Abstract
Background
The endoscopic placement of a self‐expandable metal stent (SEMS), an alternative to surgical bypass for the palliation of malignant gastric outlet obstruction (GOO), is commonly performed using a forward‐viewing endoscope with a wide therapeutic channel; however, due to limited availability, most Indian centers use a side‐viewing duodenoscope. We studied the feasibility and outcome of SEMS placement using side‐ and forward‐viewing endoscopes.
Method
Data of patients undergoing SEMS placement using side‐ and forward‐viewing endoscopes with a therapeutic channel for the palliation of malignant GOO presenting during a 5‐year period were analyzed retrospectively. Follow‐up data were obtained from records and telephonic interviews, and technical and clinical success, complications, and survival were evaluated.
Results
Of 114 patients (age 56.5 ± 11.6 years, 59 [52%] female), 90 (79%) and 24 (21%) underwent SEMS placement using side‐ and forward‐viewing endoscopes, respectively. Technical (89, 98.9% vs. 24, 100%, P = ns) and clinical success (84, 93.3% vs. 23, 95.8%, P = ns) and complication rate (3, 3.3% vs. 0, P = ns) between side‐ and forward‐viewing endoscopes were comparable. However, SEMS could be placed in a shorter time using a forward‐ rather than side‐viewing endoscope (21 min [inter‐quartile range 19.5–35] vs. 34 min [25–45], P = < 0.001). SEMS could be deployed successfully with a forward‐viewing endoscope in two patients in whom an initial attempt using side‐viewing endoscope failed. Gastric outlet obstruction scoring system (GOOSS) improved following stent placement (median 0, range 0–2 vs. 2, 0–3, P = 0.0001). The survival of patients undergoing SEMS placement using side‐ and forward‐viewing endoscopes was comparable.
Conclusion
Although side‐ and forward‐viewing endoscopes are equally effective for antroduodenal SEMS placement, the procedure can be performed faster using the latter.
Keywords: enteral nutrition, gallbladder cancer, gastric cancer, gastric outlet obstruction, vomiting
Introduction
Malignant gastric outlet obstruction (GOO) is a late complication of pancreaticobiliary and gastroduodenal malignancy.1, 2 Typical symptoms are abdominal pain, postprandial epigastric fullness, and recurrent vomiting, which lead to severe malnutrition and dehydration.1, 3 These patients are also at risk of aspiration pneumonia. All these factors diminish the quality of life and limit life expectancy.4 Treatment of malignant GOO is mainly palliative, aimed to improve the quality of life. Patients with malignant GOO have traditionally been treated with surgical bypass.4, 5 However, these patients tend to be poor surgical candidates, with perioperative complication rates of 25–35% and mortality of up to 2%.5, 6
Endoscopic self‐expandable metal stent (SEMS) placement is an effective and safe method for palliation of malignant GOO.7, 8, 9, 10 Antroduodenal SEMSs are typically placed using a forward‐viewing endoscope with a wide therapeutic channel.11, 12 Therapeutic forward‐viewing endoscopes are not widely available in most centers in developing countries, including India. Therefore, side‐viewing duodenoscopes are frequently used for this purpose. There are, however, limited data on the feasibility and outcome of gastroduodenal stent placement using side‐viewing duodenoscopes compared to forward‐viewing endoscope with a wide therapeutic channel.11 Accordingly, we studied the feasibility and outcome of SEMS placement using either side‐ or forward‐viewing endoscopes.
Patients
Data of consecutive patients undergoing endoscopic SEMS placement for palliation of malignant GOO presenting to two units of the Gastroenterology Department of a multilevel teaching hospital during a 5‐year period (February 2012 to January 2017) were analyzed retrospectively. Antroduodenal SEMS placement was performed in patients with symptomatic GOO due to unresectable pancreaticobiliary or gastroduodenal malignancy, confirmed by endoscopy and/or contrast imaging. Antroduodental stent placement was avoided in patients with poor performance status (Karnofsky performance status [KPS] score < 50)13 and limited life expectancy. All patients provided written informed consent before the procedure.
