Abstract
Approximately 20% of patients with acute pancreatitis develop pancreatic necrosis. The presence of necrosis in a pancreatic collection significantly worsens the prognosis. Pancreatic necrosis is associated with high mortality and morbidity. In the last few decades, there has been a significant revolution in the treatment of infected pancreatic necrosis. A step‐up approach has been proposed, from less invasive procedures to the operative intervention. Minimally invasive treatment modalities such as endoscopic drainage and necrosectomy, percutaneous drainage, and minimally invasive surgery have recently replaced open surgical necrosectomy as the first‐line treatment option. Endoscopic intervention for pancreatic necrosis is being increasingly performed with good success and a lower complication rate. However, techniques of endotherapy are still not uniform and vary as per local expertise, and there are still many unresolved questions with regard to the interventions in patients with pancreatic necrosis. The objective of this paper is to critically review the literature and update the concepts of endoscopic interventional therapy of pancreatic necrosis.
Keywords: acute necrotizing pancreatitis, acute pancreatitis, direct endoscopic necrosectomy, endotherapy, minimal access retroperitoneal pancreatic necrosectomy, open necrosectomy, walled‐off pancreatic necrosis
Introduction
Approximately 20% of patients with acute pancreatitis develop pancreatic necrosis. The presence of necrosis in a pancreatic collection significantly worsens the prognosis. About 30% of patients with acute necrotizing pancreatitis develop infected pancreatic necrosis. The mortality rate in patients with infected pancreatic necrosis has been reported to be as high as 39%.1, 2 Traditionally, laparotomy with complete necrosectomy was performed early in the clinical course of acute necrotizing pancreatitis. However, it was associated with very high morbidity (up to 95%) and mortality (11–39%).3 Of late, there has been a significant revolution in the treatment of infected pancreatic necrosis. Delayed intervention and use of minimally invasive treatment modalities such as endoscopic drainage and necrosectomy, percutaneous catheter drainage (PCD), and minimally invasive surgery have recently replaced surgical debridement as the first‐line treatment option. The indication for laparotomy has sharply diminished in recent years and is limited to rare complications of acute pancreatitis, such as abdominal compartmental syndrome, bowel ischemia, and bowel perforation. Endoscopic intervention for pancreatic necrosis is being increasingly performed with comparable success and an acceptable complication rate. This review provides an overview and recent development in the field of interventional therapy in patients with pancreatic necrosis.
Definition
The 2012 revised Atlanta classification for acute pancreatitis addressed several key controversial issues and redefined terminology for acute pancreatitis and its sequelae.4 According to the revised Atlanta classification (2012), acute necrotizing pancreatitis is subdivided into three categories: parenchymal necrosis, peripancreatic necrosis, and combined necrosis. In the setting of acute necrotizing pancreatitis, a collection of fluid and necrotic materials involving the pancreatic parenchyma or the peripancreatic tissues is termed an acute necrotic collection (ANC) when seen within the first 4 weeks of the disease. ANCs lack a well‐defined wall. When an ANC persists beyond 4 weeks and becomes encapsulated, the term walled‐off pancreatic necrosis (WOPN) is used. ANCs or WOPN may be infected or sterile. The natural history of ANC and WOPN is not well described. The presence of necrosis in a pancreatic collection is considered an important prognostic marker. Pancreatic or peripancreatic infection is the second most common cause of death (next to early organ failure). Infected pancreatic necrosis causes sepsis and delayed multiple organ failure.1, 2 Infected pancreatic necrosis has a high mortality and therefore usually requires adequate debridement and drainage.
Management overview
Initial management in the case of proven or suspected infected pancreatic necrosis should include intravenous broad‐spectrum antibiotics. Antibiotics known to penetrate into the pancreas and have an effect on gut‐derived bacteria such as quinolones, carbapenems, and metronidazole should be chosen as empirical therapy. A positive culture of infected collections may result in the narrowing down of antibiotic spectrum aimed at those microorganisms. Recovery with antibiotic alone has been described in about half of cases; however, in the remaining patients, drainage and necrosectomy are needed.5, 6 Interventions are postponed whenever feasible until inflammatory reaction becomes better organized, and a WOPN is seen. As of now, the evidence is not sufficient enough to recommend an optimal technique for drainage and necrosectomy. Recent data seem to suggest that minimally invasive procedures are superior to open necrosectomy, although no trial has yet directly compared these two approaches. Currently, the most accepted interventional treatment is a “step‐up approach,” which involves initial PCD followed by minimally invasive necrosectomy by radiological, endoscopic, video‐assisted retroperitoneal (VARD), laparoscopic, or open surgical methods. Of late, endoscopic drainage and necrosectomy have gained popularity. A study (PENGUIN trial) has demonstrated endoscopic necrosectomy (EN) to be superior to VARD.7 In a recent systematic review of seven studies (total 490 patients), endoscopic drainage was found to be better compared to PCD as it was associated with significantly better clinical success, a lower reintervention rate, and a shorter hospital length of stay.8 Endoscopic drainage is also preferred over PCD due to decreased risk of pancreatic fistula.
Indications of pancreatic endotherapy
Endotherapy of pancreatic necrosis is indicated in the following conditions.
Infected necrosis
Signs of infected pancreatic necrosis include new‐onset or persistent sepsis, fever, the presence of systemic inflammatory response syndrome, elevated or increasing C‐reactive protein or progressive leukocytosis, the clinical deterioration of a stable patient on adequate support, and new/prolonged organ failure. These features are suggestive of infected pancreatic necrosis in about 80% of cases.9 In the absence of documented infection, ongoing organ failure or persisting unwellness (“failure to thrive”) for several weeks after the onset of acute pancreatitis is also suggestive of infected pancreatic necrosis. A retrospective study showed the presence of infection in 42% of these patients.10 A recent systematic review suggested that procalcitonin with a cut‐off value of 3.5 ng/mL is the best laboratory predictor of infected pancreatic necrosis (sensitivity and specificity of 0.90 and 0.89, respectively).11 The presence of gas in parenchymal or extrapancreatic necrosis on computed tomography scan (CT‐Scan) showed poor sensitivity for assessing infection of necrotic collections (sensitivity 45.9%; specificity 81.5%; accuracy 50.5%). Fine‐needle aspiration is not routinely advocated for the diagnosis of infected pancreatic necrosis.9
Infected pancreatic necrosis can be treated with conservative treatment without necrosectomy. Conservative treatment consists of intensive care, antimicrobial agents, and nutritional support, with or without drainage of the infected fluid. In a systematic review, Mouli et al. have demonstrated the successful treatment of infected pancreatic necrosis with conservative management in 64% of patients. Necrosectomy or surgery was required in 26% of patients. Mortality was seen in 12% of patients.5 Infected pancreatic necrosis not responding to medical therapy is a definite indication of endotherapy. Intervention is indicated in these patients, especially when the necrosis has become walled off. Before any invasive interventions, magnetic resonance imaging (MRI) is preferred to assess the condition of WOPN because it is better at detecting nonliquefied necrotic material than CT‐Scan and better delineates the status of pancreatic duct (Fig. 1).12 Endoscopic ultrasound (EUS) is also accurate in assessing the content of WOPN.13
Figure 1.
