Abstract
Background and Aim
To evaluate the role of multiplex polymerase chain reaction (PCR) for diagnosis of gastrointestinal tuberculosis (GITB).
Methods
This was a prospective observational study conducted from July 2015 to November 2016 at a tertiary care teaching institution in north India. Fifty individuals with clinically suspected GITB and older than 18 years of age were recruited. Patients underwent radiological investigations, esophagogastroduodenoscopy, or colonoscopy as clinically indicated. Multiple biopsies for tissue diagnosis and PCR were taken. All specimens were subjected to Ziehl Neelsen staining, histopathology, and multiplex PCR using specific primers for genes IS6110, MPB64, and Protein b. The performance of the assay was assessed using a composite reference standard for diagnosis of tuberculosis. It comprised a combination of clinical characteristics and microbiological methods, including smear, Bactenecin (BACTEC) culture, histopathology, and response to antitubercular therapy.
Results
A final diagnosis of tuberculosis was made in 32 cases (Duodenal‐4, Ileo‐colonic‐28). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of histopathology for the diagnosis of tuberculosis was 28.12, 100, 100, and 43.9%, respectively. The sensitivity, specificity, PPV and NPV of BACTEC Mycobacteria Growth Indicator Tube (MGIT) culture for the diagnosis of tuberculosis was 9.3, 100, 100, and 38.29%, respectively. The sensitivity, specificity, PPV, and NPV of multiplex PCR for the diagnosis of tuberculosis was 87.5, 100, 100, and 86.2%, respectively.
Conclusion
Multiplex PCR using specific primers for genes IS6110, MPB64, and Protein b had a higher sensitivity compared to conventional techniques for the diagnosis of GITB.
Keywords: colonoscopy, gastrointestinal tuberculosis, granuloma, multiplex PCR
Introduction
According to World Health Organization report 2017, there were 2.8 million new cases of tuberculosis in India, with an incidence rate of 217 per 100 000 population per year.1 It can involve the gastrointestinal tract from oral cavity to anal canal, pancreas, hepatobiliary system, or the peritoneum. Gastrointestinal tuberculosis (GITB) is a major health problem in developing countries like India. The diagnosis is often difficult to establish immediately and accurately. Histological and microbiological results are often inconclusive regarding GITB due to the paucibacillary nature of the disease. The reported sensitivity rates of endoscopic biopsy in the diagnosis of tuberculosis are in the range of 22–30, 7–10, and 0–20% for histopathology, AFB smear, and culture, respectively.2, 3, 4, 5, 6, 7, 8
Amplification techniques like various types of PCR have attracted considerable interest in the diagnosis of tuberculosis, particularly extrapulmonary tuberculosis (EPTB), with the hope of shortening the time required for detection. Insertion sequence, IS6110, is the most commonly used target due to its multiple‐copy presence in the genome of Mycobacterium tuberculosis. Most studies using IS6110 as a single target reported variable success.7, 9, 10 More importantly, studies have shown that IS6110 may be absent in around 10–15% isolates of M. tuberculosis in India.11 Studies that have evaluated a combination of target genes have reported higher sensitivity and specificity.12, 13 Few studies have reported a promising role of Protein b, MPB64, and IS6110 genes when used in combination to confer a higher sensitivity and specificity for EPTB.12 Thus, it is logical to hypothesize that a multiple PCR using multiple targets may provide a better sensitivity for the diagnosis of GITB, which is generally a paucibacillary disease. However, very few studies have evaluated the role of multiplex PCR in GITB, and there is still a need to find an ideal gene target.14, 15 In the present study, we evaluated the role of multiplex PCR using these three primers (Protein b, MPB64, and IS6110) for the diagnosis of GITB and compared it with conventional bacteriological techniques like smear, histology, and culture.
