Abstract
Background: There is no effective therapy for BK virus (BKV) nephropathy (BKVN). Cyclosporine A (CsA) has a lower immunosuppressive effect than tacrolimus. In vitro studies have shown that CsA inhibits BKV replication. The present study aimed to evaluate the effectiveness of switching from tacrolimus to low-dose CsA in renal transplant recipients with BKVN. Methods: Twenty-four patients diagnosed with BKVN between January 2015 and December 2016 were included. Tacrolimus was switched to low-dose CsA, and patients were followed for 24 months. Primary end points were BKV clearance in blood and graft. Secondary end points were urine specific gravity, serum creatinine, and graft loss. Results: The viremia in all patients cleared at a mean of 2.7 ± 2.0 months after switching to CsA. Urine specific gravity at 3 months after switching to CsA increased significantly compared with that at diagnosis (P=0.002). The timing and trend of urine specific gravity increase was consistent with the timing and trend of blood and urine viral load decrease. Repeated biopsies at a median of 11.2 months (range: 9.1–12.5 months) after switching to CsA showed that 8 patients (42.1%) were negative for BKV, and 11 patients (58.9%) had a decrease in BKV load (P<0.001). There was no statistical difference in the serum creatinine level between the time of diagnosis and 24 months of CsA therapy (P=0.963). The graft survival rate was 100%. Only two patients (8.3%) suffered from acute rejection. Conclusion: Switching from tacrolimus to low-dose CsA may be an effective therapy for BKVN.
Keywords: BK virus, BK virus nephropathy, cyclosporine A, conversion of immunosuppression, tacrolimus
Introduction
BK virus (BKV) nephropathy (BKVN) is one of the main complications affecting renal transplant function and prognosis [1–4]. BKVN occurs in 1–10% of renal transplant recipients [5] and 15–50% of patients with BKVN will develop to graft loss [5]. BKVN occurs at a higher rate during the period of tacrolimus administration than during the period of administration of cyclosporine A (CsA) [6].
BKVN is difficult to treat because there are no effective antiviral agents [3,7–9]. Published guidelines advocate regular monitoring and timely reduction in immunosuppression as the most optimal practices for BKVN [5]. Different protocols have been reported, including reducing the dose of calcineurin inhibitors, mycophenolic acid (MPA), or azathioprine, switching from tacrolimus to CsA or sirolimus, switching from MPA to azathioprine, or complete discontinuation of the antimetabolite [10]. Prospective clinical trials to validate different strategies are lacking, and published reports are unsatisfactory [3].
A new approach of conversion from tacrolimus into low-dosage CsA appears promising for several reasons. First, the immunosuppressive strength of CsA is weaker than that of tacrolimus [11]. Second, the CsA regimen has a significantly lower incidence of BK viremia and BKVN compared with the tacrolimus regimen [12–16]. Finally, in vitro studies indicate that CsA inhibits BKV replication by binding to cyclophilins [17,18], whereas tacrolimus activates BKV replication via FK-binding protein 12 in primary human tubular epithelial cells [19]. At present, there is only one single-center retrospective study suggesting that addition of intravenous immunoglobulin after switching from tacrolimus to CsA is helpful for clearing BK viremia [20].
Thus, the purpose of the prospective clinical study was to evaluate the efficacy and safety of conversion from tacrolimus into low-dose CsA in renal transplant recipients with BKVN.
Materials and methods
Study design
This was a single-center prospective clinical study with a follow-up of 24 months. The study was performed in accordance with the Declaration of Helsinki and approved by the institutional review board. The present study was registered in the Chinese Clinical Trial Registration database (No. ChiCTR-IOR-17010993).
Patients who received a renal transplant and were diagnosed with biopsy-proved BKVN between January 2015 and December 2016 were selected as candidates. Patients receiving triple immunosuppressive drugs (tacrolimus + MPA + steroid) were included. The exclusion criteria were: (i) serum creatinine > 300 µmol/l when diagnosed with BKVN; (ii) received large-dose methylprednisolone treatment within 1 month before diagnosis of BKVN; (iii) receiving antiviral drug treatment; (iv) receiving other immunosuppressant drugs. Written informed consent was obtained from all the patients.
Clinical follow-up and data collection
All patients were followed for 24 months. The follow-up points included before switching to CsA, and then 2 weeks, and 1, 3, 6, 9, 12, 18, and 24 months after switching to CsA. Clinical indicators were recorded at each follow-up time-point. The estimated glomerular filtration rate (eGFR) was calculated with MDRD formula [21]. Repeated renal biopsies were performed 6–12 months after switching to CsA, or when the serum creatinine had increased by more than 30%.
Urine cytological studies and virological studies
Urine cytology smears were stained by the Papanicolaou method, and evaluated for the presence of cells with intranuclear viral inclusions (decoy cells), which were counted as number per ten high-power fields [HPF] [22–24]. Quantitation of the urine and plasma BK viral load was performed by quantitative PCR (Q-PCR) and reported as copies/ml [22,23].
Diagnosis of BKVN
BKVN was defined by positive immunohistochemical nuclear staining with anti-SV40 large T antigen monoclonal antibody, as previously described [23]. The histological features of BKVN were classified using the American Society of Transplantation (AST) schema, and assigned to BKVN categories A, B, and C based on the guidelines published by Hirsch and Randhawa [25]. Histological viral load was assessed semi-quantitatively as the percentage of tubules positive for polyomavirus using a four-tier system (<10, 10–25, 25–50, and >50%) [26]. Histologic lesions, T-cell-mediated rejection (TCMR), and antibody-mediated rejection (ABMR) were defined using the Banff 2013 schema of renal allograft pathology [27].
