Using the structure of hCD36, CIDRα binding peptides, and the differential binding of CD36 orthologs to Plasmodium falciparum-infected erythrocytes (IEs), we investigated how the structure of hCD36 dictates its interaction with intact IEs.
Keywords: host-parasite interaction, infected erythrocytes, ortholog swap mutagenesis, scavenger receptor
Abstract
Background
The interaction of Plasmodium falciparum-infected erythrocytes (IEs) with the host receptor CD36 is among the most studied host-parasite interfaces. CD36 is a scavenger receptor that binds numerous ligands including the cysteine-rich interdomain region (CIDR)α domains of the erythrocyte membrane protein 1 family (PfEMP1) expressed on the surface of IEs. CD36 is conserved across species, but orthologs display differential binding of IEs.
Methods
In this study, we exploited these differences, combined with the recent crystal structure and 3-dimensional modeling of CD36, to investigate malaria-CD36 structure-function relationships and further define IE-CD36 binding interactions.
Results
We show that a charged surface in the membrane-distal region of CD36 is necessary for IE binding. Moreover, IE interaction with this binding surface is influenced by additional CD36 domains, both proximal to and at a distance from this site.
Conclusions
Our data indicate that subtle sequence and spatial differences in these domains modify receptor conformation and regulate the ability of CD36 to selectively interact with its diverse ligands.
Plasmodium falciparum malaria remains a leading cause of childhood mortality responsible for an estimated 445 000 deaths in 2016 [1–3]. Plasmodium falciparum virulence depends on the expression of a diverse var gene family encoding the erythrocyte membrane protein 1 family (PfEMP1), which is exposed on the infected erythrocyte (IE) surface. The var gene family undergoes transcriptional switching to modify the antigenic properties and adhesive phenotype of the IE and confers its ability to sequester in the microvasculature [4–6].
PfEMP1 are large proteins that contain 2 to 10 adhesive domains termed Duffy binding-like and cysteine-rich interdomain region (CIDR) domains [4–6]. Of the various host receptors that engage PfEMP1 proteins, the interaction with the scavenger receptor CD36 is among the most studied. CIDRα2–6 domains have been reported to mediate the interaction between PfEMP1 and CD36 [7–9].
CD36 is a class B scavenger receptor that belongs to a larger superfamily of pattern recognition receptors (PRRs). CD36 binds a diverse range of ligands including both pathogen-associated molecular patterns (PAMPs) and modified self-molecules [10]. CD36 can recognize PAMPs present directly on pathogens or expressed on their infected host cells including specific lipids in bacterial cell walls and parasite proteins embedded in the IE surface [10, 11]. These CD36-pathogen interactions mediate opsonin-independent uptake by monocytic cells [12]. CD36 also recognizes and internalizes endogenously derived ligands including modified lipoproteins such as oxidized low-density lipoprotein (oxLDL) [11]. CD36 has been shown to form complexes with multiple coreceptors and membrane proteins including Toll-like receptors ([TLR]2, TLR4, TLR6) [11, 13].
The role of CD36 in both homeostasis and disease has been studied for over 40 years, but the relationship between CD36 and malaria has been controversial [14]. Recent evidence indicates that CD36 binding is generally associated with milder infection, whereas binding to the endothelial protein C receptor (EPCR) is linked to severe disease [15].
The lack of structural data on this class of receptors has limited an understanding of how CD36 selectively engages and differentially responds to its diverse repertoire of ligands. Insights into these processes have been advanced by solving the crystal structure of the first member of this class of scavenger receptors (lysosomal integral membrane protein [LIMP]-2) and inferring the structure of CD36 by homology modeling [16]. CD36 forms a “hairpin-like” structure with a large ectodomain and 2 transmembrane domains. The ectodomain of CD36 is capped by a 3-α-helix bundle and apex region that contains an accumulation of cationic residues, proposed to function as a binding site for its polyanionic ligands, such as modified lipoproteins [16].
Cocrystallization studies of CIDRα2–6 domains and the CD36 ectodomain identified a conserved hydrophobic pocket on CIDRα peptides that bind a phenylalanine residue (F153) on CD36 [9]. However, these studies did not examine intact IE-CD36 interactions. Moreover, orthologs such as bovine CD36 (bCD36) express conserved cationic residues and F153 in the proposed binding surface, yet bind IEs poorly compared with human CD36 (hCD36). We hypothesized that the differential IE binding displayed by mammalian orthologs could be used to further refine CD36-malaria structure-function relationships [11]. In this study, we use an ortholog-substitution approach combined with recent structural data and CIDR peptides, to define regions important for IE binding.
METHODS
Sequences and Alignments
CD36 ortholog sequences were acquired from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) and aligned using ClustalW (www.ebi.ac.uk/Tools/msa/clustalw2/): AAA16068.1 (Homo sapiens), AAH10262.1 (Mus musculus), AAH72543.1 (Rattus norvegicus), and AAI03113.1 (Bos taurus).
Cloning and Sequencing
Full-length hCD36 and mutants were cloned and sequenced as previously described [16]. hCD36 was used as template for cloning most human constructs with bovine-sequence insertions (See Supplementary Table 1 for templates and primer sequences) with a polymerase chain reaction (PCR) strategy using overlapping primers encompassing the desired mutation and the kanamycin cassette and amplified with CloneAmp HiFi PCR Premix (Clontech). The PCR products were purified, DpnI (NEB) digested, and ligated using In-Fusion HD cloning kit (Clonetech). bCD36 was amplified from the clone Image ID 7985341 (BioScience) and cloned into pEGFP-N1 vector. This construct was used as template for cloning bovine constructs with human-sequence insertions (see Supplementary Table 1). All constructs were sequence verified. All tested mutations, their backbone and associated figures are listed in Table 1.
Table 1.
