Abstract
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer, which has poor outcome. The present study aimed to investigate the key genes implicated in the progression and prognosis of HCC. The RNA-sequencing data of HCC was extracted from The Cancer Genome Atlas (TCGA) database. Using the R package (DESeq), the differentially expressed genes (DEGs) were analyzed. Based on the Cluepedia plug-in in Cytoscape software, enrichment analysis for the protein-coding genes amongst the DEGs was conducted. Subsequently, protein–protein interaction (PPI) network was built by Cytoscape software. Using survival package, the genes that could distinguish the survival differences of the HCC samples were explored. Moreover, quantitative real-time reverse transcription-PCR (qRT-PCR) experiments were used to detect the expression of key genes. There were 2193 DEGs in HCC samples. For the protein-coding genes amongst the DEGs, multiple functional terms and pathways were enriched. In the PPI network, cyclin-dependent kinase 1 (CDK1), polo-like kinase 1 (PLK1), Fos proto-oncogene, AP-1 transcription factor subunit (FOS), serum amyloid A1 (SAA1), and lysophosphatidic acid receptor 3 (LPAR3) were hub nodes. CDK1 interacting with PLK1 and FOS, and LPAR3 interacting with FOS and SAA1 were found in the PPI network. Amongst the 40 network modules, 4 modules were with scores not less than 10. Survival analysis showed that anterior gradient 2 (AGR2) and RLN3 could differentiate the high- and low-risk groups, which were confirmed by qRT-PCR. CDK1, PLK1, FOS, SAA1, and LPAR3 might be key genes affecting the progression of HCC. Besides, AGR2 and RLN3 might be implicated in the prognosis of HCC.
Keywords: differentially expressed genes, hepatocellular carcinoma, protein-coding genes, protein-protein interaction WORK, survival analysis
Background
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and causes the most deaths in cirrhosis patients [1]. HCC usually occurs in people with chronic liver inflammation, which is closely related to virus infection or alcohol and aflatoxin exposure [2]. Epidemiological data show that the main risk factors for HCC are as follows: (i) Hepatitis B or hepatitis C virus infection. (ii) Aflatoxin. (iii) Drinking wastewater or pond water containing a large amount of organochlorine compounds and algae toxins. (iv) Other factors such as family aggregation, selenium deficiency, alcoholic and nutritional cirrhosis [2,3]. The therapeutic schedules of HCC depend on disease stage, operative tolerance, and possibility of liver transplant [3,4]. The outcome of HCC patients is usually poor, because 80–90% HCCs cannot be resected completely and leads to death in 3–6 months [5,6]. HCC is amongst the most common tumors and results in more than 670000 deaths per year globally [7]. Therefore, the mechanisms of HCC needed to be explored to improve its therapies.
A lot of studies indicated that Forkhead box C1 (FoxC1), αB-crystallin, Zinc finger and BTB domain containing 20 (ZBTB20), Dysregulated B-cell translocation gene 1 (BTG1), Homeobox A13b (HOXA13), DEK proto-oncogene (DEK), Ubiquitin-specific protease 7 (USP7), and Acyl-CoA Ligase 4 (ACSL4) played important roles in the occurrence and progression of HCC, and they may serve as novel prognostic factors and therapeutic targets for HCC [8–18]. FoxC1 may facilitate HCC metastasis via inducing epithelial–mesenchymal transition (EMT) and up-regulating neural precursor cell expressed, developmentally down-regulated 9 (NEDD9) [8,9]. The expression of αB-crystallin has correlations with the invasion and metastasis of HCC cells [10]. ZBTB20 expression was up-regulated in HCC and related to adverse prognosis in HCC patients [11,12]. BTG1 may be involved in hepatocarcinogenesis and may be taken as a biomarker for the carcinogenesis and progression of HCC [13]. HOXA13 may affect angiogenesis, progression, and outcome of HCC, and serum HOXA13 may be utilized for early diagnosis and prognosis prediction of HCC patients [14]. DEK is reported to be implicated in hepatocyte differentiation and acts as a candidate marker for the prognosis and staging of HCC [15,16]. USP7 and ACSL4 are up-regulated in HCC samples, which are related to a poor survival [17,18]. Despite these findings, the key genes having influences on the progression and prognosis of HCC patients have not been comprehensively revealed.
Various in silico tools of bioinformatics are widely applied for assessing gene expression levels and screening outstanding genes from RNA-sequencing data and next-generation sequencing data and their possible implication in growth of different types of cancer [19]. In the present study, differential expression analysis, enrichment analysis, network analysis, and survival analysis successively were conducted to find out the key genes that affected the prognosis of HCC. In addition, quantitative real-time reverse transcription-PCR (qRT-PCR) experiments were conducted to confirm the expression of key genes. The present study might help to predict the outcome of HCC patients and develop novel therapies.