Methods
Before placing an antroduodenal SEMS, gastric lavage was performed using a nasogastric tube for better endoscopic visibility. Antroduodenal SEMSs were placed by three experienced endoscopists (UCG, SM, and VAS) under fluoroscopic and endoscopic guidance with the patient under conscious sedation using intravenous midazolam (Injection Midzol 1 mg/mL, Themis Medicare Limited, Uttarakhand, India) and/or propofol (Injection Neorof 10 mg/mL, 1% W/V, Neon Lab, Mumbai, India) by standard technique (www.spreadhealth.in, video; https://youtu.be/ZkI0wn7ZxQ8) using either a side‐viewing duodenoscope (Olympus TJF‐150 endoscope, Olympus Medical Systems Corp, Tokyo, Japan with instrument channel diameter of 4.2 mm) or a double‐channel forward‐viewing endoscope with therapeutic channel (Olympus GIF type 2TH180, Olympus Medical Systems Corp, Tokyo, Japan with instrument channel diameter of 2.8/3.7 mm). Briefly, after introducing the endoscope into the stomach, a biliary catheter (GLO‐TIP‐1‐T, 4.5Fr, Cook Medical, Bloomington, IN, USA) over a guidewire (initially a soft tip [Terumo guidewire, 0.032 in. diameter with hydrophilic tip, Terumo Europe NV, Belgium] that was exchanged with a stiff wire [Metro stiff guide wire, 0.035 in. with radio‐opaque tip, Cook Medical]) was negotiated through the stricture. Subsequently, the exact site and length of the stricture were assessed after injecting a water‐soluble contrast agent. The SEMS delivery system was then advanced over the stiff guide wire, was positioned across the stricture, and stent was deployed under endoscopic and fluoroscopic vision. After placement of the stent, patients were kept in hospital for at least 48 h. Abdominal radiograph was obtained after 24 h to look for the position and expansion of stent before initiating feeding. As the forward‐viewing endoscope was available later, all the procedures were performed using a side‐viewing endoscope before February 2015.
Data collection
Data were collected using a standard questionnaire from electronic as well as printed hospital records and endoscopy laboratory data. Follow‐up data were also obtained by telephonic interview of patients and their relatives. Variables obtained included demographic and clinical profile, chemotherapeutic agents administered, and KPS and gastric outlet obstruction scores (GOOS), assessed before and after SEMS placement. The duration of stent patency and survival was also assessed. Procedure‐related measures included the type of stents, site and length of stricture, procedure time, complications, reintervention, simultaneous biliary SEMS placement, and type of endoscope (forward‐ or side‐viewing).
Study outcomes
The primary outcome measures were technical and clinical success. Secondary outcomes measures were procedure‐related complications, procedure time, stent patency, and survival. Technical success was defined as successful stent deployment across stricture and radiological relief of obstruction. For the assessment of clinical success, a standardized gastric outlet obstruction scoring system (GOOSS) was used,2, 8, 14 in which grade 0 indicates no oral intake, 1: intake of liquids only, 2: intake of soft solids, and 3: intake of full diet. Clinical success was defined as either relief of obstructive symptoms or at least one grade improvement in GOOSS score 5 days after stent placement.15 Complications detected within 24 h of procedure were labeled as procedure‐related complications. Procedure time was defined as time between endoscope insertion and SEMS deployment. Stent patency time was calculated as the period between stent placement and recurrence of obstruction assessed clinically, or on radiology and endoscopy. The endoscopist's comfort during the procedure was also assessed on a visual analogue scale ranging from 1 to 10 (1: minimum level of discomfort and 10 maximum).16 This scale was further categorized into three groups: easy (score 1–3), satisfactory (score 4–7), and difficult (score 7–10). The authors affirm that the study was performed in a manner that conformed to the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning Human and Animal Rights.
Statistical analysis
Categorical and continuous data were presented as proportion, median and range or mean, and standard deviation depending on the distribution. Categorical variables were analyzed using the Chi‐squared test with Yate's correction as applicable. Continuous parametric and nonparametric data were analyzed by unpaired t and Mann–Whitney U tests, respectively. P values less than 0.05 were considered significant. All statistical analyses were performed using statistical software SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA) and R, R‐studio, and Epicalc software (R development core team, Vienna, Austria).