Treatment of BKVN
All patients in the present study were switched to low-dose cyclosporine immediately after the diagnosis of BKVN, and no other immunosuppressant adjustment or antiviral therapy was performed. The initial dose of CsA was 3 mg/kg/day. The trough concentration of CsA was measured every 3 days, and the dose was adjusted if needed. The target trough concentration was 75–125 ng/ml. Mycophenolate mofetil (MMF) was continued at a dosage of 500 mg twice daily, the enteric-coated mycophenolate sodium (EC-MPS) was 360 mg twice daily, and the oral steroid dosage was 5 mg/d.
Efficacy evaluation
The primary study end points were BKV clearance in blood and renal tissues. BKV clearance in blood was defined as a BKV load of <1000 copies/ml at each test (three or more tests were performed over an interval of 3 months). BKV clearance in renal tissues was defined as negative SV40-T antigen immunohistochemical staining. The secondary end points were urine specific gravity, serum creatinine, and graft loss. The normal range of urine specific gravity was 1.005–1.015. Graft loss was defined as patient death, return to dialysis, or re-transplantation.
Safety evaluation
The safety evaluation included rejection events, proteinuria, and CsA-related adverse events.
The CsA adverse events, including gingival hyperplasia, hair hyperplasia, hypertension, CNI nephrotoxicity and liver damage was recorded.
Statistical analysis
All statistical analyses and calculations were performed using SPSS version 20.0 (IBM, U.S.A.). Categorical variables were presented as count and percentage. Normally distributed continuous data were presented as mean and S.D.; non-normally distributed data were presented as median (interquartile range). Normality was determined using the Kolmogorov–Smirnov test. The McNemar test was used for categorical variables. The paired-samples t test was used to compare normally distributed continuous variables, and the Wilcoxon signed-rank test was used for non-normally distributed continuous variables. Kaplan–Meier analysis was used for calculating the cumulative virus clearance rate after switching immunosuppression. All P-values were two-tailed, and <0.05 were considered significant.
Results
Patient baseline characteristics
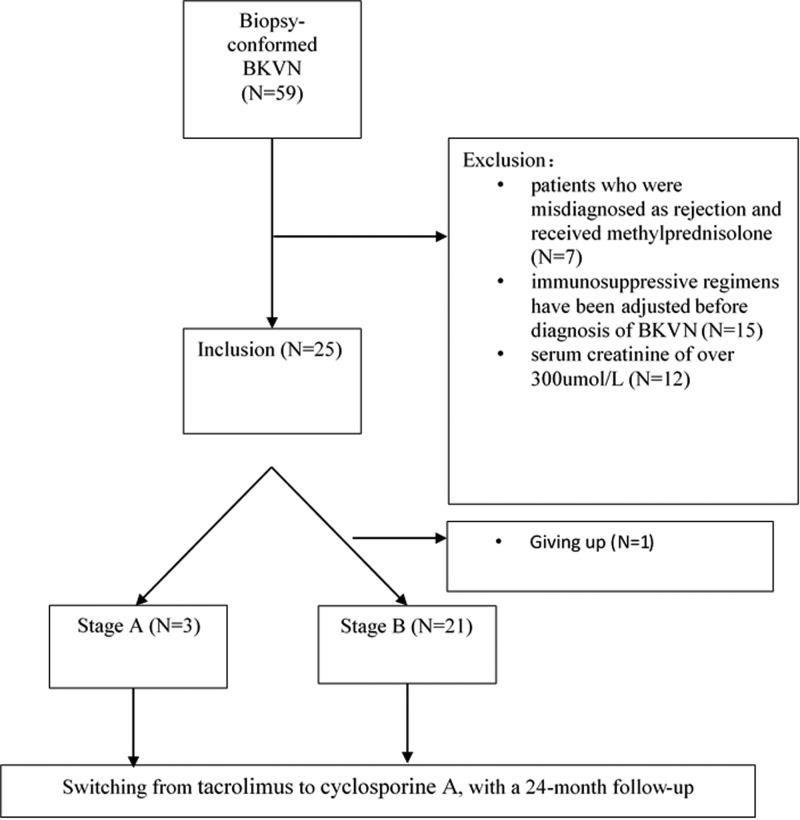
A total of 299 renal transplant recipients underwent 345 renal transplant biopsies between January 2015 and December 2016, and 59 patients were diagnosed with BKVN. Amongst them, 24 patients were included in the present study. The research procedure is summarized in Figure 1. Baseline data are summarized in Table 1. Amongst the 24 patients, three patients were diagnosed as stage A BKVN, and 21 patients were diagnosed as stage B BKVN. The time interval from transplantation to diagnosis of BKVN was 16.3 ± 9.4 months. Transplant data are summarized in Table 2.
Figure 1. Flow diagram of patient inclusion.