Materials and methods
Microarray data
The RNA-sequencing data of HCC was obtained from The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) database, which was based on the platform of llumina HiSeq 2000 RNA Sequencing. The TCGA dataset contained 419 samples, including 369 HCC samples and 50 normal control samples [20]. Meanwhile, the corresponding clinical data of the HCC samples were downloaded from the TCGA database.
Data preprocessing and differential expression analysis
The genes with counts per million (cpm) < 1 in more than 10% samples were taken as low expressed genes and removed. According to the annotation files in GENCODE database (version 22) [21], ensemble gene IDs (ENSGs) were mapped to gene symbols and gene types (coding genes or non-coding genes). Using the R package DESeq (http://www.bioconductor.org/packages/release/bioc/html/DESeq.html) [22], the differentially expressed genes (DEGs) between HCC samples and normal samples were analyzed. The thresholds for differential expression analysis were set as |log fold-change (FC)| ≥ 2 and false discovery rate (FDR) < 0.01.
Functional and pathway enrichment analyses
Gene Ontology (GO) database introduces the biological process (BP), cellular component (CC), and molecular function (MF) for proteins [23]. Kyoto Encyclopedia of Genes and Genomes (KEGG) database included the functions of genes and can be utilized for the functional prediction of gene lists [24]. Using Cluepedia plug-in (http://apps.cytoscape.org/apps/cluepedia) [25] in Cytoscape software, the protein-coding genes amongst the DEGs were performed with GO and KEGG enrichment analyses. The terms with FDR < 0.05 were significant results. Besides, the significant KEGG pathways were presented by the R package pathview (http://r-forge.r-project.org/projects/pathview/) [26].
Protein–protein interaction network analysis
Protein–protein interaction (PPI) pairs were predicted for the protein-coding genes amongst the DEGs using the STRING (http://www.string-db.org/) [27] database, with the combined score set as 700. Afterward, the PPI network was visualized using Cytoscape software (http://www.cytoscape.org) [28]. Moreover, the MCODE plug-in (http://apps.cytoscape.org/apps/MCODE) [29] in Cytoscape software was used for identifying the significant modules in PPI network.
Patient samples
Clinical samples were collected from 26 HCC patients who underwent surgery at the Central South University Xiangya School of Medicine Affiliated Haikou Hospital from 2013 to 2017. Meanwhile the adjacent non-tumor liver tissues from the HCC patients were obtained as the normal controls. None of the patients accepted radiation therapy and/or chemotherapy before surgery and all of them have signed the informed consent. Ethical approval for the study was provided by the ethics committee of Central South University Xiangya School of Medicine Affiliated Haikou Hospital, and the research has been carried out in accordance with the World Medical Association Declaration of Helsinki.
qRT-PCR analysis
The total RNAs of the samples were extracted using TRIzol (Thermo Fisher Scientific, Waltham, MA, U.S.A.), detected by UV absorbance (A260/280) and agarose gel. qRT-PCR was carried out in the ABI Prism 7500 PCR system (Applied Biosystems, Foster City, CA, U.S.A.) following the standard instructions. GADPH acted as the internal criterion for the targetted genes. 2−ΔΔct method was taken to calculate the relative expressions of the targetted genes. The primers’ sequences for GADPH were (F) 5′-GCACCGTCAAGGCTGAGAAC-3′ and (R) 5′-GCCTTCTCCATGGTGGTGAA-3′. PCR was performed in three parallel holes.
Survival analysis
Based on univariate COX regression analysis [30], the correlations between the DEG and overall survival (OS) were analyzed. Combined with the R package survival (https://CRAN.R-project.org/view=Survival) [31], the HCC samples were divided into high- and low-risk groups and then the OS differences between the two groups were analyzed using Kaplan–Meier (KM) method [32]. Student’s t tests and one-way ANOVAs were used in either two or multiple groups for statistical significance, with P<0.05 considered significant.
Results
Differential expression analysis
Relative to normal samples, a total of 2193 DEGs (including 1964 up-regulated genes and 229 down-regulated genes) in HCC samples were screened. Amongst these DEGs, there were 1800 protein-coding genes and 232 long non-coding RNAs (lncRNAs).
