Abstract
Aim
We aimed to assess the safety and efficacy of propofol versus midazolam in cirrhotic patients undergoing upper GI endoscopy.
Methods
Ninety compensated cirrhotic patients (all met class I–III criteria according to the American Society of Anesthesia) were enrolled in this comparative study. They were classified into three groups according to scheduled pre‐endoscopy sedation drugs; the midazolam group, which included 30 patients who received IV weight‐dependent midazolam (0.05 mg/kg with additional doses of 1 mg every 2 min when necessary, up to a maximum dose of 0.1 mg/kg or 10 mg); the propofol group, which included 30 patients who received a propofol bolus dose according to age and weight (0.25 mg/kg with additional doses of 20–30 mg every 30–60 s when necessary, up to a maximum dose of 400 mg); and the combined group, which included 30 patients who received half a dose of midazolam and of propofol.
Results
Prolonged postendoscopy recovery times were reported in the midazolam group, while shorter recovery times were reported in the propofol and combined groups. All patients in the propofol and combined groups gained consciousness shortly postendoscopy; however, only half of the midazolam group's patients gained consciousness after the standard recovery time (10–30 min). Highly significant differences were found among the three groups regarding consciousness level according to the Glasgow coma scale, as well as regarding the occurrence of hypoxia during endoscopy.
Conclusion
Considering safety and efficacy issues, propofol is better than midazolam in gastrointestinal endoscopy, especially in patients with liver cirrhosis.
Keywords: endoscopy, liver cirrhosis, midazolam, pre‐endoscopy sedation, propofol
Introduction
Liver cirrhosis is most usually caused by viral hepatitis, mainly HBV and HCV, alcoholism, and steatohepatitis, but has many other possible causes.1
Portal hypertension is a frequent clinical and radiological syndrome, often accompanying liver cirrhosis, and is defined as a pathological increase in the portal venous pressure that reflected the increase in pressure gradient between the portal vein and the inferior vena cava (normal level = 1–5 mmHg). When the portal pressure gradient rises above 10–12 mmHg, complications of portal hypertension can arise, including esophageal varices, ascites, and hepatorenal syndrome.2
Cirrhotic patients commonly undergo upper gastrointestinal endoscopy (UGIE) for screening and/or treatment of portal hypertension‐related complications. These endoscopic procedures can often cause pain or discomfort, and sedation is recommended to minimize anxiety and provide optimum conditions to perform the examination safely, and it also increases willingness to undergo a repeat procedure.3
Four stages of sedation have been described, ranging from minimal to moderate, deep, and general anesthesia. In general, most GI endoscopy procedures are performed under moderate sedation, a practice that was formerly referred to as “conscious sedation”.3 Although liver cirrhosis impairs protein synthesis, alters drug metabolism pathways, and compromises hepatic blood flow, all these factors may affect the pharmacokinetics of the sedative drugs; however, there are no current pre‐endoscopy sedation guidelines for cirrhotic patients.4
Benzodiazepines alone or in combination with opioids are still the most commonly used drugs for pre‐endoscopy conscious sedation in general and in cirrhotic patients during UGIE. Currently, over 90% of UGIEs in the United Kingdom and United States are conducted under intravenous conscious sedation, usually with a benzodiazepine. Despite recent advances, there is no tradition of formal training in techniques of sedating patients for endoscopic procedures. All practitioners need proper instruction on sedation techniques in cirrhotic patients, and refresher courses are indicated. Increased provision of training in sedation should be considered.5
Sedation during GI endoscopy is often achieved using propofol or midazolam in the general population. However, impaired protein synthesis, altered drug metabolism, and compromised hepatic blood flow in patients with liver cirrhosis might affect the pharmacokinetics of sedatives, placing cirrhotic patients undergoing endoscopy at a greater risk of adverse events. The objective of this study was to compare the safety and efficacy of propofol versus midazolam in patients with liver cirrhosis undergoing UGIE.
Methods
Study design and settings
This comparative study had been carried out in GI endoscopy units of the Internal Medicine and Tropical Medicine departments, Zagazig University Hospitals, Egypt, for a 6‐month period from November 2015 to April 2016.
Target population
Out of 2000 cirrhotic attendants of our GI endoscopy units for diagnostic and/or therapeutic UGIE (diagnostic screening for esophageal varices and therapeutic interventions for band ligation and sclerotherapy of the varices), 90 compensated cirrhotic patients were eligible to be included in the present work, satisfying our inclusion and exclusion criteria.
Inclusion criteria
Those educated, able to pass number connection test (NCT‐A), compensated cirrhotic patients eligible for diagnostic and/or therapeutic UGIE.
