Abstract
Interleukin 7 (IL-7) and its receptor (IL-7R, a heterodimer of IL-7Rα and γc) are essential for normal lymphoid development. In their absence, severe combined immunodeficiency occurs. By contrast, excessive IL-7/IL-7R-mediated signaling can drive lymphoid leukemia development, disease acceleration and resistance to chemotherapy. IL-7 and IL-7R activate three main pathways: STAT5, PI3K/Akt/mTOR and MEK/Erk, ultimately leading to the promotion of leukemia cell viability, cell cycle progression and growth. However, the contribution of each of these pathways towards particular functional outcomes is still not completely known and appears to differ between normal and malignant states. For example, IL-7 upregulates Bcl-2 in a PI3K/Akt/mTOR-dependent and STAT5-independent manner in T-ALL cells. This is a ‘symmetric image’ of what apparently happens in normal lymphoid cells, where PI3K/Akt/mTOR does not impact on Bcl-2 and regulates proliferation rather than survival. In this review, we provide an updated summary of the knowledge on IL-7/IL-7R-mediated signaling in the context of cancer, focusing mainly on T-cell acute lymphoblastic leukemia, where this axis has been more extensively studied.
Keywords: T-ALL, T-cell acute lymphoblastic leukemia, B-cell acute lymphoblastic leukemia, Interleukin 7, IL-7R, PI3K/Akt/mTOR pathway, JAK/STAT pathway
1. Introduction
Interleukin 7 (IL-7), a cytokine produced by stromal cells in the bone marrow, thymus and a range of other organs, and its receptor (IL-7R), expressed mainly in lymphoid cells, are absolutely required for normal T-cell development and homeostasis of mature T-cells. For years, it has been known that, in both mice and humans, inactivation of any of the elements that constitute the IL-7/IL-7R signaling machinery would lead to immunodeficiency. On the other hand, numerous studies have reported the association of this axis with, and involvement in, different autoimmune and chronic inflammatory conditions, such as diabetes, multiple sclerosis or rheumatoid arthritis. However, the most striking evidence for the fundamental need to maintain this axis under tight physiological regulation comes from more recent knowledge that driver gain-of-function mutations in IL7R exist in a subset of patients with acute lymphoblastic leukemia (ALL) of T- and B-cell origin. We previously reviewed the importance of IL-7 and IL-7R for normal T-cell development and homeostasis, the role of IL-7 as an anti-cancer agent, and the involvement of IL-7/IL-7R-mediated signaling in T-ALL (Ribeiro et al., 2013). In the following sections we provide a brief recall on these topics and then focus mainly on updating the knowledge on the participation of IL-7 and IL-7R in T-ALL, with a glimpse on therapeutic implications and opportunities.
2. The good… IL-7/IL-7R in normal T-cell biology and clinical potential of IL-7 administration
IL-7, a four helix-bundle cytokine, is produced in different organs, including the thymus, bone marrow and liver (Jiang et al., 2005; Oliveira et al., 2017; Ribeiro et al., 2013). The IL-7 receptor (IL-7R) is expressed essentially in hematopoietic cells, namely of the lymphoid lineage, and is constituted by the ‘specific’ IL-7Rα (CD127) subunit (which is actually shared by the receptor for another cytokine - TSLP) and the common gamma chain (γc; CD132), which is shared by the receptors for IL-2, -4, -9, -15 and −21. A few years after it was first cloned - 3 decades ago (Namen et al., 1988) - IL-7 and its receptor were found to be essential for normal lymphoid development in mice (Boyman et al., 2008; Peschon et al., 1994; von Freeden-Jeffry et al., 1995). In humans, IL-7R inactivating mutations result in severe T-cell lymphopenia with normal, yet non functional, numbers of B-cells (Noguchi et al., 1993; Puel et al., 1998). Additionally, IL-7 is involved on the homeostasis, differentiation and functioning of mature T-cells (Azevedo et al., 2009; Lenz et al., 2004; Pellegrini et al., 2011; Prlic et al., 2002; Schluns et al., 2000; Seddon et al., 2003; Soares et al., 1998; Swainson et al., 2007). In fact, the importance of IL-7 availability for T-cells is hinted from studies showing that IL-7-mediated signaling leads to IL-7Rα rapid internalization (Henriques et al., 2010) and subsequent transcriptional downregulation (Fry et al., 2003; Park et al., 2004), in what may be a biological strategy that has been selected to maximize the number of T-cells that gain access to this vital resource (Fry et al., 2003; Mazzucchelli and Durum, 2007; Park et al., 2004).
Given what we have just summarized, it is not surprising that IL-7 can have an important role in boosting the immune system. This is especially relevant in the context of cancer, since chemotherapy and radiotherapy frequently induce long-lasting lymphopenia (Mackall et al., 2011). Consequently, recombinant human IL-7 (rhIL7) has been tested in patients with refractory cancer, with results indicating that treatment with rhIL7 promoted sustained peripheral CD4+ and CD8+ T-cell expansion, and increased T-cell survival and diversity of the TCR repertoire, independently of the age of the subject (Sportes et al., 2010). Although the clinical evidence is still limited, the use of IL-7 in the context of anti-cancer therapies seems promising, in the least as a booster of T-cell numbers and consequent improvement of immune reconstitution. Moreover, creative ways of exploring the beneficial impact of IL-7 on T-cells may lead to new therapeutic developments. For example, in a recent study chimeric antigen receptor (CAR)-T cells were engineered to express IL-7 and CCL19. These cells showed superior anti-tumor activity compared to conventional CAR-T cells, with improved immune cell infiltration and CAR-T cell survival in mouse pre-established solid tumors. These enhanced features ultimately resulted in complete tumor regression and extended survival of the mice (Adachi et al., 2018).
3. The bad … IL-7 and IL-7R in autoimmunity, chronic inflammation and cancer
The knowledge that absent IL-7/IL-7R-mediated signaling results in lymphopenia stresses the importance of maintaining the levels of IL-7 and IL-7R above a certain physiological threshold. Below this, T-cell development and homeostasis are severely compromised. One may then ask whether an upper limit exists as well, above which excessive signaling may lead to T-cell hyperproliferation and/or excessive activation. In line with this possibility, deregulation of the IL-7/IL-7R axis has been implicated in autoimmune diseases such as diabetes and multiple sclerosis (Lee et al., 2012; Mazzucchelli et al., 2012; Monti and Bonifacio, 2014), and chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease (Churchman and Ponchel, 2008; Krzystek-Korpacka et al., 2017; Nemoto et al., 2013). The link between chronic inflammation and cancer is well established and, in agreement, elevated IL-7 levels can be found not only in patients with Crohn's disease and ulcerative colitis but also in those with metastatic colorectal cancer (Krzystek-Korpacka et al., 2017). The potential contribution of IL-7/IL-7R-mediated signaling extends to other solid cancers, including breast, lung, melanoma, bladder and colon cancer (Al-Rawi et al., 2003, 2004; Boesch et al., 2018; Cosenza et al., 2002; Li et al., 2014; Park et al., 2014; Suzuki et al., 2013; Yang et al., 2014), and different lymphoid malignancies, ranging from Hodgkin's lymphoma to chronic lymphocytic leukemia (Adachi et al., 2015; Bachireddy et al., 2015; Brown et al., 2003; Cattaruzza et al., 2009; Digel et al., 1991; Foss et al., 1994; Frishman et al., 1993; Long et al., 1995; Sasson et al., 2010). The exact mechanisms by which IL-7/IL-7R may contribute to cancer progression remain poorly explored but may be multiple, including indirect effects on the immune system or on cells from the cancer microenvironment (such as endothelial cells or fibroblasts) and direct effects on cancer cells themselves, which in some cases appear to ectopically express the IL-7R.
4. The ugly… IL-7 and IL-7R in T-ALL
Whereas reports of participation of the IL-7/IL-7R signaling axis in other cancers are still limited and often restricted to mere associations or studies in vitro with cell lines, there is compelling evidence of its involvement in ALL. In the remainder of this review, we will highlight mainly the knowledge on T-ALL, for which the most convincing findings exist of the tumorigenic potential of the IL-7/IL-7R axis.
T-cell precursors undergoing malignant transformation are exposed to, and likely compete for, IL-7 in their microenvironment, since they are believed to originate from the thymus and subsequently home to the bone marrow. This means that IL-7 should have the potential to modulate leukemogenesis. In agreement, IL-7 transgenic mice display accelerated mortality due to T- and B-cell lymphoma development (Abraham et al., 2005; Osborne et al., 2010; Rich et al., 1993). However, this constitutes a peculiar model, in that IL-7 is ectopically expressed in lymphoid cells (rather than in stromal cells), thus generating an IL-7 autocrine loop that appears to be a rare event at least in human T-ALL. On the other hand, AKR/J mice, which naturally overexpress IL-7Rα, tend to spontaneously develop thymic T-cell lymphomas. Overexpression of IL-7Rα in thymocytes from AKR/J mice associates with increased survival and selective advantage in competitive transplantation assays (Laouar et al., 2004). This suggests that T-cell precursors that have higher levels of IL-7R, and somehow ‘break the rule’ (mentioned above) that IL-7 stimulation must lead to transcriptional downregulation of IL7R, will outcompete those with lower levels of the receptor, with the increased capacity to respond to IL-7 potentially degenerating into lymphoma development. These observations are in line with the knowledge that T-ALL cells transplanted into Rag2−/− IL2rg−/- mice lacking IL-7 develop disease significantly slower than those transplanted into Rag2−/− IL2rg−/- control mice (Silva et al., 2011a). Moreover, NOTCH1, one of the most commonly mutated genes in T-ALL, is known to transcriptionally upregulate IL7R (Weng et al., 2004), and IL-7Rα appears to be involved in Notch-mediated leukemia cell maintenance (Gonzalez-Garcia et al., 2009). Also, R98S mutation in ribosomal protein L10 (RPL10-R98S), which is found in 8% of pediatric T-ALL cases, promotes the expression of IL-7Rα and elements of downstream signaling, as well as IL-7R signaling pathway activation upon IL-7 stimulation (Girardi et al., 2018).