Methods
This was a prospective observational study conducted in the department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India from July 2015 to December 2016. Patients aged older than 18 years with suspected GITB were recruited. Patients with suspected or proven gastrointestinal malignancy, celiac disease, proven inflammatory bowel disease (Crohn's disease and ulcerative colitis), pregnant or lactating females, and subjects with congestive heart failure and/or chronic kidney disease were excluded. However, patients with Human Immunodeficiency Virus (HIV) infection and past history of tuberculosis were not excluded. Clinical history recording and detailed systemic examination were carried out for all the patients. Complete blood count, erythrocyte sedimentation rate, liver and renal function tests, Mantoux test, chest X‐ray, ultrasound abdomen, and contrast enhanced computed tomography (CECT) abdomen were performed in all patients. Patients with suspected upper or lower GITB underwent esophagogastroduodenoscopy (EGD) or colonoscopy, respectively, using OLYMPUS GIF H 190 scope (Olympus Medical Systems Corp, Shinjuku Monolith, Tokyo, Japan) and OLYMPUS CF H 190L scope (Olympus Medical Systems Corp, Shinjuku Monolith, Tokyo, Japan), respectively. The OLYMPUS EVIS EXERA II CV190 video processor with CLV190 light source system was used (Olympus Medical Systems Corp, Shinjuku Monolith, Tokyo, Japan). Informed consent was obtained from all patients before the procedure after explaining the risks and benefits involved. Patients were given intravenous midazolam or pentazocine for sedation/analgesia before the procedure, as considered appropriate by the endoscopist. Colonic preparation was conducted with polyethylene glycol electrolyte‐based solution using a split‐dose schedule.
The findings and lesions found during EGD and colonoscopy were recorded. Guided biopsies were obtained from lesions found and were sent for histopathological and microbiological examination, including PCR and mycobacterial culture. HE staining was used to stain biopsy specimens. Ziehl‐Neelsen staining was performed to identify acid‐fast bacilli in tissue. Mycobacterial culture was performed using the BACTEC MGIT system, and tubes were incubated for 6 weeks before being reported as sterile. Other special stains like auramine were used as considered appropriate by the histopathologist. Histopathological examination was performed by an expert gastrointestinal pathologist with more than 20 years of experience.
Tissue samples for PCR were obtained from involved areas of the gastrointestinal tract at the time of endoscopy or colonoscopy and were transferred to a microbiology laboratory as per standard protocol. DNA was extracted from tissue samples using the chloroform:isoamyl alcohol extraction method and was stored at −20°C. Multiplex PCR, using primers against IS6110, Protein b, and MPB64, were used for the detection of M. tuberculosis DNA, as has been described previously.12 For the processing of each tissue sample, the assay used a positive and a negative control. A sequence of nucleic acid from H37Rv acted as a positive control.
Composite reference standard: Past studies have used histopathology and culture as the gold standard for tuberculosis diagnosis. However, the yield of culture and histopathology is generally quite low in GITB, which is generally a paucibacillary disease. Response to empirical antitubercular therapy or a therapeutic trial has also been considered as an important diagnostic tool in studies.16 To deal with these problems and to compare recent upcoming molecular methods, recent studies have used a composite diagnostic standard for the evaluation of new diagnostic tests.16 We used similar criteria for the diagnosis of GITB. A confirmed diagnosis of GITB was made if any one of the following four criteria were met, that is, (i) histopathology of intestinal tissue showed caseating granulomas, granulomas with acid‐fast bacilli; (ii) granulomatous inflammation without caseation or acid‐fast bacilli (AFB) but good clinical response to antitubercular therapy (ATT); (iii) isolation of AFB bacilli in samples obtained from extraintestinal sites like sputum, resected tissues, lymph nodes, etc.; and (iv) clinical, radiological, or operative findings were considered suggestive of tuberculosis, and there was a good therapeutic response to treatment with antitubercular therapy.
Patients were advised on antitubercular therapy based on the laboratory evidence of tuberculosis or the clinical judgment of treating physician. Response to therapy was assessed during follow up in out‐patient department (OPD) clinically by weight gain and resolution of symptoms like fever, pain abdomen, and subjective well‐being during 4–6 months of follow up while taking treatment. The study was approved by the institute's ethics committee.
Statistical analysis
All data and information were acquired prospectively. The data were analyzed using SPSS version 15.0 (Chicago, IL, USA). During analysis of data, continuous variables were compared using the Student t test, and dichotomous variables were compared using the Chi square test. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) was calculated for different diagnostic tests, including smear, MGIT culture, histopathology, and multiplex PCR. P value of less than 0.05 was considered statistically significant.