CD36 Mutations Included in this Manuscript
| Backbone | Introduced Mutations | Associated Figures |
|---|---|---|
| hCD36 wild type | L158E, K164E, K166E, K164/166E | Figure 2 |
| hCD36 wild type | Bovine Domains I and II | Figure 3 |
| Bovine Domain I: PQLPTMG, PQLP, P146 | Figures 4–6 | |
| Bovine Domain II: HGELAESSS, ESSS, S359 | Figures 4–6 | |
| bCD36 wild type | Human Domains I and II | Figure 3 |
| Human Domain I: SHIQQVM, SHIQ, S146 | Figures 4–6 | |
| Human Domain II: YADVSDGNR, DGNR, G359 | Figures 4–6 |
Parasite Culture
Plasmodium falciparum MR4 strains E8B and CS2 strains were cultured in T-25 flasks with 5% hematocrit in complete Dulbecco’s modified Eagle’s medium (DMEM) with a microaerophilic environment (1% O2, 5% CO2, and rest N2) as previously described [17].
Cell Culture and Transfection
CHO and Cos-7 cells were cultured to confluence in DMEM (Gibco) media with penicillin/streptomycin (Sigma) and 10% fetal bovine serum (Sigma) at 37°C and 5% CO2. One million cells were plated in T25 flasks or 12-well plates with coverslips and transfected the next day using Polyethylenimine (PEI) (Polysciences, Inc.) as previously described [18]. Here, PEI was used in a 3:1 ratio with the plasmid deoxyribonucleic acid of interest. After 15-minute incubation, the mixture was added dropwise to the cells and incubated for 4 hours. Media was exchanged and the flasks were incubated for 24 hours. Experiments were standardized using both CHO and Cos-7 cell lines. CHO was used for flow cytometry experiments, and Cos-7 was used for cytoadherence assays and confocal microscopy.
Surface Expression Determination
Surface expression of all the constructs was measured by flow cytometry using different anti-CD36 antibodies (Supplementary Figure 2): FA6-152 mouse immunoglobulin (Ig)G1 monoclonal antibody (mAb) (Beckman Coulter) and the phycoerythrin (PE)-conjugated IgM mAb (BD Biosciences). A CD36-binding peptide (CIDRa6_D3_IT4var12) specifically bound to all CD36 constructs and was also used to normalize expression of constructs.
Cytoadhesion Assay
Binding assays using non-IEs, CS2 (CSA binder) IEs, and E8B (CD36 binder) IEs were conducted. No binding was observed with any construct using noninfected and CS2 IEs (data not shown). E8B is a clone of ItG2 selected for adhesion to CD36, and A4tres var is reported as a dominant var transcript [19]. Any reference to IE is related to assays performed with E8B parasites. Mutant constructs are listed in Supplementary Table 1. Results are shown only for those mutations that showed significant differences compared with wild type (WT). Cytoadhesion assays were performed as described previously [20]. In brief, 24 hours after transfection, late-stage IEs were washed 3 times with Bis Tris saline (BTS) buffer (pH 6.8) and quantified. Culture was adjusted to 10% parasitemia in BTS, and 300 μL were added per well and continuously agitated for 90 minutes. Nonadherent cells were removed by aspiration, and coverslips were rinsed by submersion in phosphate-buffered saline (PBS). Cells were then fixed with 4% formaldehyde in PBS for 10 minutes and washed 3 times with PBS alone. Phycoerythrin-conjugated glycophorin A (BioLegend) was used to stain the IEs by 30-minute incubation. Coverslips were rinsed, and 4’,6-diamidino-2-phenylindole was added for 5 minutes. Coverslips were mounted with fluoromount (Dako). Tiles were acquired for quantification using a ×25 0.8 numerical aperture (NA) oil-immersion lens using a Zeiss AxioObserver Apotome equipped with an AxioCam camera, and images were obtained using the Zeiss ZEN software. Cells were visualized with a Zeiss ×63 1.4 NA oil-immersion lens using a Quorum spinning-disk confocal equipped with a back-thinned Hamamatsu EM-CCD camera (C9100-13); images were acquired by and processed with Perkin-Elmer Volocity software.
Cysteine-Rich Interdomain Region Peptide Binding
His-tagged CIDR peptides CIDRa1.1_D3_IT4var19, CIDRa5_D3_IT4var14, and CIDRa2.10_D2_IT4var30 were used as previously described [9]. Cells (0.5–1 million) were incubated with 100 μg/mL peptide in 2% bovine serum albumin for 60 minutes on ice. Cells were washed with PBS and incubated with 20 μg/mL anti-His mouse mAb (Abgent) for 30 minutes. Cells where then washed and incubated with 20 μg/mL anti-mouse Alexa Fluor 594 (Molecular Probes). After a final wash, cells were fixed with 1.6% formaldehyde in PBS for 10 minutes. Stained cells were washed and analyzed by flow cytometry.
Oxidized Low-Density Lipoprotein Binding
Cells (0.5–1 million) were stained with DiI-oxLDL (Intracel) for 30 minutes on ice. Cells where washed and fixed with 1.6% formaldehyde in PBS for 10 minutes. Stained cells were washed and transferred into polystyrene tubes for analysis by flow cytometry.
Flow Cytometry
Twenty-four hours after transfection, cells were detached from the flasks using 10 mM ethylenediaminetetraacetic acid (EDTA) for 10 minutes. Cells were stained for surface expression, CIDRα, or oxLDL binding, and 50 000 events were analyzed with a Becton Dickinson LSRII-CFI BGRV flow cytometer and Diva software. AbC Anti-Mouse Bead Kit (Invitrogen) was used for Alexa Fluor 594 compensation, and DiI (Molecular Probes) was used for DiI-oxLDL compensation.
Result Interpretation and Statistical Analysis
Flow cytometry data were analyzed using FlowJo X. Data were analyzed and graphed using GraphPad Prism 5 software. Graphs are presented with mean and standard deviation from a minimum of 3 independent experiments. All results were normalized to CD36 surface expression as indicated on the figure legend.
