Abstract
Background and Aim
Given the use of direct‐acting antivirals (DAAs) to treat hepatitis C virus (HCV), their effects on hepatocarcinogenesis should be determined.
Methods
This study enrolled 349 patients with HCV who underwent DAA treatment at our hospital between 2014 and 2018. Their median age was 65 years, and 184 were male; 301 cases were of HCV serotype 1, and 48 were of serotype 2. The DAA treatment was daclatasvir/asunaprevir in 107 cases, sofosbuvir (SOF)/ledipasvir in 147 cases, ritonavir‐boosted ombitasvir/paritaprevir in 28 cases, elbasvir/grazoprevir in 19 cases, and SOF/ribavirin in 48 cases. The patients’ histories included hepatocellular carcinoma (HCC) in 45 cases, liver transplant (LT) in 10 cases, and kidney transplant (KT) in 17 cases.
Results
Sustained virological responses occurred in 335 cases (96%). DAA treatment was initiated a median of 16.3 months after HCC treatment. After DAA treatment, 15 cases (33%) had recurrence of HCC after a median of 11.6 months, and 3 cases (1%) developed de novo HCC. Six LT patients and one KT patient had HCC; however, no HCC was observed after DAA. The incidence of HCC was significantly higher in patients with multiple HCC treatments in the Cox hazard model (hazard ratio 1.664, 95% confidence interval 1.134–2.441, P < 0.01). Surgical resection or LT reduced the risk of HCC.
Conclusions
DAA did not increase the rate of HCC, even in immunosuppressed patients. However, careful follow‐up for HCC recurrence is required in previously treated cases.
Keywords: direct‐acting antivirals, hepatocarcinogenesis, hepatocellular carcinoma
Introduction
Hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, which leads to cirrhosis and hepatocellular carcinoma (HCC).1 Hepatocarcinogenesis due to HCV infection is thought to be initiated via direct and indirect mechanisms.2 It is mainly triggered by indirect immune‐mediated chronic inflammation. During HCV infection, liver‐infiltrating lymphocytes fail to clear the virus, and chronic inflammation occurs. A high CD8+ T cell count is correlated with HCC occurrence and is considered a predictor of recurrence after surgical resection.3 Many inflammatory cytokines have been implicated in chronic liver inflammation and HCC progression in HCV‐infected patients, including tumor necrosis factor‐α, interleukin (IL)‐1, IL‐23, IL‐6, and lymphotoxins.4, 5 In terms of a direct effect of HCV on HCC development, HCV proteins are associated with an increased epithelial/mesenchymal transition and reactive oxygen species expression, which lead to chronic inflammation and hepatocarcinogenesis. Host tumor suppressers and proto‐oncogenes are the direct targets of HCV. The HCV core, E2, NS3, and NS5 proteins stimulate cellular proliferation by interfering with the rapidly accelerated fibrosarcoma/mitogen‐activated protein kinase/extracellular signal‐regulated kinase pathway.6, 7
The eradication of HCV was expected to reduce HCC development because HCC develops frequently in HCV‐based liver disease.1 Antiviral therapy and interferon (IFN) reduce hepatocarcinogenesis by decreasing inflammation and fibrosis.8, 9
Direct‐acting antivirals (DAA) were developed to treat HCV infection and can achieve high sustained virological response (SVR) rates of >90%, similar to conventional IFN therapy.10 Regimens approved by the US Food and Drug Administration are available for genotype 1, including daclatasvir (DCV)/asunaprevir (ASV), sofosbuvir (SOF)/ledipasvir (LDV), SOF/simeprevir (not in Japan), ritonavir (r)‐boosted ombitasvir (OBV)/paritaprevir (PTV), DCV/ASV/beclabuvir, and elbasvir/grazoprevir (EBR/GZR); in Japan, SOF/ribavirin (RBV), SOF/LDV, and OBV/PTV/r have been approved for genotype 2.11, 12, 13 Recently, a DAA with pangenotypic activity, glecaprevir/pibrentasvir, was introduced, and HCV eradication is being achieved more frequently.14
Although IFN therapy reduces HCC,15, 16 it is still unclear whether DAA reduces the risk of de novo or recurrent HCC. Here, we examined hepatocarcinogenesis in DAA‐treated patients and considered the risk of HCC and the timing of DAA therapy following HCC treatment.
Methods
Patients and study design
This retrospective study enrolled 349 patients with HCV infection, including 10 liver transplant (LT) recipients and 17 kidney transplant (KT) recipients. The treatment of HCV serotype 1 included a 24‐week combined regimen of NS5A and NS3 protease‐targeted DAAs (DCV; Daklinza) at 60 mg once a day and ASV (Sunvepra) at 100 mg twice a day (Bristol‐Myers Squibb, New York, NY, USA) in 107 patients and a 12‐week treatment with NS5A protease‐ and NS5B polymerase‐targeted DAAs (400 mg SOF/90 mg LDV [Harvoni] once a day [Gilead Sciences, Foster City, CA, USA]) in 147 patients; a 12‐week combined regimen of NS5A and NS3 protease‐targeted DAAs (Viekirax, OBV 25 mg/PTV 150 mg/r 100 mg once a day [AbbVie, North Chicago, IL, USA]) in 28 cases; and EBR/GZR, Erelsa 50 mg/Grazina 100 mg once a day (Merck Sharp & Dohme, Kenilworth, NJ, USA) in 19 cases. HCV serotype 2 was treated with SOF + RBV (Sovaldi, 400 mg once a day [Gilead Sciences] and Rebetol at 800–1400 mg orally according to patient body weight [Merck Sharp & Dohme, Kenilworth, NJ, USA]) for 12 weeks.
