Abstract
One Health is an effective approach for the management of zoonotic disease in humans, animals and environments. Examples of the management of bacterial zoonoses in Europe and across the globe demonstrate that One Health approaches of international surveillance, information-sharing and appropriate intervention methods are required to successfully prevent and control disease outbreaks in both endemic and non-endemic regions. Additionally, a One Health approach enables effective preparation and response to bioterrorism threats.
Keywords: Anthrax, Brucella, Brucellosis, Coxiella, Q fever, Tularaemia
1. Introduction
Six in ten human cases of infectious disease arise from animal transmission [1]. These so-called “zoonotic” pathogens, transmitted to humans from animals, are found globally. Wherever humans live, in both urban and rural settings, disease transmission from animals can occur [2]. The relevance of zoonoses to human health has been particularly highlighted by recent highly virulent infections that threatened to become pandemic, with the potential for high mortality. Such incidents include the 2005 H5/N1 avian influenza outbreak, the 2009 “swine flu” H1/N1 influenza pandemic, and the 2013–2016 West African Ebola outbreak [3], [4]. Although zoonotic viruses were responsible for these incidents, bacteria and parasites also pose threats for wide-spread zoonotic incidents [5]. Whilst lacking the global systemic threat of some viral zoonoses, these ‘forgotten neglected zoonoses’ have more frequent local outbreaks that can have significant consequences [6].
The 2005 H5/N1 avian influenza outbreak was the first zoonotic epidemic with high threat potential to unite global bodies in a network to address the threat of zoonoses [3]. The recognition of this zoonotic influenza as a potential global threat led to the establishment of surveillance networks; multiple national and international networks were set in motion to direct research. A key output of these networks was the One Health Initiative, founded in 2006 [7]. The concept of a One Health approach sees the health of humans, animals and ecosystems as an interconnected network, rather than problems to be tackled individually [1], [7]. Key concepts of One Health include: viewing the health of all species as needing to be balanced; focusing on health assessment and disease prevention rather than exclusively on treatment; and promoting a strong collaborative between the human medicine and veterinary sectors [7]. Under a single operative structure, the activities of both public health and veterinary services, along with others by extension, can be focussed together. Employing an “ecosystem approach” in a global context assists in mitigating health risks to both humans and animals [8]. Indeed, employing a pragmatic, preventative One Health approach to endemic zoonoses has been proposed to both be more equitable and have more effective benefits, compared to exclusively treating human cases of disease [9].
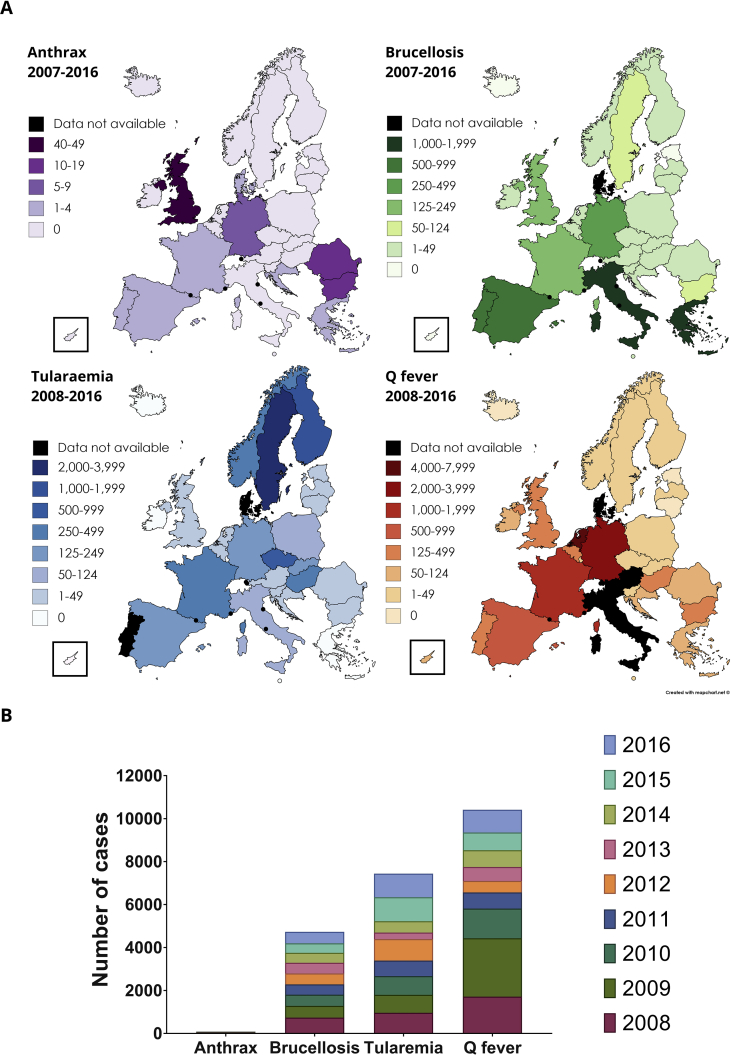
Here, we review key aspects of four bacterial zoonoses, all of which have natural reservoirs or endemic areas across Europe. Anthrax, brucellosis, tularaemia and Q fever are caused by Bacillus anthracis, Brucella species, Francisella tularensis and Coxiella burnetii, respectively. These are all currently rare human diseases (respectively causing approximately 2, 105, 155 and 230 cases per 100 million people per year in the European Union/European Economic Area (EU/EEA), Fig. 1) [10], [11]; however, sporadic outbreaks have devastating impacts for public health, animal health, and animal industries. Common salient features of these zoonoses are: each causes debilitating, potentially fatal disease in both animals and humans; infectious doses are low (in some cases a single bacterium [12]); and zoonotic transmission is a risk for those working/living in proximity to animals, in addition to those consuming untreated animal products [13], [14], [15], [16]. Consequently, the bacteria that cause each of these zoonoses consistently appear on select biological agent threat watch-lists across the globe [13], [17], [18], [19]. The principal routes of infection transmission and human risk groups for these diseases are summarised in Table 1. Contamination of land is also of concern for these pathogens, especially for C. burnetii and spores of B. anthracis which are highly resilient to external environments [19], [20].
Fig. 1.
Reported cases of anthrax, brucellosis, tularaemia and Q fever in the EU/EEA between 2008–2016. A) Maps of the EU/EEA colour-coded by the total number of cases of each zoonosis reported where data is available. Data on Q fever occurrence in Italy is not available for 2008–2015, therefore it is omitted here. B) Reported annual cases of brucellosis, Q fever and tularaemia; Anthrax is omitted here due to the much smaller number of cases (on average fewer than 10 per year). Dataset provided by ECDC based on data provided by WHO and Ministries of Health from the affected countries [10]. Figure generated using mapchart.net (https://mapchart.net/europe.html), GraphPad Prism v.6.0.1 and gravit. io (https://gravit.io/).
Table 1.
Principal routes of transmission of bacterial zoonoses. Occupational exposure relates most specifically to veterinarians, farm workers and abattoir workers. Wildlife leisure refers to hunters/hikers.
| Route of transmission | People most at risk | Prevention measures | References |
|---|---|---|---|
| Consumption of contaminated food or water | Consumers of meat/dairy products from infected animals | Consume only pasteurised dairy products and meat form healthy animals; drink only treated water | [13], [14], [15], [16] |
| Exposure to animal fluids e.g. urine/blood/faecal matter | Occupational/wildlife leisure | Protective clothing, safe waste of disposal; decontamination of exposed material and areas; store food away from rodents | [13], [14], [16], [19] |
| Direct blood entry-mosquito/tick bites or wound contamination | Occupational/wildlife leisure | Cover wounds; use insect repellent | [13], [14], [16], [21], [22] |
| Breathing in aerosolised bacteria | Anyone in proximity to a contaminated area, in addition to occupational/wildlife leisure | Surveillance by public health authorities; following confirmed local outbreaks use appropriate PPE and seek medical advice | [13], [14], [16], [23], [24] |
Data from the Surveillance Atlas of Infectious Diseases, a tool hosted at the European Centre for Disease Prevention and Control (ECDC), have been analysed for this review to discuss disease occurrence and trends in select EU/EEA Member States over a decade (2007–2016)1 [10]. This review discusses the European disease trends and global context of each disease, along with the characteristics of presentation and the medical interventions available. One Health approaches to disease management are highlighted, considering infection events in the context of ecosystem health. A key benefit of this approach is the integrated assessment of the interlinked challenges of food safety, global health, antimicrobial resistance and biological security threats [7]. These four zoonoses highlight important One Health lessons, and provide models of One Health principals in action, which can be applied more broadly to global zoonoses.