Table 1. Baseline data of 24 patients before diagnosis of BKVN.
| Total (n=24) | |
|---|---|
| Male gender, n (%) | 18 (75.0%) |
| Age at transplant (years), n (%) | 37.5 (31.3–45.4) |
| Body weight (kg) | 51.5 (50.0–57.0) |
| ESRD etiology, n (%) | |
| Glomerulonephritis | 14 (58.3%) |
| IgA nephrology | 6 (25.0%) |
| Hypertensive nephropathy | 2 (8.3%) |
| Nephrotic syndrome | 1 (4.2%) |
| Lupus nephritis | 1 (4.2%) |
| Induction regimen, n (%) | |
| Polyclonal antibody/ATG | 12 (50.0%) |
| Monoclonal antibody/Basiliximab | 12 (50.0%) |
| Maintenance regimen, n (%) | |
| Tacrolimus-MPA-Steroid | 24 (100%) |
| MPA-AUC (ng/ml) | 68.0 ± 30.2 |
| Transplant donor type, n (%) | |
| Deceased | 17 (70.8%) |
| Living | 7 (29.2%) |
Abbreviations: ATG, antithymocyte globulin; MPA-AUC, MPA concentration-area under curve.
Table 2. Clinical data of 24 patients at the time of diagnosis of BKVN and 12 and 24 months after follow-up.
| Baseline at diagnosis | Follow-up of 12 months | P* | Follow-up of 24 months | P** | |
|---|---|---|---|---|---|
| Decoy cells (/HPF) | 28.0 (13.8–56.8) | 0 (0–0) | <0.001 | 0 (0–0) | <0.001 |
| Urine BKV-DNA (copies/ml) | 2.1 × 109 (7.3 × 108 to 5.9 × 109) | 5.9 × 106 (1.5 × 105 to 3.2 × 107) | <0.001 | 3.7 × 105 (1.0 × 106 to 5.9 × 107) | <0.001 |
| Blood BKV-DNA (copies/ml) | 1.4 × 104 (4.5 × 103 to 9.0 × 104) | 0 (0–0) | <0.001 | 0 (0–0) | <0.001 |
| Serum creatinine (umol/l) | 160.6 ± 47.3 | 166.5 ± 75.7 | 0.748 | 159.8 ± 88.9 | 0.963 |
| eGFR (ml/min) | 46.0 ± 15.5 | 47.4 ± 17.2 | 0.774 | 51 ± 18.3 | 0.252 |
*, P-value of the comparison between the time of 12 months after follow-up and diagnosis of BKVN.
**, P-value of the comparison between the time of 24 months after follow-up and diagnosis of BKVN.
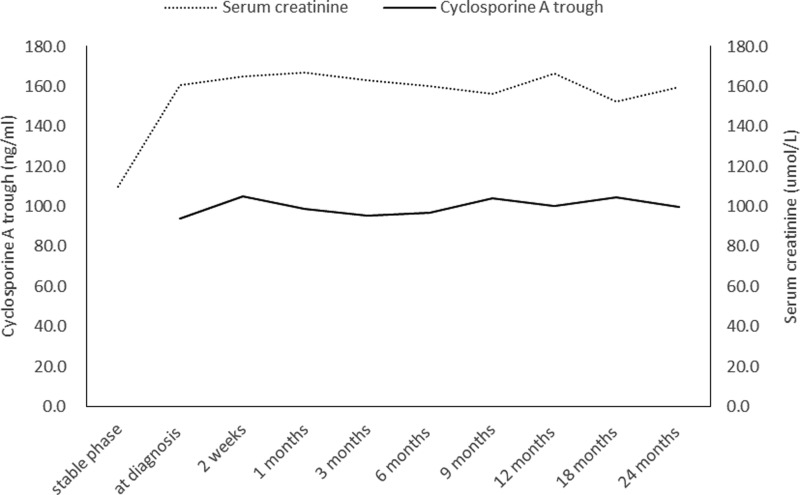
Switching from tacrolimus to low-dose CsA
All patients started with cyclosporine at a mean dose of 162.1 ± 27.2 mg/day, orally twice daily. Two weeks after switching to CsA, the mean trough concentration of CsA was 94.3 ± 33.6 ng/ml. The trough concentration remained stable during the follow-up period (Figure 2). Three patients were switched back to tacrolimus at 9, 9, and 12 months after switching to CsA. The trough concentration of tacrolimus in three patients switched back to tacrolimus set at 4–5 ng/ml, which was slightly lower than the previous trough concentration. One patient developed ABMR, and in the other two patients, the clinicians were concerned that immunosuppression was not sufficient. Before switching back to tacrolimus, blood tests for BKV had been negative for more than 3 months. None of the patients developed BK viremia or BKVN recurrence after switching back to tacrolimus.
Figure 2. Trough concentration of CsA and serum creatinine level at each follow-up point after switching from tacrolimus to CsA were stable.
