHD1297 is provided in Dataset S2. Raw peptide data are available in a PATRIC public workspace (https://patricbrc.org/workspace/public/shb360@patricbrc.org/shb2018).
In Vitro Pupylation of HrcA.
Pupylation assays were performed as described previously (17). Briefly, reaction mixtures contained 1 µM His6-PupGlu, 1 µM HrcATAP, and 0.5 µM PafA-His6 at pH 8.0 in the presence of 5 mM ATP, 50 mM Tris, 20 mM magnesium chloride, 10% glycerol, 1 mM DTT, and 150 mM sodium chloride. Reactions were incubated overnight at 25 °C.
Quantification of NAD.
Total NAD in M. tuberculosis lysates was quantified using the NAD/NADH Quantitation Kit (Sigma-Aldrich). Preparation of protein-free bacterial extracts and NAD quantification were performed according to the manufacturer’s instructions.
NadD Kinetics and Stability Assays.
Thermal stability of NadD variants was measured using differential scanning fluorimetry (thermal shift assay). Differential scanning fluorimetry was performed using a CFX96 Touch Real-Time PCR detection system and the fluorescent dye SYPRO Orange stock concentration at a final concentration of 2× in 96-well PCR plates. The initial fluorescence signal was measured after 5 min of temperature equilibration at 25 °C followed by measurements at every 1 °C⋅min−1 until reaching 95 °C. The wavelengths of excitation and emission were 490 and 580 nm, respectively. For each experiment, the protein was run alone and in the presence of 10 mM Mg-ATP. Experiments were carried out with at least three samples per condition; results were expressed as mean values ± SEM. Melting temperatures were calculated using CFX Manager 3.1 software’s d(RFU)/dT peak finder.
Reaction mixtures for the assay of nicotinic acid adenylyl transferase activity of NadD contained 100 mM Hepes-NaOH, pH 7.4, 10 mM magnesium chloride, 1 mM NaMN, 0.1 mM ATP (Sigma), 5 mU of inorganic pyrophosphatase (Sigma), and 20 µg of purified NadD in a total volume of 0.1 mL. Reactions were performed in a clear, flat-bottomed, 96-well plate at room temperature. After incubation for 10 min, inorganic phosphate was detected using the Malachite Green assay (72).
Supplementary Material
Acknowledgments
We thank A. Osterman for providing the NadD expression construct; C. Kenner, J. Li, and R. Reed for assistance with NadD purifications; S. Ehrt for the prcBA mutant; R. Copin for assistance in analysis of whole-genome sequencing data; and M. Samanovic, S. Zhang, A. Darwin, and members of the A. Darwin laboratory for helpful discussions. This work was supported by NIH Grants R01 HL092774 and AI088075 (to K.H.D.), T32 AT007180 (to S.H.B. and J.B.J.), and R03AI117361 (to K.V.K.). S.H.B. also received support from the Jan T. Vilcek Endowed Fellowship Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819468116/-/DCSupplemental.
References
- 1.Darwin KH, Ehrt S, Gutierrez-Ramos JC, Weich N, Nathan CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science. 2003;302:1963–1966. doi: 10.1126/science.1091176. [DOI] [PubMed] [Google Scholar]
- 2.Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat Med. 2007;13:1515–1520. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin G, et al. Mycobacterium tuberculosis prcBA genes encode a gated proteasome with broad oligopeptide specificity. Mol Microbiol. 2006;59:1405–1416. doi: 10.1111/j.1365-2958.2005.05035.x. [DOI] [PubMed] [Google Scholar]
- 4.Groll M, et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 5.Knipfer N, Shrader TE. Inactivation of the 20S proteasome in Mycobacterium smegmatis. Mol Microbiol. 1997;25:375–383. doi: 10.1046/j.1365-2958.1997.4721837.x. [DOI] [PubMed] [Google Scholar]
- 6.Becker SH, Darwin KH. Bacterial proteasomes: Mechanistic and functional insights. Microbiol Mol Biol Rev. 2016;81:e00036-16. doi: 10.1128/MMBR.00036-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann G, Udasin RG, Livneh I, Ciechanover A. Identification of UBact, a ubiquitin-like protein, along with other homologous components of a conjugation system and the proteasome in different gram-negative bacteria. Biochem Biophys Res Commun. 2017;483:946–950. doi: 10.1016/j.bbrc.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 8.MacMicking JD, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce MJ, Mintseris J, Ferreyra J, Gygi SP, Darwin KH. Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. Science. 