Exclusion criteria
Exclusion criteria were: known allergy or previous adverse reactions to midazolam and/or propofol, patients with significant respiratory airway disease or cardiac morbidity, and cirrhotic patients under categories Child B and C.
Patient's classification
Participants were classified into three equal groups according to the scheduled pre‐endoscopy sedative drugs: the midazolam group, which included 30 patients who received i.v. weight‐dependent midazolam (0.05 mg/kg) with additional doses of 1 mg every 2 min when necessary up to a maximum dose of 0.1 mg/kg or 10 mg; the propofol group, which included 30 patients who received a propofol IV bolus dose according to age and weight (0.25 mg/kg) with additional doses of 20–30 mg every 30–60 s when necessary, up to a maximum dose of 400 mg; and the combined group, which included 30 patients who received a half dose of the previous midazolam and of propofol (Fig. 1).
Figure 1.
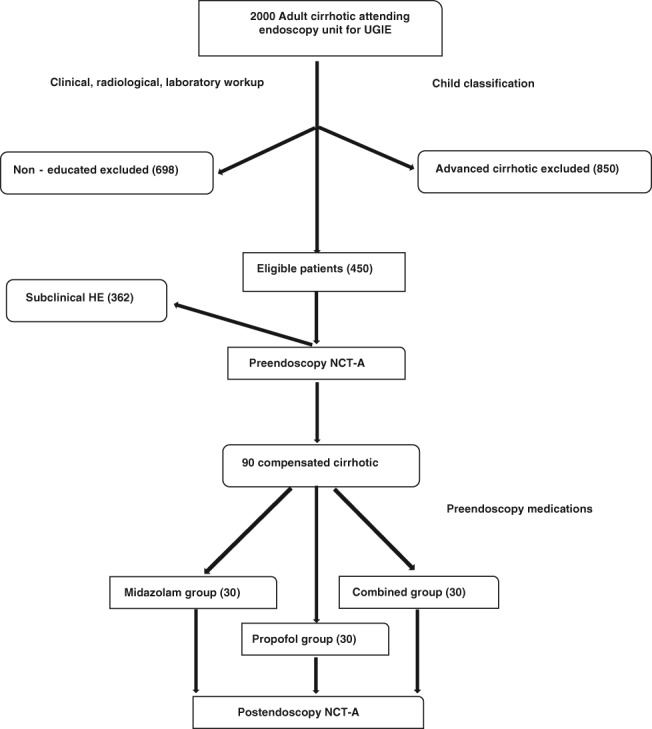
Patient flow chart.
Drugs’ efficacy, recovery time, and endoscopy time
The sedation scheme was considered ineffective when UGIE was interrupted by agitation or intolerance by the patient despite reaching the maximum sedative dose. Recovery time is the time lapse between the end of endoscopic procedure and full gain of consciousness. Endoscopy time is the time passed from start till the end of UGIE.6
Study tools
Pre‐endoscopy workup
Written consent was obtained from all enrolled participants; oral explanation of the procedure and its complications regarding their participation in the study was clearly delivered to them. Complete clinical examination of all patients aimed to exclude cardiac, respiratory, or advanced cirrhotic cases (Child B &C). Laboratory tests like complete blood count (CBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin, prothrombin time (PT), international normalized ratio (INR), and direct bilirubin were ordered, as well as serum creatinine and blood urea. Real‐time abdominal ultrasound was used to confirm liver cirrhosis, splenomegaly, evidence of portal hypertension, and presence of ascites if any. The NCT‐A test was used to detect subclinical hepatic encephalopathy before UGIE. This psychometric test measures concentration, motor speed, and visuospatial control (Normal NCT‐A time = 30 s). The patient was instructed to join the numbered circles in order on a piece of paper, and the time required to complete the task was scored, as well as the shape of line connection.
Endoscopy workup
We aimed to assess the ease of UGIE by the endoscopist (who was not aware about the type of drug used). During the endoscopy, the patient was monitored with pulse oximetry and noninvasive blood pressure measurement. Heart rate, respiratory frequency, oxygen saturation, and blood pressure were also monitored and recorded. Efficacy of the sedative drugs used in the study, the ease of performing the procedure by the endoscopist, cooperation of the patient (comfort of the endoscopist), and comfort of the patient were assessed by watching the patient's facial expression and gagging reflex.