Functional and pathway enrichment analyses
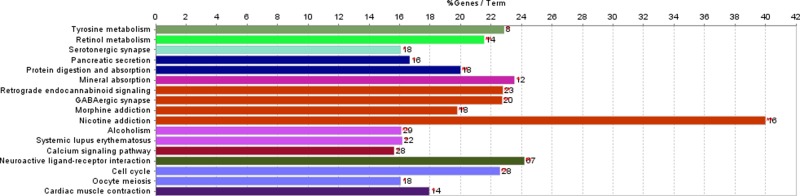
Enrichment analysis showed that multiple GO terms and 17 KEGG pathways were enriched for the protein-coding genes amongst the DEGs. The obtained GO terms mainly were multicellular organismal development (GO_BP, FDR = 1.09E-20), neurone part (GO_CC, FDR = 1.19E-08), and ion channel activity (GO_MF, FDR = 1.25E-10) (Table 1). Besides, the enriched pathways mainly included neuroactive ligand–receptor interaction (FDR = 7.13E-14), nicotine addiction (FDR = 2.62E-06), and cell cycle (FDR = 4.10E-05) (Figure 1).
Table 1. The top five GO terms enriched for the protein-coding genes amongst the DEGs.
| Category | Description | Count | FDR |
|---|---|---|---|
| GO_BP | Multicellular organismal development | 645 | 1.09E-20 |
| GO_BP | System development | 581 | 1.29E-19 |
| GO_BP | Cell–cell signaling | 217 | 2.10E-16 |
| GO_BP | Organ development | 431 | 2.27E-16 |
| GO_BP | Synaptic transmission | 145 | 5.63E-15 |
| GO_CC | Neurone part | 189 | 1.19E-08 |
| GO_CC | Ion channel complex | 62 | 1.87E-08 |
| GO_CC | Extracellular space | 203 | 2.22E-08 |
| GO_CC | Integral component of plasma membrane | 228 | 7.65E-08 |
| GO_CC | Transmembrane transporter complex | 65 | 9.23E-08 |
| GO_MF | Ion channel activity | 83 | 1.25E-10 |
| GO_MF | Sequence-specific DNA binding | 167 | 1.38E-10 |
| GO_MF | Gated channel activity | 71 | 1.43E-10 |
| GO_MF | Substrate-specific channel activity | 86 | 1.45E-10 |
| GO_MF | Channel activity | 89 | 1.45E-10 |
Figure 1. The pathways enriched for the protein-coding genes amongst the DEGs.
PPI network analysis
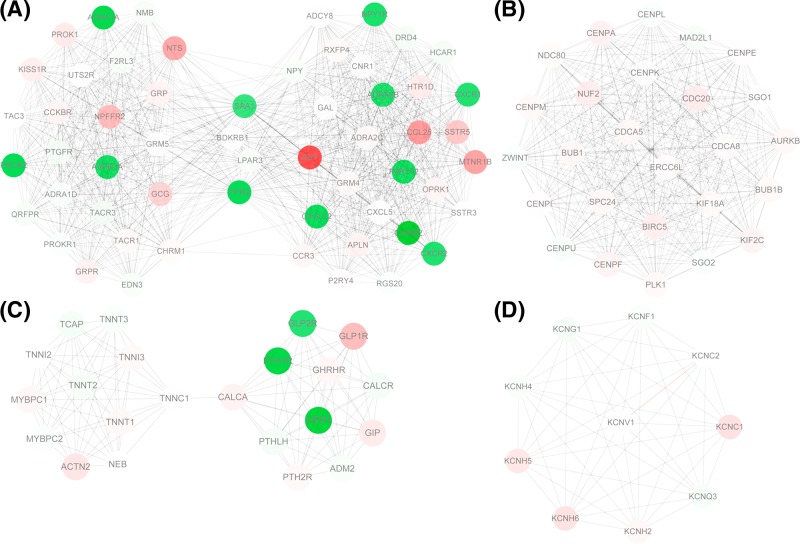
The PPI network for the protein-coding genes amongst the DEGs was constructed, which had 926 nodes and 3748 edges. The degrees of network nodes obeyed exponential distribution (r-squared = 0.849), therefore, the PPI network was a scale-free network and some network nodes had higher degrees. According to node degrees, cyclin-dependent kinase 1 (CDK1, degree = 76), polo-like kinase 1 (PLK1, degree = 72), Fos proto-oncogene, AP-1 transcription factor subunit (FOS, degree = 63), serum amyloid A1 (SAA1, degree = 59), and lysophosphatidic acid receptor 3 (LPAR3, degree = 59) were the top five nodes. Especially, CDK1 had interactions with both PLK1 and FOS, and LPAR3 could interact with FOS and SAA1 in the PPI network. Besides, FOS also had interaction with relaxin 3 (RLN3) in the network. Furthermore, a total of 40 network modules were identified. The modules with scores no less than 10 were presented in Figure 2, including module 1 (score = 32.036, involving 56 nodes and 881 edges), module 2 (score = 26, involving 26 nodes and 325 edges), module 3 (score = 10.571, involving 22 nodes and 111 edges), and module 4 (score = 10, involving 10 nodes and 45 edges) (Table 2).