Results
A total of 50 cases with mean age of 36.4 ± 14.6 years (29 males) were initially evaluated for study. A final diagnosis of GITB was made in 32 cases. Lower gastrointestinal tract involvement was seen in 27 patients, duodenal tuberculosis was diagnosed in four patients, and one patient had both duodenal and ileocecal involvement. The mean duration of symptoms at presentation was 11.7 ± 4.5 months. The symptoms at presentation were pain abdomen in 25 (78.1%) patients, weight loss in 20 (62.5%), loss of appetite in 13 (40.6%), vomiting in 11 (34.3%), fever in 12 (37.5%), diarrhea in seven (21.8%), partial intestinal obstruction in 7 (21.8%), lump abdomen in 3 (9.3%), and ascites in 2 (6.25%) patients.
Tables 1 and 2 describe the radiological (Fig. 1) and endoscopic findings, respectively, in patients of GITB. The diagnosis of tuberculosis was established using the composite reference standard as mentioned above. Smear for AFB, MGIT culture, and histopathology taken individually helped in the diagnosis of two, three, and nine patients, respectively. Considering histopathology alone for the diagnosis of GITB, presence of caseation and/or AFB, and/or granulomas combined with response to ATT, the sensitivity and specificity was 28.1 and 100%, respectively. Four patients with GITB had concomitant active pulmonary tuberculosis, and a confirmed diagnosis in these cases was established based on sputum smear positivity for AFB. Three patients had cervical and supraclavicular lymphadenopathy, and fine‐needle aspiration cytology from lymph nodes demonstrated granulomatous inflammation with AFB positivity. Response to antitubercular therapy or a therapeutic trial helped in establishing the diagnosis in 12 patients. Multiplex PCR was positive in 28 of these patients. In 12 patients diagnosed based on response to treatment, 8 were found to be PCR positive (Table 3).
Table 1.
Radiological and findings in patients of gastrointestinal tuberculosis
| CECT abdomen and chest findings | n (%) |
|---|---|
| Bowel involvement† | 25 (78.1) |
| Mesenteric lymphadenopathy | 30 (93.7) |
| Ascites | 6 (18.7) |
| Peritoneal thickening | 4 (12.5) |
| Evidence of pulmonary involvement‡ (present or past) | 9 (28.1) |
Mural thickening, luminal narrowing, or distorted ileocecal valve.
Pleural effusion, collapse lung, traction bronchiectasis, ground glass opacities, mediastinal lymph nodes.
CECT, Contrast Enhanced Computed Tomography.
Table 2.
Endoscopic findings in patients of gastrointestinal tuberculosis
| Endoscopic findings | n (%) |
|---|---|
| Ileum involvement (stricture or ulcerations) | 16 (50) |
| Ileocecal valve involvement† | 18 (56.2) |
| Colon involvement (stricture or ulcerations) | 17 (53.1) |
| Duodenal involvement (stricture or ulcerations) | 5 (15.6) |
| Mucosal changes‡ | 15 (47) |
Hypertrophied, narrowed, or gaping.
hyperemia, nodularity, friability.
Figure 1.
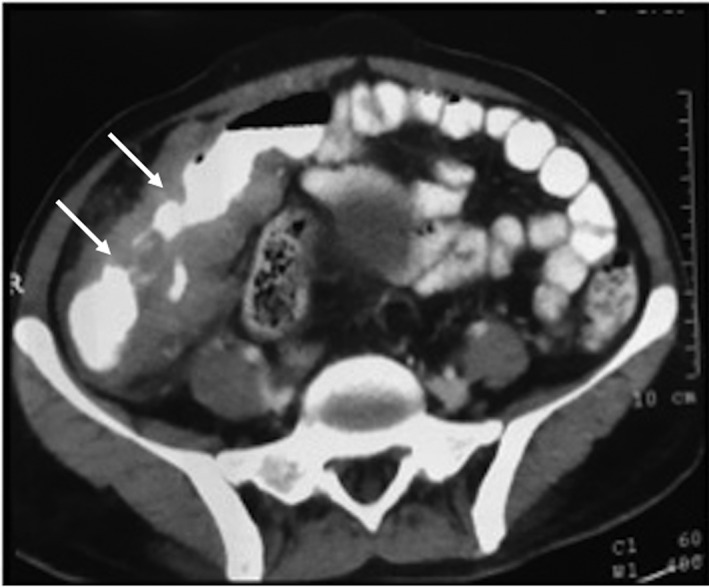
Computed Tomography (CT) film showing circumferential mural thickening and narrowing of terminal ileum and ileocecal valve, cecum, and ascending colon (white arrows).
Table 3.