Malignant cells collected from human T-ALL patients at diagnosis frequently express IL-7Rα and IL-7 promotes the survival and proliferation of leukemia cells (Barata et al., 2001, 2004a; Digel et al., 1991; Eder et al., 1990; Karawajew et al., 2000; Scupoli et al., 2007; Silva et al., 2011b; Touw et al., 1990) in a majority of T-ALL cases (more than 70%), independently of their developmental stage (Barata et al., 2001, 2004a; Touw et al., 1990). Importantly, thymic epithelial cells and bone marrow stromal cells cultured in vitro also promote T-ALL cell survival by producing IL-7 (Scupoli et al., 2003, 2007)), and chimeric fetal thymus organ cultures provide evidence that IL-7 produced in the thymus contributes to T-ALL cell proliferation (Barata et al., 2006).
5. And the really ugly… IL-7R mutational activation in T-ALL
The most striking and direct evidence for the involvement of IL-7R signaling in T-ALL stems from the identification, in 2011, of somatic gain-of-function mutations in IL-7Rα, which lead to constitutive activation of the receptor promoting cell transformation in vitro and tumor formation in vivo (Bains et al., 2012; Porcu et al., 2012; Shochat et al., 2011; Zenatti et al., 2011; Zhang et al., 2012). The mutations are all located in exon 6, and the majority originates an unpaired cysteine in the extracellular juxtamembrane-transmembrane portion of IL-7Rα, leading to disulfide bond-dependent receptor homodimerization of two mutant α chains and constitutive, ligand-independent signaling. The few exceptions to this ‘rule’ insert other residues within the transmembrane region closer to the cytosolic domain, including a tryptophan residue, an SxxxG related motif, both of which have been implicated in facilitating homo- or heterodimer formation of other receptors (Ridder et al., 2005; Russ and Engelman, 2000), or other residues that can also lead to ligand-independent IL-7Rα homodimerization (Shochat et al., 2014). Finally, some non-cysteine mutations may not promote homodimerization but instead increase responsiveness to IL-7 (Cante-Barrett et al., 2016).
Importantly, it should be noted that gain-of-function mutations occur not only in IL-7Rα itself (although not, curiously, in γc) but also in downstream effectors of IL-7/IL-7R-mediated signaling, namely in elements of JAK/STAT, PI3K/Akt/mTOR and RAS/MEK/Erk pathways, which are found in 30–50% of T-ALL cases (Cante-Barrett et al., 2016; Li et al., 2016; Oliveira et al., 2017; Ribeiro et al., 2013). IL-7R pathway mutations were reported to be enriched in patients overexpressing HOXA and TLX (Cante-Barrett et al., 2016; Liu et al., 2017; Vicente et al., 2015; Zenatti et al., 2011) or with early T-cell precursor ALL (ETP-ALL) (Goossens et al., 2015; Zhang et al., 2012), and to negatively associate with the TAL-LMO subgroup (Zenatti et al., 2011). In line with the latter, TAL1 ChIP-seq data indicate that TAL1 may directly repress IL7R (Bornschein et al., 2018), and overexpression of TAL1 combined with Pten deletion (which commonly co-occur in T-ALL) in a mouse pro-T-cell ex vivo culture system led to the downregulation of the IL-7R/JAK/STAT signaling cascade, compensated by activation of Akt and expression of E2f and Myc gene clusters (Bornschein et al., 2018). Mutations in IL7R, JAK1 and JAK3 were also shown to associate with genetic lesions in WT1, PRC2 or PHF6 epigenetic regulators (Vicente et al., 2015). This notwithstanding, IL7R mutations may actually occur in all subtypes of T-ALL (Liu et al., 2017).
In vivo studies on the impact of mutant IL7R in hematopoietic precursors highlight its leukemogenic potential, although the effects observed depend on the differentiation stage at which the mutation is expressed (Barata, 2013; Treanor et al., 2014; Yokoyama et al., 2013). Yokoyama and colleagues found that ectopic expression of mutant IL-7Rα alone in primary hematopoietic stem and progenitor cells induces oligoclonal myeloproliferation. However, when the same mutation was expressed in combination with a constitutively active form of intracellular Notch1 (ICN1), it accelerated ICN1-induced T-ALL. Furthermore, forced expression of the IL7R mutant in common lymphoid progenitors caused mature B-cell acute leukemia/lymphoma that, curiously, did not really recapitulate the B-cell precursor phenotype that is observed in IL7R mutant B-ALL patients (Roberts et al., 2012; Shochat et al., 2011). The most unexpected observation, however, came with the expression of the mutant in T-cell precursors, which did not originate T-cell leukemia or lymphoproliferation. This is possibly due to more stringent mechanisms of IL7R regulation in T- than in B-cell precursors, and may suggest the need for longer latency periods in IL7R mutant-derived T-ALL because of the requirement of additional transforming events (Barata, 2013; Yokoyama et al., 2013). In line with this possibility, Treanor et al. showed that mutant IL7R overexpression in CD4/CD8 double-negative T-cell progenitors with inactivation of the Ink4/Arf tumor suppressor locus (from Arf−/− mice), induced a block in thymocyte development (at a stage that retains both myeloid and T-cell differentiation potential), and induced monoclonal leukemia that resembled human ETP-ALL (Treanor et al., 2014).
6. The good, the bad and the ugly dissected… peculiarities of IL-7/IL-7R-mediated signaling in normal T cell precursors and T-ALL cells
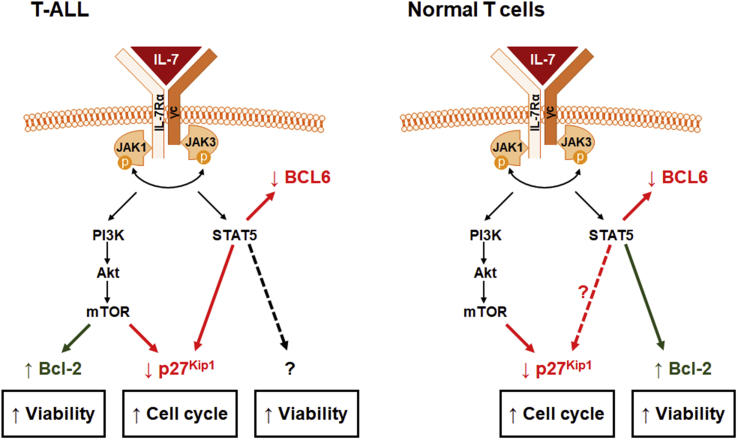
IL-7/IL-7R-mediated binding is initiated by binding of IL-7 to the receptor and the formation of a trimeric complex between the cytokine, IL-7Rα and γc (Gonnord et al., 2018; McElroy et al., 2012), leading to JAK1 and JAK3 activation, trans-phosphorylation and subsequent phosphorylation of tyrosine residues in the cytoplasmic tail of IL-7Rα that constitute docking sites for effector molecules such as PI3K and STAT5 (reviewed in (Kittipatarin and Khaled, 2007; Mazzucchelli and Durum, 2007; Ribeiro et al., 2013)). At least three main pathways are then activated: STAT, MEK/Erk and PI3K/Akt/mTOR in both T-ALL and normal T-cells (Barata et al., 2005; Jiang et al., 2005). However, the manner in which they appear to be wired is not identical in healthy and malignant cells. PI3K/Akt/mTOR signaling is essential for IL-7-mediated proliferation and survival of T-ALL cells by promoting p27kip1 downregulation and Bcl-2 upregulation (Barata et al., 2001, 2004b; Silva et al., 2011b). By contrast, in healthy T-cells, PI3K signaling activation by IL-7 induces cell cycle progression (Swainson et al., 2007) without impacting on cell survival or Bcl-2 expression (Rathmell et al., 2001). These differences should be highlighted, since they may constitute a therapeutic ‘window of opportunity’ in that PI3K/Akt/mTOR pathway inhibitors should preferentially and selectively eliminate IL-7-dependent leukemia cells while being merely cytostatic to their normal counterparts. Extending these observations to the JAK/STAT pathway, we have recently shown that STAT5 is required for increased T-ALL cell viability, growth and cell cycle progression induced by IL-7. However, contrary to what happens in normal T-cells, we found that IL-7-mediated upregulation of Bcl-2 in T-ALL is independent of STAT5 activity. Interestingly, we also observed that, in response to IL-7 stimulation, STAT5 directly downregulates BCL6 in T-ALL cells and promotes the expression of PIM1, which in turn plays a role in mediating the proliferative effects of IL-7 (Ribeiro et al., 2018). These results are in line with the fact that PIM1 expression levels are higher in cases with IL-7R pathway mutations and in the HOXA+ T-ALL patient subgroup, as well as with studies on mouse T-ALL cells indicating PIM1 as a JAK-STAT pathway target downstream from IL-7 (de Bock et al., 2018). In short, normal and malignant T-cells activate the same signaling pathways downstream from IL-7/IL-7R, but appear to utilize them to achieve a certain functional outcome in different ways (Fig. 1). More IL-7/IL-7R-mediated signaling peculiarities of normal versus malignant T-cell precursors can be found in our previous review on this topic (Ribeiro et al., 2013).