2. Anthrax
Anthrax is caused by the soil-residing Bacillus genus. B. anthracis is the main causative agent, however, recently characterised isolates of Bacillus cereus from human infections have now been found to possess anthrax-linked virulence factors [25]. B. anthracis is known for its spore-forming ability, and the highly resilient nature of these spores [13]. B. anthracis spores are resistant to temperature extremes, drought and UV light, possibly due to protection of DNA in a crystalline core [26]. This makes decontamination of material and surfaces difficult.
There were on average fewer than ten human anthrax infections per year in the EU/EEA between 2007 and 2016 (Figs. 1B and 2) [10]. However, historically, anthrax was a relatively common disease among humans and animals. In Victorian Britain, anthrax was described as ‘woolsorters’ disease’; a disease experienced by wool-workers that could be fatal in as little as 24–36 h [27]. The study of woolsorters' disease identified B. anthracis as the causative agent, capable of infection by inhalation. Consequently, control measures such as fans and ventilation systems were implemented in factories “so arranged as to carry the dust away from the worker” [28]. This demonstrated an early awareness of the risk of inhaling contaminated aerosols in occupations where animal material is handled.
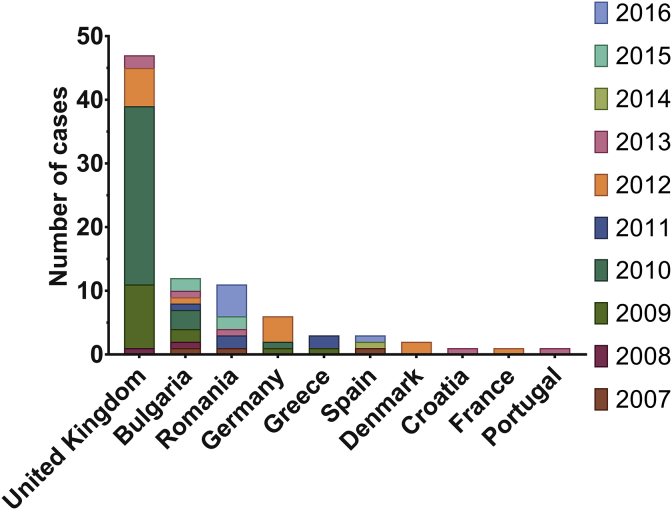
Fig. 2.
Number of cases of anthrax reported each year in the EU/EEA. Data is shown for every country with at least one case reported between 2007 and 2016. Peaks in cases reported to the ECDC have been attributed to injectional anthrax, caused by the use of contaminated heroin. 14 cases were reported to the ECDC in 2009 and 32 in 2010. It should be noted that there is a discrepancy between the ECDC data and original literature reported in December 2011 for the injectional anthrax outbreak, reflecting under-reporting by approximately 20% in the data shown here [37]. 2012 then saw a second episode of injectional anthrax cases in the UK and Germany again, with an additional report in France and two in Denmark. Dataset provided by ECDC based on data provided by WHO and Ministries of Health from the affected countries [10]. Figure generated using GraphPad Prism v.6.0.1.
Most modern-day zoonotic incidences of anthrax in humans are due to bacterial contamination of skin abrasions, causing cutaneous anthrax. If diagnosed and treated appropriately this is rarely fatal, and largely non-contagious. Without treatment, the bacteria can disseminate to cause systemic infection, and mortality of inappropriately treated cutaneous anthrax is 20% [13]. However, infections occurring through ingestion or inhalation of bacteria have much higher mortality rates (25–100% for gastrointestinal anthrax, and 86–89% for inhalational anthrax) [13]. Human-to-human transmission of anthrax has not been reported.
The level of treatment required depends on the severity of infection and can range from oral antibiotics to intravenous antibiotics and surgery or amputation as appropriate. All cases of inhalational anthrax require respiratory support in an intensive care unit. In some cases, anti-toxin antibodies or vaccine doses can be administered post-exposure [29], [30]. The frontline drugs for anthrax treatment are ciprofloxacin and doxycycline, which are usually administered together [31]. Daptomycin, of the cyclic lipopeptide class of antibiotics, is being investigated for prophylactic/post-exposure treatment of B. anthracis infection; results from in vivo trials in non-human primates will confirm if this new class of antibiotic will be effective [32].
One of the vaccines used routinely for livestock is the toxin-producing, but non-capsule-forming Sterne strain vaccine. This live-attenuated vaccine (LAV) still carries some virulence, particularly in goats and llamas, where vaccine-associated mortality can occur [33]. In addition to veterinary vaccines, there are several options for human vaccines, offered to those with occupational risks. The cell-free human vaccines Anthrax Vaccine Precipitated (AVP) and Anthrax Vaccine Adsorbed (AVA, also known as Biothrax™) are available in the UK and USA [34]. Both are derived from sterile filtrate preparations of the Sterne strain. AVA has recently been licensed for post-exposure prophylactic use by applying the “Animal Rule” regulations of the U.S. Food and Drug Administration (FDA) [30]. In addition to this, a live attenuated Salmonella spp. expressing the anthrax antigen Ty21a-PA-01 is currently being developed [35]. This aims to achieve a human vaccine that is stable at room temperature, and can be administered orally over a much-reduced immunisation period (approximately seven days compared to 18 months with AVA). These features would make this vaccine well-suited for use in response deliberate release of the pathogen.
In addition to the principal routes of transmission highlighted in Table 1, anthrax has also been found in cases of transmission linked to illegal drug use [36]. The first cases of injectional anthrax were documented in 2009 in heroin users in Scotland [37]. The outbreak continued for one year, with fourteen fatalities recorded in Scotland, and further cases confirmed in England and Germany (Figs. 1B and 2) [38]. A second outbreak of anthrax as a result of transmission by injection was experienced by the UK and Germany in 2012, with small numbers of cases additionally in Denmark and France [38]. It was notable that the ECDC data showed fewer cases than were reported retrospectively by Health Protection Scotland [10], [37]. This discrepancy highlights that data from collated international databases should be interpreted as general trends, and that sources of primary literature are required to verify the data. The source of contamination was concluded to be from goat skins used to transport the heroin [37]. The fact that the spores were able to survive the drug preparation process highlights the extent of their resilience to external stressors [36].
Attesting to the resilience of anthrax spores was an anthrax outbreak in Italy in 2004, killing 124 grazing animals, that portrayed a particularly unusual pattern of transmission [39]. After the removal of infected carcasses, which previously were left exposed to insects and wild animals, the rate of fatalities decreased. This led to the hypothesis that the pathogen was spread by flies, both necrophilic and haematophagic [39]. Due to the highly resistant nature of anthrax spores to low pH, insects that feed on infected animals and carcasses are a possible vector for further transmission. Some flying insects are able to transmit bacteria for at least 4 hours after contact with an infected animal, e.g. the house fly Musca domestica [21].
When taking into account the injectional anthrax cases of 2009–2010 and 2012, it is clear that environmental transmission of B. anthracis in the EU/EEA is low (Fig. 2). Bulgaria and Romania are the only countries in this dataset which experience yearly cases due to environmental exposure (on average one case per year for each). Two events, in Romania and Bulgaria, were the result of the slaughter and consumption of infected cattle [40], [41]. In both countries, the One Health approach to managing anthrax is adopted. Such measures include robust reporting, rapid confirmation by laboratory diagnostics, appropriate medical interventions, and screening and prophylaxis where appropriate for those suspected of exposure. Furthermore, for animals, quarantine, transport bans, vaccination of local livestock and domestic pets, tracing and destroying contaminated meat and animal products and disinfection of slaughter sites, processing factories and retail outlets are enforced [40], [41]. Part of the One Health strategy is also the implementation of laws that prohibit the slaughter and consumption of meat and animal products from sick animals to prevent contaminated products entering the food chain [40].
Anthrax illustrates the One Health challenges of eradication of robust environmental pathogens. Due to the resilience of bacterial spores, the risk for environmental contamination from abandoned animal carcases, or even soil-disturbance over historic animal graves, is significant [39], [42]. Direct eradication in the environment, requiring removal of vegetation [20], is impractical. Restricting re-emergence of veterinary and human disease requires vigilant surveillance to rapidly identify cases; vaccination of local livestock to prevent further disease; and swift disposal of infected animals/carcasses to prevent contamination of the environment and vector borne dispersal.
