Here, we compared the impact of endogenous sexual hormones on the mucosal immune response. Our study shows that the follicular phase of the menstrual cycle was associated with elevated level of cervical CCL2 and retention of resident memory CD4+ T cells.
Keywords: Genital inflammation, HIV, menstrual cycle, immune activation
Abstract
Background
Inflammation and immune activation are key factors in sexual transmission of human immunodeficiency virus (HIV). We sought to define the impact of hormonal cycling on the mucosal immune environment and HIV risk in sex workers with a natural menstrual cycle.
Methods
We compared soluble mucosal immune factors and cervical mononuclear cells during hormone titer–defined phases of the menstrual cycle among 37 sex workers from Nairobi, Kenya. Systemic and mucosal samples were collected 14 days apart to distinguish the follicular and luteal phases of the menstrual cycle, and phases were confirmed by hormone measurements. Vaginal concentrations of 19 immune modulators and cervical T-cell activation markers were measured.
Results
The follicular phase signature was characterized by an elevated CCL2 level, decreased interleukin 1α and interleukin 1β cervical concentrations, and a significant increase in the proportion of CD4+ T cells that expressed CD69. The genital concentration of CCL2 was the best marker to distinguish the follicular from the luteal phase in univariate and multivariate analyses and remained independent of elevated genital inflammation and bacterial vaginosis.
Conclusion
The follicular phase of the menstrual cycle was associated with an elevated CCL2 level and retention of resident memory CD4+ T cells, which has implications for increased susceptibility to HIV infection.
Human immunodeficiency virus type 1 (HIV-1) is a public health challenge, disproportionally affecting women of reproductive age [1]. Genital immune parameters are key factors that drive the sexual transmission of HIV in women [2–5]. During an inflammatory response, the elevation of proinflammatory cytokine and chemokine levels at the mucosal site triggers the recruitment and activation of HIV targets (CCR5+CD4+ T cells) and the disruption of the epithelial barrier [4, 6, 7]. HIV replicates more efficiently in activated CD4+ T cells [8], and the environment established by an inflammatory response creates favorable conditions for HIV infection [9]. While evidence supports the role of bacterial sexually transmitted infections (STIs) and vaginal dysbiosis in altering the vaginal environment toward a proinflammatory milieu [10–12], the influence of reproductive hormones, natural or synthetic, on the vaginal microenvironment and HIV risk remains unclear.
Studies with nonhuman primates and cervical tissue explants have supported the existence of a window of susceptibility for HIV infection during the progesterone-dominated luteal phase of the menstrual cycle [13–16]. However, it has remained extremely difficult to robustly demonstrate this hypothesis in humans. No prospective cohort study has been able to associate HIV acquisition with a specific phase of the menstrual cycle, and only a few cross-sectional studies support this hypothesis [17, 18]. As a result, controversies remain about how the natural hormonal cycle influences the mucosal environment and the recruitment of HIV-target cells.
In the present study, we characterized the variation in the immune and cell activation profiles associated with different phases of the menstrual cycle in Kenyan female sex workers with a natural hormone cycle. We compared the mucosal immune milieu between strictly defined follicular and luteal phases of the menstrual cycle. A better understanding of the timing and consequence of the natural hormonal cycle on vaginal immune environment is crucial to improve female reproductive health and better comprehend how it influences HIV sexual transmission in women.
METHODS
Participants and Study Design
We followed 37 HIV-negative female sex workers from the Pumwani Sex Worker Cohort in Nairobi, Kenya [19]. Informed written consent was obtained from all participants, and institutional review boards from Kenyatta National Hospital/University of Nairobi and the University of Manitoba approved the study. Self-declared duration of sex work was 3–10 years. Peripheral CD4+ T-cell counts (measured by FACScount flow cytometry) were determined and vulvovaginal swab specimens collected at every visit. The Nugent score was used to define bacterial vaginosis. The presence of yeast was determined by observation of candidal pseudohyphae or spores and confirmed by microscopy with potassium hydroxide. Trichomonas vaginalis was detected in vaginal swab specimens by using normal saline microscopy. Urine samples were collected for detection of Neisseria gonorrhea and Chlamydia trachomatis by polymerase chain reaction analysis (Roche Amplicor kits, Pleasanton, New Jersey). At each visit, a rapid plasma reagin serologic test was performed for Treponema pallidum detection, and a clinical, demographic, and behavioral questionnaire was completed. HIV serologic analysis with the Determine rapid test (Inverness Medical, Shinjuku-ku, Japan) was performed at the first and last visits for all participants. Women with a STI (n = 3) were excluded from the study.
Defining Phases of the Menstrual Cycle
Stringent criteria were used to define the menstrual cycle. The use of hormonal contraception was an exclusion criteria, and all participants had a natural menstrual cycle. Menstrual cycle phases were defined using self-reported days since the last menstrual period, with day 1 representing the first day of menses. The phases were confirmed by measuring the plasma concentrations of progesterone and estradiol. Their levels were measured using the Milliplex Map Steroid/Thyroid Hormone Magnetic Bead Panel (Millipore, Merck, Darmstadt, Germany). Only women in whom the ratio of the progesterone level in the luteal phase to that in the follicular phase was >2 (n = 37) were included in the study; this inclusion criterion matched the definition by Byrne et al of the menstrual cycle (progesterone level, ≥0.3 ng/mL during the follicular phase and ≥1.2 ng/mL during the luteal phase) [18]. Samples were collected between days 4 and 19 (median, day 8; interquartile range [IQR], days 6–9) for the follicular phase and between days 16 and 31 (median, day 22; IQR, days 20–24) for the luteal phase. Cycle lengths ranged from 19 to 48 days (median, 27 days; IQR, 25–31 days).
Sample Collection and Processing
Blood and cervical cytobrush specimens were obtained from all participants. Cervical samples were collected in the following order: (1) cervicovaginal lavage of the vaginal cavity with 2 mL of sterile phosphate-buffered saline (PBS) and collection of fluid from the posterior fornix region, (2) 1 swab of the vaginal vault, and (3) cervical spatula scraping of the ectocervix and cervical brush scraping of the endocervix to obtain cervical mononuclear cells. Briefly, cytobrushes were inserted into cervical os and rotated 360°, gently scraping the ectocervix. Cytobrushes were transferred into sterile PBS, kept on ice, and transported to the laboratory as described previously [20]. Blood specimens were collected in tubes by venipuncture, using heparin. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation.