Fig. 1.
Differences in the functional impact of IL-7/IL-7R-mediated signaling in T-ALL cells and their normal counterparts. In T-ALL cells, Bcl-2 is upregulated by IL-7 via PI3K/Akt/mTOR pathway, which also regulates cell cycle progression. STAT5 is also required for IL-7-mediated cell cycle progression and viability. However, STAT5 does not upregulate Bcl-2 downstream from IL-7. By contrast, IL-7 recruits PI3K/Akt/mTOR pathway strictly for cell cycle progression in normal T-cells, whereas STAT5 appears to transcriptionally activate Bcl-2 and upregulate viability.
7. A promise… targeting IL-7R-mediated signaling in T-ALL for therapeutic purposes
Given the high frequency of T-ALL patients (around 70% of the cases) whose blasts express the IL-7R and respond to IL-7, on top of which around 10% display IL7R gain-of-function mutations, which associate with very high risk in relapsed patients (Richter-Pechanska et al., 2017), there is strong basis to try and therapeutically target the IL-7/IL-7R pathway in T-ALL. This can be done in multiple ways.
An obvious strategy is to use small molecules to inhibit specific downstream signaling elements. We and others have generated in vitro and in vivo preclinical data in this direction by showing that clinical-stage JAK small molecule inhibitors are able to kill IL7R-mutant (Senkevitch et al., 2018; Shochat et al., 2011; Zenatti et al., 2011) and IL-7-dependent (Barata et al., 2004b; Melao et al., 2016) cells. Moreover, studies with a limited number of ETP-ALL patient samples showed that they were highly sensitive to IL-7 stimulation in vitro and mouse xenograft models using those samples demonstrated that the JAK1/2 inhibitor ruxolitinib could delay disease progression in vivo (Maude et al., 2015). Anti-leukemia effects of ruxolitinib treatment in vivo had been previously reported, albeit with more heterogenous, less pronounced effects, using a murine IL-7R mutant-induced ETP-ALL model mentioned above (Treanor et al., 2014). Notably, it was recently shown that a subset of glucocorticoid-resistant T-ALL cells display strong induction of JAK/STAT signaling in response to IL-7 and that IL-7 removal or treatment with ruxolitinib sensitizes the leukemia cells to glucocorticoids (Delgado-Martin et al., 2017). These observations are in line with previous studies showing that cysteine mutations in IL7R, or mutations in downstream signaling elements (such as JAK1 and KRAS), confer glucocorticoid resistance and poor clinical outcome in childhood T-ALL, working as biomarkers of reduced steroid response (Li et al., 2016). Steroid resistance was partially explained by strong activation of RAS/MEK/Erk and/or PI3K/Akt signaling pathways, which resulted in reduction of steroid-induced cell death and powerful leukemia cell survival responses. Therefore, resistance could be possibly overcome by therapeutic combinations with MEK/Erk or PI3K/Akt/mTOR signaling-specific inhibitors (Cante-Barrett et al., 2016; Li et al., 2016), the latter having demonstrated clear potential against T-ALL in pre-clinical studies (Chiarini et al., 2010; Evangelisti et al., 2011; Grimaldi et al., 2012; Hall et al., 2016; Simioni et al., 2012).
A less obvious approach may involve targeting the hedgehog signaling pathway, whose activation occurs in around 22% of T-ALL cases, with a strong positive correlation with JAK3 levels (Dagklis et al., 2015, 2016; Vicente et al., 2015). The link to the IL-7/IL-7R axis stems essentially from the fact that activation of hedgehog pathway in the T-ALL cells stimulates thymic epithelial cells to produce T-cell stimulatory molecules, such as IL-7, to promote leukemia cell survival and proliferation. Primary T-ALL patient samples presenting high expression levels of the transcription factor GLI1, a downstream effector of hedgehog pathway, were shown to be sensitive to specific inhibitors of the pathway, such as GANT61 or vismodegib. Moreover, patient-derived xenograft mouse models confirmed the potential of those inhibitors to delay in vivo disease progression. The same inhibitors were also able to increase the sensitivity of T-ALL cells to conventional chemotherapy (Dagklis et al., 2016).
Also perhaps not evident from a superficial standpoint but potentially promising strategy may involve targeting the cell cycle checkpoint kinase 1 (CHK1), which, in an apparent paradox, is overexpressed in T- and B-ALL cells (Iacobucci et al., 2015; Sarmento and Barata, 2016; Sarmento et al., 2015). It so happens that CHK1 increased expression in ALL cells is essential for preventing apoptosis arising from the high replication stress levels within leukemia cells (Sarmento and Barata, 2016; Sarmento et al., 2015). As such, CHK1 and/or ATR inhibitors are particularly appealing therapeutic options for highly proliferative T-ALL cases, including those that are responsive to IL-7 stimulation. In fact, we have shown that PF-004777736, a clinical-grade CHK1 inhibitor, efficiently promotes T-ALL cell death even in the presence of IL-7 (Sarmento et al., 2015).
Another potential target is the serine/threonine protein kinase CK2, which is a posttranslational activator of constitutive PI3K/Akt signaling in T-ALL cells by inhibiting PTEN (Silva et al., 2008). CK2 was shown to play a key role in promoting the proliferation of ALL cells of both B and T cell origin (Gomes et al., 2014; Gowda et al., 2017a, 2017b; Silva et al., 2008) and was recently shown to be required for optimal IL-7-mediated signaling, and consequent IL-7-dependent T-ALL cell cycle progression and viability (Melao et al., 2016). CK2 was also required for the viability of mutant IL-7R-expressing leukemia T-cells. Of potential clinical interest, CX-4945 (silmitasertib), a clinical-grade CK2-specific pharmacological inhibitor, synergized with ruxolitinib in promoting the death of both IL-7-dependent and mutant IL7R-expressing T-ALL cells (Melao et al., 2016).
Because IL-7/IL-7R-triggered signaling leads to the upregulation of Bcl-2, which is required for IL-7-mediated T-ALL cell survival (Barata et al., 2001), the use of Bcl-2 pharmacological inhibitors, whose preclinical efficacy has already been shown, constitutes also an appealing strategy to obviate leukemia expansion. In this regard, it is interesting to mention that JAK3 mutant T-ALL cells, whose phosphoproteomic profiling showed regulation of PI3K/Akt, RAS/MAPK and apoptotic signaling pathways downstream of the mutation, displayed increased sensitivity, both in vitro and in vivo, to combined treatment with the JAK1/3 inhibitor tofacitinib plus the Bcl-2 inhibitor venetoclax than to each inhibitor alone (Degryse et al., 2018), and that mutant IL7R-expressing mouse T-cells showed similar results for ruxolitinib and venetoclax (Senkevitch et al., 2018). Notably, the efficacy of venetoclax as a single agent has been demonstrated in immature T-ALL patients presenting high levels of Bcl-2 (Peirs et al., 2014). Still in the context of JAK3 mutant T-ALL cells, having present that PIM1 is a downstream target of the JAK/STAT pathway and that the majority of JAK3 mutants are strongly dependent on JAK1 to transform cells (Degryse et al., 2014), a recent study highlighted the therapeutic benefit of targeting both JAK1 and PIM1 with ruxolitinib and AZD1208, respectively (de Bock et al., 2018).
Targeting the IL-7R itself is another valid therapeutic option. Since most T-ALL-related IL7R mutations lead to constitutive signaling via aberrant disulfide bond formation between the IL-7Rα-mutated chains, antioxidants may also represent an interesting therapeutic strategy. The reducing agent N-acetylcysteine (NAC), although not selective, has been used to disrupt IL-7Rα homodimerization, inhibiting downstream signaling and thus promoting apoptosis of IL-7Rα mutant cells in vitro, and decreasing leukemia progression in vivo (Mansour et al., 2015).