Figure 2. PPI network analysis.
The module 1 (A), module 2 (B), module 3 (C), and module 4 (D) identified from the PPI network. Red and green circles represent up-regulated and down-regulated genes, respectively.
Table 2. The 12 genes that could differentiate the survival differences of HCC samples.
| Gene | P-value |
|---|---|
| AGR2 | 0.024 |
| CRISP2 | 0.04 |
| IL31RA | 0.0044 |
| KCNC2 | 0.026 |
| LINC01477 | 0.013 |
| LOC105372556 | 0.023 |
| PLVAP | 0.011 |
| RLN3 | 0.043 |
| SOX14 | 0.011 |
| TEDDM1 | 0.023 |
| VWA5B2 | 0.048 |
| ZNF280A | 0.016 |
Abbreviations: AGR2, anterior gradient 2; CRISP2, cysteine-rich secretory protein 2; IL31RA, interleukin 31 receptor A; KCNC2, potassium voltage-gated channel subfamily C member 2; LINC01477, long intergenic non-protein coding RNA 1477; PLVAP, plasmalemma vesicle-associated protein; SOX14, SRY-box 14; TEDDM1, transmembrane epididymal protein 1; VWA5B2, von Willebrand factor A domain containing 5B2.
Survival analysis
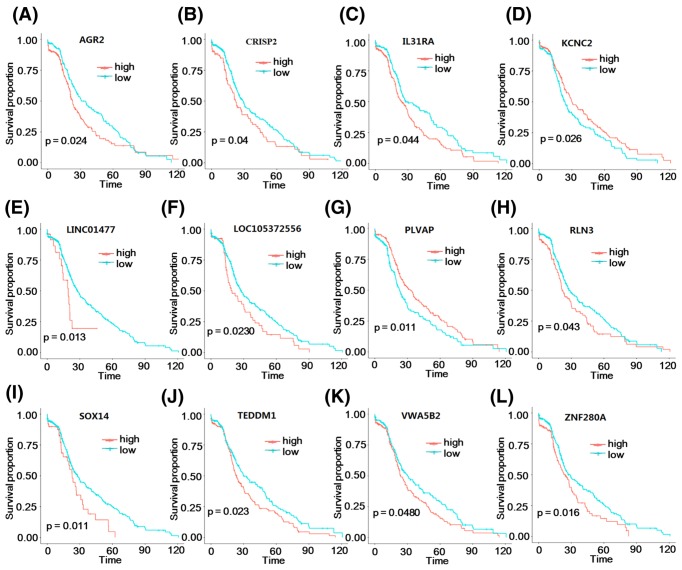
Based on univariate COX regression analysis, a total of 116 DEGs were found to be correlated with the OS of HCC samples (P-value <0.05). According to the median value of gene expression, the HCC samples were divided into high- and low-risk groups. Subsequently, the OS differences between the two groups were analyzed. KM survival curves showed that 12 genes (including anterior gradient 2, AGR2; cysteine-rich secretory protein 2, CRISP2; interleukin 31 receptor A, IL31RA; long intergenic non-protein coding RNA 1477, LINC01477; RLN3; SRY-box 14, SOX14; transmembrane epididymal protein 1, TEDDM1; von Willebrand factor A domain containing 5B2, VWA5B2; zinc finger protein 280A, ZNF280A; potassium voltage-gated channel subfamily C member 2, KCNC2; LOC105372556; and plasmalemma vesicle-associated protein, PLVAP) could divide the HCC samples into two groups that had OS differences (P-value <0.05) (Figure 3).
Figure 3. Survival analysis.
The KM survival curves for AGR2 (A), CRISP2 (B), IL31RA (C), KCNC2 (D), LINC01477 (E), LOC105372556 (F), PLVAP (G), RLN3 (H), SOX14 (I), TEDDM1 (J), VWA5B2 (K), and ZNF280A (L). Red and blue separately represent high- and low-risk groups.