RESULTS
CD36-Infected Erythrocyte Interactions: The Role of Residues Previously Implicated in Ligand Binding
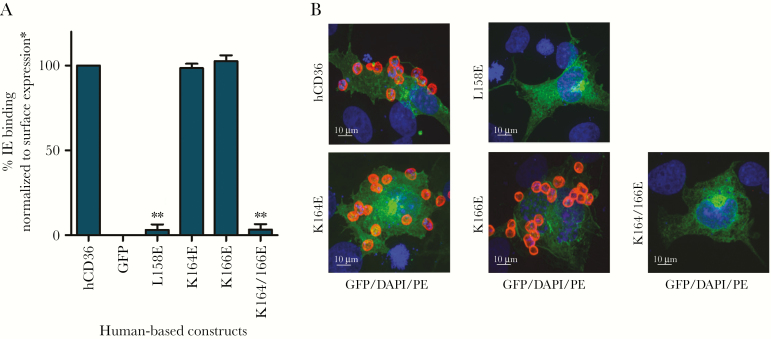
The putative tertiary structure and spatial relation between domains of CD36 have been modeled (Figure 1) [16]. A previously defined membrane-distal immunodominant region (155 to 183) lies in the helical bundle and apex region of the ectodomain [21]. To investigate a role for this region in binding IEs, we conducted a mutagenesis study of residues previously implicated in binding oxLDL [16]. We compared IE binding to WT and mutant CD36 molecules after correcting for any differences in receptor surface expression (Figure 2; Supplementary Figure 1). Mutation of the leucine residues at positions 158 (L158) and/or 161 (L161) to glutamic acid has previously been reported to abrogate oxLDL binding [16], whereas in our studies only L158E disrupted IE binding. Likewise, mutation of the cationic lysine residues at positions 164 and 166 (K164E and K166E) of CD36 eliminated IE binding, whereas single mutations did not (Figure 2, Supplementary Figure 1). Of note, point mutation of K164 also abrogated CD36 binding by the mAb FA6-152, which blocks IE binding by CD36 (Supplementary Figure 1).
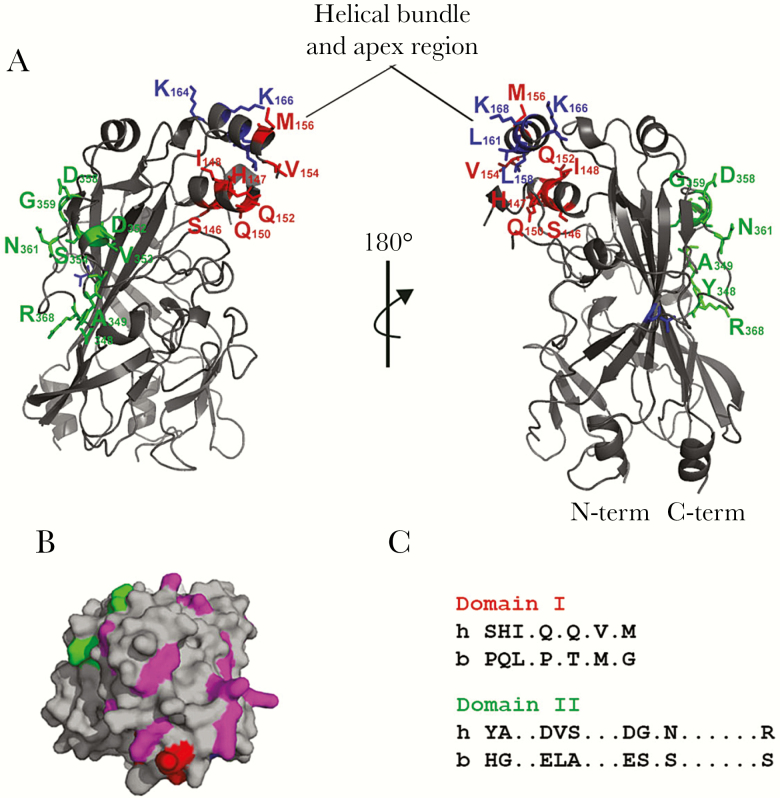
Figure 1.
Human CD36 model. (A) Three-dimensional model based on the crystal structure of lysosomal integral membrane protein (LIMP)-2 [16]. Structure depicted in gray and domains of interest color coded. Domain I in red: SHIQQVM; domain II in green: YADVSDGNR; and other amino acids of interest in blue: T, LLKK. Model generated using PyMol. Helical bundle and apex region marked. (B) The cationic patch on the surface of CD36 viewed from the top. Cationic residues in the area depicted in magenta, whereas flanking domain I (red) and domain II (green) are highlighted. The rest of the structure is shown in gray. (C) Domain I and II alignment between human and bovine CD36 sequences.
Figure 2.
Infected erythrocyte (IE) binding by human CD36 mutants. (A) Quantification of IE binding assays on mutant CD36-green fluorescent protein (GFP). Infected erythrocyte binding of a selected experiment in triplicate is shown (mean + standard deviation), and statistical significance compared with hCD36 wild type (**, P < .01) is marked. Binding experiments were fixed and mounted for analysis using Zeiss Apotome. Tiles (10 × 10) were recorded using ×25 objective. (B) Confocal images (×63) of representative IE binding experiments of GFP fusion expressing cell lines (green). For fluorescent visualization, IEs were stained with phycoerythrin (PE)-conjugated antiglycophorin A as surface marker (red) and 4’,6-diamidino-2-phenylindole (DAPI) to stain the nucleus (blue). A 10-μm size bar is shown. Surface expression was normalized to BD555455 monoclonal antibody binding.