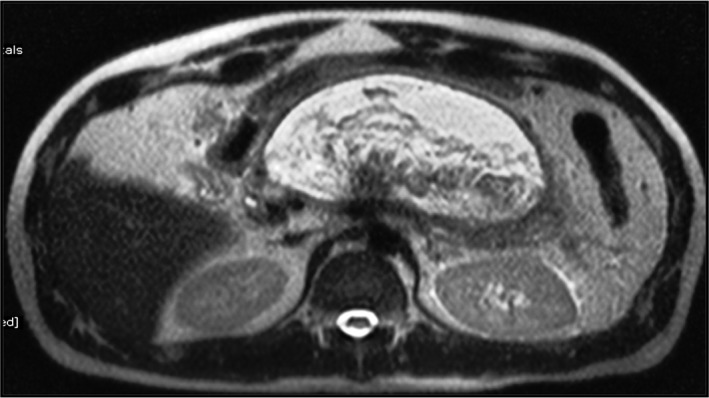
Magnetic resonance imaging (T2W) image showing a large, thick‐walled heterogenous cystic lesion with internal hypointense solid necrotic material and hyperintense fluid component indenting the posterior gastric wall, suggestive of walled‐off pancreatic necrosis.
Noninfected necrosis
Endotherapy is indicated in noninfected necrosis in the presence of the following situations: (i) an enlarging collection imposing pressure on contiguous organs, such as stomach, duodenum, or bile duct, resulting in the development of symptomatic gastric outlet obstruction or bile duct obstruction; (ii) fistulization into contiguous organs; (iii) vascular compression and erosion of vascular structures; and (iv) although debatable, symptomatic collections such as refractory abdominal pain, ongoing systemic illness, anorexia, or weight loss lasting more than 8 weeks after the onset of acute pancreatitis are indications of endotherapy.14
Contraindications of pancreatic endotherapy
Apart from hemodynamic instability and deranged coagulation profile, endotherapy is contraindicated in the necrosis cavity without adequate rim, those with >1 cm thickness between the gastroduodenal lumen and necrosis cavity, and those with pancreatic necrosis with vascular pseudoaneurysm. The presence of extensive collaterals with or without portal hypertension is also considered a relative contraindication.15
Timing of intervention
Ideally, endoscopic treatment or other interventions should be considered after 4 weeks of onset of disease. In patients with clinical deterioration, it can be carried out after the third week. However, in patients with progressive clinical deterioration requiring invasive treatment before the third week, less invasive techniques, such as PCD via retroperitoneal route, is preferred. The endoscopic or surgical method is attempted only if there is no clinical improvement.14, 16 In a study, mortality in the early surgery (within the first 48–72 h of admission) group reached 56% compared to 27% of the group managed more conservatively with delayed surgery (>12 days of admission). The main reason of postoperative deaths was multiple organ failure.17 In a study, Besselink et al. found that the mortality in patients after necrosectomy was the lowest after 30 days of onset of acute pancreatitis (8 vs 75% in the <15‐day group and 45% in the 15–29‐day group, P < 0.001).18
Complications of endotherapy
The endotherapy of WOPN is associated with a high rate of mortality and morbidity. In a systemic review, complications and mortality occurred in 36 and 6% of patients, respectively.19 The most frequent complications of endotherapy are bleeding, perforation, postprocedure infection, and migration of the stents.20
Bleeding is a very common complication of endotherapy seen in up to 18% of patients.19 The risk is higher during debridement of necrotic materials. EUS‐guided procedures have a lower risk of bleeding compared to conventional methods. Usually, bleeding is minor and stops spontaneously or is controlled with intracystic washing with diluted epinephrine. Hemoclips and sclerosants are an effective tool for the control of acutely bleeding vessels. Treatments by interventional radiology or surgery are the other effective options for uncontrolled massive bleed.
Perforation is another common serious complication seen in 4% of patients. To prevent perforation, the use of electrocautery during the creation of the fistula should be avoided. Perforation mostly occurred during the dilatation of the tract. Gradual mechanical dilation with a CRE™ balloon is the preferred approach. Endotherapy in the absence of an adequate rim around the necrotic cavity can also lead to peritonitis and leakage of the debris. Waiting for ≥4 weeks is beneficial to avoid these complications. The majority of these perforations can be treated with conservative treatment.
Stent migration is another complication, and its incidence ranges from less than 1–2%. Newer lumen‐apposing metal stents (LAMS) are designed to prevent migration. Internal migration of a stent is a serious therapeutic issue. Postprocedure infection is not an uncommon complication, which can be avoided with adequate drainage.
Air embolism is another serious complication that can cause the death of patients. Carbon dioxide insufflation during necrosectomy reduces the risk of this potentially fatal complication.