Expressions of genes differentiated the survival differences in HCC in clinical samples
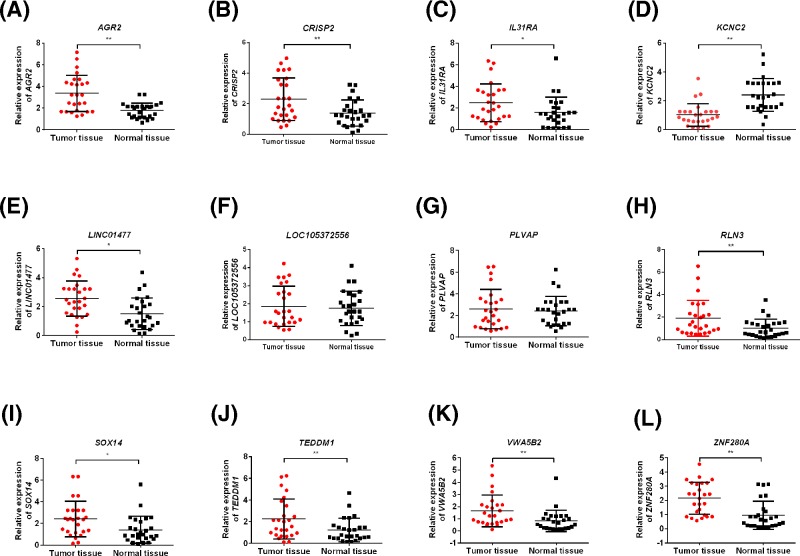
We, then, examined the expressions of genes differentiating the survival differences of HCC in HCC tissues and adjacent normal tissues by qRT-PCR. As shown in Figure 4, the expressions of AGR2, CRISP2, IL31RA, LINC01477, RLN3, SOX14, TEDDM1, VWA5B2, and ZNF280A were significantly increased in HCC tissues compared with the normal tissues (P-value <0.05) (Figure 4), which were consistent with the results of differential expression analysis.
Figure 4. Expressions of genes differentiated the survival differences in HCC in clinical samples.
The expressions of AGR2 (A), CRISP2 (B), IL31RA (C), KCNC2 (D), LINC01477 (E), LOC105372556 (F), PLVAP (G), RLN3 (H), SOX14 (I), TEDDM1 (J), VWA5B2 (K), and ZNF280A (L) in HCC tissues and adjacent normal tissues. Data were shown as mean ± S.D., n=26. *P<0.05 and **P<0.01.
Discussion
In the present study, a total of 2193 DEGs (including 1800 protein-coding genes and 232 lncRNAs) in HCC samples were identified. For the protein-coding genes amongst the DEGs, functional and pathway enrichment analyses were carried out. In the PPI network, CDK1 (degree = 76), PLK1 (degree = 72), FOS (degree = 63), SAA1 (degree = 59), and LPAR3 (degree = 59) were key nodes. A total of 40 network modules were identified, amongst which 4 modules were with scores no less than 10. Survival analysis suggested that 12 genes (including AGR2 and RLN3) could divide the HCC samples into high- and low-risk groups. Furthermore, the expressions of nine genes (including AGR2 and RLN3) were confirmed by qRT-PCR experiments.
CDK1 interacts with apoptin during HCC tumorigenesis, and their link may play a role in mediating tumor cell apoptosis [33]. Via directly suppressing CDK1 and v-akt murine thymoma viral oncogene homolog 3 (AKT3) expression and indirectly inhibiting cyclinD1 expression; miR-582-5p functions in the development and progression of HCC [34]. PLK1 expression is significantly up-regulated in HCC tissues, therefore, PLK1 may be a potential marker for the prognosis of HCC and targetting PLK1 may be applied for the diagnosis and therapy of the disease [35,36]. PLK1 is overexpressed in HCC samples relative to normal controls, and its knockdown can induce apoptosis of tumor cells via the endonuclease-G pathway [37]. FOS expression is inhibited by miR-139 down-expression in HCC cells with high metastatic potency, which promotes the metastasis in HCC [38]. Through suppressing the expression of the oncogene FOS, dysregulated miR-101 is implicated in the pathogenesis of HCC [39]. CDK1 had interactions with both PLK1 and FOS in the PPI network, suggesting that CDK1 might be involved in the development and progression of HCC through interacting with PLK1 and FOS.
The preoperative serum level of SAA is closely correlated with tumor size and tumor stage, implicating that SAA overexpression can serve as a promising prognostic factor for HCC patients [40,41]. The LPAR1/LPAR3 expression is increased in hepatoma cell line SKHep1, and the LPA-LPAR3 signaling may play an essential role in tumor invasiveness/expansion [42]. Several LPAR subtypes are detected in HCC samples, and the suppression of LPA-LPAR signaling represses the motility and proliferation of HCC cells [43]. LPAR3 could also interact with FOS and SAA1 in the PPI network, indicating that LPAR3 might function in pathogenesis of HCC via interacting with FOS and SAA1.
High AGR2 expression level contributes to the metastasis of HCC cells through acting on mitogen-activated protein kinase (MAPK) and Caspase pathway, which results in the unfavorable prognosis of HCC patients [44]. AGR2 overexpression is found in various tumors including fibrolamellar HCC, and the dysregulated AGR2 is a phenotypic characteristic of cholangiocytes [45,46]. RLN2 expression is found to be up-regulated in HCC tissues, which can be taken as a predictor for tumor progression and adverse prognosis [47]. Therefore, AGR2 and RLN3 might be correlated to the prognosis of HCC patients.