CD36 Conservation: Mapping the Infected Erythrocyte Interaction Site by Ortholog Substitution
We examined the binding of P falciparum IEs by different CD36 orthologs. hCD36, mouse, and rat CD36 all supported IE binding, whereas bCD36 displays markedly reduced binding [20, 22] (Figure 3). These orthologs share more than 80% sequence identity. This sequence conservation, combined with differential IE binding, was used to investigate IE-CD36 binding interactions. Using multispecies sequence alignment, 2 areas of key divergence between bovine and the IE-binding orthologs were identified: domain I (residues 146–156, SHIQQVM) and domain II (residues 348–368, YADVSDGNR) (Figure 1 and Supplementary Figure 2). Secondary structure prediction tools [23] predict helixes in the hCD36 sequence within those regions of interest but not in bCD36, suggesting differential secondary structure. Domains I and II are predicted to be opposite each other on the hCD36 model with domain I buried within the helical bundle and domain II in an exposed region of the ectodomain (Figure 1).
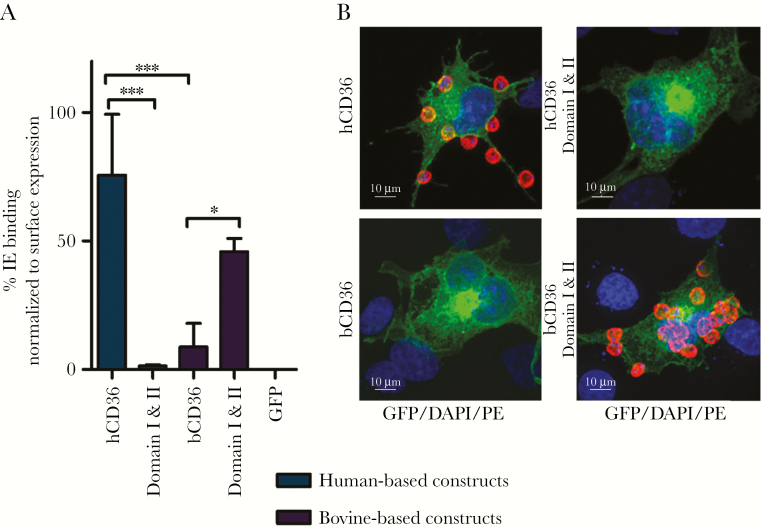
Figure 3.
Infected erythrocyte binding by human and bovine CD36. (A) Quantification of IE binding of a representative experiment in triplicate (mean + standard deviation). Statistical significance compared with hCD36 (blue) and bCD36 (purple) wild type are shown (*, P < .05; ***, P < .0001). (B) Confocal images (×63) of representative parasite binding experiments of green fluorescent protein (GFP) fusion expressing cell lines (blue, 4’,6-diamidino-2-phenylindole [DAPI]; green, GFP fusion construct; red, antiglycophorin A-phycoerythrin [PE]). A 10-μm size bar is shown. hCD36 surface expression was normalized to FA6-152 monoclonal antibody binding, and bCD36 surface expression was normalized to CIDRα6_D3_IT4var12 binding (Supplementary Figure 2).
Two Domains Regulate Differential Binding of Infected Erythrocytes by hCD36 Versus bCD36
An ortholog swap mutagenesis approach was used to examine the functional relevance of domains I and II. Amino acids of interest were exchanged between hCD36 and bCD36 to determine whether substituting residues between these orthologs would disrupt IE binding by hCD36 and, conversely, confer it to bCD36, while preserving the overall tertiary structure and surface expression (Figure 1, Supplementary Figures 1 and 2). Two anti-CD36 mAbs and a specific CD36-binding CIDRα peptide were used to confirm surface expression (Supplementary Figure 1). The exchange of hCD36 domains I and II with the comparable regions from bCD36 resulted in the complete loss of IE binding (Figure 3). In contrast, replacing the bovine domains with the hCD36 domains I and II conferred IE binding to the bCD36 backbone (Figure 3). Independent examination of each domain showed that either bCD36 domain I or II disrupted IE binding, when used to replace the corresponding region in hCD36. Whereas substituting either domain I or II of bCD36, with the analogous region from hCD36, resulted in a significant increase in IE binding to bCD36 (Figure 4). Domain I exchange between the orthologs also disrupted binding of the hCD36 mAb BD555455 to hCD36 and conferred antibody binding to bCD36 (Supplementary Figure 2). These data indicate that exchanging these small domains between orthologs is sufficient to abrogate IE binding by hCD36 and to confer binding to bCD36.