Standard endotherapy technique
Endotherapy is preferably performed with patients under deep sedation or general anesthesia. Broad‐spectrum antibiotics coverage is recommended. Anticoagulant or antiplatelets drugs should be discontinued, ideally 5 days before the procedure. Necrosectomy should be preferably performed in an endoscopic suite with the facility for carbon dioxide insufflation to avoid air embolism. The availability of a surgeon and interventional radiologist should be ensured. The presence of a necrotic collection seen on CT‐Scan or MRI should be assessed by EUS for the feasibility of endotherapy. EUS has been used to measure the exact distance between enteric and necrosis cavity lumen, to identify vessel interposition, and to decide the optimal site for drainage. A linear array echoendoscope with color Doppler is used for localization of pancreatic necrosis and identification of surrounding vasculature. Drainage can be performed using the Seldinger technique. A needle knife or 19‐gauge fine‐aspiration needle is used to create a fistula for the transenteric access of the necrosis cavity. Correct positioning of the needle can also be confirmed by the aspiration of fluid or contrast injection into the collection under fluoroscopy. After accessing the necrotic area and aspiration of fluid for microbiology analysis, a 0.035‐inch guidewire is advanced through the needle into the cavity under fluoroscopic guidance. Coiling of guidewire into the cavity should be confirmed (Fig. 2). The tract is dilated with either a 10F cystotome, or a balloon of between 8 and 15 mm, or both. For the endoscopic drainage of WOPN, tract dilation is followed by placement of at least two double pigtail stents and a nasocystic catheter into the collection (Fig. 3). The nasocystic catheter is used for continuous daily flushing with 1 L of normal saline. The adequacy of drainage should be assessed on repeat CT‐Scan after 2–3 days of the procedure. In case of failure of therapy, direct EN should be performed. For direct EN, dilatation of transenteric access is performed using a CRE™ balloon of 15–18 mm. A gastroscope is passed through the dilated tract into the necrotic cavity for irrigation and debridement of necrotic materials. The debridement of necrotic materials is conducted for several sessions using biopsy forceps, Dormia basket, Roth nets, and polypectomy snares (Fig. 4a). The procedure is completed when the most adherent necrotic tissue is removed and a vital, pink wall becomes visible (Fig. 4b). At the end of the procedure, a self‐expandable metallic stent (SEMS) (esophageal/billiary stents of small length), NAGI™ stent, LAMS (Axios™ stents), or multiple plastic stents are inserted to keep the fistula open. Sometimes, keeping the nasocystic drain to irrigate the fluid collection (1 L normal saline/24 h) is useful. Repeat sessions of necrosectomy can be performed usually every second or third day.
Figure 2.
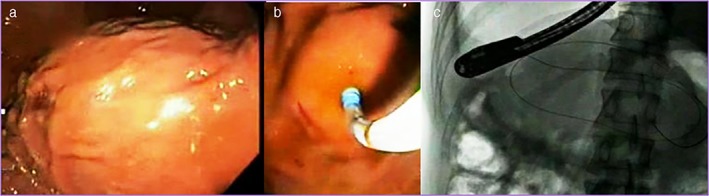
Images showing gastric bulge, access into the cavity, and coiling of guidewire into the cavity.
Figure 3.
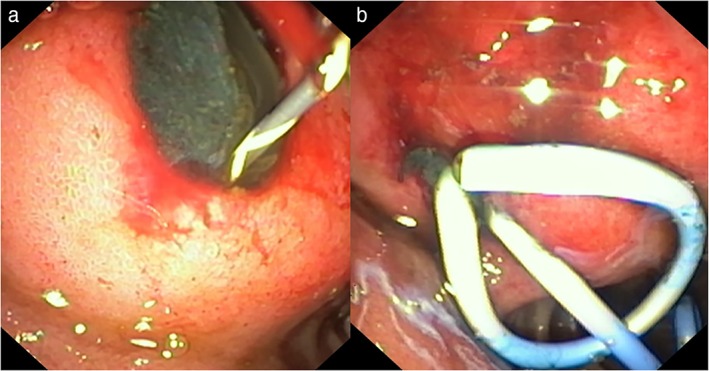
Images showing endoscopic drainage of walled‐off pancreatic necrosis with placement of two double‐pigtail plastic stents (cystoduodenostomy).
Figure 4.
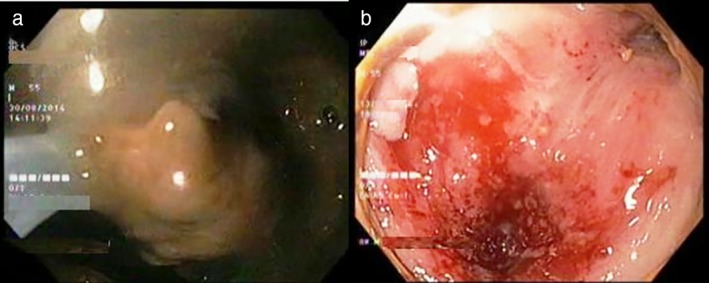
Images showing direct endoscopic necrosectomy of walled‐off pancreatic necrosis.
The conventional non‐EUS‐guided technique for pseudocyst drainage is still practiced in some medical centers. The endoscopic drainage of WOPN can rarely be performed without EUS guidance by blind puncture at the site of maximum impression on the gastric or duodenal wall. Complication rate is high with this technique compared to the EUS‐guided technique. In one study, the use of EUS during endotherapy of WOPN significantly reduced the number of procedure‐related complications (14 vs 25.9%), mainly gastrointestinal bleeding. However, the duration of treatment or the success rate of therapy was comparable in both groups (93.75 vs 92.9%).21 The uses of hydrogen peroxide without external irrigation in the non‐EUS‐guided drainage of WOPN have recently been described.22, 23
Modifications in standard endoscopic technique
The standard endoscopic technique is also known as the conventional drainage technique (CDT) and the single transluminal gateway technique (SGT) in which two plastic stents with a nasocystic catheter are deployed via one transmural tract. Several variations of the technique for the drainage of WOPN have recently been described. The uses of the multiple transluminal gateway technique (MTGT) and single transluminal gateway transcystic multiple drainages (SGTMD) for complicated WOPN have recently been reported. In MTGT, under EUS guidance, two or three transmural tracts are created between the necrotic cavity and the gastrointestinal lumen. While one tract is used to flush normal saline solution via a nasocystic catheter, multiple plastic stents are placed in others tracts to facilitate the drainage of necrotic contents. In one study (n = 60), MTGT was compared with standard technique in patients of symptomatic WOPN. Treatment was successful in 91.7 and 52.1% of patients managed by MTGT and standard technique (P = 0.01), respectively.24 In another study, treatment success was higher in patients undergoing MTGT as compared to conventional drainage of WOPN (94.4 vs 62.1%, P = 0.009).25
Mukai et al. have advocated the use of a combination of endotherapies (SGT in combination with MTGT or SGTMD) for the treatment of complicated pseudocyst and WOPN. SGTMD was useful if the subcavities with insufficient drainage were connected to the main cavity identified by CT‐Scan. The connections within the main cavity to the subcavities were identified using an endoscopic retrograde cholangiopancreatography (ERCP) catheter and soft guidewire (0.032 inch). Placement of one or more 7F plastic stents and a 5F or 6F nasocystic catheter in the subcavities, followed by thorough irrigation with saline, was performed. The technical success rate and final clinical success rate were 100 and 97.8%, respectively. Early adverse event rate and mortality rate were 12.4 and 2.2%, respectively.26
The conventional EUS‐guided endoscopic technique under fluoroscopy is the preferred standard method for pseudocyst/WOPN drainage; however, it is associated with radiation exposure. Studies have shown the successful EUS‐guided placement of plastic stents and SEMS without fluoroscopic assistance. In a study, EUS‐guided (without fluoroscopy) transgastric fully covered self‐expanding metal stent (FCSEMS) insertion was technically successful in 92.6%. All steps of the procedure were clearly visualized by EUS.27, 28 Yoo et al. performed a single‐step EUS‐guided drainage of WOPN without fluoroscopy using an electrocautery‐enhanced LAMS. Technical success with the placement of the electrocautery‐enhanced LAMS was achieved in all 25 patients.29 Further studies are warranted to address the ease of performing the procedure and the duration of the procedure without fluoroscopic assistance.