Results
Patient characteristics and intervention
A total of 114 patients were included in this study (prospectively maintained data of 58 and retrospectively included 56 patients). SEMS placement was performed using a side‐viewing duodenoscope in 90 patients (SV group) and forward‐viewing endoscope (FV group) in 24 patients. Table 1 shows the baseline characteristics of all the patients. Mean age, gender, baseline KPS, cause and site of gastroduodenal obstruction, presence of ascites, history of biliary drainage, and chemotherapy were comparable between the two groups. Gallbladder carcinoma was the most common diagnosis (n = 48, 42.1%), followed by gastric (n = 29, 25.4%), pancreatic (n = 20, 17.5%), ampullary (n = 10, 8.8%), cholangiocarcinoma (n = 6, 5.3%), and duodenal adenocarcinoma (n = 1, 0.9%). In both groups, the junction of the first and second part of the duodenum was the most common site of obstruction (n = 56, 49.1%) followed by the antropyloric (n = 36, 31.6%) and ampullary regions (n = 21, 18.4%). The patient with duodenal carcinoma had obstruction at the third part of duodenum. Ascites was present in 21 (18.4%) patients. Prior biliary drainage was performed in 52 patients (42 in SV group and 10 patients in FV group), mostly through the endoscopic transpapillary route (n = 44, 84.6%) and the per‐cutaneous transhepatic route (PTBD) in the remaining patients; 21 (18.4%) patients received chemotherapy. The various types of SEMS used included Wallflex (Boston Scientific Asia Pacific Pte Ltd., 9 North Buona Vista Drive, Singapore 138588, n = 90), Hanaro (M. I. Tech 174, Habuk2‐Gil, Jinwi‐Myeon, Pyeongtaek‐SI, Gyeonggi‐DO, 17706, South Korea, n = 7), Niti‐S (Taewoong Medical, 14, Gojeong‐ro, Wolgot‐myeon, Gimpo‐si, Gyeonggi‐do, South Korea 10 022, n = 12), Bonastent (Standard Sci‐Tech Inc. 3F Sewoon Bldg, 46 WangSan‐Ro, DongDaeMun‐Gu, South Korea 02583, n = 4), and Ottomed (Mitra Medical Services LLP, B‐226, Okhla Industrial Area, Phase 1, New Delhi‐110020, India, n = 1).
Table 1.
Characteristics of patients undergoing antroduodenal stent placement using side‐ and forward‐viewing endoscopes
| Patient characteristics | SV group (N = 90) | FV group (N = 24) | P‐ value |
|---|---|---|---|
| Age (years) (mean ± SD) | 56.4 ± 11.7 | 56.9 ± 11.6 | 0.854 |
| Gender (male) | 43 (47.8%) | 12 (50.0%) | 1.0 |
| Diagnosis | |||
| Gall bladder carcinoma (%) | 36 (40%) | 11 (50%) | 0.582 |
| Gastric carcinoma (%) | 22 (24.4%) | 7 (29.1%) | |
| Pancreatic cancer (%) | 18 (20%) | 2 (8.3%) | |
| Ampullary carcinoma (%) | 8 (8.9%) | 2 (8.3%) | |
| Cholangiocarcinoma (%) | 6 (6.7%) | 0 (0%) | |
| Duodenal (D3) carcinoma (%) | 0 | 1 (4.2%) | |
| Site of obstruction | |||
| D1/D2 Junction (%) | 44 (48.9%) | 12(50%) | 0.991 |
| Antropyloric region (%) | 29 (32.2%) | 7 (29.2%) | |
| D2 (Ampullary region) (%) | 17 (18.9%) | 4 (16.7%) | |
| Duodenal (D3) carcinoma (%) | 0 | 1 (4.2%) | |
| Biliary drainage | |||
| Yes | 42 (46.7%) | 10 (41.7%) | 0.837 |
| Not done/not indicated | 48 (53.3%) | 14 (58.13%) | |
| Baseline KPS (IQR) | 60 (50–70) | 70 (57.5–70) | 0.147 |
| Chemotherapy, Yes (%) | 18 (20%) | 3 (12.5%) | 0.557 |
FV, forward‐viewing endoscope; IQR, interquartile range; KPS, Karnofsky performance status score; SV, side‐viewing duodenoscope.