Performance of conventional methods and multiplex PCR for diagnosis of tuberculosis
| Diagnostic test | Number of cases with a positive diagnosis (%) |
|---|---|
| Smear for AFB | 2 (6.2) |
| MGIT culture | 3 (9.3) |
| Histopathology | 9 (28.1) |
| Response to antitubercular therapy | 12 (37.5) |
| Tuberculosis elsewhere | |
| Lung | 4 (12.5) |
| Lymph node | 3 (9.3) |
| Multiplex PCR | |
| IS6110 | 22 (68.7) |
| MPB64 | 26 (81.2) |
| Protein b | 24 (75) |
AFB, Acid‐fast bacilli; MGIT, Mycobacteria Growth Indicator Tube; PCR, Polymerase chain reaction.
In the present study, 9 months of antitubercular therapy was given to all patients. Four patients underwent surgery for intestinal obstruction. The therapy was continued postoperatively in all four, and they are doing fine. Two patients developed ATT‐induced hepatitis. Both were initially put on modified ATT. All 32 patients completed therapy and were asymptomatic.
Among the 50 study subjects, 18 patients were not found to have GITB. In all these patients, biopsy imprint smear, MGIT culture, and multiplex PCR were negative for tuberculosis. Two patients turned out to have adenocarcinoma colon. Two patients were diagnosed with Crohn's disease. In eight patients, colonoscopy demonstrated few aphthous ulcerations in the terminal ileum. Histopathology from ulcer margin was suggestive of acute inflammation. They were treated with 1 week of antibiotics, and they responded. In six patients, colonoscopy was suggestive of edematous ileocecal valve with hyperemia and nodularity in the terminal ileum. Histopathology indicated the presence of mild to moderate lymphoplasmacytic infiltrate. These patients also improved with 7–10 days of antibiotics, with resolution of pain abdomen.
Histopathological and microbiological findings in GITB
In patients with GITB, biopsy imprint smear was positive for AFB in two patients, and BACTEC MGIT culture was positive in three cases. The sensitivity, specificity, PPV and NPV for smear for AFB were 6.2, 100, 100, and 37.5%, respectively. The sensitivity, specificity, PPV, and NPV for MGIT culture for AFB were 9.3, 100, 100, and 38.29%, respectively. Granulomas were found in nine patients, but caseation was seen in only one patient, and AFB were seen in biopsies of five patients (Fig. 2). The detailed histopathological findings in patients of GITB are described in Table 4.
Figure 2.
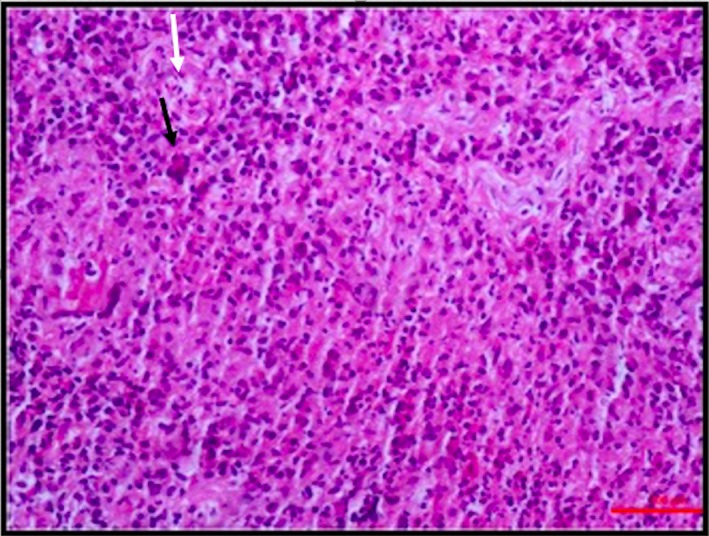
Photomicrograph of an endoscopic biopsy taken from cecum showing a loose epithelioid cell granuloma (white arrow) intermixed with many neutrophils, scattered lymphomononuclear cells, and the occasional Langhans type of giant cells (black arrow) (HE, ×200).
Table 4.