Use of self‐expanding metal stents
Metallic stents are being increasingly used to establish transgastric or transenteric tracts in WOPN. The endoscope is passed through the SEMS into the cavity for mechanical debridement of necrosis. This appears to be better than multiple plastic stents in keeping the tract open. Moreover, this also facilitates improved drainage of the lesser sac collections. Displacement of SEMS, nonavailability of a stent of appropriate size, and cost of stent are drawbacks of its use. Fully covered esophageal and biliary SEMS of different sizes are used by endoscopists. However, longer size and high migration rate are the limitations of their use. In addition, biliary SEMS does not permit the passage of endoscope for debridement. The new, fully covered SEMS (NAGI™ stent [Taewoong Medical Co., Ltd., Seoul, Korea]) is currently being used. The NAGI stent consists of a fully covered stent, 2 cm in length and 16 mm in diameter, with bilateral anchor flanges and string for the removal of SEMS. The most effective of these SEMS are called yoyo‐type stents. These stents have very broad flanges to keep them from being pulled in and out of the cyst when working through the prosthesis. The yoyo‐like design of the new SEMS, such as the Axios™ stent (Xlumena Inc., Mountain View, CA, USA), has a lumen‐apposing effect and appears promising in the management of pancreatic fluid collections. In a recent multicentric study (n = 124), Sharaiha et al. have used LAMS for the treatment of WOPN in 124 patients and demonstrated technical and clinical success in 100 and 86.3% patients, respectively, at 3 months’ follow‐up. The stents patency rate was 94%, with a migration rate of 5.6%.30 In another study, endotherapy using the LAMS was successful in 88.2% of patients with WOPN.31 In another study by Abu Dayyeh et al., WOPN was more likely to resolve without subsequent direct EN in the large‐caliber, fully covered SEMS group than the plastic stents group (60.4 vs 30.8%; P = 0.01).32 In a meta‐analysis of 11 studies with 688 patients, Hammand et al. found LAMS to be better than multiple plastic stents in terms of clinical success and adverse events.33 Another recent meta‐analysis performed by Bazerbachi et al. included 41 studies with 2213 patients with WOPN.34 WOPN resolution was more likely with SEMS compared with plastic stents (P < 0.001). The SEMS group required fewer interventions (in those cases requiring more than one intervention) and lower bleeding (5.6 vs 12.6%; P = 0.02). In a study by Siddiqui et al., comparison of plastic stents, FCSEMSs, or the novel LAMSs for treatment of WOPN showed comparable technical success.35 Complete resolution of WOPN was lower with plastic stents compared with FCSEMSs and LAMSs (81 vs 95% vs 90%; P = 0.001). The mean number of procedures required was lower in the LAMS group compared with the other two groups (2.2 vs 3 vs 3.6, respectively; P = 0 0.04). Early adverse events were lower in the FCSEMS group compared with the other two groups (1.6, 7.5, and 9.3%; P < 0.01). LAMSs are more effective in achieving successful endoscopic drainage (92 vs 84%) but are also costlier (20.029 UD dollars vs 15 941 US dollars) than plastic stents in managing WOPN.36
Endoscopic necrosectomy‐evidences
In a systemic review, 455 patients of acute necrotizing pancreatitis undergoing EN were analyzed. A total of 57% patients had infected pancreatic necrosis. On average, four (range 1–23) endoscopic interventions were performed per patient. With EN alone, treatment success was achieved in 81% of patients. Complications and mortality occurred in 36 and 6% of patients, respectively.19 In one study, Gardner et al. compared the success of direct EN with CDT. The success rate of direct EN and CDT were 88 and 45% (P < 0.01), respectively. The total number of procedures and complications were equivalent in both groups.37 Evidence regarding the endotherapy of pancreatic necrosis is summarized in Table 1.
Table 1.
Endoscopic necrosectomy—summary of major studies
| References | n | Endotherapy sessions† | Success rate (%) | Complication (%) | Mortality (%) |
|---|---|---|---|---|---|
| Thompson et al.,38 | 60 | 1.58 | 86.7 | 3.3 | 0 |
| Jagielski et al.,21 | 176 | 2.88 | 93.18 | 40 | 0.56 |
| Yasuda et al.,39 | 57 | 5 | 75 | 33 | 11 |
| Bang et al.,25 | 76 | 1.4 | 69.7 | 14.5 | 5.26 |
| Jürgensen et al.,40 | 35 | 6.2 | 100 | 9 | 2.85 |
| Gardner et al.,41 | 104 | 3 | 91 | 14 | 4.8 |
| Gardner et al.,37 | 25 | 3.6 | 88 | 32 | 0 |
| Seifert et al., 200942 | 93 | 6 | 80 | 26 | 7.5 |
| Coelho et al.,43 | 56 | 4 | 87 | 11 | 4‡ |
| Escourrou et al.,44 | 13 | 1.8 | 100 | 46 | 0 |
| Schrover et al.,45 | 8 | 4 | 75 | 0 | 12.1 |
| Mathew et al.,46 | 6 | 1 | 100 | 0 | 0 |
| Papachristou et al.,47 | 53 | 3 | 81 | 21 | 6 |
| Voermans et al.,48 | 24 | — | 93 | 7 | 0 |
| Charnley et al.,49 | 13 | 4 | 92.3 | — | 15.38‡ |
Mean/median.