Primary outcomes
Technical success was comparable between SV and FV groups (89/90, 99% vs. 24/24, 100%, P = ns; Table 2). SEMS could be deployed successfully with a forward‐viewing endoscope in two patients in whom initial attempts using a side‐viewing duodenoscope failed. The patients were diagnosed with antropyloric obstruction due to adenocarcinoma of the stomach and duodenal carcinoma causing obstruction in the third part of the duodenum. In another patient, an attempt at SEMS placement using a side‐viewing endoscope failed, and the patient died of massive tumor‐related bleed on the fifth day before reattempt. Clinical success was comparable between the SV and FV groups (84/90, 93.3% vs. 23/24, 95.8%, P = ns). GOOS improved following stent placement (median 0, range 0–2 vs. 2, 0–3, P = 0.0001; Figure 1). Characteristics of the patients in whom clinical success was not achieved (six in SV group and one in FV group) are shown in Table 3. Complications in all the three patients occurred with the side‐viewing endoscope; one had fatal postprocedure bleed, and the other two had aspiration pneumonia. Technical and clinical success did not differ in relation to the types of stents used.
Table 2.
Success and complication rates according of antroduodenal SEMS placement and type of endoscopes used
| Outcomes | SV group (N = 90) | FV group (N = 24) | P‐value |
|---|---|---|---|
| Technical success (%) | 89 (98.8%) | 24 (100%) | 1.0 |
| Clinical success (%) | 84 (93.3%) | 23 (95.8%) | 1.0 |
| GOOSS (median, IQR) | |||
| Baseline | 0 (0–1) | 0 (0–1) | 0.696 |
| Postprocedure | 2 (2–3) | 2 (2–3) | 0.618 |
| Procedure‐related complications (%) | 3 (3.3%) | 0 (0%) | 0.622 |
FV, forward viewing endoscope; GOOSS, gastric outlet obstruction scoring system; IQR, interquartile range; SEMS, self‐expandable metal stent; SV, side viewing duodenoscope.
Figure 1.
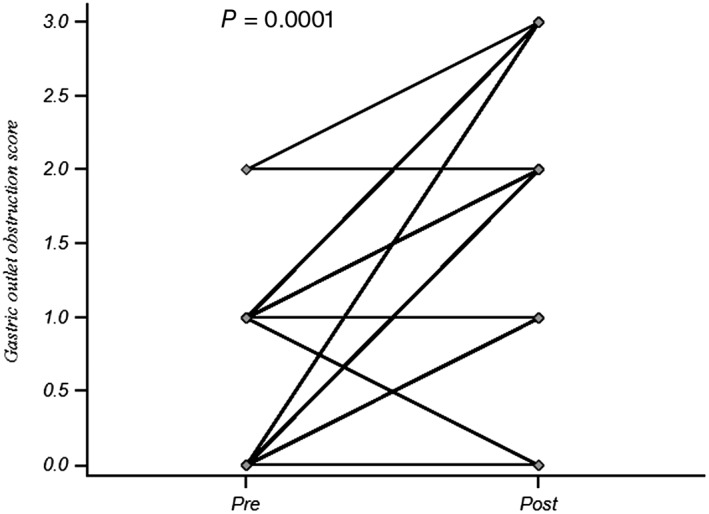
Gastric outlet obstruction score (GOOS) before and after antroduodenal SEMS placement.
Table 3.
Characteristics of patients in whom clinical success was not achieved
| Patient (SV/FV) | Age/Gender | Diagnosis/Site of obstruction | Baseline KPS | Baseline GOOSS | Biliary drainage | Chemotherapy | Ascites | Complication |
|---|---|---|---|---|---|---|---|---|
| SV‐1 | 50, M | Ca Stomach/Antropyloric | 50 | 1 | No | Yes | Yes | No |
| SV‐2 | 63, F | Ca GB/D1,D2 | 50 | 0 | Yes | No | Yes | Yes |
| SV‐3 | 41, M | Ca GB/D1,D2 | 50 | 0 | No | No | Yes | No |
| SV‐4 | 52, F | Ca GB/D1,D2 | 60 | 1 | Yes | No | No | Yes |
| SV‐5 | 60, F | Ca Stomach/Antropyloric | 60 | 1 | No | No | Yes | No |
| SV‐6 | 50, F | Ca GB/D1,D2 | 50 | 0 | Yes | No | No | No |
| FV‐1 | 48, F | Ca GB/D1,D2 | 50 | 0 | No | Yes | No | No |
SV group (N = 6), FV group (N = 1).