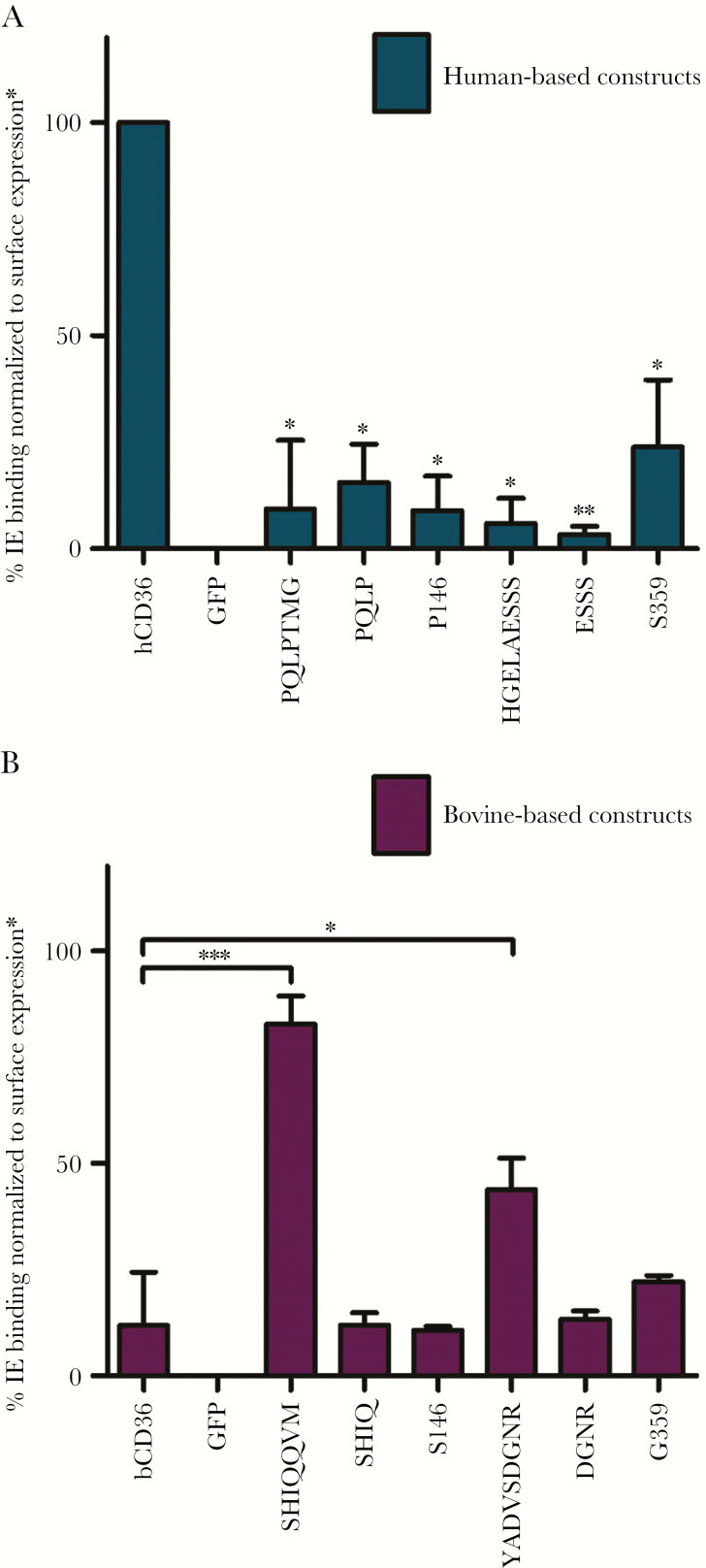
Figure 4.
Infected erythrocyte (IE) binding to human- and bovine-based CD36 mutants. (A) Human-based constructs (blue). Quantification of IE binding (mean + standard deviation [SD]) of experiments done in triplicate with statistical significance based on comparison to hCD36 wild type (*, P < .05). (B) Bovine-based constructs (purple). Quantification of IE binding (mean + SD) of experiments done in triplicate, and statistical significance compared with bCD36 wild type (*, P < .05 and ***, P < .0001). hCD36 mutants surface expression were normalized to FA6-152 monoclonal antibody binding, and bCD36 mutants surface expression were normalized to CIDRα6_D3_IT4var12 binding (Supplementary Figure 2). GFP, green fluorescent protein.
Role of Serine 146 and Glycine 359 in Infected Erythrocyte Binding
The alignment and binding data suggest that the serine in domain I at position 146 (S146) and the glycine (G359) in domain II may be important for the binding phenotype of hCD36. In support of this hypothesis, IE binding by hCD36 was abolished when this serine was replaced with the proline found in bCD36 (Figure 4A). However, the reciprocal exchange of serine with proline at this position in bCD36 did not confer IE binding (Figure 4B). Likewise, point mutation of the glycine at position 359 of the hCD36 domain II to the serine found in bCD36 significantly decreased binding (Figure 4A), but the converse exchange in bCD36 (S359G) did not increase IE binding (Figure 4B). Collectively, these ortholog mutational studies identified residues within domains I and II that are necessary to maintain IE binding in hCD36 but were not sufficient to confer binding to bCD36. These data suggest that in addition to critical residues, conformational constraints of domains I and II also modify IE interactions.
Analysis of Cysteine-Rich Interdomain Region-α Peptide-CD36 Interactions
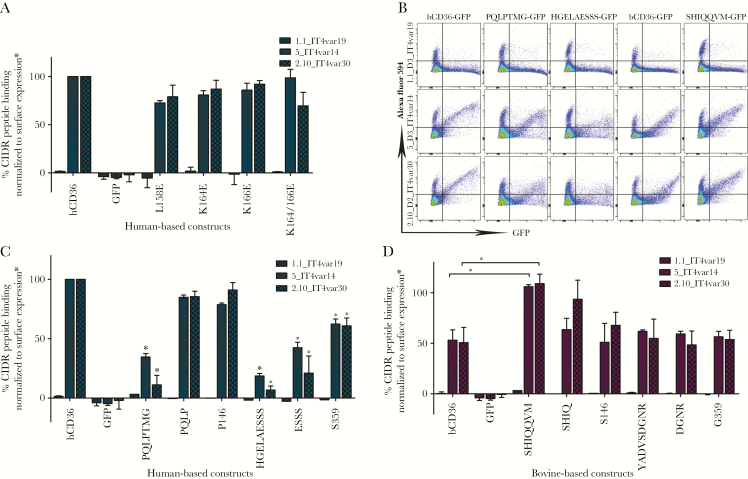
As a complementary approach to explore CD36-IE interactions, we used flow cytometry to assess binding by WT and mutant CD36, of CIDRα2 peptides that interact specifically with CD36 or, as a control, the EPCR [24]. In our binding assays, EPCR binding CIDRα1 peptides displayed no affinity for WT or mutant CD36 and served as a negative control for the transfection and binding assays (Figure 5). It is notable that mutations to modify the cationic region in hCD36 that abrogated IE binding did not affect CD36 binding of CIDRα2 peptides (Figure 5A). However, hCD36 mutants containing domains I and II from bCD36, as well as G359S, displayed significantly reduced binding of CIDRα2 peptides similar to that observed with IEs (Figure 5B). CIDRα2 peptides displayed reduced binding to bCD36 (Figure 5C and D). Binding was significantly increased when the bCD36 domain I was replaced with the corresponding human domain (Figure 5C and D).