Detailed histopathological findings of patients with gastrointestinal tuberculosis
| Histopathological finding | n (%) |
|---|---|
| Ulceration | 15 (46.8) |
| Granuloma location and characteristics | |
| Mucosa | 5 (15.6) |
| Submucosa | 3 (9.3) |
| Both | 1 (3.1) |
| Lymphocyte cuffing | 1 (3.1) |
| Small (microgranuloma) (<200 micron) | 4 (12.5) |
| Medium size (200–500 micron) | 5 (15.6) |
| Large (>500 micron) | 0 |
| Few (<4/HPF) | 5 (15.6) |
| Many (≥4/HPF) | 4 (12.5) |
| Caseation | 1 (3.1) |
| Confluence | 1 (3.1) |
| Architectural distortion | 6 (18.7) |
| Chronic inflammation | 21 (65.6) |
| Focal activity | |
| Cryptitis | 10 (31.2) |
| Crypt abscess | 9 (28.1) |
| Focally enhanced colitis | 0 |
| Submucosal inflammation | 4 (12.5) |
| AFB | 5 (15.6) |
HPF, High power field; AFB, Acid‐fast bacilli.
Multiplex PCR results in patients of GITB
All patients underwent multiplex PCR using the method described above; 28 patients had a positive multiplex PCR. IS6110, Protein b, and MPB64 primer bands were present in 22, 24, and 26 patients, respectively (Fig. 3). Twenty patients demonstrated the presence of all three bands. Use of MPB64 helped to diagnose four additional patients who had absence of IS6110 primer and would have been missed had only single IS6110 primer been used. Only one patient was Protein b positive, with the absence of the other two primers.
Figure 3.
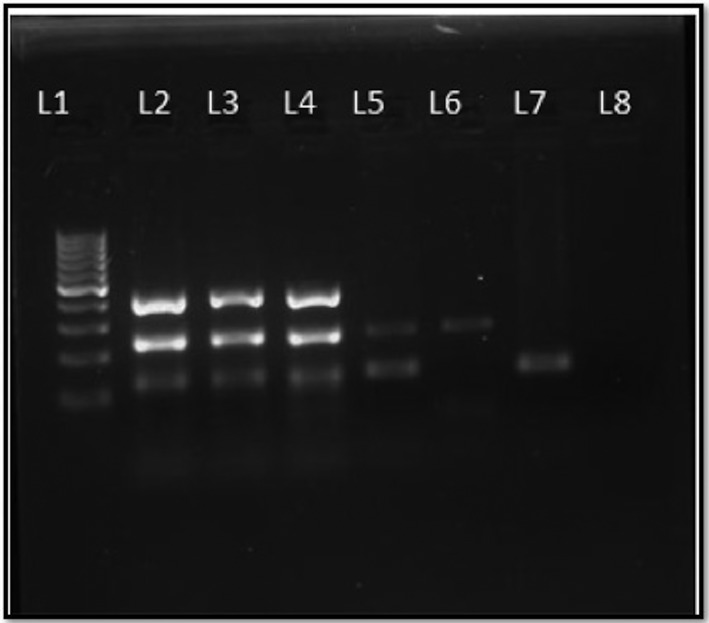
Gel picture of MPCR for diagnosis of MTB. L1—100 bp molecular marker, L2—positive control H37RV DNA showing three bands, Protein b—419 base pairs (top), MPB64—240 base pairs (middle), IS6110—123 base pairs (lower), L3, 4, 5, 6, 7—DNA extracted from patient sample positive for MTB, L8—negative control. MPCR, Multiplex Polymerase chain reaction; MTB, Mycobacterium tuberculosis; DNA, Deoxyribonucleic acid.
The sensitivity of each primer, if observed individually, is 68.75, 75, and 81.25%, respectively, the specificity being 100% for each. However, the sensitivity rose to 87.5% when a combination of three primers was considered (Table 5). Figure 3 describes a gel picture of multiplex PCR for diagnosis of tuberculosis.
Table 5.
Diagnostic performance of conventional methods and individual primers for gastrointestinal tuberculosis
| Primer | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Diagnostic accuracy |
|---|---|---|---|---|---|
| Biopsy imprint smear | 6.25 | 100 | 100 | 37.5 | 40 |
| MGIT culture | 9.3 | 100 | 100 | 38.29 | 42 |
| Histopathology | 28.12 | 100 | 100 | 43.9 | 54 |
| IS6110 | 68.75 | 100 | 100 | 90.9 | 80 |
| MPB64 | 81.25 | 100 | 100 | 94.33 | 88 |
| Protein b | 75 | 100 | 100 | 92.9 | 84 |
| Multiplex PCR (Any one) | 87.5 | 100 | 100 | 86.2 | 100 |
MGIT, Mycobacteria Growth Indicator Tube; PCR, Polymerase chain reaction.