Cytokine/Chemokine Detection
Cervicovaginal lavage samples were centrifuged and stored at −70°C until they were transferred in liquid nitrogen to the University of Manitoba. Cervical concentrations of the proinflammatory cytokines and chemokines interferon γ (IFN-γ), interleukin 12p70 (IL-12p70), sCD40LG, interleukin 10 (IL-10), interleukin 17A (IL-17A), interleukin 1α (IL-1α), interleukin 1β (IL-1β), interleukin 2 (IL-2), CXCL8 (also known as interleukin 8), interleukin 15 (IL-15), CXCL10 (also known as IFN-γ–induced protein 10), CCL2 (also known as monocyte chemoattractant protein 1), CCL3 (also known as macrophage inflammatory protein 1α [MIP-1α]), CCL4 (also known as MIP-1β), tumor necrosis factor (TNF), IL-1 receptor antagonist (IL-1RN), CXCL9 (also known as monocyte induced by IFN-γ), CCL20 (also known as MIP-3α), and interleukin 2 receptor agonist (IL-2RA) were measured by the Milliplex panel (Millipore, Merck, Burlington, Massachusetts) according to the manufacturer’s instructions and were analyzed on the BioPlex-200 (Bio-Rad, Mississauga, Canada). Cervicovaginal lavage specimens were incubated overnight described by Lajoie et al [5]. Samples with undetectable levels of cytokines or chemokines, defined as those with a value below the lower limit of detection (LLD), were assigned the value of LLD/2. Most analytes showed a skewed distribution of levels. Values were log10 transformed for further analyses. We were unable to detect the full panel of cytokines/chemokines in 5 participants.
Flow Cytometry
Freshly isolated PBMCs and cervical cells were washed with 2% fetal bovine serum–1× PBS and stained for ex vivo phenotyping. PBMCs (106) and cervical cells were incubated with antibodies coupled to PE.Cy5-CD3, FITC-CD4, V500-CD8, PE-CD95, APC.H7-HLA-DR, APC-CD161, Alexa700-CD45RA, V450-CCR5, PE.Cy7-CD69, and PE-CF594-CCR7 (BD Biosciences, Mississauga, Canada) or were stained with Far Red Live/Dead discriminant (Invitrogen, Carlsbad, California) for ex vivo phenotyping. Data were acquired on an LSRII flow cytometer (BD System, Mississauga, Canada) and analyzed using FlowJo, version 10.0.8r1 (TreeStar, Ashland, Oregon).
Statistical Analysis
Statistical analyses were performed in collaboration with the Data Sciences Platform at the George and Fay Yee Centre for Healthcare Innovation, University of Manitoba. Continuous variables were summarized using medians and interquartile ranges. D’Agostino-Pearson normality tests were performed to compute the skewness and quantify how far the distribution is from a Gaussian distribution in terms of asymmetry and shape. Cytokine and chemokine data were log10 transformed for further analysis. Paired groups were compared using the parametric paired t test on log10-transformed values. Data sets that still markedly violated symmetry were analyzed with the Wilcoxon signed rank test (paired test), using untransformed data. Proportions of cervical cells that were not normally distributed were compared using the Wilcoxon signed rank test (Prism 7; GraphPad Software). McNemar tests were used to compare paired binary variables.
Partial least squares discriminant analysis (PLS-DA) was used to identify combinations of log10-transformed cytokine/chemokine concentrations that best predicted the phase of the menstrual cycle. Briefly, PLS-DA is a generalization of principal components analysis that captures not only the covariance among the predictors, but also their joint association with an outcome. It produces weighted linear combinations of independent variables, or principal components, that best differentiate individuals on the basis of an assigned class. Every patient sample was assigned a score for each component, which can be visualized in score plots. Variable loadings were then used to identify cytokine profiles associated with different phases. Variable importance measures indicate which factors are most relevant for predicting the phase of the menstrual cycle. To assess the predictive ability of the PLS-DA model, the area under the curve (AUC) was calculated using leave-one-out cross-validation. The model was refitted multiple times, each time omitting 1 observation as a holdout for prediction. The diagnostic threshold was determined using the left-upper-corner criterion, which maximizes the sum of the sensitivity and specificity. AUC confidence intervals [CIs] that do not cross 0.5 (the diagonal line of chance agreement) are statistically significant, which was confirmed in all cases via bootstrapped 95% CIs on 2000 replicates. PLS-DAs were performed using the caret package in R, version 3.2.5 (available at: https://www.jstatsoft.org/article/view/v028i05).
Paired data (log10 transformed for cytokine/chemokine concentrations) were analyzed using a linear mixed-effect model to estimate the effect of menstrual cycle on continuous variables. Variance components were estimated using residual maximum likelihood. Results are presented in terms of the size effect (using the regression coefficient and standard CI). Crude P values and P values adjusted for age, genital inflammation, and bacterial vaginosis are reported. Analyses were performed using Stata, version 13.1 (StataCorp, College Station, Texas). P values of <.05 were considered statistically significant.
RESULTS
Study Participants
Demographic and clinical characteristics of the 37 study participants are summarized in Table 1. The plasma progesterone concentration increased by a median of 7-fold (IQR, 5–15-fold) during the luteal phase (P < .0001; Supplementary Figure 1), with a median follicular progesterone concentration of 940 pg/mL (IQR, 495–1660 pg/mL) and a median luteal concentration of 7500 pg/mL (IQR, 4035–11295 pg/mL). Estradiol levels were also elevated during the luteal phase (P = .003; Table 1), confirming that women underwent sampling during the mid–follicular phase, just before the peak in the estradiol level, and during mid–luteal phase, when the second peak in the estradiol level overlaps with the peak progesterone level [21]. No difference was observed in clinical variables between phases (Table 1).
Table 1.