Patients with severe liver dysfunction were excluded, such as those with decompensated cirrhosis or residual HCC. Patients with severe renal impairment were not given the SOF‐based regimen (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2) or RBV (eGFR < 50 mL/min/1.73 m2).
Detailed clinical and demographic information was collected, including patient age, gender, and body mass index (BMI), and complications due to lifestyle‐related diseases. Laboratory data were evaluated at the time of DAA treatment, including the tumor markers alpha fetoprotein (AFP) and des‐gamma‐carboxy pro‐thrombin (DCP). The Child–Turcotte–Pugh score and fibrosis‐4 index were evaluated.
The diagnosis of fatty liver was based on fat accumulation as seen on abdominal ultrasound or computed tomography (CT). Liver cirrhosis (LC) was defined as stage 4 fibrosis on a liver biopsy or typical clinical findings on liver imaging, esophageal and gastric varices formation, and splenomegaly. HCC was diagnosed histologically or based on imaging findings consistent with the diagnosis, using at least two of the following methods: abdominal ultrasound, CT, magnetic resonance imaging, and selective hepatic arteriography.17 The macroscopic classification, histological differentiation, and architectural pattern were evaluated according to the classification based on the general rules for the clinical and pathological studies of primary liver cancer.18, 19, 20 For HCC therapy, surgical resection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), and LT were conducted according to guidelines,21 and treatment selection was dependent on each doctor in charge. Before the DAA treatment, the HCC was completely treated and was diagnosed as healing HCC by imaging.
This study was conducted according to the principles of the 1964 Declaration of Helsinki and the ethical guidelines of the Tokyo Women's Medical University Hospital (TWMU, Tokyo, Japan). The TWMU Institutional Review Board approved the study protocol.
Statistical analysis
We compared the clinical features of patients with and without HCC before the DAA treatment. Data are presented as medians and range for continuous variables and as proportions for categorical variables. Significant differences between the two groups were assessed using the Mann–Whitney U test and χ 2 test. A P value <0.05 was considered to indicate statistical significance. The occurrence of HCC after DAA treatment was evaluated by constructing Kaplan–Meier curves in patients with and without a history of HCC, as well as according to the HCC treatment regimen, number of treatments, and transplantation. Univariate and multivariate Cox regression analyses were used to evaluate the occurrence of HCC after DAA treatment in patients with and without a history of HCC, as well as according to the HCC treatment regimen, number of treatments, and transplantation. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated. The SPSS software package (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses.
Results
Patient characteristics and comparison of those with and without a history of HCC
Of the 349 DAA‐treated cases, 45 had a history of HCC (Fig. 1, Table 1). Comparing patients with and without a history of HCC, the HCC cases were older (median age, 65 years [range: 22–86 years]) and more likely to be male (both P < 0.01). Advanced fibrosis was more frequent in the HCC cases (P < 0.01). IFN therapy was administered more often in HCC cases; however, the difference was not significant after adjusting for age and fibrosis. BMI and the rates of complications of lifestyle‐related diseases did not differ significantly between patients with and without a history of HCC. After DAA treatment, the SVR rate was ≥93%, and 18 cases developed HCC, including 15 cases of recurrent HCC and 3 of de novo HCC. Recurrent HCC (n = 15) (Table 2) occurred after a median of 22.0 (2.9–46.3) months of DAA treatment, whereas those with no HCC (n = 30) had 16.1 (2.1–242.5) months of DAA treatment. No significant difference was observed in the starting time of DAA between HCC‐recurrent and nonrecurrent cases. However, the patients with multiple HCC treatments were administered DAA soon after HCC therapy (cases 5 and 13).
Figure 1.
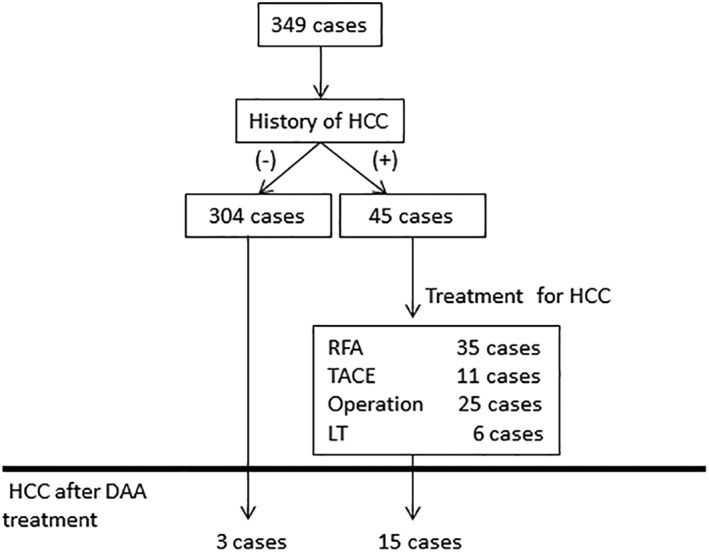
Flow chart of the study. In total, 349 cases were analyzed, and 45 cases had a history of hepatocellular carcinoma (HCC) before direct‐acting antiviral drug (DAA) treatment. After DAA treatment, 18 cases were complicated with HCC.
Table 1.