2008;322:1104–1107. doi: 10.1126/science.1163885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerda-Maira FA, et al. Reconstitution of the Mycobacterium tuberculosis pupylation pathway in Escherichia coli. EMBO Rep. 2011;12:863–870. doi: 10.1038/embor.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns KE, Liu WT, Boshoff HI, Dorrestein PC, Barry CE., 3rd Proteasomal protein degradation in Mycobacteria is dependent upon a prokaryotic ubiquitin-like protein. J Biol Chem. 2009;284:3069–3075. doi: 10.1074/jbc.M808032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darwin KH, Lin G, Chen Z, Li H, Nathan CF. Characterization of a Mycobacterium tuberculosis proteasomal ATPase homologue. Mol Microbiol. 2005;55:561–571. doi: 10.1111/j.1365-2958.2004.04403.x. [DOI] [PubMed] [Google Scholar]
- 13.Burns KE, et al. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Mol Cell. 2010;39:821–827. doi: 10.1016/j.molcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imkamp F, et al. Deletion of dop in Mycobacterium smegmatis abolishes pupylation of protein substrates in vivo. Mol Microbiol. 2010;75:744–754. doi: 10.1111/j.1365-2958.2009.07013.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, et al. Mycobacterium tuberculosis proteasome accessory factor A (PafA) can transfer prokaryotic ubiquitin-like protein (pup) between substrates. MBio. 2017;8:e00122-17. doi: 10.1128/mBio.00122-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Festa RA, et al. Prokaryotic ubiquitin-like protein (Pup) proteome of Mycobacterium tuberculosis [corrected] PLoS One. 2010;5:e8589. doi: 10.1371/journal.pone.0008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samanovic MI, et al. Proteasomal control of cytokinin synthesis protects Mycobacterium tuberculosis against nitric oxide. Mol Cell. 2015;57:984–994. doi: 10.1016/j.molcel.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watrous J, et al. Expansion of the mycobacterial “PUPylome.”. Mol Biosyst. 2010;6:376–385. doi: 10.1039/b916104j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulsen C, et al. Proteome-wide identification of mycobacterial pupylation targets. Mol Syst Biol. 2010;6:386. doi: 10.1038/msb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Küberl A, et al. Pupylated proteins in Corynebacterium glutamicum revealed by MudPIT analysis. Proteomics. 2014;14:1531–1542. doi: 10.1002/pmic.201300531. [DOI] [PubMed] [Google Scholar]
- 21.Fascellaro G, et al. Comprehensive proteomic analysis of nitrogen-starved Mycobacterium smegmatis Δpup reveals the impact of pupylation on nitrogen stress response. J Proteome Res. 2016;15:2812–2825. doi: 10.1021/acs.jproteome.6b00378. [DOI] [PubMed] [Google Scholar]
- 22.Küberl A, Polen T, Bott M. The pupylation machinery is involved in iron homeostasis by targeting the iron storage protein ferritin. Proc Natl Acad Sci USA. 2016;113:4806–4811. doi: 10.1073/pnas.1514529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamichhane G, et al. Deletion of a Mycobacterium tuberculosis proteasomal ATPase homologue gene produces a slow-growing strain that persists in host tissues. J Infect Dis. 2006;194:1233–1240. doi: 10.1086/508288. [DOI] [PubMed] [Google Scholar]
- 24.Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science. 2005;310:1960–1963. doi: 10.1126/science.1121925. [DOI] [PubMed] [Google Scholar]
- 25.Suraweera A, Münch C, Hanssum A, Bertolotti A. Failure of amino acid homeostasis causes cell death following proteasome inhibition. Mol Cell. 2012;48:242–253. doi: 10.1016/j.molcel.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elharar Y, et al. Survival of mycobacteria depends on proteasome-mediated amino acid recycling under nutrient limitation. EMBO J. 2014;33:1802–1814. doi: 10.15252/embj.201387076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viljoen AJ, Kirsten CJ, Baker B, van Helden PD, Wiid IJ. The role of glutamine oxoglutarate aminotransferase and glutamate dehydrogenase in nitrogen metabolism in Mycobacterium bovis BCG. PLoS One. 2013;8:e84452. doi: 10.1371/journal.pone.0084452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouzy A, Poquet Y, Neyrolles O. Nitrogen metabolism in Mycobacterium tuberculosis physiology and virulence. Nat Rev Microbiol. 2014;12:729–737. doi: 10.1038/nrmicro3349. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Niederweis M. Uptake of sulfate but not phosphate by Mycobacterium tuberculosis is slower than that for Mycobacterium smegmatis. J Bacteriol. 