3. Brucellosis
Brucellosis is considered to be the most prevalent zoonosis globally [43], yet is classed by the WHO as a ‘forgotten neglected zoonosis’ [5]. Members of the Brucella genus are non-spore-forming, Gram-negative bacteria. This genus consists of twelve species, four of which (Bacillus melitensis, Bacillus abortus, Bacillus suis and Bacillus canis) are relevant to human disease [44]. The most common routes of human infection are related to occupational contact with animals, with transmission through inhalation of aerosols and contact with animal secretions [14]. Consumption of animal products can also lead to contraction of brucellosis [45], [46]. Indeed, it was a link between disease sufferers consuming raw goat milk, and later detection of B. melitensis in goat blood, that led to the recognition of it as the causative agent of ‘Malta fever’ [45]. Human–human transmission of brucellosis is rare, but has been documented [47].
As brucellosis is highly contagious between animals, can cause disease by aerosol inhalation, and has a low infectious dose, species of Brucella are commonly included on bioterrorism watch lists [18]. Furthermore, although this genus of bacteria are non-spore-forming, and less capable of survival in extreme environments than B. anthracis, Brucella can persist for many weeks in wet soil and ambient-temperature farm slurry [14].
Brucellosis in humans, despite causing debilitating disease, is rarely fatal. In 2013, out of 357 confirmed cases in the EU, 70% required hospital treatment, but only one fatality was recorded [48]. Symptoms in humans can reflect both acute, febrile illness and chronic systemic disease, and there can be an incubation period of up to six months before symptoms appear [31]. Treatment for brucellosis requires a course of antibiotics for at least six weeks, usually a doxycycline and rifampicin combination therapy [18]. In animals, brucellosis symptoms include abortion, infertility, decreased milk production, weight loss, and lameness [49], all of which impact on the economics of farming. Although there are a number of livestock vaccines available for Brucella species, none are licensed for use in humans [44]. It is important for disease surveillance and diagnosis to be able to distinguish between vaccinated and infected animals. The cattle vaccine B. abortus RB51 has a rough phenotype which enables serological differentiation between vaccinated and diseased animals because animals vaccinated with RB51 do not make antibodies against Brucella's lipopolysaccharide [44]. However, the similar antibody profile generated in vaccinated small ruminants (B. melitensis Rev. 1 vaccine) to that of live Brucella exposure makes herd-surveillance for infection challenging where vaccination is common-place. Recently, new insights into the specific antigenic structure of the bacterial cell wall O-polysaccharide (OPS) have offered a resolution to this issue, revealing potential for new diagnostic markers for herd surveillance [49]. Additionally, OPS research is paving the way towards development of a synthetic glycoconjugate vaccine for use in humans and animals, which would be unreactive in serodiagnostic tests [49].
Between 2007 and 2016 Greece reported the highest prevalence of brucellosis in its population, with on average 12 in 100,000 inhabitants contracting the disease annually (Fig. 3) [11]. This is unsurprising as Greece also has the most abundant population of sheep and goats in the EU/EEA. An eradication program started in 1975 with the vaccination of young sheep and goats, on both the islands and mainland Greece [50]. A 2006 report from the UN highlights difficulties in quantifying incidence in human cases [14]. Italy alone consistently reports the highest average cases per year in countries reporting to the ECDC (Fig. 3). However, despite this it is estimated that brucellosis could be over 20-fold under-reported within the country [51].
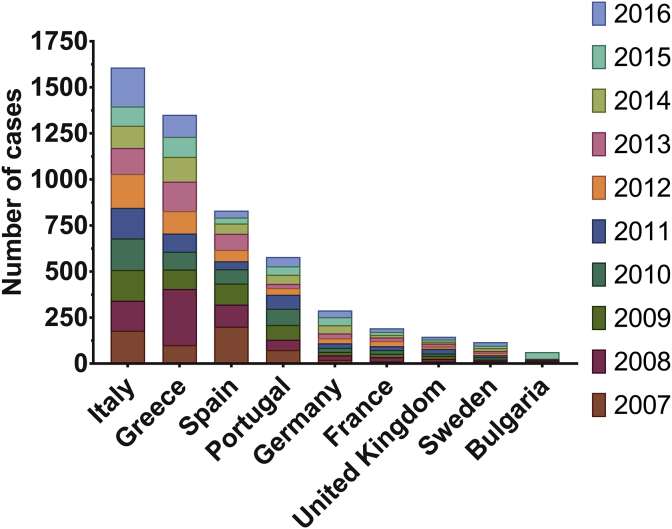
Fig. 3.
Number of cases of brucellosis reported each year in the EU/EEA. Data is shown for every country with >50 total cases reported between 2007 and 2016. In most European Member States, the notification of brucellosis in humans is mandatory. The exceptions are the UK (where only animal infection is notifiable), Belgium, and Denmark. Voluntary surveillance systems have full national coverage in the former two, but in Denmark brucellosis remains non-notifiable, with no surveillance system in place [48]. Brucellosis prevalence is highest in Italy and Greece; Italy consistently reports the highest average cases per year, but Greece has the highest incidence in its population, with on average 12 in 100,000 Greeks reporting a case of brucellosis each year, four times more than Italians. Despite high incidence of brucellosis in Spain at the start of Atlas data records, this has generally fallen from over 200 reported cases in 2007 to only 37 cases reported in 2016. Bulgaria had an outbreak in 2015 with 36 cases, compared to the yearly average of just six. 2008 had the highest number of cases of brucellosis across the EU/EEA between 2007 and 2016, with a total of 735 cases. This is 37% higher than the average total number of cases per year over that period. Dataset provided by ECDC based on data provided by WHO and Ministries of Health from the affected countries [10]. Figure generated using GraphPad Prism v.6.0.1.
In Bulgaria, after a period of 50 years free from brucellosis, the disease has started to re-emerge [52] with the most recent epidemic occurring in 2015 (Fig. 3). This was hypothesised to be the result of unauthorised import of infected animals from neighbouring endemic countries [46]. Cross-border transmission of zoonoses threatens to re-instate endemicity in countries that had previously been declared free of disease. France was declared officially free from bovine brucellosis according to the criteria of the World Organisation for Animal Health (OIE) in 2005, yet through human surveillance, re-emergence of the disease in cattle was detected [53]. The specific risks of cross-border transmission of brucellosis into Europe have been studied in the context of transmission-risk from middle-eastern countries, where there are some of the highest incidences of brucellosis in the world. Turkey has more than 15,000 new cases per year [54], and Syria has an incidence of >1000 in 100,000 [43]. In a recent case of brucellosis in a Syrian refugee in Germany, one of the ‘lessons learnt’ was that gaining a travel history from patients presenting with an undiagnosed ailment is of high import [55]. Molecular epidemiology tracing B. melitensis in Germany to immigrants and German travellers identified similar concerns for correct identification of non-endemic disease [54]. To better understand disease patterns and trends, and monitor outbreaks in real time, up to date mapping approaches can be used that harness new computer technologies [56]. This would rely on cooperative data exchange between monitoring agencies. These observations highlight that threats posed by biological agents are not confined by geographical barriers or political boundaries. Brucellosis highlights the need for non-endemic or “infection-free” countries to remain aware of the risks of global zoonoses.
4. Tularaemia
Tularaemia is a zoonotic disease caused by F. tularensis. Although there are four subspecies, only two are clinically relevant: F. tularensis subsp. tularensis (type A) and F. tularensis subsp. holarctica (type B). Whilst type A strains cause the most severe disease, with an infectious dose of fewer than ten organisms, natural reservoirs are restricted to North America [15], [57]. F. tularensis subsp. holarctica is relevant in Europe, with prevalence across the Northern hemisphere and an infectious dose of 10–50 bacteria [15], [31]. Clinical presentation of tularaemia in humans is highly dependent on the route of transmission, in a similar manner to cutaneous/gastrointestinal anthrax (Table 1). Ingestion of food or water contaminated with F. tularensis causes oropharyngeal disease [16]. Blood contact with infected animals from scratches/cuts or insect bites more often results directly in glandular presentation, causing swelling and ulcers. Finally, transmission through inhalation of aerosols in contaminated dust leads to a pneumonic presentation [16]. The latter two modes have the highest risk of environmental transmission for hunters and farmers. Pneumonic tularaemia is also the most relevant disease presentation in the context of bioterrorism [17]. The incubation period ranges from 1 to 14 days, and is generally 2–5 days [57]. Without treatment, both glandular and oropharyngeal infections can persist for weeks or months and may progress to the more serious and potentially fatal pneumonic or septicaemic tularaemia [57].