In alternative, other ways of targeting IL-7R-expressing T-ALL cells may rely on the administration of chimeric antigen receptors (CAR T-cells) against IL-7Rα or the use of anti- IL-7Rα antibodies (Cramer et al., 2016). While there are no reports as yet on the former, the latter strategy has led already to promising results. It has been very recently shown that high IL-7Rα expression directly correlated with central nervous system involvement in B-ALL patients. The authors then used a commercially available monoclonal anti-IL7Rα antibody to abrogate leukemia development in xenografted mice, which outperformed ruxolitinib (Alsadeq et al., 2018). In another study, glucocorticoid resistance in T-ALL was tackled by using a murine anti-IL-7Rα antibody conjugated to a cytotoxic agent. Although this antibody is clinically inconsequential, results highlight the potential of targeting IL-7R using monoclonal antibodies (Yasunaga et al., 2017). Following similar lines, we have generated a fully human antibody against IL-7Rα using phage display with the ability to inhibit IL-7/IL-7R-dependent signaling and anti-tumor potential against IL-7R-expressing T-ALL cells both in vitro and in vivo (Akkapeddi et al., unpublished data).
8. The not so bad… IL-7 and IL-7R as tumor suppressors?
The studies we highlighted above indicate that over-activation of the IL-7/IL-7R signaling axis can contribute to leukemia/lymphoma establishment and progression; however, there is also some evidence pointing out to the contrary. For example, restraining IL-7R-mediated signaling can accelerate T-cell lymphomagenesis in the context of p53 deficiency (Kibe et al., 2012), due to the ability of the IL-7/IL-7Rα axis to maintain telomere integrity via POT1 expression during T-cell development. Il7r −/− p53 −/− mice displayed a marked reduction of apoptosis in T-cell precursors and increased thymic lymphomagenesis, with telomere erosions and exacerbated chromosomal abnormalities, including chromosome duplications, breaks, and translocations. IL-7/IL-7Rα signaling withdrawal led to telomere erosion and activation of the p53 pathway (Kibe et al., 2012). Obviously, in the absence of p53 this did not drive apoptosis and thus likely potentiated the formation of T-cell tumors via genomic instability. Another example of potential (although not formally tested) anti-tumor effects of IL-7-mediated signaling come from the demonstration that IL-7R-mediated signaling in pre-B cells serves as a safeguard against premature activation of AID, preventing concomitant activation of AID and RAGs - which in turn are required for B-ALL development (Swaminathan et al., 2015). This feature may, however, be counterbalanced by the fact that IL-7 contributes to the survival and proliferation of B-cell precursors (Corfe and Paige, 2012), which may also justify why IL7R mutations have been identified in high-risk B-ALL cases (Roberts et al., 2012, 2014), and the IL-7/IL-7R signaling axis has been shown to contribute to the proliferation and/or survival of B-ALL cells, to steroid resistance and poor outcome in B-ALL (Brown et al., 2003; Li et al., 2016; Morishita et al., 2012; Mullighan, 2013; Roberts and Mullighan, 2015; Touw et al., 1990; van der Plas et al., 1996), and, as mentioned above, to associate with central nervous system infiltration and relapse (Alsadeq et al., 2018).
Conflicts of interest
The authors have no conflict of interest to declare. Funding agencies, mentioned in the Acknowledgements section, did not have any influence on the planning, execution or writing of the manuscript.
Acknowledgements
Publication costs were supported by LISBOA-01-0145-FEDER-007391, project cofunded by FEDER, through POR Lisboa2020 Programa Operacional Regional de Lisboa, PORTUGAL 2020, and Fundação para a Ciência e a Tecnologia (FCT, Portugal). The research work in JTB's lab related to the present review was supported by the grants FAPESP/20015/2014 and PTDC/MEC-HEM/31588/2017, from FCT; and by the consolidator grant ERC CoG-648455 from the European Research Council, under the European Union's Horizon 2020 research and innovation programme. JTB is an FCT investigator (consolidator). MLO is a LisbonBioMed PhD student and received a fellowship from FCT. PA received a PhD fellowship from the EU Marie Sklodowska-Curie ITN Protein Conjugates.
References
- Abraham N., Ma M.C., Snow J.W., Miners M.J., Herndier B.G., Goldsmith M.A. Haploinsufficiency identifies STAT5 as a modifier of IL-7-induced lymphomas. Oncogene. 2005;24(33):5252–5257. doi: 10.1038/sj.onc.1208726. [DOI] [PubMed] [Google Scholar]
- Adachi K., Kano Y., Nagai T., Okuyama N., Sakoda Y., Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 2018;36(4):346–351. doi: 10.1038/nbt.4086. [DOI] [PubMed] [Google Scholar]
- Adachi T., Kobayashi T., Sugihara E., Yamada T., Ikuta K., Pittaluga S., Saya H., Amagai M., Nagao K. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015;21(11):1272–1279. doi: 10.1038/nm.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rawi M.A., Mansel R.E., Jiang W.G. Interleukin-7 (IL-7) and IL-7 receptor (IL-7R) signalling complex in human solid tumours. Histol. Histopathol. 2003;18(3):911–923. doi: 10.14670/HH-18.911. [DOI] [PubMed] [Google Scholar]
- Al-Rawi M.A., Rmali K., Watkins G., Mansel R.E., Jiang W.G. Aberrant expression of interleukin-7 (IL-7) and its signalling complex in human breast cancer. Eur. J. Canc. 2004;40(4):494–502. doi: 10.1016/j.ejca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Alsadeq A., Lenk L., Vadakumchery A., Cousins A., Vokuhl C., Khadour A., Vogiatzi F., Seyfried F., Meyer L.H., Cario G., Hobeika E., Debatin K.M., Halsey C., Schrappe M., Schewe D.M., Jumaa H. IL7R is associated with CNS infiltration and relapse in pediatric B-cell precursor acute lymphoblastic leukemia. Blood. 2018 Aug 28 doi: 10.1182/blood-2018-04-844209. pii: blood-2018-04-844209. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo R.I., Soares M.V., Barata J.T., Tendeiro R., Serra-Caetano A., Victorino R.M., Sousa A.E. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood. 2009;113(13):2999–3007. doi: 10.1182/blood-2008-07-166223. [DOI] [PubMed] [Google Scholar]
- Bachireddy P., Burkhardt U.E., Rajasagi M., Wu C.J. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat. Rev. Canc. 2015;15(4):201–215. doi: 10.1038/nrc3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains T., Heinrich M.C., Loriaux M.M., Beadling C., Nelson D., Warrick A., Neff T.L., Tyner J.W., Dunlap J., Corless C.L., Fan G. Newly described activating JAK3 mutations in T-cell acute lymphoblastic leukemia. Leukemia. 2012;26(9):2144–2146. doi: 10.1038/leu.2012.74. [DOI] [PubMed] [Google Scholar]
- Barata J.T. IL-7Ralpha: Mr Hyde's twists and turns. Blood. 2013;122(26):4151–4152. doi: 10.1182/blood-2013-11-536987. [DOI] [PubMed] [Google Scholar]
- Barata J.T., Cardoso A.A., Boussiotis V.A. Interleukin-7 in T-cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk. Lymphoma. 2005;46(4):483–495. doi: 10.1080/10428190400027852. [DOI] [PubMed] [Google Scholar]
- Barata J.T., Cardoso A.A., Nadler L.M., Boussiotis V.A. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1) Blood. 2001;98(5):1524–1531. doi: 10.1182/blood.v98.5.1524. [DOI] [PubMed] [Google Scholar]
- Barata J.T., Keenan T.D., Silva A., Nadler L.M., Boussiotis V.A., Cardoso A.A. Common gamma chain-signaling cytokines promote proliferation of T-cell acute lymphoblastic leukemia. Haematologica. 2004;89(12):1459–1467. [PubMed] [Google Scholar]