FV, forward viewing endoscope; GOOSS, gastric outlet obstruction scoring system; KPS, Karnofsky performance status score; SV, side viewing duodenoscope.
Secondary outcomes
SEMS could be placed in a shorter time using the forward‐ rather than side‐viewing endoscope (21 min [inter‐quartile range 19.5–35] vs. 34 min [inter‐quartile range 25–45], P ≤ 0.001; Table 4). Median stent patency (76.5 days, IQR 40–103.8 vs. 51.5 days, IQR 21–98.8, P = ns) and survival time (93 days, IQR 47–110.5 vs. 59.5 days, IQR 26–109.5, P = ns) were comparable between the FV and SV groups (Fig. 2). Procedure time and the postprocedure GOOSS did not differ in relation to the types of stent used. Figure 3 shows some endoscopic and radiological photographs of the stents.
Table 4.
Procedure time, stent patency, and survival according to type of scope used
| Outcomes | SV group (N = 90) | FV group (N = 24) | P‐value |
|---|---|---|---|
| Procedure time, median (IQR) | 34 min (25–45) | 21 min (19.5–35) | 0.001 |
| Reintervention (%) | 5 (5.6%) | 0 | 0.582 |
| Stent patency, median (IQR) | 51.5 (21–98.8) | 76.5 (40–103.8) | 0.269 |
| Overall survival, median (IQR) | 59.5 days (26–109.5) | 93 days (47 110.5) | 0.162 |
FV, forward viewing endoscope; IQR, interquartile range; SV, side viewing duodenoscope.
Figure 2.
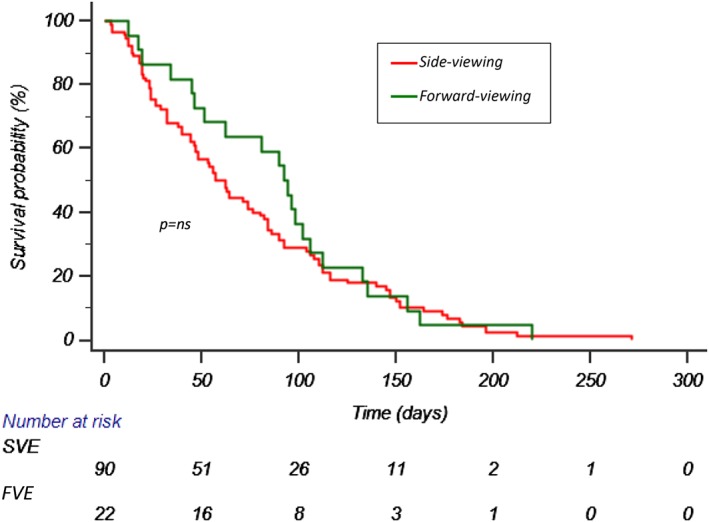
Kaplan–Meier curve showing overall survival.
Figure 3.

Endoscopic view (image a, b and c), abdominal radiograph (antroduodenal with biliary SEMS in image d and f), only antroduodenal SEMS in image e), Barium contrast X‐ray (image g) showing passage of contrast through self‐expandable metal stent.
Five patients (all in SV group) underwent CRE balloon dilatation within 3 days after stent placement due to nonexpansion. Stent migration occurred distally in two patients, one each with gastric carcinoma (SV group) and carcinoma gallbladder (FV group) at day 80 and day 15 after placement, respectively. One patient with gastric carcinoma had stent occlusion due to tumor ingrowth on day 64 (SV group). All three patients having stent migration and stent occlusion were managed with nasojejunal tube placement. A total of 32.4, 44.1, and 23.5% of the procedures performed with side‐viewing endoscope were scored as easy, satisfactory, and difficult, respectively; the corresponding values performed using forward‐viewing endoscopes were as follows: easy in 62.5%, satisfactory in 33.3%, and difficult in 4.2% of patients (P = 0.009).