As with inhalational anthrax, due to the potential severity of symptoms and risk of mortality, a dual antibiotic approach is recommended for treatment of pneumonic tularaemia, for example gentamicin and ciprofloxacin [31]. In 2013, information on the outcome of confirmed tularaemia cases in Europe (covering almost 50% of reported cases) showed that approximately 52% of cases required hospital treatment, however no deaths were reported [48]. Due to the nature of the undulating fever associated with tularaemia it is expected that the number of cases will be under-reported [58]. No human vaccine for tularaemia is licenced yet in the EU/EEA. A live vaccine strain (LVS) was produced in the Soviet Union through serial passaging, from F. tularensis subsp. holarctica. This has been in clinical trials, but currently safety and efficacy concerns have prohibited licensure [57], [59]. A modern LAV showing promise is based on Francisella novicida, a bacterial species avirulent in healthy humans [60]. Further to this, a new vaccine strategy is also in development, employing a glycoconjugate subunit vaccine, in a similar approach to that being used for brucellosis [61].
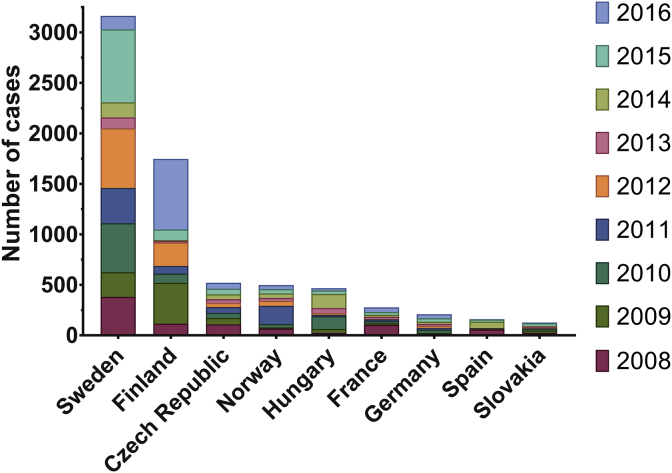
Across all EU/EEA Member States, Sweden, Finland and Norway had the highest reported prevalence of tularaemia in their populations between 2008 and 2016 (Figs. 1A and 4). Sweden alone was responsible for 43% of the average yearly cases of tularaemia in the EU/EEA, with on average four in every 100,000 people reporting a case each year [10], [11]. F. tularensis subsp. holarctica is able to infect a range of animal hosts: recently identified wild hosts include the red fox (Vulpes vulpes), wild boar (Sus scrofa) and raccoon dog (Nyctereutes procyonoides). However, most tularaemia surveillance in European animals comes from recording dead/diseased farmed rabbits/hares [16]. Infection of such forest mammals, and even fish, with F. tularensis subsp. holarctica leads to a risk of zoonotic transmission for any activities which involve contact with wildlife in endemic areas, most notably hunting (Table 1) [62]. The peaks of tularaemia outbreaks in the EU occur over the end of the summer, coinciding with the peak in mosquito populations [16]. It is therefore widely accepted that mosquitos are responsible for the transmission of F. tularensis subsp. holarctica between animals, and to humans (Table 1). A single contaminated water source can lead to mosquito-borne transmission of tularaemia [15], [22]. Furthermore, as the taiga forest covers the three European countries with highest reported prevalence of tularaemia, it is not surprising that they share natural sources for infection. Therefore, the relationship between humans and animals with parasites and vectors plays a key role in the spread of infection [63].
Fig. 4.
Number of cases of tularaemia reported each year in the EU/EEA. Data is shown for every country with >100 total cases reported between 2008 and 2016. Human tularaemia is not a notifiable disease in Denmark, Portugal and Liechtenstein, however, notification is mandatory in most EU/EEA member states [16] (Fig. 4). A voluntary surveillance system is in place for Belgium and the United Kingdom [48]. Sweden reported the highest total number of cases, 3164, followed by Finland, Czech Republic, Norway and Hungary. France, Germany, Spain and Slovakia experienced much lower incidences, fewer than 1 in 100,000 cases reported each year on average. 2015 saw the highest number of reported cases of tularaemia over 2008–2016, with 64% of these occurring in Sweden. Sweden generally reported more cases each year than any other country, except in 2009 when Finland saw twice its average yearly cases, and in 2016 when Finnish cases reached a peak of 699, 3.6 times its yearly average. In 2011 Norway also saw three times its average number of cases, affecting almost 4 in every 100,000 people. In both 2010 and 2014 Hungary experienced outbreaks, reporting 126 and 140 cases respectively, compared to the yearly average of 56. Dataset provided by ECDC based on data provided by WHO and Ministries of Health from the affected countries [10]. Figure generated using GraphPad Prism v.6.0.1.
The survival and propagation of F. tularensis subsp. holarctica in natural fresh and brackish water has been well studied, however, there have been fewer studies on the environmental survival of F. tularensis subsp. tularensis [15], [62]. An unusual outbreak of tularaemia on an island off the coast of Cape Cod, USA led to establishing that F. tularensis subsp. tularensis can indeed survive in brackish water [64]. This outbreak on Martha's Vineyard, spanning from 2000 to 2008, was unusual due to the skew of disease presentation to pneumonic, rather than the glandular presentation associated with bites from parasites, and contamination of skin wounds [23]. Two thirds of the 90 reported cases displayed pneumonic symptoms. The observation of pneumonic presentation led to investigations to track the source of infection, to ensure that this was a natural event and not bioterrorism [17]. However, no environmental samples were positive for either of the disease-causing species of F. tularensis [23], [64]. It remains unknown what the true reservoir for F. tularensis subsp. tularensis is on Martha's Vineyard; without definition of this, intervention methods are limited. However, links have been made with landscaping activities increasing likelihood for infection, thus is it advised to wear personal protective equipment, e.g. masks [23].
The management of tularaemia outbreaks highlights the need for human, animal and whole ecosystem surveillance systems to achieve an efficient One Health approach [6], [7], [58]. Understanding the source of infection is important for deployment of the most effective response to minimise disease. For example, if a parasite/rodent source is suspected, methods for pest control would be advised, however, if the source was a water system then disease management should focus on personal protection, for example vaccination [65]. In addition to the need of vaccines for ecosystem health in endemic areas, vaccine development strategies are also important to address F. tularensis as a potential bioterror agent [17].
5. Q fever
Query fever, or Q fever as it is more commonly known, is the zoonosis caused by C. burnetii, an obligate intracellular bacterium that is globally prevalent (except in New Zealand) [66]. C. burnetii, similar to F. tularensis, infects a wide range of species, including terrestrial mammals such as cats and dogs, and even aquatic mammals [66], [67]. However, Q fever is of particular economic significance in ruminants, such as cows, sheep and goats [68]. In such animals, symptoms are similar to those of brucellosis, with spontaneous abortion of pregnancies being the main clinical symptom. Again, this causes a substantial economic impact for animal industries [68]. The material shed from animal infections (e.g. abortive material, milk, faeces and urine) contaminates dirt and dust in the environment with C. burnetii. Here, C. burnetii cells adapt to the harsh environment outside of a host by adopting a highly resilient spore-like state [66]. These highly resistant cells behave similarly to anthrax spores, remaining viable for years and easily becoming aerosolised in wind, for example in dust clouds, where they can spread to new areas and infect new hosts [69].
Inhalation of bacteria is the most common route of Q fever transmission to humans. As few as 1–10 aerosolised C. burnetii cells can result in zoonotic transmission, therefore occupation is a key risk-factor for disease; individuals at highest risk of Q fever exposure are farmers, abattoir workers and vets [12], [70]. In Australia, prior to an increase in Q-fever vaccination as many as 60% of meat and agricultural workers were seropositive after 25 years in the industry [70]. In addition to occupational risks, the presence of C. burnetii in ruminant milk, as with Brucella, also poses a risk for disease transmission [71], [72], [73], [74] (Table 1). Humans generally present with acute infections, causing symptoms of an undifferentiated febrile illness after an incubation period of 2–40 days (most commonly 18–21 days) [31], [75]. However, patients can develop life-changing complications from persistent focalised infections, such as hepatitis, chronic fatigue, and endocarditis [76]. A quick and accurate diagnosis for Q fever is important as although little is known about the development of persistent infections, and post–Q fever fatigue, the severity of the initial infection is a known risk factor [66]. Doxycycline, often administered as a monotherapy, is the primary antibiotic used in the treatment of acute Q fever in humans, and swift administration should minimise complications [31], [66]. For animals, a whole-cell inactivated vaccine, Coxevac, can be used to prevent infection, and has been shown to reduce shedding of bacteria when applied in combination with antibiotic therapy for dairy herds already affected by Q fever [77]. While a similar formalin-inactivated whole-cell vaccine is available for human use in Australia, there is currently no Q fever vaccine licensed in the UK/EU/US, but research programs are on-going [78].