- Barata J.T., Silva A., Abecasis M., Carlesso N., Cumano A., Cardoso A.A. Molecular and functional evidence for activity of murine IL-7 on human lymphocytes. Exp. Hematol. 2006;34(9):1133–1142. doi: 10.1016/j.exphem.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Barata J.T., Silva A., Brandao J.G., Nadler L.M., Cardoso A.A., Boussiotis V.A. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 2004;200(5):659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch M., Onder L., Cheng H.W., Novkovic M., Morbe U., Sopper S., Gastl G., Jochum W., Ruhstaller T., Knauer M., Ludewig B. Interleukin 7-expressing fibroblasts promote breast cancer growth through sustenance of tumor cell stemness. OncoImmunology. 2018;7(4) doi: 10.1080/2162402X.2017.1414129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornschein S., Demeyer S., Stirparo R., Gielen O., Vicente C., Geerdens E., Ghesquiere B., Aerts S., Cools J., de Bock C.E. Defining the molecular basis of oncogenic cooperation between TAL1 expression and Pten deletion in T-ALL using a novel pro-T-cell model system. Leukemia. 2018;32(4):941–951. doi: 10.1038/leu.2017.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O., Ramsey C., Kim D.M., Sprent J., Surh C.D. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J. Immunol. 2008;180(11):7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- Brown V.I., Fang J., Alcorn K., Barr R., Kim J.M., Wasserman R., Grupp S.A. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proc. Natl. Acad. Sci. U. S. A. 2003;100(25):15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cante-Barrett K., Spijkers-Hagelstein J.A., Buijs-Gladdines J.G., Uitdehaag J.C., Smits W.K., van der Zwet J., Buijsman R.C., Zaman G.J., Pieters R., Meijerink J.P. MEK and PI3K-AKT inhibitors synergistically block activated IL7 receptor signaling in T-cell acute lymphoblastic leukemia. Leukemia. 2016;30(9):1832–1843. doi: 10.1038/leu.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza L., Gloghini A., Olivo K., Di Francia R., Lorenzon D., De Filippi R., Carbone A., Colombatti A., Pinto A., Aldinucci D. Functional coexpression of Interleukin (IL)-7 and its receptor (IL-7R) on Hodgkin and Reed-Sternberg cells: involvement of IL-7 in tumor cell growth and microenvironmental interactions of Hodgkin's lymphoma. Int. J. Canc. 2009;125(5):1092–1101. doi: 10.1002/ijc.24389. [DOI] [PubMed] [Google Scholar]
- Chiarini F., Grimaldi C., Ricci F., Tazzari P.L., Evangelisti C., Ognibene A., Battistelli M., Falcieri E., Melchionda F., Pession A., Pagliaro P., McCubrey J.A., Martelli A.M. Activity of the novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 against T-cell acute lymphoblastic leukemia. Canc. Res. 2010;70(20):8097–8107. doi: 10.1158/0008-5472.CAN-10-1814. [DOI] [PubMed] [Google Scholar]
- Churchman S.M., Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology. 2008;47(6):753–759. doi: 10.1093/rheumatology/ken053. [DOI] [PubMed] [Google Scholar]
- Corfe S.A., Paige C.J. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin. Immunol. 2012;24(3):198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Cosenza L., Gorgun G., Urbano A., Foss F. Interleukin-7 receptor expression and activation in nonhaematopoietic neoplastic cell lines. Cell. Signal. 2002;14(4):317–325. doi: 10.1016/s0898-6568(01)00245-5. [DOI] [PubMed] [Google Scholar]
- Cramer S.D., Aplan P.D., Durum S.K. Therapeutic targeting of IL-7Ralpha signaling pathways in ALL treatment. Blood. 2016;128(4):473–478. doi: 10.1182/blood-2016-03-679209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagklis A., Demeyer S., De Bie J., Radaelli E., Pauwels D., Degryse S., Gielen O., Vicente C., Vandepoel R., Geerdens E., Uyttebroeck A., Boeckx N., de Bock C.E., Cools J. Hedgehog pathway activation in T-cell acute lymphoblastic leukemia predicts response to SMO and GLI1 inhibitors. Blood. 2016;128(23):2642–2654. doi: 10.1182/blood-2016-03-703454. [DOI] [PubMed] [Google Scholar]
- Dagklis A., Pauwels D., Lahortiga I., Geerdens E., Bittoun E., Cauwelier B., Tousseyn T., Uyttebroeck A., Maertens J., Verhoef G., Vandenberghe P., Cools J. Hedgehog pathway mutations in T-cell acute lymphoblastic leukemia. Haematologica. 2015;100(3):e102–105. doi: 10.3324/haematol.2014.119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bock C.E., Demeyer S., Degryse S., Verbeke D., Sweron B., Gielen O., Vandepoel R., Vicente C., Vanden Bempt M., Dagklis A., Geerdens E., Bornschein S., Gijsbers R., Soulier J., Meijerink J.P., Heinaniemi M., Teppo S., Bouvy-Liivrand M., Lohi O., Radaelli E., Cools J. HOXA9 cooperates with activated JAK/STAT signaling to drive leukemia development. Canc. Discov. 2018;8(5):616–631. doi: 10.1158/2159-8290.CD-17-0583. [DOI] [PubMed] [Google Scholar]
- Degryse S., de Bock C.E., Cox L., Demeyer S., Gielen O., Mentens N., Jacobs K., Geerdens E., Gianfelici V., Hulselmans G., Fiers M., Aerts S., Meijerink J.P., Tousseyn T., Cools J. JAK3 mutants transform hematopoietic cells through JAK1 activation, causing T-cell acute lymphoblastic leukemia in a mouse model. Blood. 2014;124(20):3092–3100. doi: 10.1182/blood-2014-04-566687. [DOI] [PubMed] [Google Scholar]
- Degryse S., de Bock C.E., Demeyer S., Govaerts I., Bornschein S., Verbeke D., Jacobs K., Binos S., Skerrett-Byrne D.A., Murray H.C., Verrills N.M., Van Vlierberghe P., Cools J., Dun M.D. Mutant JAK3 phosphoproteomic profiling predicts synergism between JAK3 inhibitors and MEK/BCL2 inhibitors for the treatment of T-cell acute lymphoblastic leukemia. Leukemia. 2018;32(3):788–800. doi: 10.1038/leu.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Martin C., Meyer L.K., Huang B.J., Shimano K.A., Zinter M.S., Nguyen J.V., Smith G.A., Taunton J., Winter S.S., Roderick J.R., Kelliher M.A., Horton T.M., Wood B.L., Teachey D.T., Hermiston M.L. JAK/STAT pathway inhibition overcomes IL7-induced glucocorticoid resistance in a subset of human T-cell acute lymphoblastic leukemias. Leukemia. 2017;31(12):2568–2576. doi: 10.1038/leu.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digel W., Schmid M., Heil G., Conrad P., Gillis S., Porzsolt F. Human interleukin-7 induces proliferation of neoplastic cells from chronic lymphocytic leukemia and acute leukemias. Blood. 1991;78(3):753–759. [PubMed] [Google Scholar]
- Eder M., Ottmann O.G., Hansen-Hagge T.E., Bartram C.R., Gillis S., Hoelzer D., Ganser A. Effects of recombinant human IL-7 on blast cell proliferation in acute lymphoblastic leukemia. Leukemia. 1990;4(8):533–540. [PubMed] [Google Scholar]
- Evangelisti C., Ricci F., Tazzari P., Chiarini F., Battistelli M., Falcieri E., Ognibene A., Pagliaro P., Cocco L., McCubrey J.A., Martelli A.M. Preclinical testing of the Akt inhibitor triciribine in T-cell acute lymphoblastic leukemia. J. Cell. Physiol. 2011;226(3):822–831. doi: 10.1002/jcp.22407. [DOI] [PubMed] [Google Scholar]
- Foss F.M., Koc Y., Stetler-Stevenson M.A., Nguyen D.T., O'Brien M.C., Turner R., Sausville E.A. Costimulation of cutaneous T-cell lymphoma cells by interleukin-7 and interleukin-2: potential autocrine or paracrine effectors in the Sezary syndrome. J. Clin. Oncol. 1994;12(2):326–335. doi: 10.1200/JCO.1994.12.2.326. [DOI] [PubMed] [Google Scholar]
- Frishman J., Long B., Knospe W., Gregory S., Plate J. Genes for interleukin 7 are transcribed in leukemic cell subsets of individuals with chronic lymphocytic leukemia. J. Exp. Med. 1993;177(4):955–964. doi: 10.1084/jem.177.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry T.J., Moniuszko M., Creekmore S., Donohue S.J., Douek D.C., Giardina S., Hecht T.T., Hill B.J., Komschlies K., Tomaszewski J., Franchini G., Mackall C.L. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101(6):2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- Girardi T., Vereecke S., Sulima S.O., Khan Y., Fancello L., Briggs J.W., Schwab C., de Beeck J.O., Verbeeck J., Royaert J., Geerdens E., Vicente C., Bornschein S., Harrison C.J., Meijerink J.P., Cools J., Dinman J.D., Kampen K.R., De Keersmaecker K. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling. Leukemia. 2018;32(3):809–819. doi: 10.1038/leu.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A.M., Soares M.V., Ribeiro P., Caldas J., Povoa V., Martins L.R., Melao A., Serra-Caetano A., de Sousa A.B., Lacerda J.F., Barata J.T. Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels. Haematologica. 2014;99(6):1062–1068. doi: 10.3324/haematol.2013.096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnord P., Angermann B.R., Sadtler K., Gombos E., Chappert P., Meier-Schellersheim M., Varma R. A hierarchy of affinities between cytokine receptors and the common gamma chain leads to pathway cross-talk. Sci. Signal. 2018;11(524) doi: 10.1126/scisignal.aal1253. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia S., Garcia-Peydro M., Martin-Gayo E., Ballestar E., Esteller M., Bornstein R., de la Pompa J.L., Ferrando A.A., Toribio M.L. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R{alpha} gene expression in early human thymopoiesis and leukemia. J. Exp. Med. 2009;206(4):779–791. doi: 10.1084/jem.20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens S., Radaelli E., Blanchet O., Durinck K., Van der Meulen J., Peirs S., Taghon T., Tremblay C.S., Costa M., Farhang Ghahremani M., De Medts J., Bartunkova S., Haigh K., Schwab C., Farla N., Pieters T., Matthijssens F., Van Roy N., Best J.A., Deswarte K., Bogaert P., Carmichael C., Rickard A., Suryani S., Bracken L.S., Alserihi R., Cante-Barrett K., Haenebalcke L., Clappier E., Rondou P., Slowicka K., Huylebroeck D., Goldrath A.W., Janzen V., McCormack M.P., Lock R.B., Curtis D.J., Harrison C., Berx G., Speleman F., Meijerink J.P., Soulier J., Van Vlierberghe P., Haigh J.J. ZEB2 drives immature T-cell lymphoblastic leukaemia development via enhanced tumour-initiating potential and IL-7 receptor signalling. Nat. Commun. 2015;6:5794. doi: 10.1038/ncomms6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda C., Soliman M., Kapadia M., Ding Y., Payne K., Dovat S. Casein kinase II (CK2), glycogen synthase Kinase-3 (GSK-3) and ikaros mediated regulation of leukemia. Adv Biol Regul. 2017;65:16–25. doi: 10.1016/j.jbior.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda C., Song C., Kapadia M., Payne J.L., Hu T., Ding Y., Dovat S. Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros. Adv Biol Regul. 2017;63:71–80. doi: 10.1016/j.jbior.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi C., Chiarini F., Tabellini G., Ricci F., Tazzari P.L., Battistelli M., Falcieri E., Bortul R., Melchionda F., Iacobucci I., Pagliaro P., Martinelli G., Pession A., Barata J.T., McCubrey J.A., Martelli A.M. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: therapeutic implications. Leukemia. 2012;26(1):91–100. doi: 10.1038/leu.2011.269. [DOI] [PubMed] [Google Scholar]
- Hall C.P., Reynolds C.P., Kang M.H. Modulation of glucocorticoid resistance in pediatric T-cell acute lymphoblastic leukemia by increasing BIM expression with the PI3K/mTOR inhibitor BEZ235. Clin. Canc. Res. 2016;22(3):621–632. doi: 10.1158/1078-0432.CCR-15-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques C.M., Rino J., Nibbs R.J., Graham G.J., Barata J.T. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood. 2010;115(16):3269–3277. doi: 10.1182/blood-2009-10-246876. [DOI] [PubMed] [Google Scholar]
- Iacobucci I., Di Rora A.G., Falzacappa M.V., Agostinelli C., Derenzini E., Ferrari A., Papayannidis C., Lonetti A., Righi S., Imbrogno E., Pomella S., Venturi C., Guadagnuolo V., Cattina F., Ottaviani E., Abbenante M.C., Vitale A., Elia L., Russo D., Zinzani P.L., Pileri S., Pelicci P.G., Martinelli G. In vitro and in vivo single-agent efficacy of checkpoint kinase inhibition in acute lymphoblastic leukemia. J. Hematol. Oncol. 2015;8:125. doi: 10.1186/s13045-015-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Li W.Q., Aiello F.B., Mazzucchelli R., Asefa B., Khaled A.R., Durum S.K. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16(4–5):513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Karawajew L., Ruppert V., Wuchter C., Kosser A., Schrappe M., Dorken B., Ludwig W.D. Inhibition of in vitro spontaneous apoptosis by IL-7 correlates with bcl-2 up-regulation, cortical/mature immunophenotype, and better early cytoreduction of childhood T-cell acute lymphoblastic leukemia. Blood. 2000;96(1):297–306. [PubMed] [Google Scholar]
- Kibe R., Zhang S., Guo D., Marrero L., Tsien F., Rodriguez P., Khan S., Zieske A., Huang J., Li W., Durum S.K., Iwakuma T., Cui Y. IL-7Ralpha deficiency in p53null mice exacerbates thymocyte telomere erosion and lymphomagenesis. Cell Death Differ. 2012;19(7):1139–1151. doi: 10.1038/cdd.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittipatarin C., Khaled A.R. Interlinking interleukin-7. Cytokine. 2007;39(1):75–83. doi: 10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystek-Korpacka M., Zawadzki M., Neubauer K., Bednarz-Misa I., Gorska S., Wisniewski J., Witkiewicz W., Gamian A. Elevated systemic interleukin-7 in patients with colorectal cancer and individuals at high risk of cancer: association with lymph node involvement and tumor location in the right colon. Cancer Immunol. Immunother. 2017;66(2):171–179. doi: 10.1007/s00262-016-1933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y., Crispe I.N., Flavell R.A. Overexpression of IL-7R alpha provides a competitive advantage during early T-cell development. Blood. 2004;103(6):1985–1994. doi: 10.1182/blood-2003-06-2126. [DOI] [PubMed] [Google Scholar]
- Lee L.F., Logronio K., Tu G.H., Zhai W., Ni I., Mei L., Dilley J., Yu J., Rajpal A., Brown C., Appah C., Chin S.M., Han B., Affolter T., Lin J.C. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc. Natl. Acad. Sci. U. S. A. 2012;109(31):12674–12679. doi: 10.1073/pnas.1203795109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz D.C., Kurz S.K., Lemmens E., Schoenberger S.P., Sprent J., Oldstone M.B., Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc. Natl. Acad. Sci. U. S. A. 2004;101(25):9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liu J., Mao X., Tang Q., Lu H. IL-7 receptor blockade inhibits IL-17-producing gammadelta cells and suppresses melanoma development. Inflammation. 2014;37(5):1444–1452. doi: 10.1007/s10753-014-9869-2. [DOI] [PubMed] [Google Scholar]
- Li Y., Buijs-Gladdines J.G., Cante-Barrett K., Stubbs A.P., Vroegindeweij E.M., Smits W.K., van Marion R., Dinjens W.N., Horstmann M., Kuiper R.P., Buijsman R.C., Zaman G.J., van der Spek P.J., Pieters R., Meijerink J.P. IL-7 receptor mutations and steroid resistance in pediatric T cell acute lymphoblastic leukemia: a genome sequencing study. PLoS Med. 2016;13(12) doi: 10.1371/journal.pmed.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Easton J., Shao Y., Maciaszek J., Wang Z., Wilkinson M.R., McCastlain K., Edmonson M., Pounds S.B., Shi L., Zhou X., Ma X., Sioson E., Li Y., Rusch M., Gupta P., Pei D., Cheng C., Smith M.A., Auvil J.G., Gerhard D.S., Relling M.V., Winick N.J., Carroll A.J., Heerema N.A., Raetz E., Devidas M., Willman C.L., Harvey R.C., Carroll W.L., Dunsmore K.P., Winter S.S., Wood B.L., Sorrentino B.P., Downing J.R., Loh M.L., Hunger S.P., Zhang J., Mullighan C.G. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat. Genet. 2017;49(8):1211–1218. doi: 10.1038/ng.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B.W., Witte P.L., Abraham G.N., Gregory S.A., Plate J.M. Apoptosis and interleukin 7 gene expression in chronic B-lymphocytic leukemia cells. Proc. Natl. Acad. Sci. U. S. A. 1995;92(5):1416–1420. doi: 10.1073/pnas.92.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall C.L., Fry T.J., Gress R.E. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011;11(5):330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M.R., Reed C., Eisenberg A.R., Tseng J.C., Twizere J.C., Daakour S., Yoda A., Rodig S.J., Tal N., Shochat C., Berezovskaya A., DeAngelo D.J., Sallan S.E., Weinstock D.M., Izraeli S., Kung A.L., Kentsis A., Look A.T. Targeting oncogenic interleukin-7 receptor signalling with N-acetylcysteine in T cell acute lymphoblastic leukaemia. Br. J. Haematol. 2015;168(2):230–238. doi: 10.1111/bjh.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S.L., Dolai S., Delgado-Martin C., Vincent T., Robbins A., Selvanathan A., Ryan T., Hall J., Wood A.C., Tasian S.K., Hunger S.P., Loh M.L., Mullighan C.G., Wood B.L., Hermiston M.L., Grupp S.A., Lock R.B., Teachey D.T. Efficacy of JAK/STAT pathway inhibition in murine xenograft models of early T-cell precursor (ETP) acute lymphoblastic leukemia. Blood. 2015;125(11):1759–1767. doi: 10.1182/blood-2014-06-580480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli R., Durum S.K. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 2007;7(2):144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli R.I., Riva A., Durum S.K. The human IL-7 receptor gene: deletions, polymorphisms and mutations. Semin. Immunol. 2012;24(3):225–230. doi: 10.1016/j.smim.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy C.A., Holland P.J., Zhao P., Lim J.M., Wells L., Eisenstein E., Walsh S.T. Structural reorganization of the interleukin-7 signaling complex. Proc. Natl. Acad. Sci. U. S. A. 2012;109(7):2503–2508. doi: 10.1073/pnas.1116582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melao A., Spit M., Cardoso B.A., Barata J.T. Optimal interleukin-7 receptor-mediated signaling, cell cycle progression and viability of T-cell acute lymphoblastic leukemia cells rely on casein kinase 2 activity. Haematologica. 2016;101(11):1368–1379. doi: 10.3324/haematol.2015.141143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti P., Bonifacio E. Interleukin-7 and type 1 diabetes. Curr. Diabetes Rep. 2014;14(9):518. doi: 10.1007/s11892-014-0518-9. [DOI] [PubMed] [Google Scholar]