2012;194:956–964. doi: 10.1128/JB.06132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouzy A, et al. Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathog. 2014;10:e1003928. doi: 10.1371/journal.ppat.1003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouzy A, et al. Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat Chem Biol. 2013;9:674–676. doi: 10.1038/nchembio.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieck B, et al. PknG senses amino acid availability to control metabolism and virulence of Mycobacterium tuberculosis. PLoS Pathog. 2017;13:e1006399. doi: 10.1371/journal.ppat.1006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stermann M, Sedlacek L, Maass S, Bange FC. A promoter mutation causes differential nitrate reductase activity of Mycobacterium tuberculosis and Mycobacterium bovis. J Bacteriol. 2004;186:2856–2861. doi: 10.1128/JB.186.9.2856-2861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malm S, et al. The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD and the response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis. Microbiology. 2009;155:1332–1339. doi: 10.1099/mic.0.023275-0. [DOI] [PubMed] [Google Scholar]
- 35.Narberhaus F. Negative regulation of bacterial heat shock genes. Mol Microbiol. 1999;31:1–8. doi: 10.1046/j.1365-2958.1999.01166.x. [DOI] [PubMed] [Google Scholar]
- 36.Stewart GR, et al. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology. 2002;148:3129–3138. doi: 10.1099/00221287-148-10-3129. [DOI] [PubMed] [Google Scholar]
- 37.Martin J, Mayhew M, Langer T, Hartl FU. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature. 1993;366:228–233. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- 38.Langer T, Pfeifer G, Martin J, Baumeister W, Hartl FU. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992;11:4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayer-Hartl M, Bracher A, Hartl FU. The GroEL-GroES chaperonin machine: A nano-cage for protein folding. Trends Biochem Sci. 2016;41:62–76. doi: 10.1016/j.tibs.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Kong TH, Coates AR, Butcher PD, Hickman CJ, Shinnick TM. Mycobacterium tuberculosis expresses two chaperonin-60 homologs. Proc Natl Acad Sci USA. 1993;90:2608–2612. doi: 10.1073/pnas.90.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeJesus MA, et al. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. MBio. 2017;8:e02133-16. doi: 10.1128/mBio.02133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Susin MF, Baldini RL, Gueiros-Filho F, Gomes SL. GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J Bacteriol. 2006;188:8044–8053. doi: 10.1128/JB.00824-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman E, et al. Global aggregation of newly translated proteins in an Escherichia coli strain deficient of the chaperonin GroEL. Proc Natl Acad Sci USA. 2006;103:15800–15805. doi: 10.1073/pnas.0607534103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maisnier-Patin S, et al. Genomic buffering mitigates the effects of deleterious mutations in bacteria. Nat Genet. 2005;37:1376–1379. doi: 10.1038/ng1676. [DOI] [PubMed] [Google Scholar]
- 45.Kerner MJ, et al. Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell. 2005;122:209–220. doi: 10.1016/j.cell.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Coleman KJ, Cornish-Bowden A, Cole JA. Purification and properties of nitrite reductase from Escherichia coli K12. Biochem J. 1978;175:483–493. doi: 10.1042/bj1750483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Gerdes SY, et al. From genetic footprinting to antimicrobial drug targets: Examples in cofactor biosynthetic pathways. J Bacteriol. 2002;184:4555–4572. doi: 10.1128/JB.184.16.4555-4572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodionova IA, et al. Metabolic and bactericidal effects of targeted suppression of NadD and NadE enzymes in mycobacteria. MBio. 2014;5:e00747-13. doi: 10.1128/mBio.00747-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kemp JD, Atkinson DE. Nitrite reductase of Escherichia coli specific for reduced nicotinamide adenine dinucleotide. J Bacteriol. 1966;92:628–634. doi: 10.1128/jb.92.3.628-634.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodionova IA, et al. Mycobacterial nicotinate mononucleotide adenylyltransferase: Structure, mechanism, and implications for drug discovery. J Biol Chem. 2015;290:7693–7706. doi: 10.1074/jbc.M114.628016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns KE, Pearce MJ, Darwin KH. Prokaryotic ubiquitin-like protein provides a two-part degron to Mycobacterium proteasome substrates. J Bacteriol. 2010;192:2933–2935. doi: 10.1128/JB.01639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aly S, et al. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J Pathol. 2006;210:298–305. doi: 10.1002/path.2055. [DOI] [PubMed] [Google Scholar]