Sociodemographic and Behavioral Characteristics of 37 Female Sex Workers
| Characteristic | Value | ||
|---|---|---|---|
| Fixed | |||
| HIV negative | 100 (37/37) | ||
| Age, y | 34 (31–39) | ||
| Marital status | |||
| Not married, not living with a man | 69 (25/36) | ||
| Not married, living with a man | 3 (1/36) | ||
| Married, not living with a man | 28 (10/36) | ||
| Self-reported sex work duration, y | 3 (3–10) | ||
| Education duration, y | 10 (8–13) | ||
| Pregnancies, no. | 2 (2–3) | ||
| Ratio of luteal to follicular progesterone levels | 7:1 (5:1 to 15:1) | ||
| Time between visits, d | 14 (13–14) | ||
| Cycle length, d | 27 (25–31) | ||
| Varying | Follicular Phase | Luteal Phase | P |
| Time since last menses, d | 8 (6–9) | 22 (20–24) | <.0001a |
| Reported having a regular sex partner at study visit | 65 (24/37) | 59 (22/37) | .157b |
| Clients in past 7 d, no. | 4 (2–8) | 4 (3–8) | .241a |
| Condom use with clients in past 7 d | .357c | ||
| Never | 0 (0/37) | 5 (2/37) | |
| Sometimes | 32 (12/37) | 30 (11/37) | |
| Always | 68 (25/37) | 65 (24/37) | |
| Detection of PSA (unprotected sex in the past 48 h) | 21 (7/33) | 32 (11/34) | .303c |
| Reported vaginal douching | 34 (12/35) | 22 (8/37) | |
| Presence of yeast infection | 18 (6/33) | 34 (11/32) | |
| Bacterial vaginosis status (Nugent score) | .096c | ||
| Normal (0–3) | 53 (19/36) | 49 (18/37) | |
| Intermediate (4–6) | 25 (9/36) | 27 (10/37) | |
| Bacterial vaginosis (7–10) | 22 (8/36) | 24 (9/37) | |
| Plasma progesterone level, pg/mL | 940 (495–1660) | 7500 (4035–11295) | <.0001a |
| Plasma estradiol level, pg/mL | 130 (55–185) | 240 (140–310) | .003a |
| Genital inflammationd | 28 (9/32) | 22 (7/32) | .480b |
Data are median (interquartile range) or % (no. with characteristic/no. analyzed).
Abbreviations: HIV, human immunodeficiency virus; PSA, prostate-specific antigen.
aBy the paired t test.
bBy the McNemar test for binary variables.
cBy the Fisher exact test.
dGenital inflammation is defined as ≥5 cytokines/chemokines with levels in the upper quartile of the 12 cytokines/chemokines expressed in at least 30% of the participants (CCL2, interleukin 12p70, CCL4, interleukin 17A, CCL3, interleukin 1α, interleukin 1β, CXCL10, interleukin 1 receptor antagonist, CXCL9, CXCL8, and CCL20).
Genital Inflammatory Profile Differs Between the Follicular and Luteal Phases of the Menstrual Cycle
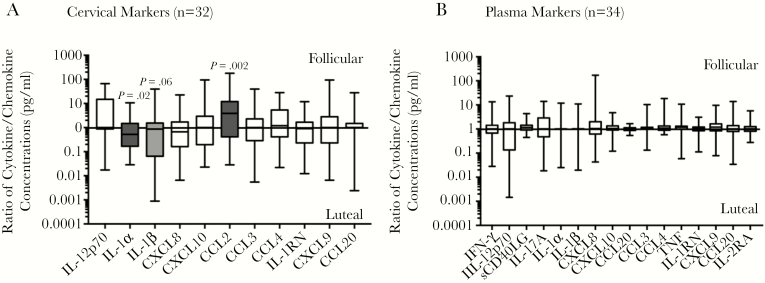
We compared the genital and plasma concentrations of 19 cytokines/chemokines between both phases of the menstrual cycle. Of the 19 markers, 12 (CCL2, IL-12p70, CCL3, CCL4, IL-17A, IL-1α, IL-1β, CXCL10, IL-1RN, CXCL9, CXCL8, and CCL20) were classified as proinflammatory and could be detected in at least 30% of participants (Table 2). The cervical concentration of CCL2 significantly decreased between the follicular and luteal phases (P = .002) in univariate analysis (Figure 1A and Table 2). The plasma concentration of sCD40LG significantly decreased (P = .032) between the follicular and luteal phases of the natural menstrual cycle (Figure 1B and Supplementary Table 1).
Table 2.
Paired Comparison Between Cervical Cytokine and Chemokine Concentrations Between the Follicular and Luteal Phases of the Menstrual Cycle
| Variable | Level During Follicular Phase | Level During Luteal Phase | P | ||
|---|---|---|---|---|---|
| Greater Than LLD, % | Overall, pg/mL, Median (IQR) | Less Than LLD, % | Overall, pg/mL, Median (IQR) | ||
| Antiinflammatory cytokines | |||||
| IL-10 | 9 | 0.55 (0.55–0.55) | 15 | 0.55 (0.55–0.55) | .4142a |
| sCD40LG | 32 | 2.55 (2.55–3.49) | 24 | 2.55 (2.55–2.55) | .2482a |
| Proinflammatory cytokines | |||||
| IL-2 | 23 | 0.50 (0.50–0.50) | 18 | 0.50 (0.50–0.50) | .2568a |
| IL-15 | 15 | 0.60 (0.60–0.60) | 13 | 0.60 (0.60–0.60) | .999a |
| IL-1α | 94 | 71.16 (16.4–231.6) | 97 | 122.3 (59.63–277.7) | .020b |
| IL-1β | 59 | 4.35 (0.40–31.75) | 74 | 15.12 (0.40–74.85) | .208c |
| IL-12p70 | 62 | 4.36 (0.30–8.65) | 41 | 0.30 (0.30–5.95) | .248c |
| TNF | 21 | 0.35 (0.35–0.35) | 24 | 0.35 (0.35–0.35) | 1.000a |
| IL-17A | 31 | 0.35 (0.35–3.68) | 25 | 0.35 (0.35–2.58) | .5637a |
| IFN-γ | 26 | 0.40 (0.40–0.40) | 24 | 0.40 (0.40–0.40) | .4795a |
| Proinflammatory chemoattractants | |||||
| CCL3 | 50 | 2.61 (1.45–18.41) | 62 | 5.79 (1.45–19.32) | .649b |
| CCL20 | 35 | 0.8 (0.8–16.4) | 38 | 0.80 (0.80–29.41) | .890c |
| CXCL8 | 100 | 408.6 (198.7–1976) | 100 | 672.6 (204.8–3115) | .158b |
| CCL4 | 79 | 12.88 (3.66–21.37) | 74 | 6.69 (1.50–19.95) | .290b |
| CXCL10 | 79 | 77.74 (6.29–143.1) | 76 | 61.52 (6.66–151.6) | .880b |
| CCL2 | 97 | 53.31 (14.03–152.7) | 79 | 18.79 (3.46–62.35) | .002b |
| CXCL9 | 79 | 202.4 (56.33–512.7) | 74 | 278.1 (5.15–792.8) | .696b |
| Cytokine receptors | |||||
| IL-1RN | 100 | 11000 (6578–11000) | 100 | 11000 (10208–11000) | .231c |
| IL-2RA | 25 | 3.0 (3.0–19.55) | 19 | 3.0 (3.0–3.0) | .480a |
Data are for samples from 32 sex workers. Samples with undetectable levels, defined as levels below the LLD, were assigned the value of LLD/2. We were unable to detect the full panel of cytokines/chemokines in 5 participants.