Efficacy evaluation
Switching from tacrolimus to CsA reduced the blood and urine BKV load
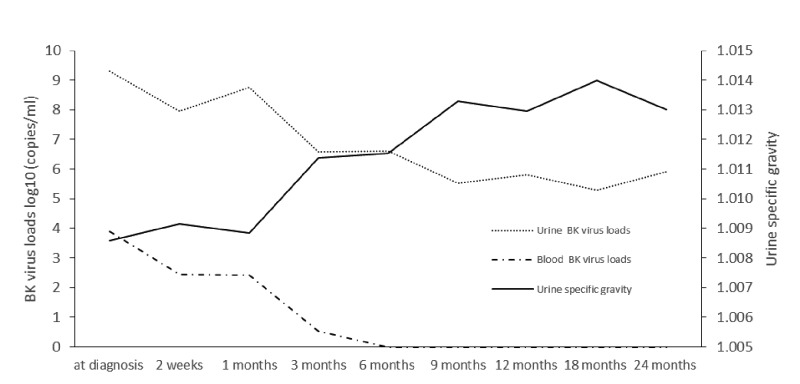
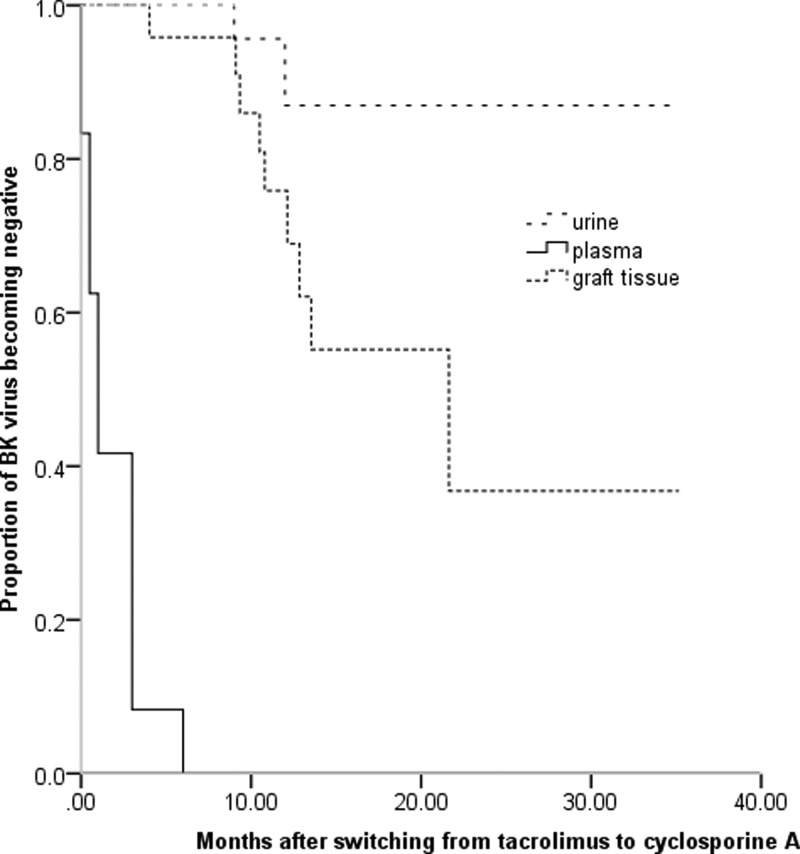
BKV clearance is shown in Figure 3. At 3 months after switching from tacrolimus to CsA, decoy cells and blood and urine BKV load were significantly lower than those at diagnosis (all, P<0.001). At a mean of 2.7 ± 2.0 months after switching to CsA, all patients were negative for viremia. At a mean of 1.8 ± 1.9 months after switching, decoy cells were not present in any of the patients. Decoy cells were cleared 0.8 ± 0.8 months earlier than when viremia was cleared. Viruria was cleared in only 3 patients during follow-up.
Figure 3. The BKV load in blood and tissues was reduced after switching from tacrolimus to CsA.
The timing and trend of BKV load decrease was consistent with the timing and trend of urine specific gravity increase. After log conversion, the BKV load in blood and urine, and urine specific gravity were expressed as mean.
Switching from tacrolimus to CsA promoted urine specific gravity recover
The mean value of morning urine specific gravity was 1.0086 ± 0.0027 at diagnosis of BKVN. In contrast, the mean morning urine specific gravity at 3 months after switching to CsA increased significantly (1.0114 ± 0.0032 compared with 1.0086 ± 0.0027, P=0.002). The timing and trend of urine specific gravity increase was consistent with the timing and trend of blood and urine viral load decrease (Figure 3).
Renal histopathological changes after switching from tacrolimus to CsA
At a median of 11.2 months (range: 9.1–12.5 months) after switching to CsA, 19 patients received 22 renal biopsies. The pathological scores of the first and last renal biopsies are shown in Table 3. The last biopsies showed that SV40-T staining was negative in 8 patients (42.1%), and was decreased in the other 11 patients (58.9%). Representative Hematoxylin and Eosin staining and immunohistochemical staining results before and after treatment are shown in Figure 4. The time of clearance of SV40-T staining was 10.7 months (range: 8.2–21.6 months). The timeline of virus clearance is shown in Figure 5. Five patients did not receive repeated renal biopsies, but their renal function was stable. Their median serum creatinine at the last follow-up was 102.0 µmol/l (range: 87.0–118.0 µmol/l).
Table 3. Pathological characteristics at BKVN diagnosis and last repeated biopsies.
| Initial biopsy, n=24 | Last biopsy, n=19 | P | |
|---|---|---|---|
| SV40-T, n (%) | 24 (100%) | 11 (57.9%) | 0.002 |
| Banff t score | 1.4 ± 0.8 | 0.9 ± 0.6 | 0.056 |
| Banff i score | 1.0 ± 1.0 | 0.3 ± 0.7 | 0.005 |
| Banff ci score | 1.2 ± 0.7 | 2.1 ± 0.9 | 0.001 |
| Banff ct score | 1.2 ± 0.5 | 2.1 ± 0.9 | 0.001 |
| Extent of SV40-T | 1.8 ± 0.8 | 0.6 ± 0.5 | <0.001 |
Abbreviations: ci, interstitial fibrosis; ct, tubular atrophy; i, interstitial inflammation; t, tubulitis.