- Morishita N., Tsukahara H., Chayama K., Ishida T., Washio K., Miyamura T., Yamashita N., Oda M., Morishima T. Activation of Akt is associated with poor prognosis and chemotherapeutic resistance in pediatric B-precursor acute lymphoblastic leukemia. Pediatr. Blood Canc. 2012;59(1):83–89. doi: 10.1002/pbc.24034. [DOI] [PubMed] [Google Scholar]
- Mullighan C.G. Genome sequencing of lymphoid malignancies. Blood. 2013;122(24):3899–3907. doi: 10.1182/blood-2013-08-460311. [DOI] [PubMed] [Google Scholar]
- Namen A.E., Lupton S., Hjerrild K., Wignall J., Mochizuki D.Y., Schmierer A., Mosley B., March C.J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Nemoto Y., Kanai T., Takahara M., Oshima S., Nakamura T., Okamoto R., Tsuchiya K., Watanabe M. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut. 2013;62(8):1142–1152. doi: 10.1136/gutjnl-2012-302029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M., Yi H., Rosenblatt H.M., Filipovich A.H., Adelstein S., Modi W.S., McBride O.W., Leonard W.J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73(1):147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- Oliveira M.L., Akkapeddi P., Alcobia I., Almeida A.R., Cardoso B.A., Fragoso R., Serafim T.L., Barata J.T. From the outside, from within: biological and therapeutic relevance of signal transduction in T-cell acute lymphoblastic leukemia. Cell. Signal. 2017;38:10–25. doi: 10.1016/j.cellsig.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Osborne L.C., Duthie K.A., Seo J.H., Gascoyne R.D., Abraham N. Selective ablation of the YxxM motif of IL-7Ralpha suppresses lymphomagenesis but maintains lymphocyte development. Oncogene. 2010;29(26):3854–3864. doi: 10.1038/onc.2010.133. [DOI] [PubMed] [Google Scholar]
- Park J.H., Yu Q., Erman B., Appelbaum J.S., Montoya-Durango D., Grimes H.L., Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Park S.L., Lee E.J., Kim W.J., Moon S.K. p27KIP1 is involved in ERK1/2-mediated MMP-9 expression via the activation of NF-kappaB binding in the IL-7-induced migration and invasion of 5637 cells. Int. J. Oncol. 2014;44(4):1349–1356. doi: 10.3892/ijo.2014.2290. [DOI] [PubMed] [Google Scholar]
- Peirs S., Matthijssens F., Goossens S., Van de Walle I., Ruggero K., de Bock C.E., Degryse S., Cante-Barrett K., Briot D., Clappier E., Lammens T., De Moerloose B., Benoit Y., Poppe B., Meijerink J.P., Cools J., Soulier J., Rabbitts T.H., Taghon T., Speleman F., Van Vlierberghe P. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Calzascia T., Toe J.G., Preston S.P., Lin A.E., Elford A.R., Shahinian A., Lang P.A., Lang K.S., Morre M., Assouline B., Lahl K., Sparwasser T., Tedder T.F., Paik J.H., DePinho R.A., Basta S., Ohashi P.S., Mak T.W. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144(4):601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Peschon J.J., Morrissey P.J., Grabstein K.H., Ramsdell F.J., Maraskovsky E., Gliniak B.C., Park L.S., Ziegler S.F., Williams D.E., Ware C.B., Meyer J.D., Davison B.L. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180(5):1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu M., Kleppe M., Gianfelici V., Geerdens E., De Keersmaecker K., Tartaglia M., Foa R., Soulier J., Cauwelier B., Uyttebroeck A., Macintyre E., Vandenberghe P., Asnafi V., Cools J. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood. 2012;119(19):4476–4479. doi: 10.1182/blood-2011-09-379958. [DOI] [PubMed] [Google Scholar]
- Prlic M., Lefrancois L., Jameson S.C. Multiple choices: regulation of memory CD8 T cell generation and homeostasis by interleukin (IL)-7 and IL-15. J. Exp. Med. 2002;195(12):F49–F52. doi: 10.1084/jem.20020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A., Ziegler S.F., Buckley R.H., Leonard W.J. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 1998;20(4):394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- Rathmell J.C., Farkash E.A., Gao W., Thompson C.B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 2001;167(12):6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Ribeiro D., Melao A., Barata J.T. IL-7R-mediated signaling in T-cell acute lymphoblastic leukemia. Adv Biol Regul. 2013;53(2):211–222. doi: 10.1016/j.jbior.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro D., Melao A., van Boxtel R., Santos C.I., Silva A., Silva M.C., Cardoso B.A., Coffer P.J., Barata J.T. STAT5 is essential for IL-7-mediated viability, growth, and proliferation of T-cell acute lymphoblastic leukemia cells. Blood Adv. 2018;2(17):2199–2213. doi: 10.1182/bloodadvances.2018021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B.E., Campos-Torres J., Tepper R.I., Moreadith R.W., Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J. Exp. Med. 1993;177(2):305–316. doi: 10.1084/jem.177.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Pechanska P., Kunz J.B., Hof J., Zimmermann M., Rausch T., Bandapalli O.R., Orlova E., Scapinello G., Sagi J.C., Stanulla M., Schrappe M., Cario G., Kirschner-Schwabe R., Eckert C., Benes V., Korbel J.O., Muckenthaler M.U., Kulozik A.E. Identification of a genetically defined ultra-high-risk group in relapsed pediatric T-lymphoblastic leukemia. Blood Canc. J. 2017;7(2):e523. doi: 10.1038/bcj.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder A., Skupjen P., Unterreitmeier S., Langosch D. Tryptophan supports interaction of transmembrane helices. J. Mol. Biol. 2005;354(4):894–902. doi: 10.1016/j.jmb.2005.09.084. [DOI] [PubMed] [Google Scholar]
- Roberts K.G., Li Y., Payne-Turner D., Harvey R.C., Yang Y.L., Pei D., McCastlain K., Ding L., Lu C., Song G., Ma J., Becksfort J., Rusch M., Chen S.C., Easton J., Cheng J., Boggs K., Santiago-Morales N., Iacobucci I., Fulton R.S., Wen J., Valentine M., Cheng C., Paugh S.W., Devidas M., Chen I.M., Reshmi S., Smith A., Hedlund E., Gupta P., Nagahawatte P., Wu G., Chen X., Yergeau D., Vadodaria B., Mulder H., Winick N.J., Larsen E.C., Carroll W.L., Heerema N.A., Carroll A.J., Grayson G., Tasian S.K., Moore A.S., Keller F., Frei-Jones M., Whitlock J.A., Raetz E.A., White D.L., Hughes T.P., Guidry Auvil J.M., Smith M.A., Marcucci G., Bloomfield C.D., Mrozek K., Kohlschmidt J., Stock W., Kornblau S.M., Konopleva M., Paietta E., Pui C.H., Jeha S., Relling M.V., Evans W.E., Gerhard D.S., Gastier-Foster J.M., Mardis E., Wilson R.K., Loh M.L., Downing J.R., Hunger S.P., Willman C.L., Zhang J., Mullighan C.G. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N. Engl. J. Med. 2014;371(11):1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K.G., Morin R.D., Zhang J., Hirst M., Zhao Y., Su X., Chen S.C., Payne-Turner D., Churchman M.L., Harvey R.C., Chen X., Kasap C., Yan C., Becksfort J., Finney R.P., Teachey D.T., Maude S.L., Tse K., Moore R., Jones S., Mungall K., Birol I., Edmonson M.N., Hu Y., Buetow K.E., Chen I.M., Carroll W.L., Wei L., Ma J., Kleppe M., Levine R.L., Garcia-Manero G., Larsen E., Shah N.P., Devidas M., Reaman G., Smith M., Paugh S.W., Evans W.E., Grupp S.A., Jeha S., Pui C.H., Gerhard D.S., Downing J.R., Willman C.L., Loh M., Hunger S.P., Marra M.A., Mullighan C.G. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Canc. Cell. 2012;22(2):153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K.G., Mullighan C.G. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat. Rev. Clin. Oncol. 2015;12(6):344–357. doi: 10.1038/nrclinonc.2015.38. [DOI] [PubMed] [Google Scholar]
- Russ W.P., Engelman D.M. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 2000;296(3):911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- Sarmento L.M., Barata J.T. CHK1 and replicative stress in T-cell leukemia: can an irreverent tumor suppressor end up playing the oncogene? Adv Biol Regul. 2016;60:115–121. doi: 10.1016/j.jbior.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Sarmento L.M., Povoa V., Nascimento R., Real G., Antunes I., Martins L.R., Moita C., Alves P.M., Abecasis M., Moita L.F., Parkhouse R.M., Meijerink J.P., Barata J.T. CHK1 overexpression in T-cell acute lymphoblastic leukemia is essential for proliferation and survival by preventing excessive replication stress. Oncogene. 2015;34(23):2978–2990. doi: 10.1038/onc.2014.248. [DOI] [PubMed] [Google Scholar]