- 54.Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sohaskey CD, Wayne LG. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol. 2003;185:7247–7256. doi: 10.1128/JB.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wayne LG, Hayes LG. Nitrate reduction as a marker for hypoxic shiftdown of Mycobacterium tuberculosis. Tuber Lung Dis. 1998;79:127–132. doi: 10.1054/tuld.1998.0015. [DOI] [PubMed] [Google Scholar]
- 57.Akhtar S, Khan A, Sohaskey CD, Jagannath C, Sarkar D. Nitrite reductase NirBD is induced and plays an important role during in vitro dormancy of Mycobacterium tuberculosis. J Bacteriol. 2013;195:4592–4599. doi: 10.1128/JB.00698-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cunningham-Bussel A, Bange FC, Nathan CF. Nitrite impacts the survival of Mycobacterium tuberculosis in response to isoniazid and hydrogen peroxide. MicrobiologyOpen. 2013;2:901–911. doi: 10.1002/mbo3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cunningham-Bussel A, Zhang T, Nathan CF. Nitrite produced by Mycobacterium tuberculosis in human macrophages in physiologic oxygen impacts bacterial ATP consumption and gene expression. Proc Natl Acad Sci USA. 2013;110:E4256–E4265. doi: 10.1073/pnas.1316894110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roncarati D, Danielli A, Scarlato V. The HrcA repressor is the thermosensor of the heat-shock regulatory circuit in the human pathogen Helicobacter pylori. Mol Microbiol. 2014;92:910–920. doi: 10.1111/mmi.12600. [DOI] [PubMed] [Google Scholar]
- 61.Hitomi M, Nishimura H, Tsujimoto Y, Matsui H, Watanabe K. Identification of a helix-turn-helix motif of Bacillus thermoglucosidasius HrcA essential for binding to the CIRCE element and thermostability of the HrcA-CIRCE complex, indicating a role as a thermosensor. J Bacteriol. 2003;185:381–385. doi: 10.1128/JB.185.1.381-385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Houry WA, Frishman D, Eckerskorn C, Lottspeich F, Hartl FU. Identification of in vivo substrates of the chaperonin GroEL. Nature. 1999;402:147–154. doi: 10.1038/45977. [DOI] [PubMed] [Google Scholar]
- 63.Chaudhuri TK, Farr GW, Fenton WA, Rospert S, Horwich AL. GroEL/GroES-mediated folding of a protein too large to be encapsulated. Cell. 2001;107:235–246. doi: 10.1016/s0092-8674(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 64.Farr GW, et al. Folding with and without encapsulation by cis- and trans-only GroEL-GroES complexes. EMBO J. 2003;22:3220–3230. doi: 10.1093/emboj/cdg313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorci L, et al. Targeting NAD biosynthesis in bacterial pathogens: Structure-based development of inhibitors of nicotinate mononucleotide adenylyltransferase NadD. Chem Biol. 2009;16:849–861. doi: 10.1016/j.chembiol.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anandan T, et al. Phosphorylation regulates mycobacterial proteasome. J Microbiol. 2014;52:743–754. doi: 10.1007/s12275-014-4416-2. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y, et al. Mycobacterium tuberculosis proteasomal ATPase Mpa has a β-grasp domain that hinders docking with the proteasome core protease. Mol Microbiol. 2017;105:227–241. doi: 10.1111/mmi.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atlas RM, Snyder JW. Handbook of Media for Clinical Microbiology. CRC Press; Boca Raton, FL: 1995. [Google Scholar]
- 69.van Soolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: Evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: Mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Festa RA, et al. A novel copper-responsive regulon in Mycobacterium tuberculosis. Mol Microbiol. 2011;79:133–148. doi: 10.1111/j.1365-2958.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherwood AR, Paasch BC, Worby CA, Gentry MS. A malachite green-based assay to assess glucan phosphatase activity. Anal Biochem. 2013;435:54–56. doi: 10.1016/j.ab.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.