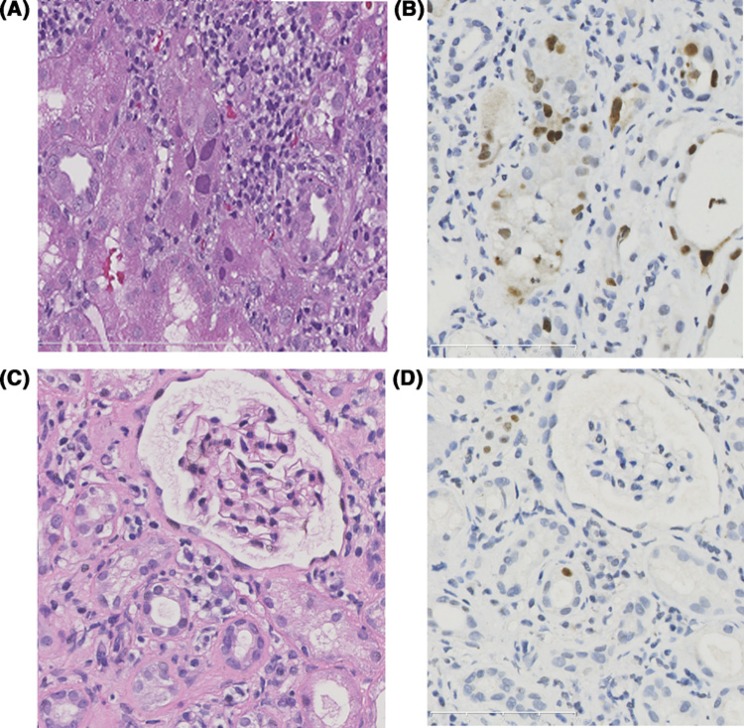
Figure 4. Comparison of representative pathological findings between initial biopsy and repeated biopsy.
BKV cytopathic changes and SV40 large T antigen staining before (A,B) and after (C,D) treatment. Initial biopsy show nuclear enlargement and nuclear inclusions within tubular epithelial cells (A). The SV40 large T antigen staining (B) shows extensive staining in the nuclei in the infected tubules. After switching to low-dose cyclosporine, the viral cytopathic changes become sparse or unrecognizable on Hematoxylin and Eosin staining. SV40 large T antigen staining (D) shows infected nuclei in isolated cells.
Figure 5. The BKV clearance in urine, blood, and tissues after switching from tacrolimus to CsA.
Switching from tacrolimus to CsA postponed renal transplant function deterioration and graft loss
The mean serum creatinine level and eGFR of all 24 patients was relatively stable at each follow-up time-point (Figure 2). As compared with the time of diagnosis, serum creatinine, and eGFR were not significantly different at 12 and 24 months after switching to CsA (Table 2). The renal survival rate was 100% at last follow-up.
Safety evaluation
Acute rejection events
The serum creatinine level of one patient increased to 300 µmol/l at 9 months after switching to CsA (viremia had become negative). Renal biopsy revealed ABMR combined with TCMR Banff IIa. After addition of methylprednisolone, and increasing the dose of both MMF and CsA, the serum creatinine level was 323 µmol/l at the 24-month follow-up. Another patient was confirmed as chronic active ABMR by the protocol biopsy at 12 months. The patient was treated with methylprednisolone, and the serum creatinine was 154 µmol/l at the 24-month follow-up. Rejection in both patients was due to non-adherence documented by missing medication, including cyclosporin, MPA, and prednisone. Neither patient developed a recurrence of viremia.
Other adverse events
Five patients developed proteinuria. Amongst them, four patients were confirmed as having recurrent IgA nephropathy through biopsy, and one patient was diagnosed with mixed rejection mentioned above. There was no CsA-related adverse event.
Discussion
There are few controlled studies available to guide the management of BKVN in kidney transplant recipients [3]. Reducing immunosuppression is a relatively effective treatment. In a retrospective study, maintenance immunosuppression was reduced in 52 patients with biopsy-proven BKVN. Eight patients (15.3%) developed acute rejection, and eight patients (16.4%) lost their grafts [28]. Schaub et al. [29] treated 13 patients with definitive BKVN, 17 patients with presumptive BKVN, and 8 patients with low BKV-viremia by reducing the dosage of tacrolimus, and subsequently reducing the dose of MPA. Clearance of BKV-viremia was achieved in 35 of the 38 patients (92%), but 7 patients (18%) required treatment for presumed concurrent rejection while BKV-viremia was present. Only reducing immunosuppressive dosage might be insufficient or excessive. As compared with above studies, the therapeutic regimen in the present study was associated with a higher virus clearance rate, lower secondary rejection rate, and lower renal allograft loss rate.