Diagnosis of gastric cancer, baseline KPS 70–80, and baseline GOOSS of ≥1 were associated with better survival (Table 5). Age, gender, and history of biliary drainage had no relationship with survival after antroduodenal SEMS placement.
Table 5.
Association between clinical and demographic factors with survival time
| Variable | Survival in days (median, inter‐quartile range) | P value |
|---|---|---|
| Age (years) | ||
| <65 | 62 (25–110) | 0.894 |
| ≥65 | 62 (32–96) | |
| Gender | ||
| Male | 84 (39–114) | 0.018 |
| Female | 48 (24–90) | |
| Biliary drainage | ||
| Done | 56 (26–92) | 0.125 |
| Not done | 76 (30–136) | |
| Diagnosis | ||
| Ca gall bladder | 42 (19–76) | <0.001 |
| Gastric carcinoma | 125 (71–154) | |
| Pancreatic carcinoma | 60 (40–105) | |
| Ampullary carcinoma | 74(41–99) | |
| Cholangiocarcinoma | 65 (23–97) | |
| KPS | ||
| 50–60 | 44(19–64) | <0.001 |
| 70–80 | 116 (91–163) | |
| GOOS score (baseline) | ||
| 0 | 46 (23–81) | <0.001 |
| 1 | 106 (47–146) | |
Ca, carcinoma; GOOS, gastric outlet obstruction scores; KPS, Karnofsky performance status score.
Discussion
This study showed that (i) the technical and clinical success and complication rates of antroduodenal metal stent placement using side‐ and forward‐viewing endoscopes were comparable; (ii) however, SEMS could be placed in a shorter time using forward‐ rather than side‐viewing endoscope; (iii) the endoscopist's comfort level was better when placing antroduodenal stent using the forward‐viewing endoscope with therapeutic channel; (iv) gallbladder followed by gastric and pancreatic cancers were among the most common causes of malignant GOO overall in this northern Indian hospital; and (v) diagnosis of gastric cancer, baseline KPS 70–80, and GOOSS of ≥1 were factors associated with better survival.
Side‐viewing duodenoscopes are frequently used for inserting antroduodenal SEMS with therapeutic channel because of unavailability of forward‐viewing endoscopes in many centers of developing countries, including India. However, there are scanty data on the feasibility and outcome of antroduodenal stent placement using side‐viewing duodenoscopes.
In the previous studies on antroduodenal metal stent placement for malignant GOO, technical success rates varied from 92 to 100%, and clinical success rates were in the range of 67–97%.9, 17, 18 In our study, overall technical and clinical success rates of antroduodenal stent placement are 99.1 and 93.8%, respectively. In a study on 108 patients undergoing antroduodenal metal stent placement for malignant GOO using either side‐ (66.7%) or forward‐viewing endoscopes (33.3%), overall technical and clinical success rates were 99.1 and 84.5%, respectively,2 although the differences in success rates with side‐ or forward‐viewing endoscopes were not separately reported. In a South Korean study11 that compared, for the first time, the feasibility and outcomes of endoscopic SEMSs placement using either a side‐ (31 patients) or forward‐viewing endoscopes (15 patients), a majority of patients had an obstruction at the level of the third part of duodenum. Technical and clinical success was achieved in all patients in both the groups. Procedure‐related complications (microperforation) occurred in one patient in the side‐viewing endoscope group. Median survival was comparable in the two groups (134 days, 95% CI 77–191 days in SV group and 108 days, 95% CI 73–143 days in the FV group). The limitations of this study include small sample size; lack of inclusion of patients with more proximal obstruction, which is more common in practice; and a lack of data on procedure time and endoscopist's comfort levels. Hence, our study is important as some of the above mentioned limitations have been overcome.
In our study, no statistically significant difference was found in the technical and clinical success rates between SV and FV groups. However, interestingly, we found a significant shorter procedure time in FV group. The forward‐viewing endoscope has better coverage of the forward field of vision as well as better angulation control of the distal bending section in comparison with the side‐viewing duodenoscope. All these factors may help in the placement of a guide wire across the stricture. Survival time in our study was less in comparison with previous studies, partially related to advanced disease at the time of presentation.