Figure 5.
CIDR peptides binding to CD36 mutants. (A) Quantification of peptide binding to hCD36 and point mutants by flow cytometry performed in duplicate (mean + standard deviation). Statistical significance compared with hCD36 wild type (*, P < .05). (B) Flow cytometry charts for selected constructs. (C) Quantification of peptide binding to hCD36-based constructs (blue). Statistical significance compared with hCD36 wild type (*, P < .05). (D) Quantification of peptide binding to bCD36-based constructs (purple). Statistical significance compared with bCD36 wild type (*, P < .05). Surface expression of A was normalized to BD555455 monoclonal antibody binding, hCD36 mutants in C were normalized to FA6-152 monoclonal antibody binding, and bCD36 mutants in D were normalized to CIDRα6_D3_IT4var12 binding (Supplementary Figure 2). GFP, green fluorescent protein.
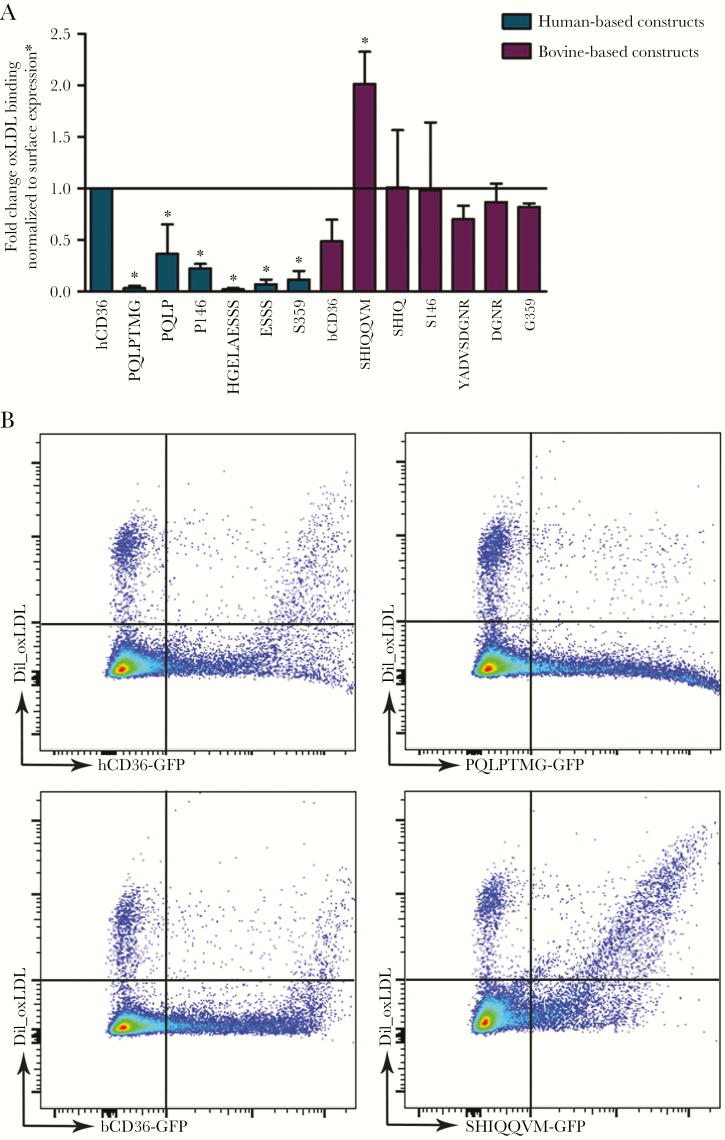
Binding of Oxidized Low-Density Lipoprotein, Like Infected Erythrocytes, Is Influenced by hCD36 Domain I and II
As an alternative ligand to IEs, we examined oxLDL binding by the CD36 constructs generated (Figure 6). Exchanging domain I or II in hCD36 with the comparable bovine domains, as well as introducing bovine point mutations into hCD36 domain I or II, all resulted in significantly reduced oxLDL binding by hCD36 (Figure 6). In contrast, replacing bCD36 domain I with the hCD36 domain increased oxLDL binding to bCD36 by 2-fold. Exchange of domain II or other residues within domain II conferred levels of oxLDL binding similar to, or greater than, that of hCD36 WT (Figure 6).
Figure 6.
DiI_OxLDL binding. (A) Quantification of oxidized low-density lipoprotein (oxLDL) binding to human-based (blue) and bovine-based (purple) constructs by flow cytometry performed in duplicate (mean + standard deviation) (*, P < .05). (B) Flow cytometry charts for selected constructs. hCD36 mutant results were surface normalized to FA6-152 monoclonal antibody binding, and bCD36 mutants were normalized to CIDRα6_D3_IT4var12 binding (Supplementary Figure 2). GFP, green fluorescent protein.
DISCUSSION
Solving the crystal structure of LIMP-2 has enabled an improved understanding of the structure of this family of PRRs and how they functionally interact with diverse ligands. Furthermore, the recent cocrystallization of the CD36 ectodomain with PfEMP1 CIDRα2–6 peptides identified a phenylalanine residue at position 153 in hCD36 as critical for binding both the oxLDL and CIDRα2–6 domains [9]. In this study, we used the predicted structure of CD36 combined with differential ligand binding by CD36 orthologs to further characterize binding interactions between the biologically relevant ligand (ie, intact IEs) and CD36. Our results provide evidence that the IE interaction with CD36 is dependent on a cationic residue cluster that is influenced by at least 2 additional domains, 1 in proximity and 1 at a distance from this interaction surface.