Patient characteristics
| HCC (−) vs HCC (+) P value | ||||||
|---|---|---|---|---|---|---|
| Total (n = 349) | HCC (−) (n = 304) | Previous HCC (+) (n = 45) | HCC (+) Post DAA (n = 15) | HCC (−) vs Previous HCC (+) | Post‐DAA among previous HCC (+) | |
| Age (years) | 65 (22–86) | 65 (22–86) | 69 (48–82) | 70 (52–81) | <0.01 | 0.78 |
| Males (% male) | 184 (53) | 152 (50) | 32 (71) | 11 (73) | <0.01 | 0.82 |
| HCV serotype 1/2 | 301/48 | 262/42 | 39/6 | 13/2 | 0.93 | 1.00 |
| HCV‐RNA (logIU/mL) | 6.2 (2.5–8.0) | 6.2 (2.5–7.5) | 6.0 (3.8–8.0) | 6.2 (4.1–6.7) | 0.30 | 0.47 |
| Body mass index (kg/m2) | 22.6 (13.9–40.4) | 22.5 (13.9–40.4) | 22.8 (17.4–28.0) | 23.0 (17.4–25.3) | 0.71 | 0.76 |
| Complications | ||||||
| Diabetes (%) | 72 (21) | 60 (20) | 12 (27) | 4 (27) | 0.29 | 1.00 |
| Dyslipidemia (%) | 61 (17) | 55 (18) | 6 (13) | 0 (0) | 0.42 | 0.06 |
| Fatty liver (%) | 35 (10) | 32 (11) | 3 (7) | 1 (7) | 0.44 | 0.98 |
| Liver cirrhosis (%) | 56 (16) | 41 (13) | 15 (33) | 6 (40) | <0.01 | 0.50 |
| Hemoglobin A1c (%) | 5.8 (4.5–9.7) | 5.8 (4.6–9.7) | 5.9 (4.5–7.6) | 6.0 (5.2–7.6) | 0.41 | 0.40 |
| CTP score | 5 (5–8) | 5 (5–8) | 5 (5–8) | 5 (5–6) | 0.20 | 0.35 |
| FIB‐4 index | 2.63 (0.28–23.91) | 2.50 (0.28–23.91) | 5.33 (1.64–15.40) | 6.71 (2.10–15.40) | <0.01 | 0.20 |
| AFP (ng/mL) | 5 (1–230) | 5 (1–230) | 8 (2–140) | 11.5 (2–48) | 0.37 | 0.98 |
| DCP (mAU/mL) | 17 (6–74) | 17 (7–74) | 15 (6–55) | 17 (14–55) | 0.93 | 0.34 |
| Previous IFN therapy (%) | 126 (36) | 102 (34) | 24 (53) | 6 (40) | <0.01 | 0.09 |
| Previous HCC treatment | — | — | <0.05 | |||
| Surgical resection | 25 | 25 | 8 | |||
| RFA | 35 | 35 | 20 | |||
| TACE | 11 | 11 | 3 | |||
| LT | 6 | 6 | 0 | |||
| Number of previous HCC treatments | 1 (1–6) | — | 1 (1–6) | 2 (1–6) | — | 0.13 |
| DAA treatment | 0.88 | 0.30 | ||||
| DCV/ASV | 107 | 91 | 16 | 7 | ||
| SOF/LDV | 147 | 129 | 18 | 6 | ||
| OBV/PTV/r | 28 | 25 | 3 | 0 | ||
| EBR/GZR | 19 | 17 | 2 | 0 | ||
| SOF + RBV | 48 | 42 | 6 | 2 | ||
| SVR (%) | 335 (96) | 292 (96) | 43 (96) | 14 (93) | 0.85 | 0.87 |
| Initiation of DAA after HCC treatment (months) | 16.3 (2.1–242.5) | — | 16.3 (2.1–242.5) | 22.0 (2.9–46.3) | — | 0.25 |
| Observation period (months) | 25.5 (0.3–41.3) | 25.5 (0.3–41.1) | 25.9 (2.7–41.3) | 25.9 (13.0–41.3) | 0.32 | 0.07 |
| Posttransplantation | <0.05 | 0.10 | ||||
| Liver | 10 | 4 | 6 | 0 | ||
| Kidney | 17 | 16 | 1 | 0 | ||
| HCC occurrence following DAA treatment | 18 | 3 | 15 | 15 | <0.01 | — |
| Time of HCC occurrence after DAA (months) | 15.6 (2.2–34.2) | 20.5 (18.8–23.5) | 11.6 (2.2–34.2) | 11.6(2.2–34.2) | <0.05 | — |
AFP, alpha fetoprotein; CTP, Child–Turcotte–Pugh; DAA, direct‐acting antiviral; DCP, des‐gamma‐carboxy pro‐thrombin; DCV/ASV, daclatasvir/asunaprevir; EBR/GZR, elbasvir/grazoprevir; FIB‐4, fibrosis‐4; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon; LT, liver transplantation; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; RBV, ribavirin; RFA, radiofrequency ablation; SOF/LDV, sofosbuvir/ledipasvir; SVR, sustained virological response; TACE, transcatheter arterial chemo‐ embolization.
Table 2.