Abbreviations: IFN-γ, interferon γ; IL-1RN, interleukin 1 receptor antagonist; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-2, interleukin 2; IL-2RA, interleukin 2 receptor agonist; IL-10, interleukin 10; IL-12p70, interleukin 12p70; IL-15, interleukin 15; IL-17A, interleukin 17A; IQR, interquartile range; LLD, lower limit of detection; TNF, tumor necrosis factor.
aCytokines expressed in <30% of participants were analyzed as paired binary variables using McNemar test.
bParametric paired t tests were used to compare means between follicular and luteal phases on log10 transformed values.
cWilcoxon signed rank tests were used to compare untransformed ranked values between follicular and luteal phases.
Figure 1.
Ratios of cytokine/chemokine concentrations in picograms/milliliter during the follicular phase to those during the luteal phase (ie, fold change) in paired cervicovaginal lavage and plasma specimens from women who underwent sampling during both phases of the menstrual cycle (1, no change in level; >1, elevated level during the follicular phase; and <1, elevated level during the luteal phase). Only ratios of markers for which concentrations were detectable in at least 30% of the participants are presented in this figure. The ratios are presented as box and whisker plots showing the medians (middle lines), interquartile ranges (upper and lower limits of boxes), and minimum and maximum values (upper and lower whiskers). The parametric paired t test was used for comparing the log10-transformed cytokine/chemokine concentrations between phases. Levels of cytokines/chemokines that were significantly altered by the menstrual cycle are indicated by shaded bars. IFN-γ, interferon γ; IL-1RN, interleukin 1 receptor antagonist; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-2RA, interleukin 2 receptor agonist; IL-12p70, interleukin 12p70; IL-17A, interleukin 17A; TNF, tumor necrosis factor.
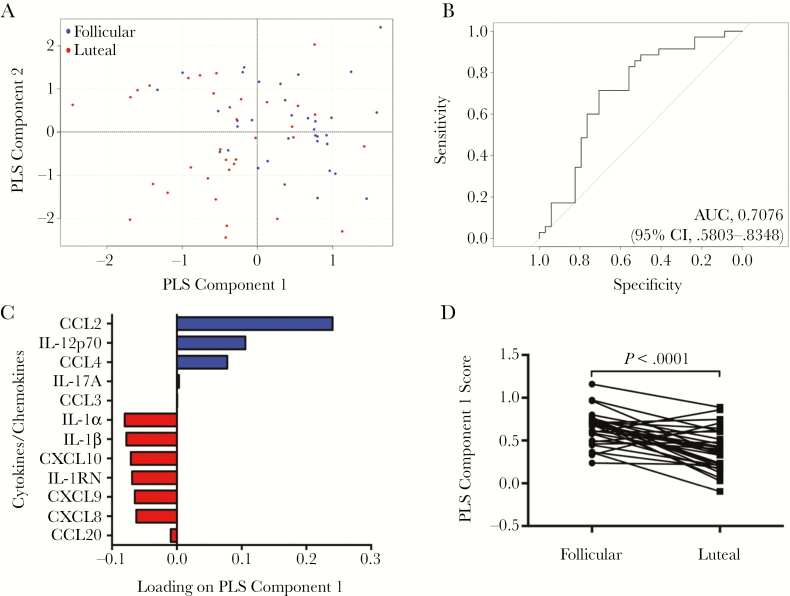
We used a PLS-DA to determine the multivariate combinations of cervical cytokines that best distinguished between the menstrual cycle phases. Briefly, PLS-DA captures the covariance among predictors in addition to their joint association with an outcome. PLS component 1 of our PLS-DA best separated the follicular phase from the luteal phase (Figure 2A), and the optimal 2-component model remained predictive after cross-validation, with an AUC of 0.71 (95% CI, .58–.83; sensitivity, 0.71; specificity, 0.71; Figure 2B). Absolute regression coefficients of the predictive equation are presented in Figure 2C, and the sensitivity and specificity of the PLS-DA for predicting subjects’ menstrual phases are presented in Supplementary Table 2 for various cutoff values of the PLS-DA score. Levels of factors positively loaded on component 1 (CCL2, IL12 p70, CCL4, IL17A, and CCL3) were elevated during the follicular phase, whereas factors negatively loaded (IL-1α, IL-1β, CXCL10, IL-1RN, CXCL9, CXCL8, and CCL20) were comparatively lower (Figure 2C). The variable importance score demonstrated that CCL2 was the most critical of the 12 cytokines for classifying the phases, with twice the score of the next most predictive marker (Table 3). PLS component 1 scores were significantly higher in the follicular phase as compared to the luteal phase (P < .0001; Figure 2D).
Figure 2.
Identification of multivariate cytokine profiles associated with distinct phases of the menstrual cycle. A, Partial least squares discriminant analysis (PLS-DA) model of all 12 cytokines classified in individuals (blue, follicular phase; red, luteal phase). B, The area under the curve (AUC) was 0.79 (95% confidence interval [CI], .68–.90), with a sensitivity of 0.74 and a specificity of 0.74, for the autopredictive model; the AUC was 0.71 (95% CI, .58–.83), with a sensitivity of 0.71 and a specificity of 0.71, for the cross-validated model. C, Loading plots of the 12 cytokines/chemokines measured. PLS component 1 best separated the follicular phase from the luteal phase of the menstrual cycle. The follicular phase clustered in the positive region of PLS component 1 (A). Levels of cytokines positively loaded on PLS component 1 (CCL2, interleukin 12p70 [IL-12p70], CCL4, interleukin 17A [IL-17A], and CCL3) are elevated in the follicular phase profile, whereas levels of cytokines negatively loaded (interleukin 1α [IL-1α], interleukin 1β [IL-1β], CXCL10, interleukin 1 receptor antagonist [IL-1RN], CXCL9, CXCL8, and CCL20) were comparatively reduced during the luteal phase. D, PLS component 1 scores were significantly higher in the follicular phase of the menstrual cycle as compared to the luteal phase (P < .0001). Model-fitted values calculated were compared by the parametric paired t test (n = 32).