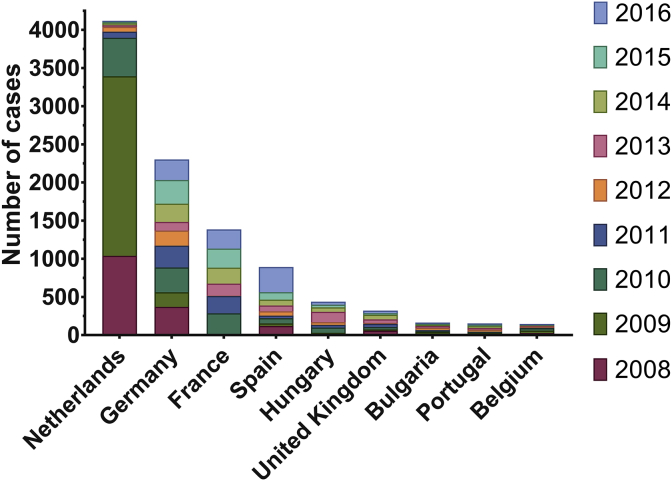
Between 2007 and 2010 the Netherlands experienced the biggest Q fever epidemic in recorded history (Fig. 5). Over 4000 human cases were confirmed during this outbreak; additionally, over 50,000 dairy goats were culled [79]. A cross-sectional population-based serological survey later confirmed that airborne bacteria carried on the wind from infected goat farms were responsible for zoonotic transmission [69]. Real-time PCR for acute-phase diagnostics was pivotal to the outbreak assessment, contributing to the ability to confirm a Q fever diagnosis in cases where serology was inconclusive [80]. Directly following the outbreak only six fatalities were reported but by May 2016 the death toll had risen to 74 [81]. The rise to 74 by 2016 reflects that Q fever infections can remain dormant, with persistent focalised infections causing symptoms long after exposure [76], [82]. As a result of the epidemic, seroprevalence to C. burnetii antibodies in the general population of the Netherlands rose from 2.4% in 2006 to 6.1% in 2015 [69]. One key output of the Netherlands epidemic was the establishment of a national zoonosis structure with a monthly signalling forum [68].
Fig. 5.
Number of cases of Q fever reported each year in the EU/EEA. Data is shown for every country with >125 total cases reported between 2008 and 2016. The 2007–2010 Q fever epidemic was contained within southern areas of the Netherlands, affecting small ruminant farms in the direction of the prevailing wind from the affected goat farms. This accounted for 37% of the total cases of Q fever in the EU/EEA between 2008 and 2016, with on average 1300 cases reported per year. After this was resolved, the country with the highest prevalence of Q fever was Germany, with on average 240 cases/year between 2011 and 2016 (incidence of 2 in 100,000), followed by France, Spain and Hungary, with 180, 110 and 60 cases/year, respectively. In the six years following the epidemic resolution, the Netherlands experienced a much-reduced average of 37 cases reported per year. Additionally, in 2013 Hungary experienced an epidemic of 135 cases, this was resolved within two years. Dataset provided by ECDC based on data provided by WHO and Ministries of Health from the affected countries [10]. Figure generated using GraphPad Prism v.6.0.1.
In the Netherlands, as a response to the large epidemic, government measures were put in place to vaccinate all dairy goats and sheep, and to test and cull pregnant animals testing positive for C. burnetii. One of the methods for detection was the presence or absence of C. burnetii DNA in bulk tank milk (BTM) tested by PCR [72]. However, up to nine days after immunisation, vaccine-derived C. burnetii DNA can be detected in the milk of dairy goats which have not had live pathogen exposure. Consequently, a two-week post-vaccination interval was introduced to the test-and-cull control measures, in order to avoid unnecessary culling due to vaccine-derived false-positive detection [71].
Globally, the proportion of community-acquired pneumonia related to acute Q fever is highest in French Guiana [83], followed by Canada, Northern Spain, Croatia and the Netherlands [66]. In Cayenne, French Guiana, Q fever is a hyperendemic disease, with the incidence of cases in 2005 reaching 150 cases per 100,000 inhabitants [84]. A retrospective cohort study recently linked two independent risk factors to a 2013 epidemic in Cayenne: cleaning the house; and carrying a three-toed sloth. Both of these activities correlate to inhalational disease acquisition [85].
In 2013, Hungary experienced a Q fever outbreak, albeit on a smaller scale (Fig. 5). The source of this epidemic was tracked to a flock of Merino sheep, where, as with the previous Netherlands epidemic, dried contaminated material was carried by the wind causing human infections by inhalation [86]. The epidemic was resolved after all manure from the infected farm was eliminated and the farm disinfected. Furthermore, for the management of C. burnetii infection spread within a herd, good farm practices such as regular litter-cleaning have been recommended as simple measures prior to whole-farm disinfection [87].
Generally, Q fever infection in humans is controllable by good hygiene practices when dealing with animals, particularly ruminants. From a One Health perspective, Q fever represents one example of a wide range of conditions that cause febrile disease. Rapid diagnostics that can differentiate these (often rare) underlying diseases offer the opportunity to avoid unnecessary antimicrobial use and to take early, specific actions to prevent development of disease [24], [80]. Surveillance of enzootic pathogens using seroprevalence in livestock assists in informing the risk of transmission of zoonoses to humans.
6. Conclusions
Bacterial zoonoses are often omitted from discussions on priority global pathogens. Nevertheless, while natural reservoirs for disease remain, they retain relevance to acheiving success in One Health approaches worldwide [9]. Anthrax is enzootic to Eastern Europe, with consistent yearly cases of zoonotic transmission in Bulgaria and Romania (Fig. 2) [10]. While brucellosis eradication programmes are being employed across Europe, the disease remains endemic in both Greece and Italy [50], [51]. However, the main threat for brucellosis re-emergence in Europe arises from countries such as Syria, which has an incidence 100-times greater than that of endemic European countries [43]. Sweden has the highest endemic prevalence of F. tularensis subsp. holarctica, with 43% of tularaemia cases reported to the ECDC occurring there. For a zoonosis like this, where >50% of cases can require hospital treatment, applying One Health control and prevention measures in an eco-system approach offers an attractive model for lessening the economic burden of disease [9]. Whilst endemic globally, it was the Q fever epidemic experienced by the Netherlands that drew global attention to the disease [79]. The networks in place for a One Health approach to endemic disease management apply also in response to epidemics [88]; analysis here shows that 67% of all Q fever cases reported to the ECDC between 2008 and 2010 occurred in the Netherlands (the latter three years of the 2007–2010 epidemic) (Fig. 5) [10]. However, in the six years following, only 5% of the total cases across the EU/EEA were of Dutch origin, showing an effectively maintained response.
One Health intervention methods include surveillance, medical interventions (post-exposure therapeutics and prophylactic vaccines), and sanitation. The case for employing One Health initiatives, and engaging communities to partake in them, clearly highlights the potential for much improved efficacy, and more equitable health and livelihood benefits [9]. In addition to monitoring and controlling endemic disease epidemics, it is also important to keep the global conversation updated on bacterial zoonoses due to the potential threat of their malicious misuse.
Surveillance requires accurate and reliable reporting mechanisms, so that appropriate points for intervention can be recognised [88]. Maintaining reliable information on international prevalence (both human and animal), and detailed case histories for infection incidence is paramount to One Health. These will include national reporting structures, such as that set-up after the Q fever outbreak in the Netherlands [68]. International tools for collating data, such as The ECDC Surveillance Atlas of Infectious Diseases [10], offer a broader perspective and information for professionals in all sectors working towards One Health.
Diagnostics play a key role in disease surveillance. Misdiagnosis results in inappropriate treatment, or missed opportunities to prevent further disease transmission. The zoonoses discussed here often present as undifferentiated febrile illnesses, and so a detailed history is key to diagnosis. More common ailments with similar symptoms will be initially suspected, and diagnosis may be missed altogether in self-limiting cases. While algorithm tools for disease diagnosis and management have been developed to aid medical professionals in diagnosis of zoonoses [89], there is a clear need for accurate and sensitive point-of-care diagnostic tests [9]. Emerging technologies such as high throughput sequencing and semiconductor genome analysis offer the potential for diagnosis within hours [90]. This will be of particular benefit for zoonoses where development to persistent or chronic disease is a risk [57], [76].