Although both are calcineurin inhibitors, the immunosuppressive strength of CsA is weaker than that of tacrolimus [11]. Registry analyses, single-center retrospective studies, and prospective randomized clinical trials have suggested that tacrolimus is associated with a higher risk of BKV reactivation than CsA [14,15,17,18]. In vitro studies have indicated that tacrolimus activates BKV replication via FK-binding protein 12 in primary human tubular epithelial cells [19]. CsA inhibits BKV large T-antigen and viral protein 1 expression by binding to cyclophilins [17,18]. MPA has been demonstrated to be an important factor leading to BKV activation [14]. The combination of CsA with MPA results in less intense immunosuppression than that of tacrolimus with the same dose of MPA. This is due to CsA inhibition of the enterohepatic recirculation of the major MPA metabolite, glucuronide [30]. After switching from tacrolimus to low-dose CsA, the overall immunosuppressive intensity is reduced, and with the antiviral effect of CsA virus clearance is promoted.
In the present study, there was no statistical difference in the serum creatinine level at 24 months after switching to CsA and the time of diagnosis. The graft survival rate was 100%. The stability of renal transplant function reported in the present study is superior to that in previous studies [28,29]. The incidence of rejection events was 8.3%, which was lower than that reported in two other studies (15.3 and 18.0%, respectively) [28]. In this study, two patients developed rejection, which was correlated with drug incompliance.
Regardless of the treatment strategy employed for BKVN, rapid viremia clearance has been associated with stable or improving eGFR [31]. In this study, the viremia clearance time was 2.7 ± 2.0 months, which was shorter than that reported in other studies [20,29]. Of course, the shorter viremia clearance time in this study may be partially attributed to a threshold of 1000 copies/ml for viremia rather than a lower threshold, such as 200 copies/ml reported by Tong et al. [32]. It is worth noting that the clearance rate of decoy cells was the fastest. The massive replication of BKV in renal tubular epithelium and urinary tract epithelium could cause shedding of epithelial cells, which are shed into the urine to form decoy cells [33]. Negative decoy cells indicate that BKV replication is reduced or is completely blocked in the graft. In the present study, decoy cells became negative sooner than viremia became negative, which is consistent with the kinetics of BKV clearance.
From a pathological view, the evolution of BKVN may begin with BKV activation in transitional cells, followed by retrograde infection in the medullary collecting ducts, distal tubules, and proximal tubules [2]. Early BKV infection is mainly characterized by involvement of the medullary collecting ducts [2,4]. Medullary collecting duct function is closely correlated with the ability to concentrate urine [34]. Necrotic epithelia are shed into the collecting ducts after BKV infection, leading to impairment of the ability to concentrate urine, which is characterized by decreased urine specific gravity. Findings of the present study showed that urine specific gravity was significantly lower than normal level at the time BKVN was diagnosed. At 3 months after switching to CsA urine specific gravity had increased, and the trend of urine specific gravity increase was consistent with the trend of BKV load decrease in blood and urine. We speculate that after virus clearance in the graft, the epithelia of collecting duct repair, and function normally to concentrate urine. Urine specific gravity could be used to predict renal tubular injury and repair, as well as to predict the degree of virus clearance in BKVN patients.
In addition to decreasing viremia, diminishing or absent viral cytopathological changes and SV40-T staining usually indicates resolving BKVN [35]. In the present study, repeated biopsies showed that SV40-T staining became negative in 8 patients (42.1%), and was reduced in 11 patients (58.9%), suggesting resolving BKVN. Similar to other studies, repeated biopsies in the present study showed that residual tubulointerstitial inflammation can occur after virus clearance, and it is believed that this antiviral inflammatory microenvironment promotes healing and a good prognosis [35,36]. However, if tubulointerstitial inflammation is aggravated, which occurred in the non-positive area of SV40-T staining, TCMR should be taken into account [35,36]. In this study, repeated biopsies showed aggravated tubulitis and interstitial inflammation combined with de novo end arteritis in only one patient, who was thus diagnosed with TCMR Banff IIa mixed with ABMR.
There are some limitations of the present study. The sample size was small due to strict inclusion criteria. In addition, no control group that received a standardized treatment for BKVN was included.
The present study initially reveals that switching from tacrolimus to low-dose CsA could serve as an alternative treatment for early BKVN in kidney transplant recipients. This strategy still requires more large-scale, multicenter clinical studies to confirm.
Clinical perspectives
There is no effective therapy for BKVN. CsA has a lower immunosuppressive effect than tacrolimus. In vitro studies have shown that CsA inhibits BKV replication.
We treated 24 renal transplant recipients with BKVN by switching from tacrolimus to low-dose CsA. The viremia in all patients cleared at a mean of 2.7 ± 2.0 months after switching to CsA. Renal biopsies at a mean of 11.2 months (range: 9.1–12.5 months) showed that 8 patients (42.1%) were negative for BKV, and 11 patients (58.9%) had a decrease in BKV load (P<0.001). There was no statistical differences in the serum creatinine level between the time of diagnosis and at 24 months (P=0.963). The graft survival rate was 100%. Only two patients (8.3%) had an acute rejection reaction.
Switching from tacrolimus to low-dose CsA may serve as a safe and effective therapy for BKVN.
Abbreviations
- ABMR
antibody-mediated rejection
- BKV
BK virus
- BKVN
BKV nephropathy
- CNI
Calcineurin inhibitor
- CsA
cyclosporine A
- eGFR
estimated glomerular filtration rate
- MDRD
Modification of Diet in Renal Disease
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- TCMR
T-cell-mediated rejection
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81770749]; the Natural Science Foundation of Guangdong Province [grant number 2017A030313710]; and the Fundamental Research Funds for the Central Universities [grant number 17ykpy29].