Hepatocellular carcinoma (HCC) cases after direct‐acting antiviral (DAA) treatment
| Case | Age (y) | Sex | fibrosis | IFN treatment | DAA regimen | HCC treatment pre‐DAA | DAA after last HCC treatment (months) | First HCC occurrence after DAA (months) | The number of HCC | HCC treatment post‐DAA | Prognosis and HCC control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | M | CH | — | SOF + RBV | Resection | 40.7 | 7.1 | 1 | RFA | Alive |
| RFA | |||||||||||
| 2 | 70 | M | CH | PEG‐IFN + RBV+SMV | SOF/LDV | Resection | 44.7 | 11.6 | 1 | RFA twice | Alive |
| 3 | 52 | M | LC | IFN + RBV | SOF/LDV | TACE twice | 3.1 | 4.8 | 1 | TACE twice | Death |
| 4 | 74 | M | CH | — | SOF/LDV | RFA | 12.4 | 19.1 | 2 | Resection | Alive |
| 5 | 58 | M | CH | PEG‐IFN + RBV | SOF/LDV | RFA 6 times | 6.7 | 6.3 | Multiple | TACE twice RFA | Alive and uncontrolled |
| 6 | 69 | M | LC | — | SOF/LDV | Resection | 22.2 | 9.8 | 2 | TACE | Alive |
| TACE | |||||||||||
| 7 | 70 | F | LC | — | SOF/LDV | RFA twice | 26.9 | 7.2 | 1 | RFA | Alive |
| 8 | 72 | M | LC | PEG‐IFN + RBV | DCV/ASV | Resection | 13.3 | 34.2 | 1 | RFA | Alive |
| RFA | |||||||||||
| 9 | 75 | F | CH | PEG‐IFN + RBV+SMV | DCV/ASV | Resection | 10.1 | 2.2 | Multiple | TACE | Alive |
| 10 | 70 | M | LC | IFN‐beta | DCV/ASV | RFA | 46.3 | 26.9 | unknown | RFA | Alive |
| 11 | 68 | M | CH | unknown | DCV/ASV | Resection twice | 43.8 | 19.0 | 1 | TACE | Alive |
| 12 | 65 | F | CH | PEG‐IFN + RBV | DCV/ASV | RFA twice | 14.4 | 4.8 | 1 | RFA | Alive |
| 13 | 74 | M | LC | PEG‐IFN | DCV/ASV | RFA 4 times Resection | 2.9 | 27.7 | Multiple | RFA TACE 6 times | Alive and uncontrolled |
| 14 | 74 | F | CH | PEG‐IFN + RBV | DCV/ASV | RFA | 22.0 | 22.1 | 1 | Resection | Alive |
| 15 | 81 | M | CH | — | SOF + RBV | RFA | 27.5 | 13.1 | Multiple | TACE 4 times Sorafenib | Alive and uncontrolled |
| 16 | 74 | F | CH | PEG‐IFN + RBV | SOF/LDV | — | 20.5 | 1 | RFA | Alive | |
| 17 | 76 | M | LC | PEG‐IFN + RBV | DCV/ASV | — | 18.8 | 1 | RFA | Alive | |
| 18 | 71 | M | CH | PEG‐IFN + RBV | DCV/ASV | — | 23.5 | 1 | Resection | Alive |
CH, chronic hepatitis; DCV/ASV, daclatasvir/asunaprevir; EBR/GZR, elbasvir/grazoprevir; F, female; LC, liver cirrhosis; M, male; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PEG‐IFN, pegylated interferon; RBV, ribavirin, RFA, radiofrequency ablation; SMV, simeprevir; SOF/LDV, sofosbuvir/ledipasvir; TACE, transcatheter arterial chemo‐embolization.
Previous treatment of HCC and HCC recurrence after DAA
The rate of HCC occurrence following DAA treatment was significantly increased in patients with a history of HCC on Kaplan–Meier analysis (Fig. 2, P < 0.01). The median interval until HCC occurrence after DAA therapy was 11.6 months (range: 2.2–34.2 months) in recurrent HCC and 20.5 months (range: 18.8–23.5 months) in de novo HCC (P < 0.05, Table 1).
Figure 2.
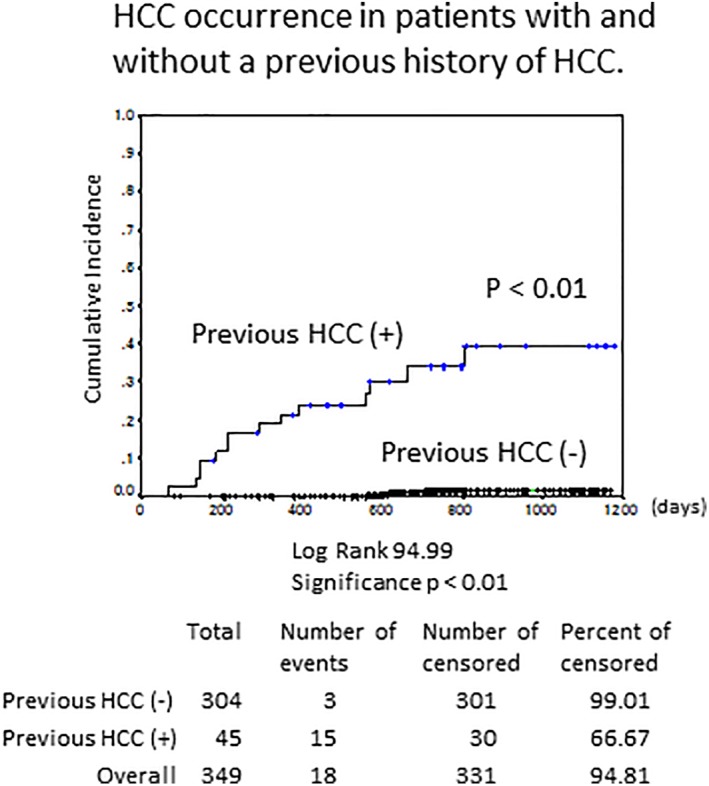
Hepatocellular carcinoma (HCC) occurrence and recurrence in patients with and without a previous history of HCC. HCC occurred significantly more often in patients with a history of previous HCC by Kaplan–Meier analysis (P < 0.01).