Died of other cause.
n, number of patients. Procedure‐related complication was nil.
Transpapillary drainage of WOPN
Central pancreatic necrosis can lead to the disruption of the main pancreatic duct. This can be diagnosed as a leak of contrast medium into the necrotic cavity during ERCP. Endoscopic stent placement is frequently successful in the transpapillary drainage of necrosis cavity. Authors have described the successful transpapillary drainage of WOPN.50 However, the management of a disconnected pancreatic duct syndrome in the setting of acute necrotizing pancreatitis is difficult and includes long‐term endoscopic transmural stent placement and/or distal pancreatectomy.
Tailoring the endotherapy approach
Tailoring the endoscopic technique as per the specific characteristics of each collection, including size, location, amount of debris, presence of internal septation, and stepwise response to intervention, appears to be the superior approach for the treatment of WOPN. A small collection (<12 cm) with minimal debris can be treated by CDT. Patients with WOPN measuring >12 cm, with extensive necrosis and extending to the paracolic gutters, require placement of multiple internal conduits under EUS guidance for the efflux of necrotic contents and better drainage. In one study, an algorithmic approach was adopted based on the size and extent of the WOPN and stepwise response to intervention.51 The treatment success rate was higher for the algorithmic approach compared with standard treatment (91 vs 60% respectively; P < 0.001). Management based on the algorithm was the only predictor of treatment success (odds ratio 6·51; P = 0.001).
Limitations of endotherapy
Apart from the risk of complications in the form of bleeding, perforation, stent migration, and air embolism, other limitations of endoscopic therapy are the need for repeated sessions, prolonged external irrigation, availability, and expertise of EUS. EN requires multiple sessions of endoscopic procedure. In a systemic review, 455 patients of acute necrotizing pancreatitis undergoing EN were analyzed.19 On average, four (range 1–23) endoscopic interventions were performed per patient. In most of the studies, the number of procedures required to endoscopically remove necrotic debris was three to six (Table 1). Scheduling patients for multiple sessions of necrosectomy is not always easy and needs a significant commitment from the patient and endoscopy suite. Concern of cost‐effectiveness is poorly addressed and needs further analysis. The recurrent fluid collection from the disconnected tail, especially after the removal of stents in patients with central pancreatic necrosis, is another problem. After any type of endoscopic treatment, there is always a risk of recurrence in the form of pseudocyst or necrotic cavity. In case of WOPN, the reported recurrence rate after endoscopic transmural drainage was 9.4%,25 after combined percutaneous and endoscopic drainage was 7.8%52 and after EN in a meta‐analysis of eight studies was 10.9%.53
Percutaneous catheter drainage
PCD of necrotic collection was introduced as a bridge to definitive surgery. Nearly all peripancreatic collections are accessible via retroperitoneal or transperitoneal route. However, the retroperitoneal route is preferred because it is associated with fewer complications, including fewer chances of peritoneal contamination. The same route can also be utilized for minimally invasive necrosectomy. PCD can be performed safely even before maturation of the wall of necrosis cavity. If additional intervention is needed after PCD, a VARD procedure can be performed as part of the surgical step‐up procedure. In a recent retrospective study, the outcome of endotherapy and PCD was compared. In comparison to PCD, treatment success was significantly higher in endotherapy (70 vs 31%). The endotherapy cohort required fewer interventions, had lower rates of residual collections, and the need for surgical intervention. The procedural complications were higher in the endotherapy cohort compared to the PCD group (10 vs 1%).54 In a systematic review of 11 studies, including one RCT, aiming to assess the role of PCD as a primary treatment for acute necrotizing pancreatitis, 55.7% patients improved without additional surgical necrosectomy. The overall complication rate was 20%, and the most common complications were the formation of pancreaticocutaneous and pancreaticoenteric fistula.55 Similarly, a systematic review evaluating the role of conservative treatment (including antibiotic + PCD) in infected pancreatic necrosis patients demonstrated successful outcomes in 64% of patients.56
Dual‐modality drainage
Dual‐modality drainage or combined modality therapy, in which endotherapy is performed in addition to PCD, has demonstrated impressive success in the treatment of WOPN.52, 57, 58 Dual‐modality drainage with endotherapy and the percutaneous route is an effective option with low mortality and morbidity. Compared to standard PCD, patients undergoing combined modality therapy have a significantly decreased length of hospitalization (26 vs 55 days, P < 0.0026), duration of external drainage (83.9 vs 189 days, P < 0.002), and number of CT‐Scans (8.95 vs 14.3, P < 0.002). Patients treated with PCD have more complications.57 A minimally invasive step‐up approach is preferred over open necrosectomy. The step‐up approach consists of PCD followed, if necessary, by minimally invasive retroperitoneal necrosectomy. In one study (n = 88), major complications or death occurring in the step‐up approach was significantly lower compared to open necrosectomy (40 vs 69%) (P = 0.006).59
Surgical necrosectomy
The techniques for pancreatic necrosectomy include minimal access techniques via laparoscopy or endoscopy, open necrosectomy, and laparostomy and closed packing with or without lavage. Unfortunately, the mortality rates for open necrosectomy range from 6 to 47%.60 The minimal access retroperitoneal pancreatic necrosectomy (MARPN), a less invasive approach, reduced the overall complications and deaths. In a retrospective study (n = 394), treatment with MARPN showed a lower mortality and morbidity rate compared to open necrosectomy in patients of pancreatic necrosis. The mortality and morbidity rates were 15.3 and 63.5%, respectively, in the MARPNs group and 23.3 and 81.7%, respectively, in the open necrosectomy group.61 In a review analysis, the MARPNs were compared with EN. The mortality and complication rates were 16 and 41%, respectively, in the MARPNs group (n = 141) and 5 and 20%, respectively, in the EN group (n = 157).62 In another study, minimally invasive surgical and endoscopic therapy for the treatment of sterile WOPN was comparable in terms of outcomes and cost‐effectiveness.63
Current guidelines
In 2012, the International Association of Pancreatology and the American Pancreatic Association jointly issued guidelines concerning a key aspect of the medical and surgical management of acute pancreatitis. Recently (2018), the European Society of Gastrointestinal Endoscopy published guidelines on the management of acute necrotizing pancreatitis.14, 64 The key points of the recommendations are as follows: (i) invasive intervention is indicated for patients with clinically suspected or proven infected pancreatic necrosis; (ii) routine percutaneous fine‐needle aspiration of peripancreatic collections to detect infection is not indicated; (iii) the first intervention should be delayed for 4 weeks if tolerated by the patient; (iv) depending on the location of WOPN and local expertise, endoscopic or percutaneous drainage of (suspected) infected WOPN should be performed as the first interventional method, and in the absence of improvement following endoscopic transmural drainage, EN or minimally invasive surgery is to be preferred over open surgery; (v) long‐term indwelling of transluminal plastic stents is recommended in patients with disconnected pancreatic duct syndrome; and (vi) LAMS should be retrieved within 4 weeks to avoid stent‐related adverse effects.