Table 3.
Scaled Variable Importance Projection Scores and Individual Diagnostic Performance of the Top 12 Contributing Analytes
| Cytokine/Chemokine | Variable Importance Projection Score | Univariate AUC (95% CI) |
|---|---|---|
| CCL2 | 100 | 0.71 (.59–.83) |
| IL-12p70 | 44.1 | 0.59 (.46–.72) |
| IL-1α | 42.3 | 0.59 (.46–.73) |
| IL-1β | 37.9 | 0.56 (.42–.69) |
| CCL4 | 31.3 | 0.60 (.46–.73) |
| IL-1RN | 27.8 | 0.60 (.46–.74) |
| CXCL8 | 21.1 | 0.54 (.41–.68) |
| CXCL10 | 9.6 | 0.50 (.37–.64) |
| IL-17A | 7.3 | 0.53 (.42–.64) |
| CXCL9 | 4.4 | 0.51 (.37–.64) |
| CCL20 | 3.8 | 0.51 (.37–.62) |
| CCL3 | 0 | 0.49 (.36–.63) |
Variable importance projection scores indicated that CCL2 was the most important cytokine to differentiate between phases.
Abbreviations: AUC, area under the curve; CI, confidence interval; IL-1RN, interleukin 1 receptor antagonist; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-12p70, interleukin 12p70; IL-17A, interleukin 17A.
The Menstrual Cycle Influences the Expression of HIV Susceptibility Markers on T Cells in the Female Genital Tract in Paired Comparisons
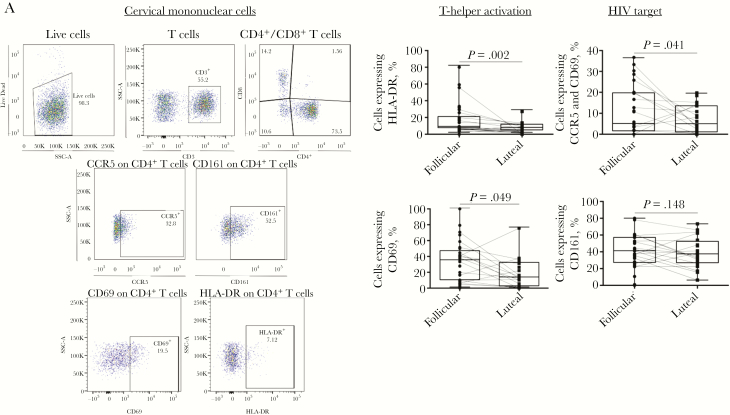
Having established the cytokine signature profile of the menstrual cycle phases, we explored the potential relationship between phases and activation profile of cervical T cells. The proportion of CD4+ T cells expressing HIV coreceptor CCR5 or activation markers CD69 and HLA-DR was higher in the follicular phase as compared to the luteal phase (Figure 3 and Supplementary Table 3).
Figure 3.
Proportion of activated T-helper cells in cervical mononuclear cells. A, Gating strategy to characterize expression of mucosal T-helper cell activation markers. For each cervical cell sample, 100000 events were collected and gated to identify lymphocytes (forward scatter [FSC-A] vs side scatter [SSC-A]). Live CD3 cells were selected and subdivided into CD4+ and CD8+ T-cell subset, and those expressing CD161, CCR5 (depicted as an example), CD69, and HLA-DR were identified. B, Comparison of the proportion of T-helper (CD4+) cells in cervical cells expressing activation markers CD69, HLA-DR, Th17 marker CD161, and human immunodeficiency virus (HIV) coreceptor CCR5 between the follicular and luteal phases of the menstrual cycle. Results are presented as box plots showing the medians (middle lines), interquartile ranges (upper and lower limits of boxes), and minimum and maximum values (upper and lower whiskers). Differences between phases were assessed using the Wilcoxon signed rank test. P values of <.05 were considered statistically significant.
Double-positive cervical CCR5+CD69+CD4+ T cells possess the highest degree of susceptibility to HIV entry when compared to CCR5+CD4+ T cells [22]. We observed that the proportion of CD4+ T cells expressing CCR5+CD69+ was also higher during the follicular phase (P = .041; Figure 3) in univariate analysis. The proportion of CD161+CD4+ T cells, which correspond to a population of T-helper type 1 (Th1)/Th17 cells in the cervical compartment and are preferentially depleted by HIV infection [19], remained unchanged between phases (Figure 3).
The Menstrual Cycle-Induced Fluctuation of Immune Factor Levels Is Independent of Elevated Genital Inflammation and Bacterial Vaginosis in Multivariate Models
A growing body of evidence supports a role for elevated genital inflammation in the sexual acquisition of HIV-1 [2, 3, 6]. In the CAPRISA 004 study, women who subsequently became HIV infected had elevated levels of several cytokines in the genital tract [2]. Genital inflammation was defined as the presence of at least 5 cytokines/chemokines with levels above the upper quartile in the genital tract prior to seroconversion, with CCL3, CCL4, CXCL10, and CXCL8 being the most critical markers of seroconversion [2]. We compared the proportion of women with genital inflammation (ie, those with levels of >5 of 12 cytokines in the upper quartile) between phases of the menstrual cycle to assess whether the hormonal cycle could contribute to the inflammatory status of women with a natural menstrual cycle and therefore affect susceptibility to HIV infection. Five women were exclusively inflamed during the follicular phase, and 3 were exclusively inflamed during the luteal phase. In 4 women, genital inflammation persisted across phases. Therefore, the proportion of women with elevated genital inflammation was not different between phases (P = .480; Table 1) and could persist over a full cycle.
Bacterial vaginosis is an important driver of genital inflammation and HIV risk [11, 23, 24]. In our study, a quarter of the women (8 of 36 during the follicular phase and 9 of 37 during the luteal phase) had a Nugent score of >7, corresponding to bacterial vaginosis (Table 1). When adjusting our analyses for the presence of genital inflammation or bacterial vaginosis in multivariate regression models, we found that the difference in cervical concentrations of CCL2, IL-1α, and IL-1β and the proportion of cervical CD69+CD4+ T cells between menstrual cycle phases remained significant (Table 4).
Table 4.