The treatments of previous HCC (n = 45) included surgical resection in 25 cases, RFA in 35 cases, TACE in 11 cases, and LT in 6 cases. The previous HCC treatment differed significantly between recurrence (n = 15) and nonrecurrence (n = 30, P < 0.05). The patients treated with surgical resection or LT had a lower rate of recurrence than those administered the other treatment regimens (P = 0.07, Fig. 3a). Ten LT recipients received DAA, of whom six had HCC at the time of transplantation. All patients were started on DAAs with immunosuppression using tacrolimus and mycophenolate mofetil (MMF) ± steroid at 18.5 months (range: 5.9–242.5 months) after transplantation. Nine patients achieved SVR (90%), and following DAA therapy, no patient developed HCC after follow‐up for 32.4 months (range: 12.3–40.7 months), even those with a history of HCC. In comparison, the 17 KT recipients were treated with DAA with azathioprine or tacrolimus and MMF ± methyl prednisone or everolimus. All patients achieved SVR, and one had a history of HCC; however, there was no recurrent or de novo HCC following DAA treatment after follow‐up for 27.9 months (range: 5.2–40.4 months). Compared with nonrecipients (n = 322), LT/KT recipients (n = 27) did not show significantly increased HCC occurrence, even under immunosuppression, during 29.0 (range: 5.2–40.7) months of follow‐up (Fig. 4).
Figure 3.
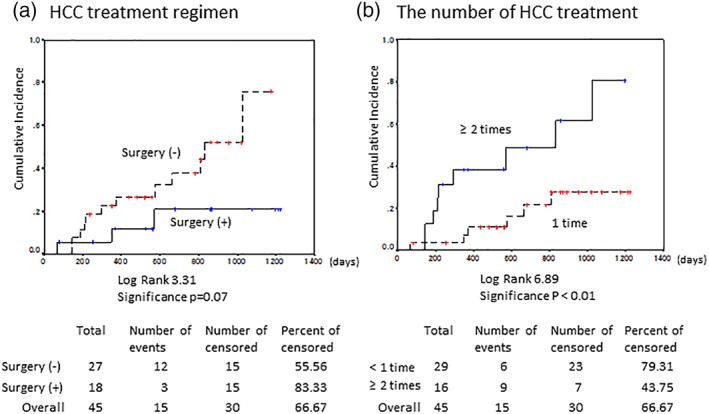
Hepatocellular carcinoma (HCC) recurrence according to type of previous HCC treatment. (a) HCC treatment regimen, (b) number of treatments. Kaplan–Meier analysis showed that the rate of HCC tended to be reduced by surgery (surgical resection or LT [a, P = 0.07]) and significantly increased with multiple treatments (b, P < 0.01).
Figure 4.
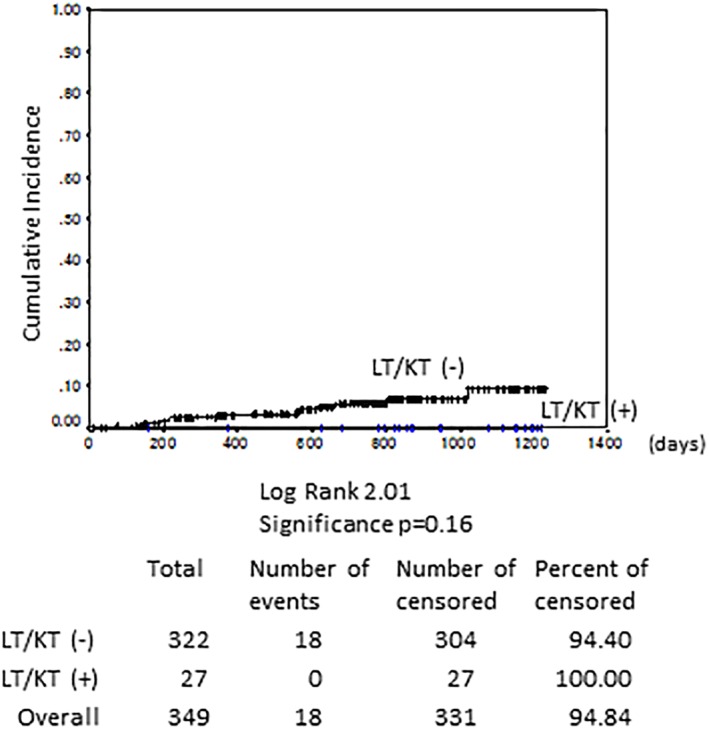
Hepatocellular carcinoma (HCC) recurrence according to liver/kidney transplant (LT/KT). Compared with nonrecipients, LT/KT recipients did not show significantly increased HCC occurrence, even under immunosuppression, during 29.0 (range: 5.2–40.7) months of follow‐up (Kaplan–Meier analysis, P = 0.16).
The median number of HCC treatments before DAA was two (range: 1–6) in recurrent HCC and one (range: 1–5) in the nonrecurrent HCC cases (Table 1, P = 0.13). The patients who were treated for HCC multiple times had significantly more recurrence after DAA (Fig. 3b, P < 0.01). They especially required multiple HCC treatments after DAA, delivered over a short period. Three patients had five or more treatments before DAA, and two of these developed multiple HCC and required several treatments after DAA (cases 5 and 13, Table 2).
The incidence of HCC after DAA was significantly higher in patients with multiple episodes of HCC treatment; this was a risk factor for developing HCC in the Cox hazard analysis (HR 1.664, 95% CI 1.134–2.441, P < 0.01). Treatment with surgical resection or LT was not a significant risk factor for developing HCC (HR 0.274, 95% CI 0.060–1.262, P = 0.097). Factors affecting the incidence of HCC included age, gender, LC, a history of HCC, number of HCC treatments, HCC and DAA treatment regimens, and HCV genotype.