Future challenges
Endotherapy appears to have comparable or better success and less morbidity and mortality than minimally invasive surgery. Although available evidence is optimal to recommend endotherapy as the first‐line therapy of pancreatic necrosis, it has not been proven yet in high‐quality comparative trials. Techniques of endotherapy are still not uniform and vary per local expertise. Ideal device for the effective removal of necrotic debris is still not available. An endoscopic, high‐pressure, water‐blasting system to pulverize the necrosis without damaging surrounding structures would be extremely helpful in necrosectomy.65 The future of endotherapy demands the advocacy of EUS‐guided necrosectomy, the innovation of more precise devices, multicentric trials, and a multidisciplinary team approach.
Conclusion
Pancreatic necrosis is associated with very high mortality and morbidity. Minimally invasive treatment modalities have recently replaced open surgical debridement as the first‐line treatment option. Endoscopic intervention for pancreatic necrosis is being increasingly performed with comparable success and a lower complication rate. Endotherapy for the treatment of pancreatic necrosis demands a tailored endoscopic approach, advocacy of EUS‐guided necrosectomy, innovation of more precise devices, multicentric trials, and a multidisciplinary team approach.
Declaration of conflict of interest: None.
Author contribution: Ashish Kumar Jha was involved in designing and writing the manuscript; Ashish Kumar Jha and Arya Suchismita were responsible for thorough literature search; and Ramesh Kumar and Mahesh Kumar Goenka were involved in editing the manuscript. All authors read and approved the final manuscript.
References
- 1. Banks PA, Freeman ML; Practice Parameters Committee of the American College of GastroenterologyPractice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006; 101: 2379–400. [DOI] [PubMed] [Google Scholar]
- 2. Freeman ML, Werner J, van Santvoort HC et al International Multidisciplinary Panel of Speakers and Moderators. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012; 41: 1176–94. [DOI] [PubMed] [Google Scholar]
- 3. Rau B, Bothe A, Beger HG. Surgical treatment of necrotizing pancreatitis by necrosectomy and closed lavage: changing patient characteristics and outcome in a 19‐year, single‐center series. Surgery. 2005; 138: 28–39. [DOI] [PubMed] [Google Scholar]
- 4. Banks PA, Bollen TL, Dervenis C et al Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62: 102–11. [DOI] [PubMed] [Google Scholar]
- 5. Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta‐analysis. Gastroenterology. 2013; 144: 333–40. [DOI] [PubMed] [Google Scholar]
- 6. Lytras D, Manes K, Triantopoulou C et al Persistent early organ failure: defining the high‐risk group of patients with severe acute pancreatitis? Pancreas. 2008; 36: 249–54. [DOI] [PubMed] [Google Scholar]
- 7. Bakker OJ, van Santvoort HC, van Brunschot S et al Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012; 307: 1053–61. [DOI] [PubMed] [Google Scholar]
- 8. Khan MA, Hammad T, Khan Z et al Endoscopic versus percutaneous management for symptomatic pancreatic fluid collections: a systematic review and meta‐analysis. Endosc. Int. Open. 2018; 6: E474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Baal MC, Bollen TC, Bakker OJ et al Dutch Pancreatitis Study Group. The role of routine fine‐needle aspiration in the diagnosis of infected necrotizing pancreatitis. Surgery. 2014; 155: 442–8. [DOI] [PubMed] [Google Scholar]
- 10. Rodriguez JR, Razo AO, Targarona J et al Debridement and closed packing for sterile or infected necrotizing pancreatitis: insights into indications and outcomes in 167 patients. Ann. Surg. 2008; 247: 294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig. Liver Dis. 2014; 46: 446–51. [DOI] [PubMed] [Google Scholar]
- 12. Kamal A, Singh VK, Akshintala VS et al CT and MRI assessment of symptomatic organized pancreatic fluid collections and pancreatic duct disruption: an interreader variability study using the revised Atlanta classification 2012. Abdom. Imaging. 2015; 40: 1608–16. [DOI] [PubMed] [Google Scholar]
- 13. Rana SS, Chaudhary V, Sharma R, Sharma V, Chhabra P, Bhasin DK. Comparison of abdominal ultrasound, endoscopic ultrasound and magnetic resonance imaging in detection of necrotic debris in walled‐off pancreatic necrosis. Gastroenterol. Rep. 2016; 4: 50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Working Group IAPAPAAPG . IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013; 13 (Suppl. 2): e1–e15. [DOI] [PubMed] [Google Scholar]
- 15. de‐Madaria E, Abad‐González A, Aparicio JR et al The Spanish Pancreatic Club's recommendations for the diagnosis and treatment of chronic pancreatitis: part 2 (treatment). Pancreatology. 2013; 13: 18–28. [DOI] [PubMed] [Google Scholar]
- 16. Baron TH, Kozarek RA. Endotherapy for organized pancreatic necrosis: perspectives after 20 years. Clin. Gastroenterol. Hepatol. 2012; 10: 1202–7. [DOI] [PubMed] [Google Scholar]
- 17. Mier J, León EL, Castillo A, Robledo F, Blanco R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am. J. Surg. 1997; 173: 71–5. [DOI] [PubMed] [Google Scholar]
- 18. Besselink MG, Verwer TJ, Schoenmaeckers EJ et al Timing of surgical intervention in necrotizing pancreatitis. Arch. Surg. 2007; 142: 1194–201. [DOI] [PubMed] [Google Scholar]