Postendoscopy workup
We aimed to detect hepatic encephalopathy and record recovery time by repeating the NCT‐A and assessing the patient's consciousness level according to Observer's Assessment of Alertness/Sedation Scale (OAAS). In the recovery room, a physician measured and recorded the same previous clinical parameters every 15 min until hospital discharge. All patients received 3 L/min of oxygen supplementation through a nasal catheter.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science version 14 (spss Inc., New York, NY, USA). For comparison of proportions, a chi‐square test was used, and a p value equal or less than 0.05 was considered significant.
Results
Participants were matched for age and gender; however, males accounted for a majority of participants. However, there was no significant difference between each gender when comparing the three groups (Table 1).
Table 1.
Demographic data of the studied groups
| Midazolam group | Propofol group | Combined group | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD | Mean ± SD | Mean ± SD | P | ||||
| Age (years) | 52.06 ± 7.9 | 51.7 ± 8.39 | 53.13 ±9.61 | NS | ||||
| Sex | No. | % | No. | % | No. | % | X 2 | P |
| 23 | 76.7 | 22 | 73.3 | 26 | 86.7 | 1.7 | NS | |
| 7 | 23.3 | 8 | 26.7 | 4 | 13.3 | |||
After prolonged endoscopy, prolonged recovery times were noticed in the midazolam group, while shorter recovery times recorded in propofol and combined groups ([31.06 ± 6.25], [6.06 ± 2.1], and [12.76 ± 2.67], respectively, and P = [< 0.001]). Intraprocedural hypoxia was less frequent in the propofol and combined groups in comparison to the midazolam group; however, no significant difference was found between the propofol and combined groups. An anova study of the same parameters showed no significant difference between the midazolam group and propofol group, but significant and highly significant differences were found between the combined group and both the propofol and midazolam groups (P ≤ 0.05 and P ≤ 0.001), respectively (Tables 2 and 3).
Table 2.
Endoscopy time, recovery time, and hypoxia among the studied groups
| Midzolam group | Propofol group | Combined group | ||
|---|---|---|---|---|
| Variable | Mean ± SD | Mean ± SD | Mean ± SD | P |
| Enodoscpy time (min.) | 3.18 ± 1.14 | 3.59 ± 0.93 | 3.21 ± 1.06 | < 0.001 |
| Reocvery time (min.) | 31.06 ± 6.25 | 6.06 ± 2.13 | 12.76 ± 2.67 | < 0.001 |
| Hypoxia (% of Pts) | 23% | 000 | 000 | < 0.001 |
Table 3.
Endoscopy time, recovery time, and oxygen saturation among groups
| Endoscopy time (min.) | Recovery time (min.) | Hypoxia % | ||
|---|---|---|---|---|
| Midazolam | Propofol | NS | < 0.001 | < 0.001 |
| Combined | < 0.001 | < 0.001 | < 0.05 | |
| Propofol | Midazolam | NS | < 0.001 | < 0.001 |
| Combined | < 0.05 | < 0.001 | NS | |
| Combined | Midazolam | < 0.001 | < 0.001 | < 0.05 |
| Propofol | < 0.05 | < 0.001 | NS | |
A highly significant difference was noted among the three groups regarding consciousness level score assessment after endoscopy using OAAS scale (Table 4): more than half of patients in the propofol and combined groups gained consciousness (score 5) after endoscopy; approximately 60% in propofol group and approximately 50% in combined groups, while only 25% did in the midazolam group, and intermediate scores (3–4 score) included 30, 33, and 20, respectively. Moreover, patients who obtained a score of 3 or less (delayed recovered time and late gain of consciousness level) in the three groups were 10, 20, and 57%, respectively (Tables 4 and 5).
Table 4.
Observer's assessment of alertness/sedation scale
| Responsiveness | Speech | Facial expression | Eye | Score |
|---|---|---|---|---|
| Responds readily to name | Normal | Normal | Clear with no ptosis | 5 |
| Lethargic response to name | Mild slowing or thickening | Mild relaxing | Glazed or mild | 4 |
| Responds only after name is called loudly and/or repeatedly | Slurring or prominent slowing | Marked relaxation | Ptosis | 3 |
| Responds only after mild prodding or shaking | Few recognizable words | — | Glazed and marked ptosis | 2 |
| Does not respond to mild prodding or shaking | — | — | — | 1 |
Table 5.
Consciousness assessment among groups according to OAAS scale
| Score | Midazolam | Propofol | Combined | X 2 | P | |||
|---|---|---|---|---|---|---|---|---|
| No | % | No. | % | No. | % | |||
| Score 5 | 7 | 23.3 | 18 | 60 | 14 | 46.7 | 14.3 | 0.001 |
| Score 3–4 | 6 | 20 | 9 | 30 | 10 | 33.3 | ||
| 3 | 17 | 56.7 | 3 | 10 | 6 | 20 | ||
| Total | 30 | 100 | 30 | 100 | 30 | 100 | ||
Intraprocedural hypoxia (O2% less than 90%) was recorded in the midazolam group (seven patients), while no hypoxia was reported in any patient in both the propofol and combined groups (Table 6 and Fig. 2).