Although structurally heterogeneous, the CD36 superfamily displays a conserved positively charged region or “cationic patch” that has been implicated in receptor interaction with polyanionic ligands such as modified lipoproteins [10, 13]. In support of this observation, mutating charges within the membrane distal helical bundle region of hCD36 (eg, K164E or K166E) have been shown to disrupt oxLDL binding [10, 16]. In this study, we show that these conserved residues are also important for CD36 binding of intact IEs. Although a single charge change in this region is insufficient to disrupt CD36-ligand interactions, a double mutant (K164E and K166E) abrogates binding of both oxLDL and IEs. Collectively, these data provide additional evidence supporting the hypothesis that despite structural diversity, a conserved region of shape and electrostatic potential contributes to the broad repertoire of ligands bound by the CD36 superfamily of receptors [10].
Residues within this putative interaction site are necessary for binding; however, their functionality appears to be conformationally constrained. Given the broad and unrelated nature of many of the ligands bound by scavenger receptors, it has been proposed that, in addition to ligand interaction surfaces, other small sequence and structural features will determine the function and regulate differential ligand recognition by this class of receptors. We provide several lines of evidence supporting this hypothesis. First, bCD36 has a conserved phenylalanine at 153 (ie, the reported binding site in hCD36 for CIDRα2–6 peptides), as well as the same conserved lysine residues at positions 164 and 166 as hCD36, but only binds oxLDL approximately 50% as well and binds IEs poorly compared with hCD36. Residues predicted to be buried in the helical bundle, L158 and L161, were previously shown to be required for binding of oxLDL and have been proposed to be important for protein-protein interactions or dimer formation [16]. Of note, L158 is required for IE binding by hCD36. Mutations in this area induce allosteric conformational changes on the cationic surface that directly or indirectly disrupt ligand binding.
Second, when the hCD36 domain I is introduced to replace the corresponding region in bCD36, IE binding to the bCD36 backbone is significantly enhanced and comparable to WT hCD36. Notably, introducing hCD36 domain I to replace the corresponding domain in bovine also results in enhanced binding of oxLDL by bCD36, increasing oxLDL binding by 2-fold over WT hCD36. However, inserting bovine mutations into domain I and II of hCD36 results in decreased oxLDL binding. Collectively, these data suggest that subtle changes in sequence or domain arrangement can alter ligand interaction and selectivity, perhaps by inducing conformation changes that make the interaction surface more or less accessible to the ligand.
Finally, we observed that CD36 binding of CIDRα2 peptides was influenced by domain I and II exchange and specific mutations within these domains. Exchanging bovine domain I with human domain I resulted in 2-fold increase in binding of CIDRα peptides by bCD36 and conversely a significant decrease in binding by hCD36. Similar to IE binding, introducing bovine domain II, or its associated point mutations, decreased CIDRα2 binding by hCD36. However, none of the mutations introduced to alter charge in the cationic region had an impact on binding of CIDRα peptides. Of note, CD36 can recognize and bind hundreds of CIDRα sequences although there is minimal sequence conservation [8]. Our data support the hypothesis that CD36, as a PRR, recognizes the conserved structure of these peptides as PAMPs, and binding is influenced by subtle changes in tertiary structure, rather than by a sequential binding process requiring specific charged residues as was observed for intact IEs.
Collectively, our data suggest a putative model where (1) a charged binding surface on CD36 binds IEs via parasite-encoded domains, and/or modified host molecules and (2) direct binding to F153 by PfEMP1 CIDRα2–6 domains. These interactions collectively increase the avidity to form a stable interaction. Precisely how conformational changes induced by domains I and II influence the selectivity and interaction of CD36 for other ligands remains to be determined.
A limitation of mutational approaches is the potential to introduce structural changes in the receptor that abrogates binding independent of informative changes in binding domains. However, to mitigate these issues, we used small domain exchanges between highly conserved and well expressed orthologs. We confirmed expression and function of CD36 mutants by normalizing for cell surface expression and by gain and loss of function experiments using IE, CIDR peptides, and oxLDL binding and antibody reactivity between the mutant CD36 receptors that underwent reciprocal domain swap exchange.
Immune pressure on CIDRα molecules seems to be responsible for their sequence diversity, but conservation of domain structure is required to bind to the same or similar sites as physiological ligands [25]. This characteristic is shared by other PPRs that recognize diverse ligands through shared structure rather than amino acid sequence. Scavenger receptors are also known for their propensity to oligomerize, favoring binding of large and multivalent ligands [10]. This could certainly be the case for multiple CIDRα domains in PfEMP1 molecules widely distributed on the red blood cell membrane.
CONCLUSIONS
Because CIDRα peptide binding is not dependent upon charge, it is currently unknown what ligand on IEs directly interacts with the charged binding surface. These candidates include other parasite-encoded erythrocyte surface proteins (eg, Clag9) [26, 27], products of oxidation, modification, and degradation of membrane proteins (eg, Band-3, hemichrome formation, CD47) [28–33], or disruption of the phospholipid asymmetry by exposure of phosphatidylserine [34–36]. Further studies are also required to examine the involvement of other IE ligands in CD36 binding, as well as the implications of sequence variations in CD36 on modifying uptake of oxLDL by monocytic cells and whether this plays a role in the pathobiology of atherosclerosis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded by Canadian Institutes of Health Research (CIHR) Foundation Grant FDN-148439 (to K. C. K.), Canada Research Chair (to K. C. K.), CIHR Postdoctoral Fellowship (to A. C.), The Natural Science Foundation of Zhejiang Province Grant 31770938 (to D. N.), and National Natural Science Foundation of China Grant LZ16C050001 (to D. N.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: American Society for Tropical Medicine and Hygiene, 2013, Washington, DC; BioMalPar, 2014, Heidelberg, Germany.