Author contribution
G.H. and C.-X.W. designed the article. X.-T.C. and J.L. interpreted data and wrote the manuscript. R.-H.D. reviewed the whole manuscript. G.H. and C.-X.W. reviewed and edited the whole manuscript. S.-C.Y. and Y.-Y.C., as pathologists, evaluated the slides. P.-S.C. performed the laboratory testing. Z.-Y.W. and Y.H. collected, analyzed and interpreted data. All authors read and approved the manuscript.
References
- 1.Wojciechowski D. and Chandran S. (2016) BK virus infection after kidney transplantation. Transplantation 100, 703–704 10.1097/TP.0000000000001067 [DOI] [PubMed] [Google Scholar]
- 2.Lamarche C., Orio J., Collette S., Senécal L., Hébert M., Renoult É. et al. (2016) BK Polyomavirus and the transplanted kidney: immunopathology and therapeutic approaches. Transplantation 100, 2276–2287 10.1097/TP.0000000000001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston O., Jaswal D., Gill J.S., Doucette S., Fergusson D.A. and Knoll G.A. (2010) Treatment of Polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation 89, 1057–1070 10.1097/TP.0b013e3181d0e15e [DOI] [PubMed] [Google Scholar]
- 4.Yi S.G., Knight R.J. and Lunsford K.E. (2017) BK virus as a mediator of graft dysfunction following kidney transplantation. Curr. Opin. Organ Transplant. 22, 320–327 10.1097/MOT.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 5.Hirsch H.H., Brennan D.C., Drachenberg C.B., Ginevri F., Gordon J., Limaye A.P.. et al. (2005) Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79, 1277–1286 10.1097/01.TP.0000156165.83160.09 [DOI] [PubMed] [Google Scholar]
- 6.Suwelack B., Malyar V., Koch M., Sester M. and Sommerer C. (2012) The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transplant. Rev. 26, 201–211 10.1016/j.trre.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Knoll G.A., Humar A., Fergusson D., Johnston O., House A.A., Kim S.J.. et al. (2014) Levofloxacin for BK virus prophylaxis following kidney transplantation. JAMA 312, 2106 10.1001/jama.2014.14721 [DOI] [PubMed] [Google Scholar]
- 8.Zaman R.A., Ettenger R.B., Cheam H., Malekzadeh M.H. and Tsai E.W. (2014) A novel treatment regimen for BK viremia. Transplantation 97, 1166–1171 10.1097/01.TP.0000441825.72639.4f [DOI] [PubMed] [Google Scholar]
- 9.Anyaegbu E.I., Almond P.S., Milligan T., Allen W.R., Gharaybeh S. and Al-Akash S.I. (2012) Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr. Transplant. 16, E19–E24 10.1111/j.1399-3046.2010.01384.x [DOI] [PubMed] [Google Scholar]
- 10.Manitpisitkul W., Wilson N.S. and Haririan A. (2010) Immunosuppressive agents as risk factors for BK virus nephropathy: an overview and update. Expert Opin. Drug Saf. 9, 959–969 10.1517/14740338.2010.495714 [DOI] [PubMed] [Google Scholar]
- 11.Azzi J.R., Sayegh M.H. and Mallat S.G. (2013) Calcineurin inhibitors: 40 years later, can’t live without. J. Immunol. 191, 5785–5791 10.4049/jimmunol.1390055 [DOI] [PubMed] [Google Scholar]
- 12.Brennan D.C., Agha I., Bohl D.L., Schnitzler M.A. and Hardinger K.L. (2005) Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am. J. Transplant. 5, 582–594 10.1111/j.1600-6143.2005.00742.x [DOI] [PubMed] [Google Scholar]
- 13.Manitpisitkul W., Drachenberg C., Ramos E., Munivenkatappa R., Philosophe B., Klassen D.. et al. (2009) Maintenance immunosuppressive agents as risk factors for BK virus nephropathy: a case-control study. Transplantation 88, 83–88 10.1097/TP.0b013e3181aa8d93 [DOI] [PubMed] [Google Scholar]
- 14.Dharnidharka V.R., Cherikh W.S. and Abbott K.C. (2009) An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 87, 1019–1026 10.1097/TP.0b013e31819cc383 [DOI] [PubMed] [Google Scholar]
- 15.Hirsch H.H., Vincenti F., Friman S., Tuncer M., Citterio F., Wiecek A.. et al. (2013) Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am. J. Transplant. 13, 136–145 10.1111/j.1600-6143.2012.04320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kizilbash S.J., Rheault M.N., Bangdiwala A., Matas A., Chinnakotla S. and Chavers B.M. (2017) Infection rates in tacrolimus versus cyclosporine-treated pediatric kidney transplant recipients on a rapid discontinuation of prednisone protocol: 1-year analysis. Pediatr. Transplant. 21, e12919 10.1111/petr.12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y.J., Weng C.W., Wu H.H., Chen Y.C. and Hung C.C. (2010) A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation 89, 299–306 10.1097/TP.0b013e3181c9b51c [DOI] [PubMed] [Google Scholar]
- 18.Acott P.D., O’Regan P.A., Lee S.H. and Crocker J.F. (2008) In vitro effect of cyclosporin A on primary and chronic BK polyoma virus infection in Vero E6 cells. Transplant. Infect. Dis. 10, 385–390 10.1111/j.1399-3062.2008.00330.x [DOI] [PubMed] [Google Scholar]