Table 6.
Intraprocedural hypoxia occurrance among the studied groups†
| Midazolam | Propofol | Combined | Total: 90 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 30 | N = 30 | N = 30 | ||||||||
| Hypoxia | No. | % | No | % | No | % | No | % | X 2 | P |
| Yes | 7 | 23.3 | 0 | 0.0 | 0 | 0.0 | 7 | 7.8 | 15.1 | <0.001 |
| No | 23 | 76.7 | 30 | 100.0 | 30 | 100.0 | 83 | 92.2 | ||
Hypoxia was preset if O2 % value(measured by pulse oximetry) was below 95% and absent if O2 > 95%.
Figure 2.
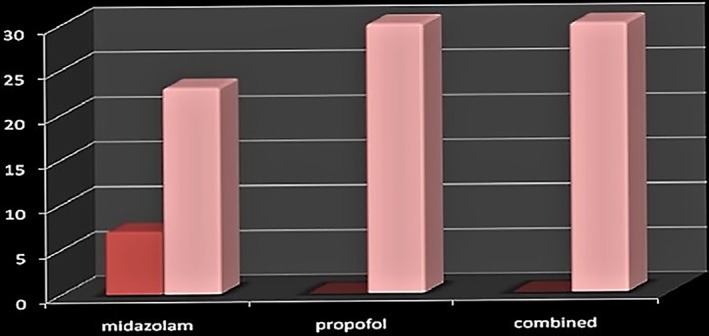
Intraprocedural hypoxia among the groups.  , hypoxic;
, hypoxic;  , normal.
, normal.
Pre‐ and postendoscopy number connection test (NCT‐A) times were prolonged in the midazolam group than in the propofol and combined groups (P < 0.001). Pre‐endoscopy NCT‐A scores in the midazolam, propofol, and combined groups were: (64.7 ± 23.6), (58.06 ± 16), and (37.7 ± 10.7), respectively, and postendoscopy NCT‐A scores were (115.5 ± 43.6), (52.7 ± 12.5), and (39.06 ± 9.3), respectively. However, a significant difference was found between the midazolam group and propofol group, but highly significant differences were reported in the combined group in comparison to both the propofol and midazolam groups (P < 0.001) Pre‐endoscopy NCT‐A was highly statistically positively correlated with postendoscopy NCT‐A and oxygen saturation in the midazolam group, while age and pre‐endoscopy NCT‐A were positively correlated with postendoscopy NCT‐A in the propofol group and the combined group. Moreover, the pre‐endoscopy NCT‐A was also positively correlated with postendoscopy NCT‐A in the combined group (Table 7).
Table 7.
Pre‐ and postendoscopy NCT‐A among the studied groups
| Midazolam group | Propofol group | Combined group | ||
|---|---|---|---|---|
| Variable | Mean ± SD | Mean ± SD | Mean ± SD | P |
| Pre‐endoscopy NCT (s) | 64.73 ± 23.65 | 48.06 ± 16.04 | 37.76 ± 10.73 | <0.001 |
| Postendoscopy NCT (s) | 115.5 ± 43.57 | 42.7 ± 12.46 | 39.06 ± 9.32 | <0.001 |
Discussion
Cirrhotic patients often undergo UGIE for screening and treatment of portal hypertension‐related complications. These endoscopic procedures usually cause pain and/or discomfort; thus, pre‐endoscopy sedation is recommended to minimize anxiety and provide conditions to perform the examination safely. It also increases willingness to undergo a repeat procedure.3 Sedation comprises a continuum of states that include minimal sedation (anxiolysis), moderate sedation (conscious), deep sedation, and general anesthesia.6 Routine UGIE can be performed successfully with either moderate or deep sedation; however, moderate sedation, “conscious sedation,” provides adequate anxiolysis, pain control, and amnesia for most patients and is safer than deep sedation.7
The use of conscious sedation for routine endoscopic procedures varies widely throughout the world. 7 Cohen LB et al. suggested that more than 98% of UGIEs and colonoscopies are performed with sedation; however, in many European countries, endoscopy is commonly performed without sedation.8
Several agents are available for the achievement of moderate sedation during UGIE in cirrhotic patients. These agents include benzodiazepines, narcotics, propofol, neuroleptic tranquilizers, antihistamines, and dopaminergic receptor antagonists (promethazine). A combination of a short‐acting benzodiazepine (midazolam) and a narcotic (pethidine) is used in approximately three fourths of procedures, and propofol alone is used in approximately 25%.9