Characteristics of HCC following DAA treatment and prognosis
Regarding the characteristics of HCC, the diameter of the HCC before DAA was 18 mm (range: 3–70 mm, Table 3). The macroscopic classification included 33 cases of the simple nodular type, 5 cases of the small nodular type with indistinct margins, 5 cases of the simple nodular type with extranodular growth, and 3 cases of the multinodular type. Histologically, 32 HCC cases were moderately differentiated, 34 had a trabecular pattern, and 2 had a tubular pattern. The diameter of the HCC that occurred after beginning DAA treatment was 18 mm (range: 4–25 mm), and five cases were of the simple nodular type, with moderate differentiation and a trabecular pattern. Overall, 9 of 31 (29%) nodules were recurrent cases. HCC after DAA was predominantly of the multiple nodule type (P < 0.05).
Table 3.
Characteristics of hepatocellular carcinoma (HCC) before and after direct‐acting antiviral (DAA) therapy
| HCC in pre‐DAA (n = 45) | HCC in post‐DAA (n = 18) | P value | |
|---|---|---|---|
| Diameter of HCC (mm) | 18 (3–70) | 18 (4–25) | 0.06 |
| Number of HCCs | <0.05 | ||
| Single | 59 | 18 | |
| Multiple | 19 | 14 | |
| Macroscopic classification | 0.14 | ||
| Small nodular type with indistinct margin | 5 | 0 | |
| Simple nodular | 33 | 13 | |
| Simple nodular type with extranodular growth | 5 | 5 | |
| Conflict multinodular type | 3 | 0 | |
| Histological differentiation | 0.49 | ||
| Well‐differentiated | 2 | 1 | |
| Moderately differentiated | 32 | 5 | |
| Poorly differentiated | 3 | 0 | |
| Architectural pattern | 0.55 | ||
| Trabecular pattern | 34 | 6 | |
| Tubular pattern | 2 | 0 |
One patient died (case 3) of HCC, and all of the other patients are alive. HCC was uncontrolled in three cases despite several treatments (cases 5, 13, and 15).
A case of massive HCC recurrence after DAA treatment
An 81‐year‐old man received RFA therapy 2.5 years before beginning DAA therapy (case 15). HCC was completely ablated (Fig. 5a,b). At the time of DAA treatment, no recurrence was observed on CT (Fig. 5c), and he was treated with SOF + RBV for 3 months. No adverse events were seen, and SVR was attained. Four months after DAA therapy, the serum DCP level was slightly increased (to 55 mAU/mL). Eight months after the DAA treatment, the serum DCP level was further elevated (174 mAU/mL), and multiple tiny HCCs were seen on CT (Fig. 5d). Hepatic angiography detected multiple staining in both liver lobes (Fig. 5e). TACE was performed three times, and sorafenib was started at 19 months. However, the HCC was not controllable even after switching to sorafenib (Fig. 5f).
Figure 5.
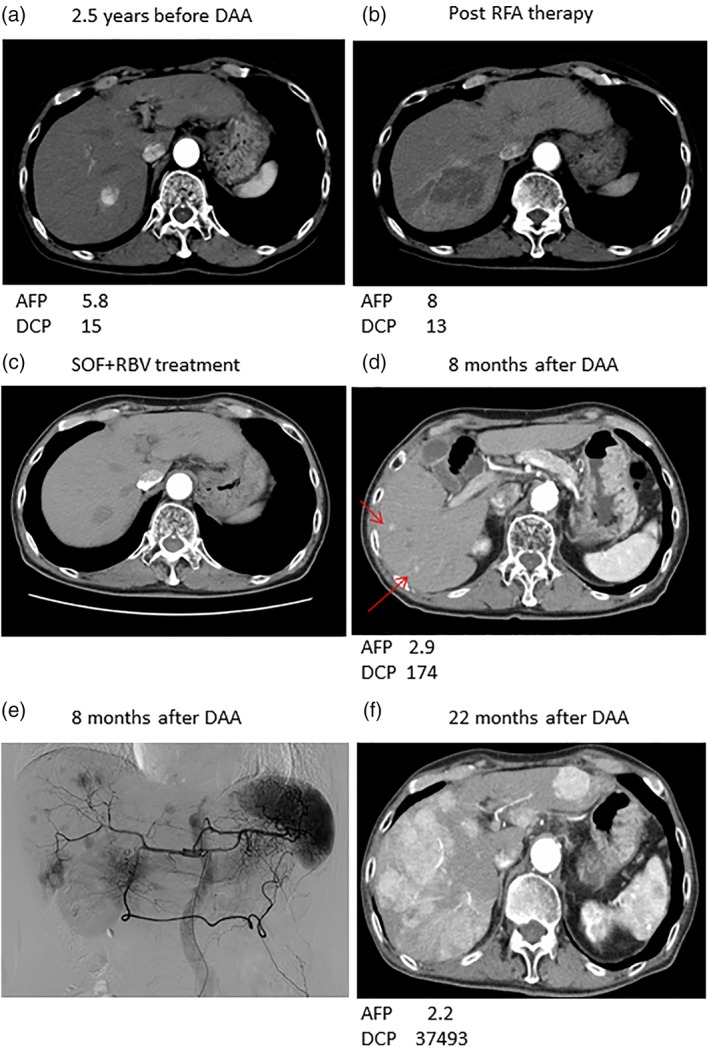
Representative computed tomography findings in case no. 15 after direct‐acting antiviral (DAA) HCC recurrence: (a) 2.5 years before DAA treatment; (b) after RFA therapy; (c) at the time of sofosbuvir + ribavirin (SOF + RBV) treatment; and (d) 8 and (f) 19 months after DAA treatment. Red arrows, hepatocellular carcinoma (HCC). (e) Abdominal angiography 8 months after DAA treatment. HCC was observed 2.5 years before DAA treatment (a) and was completely cured by radiofrequency ablation (RFA) therapy (b). SOF + RBV was administered for 3 months (c). Tiny HCCs were observed 8 months after DAA treatment (d), and hepatic arteriography showed multiple tumors with des‐gamma‐carboxy pro‐thrombin (DCP) elevation (e). HCC was uncontrollable despite many treatments (f). AFP, alpha fetoprotein.