- 19.Hirsch H.H., Yakhontova K., Lu M. and Manzetti J. (2016) BK Polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am. J. Transplant. 16, 821–832 10.1111/ajt.13541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kable K., Davies C.D., O’Connell P.J., Chapman J.R. and Nankivell B.J. (2017) Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant. Direct 3, e142 10.1097/TXD.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Froissart M., Rossert J., Jacquot C., Paillard M. and Houillier P. (2005) Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J. Am. Soc. Nephrol. 16, 763–773 10.1681/ASN.2004070549 [DOI] [PubMed] [Google Scholar]
- 22.Huang G., Chen L., Qiu J., Wang C., Fei J., Deng S.. et al. (2010) Prospective study of polyomavirus BK replication and nephropathy in renal transplant recipients in China: a single-center analysis of incidence, reduction in immunosuppression and clinical course. Clin. Transplant. 24, 599–609 10.1111/j.1399-0012.2009.01141.x [DOI] [PubMed] [Google Scholar]
- 23.Huang G., Wang C., Zhang L., Fei J., Deng S., Qiu J.. et al. (2015) Monitoring of polyomavirus BK replication and impact of preemptive immunosuppression reduction in renal-transplant recipients in China: a 5-year single-center analysis. Diagn. Microbiol. Infect. Dis. 81, 21–26 10.1016/j.diagmicrobio.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 24.Drachenberg R.C., Drachenberg C.B., Papadimitriou J.C., Ramos E., Fink J.C., Wali R.. et al. (2001) Morphological spectrum of polyoma virus disease in renal allografts: diagnostic accuracy of urine cytology. Am. J. Transplant. 1, 373–381 10.1034/j.1600-6143.2001.10414.x [DOI] [PubMed] [Google Scholar]
- 25.Hirsch H.H. and Randhawa P. (2013) BK Polyomavirus in solid organ transplantation. Am. J. Transplant. 13, 179–188 10.1111/ajt.12110 [DOI] [PubMed] [Google Scholar]
- 26.Hirsch H.H., Brennan D.C., Drachenberg C.B., Ginevri F., Gordon J., Limaye A.P.. et al. (2005) Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79, 1277–1286 10.1097/01.TP.0000156165.83160.09 [DOI] [PubMed] [Google Scholar]
- 27.Haas M., Sis B., Racusen L.C., Solez K., Glotz D., Colvin R.B.. et al. (2014) Banff 2013 Meeting Report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. 14, 272–283 10.1111/ajt.12590 [DOI] [PubMed] [Google Scholar]
- 28.Ramos E., Drachenberg C.B., Papadimitriou J.C., Hamze O., Fink J.C., Klassen D.K.. et al. (2002) Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J. Am. Soc. Nephrol. 13, 2145–2151 10.1097/01.ASN.0000023435.07320.81 [DOI] [PubMed] [Google Scholar]
- 29.Schaub S., Hirsch H.H., Dickenmann M., Steiger J., Mihatsch M.J., Hopfer H.. et al. (2010) Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am. J. Transplant. 10, 2615–2623 10.1111/j.1600-6143.2010.03310.x [DOI] [PubMed] [Google Scholar]
- 30.Naito T., Shinno K., Maeda T., Kagawa Y., Hashimoto H., Otsuka A.. et al. (2006) Effects of calcineurin inhibitors on pharmacokinetics of mycophenolic acid and its glucuronide metabolite during the maintenance period following renal transplantation. Biol. Pharm. Bull. 29, 275–280 10.1248/bpb.29.275 [DOI] [PubMed] [Google Scholar]
- 31.Schwarz A., Linnenweber-Held S., Heim A., Bröcker V., Rieck D., Framke T.. et al. (2012) Factors influencing viral clearing and renal function during Polyomavirus BK–associated nephropathy after renal transplantation. Transplant. J. 94, 396–402 10.1097/TP.0b013e31825a505d [DOI] [PubMed] [Google Scholar]
- 32.Tong C.Y., Hilton R., MacMahon E.M., Brown L., Pantelidis P., Chrystie I.L.. et al. (2004) Monitoring the progress of BK virus associated nephropathy in renal transplant recipients. Nephrol. Dial. Transplant. 19, 2598–2605 10.1093/ndt/gfh391 [DOI] [PubMed] [Google Scholar]
- 33.Ambalathingal G.R., Francis R.S., Smyth M.J., Smith C. and Khanna R. (2017) BK Polyomavirus: clinical aspects, immune regulation, and emerging therapies. Clin. Microbiol. Rev. 30, 503–528 10.1128/CMR.00074-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gueutin V., Deray G. and Isnard-Bagnis C. (2012) Renal physiology. Bull Cancer 99, 237–249 [DOI] [PubMed] [Google Scholar]
- 35.Drachenberg C.B., Papadimitriou J.C., Chaudhry M.R., Ugarte R., Mavanur M., Thomas B.. et al. (2017) Histological evolution of BK virus-associated nephropathy: importance of integrating clinical and pathological findings. Am. J. Transplant. 17, 2078–2091 10.1111/ajt.14314 [DOI] [PubMed] [Google Scholar]
- 36.Menter T., Mayr M., Schaub S., Mihatsch M.J., Hirsch H.H. and Hopfer H. (2013) Pathology of resolving polyomavirus-associated nephropathy. Am. J. Transplant. 13, 1474–1483 10.1111/ajt.12218 [DOI] [PubMed] [Google Scholar]