All complications occurred in the SV group. Two patients had aspiration pneumonia, possibly because of prolonged procedure time in the SV group. Other studies showed similar rates of major complications like duodenal perforation and bleeding.19, 20 In our study, the incidence of stent migration and occlusion was not very high, as seen in previous studies.19, 21 One possible explanation of these differences could be that patients with such advanced disease tend to have poor follow‐up, which is why stent migration and occlusion could not be picked up in many cases.
In addition, we also measured the endoscopist's comfort level during the procedure on a visual analogue scale. We observed that performing the procedure by using a forward‐viewing endoscope is relatively easier than when using a side‐viewing duodenoscope.
In this study, gallbladder carcinoma was the most common cause of malignant GOO. Etiology of malignant GOO in this study is in accordance with the other Indian studies,22, 23, 24, 25 although quite different from other parts of the world where pancreatic and gastric cancers are more common.11, 26, 27 A long‐standing gall stone is associated with higher incidence of gall bladder carcinoma, particularly in women living in the Gangetic plains of northern India.28, 29 In Chile, gallbladder cancer is a leading cause of cancer‐related death among women.30
Our study has some limitations. First, fewer patients were included in the FV group. Second, we did not study change in the quality of life after antroduodenal stent placement.
In conclusion, side‐ and forward‐viewing endoscopes are equally effective for antroduodenal SEMS placement, but the procedure can be performed faster using the latter. The forward‐viewing endoscope has additional advantage of less procedure time and a better comfort level for the endoscopist.
Declaration of conflict of interest: None.
References
- 1. Didden P, Spaander MC, de Ridder R et al Efficacy and safety of a partially covered stent in malignant gastric outlet obstruction: a prospective Western series. Gastrointest. Endosc. 2013; 77: 664–8. [DOI] [PubMed] [Google Scholar]
- 2. Tringali A, Didden P, Repici A et al Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest. Endosc. 2014; 79: 66–75. [DOI] [PubMed] [Google Scholar]
- 3. Kanno Y, Ito K, Fujita N et al Efficacy and safety of a WallFlex enteral stent for malignant gastric obstruction. Dig. Endosc. 2013; 25: 386–91. [DOI] [PubMed] [Google Scholar]
- 4. Mehta S, Hindmarsh A, Cheong E et al Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg. Endosc. 2006; 20: 239–42. [DOI] [PubMed] [Google Scholar]
- 5. Weaver DW, Wiencek RG, Bouwman DL, Walt AJ. Gastrojejunostomy: is it helpful for patients with pancreatic cancer? Surgery. 1987; 102: 608–13. [PubMed] [Google Scholar]
- 6. van Wagensveld BA, Coene PP, van Gulik TM, Rauws EA, Obertop H, Gouma DJ. Outcome of palliative biliary and gastric bypass surgery for pancreatic head carcinoma in 126 patients. Br. J. Surg. 1997; 84: 1402–6. [PubMed] [Google Scholar]
- 7. Keranen I, Udd M, Lepisto A, Halttunen J, Kylänpää L. Outcome for self‐expandable metal stents in malignant gastroduodenal obstruction: single‐center experience with 104 patients. Surg. Endosc. 2010; 24: 891–6. [DOI] [PubMed] [Google Scholar]
- 8. Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self‐expanding metal stents: experience in 36 patients. Am. J. Gastroenterol. 2002; 97: 72–8. [DOI] [PubMed] [Google Scholar]
- 9. Nassif T, Prat F, Meduri B et al Endoscopic palliation of malignant gastric outlet obstruction using self‐expandable metallic stents: results of a multicenter study. Endoscopy. 2003; 35: 483–9. [DOI] [PubMed] [Google Scholar]
- 10. Song HY, Yang DH, Kuh JH, Choi KC. Obstructing cancer of the gastric antrum: palliative treatment with covered metallic stents. Radiology. 1993; 187: 357–8. [DOI] [PubMed] [Google Scholar]