Conclusion
In conclusion, a total of 2193 DEGs in HCC samples were identified. Besides, CDK1, PLK1, FOS, SAA1, LPAR3, AGR2, and RLN3 might play important roles in the progression and prognosis of HCC. Nevertheless, lacking experiments was main limitation of the present study and more experiments should be designed to support our results.
Abbreviations
- ACSL4
acyl-CoA ligase 4
- AGR2
anterior gradient 2
- BP
biological process
- BTG1
B-cell translocation gene 1
- CC
cellular component
- CDK1
cyclin-dependent kinase 1
- CRISP2
cysteine-rich secretory protein 2
- DEG
differentially expressed gene
- FDR
false discovery rate
- FOS
Fos proto-oncogene, AP-1 transcription factor subunit
- FoxC1
Forkhead box C1
- GO
gene ontology
- HCC
hepatocellular carcinoma
- HOXA13
homeobox A13b
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KM
Kaplan–Meier
- LINC01477
long intergenic non-protein coding RNA 1477
- lncRNA
long non-coding RNA
- LPAR3
lysophosphatidic acid receptor 3
- OS
overall survival
- PLK1
polo-like kinase 1
- PPI
protein–protein interaction
- qRT-PCR
quantitative real-time reverse transcription-PCR
- RLN3
relaxin 3
- SAA
serum amyloid A1
- SOX14
SRY-box 14
- TCGA
The Cancer Genome Atlas
- TEDDM1
transmembrane epididymal protein 1
- USP7
ubiquitin-specific protease 7
- VWA5B2
von Willebrand factor A domain containing 5B2
- ZBTB20
zinc finger and BTB domain containing 20
- ZNF280A
zinc finger protein 280A
Funding
This work was supported by the Natural Science Foundation of Hainan Province grants [grant number 817379]; the Key Research and Development Project of Hainan Province [grant number ZDYF2018174]; and the Major Science and Technology Program of Hainan Province [grant number ZDKJ2017007].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Author contribution
Conceived and designed the study: Y.Z. and S.W. Performed the literature search and data extraction: Y.Z. and J.X. Analyzed the data: Y.Z. and H.Z. Drafted the manuscript: Y.Z.
References
- 1.Kim Y., Park Y H., Hwang S.. et al. (2016) Diagnostic role of blood tumor markers in predicting hepatocellular carcinoma in liver cirrhosis patients undergoing liver transplantation. Ann. Transplant. 21, 660–667 10.12659/AOT.900552 [DOI] [PubMed] [Google Scholar]
- 2.El-Serag H.B. and Rudolph K.L. (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 10.1053/j.gastro.2007.04.061 [DOI] [PubMed] [Google Scholar]
- 3.Elserag H.B., Marrero J.A., Rudolph L. and Reddy K.R. (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134, 1752 10.1053/j.gastro.2008.02.090 [DOI] [PubMed] [Google Scholar]
- 4.Bruix J. and Llovet J.M. (2010) Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 35, 519–524 10.1053/jhep.2002.32089 [DOI] [PubMed] [Google Scholar]
- 5.Stuart K.E., Anand A.J. and Jenkins R.L. (2015) Hepatocellular carcinoma in the United States: prognostic features, treatment outcome, and survival. Cancer 77, 2217–2222 [DOI] [PubMed] [Google Scholar]
- 6.Borie F., Bouvier A.-M., Herrero A., Faivre J., Launoy G., Delafosse P.. et al. (2008) Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J. Surg. Oncol. 98, 505 10.1002/jso.21159 [DOI] [PubMed] [Google Scholar]
- 7.Siu E.H., Chan A.W., Chong C.C.. et al. (2018) Treatment of advanced hepatocellular carcinoma: immunotherapy from checkpoint blockade to potential of cellular treatment. Transl. Gastroenterol. Hepatol. 3, 89 10.21037/tgh.2018.10.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia L., Huang W., Tian D., Zhu H., Qi X., Chen Z.. et al. (2013) Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology 57, 610–624 10.1002/hep.26029 [DOI] [PubMed] [Google Scholar]
- 9.Aravalli R.N. and Greten T.F. (2015) FoxC1: novel regulator of inflammation-induced metastasis in hepatocellular carcinoma. Gastroenterology 149, 861–863 10.1053/j.gastro.2015.08.032 [DOI] [PubMed] [Google Scholar]