Discussion
We found 18 cases of HCC after DAA treatment, of which 15 (93%) were patients with a history of HCC before HCV eradication by DAA treatment. DAA did not increase HCC, even with an immunosuppressive regimen, and may not influence hepatocarcinogenesis directly. However, some patients developed faster‐growing HCC with a more aggressive phenotype after DAA.
There is no DAA treatment that leads directly to HCC development, and there are also reports that it reduces, or at least does not increase, the risk of HCC.22, 23, 24 One meta‐analysis found no evidence that HCC occurrence or recurrence differs between patients receiving DAA versus IFN therapy.25 In the HEPATHER cohort, the 6‐month recurrence rate was 10.6% in patients treated with DAA, whereas it was 18.7% in untreated patients.26 Reports suggest that DAA does not increase the risk of HCC.27 Clinically, as the transaminase level decreases sharply immediately after DAA initiation, HCV clearance may suppress the inflammation caused by DAA treatment. In addition, a study of HCV‐infected patients treated with a SOF‐based regimen reported a significant reduction in the rate of liver fibrosis.28
Nevertheless, there are reports of DAA‐associated hepatocarcinogenesis. Yang et al. reported that early HCC recurrence was more frequent in DAA‐treated patients.29 We experienced a patient who developed HCC after DAA. The HCC phenotype was obviously different from the previous HCC, and intrahepatic metastasis was seen with DCP elevation (Fig. 5). An immunological mechanism is suspected to underlie the high rate of HCC occurrence with DAA. After HCV clearance, decreased levels of inflammation may lead to reduced tumor immunosurveillance by the host and promote HCC.30, 31 Moreover, a reduction in natural killer group 2 member D, which is associated with cancer immunity,32 and increased serum levels of vascular endothelial growth factor during the DAA treatment33 have both been reported. Zanetto et al. reported that tumor growth in patients with HCC while on the waiting list for LT did not differ significantly before versus after DAA treatment.34 It is not clear whether DAA increases the malignant potential of HCC. We speculate that, while DAA may not directly promote HCC, it increased the incidence and number of HCCs at the time of DAA treatment via an indirect mechanism involving the suppression of inflammation.
In this study, HCC recurrence was observed in patients treated for HCC multiple times before DAA treatment. Patients with a history of HCC are already in a precarcinogenic state and thus develop HCC more quickly, and at high rates. However, even when immunosuppressed, such as in LT cases, hepatocarcinogenesis was suppressed when the HCC and fibrosed liver were removed completely.35 Complete treatment by surgical resection or LT may reduce HCC recurrence. In the setting of KT, no report has analyzed HCC development after DAA treatment. We observed no HCC during follow‐up for up to 40.4 months after KT. In these cases, although the DAA treatment was administered with the remaining native liver under immunosuppression, no HCC was observed. Therefore, HCC development may be influenced by past hepatocarcinogenesis more so than by DAA treatment or immunosuppression.
Our study had some limitations. It had a single‐center, retrospective design, with an observation period of only 3.4 years, and we could not determine the levels of cytokines or immunological mediators. Further study of hepatocarcinogenesis after DAA treatment is required with a longer follow‐up period.
In conclusion, DAA may not increase the incidence of HCC, and the timing of initiation of DAA did not influence the rate of HCC occurrence. However, the absolute risk of HCC remained high in patients with a history of HCC. Careful monitoring for HCC with liver imaging and AFP or DCP should be performed at least every 3 months.
Acknowledgments
We express our gratitude to Prof. Kazunari Tanabe and Dr Hideki Ishida of the Department of Urology and to Dr Shohei Fuchinoue of the Department of Surgery, Kidney Center, Tokyo Women's Medical University for their collaboration after kidney transplantation.
Declaration of conflict of interest: Katsutoshi Tokushige received research funding from Sumitomo Dainippon Pharma Co., Ltd.; Astellas Pharma Inc.; Eisai Co., Ltd.; TAIHO Pharmaceutical Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; Daiichi Sankyo Pharmaceutical Co., Ltd.; AbbVie GK; Takeda Pharmaceutical Co., Ltd.; Asahi Kasei Corporation; AJINOMOTO Co., Inc.; and Otsuka Pharmaceutical.