- 11. Park JM, Min BH, Lee SH et al Feasibility of self‐expandable metal stent placement with side‐viewing endoscope for malignant distal duodenal obstruction. Dig. Dis. Sci. 2015; 60: 524–30. [DOI] [PubMed] [Google Scholar]
- 12. Lowe AS, Beckett CG, Jowett S et al Self‐expandable metal stent placement for the palliation of malignant gastroduodenal obstruction: experience in a large, single, UKcentre. Clin. Radiol. 2007; 62: 738–44. [DOI] [PubMed] [Google Scholar]
- 13. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J. Clin. Oncol. 1984; 2: 187–93. [DOI] [PubMed] [Google Scholar]
- 14. Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction. Analysis of technical and functional efficacy. Arch. Intern. Med. 1985; 145: 1443–6. [PubMed] [Google Scholar]
- 15. Maetani I, Mizumoto Y, Shigoka H et al Placement of a triple‐layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig. Endosc. 2014; 26: 192–9. [DOI] [PubMed] [Google Scholar]
- 16. Sachdeva A, Bhalla A, Sood A, Duseja A, Gupta V. The effect of sedation during upper gastrointestinal endoscopy. Saudi J. Gastroenterol. 2010; 16: 280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oh D, Lee SS, Song TJ et al Efficacy and safety of a partially covered duodenal stent for malignant gastroduodenal obstruction: a pilot study. Gastrointest. Endosc. 2015; 82: 32–6 e1. [DOI] [PubMed] [Google Scholar]
- 18. Shin YS, Choi CW, Kang DH et al Factors associated with clinical failure of self‐expandable metal stent for malignant gastroduodenal obstruction. Scand. J. Gastroenterol. 2016; 51: 103–10. [DOI] [PubMed] [Google Scholar]
- 19. Dormann A, Meisner S, Verin N, Wenk Lang A. Self‐expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004; 36: 543–50. [DOI] [PubMed] [Google Scholar]
- 20. Havemann MC, Adamsen S, Wojdemann M. Malignant gastric outlet obstruction managed by endoscopic stenting: a prospective single‐centre study. Scand. J. Gastroenterol. 2009; 44: 248–51. [DOI] [PubMed] [Google Scholar]
- 21. Yamao K, Kitano M, Kayahara T et al Factors predicting through‐the‐scope gastroduodenal stenting outcomes in patients with gastric outlet obstruction: a large multicenter retrospective study in West Japan. Gastrointest. Endosc. 2016; 84: 757–63 e6. [DOI] [PubMed] [Google Scholar]
- 22. Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy. 1998; 30: 484–6. [DOI] [PubMed] [Google Scholar]
- 23. Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N. Am. J. Med. Sci. 2015; 7: 403–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sikora SS, Kapoor R, Pradeep R, Kapoor VK, Saxena R, Kaushik SP. Palliative surgical treatment of malignant obstructive jaundice. Eur. J. Surg. Oncol. 1994; 20: 580–4. [PubMed] [Google Scholar]
- 25. Chaudhary A, Dhar P, Sachdev A, Agarwal A. Gastric outlet obstruction in carcinoma gall bladder. Indian J. Gastroenterol. 1999; 18: 101–3. [PubMed] [Google Scholar]
- 26. Telford JJ, Carr‐Locke DL, Baron TH et al Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: outcomes from a multicenter study. Gastrointest. Endosc. 2004; 60: 916–20. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki R, Sakai Y, Tsuyuguchi T et al Endoscopic management of unresectable malignant gastroduodenal obstruction with a nitinol uncovered metal stent: A prospective Japanese multicenter study. World J. Gastroenterol. 2016; 22: 3837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tewari M, Agarwal A, Mishra RR, Meena RN, Shukla HS. Epigenetic changes in carcinogenesis of gallbladder. Indian J. Surg. Oncol. 2013; 4: 356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhir V, Mohandas KM. Epidemiology of digestive tract cancers in India IV. Gall bladder and pancreas. Indian J. Gastroenterol. 1999; 18: 24–8. [PubMed] [Google Scholar]
- 30. Villanueva L. Cancer of the gallbladder‐Chilean statistics. Ecancermedicalscience. 2016; 10: 704. [DOI] [PMC free article] [PubMed] [Google Scholar]