In hepatic patients, topical anesthesia alone is not enough for pain‐free UGIE procedures. In contrast, general anesthesia, which may be of benefit for both the patient and the endoscopist, may be difficult to administer and may precipitate hepatic encephalopathy. In addition, the lack of experience in anesthesia care among endoscopy personnel might increase the risk of complications. The use of propofol for pre‐endoscopy conscious sedation in cirrhotic patients may be increasing, and propofol combined with midazolam, with or without fentanyl, has already been used in several GI endoscopic procedures. Moreover, sedation with or without propofol is safe and well tolerated by most patients, including the cirrhotic ones.10
Wang et al. concluded that propofol is safe and effective for UGIE in normal and cirrhotic patients and is associated with shorter recovery and discharge periods, higher postanesthesia recovery scores, better sedation, and greater patient cooperation than traditional sedation (midazolam), without any increase in cardiopulmonary complications or precipitating hepatic encephalopathy.11 Moreover, Poulos et al. recommended propofol as the sole pre‐endoscopy sedative agent in cirrhotic patients as it resulted in less patient time in the endoscopy unit, quicker recovery, and faster discharge than did regimens using midazolam‐based sedation. Propofol sedation also resulted in greater patient satisfaction, less pain and awareness during the procedure, and increased awareness at the end of the procedure compared with other anesthetic techniques.12
Our study showed that the use of propofol sedation in UGIE is safe and effective in cirrhotic patients regardless of the age and/or gender of the participants. The results of Correia et al.13 were in concordance with our results. Moreover, Kerker et al.14 underwent a comparative analysis of a geriatric cirrhotic patient population with a high level of comorbidity; they did not observe a significant increase in the complication rate for combined propofol/midazolam sedation for UGIE. Martínez et al.15 found that continuous propofol sedation in patients >80 years of age is also generally as safe as in younger patents, although patients >80 years showed a greater tendency for complications.
Our study showed that there were highly significant differences among the three studied groups regarding endoscopy time, recovery time, and oxygen saturation. Prolonged recovery times were reported in midazolam group [approximately five times that of propofol], while shorter recovery times were reported in the propofol and combined groups [the latter approximately twice that of the former group], illustrating the marked superiority of propofol in this context. Our results agree with those of Carlsson and Grattidge16 who reported better compliance, sedative effect, and more rapid recovery with propofol compared to midazolam, but they recorded similar anterograde amnesia and arterial blood oxygen saturation. On the other hand, however, our results disagree with those of Koo et al.,35 who found that low‐ or high‐dose midazolam and propofol combinations have a similar sedative effect as a high dose of midazolam alone, and there was no significant difference regarding recovery time, endoscopy time, or oxygen saturation among the studied groups. Differences in patient selection, group stratification, and/or patients’ randomization might underlie the apparent discrepancy in our findings.
In the same context, McQuaid and Laine compared the relative efficacy, safety, and efficiency of both midazolam and propofol for moderate sedation in UGIEs in cirrhotic patient and confirmed that moderate sedation provides a high level of physician and patient satisfaction and a low risk of serious adverse events with both agents. They found midazolam‐based regimens to have longer sedation and recovery times than propofol‐based regimens. However, they found no significant differences regarding endoscopy times and Child‐Pugh score.17 It is worth noting here that our study subjects were all within the Child A class [score 5 or 6], and accordingly, we have no input to discuss with the latter finding.