REFERENCES
- 1. Kudo M, Izumi N, Ichida T et al Report of the 19th follow‐up survey of primary liver cancer in Japan. Hepatol. Res. 2016; 46: 372–90. [DOI] [PubMed] [Google Scholar]
- 2. Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus‐induced hepatocarcinogenesis. J. Hepatol. 2009; 51: 810–20. [DOI] [PubMed] [Google Scholar]
- 3. Ramzan M, Sturm N, Decaens T et al Liver‐infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus‐related hepatocellular carcinoma. Liver Int. 2016; 36: 434–44. [DOI] [PubMed] [Google Scholar]
- 4. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haybaeck J, Zeller N, Wolf MJ et al A lymphotoxin‐driven pathway to hepatocellular carcinoma. Cancer Cell. 2009; 16: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayashi J, Aoki H, Kajino K, Moriyama M, Arakawa Y, Hino O. Hepatitis C virus core protein activates the MAPK/ERK cascade synergistically with tumor promoter TPA, but not with epidermal growth factor or transforming growth factor alpha. Hepatology. 2000; 32: 958–61. [DOI] [PubMed] [Google Scholar]
- 7. Feng DY, Sun Y, Cheng RX, Ouyang XM, Zheng H. Effect of hepatitis C virus nonstructural protein NS3 on proliferation and MAPK phosphorylation of normal hepatocyte line. World J. Gastroenterol. 2005; 11: 2157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasahara A, Hayashi N, Mochizuki K et al Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998; 27: 1394–402. [DOI] [PubMed] [Google Scholar]
- 9. Takimoto M, Ohkoshi S, Ichida T et al Interferon inhibits progression of liver fibrosis and reduces the risk of hepatocarcinogenesis in patients with chronic hepatitis C: a retrospective multicenter analysis of 652 patients. Dig. Dis. Sci. 2002; 47: 170–6. [DOI] [PubMed] [Google Scholar]
- 10. Huang AC, Mehta N, Dodge JL, Yao FY, Terrault NA. Direct‐acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local‐regional therapy or liver transplant waitlist dropout. Hepatology. 2018; 68: 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014; 146: 1176–92. [DOI] [PubMed] [Google Scholar]
- 12. Manns M, Pol S, Jacobson IM et al All‐oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014; 384: 1597–605. [DOI] [PubMed] [Google Scholar]
- 13. Suda G, Furusyo N, Toyoda H et al Daclatasvir and asunaprevir in hemodialysis patients with hepatitis C virus infection: a nationwide retrospective study in Japan. J. Gastroenterol. 2018; 53: 119–28. [DOI] [PubMed] [Google Scholar]
- 14. Zeuzem S, Foster GR, Wang S et al Glecaprevir‐Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N. Engl. J. Med. 2018; 378: 354–69. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida H, Shiratori Y, Moriyama M et al Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann. Intern. Med. 1999; 131: 174–81. [DOI] [PubMed] [Google Scholar]
- 16. Hsu CS, Chao YC, Lin HH, Chen DS, Kao JH. Systematic review: impact of interferon‐based therapy on HCV‐related hepatocellular carcinoma. Sci. Rep. 2015; 5: 9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann. Surg. 2007; 245: 909–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enggel H. Über das primäre Karzinom der Leber (in German). Beitr. Pathol. Anat. Allg. Pathol. 1901; 30: 506–604. [Google Scholar]
- 19. Kanai T, Hirohashi S, Upton MP et al Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987; 60: 810–9. [DOI] [PubMed] [Google Scholar]
- 20. The Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 5th ed, revised version edn. Kanehara, Tokyo, 2009. [Google Scholar]
- 21. Kokudo N, Hasegawa K, Akahane M et al Evidence‐based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH‐HCC Guidelines). Hepatol. Res. 2015; 45: 123–127. [DOI] [PubMed] [Google Scholar]
- 22. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Innes H, Barclay ST, Hayes PC et al The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J. Hepatol. 2017; 66: S22–3. [DOI] [PubMed] [Google Scholar]
- 24. Li M, Yu X, Li W et al Deguelin suppresses angiogenesis in human hepatocellular carcinoma by targeting HGF‐c‐Met pathway. Oncotarget. 2018; 9: 152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waziry R, Hajarizadeh B, Grebely J et al Hepatocellular carcinoma risk following direct‐acting antiviral HCV therapy: a systematic review, meta‐analyses, and meta‐regression. J. Hepatol. 2017; 67: 1204–12. [DOI] [PubMed] [Google Scholar]
- 26. ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER COCaCCcEaspaf) . Lack of evidence of an effect of direct‐acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J. Hepatol. 2016; 65: 734–40. [DOI] [PubMed] [Google Scholar]
- 27. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El‐Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct‐acting antiviral agents. Gastroenterology. 2017; 153: 996–1005.e1. [DOI] [PubMed] [Google Scholar]
- 28. Bernuth S, Yagmur E, Schuppan D et al Early changes in dynamic biomarkers of liver fibrosis in hepatitis C virus‐infected patients treated with sofosbuvir. Dig. Liver Dis. 2016; 48: 291–7. [DOI] [PubMed] [Google Scholar]
- 29. Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C‐associated hepatocellular carcinoma. J. Hepatol. 2016; 65: 859–60. [DOI] [PubMed] [Google Scholar]
- 30. Reig M, Mariño Z, Perelló C et al Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J. Hepatol. 2016; 65: 719–26. [DOI] [PubMed] [Google Scholar]
- 31. Nault JC, Colombo M. Hepatocellular carcinoma and direct acting antiviral treatments: controversy after the revolution. J. Hepatol. 2016; 65: 663–5. [DOI] [PubMed] [Google Scholar]
- 32. Chu PS, Nakamoto N, Taniki N et al On‐treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon‐free therapy for chronic hepatitis C. PLoS One. 2017; 12: e0179096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villani R, Facciorusso A, Bellanti F et al DAAs rapidly reduce inflammation but increase serum VEGF level: a rationale for tumor risk during anti‐HCV treatment. PLoS One. 2016; 11: e0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zanetto A, Shalaby S, Vitale A et al Dropout rate from the liver transplant waiting list because of hepatocellular carcinoma progression in hepatitis C virus‐infected patients treated with direct‐acting antivirals. Liver Transpl. 2017; 23: 1103–12. [DOI] [PubMed] [Google Scholar]
- 35. Charlton M, Gane E, Manns MP et al Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015; 148: 108–17. [DOI] [PubMed] [Google Scholar]