Comparison of Changes in Cervical Cytokine and Chemokine Levels CD4+ T-Cell Percentages, Using a Linear Mixed-Effect Model, Between Follicular and Luteal Phases of the Menstrual Cycle, Adjusted for Age, Genital Inflammation, and Bacterial Vaginosis Status
| Outcome Variable | Bacterial Vaginosis | Genital Inflammationa | ||||
|---|---|---|---|---|---|---|
| Regression Coefficientb (95% CI) | Crude P | Regression Coefficientb (95% CI) | Adjusted Pc | Regression Coefficientb (95% CI) | Adjusted Pd | |
| Cervical cytokines | ||||||
| IL-12p70 | −0.28 (−.58–.01) | .059 | −0.27 (−.56–.02) | .064 | −0.23 (−.53–.05) | .106 |
| IL-17A | −0.02 (−.28–.23) | .848 | 0.01 (−.24–.26) | .929 | 0.05 (−.16–.25) | .641 |
| IL-1α | 0.29 (.08–.51) | .008 | 0.30 (.09–.51) | .006 | 0.31 (.11–.52) | .003 |
| IL-1β | 0.39 (.02–.76) | .039 | 0.43 (.09–.77) | .014 | 0.50 (.18–.82) | .002 |
| IL-1RN | 0.17 (−.07–.41) | .164 | 0.13 (−.11–.38) | .293 | 0.20 (−.04–.43) | .107 |
| CCL20 | 0.02 (−.28–.31) | .915 | 0.02 (−.28–.31) | .916 | 0.13 (−.12–.38) | .302 |
| Cervical chemokines | ||||||
| CCL2 | −0.47 (−.77 to −.16) | .003 | −0.45 (−.76 to −.14) | .005 | −0.47 (−.76 to −.19) | .001 |
| CCL3 | 0.09 (−.18–.36) | .509 | 0.11 (−.17–.40) | .438 | 0.14 (−.10–.38) | .247 |
| CCL4 | −0.14 (−.39–.10) | .246 | −0.13 (−.38–.12) | .297 | −0.09 (−.31–.13) | .426 |
| CXCL8 | 0.22 (−.05–.49) | .107 | 0.22 (−.06–.50) | .118 | 0.28 (.03–.52) | .025 |
| CXCL9 | 0.09 (−.22–.39) | .586 | 0.12 (−.17–.41) | .409 | 0.12 (−.18–.42) | .430 |
| CXCL10 | 0.09 (−.21–.39) | .563 | 0.11 (−.17–.39) | .441 | 0.11 (−.18–.40) | .449 |
| Cervical cells, % of CD4+ T cells | ||||||
| CD69+ | −13.53 (−24.70 to −2.35) | .018 | −13.10 (−24.48 to −1.73) | .024 | −12.54 (−23.85 to −1.22) | .030 |
| HLA-DR+ | −4.75 (−7.47 to −2.03) | .001 | −4.77 (−10.82–1.29) | .123 | −4.79 (−10.35–.77) | .091 |
| CD69+CCR5+ | −5.13 (−11.19–.93) | .097 | −4.79 (−11.07–1.50) | .135 | −6.24 (−12.34 to −.15) | .045 |
Abbreviations: CI, confidence interval; IL-1RN, interleukin 1 receptor antagonist; IL-1α, interleukin 1α; IL-1β, interleukin 1β; IL-12p70, interleukin 12p70; IL-17A, interleukin 17A.
aGenital inflammation is defined as ≥5 cytokines/chemokines with levels in the upper quartile of the 12 cytokines/chemokines expressed in at least 30% of the participants (CCL2, IL-12p70, CCL4, IL-17A, CCL3, IL-1α, IL-1β, CXCL10, IL-1RN, CXCL9, CXCL8, and CCL20).
bEffect size, difference in the mean log concentration of cytokines/chemokines (in pg/mL), or cell proportion between follicular and luteal phases.
cAdjusted for age and bacterial vaginosis status.
dAdjusted for age and genital inflammation.
DISCUSSION
This study was designed to determine the influence of the menstrual cycle on immune activation, with a particular focus on markers relevant to HIV susceptibility. We characterized the variation in inflammatory profile induced by the natural hormonal fluctuation in paired samples from healthy, HIV-negative women from Nairobi, Kenya. We identified a signature profile of genital cytokines/chemokines that has the capacity to predict the phases of the menstrual cycle in our study participants.
During the menstrual cycle, progesterone and estradiol are secreted in a cyclic fashion to support reproductive functions [21, 25, 26]. In our study, we strictly controlled for the phase of the menstrual cycle in which samples were collected from the women. Sampling occurred during the mid–follicular phase, before the peak in the estradiol level, and during the mid–luteal phase, when the progesterone level is the highest and overlaps estradiol expression. We observed that an elevated cervicovaginal lavage concentration of CCL2 defined the follicular phase, whereas elevated IL-1α and IL-1β levels defined the luteal phase.
Cytokines/chemokines in the genital tract can be secreted by several cell types in response to different stimuli, such as virus and bacteria, but also sex hormones [21]. Published studies comparing cytokine concentrations in univariate paired-matched cervicovaginal lavage specimens collected at each phase of the menstrual cycle have yielded findings that are somewhat inconsistent. Kyogo et al observed higher cervicovaginal lavage concentrations of IL-1RN and CCL4 and a lower cervicovaginal lavage concentration of IL-1α during the follicular phase [27]; Francis et al reported high levels of CCL2 and CXCL12 (also known as stromal cell–derived factor 1β) and low levels of CXCL8, IL-10, IL-17A, and transforming growth factor β during the follicular phase in cervicovaginal lavage specimens [28]. Al-Harti et al and Keller et al both observed that the IL-6 level was higher in cervicovaginal lavage specimens during the follicular phase, with one study reporting elevated levels of IL-1β during the follicular phase and the other reporting elevated levels of CXCL8 during the luteal phase [29, 30]. Because their expressions are highly correlated, it is important to consider cytokine/chemokine concentrations not independently but as a contingent of mutually interdependent factors. Using an approach that considers the multivariate combinations of cervical cytokines, we identified CCL2 as the most influential marker differentiating the phases of the menstrual cycle and defined a signature profile for each phase.
CCL2 is one of the key chemokines that regulates migration and infiltration of monocytes/macrophages, T lymphocytes, and memory CD4+ T cells in the tissue [31]. Progesterone directly alters CCL2 expression by freely diffusing to the cell membrane and signaling through the progesterone receptor [32]. It is known that progesterone exerts its antiinflammatory effect by directly interfering with transcription of genes encoding CCL2, IL-12p70, TNF, IFN-γ, and IL-6 [32]. Here, we provide evidence that cyclic inhibition of CCL2 production by progesterone is a defining characteristic of the natural menstrual cycle and that its inhibitory effect is compartmentalized to the genital tract.