Medical interventions, including post-exposure therapeutics such as antibiotics, are essential especially for human treatment [31]. For diseased animals, post-exposure therapy is often not a viable approach due to the associated costs, risk of further transmission, and virulence of these infections potentially causing death before culling. Instead, One Health necessitates a focus on prevention, and requires cheap, effective and readily deployable prophylaxis methods, such as veterinary and human vaccines [9]. Current vaccine research directives are progressing away from LAVs or whole cell killed vaccines. Such approaches are using reverse vaccinology, subunit vaccines and conjugate vaccines (e.g. the Salmonella-Ty21a-PA-01 anthrax toxin conjugate vaccine, glycoconjugate vaccines for brucellosis and tularaemia, and epitope-selected subunit vaccines for Q fever [35], [49], [61], [78]). These minimise safety risks (such as potential animal toxicity of the anthrax Sterne strain vaccine) and enable more effective herd surveillance methods. The prospect of room-temperature-stable vaccines (e.g. anthrax toxin-conjugate vaccine [35]) offers advantages for public health and veterinary preparedness, as well as outbreak and bio-terrorism management.
Sanitation, such as basic infection control measures, should be practiced in areas of endemic zoonoses. This includes vaccination where appropriate, good hygiene practices and the use of appropriate personal protective equipment (especially where exposure to aerosols is a risk) [23], [24]. In Australia, it is recommended that clothing potentially contaminated with C. burnetii should not be washed in the presence of un-vaccinated individuals [24]. Farm sanitation is also important, as shown for Brucella which can survive in farm slurry [14], and the recommendation for regular cleaning and incineration of litter to prevent the spread of Q fever in a herd [87].
Bioterror classifications set by the United States Centers for Disease Control and Prevention (U.S. CDC) classify anthrax and tularaemia as Category A agents, the highest priority [91]. This is due to their transmissibility, potential for high mortality, potential for major impact to public health, potential to cause public panic and social disruption, and the requirement of special action for public health preparedness. Brucellosis and Q fever appear in Category B where, despite high infectiousness, mortality rates are lower [91]. One key aspect to disease threat categorisation is whether the disease exists naturally or is endemic. For example, in the UK, any confirmed case of a non-endemic biothreat should be assumed to be the result of a deliberate release until proven otherwise [31]. This is the case for pulmonary anthrax and tularaemia, in addition to other zoonoses such as smallpox, plague, glanders, Venezuelan equine encephalitis (VEE) and viral haemorrhagic fever (VHF). Appreciation of an area's endemic pathogens, in the context of global distribution, is therefore of considerable importance to threat assessment [88]. Anthrax is possibly the most high-profile modern biological threat agent, due to its weaponization and use in the late 20th century, most notably the intentional contamination of postal letters in 2001, resulting in five mortalities [92]. There has been speculative evidence of C. burnetii used maliciously in Europe in the past, including an outbreak of Q fever among army troops during World War II [19]. Indeed, F. tularensis was also suspected to have been deployed maliciously during World War II [17]. Used as weapons, Brucella species (notably B. suis), F. tularensis subsp. holarctica and C. burnetii would have low mortality rates, but carry the potential to debilitate large numbers of people and animals, contaminate the environment, and disrupt animal industries [19], [93].
While transmission of zoonotic disease in the EU/EEA is most relevant to those with occupational health risks, global threats to human, animal and environmental health security do remain from cross-border transmission, environmentally resilient pathogens and the potential for biological agent weaponization. The most poignant risks to global health is the lack of disease awareness and ignorance of the interlinked connections between global health, food safety, antimicrobial resistance and biological security threats. Thus, employing a One Health approach is vital, and local and international information-sharing on surveillance, control and prevention measures is of the utmost importance to enabling One Health for all zoonoses.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Jennifer Dow (London School of Hygiene and Tropical Medicine) for advice on the tularaemia section; and Adam Thomas and Rhys Cutlan (University of Exeter) for assistance in preparing figures.
A.R.C. is supported by a BBSRC iCASE Studentship in partnership with the University of Exeter and the Defence Science and Technology Laboratory (Dstl) (Grand no. BB/M016404/1). S.R. is supported by the BBSRC grant number BB/N001591/1.
The dataset used for this publication came from the ECDC Surveillance Atlas of Infectious Diseases. This can be accessed at https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases. These data are available under the ECDC’s terms of use. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the ECDC. The accuracy of the authors' statistical analysis and the findings they report are not the responsibility of ECDC. ECDC is not responsible for conclusions or opinions drawn from the data provided. ECDC is not responsible for the correctness of the data and for data management, data merging and data collation after provision of the data. ECDC shall not be held liable for improper or incorrect use of the data.
Footnotes
Data collected through The European Surveillance System (TESSy). Data is only available for Croatia from 2012.
References
- 1.CDC . 20/02/2018. One health.https://www.cdc.gov/onehealth/ [Google Scholar]
- 2.Morand S., McIntyre K.M., Baylis M. Domesticated animals and human infectious diseases of zoonotic origins: domestication time matters. Infect Genet Evol. 2014;24:76–81. doi: 10.1016/j.meegid.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 3.FAO/OIE/WHO . 15–17 November 2011. High-level technical meeting to address health risks at the human-animal ecosystems interfaces: Mexico city, Mexico. [Google Scholar]
- 4.Gebreyes W.A., Dupouy-Camet J., Newport M.J., Oliveira C.J., Schlesinger L.S., Saif Y.M. The global one health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Neglected Trop Dis. 2014;8:e3257. doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . 2015. The control of neglected zoonotic diseases: from advocacy to action: report of the fourth international meeting held at WHO headquarters, Geneva, Switzerland. Geneva; p. 44. [Google Scholar]
- 6.Salyer S.J., Silver R., Simone K. Barton Behravesh C. Prioritizing zoonoses for global health capacity building-themes from one health zoonotic disease workshops in 7 countries, 2014–2016. Emerg Infect Dis. 2017;23 doi: 10.3201/eid2313.170418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan L.H., Kaplan B., Monath T.P., Woodall J., Conti L.A. 26/02/2018. One health initiative.http://www.onehealthinitiative.com/ [Google Scholar]
- 8.Gyles C. One medicine, one health, one world. Can Vet J. 2016;57:345–346. [PMC free article] [PubMed] [Google Scholar]
- 9.Cleaveland S., Sharp J., Abela-Ridder B., Allan K.J., Buza J., Crump J.A. One health contributions towards more effective and equitable approaches to health in low- and middle-income countries. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160168. doi: 10.1098/rstb.2016.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ECDC . 25/05/2018. Surveillance Atlas of infectious diseases.http://atlas.ecdc.europa.eu/public/index.aspx [Google Scholar]
- 11.Eurostat . 28/11/2017. Population on 1 January.http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&plugin=1&language=en&pcode=tps00001 [Google Scholar]
- 12.Tigertt W.D., Benenson A.S., Gochenour W.S. Airborne Q fever. Bacteriol Rev. 1961;25:285–293. doi: 10.1128/br.25.3.285-293.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamal S.M., Rashid A.K., Bakar M.A., Ahad M.A. Anthrax: an update. Asian Pac J Trop Biomed. 2011;1:496–501. doi: 10.1016/S2221-1691(11)60109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbel M.J. World Health Organization; 2006. Brucellosis in humans and animals. [Google Scholar]