References
- 1. Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005; 434:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJ, Rosenfeld LC, Lim SS, et al. . Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012; 379:413–31. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. World Malaria Report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 4. Guizetti J, Scherf A. Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum. Cell Microbiol 2013; 15:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 2008; 62:445–70. [DOI] [PubMed] [Google Scholar]
- 6. Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol 2006; 9:374–80. [DOI] [PubMed] [Google Scholar]
- 7. Mo M, Lee HC, Kotaka M, et al. . The C-terminal segment of the cysteine-rich interdomain of Plasmodium falciparum erythrocyte membrane protein 1 determines CD36 binding and elicits antibodies that inhibit adhesion of parasite-infected erythrocytes. Infect Immun 2008; 76:1837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein MM, Gittis AG, Su HP, et al. . The cysteine-rich interdomain region from the highly variable Plasmodium falciparum erythrocyte membrane protein-1 exhibits a conserved structure. PLoS Pathog 2008; 4:e1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsieh FL, Turner L, Bolla JR, Robinson CV, Lavstsen T, Higgins MK. The structural basis for CD36 binding by the malaria parasite. Nat Commun 2016; 7:12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol 2013; 13:621–34. [DOI] [PubMed] [Google Scholar]
- 11. Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009; 2:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 2000; 96:3231–40. [PubMed] [Google Scholar]
- 13. Heit B, Kim H, Cosío G, et al. . Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization. Dev Cell 2013; 24:372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kobylka D, Carraway KL. Proteolytic digestion of proteins of the milk fat globule membrane. Biochim Biophys Acta 1973; 307:133–40. [DOI] [PubMed] [Google Scholar]
- 15. Cabrera A, Neculai D, Kain KC. CD36 and malaria: friends or foes? A decade of data provides some answers. Trends Parasitol 2014; 30:436–44. [DOI] [PubMed] [Google Scholar]
- 16. Neculai D, Schwake M, Ravichandran M, et al. . Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature 2013; 504:172–6. [DOI] [PubMed] [Google Scholar]
- 17. Trager W. Plasmodium falciparum in culture: improved continuous flow method. J Protozool 1979; 26:125–9. [DOI] [PubMed] [Google Scholar]
- 18. Dorrington MG, Roche AM, Chauvin SE, et al. . MARCO is required for TLR2- and Nod2-mediated responses to Streptococcus pneumoniae and clearance of pneumococcal colonization in the murine nasopharynx. J Immunol 2013; 190:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaiyaroj SC, Coppel RL, Novakovic S, Brown GV. Multiple ligands for cytoadherence can be present simultaneously on the surface of Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A 1994; 91:10805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serghides L, Crandall I, Hull E, Kain KC. The Plasmodium falciparum-CD36 interaction is modified by a single amino acid substitution in CD36. Blood 1998; 92:1814–9. [PubMed] [Google Scholar]
- 21. Baruch DI, Ma XC, Pasloske B, Howard RJ, Miller LH. CD36 peptides that block cytoadherence define the CD36 binding region for Plasmodium falciparum-infected erythrocytes. Blood 1999; 94:2121–7. [PubMed] [Google Scholar]
- 22. Ockenhouse CF, Tandon NN, Jamieson GA, Greenwalt DE. Antigenic and functional differences in adhesion of Plasmodium falciparum-infected erythrocytes to human and bovine CD36. Infect Immun 1993; 61:2229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garnier J, Gibrat JF, Robson B. GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol 1996; 266:540–53. [DOI] [PubMed] [Google Scholar]
- 24. Turner L, Lavstsen T, Berger SS, et al. . Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013; 498:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau CK, Turner L, Jespersen JS, et al. . Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 2015; 17:118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ockenhouse CF, Klotz FW, Tandon NN, Jamieson GA. Sequestrin, a CD36 recognition protein on Plasmodium falciparum malaria-infected erythrocytes identified by anti-idiotype antibodies. Proc Natl Acad Sci U S A 1991; 88:3175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holt DC, Gardiner DL, Thomas EA, et al. . The cytoadherence linked asexual gene family of Plasmodium falciparum: are there roles other than cytoadherence?Int J Parasitol 1999; 29:939–44. [DOI] [PubMed] [Google Scholar]
- 28. Winograd E, Sherman IW. Malaria infection induces a conformational change in erythrocyte band 3 protein. Mol Biochem Parasitol 2004; 138:83–7. [DOI] [PubMed] [Google Scholar]
- 29. Crandall I, Sherman IW. Plasmodium falciparum (human malaria)-induced modifications in human erythrocyte band 3 protein. Parasitology 1991; 102 Pt 3:335–40. [DOI] [PubMed] [Google Scholar]
- 30. Wolofsky KT, Ayi K, Branch DR, et al. . ABO blood groups influence macrophage-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes. PLoS Pathog 2012; 8:e1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crandall I, Collins WE, Gysin J, Sherman IW. Synthetic peptides based on motifs present in human band 3 protein inhibit cytoadherence/sequestration of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A 1993; 90:4703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Banerjee R, Khandelwal S, Kozakai Y, Sahu B, Kumar S. CD47 regulates the phagocytic clearance and replication of the Plasmodium yoelii malaria parasite. Proc Natl Acad Sci U S A 2015; 112:3062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayi K, Lu Z, Serghides L, et al. . CD47-SIRPα interactions regulate macrophage uptake of Plasmodium falciparum-infected erythrocytes and clearance of malaria In Vivo. Infect Immun 2016; 84:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Setty BN, Kulkarni S, Stuart MJ. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood 2002; 99:1564–71. [DOI] [PubMed] [Google Scholar]
- 35. Walker B, Towhid ST, Schmid E, et al. . Dynamic adhesion of eryptotic erythrocytes to immobilized platelets via platelet phosphatidylserine receptors. Am J Physiol Cell Physiol 2014; 306:C291–7. [DOI] [PubMed] [Google Scholar]
- 36. Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 2006; 203:2613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.