Owing to the suppression of immune function observed in the female reproductive tract during the luteal phase, Wira et al hypothesized that this phase of the menstrual cycle corresponds to a window of vulnerability to HIV infection and other STIs [33]. In support of this hypothesis, Kersh et al reported that pig-tailed macaques with a natural menstrual cycle were infected mainly, although inconsistently, during the luteal phase following repeated challenges by simian HIV [34]. Furthermore, ex vivo infection of cervical explants from women were productively infected by HIV only when explants were obtained during the luteal phase [15]. Cross-sectional studies reported an overabundance of proteins involved in chemotaxis, cellular movement, immune cell activation, peptidase activity, and damage to the epithelial barrier [17, 35] and an increased proportion of HIV-target cells [18] during the luteal phase. However, we observed, in a longitudinal setting, an elevated proportion of CCR5+CD69+CD4+ T cells and activated HLA-DR+CD4+ T cells during the follicular phase. When adjusting for bacterial vaginosis, we found that the difference in the proportion of activated CD4+ T cells was no longer significant across phases, indicating that the fluctuation observed was likely driven by bacterial vaginosis and not sex hormones. Furthermore, the proportion of CD161+ T helper cells, previously defined as a subset of Th1/Th17 cells [19], and CCR5+CD4+ T cells remained consistent across phases. Although we cannot exclude that we lacked power to capture a difference in CCR5+CD4+ T-cell proportions or that samples were collected too early in the luteal phase to capture the window of susceptibility [34], we found a lack of support for a menstrual cycle–induced fluctuation in the HIV-target cell proportion in the genital tract, as observed by others [36–39].
In our study, the proportion of CD4+ T cells expressing CD69 in the genital tract was higher during the follicular phase. CD69 was the only marker differently expressed between the phases of the menstrual cycle, when accounting for bacterial vaginosis infection and genital inflammation. CD69 is briefly expressed on stimulated T cells and mediates tissue retention [40, 41]. CD69 identifies tissue-resident memory T cells (TRM), which mediate rapid protection against pathogens [40, 41]. The genital tract is an immune-restricted site where T-cell trafficking is tightly regulated and circulating memory T cells fail to access the genital mucosa at steady state [42]. In the genital tract, TRM generate a tissue-wide pathogen-alert environment capable of activating multiple populations in the immune system without recirculating through lymphoid tissues [43]. CD4+ T cells in the genital tract compose a population of both resident (CD69+) and recirculating (CD69−) memory CD4+ T cells [44]. We observed an increased proportion of CD69 expression on CD4+ T cells during the follicular phase, suggesting increased retention of these cells in the cervical tissue. Recent work from Swaims-Kohlmeier et al [44] described a population of recirculating CD4+ T cells (CCR7hi) in the genital tract and suggested that heterosexual HIV transmission may occur through the infection of recirculating CCR7hi memory CD4+ T cells trafficking from the luminal surface of the genital tract across the epithelium to lymphoid tissues. Consistent with the increased tissue retention capacity we observed during the follicular phase in our study, Swaims-Kohlmeier et al reported that the proportion of recirculating cells was elevated during the luteal phase of the menstrual cycle. The immune environment induced by hormonal cycling may promote the retention of memory CD4+ T cells in the tissue during the follicular phase and their recirculation during the luteal phase. Therefore, CD69+ cells also expressing CCR5 may be more likely to become infected during the follicular phase and, when recirculating, can disseminate HIV during the luteal phase, explaining why productive infection has previously been observed during this phase [13, 14, 45].
It remains challenging to establish whether the natural changes triggered by the menstrual cycle are sufficient to influence HIV acquisition. Our study did not look at seroconversion as an end point, and the length of the eclipse phase has yet to be established in humans. It has become clearer that genital inflammation, particularly chemokine concentrations, is a determinant for HIV acquisition [2, 46]. What remains unclear is the threshold of genital inflammation that predicts HIV-acquisition and whether the changes in CCL2 induced by the menstrual cycle would even cross this threshold. A measurable range of cytokine/chemokine concentrations in the genital tract, comparable across studies, still needs to be established to answer these questions.
In summary, this study established that CCL2 is the signature chemokine in cervical lavage specimens that best distinguishes menstrual cycle phases, that its concentration is elevated during the follicular phase, and that the increase is paired with retention of CD4+ T cells in the genital tract. By further defining how hormones regulate the immune response within the genital tract and influence HIV risk, this study contributes to inform the design of HIV prevention strategies and safer contraceptive methods.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We thank the research participants, who took the time and made the effort to participate in this study; and Natasha Hollett, Kara Lipsky, Kelsie Doering, Lucy Mwangi, Domnic Ouma, and Steve Wayne, for technical assistance.
G. B.-L. participated in the study design, performed the experiments and data analysis, and wrote the manuscript. J. L. participated in the study design, acted as the study coordinator, and offered critical review for the manuscript. B. D. performed the PSL-DA analysis. K. O. performed some of the flow cytometry experiments. J. C. and J. N. were the clinical coordinators. M. Kowatsch performed some of the cytokines and hormones assays. M. Kimani proceeded to the medical examinations of the participants and collected the mucosal samples. J. O. coordinated the laboratory activities in Nairobi. J. K. manages the clinical cohort. K. R. F. is the principal investigator of the study and offered critical review of the manuscript.
Financial support. This work was supported by a grant from the Canadian Institutes of Health Research (CIHR; MOP 86721; fellowship to G. B.-L.). Fond de la recherche–Santé (fellowship to G. B.-L.), and the CIHR International Infectious Diseases and Global Health Training Program (fellowship to G. B.-L.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: STD/AIDS Collaborative Research Group Annual Meeting, Kenya, January 2016; STD/AIDS Collaborative Research Group Annual Meeting, Kenya, January 2017; Canadian Association for HIV Research, Canada, May 2016. Abstract BSP4.02; American Society for Reproductive Immunology, Canada, June 2015.