To summarize, in the present study, a confirmed diagnosis of GITB was made in 32 of 50 suspected cases of GITB. All patients were started on ATT for 9 months. All patients completed 9 months of treatment and improved. Four patients underwent surgery for intestinal obstruction. They continued ATT in the postoperative period. Two patients developed ATT‐induced hepatitis. Both of them improved on modified ATT.
Discussion
In the present study, we evaluated different diagnostic methods for making a positive diagnosis of GITB in 32 patients, which included 28 patients of ileocolonic and 5 patients of duodenal tuberculosis (one having duodenal as well as ileocolonic lesions). The diagnostic sensitivity of BACTEC MGIT culture, histopathology, and multiplex PCR was 9.3, 28, and 87.5%, respectively, specificity for each being 100%. Kim et al. studied the clinicopathological findings of 42 patients with GITB. The sensitivity of smear for AFB, tissue AFB staining, and tissue culture was 32, 32, and 36%, respectively.5 Similar low sensitivities have been reported in various studies.3, 4, 17, 18
PCR is the most promising new approach for the diagnosis of GITB. PCR assay on tissue from patients of GITB obtained using surgery or colonoscopy has been found to be highly accurate for the diagnosis of GITB.19 Various studies using PCR report a variable sensitivity from as low as 30–40% (using single primer) to as high as 80% (multiple primers) for GITB.9, 20, 21 Studies report no variation in the accuracy of assay irrespective of the presence or absence of granuloma or caseous necrosis in tissue biopsy.22, 23, 24 Other benefits of PCR include the rapidity in obtaining the results with a quick and accurate diagnosis. Kulkarni et al. used PCR for the identification of M. tuberculosis with a single primer for 340 base pair nucleotide sequence. Considering histopathology the gold standard, they reported the sensitivity, specificity, PPV, and NPV of PCR to be 77, 68, 80, and 73%, respectively.9 In the present study, we used a composite reference standard (described previously in methods) rather than histopathology alone to assess the diagnostic accuracy of multiplex PCR and found sensitivity, specificity, PPV, and NPV of 87.5, 100, 100, and 86.2%, respectively. As the sensitivity of multiple PCR was expected to be higher than histopathological examination, we used a composite reference standard that has been previously described in literature.
Gan et al. conducted a study to differentiate between Crohn's disease and tuberculosis using PCR. The sensitivity, specificity, PPV, NPV, and accuracy PCR for diagnosis of GITB were 64.1, 100, 100, 68.2, and 79.7%, respectively.10 Sekine et al. found the sensitivity and specificity of PCR to be 25 and 100% for diagnosis of GITB.7 The reason for a higher sensitivity in our study compared to the above two studies is the use of three primers in our study and only a single primer in the abovementioned two studies.
Multiplex PCR technique is a molecular technology that uses multiple primers for the identification of an organism. Recent studies have reported its use in the diagnosis of various forms of pulmonary and EPTB. In a study to evaluate multiplex PCR using Protein b, MPB64, and IS6110 primers for diagnosis of tuberculous meningitis, sensitivity of microscopy, culture, and multiplex PCR was 1.81, 16.73, and 86.63% and specificity was 100, 100, and 100%, respectively.12 A study by Wattal et al., conducted to assess multiplex PCR using IS6110 and MPB64 for EPTB found that IS6110 had a better sensitivity (90.3%) compared to MPB64 (64.5%).25 Negi et al. and Singh et al. reported better yield when IS6110 was used for tuberculous lymphadenitis.24, 26