In a meta‐analysis study conducted by Singh et al.,18 which included 20 studies on the use of midazolam and propofol for UGIEs in cirrhotic patients, the analysis showed that recovery and discharge times were shorter with the use of propofol than midazolam. There was also higher patient satisfaction with the use of propofol, although no significant differences were reported in the endoscopy time between the two agents. In another study, Levitzky et al. showed that propofol sedation used to induce moderate sedation in cirrhotic patients undergoing UGIEs results in better patient satisfaction and a shorter recovery time and less hypoxemia than standard sedation by midazolam.19
Rex et al. reported propofol administration by a nonanesthesiologist in 2000 patients and detected hypoxia of less than 90% of O2 in only four cases, all during endoscopy, which was countered by using oxygen mask. They added that propofol was safely administered by a skilled nurse under the supervision of the endoscopist.20 Moreover, Cho et al. performed UGIE using propofol in a low‐risk group with ASA classifications I and II and a high‐risk group of ASA classifications III and IV and found that the high‐risk group experienced significantly increased incidence of hypoxia and a single case of apnea during the procedure. Oxygen saturation was reduced to 90% or less in one patient in each group for a short period, less than 10 s, but immediately returned to normal after using the oxygen mask.21
Propofol, a widely employed drug for conscious sedation during UGIE because it is easy to use, has a good safety and efficacy profile due to its quick onset of action, rapid metabolism, and significantly shorter recovery time and has some antiemetic, bronchodilator, and anticonvulsant effects.22 Our study showed that there was a highly significant difference among the studied groups regarding postendoscopy consciousness level according to Glasgow coma scale. All patients of combined and propofol groups gained consciousness shortly (5–15 min) after the endoscopy; however, only half of patients in the midazolam group gained consciousness after the standard recovery time (more than 30 min). It was also noted that postendoscopy NCTs in the midazolam group were prolonged than those of the propofol and combined groups. Assy et al.,23 in a case–control study, confirmed our conclusions and demonstrated that most cirrhotic patients with subclinical hepatic encephalopathy (SHE) before sedation became worse after midazolam administration. In addition, Vasudevan et al. observed that 54.1% of cirrhotic patients presented prolonged NCT times, suggesting SHE before UGIE, and 75.4% had impaired test results after sedation with midazolam.24
A cohort study conducted by Amoros et al.36 demonstrated that even deep sedation with propofol did not precipitate subclinical or overt hepatic encephalopathy in cirrhotic patients. This observation was also confirmed by Riphaus et al.2 in a prospective, randomized study comparing propofol with midazolam for pre‐endoscopic sedation in cirrhotic patients during UGIE, and they concluded that propofol sedation results in better patient satisfaction and a shorter recovery time with less hypoxemia than standard sedation with midazolam, although no significant differences were reported in the endoscopy time. Moreover, Assy et al. confirmed a significant prolongation of the NCT results in only 10 of 40 patients (25%) with liver cirrhosis after sedation with midazolam, and it has been shown that other factors, like the level of patient education, might also influence the NCT results.23
Our study used standard patient monitoring, including recording of blood pressure, pulse rate, respiratory rate, and pulse oximetry. We noticed a significant difference between the midazolam, propofol, and combined groups regarding complications of sedation, (mainly of hypoxia) during endoscopy; hypoxia was more frequent in the midazolam group; hypoxia was recorded in 7 out of 30 patients (23%), while no hypoxia was reported in the propofol and combined groups. Heuss et al. concluded that endoscopic sedation does not increase the complication rate in comparison to the nonsedation groups, and sedation‐related complications were recorded in 0.54% of the sedated groups.25 Moreover, Correia et al. reported that sedation‐related complications were not statistically different between the studied groups and were observed in 22 of 210 patients (10.5%). In the midazolam group, 8 of 110 patients (7.3%) experienced complications compared with 14 of 100 (14%) in the propofol group. In the propofol group, simultaneous complications were detected in one patient—mild hypotension and bradycardia—and serious complications (severe hypotension requiring intravenous saline) occurred in five of 210 patients (2.4%); four were recorded in the midazolam group and one in the propofol group.13 Amornyotin et al. reported a difference in the incidence of complication rate between cirrhotic patients who received propofol‐based and non‐propofol‐based sedation during UGIE procedures. They showed that propofol‐based sedation does not increase the rate of complication during UGIEs.26 On the contrary, we reported no serious complications in our study. However, the total number of subjects in the present work was less than half of those on Correia et al.
In our study, a trained nurse administered the pre‐endoscopy medications to our participants. Rex et al. confirmed the safety of endoscopist‐directed propofol sedation (EDP) in UGIE procedures and estimated the cost of substituting anesthesia specialists for endoscopists in the delivery of sedation for UGIE. EDP sedation is safe or safer than endoscopist‐administered opioids and benzodiazepines.27 Furthermore Clark AC et al. showed that the presence of anesthesiologists for sedation in UGIE procedures is costly and not technically needed.28
In the present work, we detected better endoscopic outcomes with a combination of low‐dose midazolam and propofol. Reimann et al. and Waring et al. confirmed the same conclusion and added that low‐dose midazolam and propofol combination treatment induced a synergistic effect in sedation for EGD in patients 60 years old or younger, better comfort during the procedure, and shorter recovery time than a combination of midazolam and opioids.29, 30 In the same context, Cho et al. and Chernik et al., Rex & Khalafan reported that low‐dose midazolam and propofol combination treatment induces a better sedative effect and endoscopist's satisfaction compared to midazolam single treatment with a similar degree of complication and consciousness recovery as a sedation strategy for UGIE.31, 34
A comprehensive meta‐analysis published in Taiwan in 201532 suggested that propofol‐based sedation for UGIEs in cirrhotic patients provided more rapid sedation and recovery than midazolam did, and the risk of sedation‐related side effects for propofol did not differ significantly from that of midazolam. Moreover, Chernik et al. confirmed the efficacy of propofol in cirrhotic patients undergoing endoscopy is superior to those of midazolam.33 Testing these conclusions in our Egyptian population, as we illustrated in the discussion, led to the conclusion that, considering safety and efficacy issues, propofol is better than midazolam in GI endoscopy, especially in patients with liver cirrhosis. Propofol should replace midazolam in our endoscopy units, especially for high‐risk patients with advanced cirrhosis.