- 19. van Brunschot S, Fockens P, Bakker OJ et al Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg. Endosc. 2014; 28: 1425–38. [DOI] [PubMed] [Google Scholar]
- 20. Varadarajulu S, Christein JD, Wilcox CM. Frequency of complications during EUS‐guided drainage of pancreatic fluid collections in 148 consecutive patients. J. Gastroenterol. Hepatol. 2011; 26: 1504–8. [DOI] [PubMed] [Google Scholar]
- 21. Jagielski M, Smoczyński M, Jabłońska A, Marek I, Dubowik M, Adrych K. The role of endoscopic ultrasonography in endoscopic debridement of walled‐off pancreatic necrosisa single center experience. Pancreatology. 2015; 15: 503–7. [DOI] [PubMed] [Google Scholar]
- 22. Abdelhafez M, Elnegouly M, Hasab Allah MS, Elshazli M, Mikhail HM, Yosry A. Transluminal retroperitoneal endoscopic necrosectomy with the use of hydrogen peroxide and without external irrigation: a novel approach for the treatment of walled‐off pancreatic necrosis. Surg. Endosc. 2013; 27: 3911–20. [DOI] [PubMed] [Google Scholar]
- 23. Siddiqui AA, Easler J, Strongin A et al Hydrogen peroxide‐assisted endoscopic necrosectomy for walled‐off pancreatic necrosis: a dual center pilot experience. Dig. Dis. Sci. 2014; 59: 687–90. [DOI] [PubMed] [Google Scholar]
- 24. Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS‐guided drainage of symptomatic walled‐off pancreatic necrosis. Gastrointest. Endosc. 2011; 74: 74–80. [DOI] [PubMed] [Google Scholar]
- 25. Bang JY, Wilcox CM, Trevino J et al Factors impacting treatment outcomes in the endoscopic management of walled‐off pancreatic necrosis. J. Gastroenterol. Hepatol. 2013; 28: 1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukai S, Itoi T, Sofuni A et al Novel single transluminal gateway transcystic multiple drainages after EUS‐guided drainage for complicated multilocular walled‐off necrosis (with videos). Gastrointest. Endosc. 2014; 79: 531–5. [DOI] [PubMed] [Google Scholar]
- 27. Rana SS, Bhasin DK, Rao C, Gupta R, Singh K. Non‐fluoroscopic endoscopic ultrasound‐guided transmural drainage of symptomatic non‐bulging walled‐off pancreatic necrosis. Dig. Endosc. 2013; 25: 47–52. [DOI] [PubMed] [Google Scholar]
- 28. Braden B, Koutsoumpas A, Silva MA, Soonawalla Z, Dietrich CF. Endoscopic ultrasound‐guided drainage of pancreatic walled‐off necrosis using self‐expanding metal stents without fluoroscopy. World J. Gastrointest. Endosc. 2018; 10: 93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoo J, Yan L, Hasan R, Somalya S, Nieto J, Siddiqui AA. Feasibility, safety, and outcomes of a single‐step endoscopic ultrasonography‐guided drainage of pancreatic fluid collections without fluoroscopy using a novel electrocautery‐enhanced lumen‐apposing, self‐expanding metal stent. Endosc. Ultrasound. 2017; 6: 131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharaiha RZ, Tyberg A, Khashab MA et al Endoscopic therapy with lumen‐apposing metal stents is safe and effective for patients with pancreatic walled‐off necrosis. Clin. Gastroenterol. Hepatol. 2016; 14: 1797–803. [DOI] [PubMed] [Google Scholar]
- 31. Siddiqui AA, Adler DG, Nieto J et al EUS‐guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen‐apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest. Endosc. 2016; 83: 699–707. [DOI] [PubMed] [Google Scholar]
- 32. Abu Dayyeh BK, Mukewar S, Majumder S et al Large‐caliber metal stents versus plastic stents for the management of pancreatic walled‐off necrosis. Gastrointest. Endosc. 2018; 87: 141–9. [DOI] [PubMed] [Google Scholar]
- 33. Hammad T, Khan MA, Alastal Y et al Efficacy and safety of lumen‐apposing metal stents in management of pancreatic fluid collections: are they better than plastic stents? a systematic review and meta‐analysis. Dig. Dis. Sci. 2018; 63: 289–301. [DOI] [PubMed] [Google Scholar]
- 34. Bazerbachi F, Sawas T, Vargas EJ et al Metal stents versus plastic stents for the management of pancreatic walled‐off necrosis: a systematic review and meta‐analysis. Gastrointest. Endosc. 2018; 87: 30–42.e15. [DOI] [PubMed] [Google Scholar]
- 35. Siddiqui AA, Kowalski TE, Loren DE et al Fully covered self‐expanding metal stents versus lumen‐apposing fully covered self‐expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled‐off necrosis: clinical outcomes and success. Gastrointest. Endosc. 2017; 85: 758–65. [DOI] [PubMed] [Google Scholar]
- 36. Chen YI, Barkun AN, Adam V et al Cost‐effectiveness analysis comparing lumen‐apposing metal stents with plastic stents in the management of pancreatic walled‐off necrosis. Gastrointest. Endosc. 2018; 88: 267–276.e1. [DOI] [PubMed] [Google Scholar]
- 37. Gardner TB, Chahal P, Papachristou GI et al A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled‐off pancreatic necrosis. Gastrointest. Endosc. 2009; 69: 1085–94. [DOI] [PubMed] [Google Scholar]
- 38. Thompson CC, Kumar N, Slattery J et al A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology. 2016; 16: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yasuda I, Nakashima M, Iwai T et al Japanese multicenter experience of endoscopic necrosectomy for infected walled‐off pancreatic necrosis: the JENIPaN study. Endoscopy. 2013; 45: 627–34. [DOI] [PubMed] [Google Scholar]
- 40. Jürgensen C, Neser F, Boese‐Landgraf J, Schuppan D, Stölzel U, Fritscher‐Ravens A. Endoscopic ultrasound‐guided endoscopic necrosectomy of the pancreas: is irrigation necessary? Surg. Endosc. 2012; 26: 1359–63. [DOI] [PubMed] [Google Scholar]