Recent studies, including those mentioned above, report a high accuracy of multiplex PCR for different forms of EPTB like lymph node, skin, osteoarticular, and meningeal tuberculosis.12, 13, 25, 26 However, after a review of PubMed, we could trace only two studies that evaluated the role of multiplex PCR in abdominal tuberculosis, and more specifically, GITB.14, 15 Jin et al. performed PCR to differentiate GITB from Crohn's disease using primers against IS6110 and MPB64. The sensitivity and specificity of the PCR test was 88.9 and 100%, respectively.14 A study by Hallur et al. studied multiplex PCR using primers 16SrRNA, IS6110, and devR for the diagnosis of GITB. In patients with suspected GITB, 32 (80%), 31 (77.5%), and 24 (60%) cases were positive by devR, IS6110, and 16SrRNA PCR, respectively, while 35 (87.5%) cases were positive by multiplex PCR assay.15 The results of our study are consistent with the above two studies and highlight the highly sensitive and promising role of multiplex PCR in rapid and accurate diagnosis of GITB. Most of studies have used IS6110 as the single target; however, data of efficacy using multiple targets for GITB are scarce. However, in the present study, primer MPB64 and Protein b yielded better results than IS6110. Four patients were IS6110 negative and MPB64 positive. The absence of IS6110 copies in some of Indian M. tuberculosis strains, as observed by some earlier studies, was also evident in the present study.27, 28
The strengths of our study include a prospective analysis of cases of suspected GITB using strict inclusion and exclusion criteria using three primers, IS6110, MPB64, and Protein b, versus single or two primers used in previous studies. The present study has also made an attempt to use composite reference standard for the comparative evaluation of multiplex PCR instead of using only culture or histopathology as the gold standard. However, the study has a few limitations. First, the sample size might not represent the population in general. Second, there is the higher cost of multiplex PCR, which is in its development phase, although it is expected to decrease in future. In addition, GITB is a paucibacillary disease; hence, inhomogeneous distribution of bacilli in the tissue obtained using endoscopic biopsy may explain the false negative multiplex PCR results in a subset of patients. A previous study had shown increased yield of endoscopic biopsy if endoscopic mucosal resection (EMR) was used for tissue acquisition.8 However, EMR was not performed in any of the patients in present study, which might be a potential limitation. Another possible limitation could be the use of a single, rather than two, independent histopathologists.
In summary, the multiplex PCR assay targeting IS6110, MPB64, and Protein b has the potential to improve the diagnosis of GITB. While multiplex PCR detected M. tuberculosis in less than a day, the automated culture can take up to 42 days for results. Therefore, we suggest that multiplex PCR should be considered an important tool for the rapid and accurate diagnosis of GITB in future.
Declaration of conflict of interest: No conflict of interests to declare.
Financial support: None.
References
- 1. World Health Organization Global Tuberculosis Report . Bending the Curve ‐ Ending TB: Annual Report, New Delhi, India, 2017. [Google Scholar]
- 2. Patel N, Amarapurkar D, Agal S et al Gastrointestinal luminal tuberculosis: establishing the diagnosis. J. Gastroenterol. Hepatol. 2004; 19: 1240–6. [DOI] [PubMed] [Google Scholar]
- 3. Makharia GK, Srivastava S, Das P et al Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am. J. Gastroenterol. 2010; 105: 642–51. [DOI] [PubMed] [Google Scholar]
- 4. Misra SP, Misra V, Dwivedi M, Gupta SC. Colonic tuberculosis: clinical features, endoscopic appearance and management. J. Gastroenterol. Hepatol. 1999; 14: 723–9. [DOI] [PubMed] [Google Scholar]
- 5. Kim KM, Lee A, Choi KY, Lee KY, Kwak JJ. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am. J. Gastroenterol. 1998; 93: 606–9. [DOI] [PubMed] [Google Scholar]
- 6. Pulimood AB, Peter S, Ramakrishna B et al Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn's disease. J. Gastroenterol. Hepatol. 2005; 20: 688–96. [DOI] [PubMed] [Google Scholar]
- 7. Sekine K, Nagata N, Shindo T et al Combined identifying granuloma and biopsy culture is useful for diagnosing intestinal tuberculosis. Int. J. Colorectal Dis. 2015; 30: 939–45. [DOI] [PubMed] [Google Scholar]
- 8. Puri AS, Sachdeva S, Mittal VV et al Endoscopic diagnosis, management and outcome of gastroduodenal tuberculosis. Indian J. Gastroenterol. 2012; 31: 125–9. [DOI] [PubMed] [Google Scholar]