Declaration of conflict of interest: None.
References
- 1. Boon A, Nicholas D, Stanley RC et al Davidson's principles practice of medicine. Edinburgh: Elsevier/Churchill Livingstone, 2006); 212–13. [Google Scholar]
- 2. Riphaus A, Wehrmann T, Weber B et al S3 Guideline: sedation for gastrointestinal endoscopy 2008. Endoscopy. 2009), 2009; 41: 797–915. [Google Scholar]
- 3. Thuluvath PJ. Toward safer sedation in patients with cirrhosis; have we done enough? Gastrointest. Endosc. 2009; 70: 269–70. [DOI] [PubMed] [Google Scholar]
- 4. Aisenberg J, Cohen LB. Sedation in endoscopic practice. Gastrointest. Endosc. Clin N Am. 2006; 16: 695–708. [DOI] [PubMed] [Google Scholar]
- 5. American Society of Anesthesiologists . Task force on sedation and analgesia by non‐anesthesiologists. Practice guidelines for sedation and analgesia by non‐anesthesiologists. Anesthesiology. 2002; 96: 1004–117.11964611 [Google Scholar]
- 6. Correia L, Bonilha D, Gomes G et al Sedation during upper GI endoscopy in cirrhotic outpatients; a randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointest. Endosc. 2011; 73(1): 45–51. [DOI] [PubMed] [Google Scholar]
- 7. Rex DK. Moderate sedation for endoscopy; sedation regimens for non‐anaesthesiologists. Aliment. Pharmacol. Ther. 2006; 24: 163–71. [DOI] [PubMed] [Google Scholar]
- 8. Cohen LB, Delegge MH, Aisenberg J et al AGA institute review of endoscopic sedation. Gastroenterology. 2007; 133: 675–701. [DOI] [PubMed] [Google Scholar]
- 9. Cohen L, Wecsler J, Gaetano J et al Endoscopic sedation in the United States; results from a nationwide survey. Am. J. Gastroenterol. 2006; 101: 967–74. [DOI] [PubMed] [Google Scholar]
- 10. Faulx AL, Vela S, Das A et al The changing landscape of practice patterns regarding unsedated endoscopy and propofol use; a national web survey. Gastrointest. Endosc. 2005; 62(1): 9–15. [DOI] [PubMed] [Google Scholar]
- 11. Wang D, Chen C, Chen J et al The use of propofol as a sedative agent in gastrointestinal endoscopy; a meta‐analysis. PLoS One. 2013; 8(1): e53311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poulos JE, Kalogerinis PT, Caudle JN. Propofol compared with combination propofol or midazolam/fentanyl for endoscopy in a community setting. AANA J. 2013; 81: 31–6. [PubMed] [Google Scholar]
- 13. Correia LM, Bonilha DQ, Gomes GF et al Sedation during upper GI endoscopy in cirrhotic outpatients; a randomized, controlled trial comparing propofol and fentanylwith midazolam and fentanyl. Gastrointest. Endosc. 2011; 73(1): 45–51. [DOI] [PubMed] [Google Scholar]
- 14. Kerker A, Hardt C, Schlief HE et al Combined sedation with midazolam/propofol for gastrointestinal endoscopy in elderly patients. BMC Gastroenterol. 2010; 2010: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez JF, Aparicio JR, Compañy L et al Safety of continuous propofol sedation for endoscopic procedures in elderly patients. Rev. Esp. Enferm. Dig. 2011; 103: 76–82. [DOI] [PubMed] [Google Scholar]
- 16. Carlsson U and Grattidge P. Sedation for upper gastrointestinal endoscopy: a comparative study of propofol and midazolam. Endoscopy. 1995; 27: 240–3. [DOI] [PubMed] [Google Scholar]
- 17. McQuaid K, and Laine L. A systematic review and meta‐analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest. Endosc. 2008; 67: 910–23. [DOI] [PubMed] [Google Scholar]
- 18. Singh H, Poluha W, Cheung M et al Propofol for sedation during gastrointestinal endoscopy. Cochrane Database Syst. Rev. 2008; 4: CD006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwersen‐Bergmann S, Rösner P, Kühnau HC et al Death after excessive propofol abuse. Int. J. Legal Med. 2001; 114(4–5): 248–51. [DOI] [PubMed] [Google Scholar]