- 41. Gardner TB, Coelho‐Prabhu N, Gordon SR et al Direct endoscopic necrosectomy for the treatment of walled‐off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest. Endosc. 2011; 73: 718–26. [DOI] [PubMed] [Google Scholar]
- 42. Seifert H, Biermer M, Schmitt W et al Transluminal endoscopic necrosectomy after acute pancreatitis: a multicentre study with long‐term follow‐up (the GEPARD Study). Gut. 2009; 58: 1260–6. [DOI] [PubMed] [Google Scholar]
- 43. Coelho D, Ardengh JC, Eulálio JM, Manso JE, Mönkemüller K, Coelho JF. Management of infected and sterile pancreatic necrosis by programmed endoscopic necrosectomy. Dig. Dis. 2008; 26: 364–9. [DOI] [PubMed] [Google Scholar]
- 44. Escourrou J, Shehab H, Buscail L et al Peroral transgastric/transduodenal necrosectomy: success in the treatment of infected pancreatic necrosis. Ann. Surg. 2008; 248: 1074–80. [DOI] [PubMed] [Google Scholar]
- 45. Schrover IM, Weusten BL, Besselink MG, Bollen TL, van Ramshorst B, Timmer R. EUS‐guided endoscopic transgastric necrosectomy in patients with infected necrosis in acute pancreatitis. Pancreatology. 2008; 8: 271–6. [DOI] [PubMed] [Google Scholar]
- 46. Mathew A, Biswas A, Meitz KP. Endoscopic necrosectomy as primary treatment for infected peripancreatic fluid collections (with video). Gastrointest. Endosc. 2008; 68: 776–82. [DOI] [PubMed] [Google Scholar]
- 47. Papachristou GI, Takahashi N, Chahal P, Sarr MG, Baron TH. Peroral endoscopic drainage/debridement of walled‐off pancreatic necrosis. Ann. Surg. 2007; 245: 943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voermans RP, Veldkamp MC, Rauws EA, Bruno MJ, Fockens P. Endoscopic transmural debridement of symptomatic organized pancreatic necrosis (with videos). Gastrointest. Endosc. 2007; 66: 909–16. [DOI] [PubMed] [Google Scholar]
- 49. Charnley RM, Lochan R, Gray H, O'Sullivan CB, Scott J, Oppong KE. Endoscopic necrosectom as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006; 38: 925–8. [DOI] [PubMed] [Google Scholar]
- 50. Smoczyński M, Jagielski M, Jabłońska A, Adrych K. Transpapillary drainage of walled‐off pancreatic necrosis – a single center experience. Wideochir. Inne Tech. Maloinwazyjne. 2016; 10: 527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bang JY, Holt BA, Hawes RH et al Outcomes after implementing a tailored endoscopic step‐up approach to walled‐off necrosis in acute pancreatitis. Br. J. Surg. 2014; 101: 1729–38. [DOI] [PubMed] [Google Scholar]
- 52. Ross AS, Irani S, Gan SI et al Dual‐modality drainage of infected and symptomatic walled‐off pancreatic necrosis: long‐term clinical outcomes. Gastrointest. Endosc. 2014; 79: 929–35. [DOI] [PubMed] [Google Scholar]
- 53. Puli SR, Graumlich JF, Pamulaparthy SR, Kalva N. Endoscopic transmural necrosectomy for walled‐off pancreatic necrosis: a systematic review and meta‐analysis. Can. J. Gastroenterol. Hepatol. 2014; 28: 50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Keane MG, Sze SF, Cieplik N et al Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14‐year experience from a tertiary hepatobiliary centre. Surg. Endosc. 2016; 30: 3730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG; Dutch Pancreatitis Study GroupSystematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br. J. Surg. 2011; 98: 18–27. [DOI] [PubMed] [Google Scholar]
- 56. Shenvi S, Gupta R, Kang M et al Timing of surgical intervention in patients of infected necrotizing pancreatitis not responding to percutaneous catheter drainage. Pancreatology. 2016; 16: 778–87. [DOI] [PubMed] [Google Scholar]
- 57. Gluck M, Ross A, Irani S et al Endoscopic and percutaneous drainage of symptomatic walled‐off pancreatic necrosis reduces hospital stay and radiographic resources. Clin. Gastroenterol. Hepatol. 2010; 8: 1083–8. [DOI] [PubMed] [Google Scholar]
- 58. Yokoi Y, Kikuyama M, Kurokami T, Sato T. Early dual drainage combining transpapillary endotherapy and percutaneous catheter drainage in patients with pancreatic fistula associated with severe acute pancreatitis. Pancreatology. 2016; 16: 497–507. [DOI] [PubMed] [Google Scholar]
- 59. van Santvoort HC, Besselink MG, Bakker OJ et al Dutch Pancreatitis Study Group. A step‐up approach or open necrosectomy for necrotizing pancreatitis. N. Engl. J. Med. 2010; 362: 1491–502. [DOI] [PubMed] [Google Scholar]
- 60. Raraty MG, Halloran CM, Dodd S et al Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann. Surg. 2010; 251: 787–93. [DOI] [PubMed] [Google Scholar]
- 61. Gomatos IP, Halloran CM, Ghaneh P et al Outcomes from minimal access retroperitoneal and open pancreatic necrosectomy in 394 patients with necrotizing pancreatitis. Ann. Surg. 2016; 263: 992–1001. [DOI] [PubMed] [Google Scholar]
- 62. Babu BI, Siriwardena AK. Current status of minimally invasive necrosectomy for post‐inflammatory pancreatic necrosis. HPB. 2009; 11: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khreiss M, Zenati M, Clifford A et al Cyst gastrostomy and necrosectomy for the management of sterile walled‐off pancreatic necrosis: a comparison of minimally invasive surgical and endoscopic outcomes at a high‐volume pancreatic center. J. Gastrointest. Surg. 2015; 19: 1441–8. [DOI] [PubMed] [Google Scholar]
- 64. Arvanitakis M, Dumonceau JM, Albert J et al Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence‐based multidisciplinary guidelines. Endoscopy. 2018; 50: 524–46. [DOI] [PubMed] [Google Scholar]
- 65. Voermans RP, Besselink MG, Fockens P. Endoscopic management of walled‐off pancreatic necrosis. J. Hepatobiliary Pancreat. Sci. 2015; 22: 20–6. [DOI] [PubMed] [Google Scholar]