- 9. Kulkarni S, Vyas S, Supe A, Kadival G. Use of polymerase chain reaction in the diagnosis of abdominal tuberculosis. J. Gastroenterol. Hepatol. 2006; 21: 819–23. [DOI] [PubMed] [Google Scholar]
- 10. Gan HT, Chen YQ, Ouyang Q, Bu H, Yang XY. Differentiation between intestinal tuberculosis and Crohn's disease in endoscopic biopsy specimens by polymerase chain reaction. Am. J. Gastroenterol. 2002; 97: 1446–51. [DOI] [PubMed] [Google Scholar]
- 11. Chauhan DS, Sharma VD, Parashar D et al Molecular typing of Mycobacterium tuberculosis isolates from different parts of India based on IS6110 element polymorphism using RFLP analysis. Indian J. Med. Res. 2007; 125: 577–81. [PubMed] [Google Scholar]
- 12. Kusum S, Aman S, Pallab R et al Multiplex PCR for rapid diagnosis of tuberculous meningitis. J. Neurol. 2011; 258: 1781–7. [DOI] [PubMed] [Google Scholar]
- 13. Sharma K, Sharma A, Sharma SK, Sen RK, Dhillon MS, Sharma M. Does multiplex polymerase chain reaction increase the diagnostic percentage in osteoarticular tuberculosis? A prospective evaluation of 80 cases. Int. Orthop. 2012; 36: 255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin XJ, Kim JM, Kim HK et al Histopathology and TB‐PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn's disease. World J. Gastroenterol. 2010; 16: 2496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hallur V, Sharma M, Sethi S et al Development and evaluation of multiplex PCR in rapid diagnosis of abdominal tuberculosis. Diagn. Microbiol. Infect. Dis. 2013; 76: 51–5. [DOI] [PubMed] [Google Scholar]
- 16. Kumar S, Bopanna S, Kedia S et al Evaluation of Xpert MTB/RIF assay performance in the diagnosis of abdominal tuberculosis. Intest. Res. 2017; 15: 187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh V, Kumar P, Kamal J, Prakash V, Vaiphei K, Singh K. Clinicocolonoscopic profile of colonic tuberculosis. Am. J. Gastroenterol. 1996; 91: 565–8. [PubMed] [Google Scholar]
- 18. Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005; 37: 351–6. [DOI] [PubMed] [Google Scholar]
- 19. Gan H, Ouyang Q, Bu H et al Value of polymerase chain reaction assay in diagnosis of intestinal tuberculosis and differentiation from Crohn's disease. Chin Med J (Engl). 1995; 108: 215–20. [PubMed] [Google Scholar]
- 20. Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J. Gastroenterol. 2008; 14: 741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amarapurkar DN, Patel ND, Amarapurkar AD, Agal S, Baigal R, Gupte P. Tissue polymerase chain reaction in diagnosis of intestinal tuberculosis and Crohn's disease. J. Assoc. Physicians India. 2004; 52: 863–7. [PubMed] [Google Scholar]
- 22. Sharma K, Gupta N, Sharma A et al Multiplex polymerase chain reaction using insertion sequence 6110 (IS6110) and mycobacterial protein fraction from BCG of Rm 0.64 in electrophoresis target genes for diagnosis of tuberculous lymphadenitis. Indian J. Med. Microbiol. 2013; 31: 24–8. [DOI] [PubMed] [Google Scholar]
- 23. Sharma K, Sinha SK, Sharma A et al Multiplex PCR for rapid diagnosis of gastrointestinal tuberculosis. J. Glob. Infect. 2013; 5: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh HB, Singh P, Jadaun GP et al Simultaneous use of two PCR systems targeting IS6110 and MPB64 for confirmation of diagnosis of tuberculous lymphadenitis. J. Commun. Dis. 2006; 38: 274–9. [PubMed] [Google Scholar]
- 25. Raveendran R, Wattal C. Utility of multiplex real‐time PCR in the diagnosis of extrapulmonary tuberculosis. Braz. J. Infect. Dis. 2016; 20: 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Negi SS, Anand R, Pasha ST et al Diagnostic potential of IS6110, 38kDa, 65kDa and 85B sequence‐based polymerase chain reaction in the diagnosis of Mycobacterium tuberculosis in clinical samples. Indian J. Med. Microbiol. 2007; 25: 43–9. [DOI] [PubMed] [Google Scholar]
- 27. van Soolingen D, de Haas PE, Hermans PW, Groenen PM, van Embden JD. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J. Clin. Microbiol. 1993; 31: 1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das S, Paramasivan CN, Lowrie DB, Prabhakar R, Narayanan PR. IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, south India. Tuber. Lung Dis. 1995; 76: 550–4. [DOI] [PubMed] [Google Scholar]