- 20. Rex D, Deenadayalu V, Eid E et al Endoscopist‐directed administration of propofol a worldwide safety experience. Gastroenterology. 2009; 137: 1229–37. [DOI] [PubMed] [Google Scholar]
- 21. Cho H, Kim YM, Oh JH et al The effect of propofol for conscious sedation during colonoscopy: a prospective, randomized study. Korean J Med. 2005; 69: 30–8. [Google Scholar]
- 22. Gasparović S, Rustemović N, Opacić M et al Clinical analysis of propofol deep sedation for 1104 patients undergoing gastrointestinal endoscopic procedures; a three‐year prospective study. World J. Gastroenterol. 2006; 12: 327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Assy N, Rosser B, Grahame G et al Risk of sedation for upper GI endoscopy exacerbating subclinical hepatic encephalopathy in patients with cirrhosis. Gastrointest. Endosc. 1999; 49: 690–4. [DOI] [PubMed] [Google Scholar]
- 24. Vasudevan AE, Goh KL, Bulgiba AM. Impairment of psychomotor responses after conscious sedation in cirrhotic patients undergoing therapeutic upper GI endoscopy. Am. J. Gastroenterol. 2002; 97: 1717–21. [DOI] [PubMed] [Google Scholar]
- 25. Heuss LT, Schnieper P, Drewe J et al Safety of propofol for conscious sedation during endoscopic procedures in high‐risk patients; a prospective, controlled study. Am. J. Gastroenterol. 2003; 98: 1751–7. [DOI] [PubMed] [Google Scholar]
- 26. Amornyotin S, Srikureja W, Pausawasdi N et al Intravenous sedation for gastrointestinal endoscopy in very elderly patients of Thailand. Asian Biomed. 2011; 5: 485–91. [Google Scholar]
- 27. Rex DK, Overley C, Kinser K et al Safety of propofol administered by registered nurses with gastroenterologist supervision in 2000 endoscopic cases. Am. J. Gastroenterol. 2002; 97: 1159–63. [DOI] [PubMed] [Google Scholar]
- 28. Clarke AC, Chiragakis L, Hillman LC et al Sedation for endoscopy; the safe use of propofol by general practitioner seditionists. Med. J. Aust. 2002; 176: 158–61. [DOI] [PubMed] [Google Scholar]
- 29. Reimann FM, Derad IS, Fuchs M et al Synergistic sedation with low‐dose midazolam and propofol for colonoscopies. Endoscopy. 2000; 32: 239–44. [DOI] [PubMed] [Google Scholar]
- 30. Waring JP, Baron TH, Hirota WK et al Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest. Endosc. 2003; 58: 317–22. [DOI] [PubMed] [Google Scholar]
- 31. Cho YS, Seo E, Han J‐H et al Comparison of midazolam alone versus midazolam plus propofol during endoscopic submucosal dissection. Clin. Endosc. 2012; 45: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsiao‐Chien, Tsai1 , Lin Yu‐Cih, et al. (2015): Propofol versus midazolam for upper gastrointestinal endoscopy in cirrhotic patients: a meta‐analysis of randomized controlled trials. DOI: 10.1371/journal.pone.0117585 [DOI] [PMC free article] [PubMed]
- 33. Chernik D, Gillings D, Laine H et al Validity and reliability of OAAS: study with intravenous midazolam. J. Clin. 1990; 10: 244–90. [PubMed] [Google Scholar]
- 34. Rex DK and Khalfan HK. Sedation and the technical performance of colonoscopy. Gastrointest. Endosc. Clin. N Am. 2005; 15(4): 661–72. [DOI] [PubMed] [Google Scholar]
- 35. Koo JS, Choi JH, Jung SW et al Conscious sedation with midazolam combined with propofol for colonoscopy. Korean J Gastrointest Endosc. 2007; 34: 298–303. [Google Scholar]
- 36. Amorós A, Aparicio JR, Garmendia M et al Deep sedation with propofol does not precipitate hepatic encephalopathy during elective upper endoscopy. Gastrointest. Endosc. 2009; 70: 262–8. [DOI] [PubMed] [Google Scholar]