- Sasson S.C., Smith S., Seddiki N., Zaunders J.J., Bryant A., Koelsch K.K., Weatherall C., Munier M.L., McGinley C., Yeung J., Mulligan S.P., Moore J., Cooper D.A., Milliken S., Kelleher A.D. IL-7 receptor is expressed on adult pre-B-cell acute lymphoblastic leukemia and other B-cell derived neoplasms and correlates with expression of proliferation and survival markers. Cytokine. 2010;50(1):58–68. doi: 10.1016/j.cyto.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Schluns K.S., Kieper W.C., Jameson S.C., Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Scupoli M.T., Perbellini O., Krampera M., Vinante F., Cioffi F., Pizzolo G. Interleukin 7 requirement for survival of T-cell acute lymphoblastic leukemia and human thymocytes on bone marrow stroma. Haematologica. 2007;92(2):264–266. doi: 10.3324/haematol.10356. [DOI] [PubMed] [Google Scholar]
- Scupoli M.T., Vinante F., Krampera M., Vincenzi C., Nadali G., Zampieri F., Ritter M.A., Eeren E., Santini F., Pizzolo G. Thymic epithelial cells promote survival of human T-cell acute lymphoblastic leukemia blasts: the role of interleukin-7. Haematologica. 2003;88(11):1229–1237. [PubMed] [Google Scholar]
- Seddon B., Tomlinson P., Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- Senkevitch E., Li W., Hixon J.A., Andrews C., Cramer S.D., Pauly G.T., Back T., Czarra K., Durum S.K. Inhibiting Janus Kinase 1 and BCL-2 to treat T cell acute lymphoblastic leukemia with IL7-Ralpha mutations. Oncotarget. 2018;9(32):22605–22617. doi: 10.18632/oncotarget.25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat C., Tal N., Bandapalli O.R., Palmi C., Ganmore I., Te Kronnie G., Cario G., Cazzaniga G., Kulozik A.E., Stanulla M., Schrappe M., Biondi A., Basso G., Bercovich D., Muckenthaler M.U., Izraeli S. Gain-of-function mutations in interleukin-7 receptor-{alpha} (IL7R) in childhood acute lymphoblastic leukemias. J. Exp. Med. 2011;208(5):901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat C., Tal N., Gryshkova V., Birger Y., Bandapalli O.R., Cazzaniga G., Gershman N., Kulozik A.E., Biondi A., Mansour M.R., Twizere J.C., Muckenthaler M.U., Ben-Tal N., Constantinescu S.N., Bercovich D., Izraeli S. Novel activating mutations lacking cysteine in type I cytokine receptors in acute lymphoblastic leukemia. Blood. 2014;124(1):106–110. doi: 10.1182/blood-2013-10-529685. [DOI] [PubMed] [Google Scholar]
- Silva A., Laranjeira A.B.A., Martins L.R., Cardoso B.A., Demengeot J., Yunes J.A., Seddon B., Barata J.T. IL-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res. 2011;71(14):4780–4789. doi: 10.1158/0008-5472.CAN-10-3606. [DOI] [PubMed] [Google Scholar]
- Silva A., Girio A., Cebola I., Santos C.I., Antunes F., Barata J.T. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia. 2011;25(6):960–967. doi: 10.1038/leu.2011.56. [DOI] [PubMed] [Google Scholar]
- Silva A., Yunes J.A., Cardoso B.A., Martins L.R., Jotta P.Y., Abecasis M., Nowill A.E., Leslie N.R., Cardoso A.A., Barata J.T. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Invest. 2008;118(11):3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni C., Neri L.M., Tabellini G., Ricci F., Bressanin D., Chiarini F., Evangelisti C., Cani A., Tazzari P.L., Melchionda F., Pagliaro P., Pession A., McCubrey J.A., Capitani S., Martelli A.M. T-cell Acute Lymphoblastic Leukemia. Leukemia. 2012. Cytotoxic activity of the novel Akt inhibitor, MK-2206. [DOI] [PubMed] [Google Scholar]
- Soares M.V., Borthwick N.J., Maini M.K., Janossy G., Salmon M., Akbar A.N. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J. Immunol. 1998;161(11):5909–5917. [PubMed] [Google Scholar]
- Sportes C., Babb R.R., Krumlauf M.C., Hakim F.T., Steinberg S.M., Chow C.K., Brown M.R., Fleisher T.A., Noel P., Maric I., Stetler-Stevenson M., Engel J., Buffet R., Morre M., Amato R.J., Pecora A., Mackall C.L., Gress R.E. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin. Canc. Res. 2010;16(2):727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kadota K., Sima C.S., Nitadori J., Rusch V.W., Travis W.D., Sadelain M., Adusumilli P.S. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J. Clin. Oncol. 2013;31(4):490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson L., Kinet S., Mongellaz C., Sourisseau M., Henriques T., Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109(3):1034–1042. doi: 10.1182/blood-2006-06-027912. [DOI] [PubMed] [Google Scholar]
- Swaminathan S., Klemm L., Park E., Papaemmanuil E., Ford A., Kweon S.M., Trageser D., Hasselfeld B., Henke N., Mooster J., Geng H., Schwarz K., Kogan S.C., Casellas R., Schatz D.G., Lieber M.R., Greaves M.F., Muschen M. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat. Immunol. 2015;16(7):766–774. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touw I., Pouwels K., van Agthoven T., van Gurp R., Budel L., Hoogerbrugge H., Delwel R., Goodwin R., Namen A., Lowenberg B. Interleukin-7 is a growth factor of precursor B and T acute lymphoblastic leukemia. Blood. 1990;75(11):2097–2101. [PubMed] [Google Scholar]
- Treanor L.M., Zhou S., Janke L., Churchman M.L., Ma Z., Lu T., Chen S.C., Mullighan C.G., Sorrentino B.P. Interleukin-7 receptor mutants initiate early T cell precursor leukemia in murine thymocyte progenitors with multipotent potential. J. Exp. Med. 2014;211(4):701–713. doi: 10.1084/jem.20122727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas D.C., Smiers F., Pouwels K., Hoefsloot L.H., Lowenberg B., Touw I.P. Interleukin-7 signaling in human B cell precursor acute lymphoblastic leukemia cells and murine BAF3 cells involves activation of STAT1 and STAT5 mediated via the interleukin-7 receptor alpha chain. Leukemia. 1996;10(8):1317–1325. [PubMed] [Google Scholar]
- Vicente C., Schwab C., Broux M., Geerdens E., Degryse S., Demeyer S., Lahortiga I., Elliott A., Chilton L., La Starza R., Mecucci C., Vandenberghe P., Goulden N., Vora A., Moorman A.V., Soulier J., Harrison C.J., Clappier E., Cools J. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica. 2015;100(10):1301–1310. doi: 10.3324/haematol.2015.130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Freeden-Jeffry U., Vieira P., Lucian L.A., McNeil T., Burdach S.E., Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181(4):1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng A.P., Ferrando A.A., Lee W., Morris J.P.t., Silverman L.B., Sanchez-Irizarry C., Blacklow S.C., Look A.T., Aster J.C. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Yang J., Zeng Z., Peng Y., Chen J., Pan L., Pan D. IL-7 splicing variant IL-7delta5 induces EMT and metastasis of human breast cancer cell lines MCF-7 and BT-20 through activation of PI3K/Akt pathway. Histochem. Cell Biol. 2014;142(4):401–410. doi: 10.1007/s00418-014-1222-1. [DOI] [PubMed] [Google Scholar]
- Yasunaga M., Manabe S., Matsumura Y. Immunoregulation by IL-7R-targeting antibody-drug conjugates: overcoming steroid-resistance in cancer and autoimmune disease. Sci. Rep. 2017;7(1):10735. doi: 10.1038/s41598-017-11255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Yokoyama N., Izawa K., Kotani A., Harashima A., Hozumi K., Tojo A. In vivo leukemogenic potential of an interleukin 7 receptor alpha chain mutant in hematopoietic stem and progenitor cells. Blood. 2013;122(26):4259–4263. doi: 10.1182/blood-2012-08-451278. [DOI] [PubMed] [Google Scholar]
- Zenatti P.P., Ribeiro D., Li W., Zuurbier L., Silva M.C., Paganin M., Tritapoe J., Hixon J.A., Silveira A.B., Cardoso B.A., Sarmento L.M., Correia N., Toribio M.L., Kobarg J., Horstmann M., Pieters R., Brandalise S.R., Ferrando A.A., Meijerink J.P., Durum S.K., Yunes J.A., Barata J.T. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat. Genet. 2011;43(10):932–939. doi: 10.1038/ng.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ding L., Holmfeldt L., Wu G., Heatley S.L., Payne-Turner D., Easton J., Chen X., Wang J., Rusch M., Lu C., Chen S.C., Wei L., Collins-Underwood J.R., Ma J., Roberts K.G., Pounds S.B., Ulyanov A., Becksfort J., Gupta P., Huether R., Kriwacki R.W., Parker M., McGoldrick D.J., Zhao D., Alford D., Espy S., Bobba K.C., Song G., Pei D., Cheng C., Roberts S., Barbato M.I., Campana D., Coustan-Smith E., Shurtleff S.A., Raimondi S.C., Kleppe M., Cools J., Shimano K.A., Hermiston M.L., Doulatov S., Eppert K., Laurenti E., Notta F., Dick J.E., Basso G., Hunger S.P., Loh M.L., Devidas M., Wood B., Winter S., Dunsmore K.P., Fulton R.S., Fulton L.L., Hong X., Harris C.C., Dooling D.J., Ochoa K., Johnson K.J., Obenauer J.C., Evans W.E., Pui C.H., Naeve C.W., Ley T.J., Mardis E.R., Wilson R.K., Downing J.R., Mullighan C.G. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]