References
- 1. Joint United Nations Programme on HIV. UNAIDS data 2017. Geneva: UNAIDS, 2017:1–246. [Google Scholar]
- 2. Masson L, Passmore JA, Liebenberg LJ, et al. . Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011; 62:127–39. [DOI] [PubMed] [Google Scholar]
- 4. Naranbhai V, Abdool Karim SS, Altfeld M, et al. ; CAPRISA004 TRAPS team Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis 2012; 206:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lajoie J, Juno J, Burgener A, et al. . A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol 2012; 5:277–87. [DOI] [PubMed] [Google Scholar]
- 6. Arnold KB, Burgener A, Birse K, et al. . Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016; 9:194–205. [DOI] [PubMed] [Google Scholar]
- 7. Nazli A, Chan O, Dobson-Belaire WN, et al. . Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang ZQ, Wietgrefe SW, Li Q, et al. . Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A 2004; 101:5640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaul R, Prodger J, Joag V, et al. . Inflammation and HIV Transmission in Sub-Saharan Africa. Curr HIV/AIDS Rep 2015; 12:216–22. [DOI] [PubMed] [Google Scholar]
- 10. Anahtar MN, Byrne EH, Doherty KE, et al. . Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masson L, Mlisana K, Little F, et al. . Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Trans Infect 2014; 90:580–7. [DOI] [PubMed] [Google Scholar]
- 12. Kyongo JK, Crucitti T, Menten J, et al. . Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal microbiota in Sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol 2015; 22:526–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vishwanathan SA, Guenthner PC, Lin CY, et al. . High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 2011; 57:261–4. [DOI] [PubMed] [Google Scholar]
- 14. Kersh EN, Henning T, Vishwanathan SA, et al. . SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol 2014; 43:310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saba E, Grivel JC, Vanpouille C, et al. . HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol 2010; 3:280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris MR, Byrareddy SN, Villinger F, et al. . Relationship of menstrual cycle and vaginal infection in female rhesus macaques challenged with repeated, low doses of SIVmac251. J Med Primatol 2015; 44:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birse K, Arnold KB, Novak RM, et al. . Molecular signatures of immune activation and epithelial barrier remodeling are enhanced during the luteal phase of the menstrual cycle: implications for HIV Susceptibility. J Virol 2015; 89:8793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Byrne EH, Anahtar MN, Cohen KE, et al. . Association between injectable progestin-only contraceptives and HIV acquisition and HIV target cell frequency in the female genital tract in South African women: a prospective cohort study. Lancet Infect Dis 2016; 16:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boily-Larouche G, Omollo K, Cheruiyot J, et al. . CD161 identifies polyfunctional Th1/Th17 cells in the genital mucosa that are depleted in HIV-infected female sex workers from Nairobi, Kenya. Sci Rep 2017; 7:11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juno JA, Boily-Larouche G, Lajoie J, Fowke KR. Collection, isolation, and flow cytometric analysis of human endocervical samples. J Vis Exp 2014:e51906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 2015; 15:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joag VR, McKinnon LR, Liu J, et al. ; Toronto HIV Research Group Identification of preferential CD4+ T-cell targets for HIV infection in the cervix. Mucosal Immunol 2016; 9:1–12. [DOI] [PubMed] [Google Scholar]
- 23. Low N, Chersich MF, Schmidlin K, et al. . Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moncla BJ, Chappell CA, Debo BM, Meyn LA. The effects of hormones and vaginal microflora on the glycome of the female genital tract: cervical-vaginal fluid. PLoS One 2016; 11:e0158687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nadeau-Vallée M, Obari D, Palacios J, et al. . Sterile inflammation and pregnancy complications: a review. Reproduction 2016; 152:R277–92. [DOI] [PubMed] [Google Scholar]
- 26. Hyde KJ, Schust DJ. Immunologic challenges of human reproduction: an evolving story. Fertil Steril 2016; 106:499–510. [DOI] [PubMed] [Google Scholar]
- 27. Kyongo JK, Jespers V, Goovaerts O, et al. . Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 2012; 7:e43951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francis SC, Hou Y, Baisley K, et al. . Immune activation in the female genital tract: expression profiles of soluble proteins in women at high risk for HIV infection. PLoS One 2016; 11:e0143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Harthi L, Wright DJ, Anderson D, et al. . The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res 2000; 20:719–24. [DOI] [PubMed] [Google Scholar]
- 30. Keller MJ, Guzman E, Hazrati E, et al. . PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS 2007; 21:467–76. [DOI] [PubMed] [Google Scholar]
- 31. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol 2017; 10:1097–107. [DOI] [PubMed] [Google Scholar]
- 33. Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 2008; 22:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kersh EN, Ritter J, Butler K, et al. . Relationship of estimated SHIV acquisition time points during the menstrual cycle and thinning of vaginal epithelial layers in pigtail macaques. Sex Transm Dis 2015; 42:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vishwanathan SA, Burgener A, Bosinger SE, et al. . Cataloguing of potential HIV susceptibility factors during the menstrual cycle of pig-tailed macaques by using a systems biology approach. J Virol 2015; 89:9167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thurman AR, Chandra N, Yousefieh N, et al. . Comparison of follicular and luteal phase mucosal markers of HIV susceptibility in healthy women. AIDS Res Hum Retroviruses 2016; 32:547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brawner BM, Sommers MS, Moore K, et al. . Exploring genitoanal injury and HIV risk among women: menstrual phase, hormonal birth control, and injury frequency and prevalence. J Acquir Immune Defic Syndr 2016; 71:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaireti R, Lindahl TL, Byström B, Bremme K, Larsson A. Inflammatory and endothelial markers during the menstrual cycle. Scand J Clin Lab Invest 2016; 76:190–4. [DOI] [PubMed] [Google Scholar]
- 39. Chandra N, Thurman AR, Anderson S, et al. . Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses 2013; 29:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lugli E, Hudspeth K, Roberto A, Mavilio D. Tissue-resident and memory properties of human T-cell and NK-cell subsets. Eur J Immunol 2016; 46:1809–17. [DOI] [PubMed] [Google Scholar]
- 41. Sathaliyawala T, Kubota M, Yudanin N, et al. . Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013; 38:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014; 346:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol 2013; 34:27–32. [DOI] [PubMed] [Google Scholar]
- 44. Swaims-Kohlmeier A, Haaland RE, Haddad LB, et al. . Progesterone levels associate with a novel population of CCR5+CD38+ CD4 T cells resident in the genital mucosa with lymphoid trafficking potential. J Immunol 2016; 197:368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saba E, Origoni M, Taccagni G, et al. . Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 2013; 6:1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liebenberg LJ, Masson L, Arnold KB, et al. . Genital-systemic chemokine gradients and the risk of HIV acquisition in women. J Acquir Immune Defic Syndr 2017; 74:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.