- 15.Broman T., Thelaus J., Andersson A.C., Backman S., Wikstrom P., Larsson E. Molecular detection of persistent Francisella tularensis subspecies holarctica in natural waters. Internet J Microbiol. 2011;2011:851946. doi: 10.1155/2011/851946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hestvik G., Warns-Petit E., Smith L.A., Fox N.J., Uhlhorn H., Artois M. The status of tularemia in Europe in a one-health context: a review. Epidemiol Infect. 2015;143:2137–2160. doi: 10.1017/S0950268814002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennis D.T., Inglesby T.V., Henderson D.A., Bartlett J.G., Ascher M.S., Eitzen E. Tularemia as a biological weapon. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 18.Bossi P., Tegnell A., Baka A., Van Loock F., Hendriks J., Werner A. Bichat guidelines for the clinical management of brucellosis and bioterrorism-related brucellosis. Eur Commun Dis Bull. 2004:6. doi: 10.2807/esm.09.12.00506-en. [DOI] [PubMed] [Google Scholar]
- 19.Madariaga M.G., Rezai K., Trenholme G.M., Weinstein R.A. Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3:709–721. doi: 10.1016/s1473-3099(03)00804-1. [DOI] [PubMed] [Google Scholar]
- 20.Manchee R.J., Broster M.G., Stagg A.J., Hibbs S.E. Formaldehyde solution effectively inactivates spores of Bacillus anthracis on the Scottish island of Gruinard. Appl Environ Microbiol. 1994;60:4167–4171. doi: 10.1128/aem.60.11.4167-4171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fasanella A., Scasciamacchia S., Garofolo G., Giangaspero A., Tarsitano E., Adone R. Evaluation of the house fly Musca domestica as a mechanical vector for an anthrax. PLoS One. 2010;5:e12219. doi: 10.1371/journal.pone.0012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryden P., Bjork R., Schafer M.L., Lundstrom J.O., Petersen B., Lindblom A. Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis. 2012;205:297–304. doi: 10.1093/infdis/jir732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrada Z.L., Telford S.R. Diversity of Francisella species in environmental samples from Martha's vineyard, Massachusetts. Microb Ecol. 2010;59:277–283. doi: 10.1007/s00248-009-9568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastwood K., Massey P.D., Hutchinson P., van den Berg D., Bosward K., Graves S.R. Q fever: a rural disease with potential urban consequences. Aust J Gen Pract. 2018;47:5555. doi: 10.31128/AFP-08-17-4299. [DOI] [PubMed] [Google Scholar]
- 25.Marston C.K., Ibrahim H., Lee P., Churchwell G., Gumke M., Stanek D. Anthrax toxin-expressing Bacillus cereus isolated from an anthrax-like Eschar. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156987. e0156987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dittmann C., Han H.M., Grabenbauer M., Laue M. Dormant Bacillus spores protect their DNA in crystalline nucleoids against environmental stress. J Struct Biol. 2015;191:156–164. doi: 10.1016/j.jsb.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Stark J.F. Bacteriology in the service of sanitation: the factory environment and the regulation of industrial anthrax in late-victorian Britain. Soc Hist Med. 2011;25:343–361. [Google Scholar]
- 28.Report of the dangerous trades (anthrax) committee. Public health journal. Elsevier Inc.; 1897. pp. 379–380. [Google Scholar]
- 29.Schneemann A., Manchester M. Anti-toxin antibodies in prophylaxis and treatment of inhalation anthrax. Future Microbiol. 2009;4:35–43. doi: 10.2217/17460913.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longstreth J., Skiadopoulos M.H., Hopkins R.J. Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: the first vaccine licensed using the FDA animal rule. Expert Rev Vaccines. 2016;15:1467–1479. doi: 10.1080/14760584.2016.1254556. [DOI] [PubMed] [Google Scholar]
- 31.EMA/CHMP . European Medicines Agency; London: 2014. Guidance document on use of medicinal products for the treatment and prophylaxis of biological agents that might be used as weapons of bioterrorism. [Google Scholar]
- 32.Xing Y.H., Wang W., Dai S.Q., Liu T.Y., Tan J.J., Qu G.L. Daptomycin exerts rapid bactericidal activity against Bacillus anthracis without disrupting membrane integrity. Acta Pharmacol Sin. 2014;35:211–218. doi: 10.1038/aps.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbull P.C. Anthrax vaccines: past, present and future. Vaccine. 1991;9:533–539. doi: 10.1016/0264-410x(91)90237-z. [DOI] [PubMed] [Google Scholar]
- 34.Laws T.R., Kuchuloria T., Chitadze N., Little S.F., Webster W.M., Debes A.K. A comparison of the adaptive immune response between recovered anthrax patients and individuals receiving three different anthrax vaccines. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148713. e0148713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sim B.K.L., Li M., Osorio M., Wu Y., Wai T.T., Peterson J.W. Protection against inhalation anthrax by immunization with Salmonella enterica serovar Typhi Ty21a stably producing protective antigen of Bacillus anthracis. NPJ Vaccine. 2017;2:17. doi: 10.1038/s41541-017-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brett M.M., Hood J., Brazier J.S., Duerden B.I., Hahne S.J. Soft tissue infections caused by spore-forming bacteria in injecting drug users in the United Kingdom. Epidemiol Infect. 2005;133:575–582. doi: 10.1017/s0950268805003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Team N.A.O.C. 2011. An outbreak of Anthrax among drug users in Scotland, december 2009 to december 2010. Glasgow. [Google Scholar]
- 38.Grunow R., Verbeek L., Jacob D., Holzmann T., Birkenfeld G., Wiens D. Injection anthrax–a new outbreak in heroin users. Dtsch Arztebl Int. 2012;109:843–848. doi: 10.3238/arztebl.2012.0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fasanella A., Garofolo G., Galante D., Quaranta V., Palazzo L., Lista F. Severe anthrax outbreaks in Italy in 2004: considerations on factors involved in the spread of infection. N Microbiol. 2010;33:83–86. [PubMed] [Google Scholar]
- 40.Popescu R., Pistol A., Miltaru L., Caplan D., Cucuiu R., Popovici F. Two cases of infection with Bacillus anthracis, Romania, October 2011. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 41.ECDC/ESFA . ECDC; Stockholm: 2015. Technical report on a fatal human case of Bacillus anthracis infection and bovine meat contamination in Bulgaria. [Google Scholar]
- 42.WHO . World Health Organization; Geneva: 2008. Anthrax in animals. Anthrax in humans and animals. [Google Scholar]
- 43.Pappas G., Papadimitriou P., Akritidis N., Christou L., Tsianos E.V. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 44.Pascual D.W., Yang X., Wang H., Goodwin Z., Hoffman C., Clapp B. Alternative strategies for vaccination to brucellosis. Microb Infect. 2018;20:599–605. doi: 10.1016/j.micinf.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossetti C.A., Arenas-Gamboa A.M., Maurizio E. Caprine brucellosis: a historically neglected disease with significant impact on public health. PLoS Neglected Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005692. e0005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nenova R., Tomova I., Saparevska R., Kantardjiev T. A new outbreak of brucellosis in Bulgaria detected in July 2015–preliminary report. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.ES.2015.20.39.30031. [DOI] [PubMed] [Google Scholar]
- 47.Tuon F.F., Gondolfo R.B., Cerchiari N. Human-to-human transmission of Brucella - a systematic review. Trop Med Int Health. 2017;22:539–546. doi: 10.1111/tmi.12856. [DOI] [PubMed] [Google Scholar]
- 48.ESFA E.C.D.C. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2013. EFSA J. 2015:3991. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bundle D.R., Brucellosis McGiven J. Improved diagnostics and vaccine insights from synthetic Glycans. Acc Chem Res. 2017;50:2958–2967. doi: 10.1021/acs.accounts.7b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pappas G. Brucellosis in the world today | HCDCP. 20/02/2018. http://www2.keelpno.gr/blog/?p=2033&lang=en
- 51.Mancini F.R., Bella A., Graziani C., Marianelli C., Mughini-Gras L., Pasquali P. Trends of human brucellosis in Italy, 1998–2010. Epidemiol Infect. 2014;142:1188–1195. doi: 10.1017/S0950268813002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karcheva M.D., Birdanova V.A., Alexandrova M.L. Human brucellosis -new public health problem in Bulgaria. Int J Infect Dis Ther. 2017;2:66–71. [Google Scholar]