- 10.Tang Q., Liu Y.F., Zhu X.J., Li Y.H., Zhu J., Zhang J.P.. et al. (2009) Expression and prognostic significance of the alpha B-crystallin gene in human hepatocellular carcinoma. Hum. Pathol. 40, 300–305 10.1016/j.humpath.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 11.Wang Q., Tan Y.X., Ren Y.B., Dong L.W., Xie Z.F., Tang L.. et al. (2011) Zinc finger protein ZBTB20 expression is increased in hepatocellular carcinoma and associated with poor prognosis. BMC Cancer 11, 271 10.1186/1471-2407-11-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kan H., Huang Y., Li X., Liu D., Chen J. and Shu M. (2016) Zinc finger protein ZBTB20 is an independent prognostic marker and promotes tumor growth of human hepatocellular carcinoma by repressing FoxO1. Oncotarget 7, 14336–14349 10.18632/oncotarget.7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda M., Sugimoto H., Nomoto S., Oya H., Hibino S., Shimizu D.. et al. (2015) B-cell translocation gene 1 serves as a novel prognostic indicator of hepatocellular carcinoma. Int. J. Oncol. 46, 641–648 10.3892/ijo.2014.2762 [DOI] [PubMed] [Google Scholar]
- 14.Pan T.T., Jia W.D., Yao Q.Y., Sun Q.K., Ren W.H., Huang M.. et al. (2014) Overexpression of HOXA13 as a potential marker for diagnosis and poor prognosis of hepatocellular carcinoma. Tohoku J. Exp. Med. 234, 209–219 10.1620/tjem.234.209 [DOI] [PubMed] [Google Scholar]
- 15.Yi H.C., Liu Y.L., You P., Pan J.S., Zhou J.Y., Liu Z.J.. et al. (2015) Overexpression of DEK gene is correlated with poor prognosis in hepatocellular carcinoma. Mol. Med. Rep. 11, 1318 10.3892/mmr.2014.2781 [DOI] [PubMed] [Google Scholar]
- 16.Lin L.J. and Chen L.T. (2013) The role of DEK protein in hepatocellular carcinoma for progression and prognosis. Pakistan J. Med. Sci. 29, 778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Zhang Q., Wang Y., Zhuang H. and Chen B. (2018) Clinical significance of ubiquitin specific protease 7 (USP7) in predicting prognosis of hepatocellular carcinoma and its functional mechanisms. Med. Sci. Monit. 24, 1742–1750 10.12659/MSM.909368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X.J. and Xu G.L. (2017) Overexpression of Acyl-CoA Ligase 4 (ACSL4) in patients with hepatocellular carcinoma and its prognosis. Med. Sci. Monit. 23, 4343–4350 10.12659/MSM.906639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maekawa S., Suzuki A., Sugano S. and Suzuki Y. (2014) RNA sequencing: from sample preparation to analysis. Methods Mol. Biol. 1164, 51–65 10.1007/978-1-4939-0805-9_6 [DOI] [PubMed] [Google Scholar]
- 20.Ho D.W., Kai A.K. and Ng I.O. (2015) TCGA whole-transcriptome sequencing data reveals significantly dysregulated genes and signaling pathways in hepatocellular carcinoma. Front. Med. 9, 322–330 10.1007/s11684-015-0408-9 [DOI] [PubMed] [Google Scholar]
- 21.Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F.. et al. (2012) GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 22, 1760–1774 10.1101/gr.135350.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love M.I., Huber W. and Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tweedie S., Ashburner M., Falls K., Leyland P., Mcquilton P., Marygold S.. et al. (2009) FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 37, D555 10.1093/nar/gkn788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altermann E. and Klaenhammer T.R. (2005) PathwayVoyager: pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics 6, 60 10.1186/1471-2164-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bindea G., Galon J. and Mlecnik B. (2013) CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics 29, 661 10.1093/bioinformatics/btt019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo W. and Brouwer C. (2013) Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 29, 1830–1831 10.1093/bioinformatics/btt285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A.. et al. (2013) STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815 10.1093/nar/gks1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohl M., Wiese S. and Warscheid B. (2011) Cytoscape: software for visualization and analysis of biological networks. Methods Mol. Biol. 696, 291–303 10.1007/978-1-60761-987-1_18 [DOI] [PubMed] [Google Scholar]
- 29.Bader G.D. and Hogue C.W. (2003) An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 4, 2 10.1186/1471-2105-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H., Zhang Y. and Geng Y. (2014) Cox regression analysis of prognostic factors of advanced non-small cell lung cancer patients treated with chemotherapy. Pract. J. Cancer 29, 165–167 [Google Scholar]