- 53.Mailles A., Garin-Bastuji B., Lavigne J.P., Jay M., Sotto A., Maurin M. Human brucellosis in France in the 21st century: results from national surveillance 2004–2013. Med Maladies Infect. 2016;46:411–418. doi: 10.1016/j.medmal.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Gwida M., Neubauer H., Ilhan Z., Schmoock G., Melzer F., Nockler K. Cross-border molecular tracing of brucellosis in Europe. Comp Immunol Microbiol Infect Dis. 2012;35:181–185. doi: 10.1016/j.cimid.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Grunow R., Jacob D., Klee S., Schlembach D., Jackowski-Dohrmann S., Loenning-Baucke V. Brucellosis in a refugee who migrated from Syria to Germany and lessons learnt, 2016. Euro Surveill. 2016;21:30311. doi: 10.2807/1560-7917.ES.2016.21.31.30311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savini L., Candeloro L., Conte A., Massis D.F., Giovannini A. Development of a forecasting model for brucellosis spreading in the Italian cattle trade network aimed to prioritise the field interventions. PLoS One. 2017:12. doi: 10.1371/journal.pone.0177313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heptonstall J., Gent N. Health Protection Agency; London: 2006. CRBN incidents: clinical management & health protection. [Google Scholar]
- 58.Splettstoesser W.D., Piechotowski I., Buckendahl A., Frangoulidis D., Kaysser P., Kratzer W. Tularemia in Germany: the tip of the iceberg? Epidemiol Infect. 2009;137:736–743. doi: 10.1017/S0950268808001192. [DOI] [PubMed] [Google Scholar]
- 59.Fortier A.H., Slayter M.V., Ziemba R., Meltzer M.S., Nacy C.A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu P., Cunningham A.L., Yu J.J., Nguyen J.Q., Barker J.R., Lyons C.R. Live attenuated Francisella novicida vaccine protects against Francisella tularensis pulmonary challenge in rats and non-human primates. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004439. e1004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cuccui J., Thomas R.M., Moule M.G., D'Elia R.V., Laws T.R., Mills D.C. Exploitation of bacterial N-linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biol. 2013;3:130002. doi: 10.1098/rsob.130002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maurin M., Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis. 2016;16:113–124. doi: 10.1016/S1473-3099(15)00355-2. [DOI] [PubMed] [Google Scholar]
- 63.Shahsavari S., Baghi H., Kafil H., Leylabadlo H. Re-emerging tularemia in some Middle East countries: what are the reasons? Iran J Public Health. 2018;47:305–306. [PMC free article] [PubMed] [Google Scholar]
- 64.Berrada Z., Iii S.R. Survival of Francisella tularensis type a in brackish-water. Arch Microbiol. 2011;193:223–226. doi: 10.1007/s00203-010-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Telford S.R., Goethert H.K. Toward an understanding of the perpetuation of the agent of tularemia. Front Microbiol. 2011;1:150. doi: 10.3389/fmicb.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eldin C., Melenotte C., Mediannikov O., Ghigo E., Million M., Edouard S. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30:115–190. doi: 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duncan C., Kersh G.J., Spraker T., Patyk K.A., Fitzpatrick K.A., Massung R.F. Coxiella burnetii in northern Fur seal (Callorhinus ursinus) placentas from St. Paul Island, Alaska. Vector Borne Zoonotic Dis. 2012;12:192–195. doi: 10.1089/vbz.2011.0715. [DOI] [PubMed] [Google Scholar]
- 68.Mori M., Roest H.J. Farming, Q fever and public health: agricultural practices and beyond. Arch Publ Health. 2018;76:2. doi: 10.1186/s13690-017-0248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pijnacker R., Reimerink J., Smit L.A.M., van Gageldonk-Lafeber A.B., Zock J.P., Borlee F. Remarkable spatial variation in the seroprevalence of Coxiella burnetii after a large Q fever epidemic. BMC Infect Dis. 2017;17:725. doi: 10.1186/s12879-017-2813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ackland J.R., Worswick D.A., Marmion B.P. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Aust. 1994;160:704–708. [PubMed] [Google Scholar]
- 71.Hermans M.H., Huijsmans C.R., Schellekens J.J., Savelkoul P.H., Wever P.C. Coxiella burnetii DNA in goat milk after vaccination with Coxevac((R)) Vaccine. 2011;29:2653–2656. doi: 10.1016/j.vaccine.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 72.Muskens J., van Engelen E., van Maanen C., Bartels C., Lam T.J. Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. Vet Rec. 2011;168:79. doi: 10.1136/vr.c6106. [DOI] [PubMed] [Google Scholar]
- 73.Anastacio S., Carolino N., Sidi-Boumedine K., da Silva G.J. Q fever dairy herd status determination based on serological and molecular analysis of bulk tank milk. Transbound Emerg Dis. 2016;63 doi: 10.1111/tbed.12275. e293-300. [DOI] [PubMed] [Google Scholar]
- 74.Ryan E.D., Wrigley K., Hallinan A., McGrath G., Clegg T.A. Antibodies to Coxiella burnetii in Irish bulk tank milk samples. Vet Rec. 2018 doi: 10.1136/vr.104663. [DOI] [PubMed] [Google Scholar]
- 75.Raoult D., Marrie T., Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 76.Million M., Raoult D. No such thing as chronic Q fever. Emerg Infect Dis. 2017;23:856–857. doi: 10.3201/eid2305.151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taurel A.-F., Guatteo R., Joly A., Beaudeau F. Effectiveness of vaccination and antibiotics to control Coxiella burnetii shedding around calving in dairy cows. Vet Microbiol. 2012;159:432–437. doi: 10.1016/j.vetmic.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 78.Reeves P.M., Paul S.R., Sluder A.E., Brauns T.A., Poznansky M.C. Q-vaxcelerate: a distributed development approach for a new Coxiella burnetii vaccine. Hum Vaccines Immunother. 2017;13:2977–2981. doi: 10.1080/21645515.2017.1371377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneeberger P.M., Wintenberger C., van der Hoek W., Stahl J.P. Q fever in The Netherlands – 2007–2010: what we learned from the largest outbreak ever. Med Maladies Infect. 2014;44:339–353. doi: 10.1016/j.medmal.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Schneeberger P.M., Hermans M.H., van Hannen E.J., Schellekens J.J., Leenders A.C., Wever P.C. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol. 2010;17:286–290. doi: 10.1128/CVI.00454-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Some Pieters J. 74 people killed by Q-Fever outbreak. NL Times. 2016 https://nltimes.nl/2016/05/31/74-people-killed-q-fever-outbreak [Google Scholar]
- 82.Kampschreur L.M., Delsing C.E., Groenwold R.H.H., Wegdam-Blans M.C.A., Bleeker-Rovers C.P., de Jager-Leclercq M.G.L. Chronic Q fever in The Netherlands 5 Years after the start of the Q Fever epidemic: results from the Dutch chronic Q fever database. J Clin Microbiol. 2014;52:1637–1643. doi: 10.1128/JCM.03221-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edouard S., Mahamat A., Demar M., Abboud P., Djossou F., Raoult D. Comparison between emerging Q fever in French Guiana and endemic Q fever in Marseille, France. Am J Trop Med Hyg. 2014;90:915–919. doi: 10.4269/ajtmh.13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eldin C., Mahamat A., Demar M., Abboud P., Djossou F., Raoult D. Q fever in French Guiana. Am J Trop Med Hyg. 2014;91:771–776. doi: 10.4269/ajtmh.14-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pommier de Santi V., Briolant S., Mahamat A., Ilcinkas C., Blanchet D., de Thoisy B. Q fever epidemic in Cayenne, French Guiana, epidemiologically linked to three-toed sloth. Comp Immunol Microbiol Infect Dis. 2018;56:34–38. doi: 10.1016/j.cimid.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Gyuranecz M., Sulyok K., Balla E., Mag T., Balazs A., Simor Z. Q fever epidemic in Hungary, April to July 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.30.20863. [DOI] [PubMed] [Google Scholar]
- 87.Cantas H., Muwonge A., Sareyyupoglu B., Yardimci H., Skjerve E. Q fever abortions in ruminants and associated on-farm risk factors in northern Cyprus. BMC Vet Res. 2011;7:13. doi: 10.1186/1746-6148-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belay E.D., Kile J.C., Hall A.J., Barton-Behravesh C., Parsons M.B., Salyer S. Zoonotic disease programs for enhancing global health security. Emerg Infect Dis. 2017;23 doi: 10.3201/eid2313.170544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gunaratnam P., Massey P.D., Eastwood K., Durrhein D., Graves S., Coote D. Diagnosis and management of zoonoses - a tool for general practice. Aust Fam Physician. 2014;43:124–128. [PubMed] [Google Scholar]
- 90.Li Y., Yang X., Zhao W. Emerging microtechnologies and automated systems for rapid bacterial identification and antibiotic susceptibility testing. SLAS Technol. 2017;22:585–608. doi: 10.1177/2472630317727519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CDC . 20/02/2018. Bioterrorism agents/diseases (by category) | emergency preparedness & response.https://emergency.cdc.gov/agent/agentlist-category.asp [Google Scholar]
- 92.Dewan P.K., Fry A.M., Laserson K., Tierney B.C., Quinn C.P., Hayslett J.A. Inhalational anthrax outbreak among postal workers, Washington, D.C., 2001. Emerg Infect Dis. 2002;8:1066–1072. doi: 10.3201/eid0810.020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Croddy E.A. ABC-CLIO, ABC-CLIO; Santa Barbara, California: 2005. Volume I: chemical and biological weapons. [Google Scholar]