- 31.Ravishanker N. (2004) Survival analysis. Technometrics 46 (1), 111–112 [Google Scholar]
- 32.Stel V.S., Dekker F.W., Tripepi G., Zoccali C. and Jager K.J. (2011) Survival Analysis I: the Kaplan-Meier method. Nephron Clin. Practice 119, 83–88 10.1159/000324758 [DOI] [PubMed] [Google Scholar]
- 33.Zhao J., Han S.X., Ma J.L., Ying X., Liu P., Li J.. et al. (2013) The role of CDK1 in apoptin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 30, 253–259 10.3892/or.2013.2426 [DOI] [PubMed] [Google Scholar]
- 34.Yi Z., Wei H., Yan R., Yan X., Zhong Z., Fan X.. et al. (2015) miR-582-5p inhibits proliferation of hepatocellular carcinoma by targeting CDK1 and AKT3. Tumour Biol. 36, 8309–8316 10.1007/s13277-015-3582-0 [DOI] [PubMed] [Google Scholar]
- 35.Sun W., Su Q., Cao X., Shang B., Chen A., Yin H.. et al. (2014) High expression of Polo-like kinase 1 is associated with early development of hepatocellular carcinoma. Int. J. Genomics 2014, 312130 10.1155/2014/312130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Z.L., Zheng H., Lin H., Miao X.Y. and Zhong D.W. (2009) Overexpression of polo-like kinase1 predicts a poor prognosis in hepatocellular carcinoma patients. World J. Gastroenterol. 15, 4177–4182 10.3748/wjg.15.4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mok W.C., Wasser S., Tan T. and Lim S.G. (2012) Polo-like kinase 1, a new therapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 18, 3527 10.3748/wjg.v18.i27.3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Q., He M., Deng X., Wu W.K., Zhao L., Tang J.. et al. (2013) Derepression of c-Fos caused by microRNA-139 down-regulation contributes to the metastasis of human hepatocellular carcinoma. Cell Biochem. Funct. 31, 319–324 10.1002/cbf.2902 [DOI] [PubMed] [Google Scholar]
- 39.Li S., Fu H., Wang Y., Tie Y., Xing R., Zhu J.. et al. (2009) MicroRNA-101 regulates expression of the v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene in human hepatocellular carcinoma. Hepatology 49, 1194–1202 10.1002/hep.22757 [DOI] [PubMed] [Google Scholar]
- 40.Ni X.C., Yi Y., Fu Y.P., He H.W., Cai X.Y., Wang J.X.. et al. (2014) Serum amyloid A is a novel prognostic biomarker in hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 15, 10713–10718 10.7314/APJCP.2014.15.24.10713 [DOI] [PubMed] [Google Scholar]
- 41.Sun L. and Ye R.D. (2016) Serum amyloid A1: structure, function and gene polymorphism. Gene 583, 48–57 10.1016/j.gene.2016.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuckerman V., Sokolov E., Swet J.H., Ahrens W.A., Showlater V., Iannitti D.A.. et al. (2016) Expression and function of lysophosphatidic acid receptors (LPARs) 1 and 3 in human hepatic cancer progenitor cells. Oncotarget 7, 2951 10.18632/oncotarget.6696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokolov E., Eheim A.L., Ahrens W.A., Walling T.L., Swet J.H., Mcmillan M.T.. et al. (2013) Lysophosphatidic acid receptor expression and function in human hepatocellular carcinoma. J. Surg. Res. 180, 104 10.1016/j.jss.2012.10.054 [DOI] [PubMed] [Google Scholar]
- 44.Yu H., Jian Z., Ling L., Yang Z., Fan Z., Liu Y.. et al. (2012) Proteomic study explores AGR2 as pro-metastatic protein in HCC. Mol. Biosyst. 8, 2710–2718 10.1039/c2mb25160d [DOI] [PubMed] [Google Scholar]
- 45.Lepreux S., Bioulac-Sage P. and Chevet E. (2011) Differential expression of the anterior gradient protein-2 is a conserved feature during morphogenesis and carcinogenesis of the biliary tree. Liver Int. 31, 322–328 10.1111/j.1478-3231.2010.02438.x [DOI] [PubMed] [Google Scholar]
- 46.Brychtova V., Vojtesek B. and Hrstka R. (2011) Anterior gradient 2: a novel player in tumor cell biology. Cancer Lett. 304, 1–7 10.1016/j.canlet.2010.12.023 [DOI] [PubMed] [Google Scholar]
- 47.Pan H.Z., Dong A.B., Wang L., Tan S.S., Yang Q., Tong X.Y.. et al. (2013) Significance of relaxin-2 expression in hepatocellular carcinoma: relation with clinicopathological parameters. Eur. Rev. Med. Pharmacol. Sci. 17, 1095. [PubMed] [Google Scholar]