Abstract
The perspectives of regenerative medicine are still severely hampered by the host response to biomaterial implantation, despite the robustness of technologies that hold the promise to recover the functionality of damaged organs and tissues. In this scenario, the cellular and molecular events that decide on implant success and tissue regeneration are played at the interface between the foreign body and the host inflammation, determined by innate and adaptive immune responses. To avoid adverse events, rather than the use of inert scaffolds, current state of the art points to the use of immunomodulatory biomaterials and their knowledge-based use to reduce neutrophil activation, and optimize M1 to M2 macrophage polarization, Th1 to Th2 lymphocyte switch, and Treg induction. Despite the fact that the field is still evolving and much remains to be accomplished, recent research breakthroughs have provided a broader insight on the correct choice of biomaterial physicochemical modifications to tune the reaction of the host immune system to implanted biomaterial and to favor integration and healing.
Keywords: biomaterials, immune response, macrophages, scaffold, foreign body reaction, extra-cellular matrix
1. Introduction
Biomaterials play a central role in a wide variety of healthcare issues and have fostered great improvements in different biomedical fields, such as tissue engineering, medical implants, drug delivery, and immunotherapies [1,2,3,4,5]. This wide applicative potential relies on the ability of these materials to provide biocompatible supports (i.e., scaffolds, devices), to encapsulate and protect biological active products (i.e., cells, chemicals, and proteins), and to allow easy modification of chemical and physicochemical properties [5,6,7,8,9,10]. Biomaterials include a broad range of compounds that widely differ in function and structural features, ranging from naturally occurring biological macromolecules to fully synthetic coatings.
However, one common property of biomaterials is the induction of adverse immune reactions resulting in excessive inflammation, impairment of healing, fibrotic encapsulation, tissue destruction, or even isolation and rejection of medical devices.
A more in depth understanding of the material/biological environment interplay is greatly needed, in order to develop strategies and solutions to overcome side effects in the use of these devices, which still represent an important challenge in the biomedical field.
In this review, we detail the different cellular and molecular events characterizing biomaterial-immune system interactions. Then, we discuss how the immune response can be tuned by biomaterial properties (such as surface chemistry and topography) and by decellularized extracellular matrix. Finally, we highlight how the specific features of the different biomaterials could be exploited to control the inflammatory-immune response to implanted biomaterials and to promote tissue regeneration.
2. Immune System—Biomaterial Interplay
The immune response is a biological network in charge of protecting the host from foreign threats and maintaining homeostasis.
The human immune system comprises two arms: the innate immune system, which elicits a non-specific inflammatory response following the immediate recognition of foreign material, and the adaptive immune system, which performs highly specific antigen responses and develops a long-term memory. Each part includes different cell populations: polymorphonuclear cells, mononuclear phagocyte cells (dendritic cells—DCs, monocytes, and macrophages) and lymphocytes (natural killer cells, gamma delta T-cells, and innate lymphoid cells) belong to the innate system, whereas B and T lymphocytes belong to the adaptive one [11]. The development of an appropriate and effective immune response requires close, coordinated, and carefully controlled crosstalk between the two systems, by means of soluble factors and cellular subsets.
Implantation of a biomaterial induces a host reaction to the implant that determines the outcome of the integration and the biological performance of the implant. Degradation products released by devices (tissue engineered scaffolds, orthopedic implants, biomedical devices) and the resulting surface changes of the degrading biomaterials activate the immune system [12]. The interplay between the host immune system and the biomaterial depends on the tissue surrounding the implant, which will drive the tissue-specific innate defenses and the following induction of adaptive immune responses. In fact, it is becoming more apparent that macrophages resident in tissues or recruited from other sites play distinct roles in the healing process likewise implantation of the same material into different sites elicits distinct responses [13].
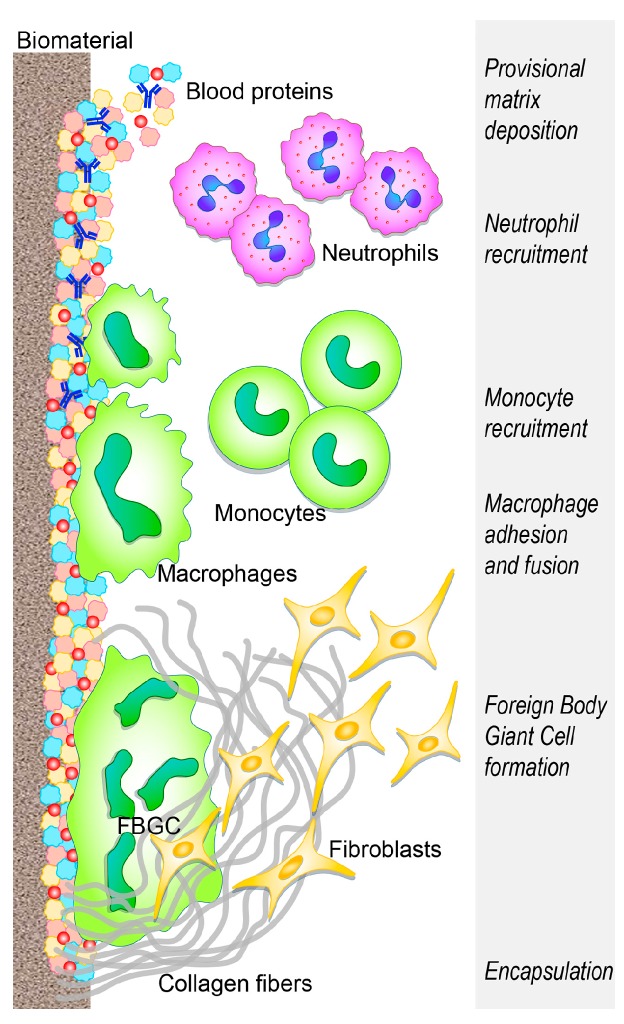
The benefit and functionality of the implanted biomaterial can be weakened by the development of an acute sterile inflammatory reaction (foreign body reaction—FBR) superimposing tissue vascularization and remodeling, and ending with a fibrotic encapsulation that prevents further interplay between the biomaterial and the host tissue (Figure 1) [14,15,16] (extensively reviewed by [1,17,18,19]).
Figure 1.
Innate immune response to biomaterials: the development of the foreign body reaction. The main cellular players in the biomaterial-immune system interaction are represented. The main events, from the initial biomaterial implantation to fibrous encapsulation, are schematically described.
Even if biomaterial implants have the ability to induce a FBR according to context specific features, the clinical manifestations widely differ for gravity and for the resulting implant outcome [6,12,19,20]. However, FBR is only one possible outcome of biomaterial implantation and the possibility to modulate this response is the key for successful implantation.
Within a few seconds from implant placement, blood from the damaged vessels surrounds the biomaterial, thus beginning the interaction with the implant. Within minutes, host plasma components, including proteins (albumin, fibrinogen, fibronectin, vitronectin, and gammaglobulins), lipids, sugars, and ions, are rapidly and spontaneously adsorbed on the implant surface [6,21]. Various characteristics of the biomaterial surface (such as energy, chemistry, topography, and roughness) influence the type, the amount, the composition, and the conformation changes of adsorbed molecules and further recruitment and adhesion of tissue-derived, inflammatory, vascular, and stromal cells, in the following hours/days [22,23,24]. These characteristics are crucial determinants of the tissue reaction to such implants [6,25].
The blood exudate also contains platelets and other components of the coagulation cascade, and the resulting clot formation defines the provisional matrix around the biomaterial [26,27], leading to further platelet activation and aggregation [28,29] and to fibrinogen cleavage into fibrin by means of thrombin [30]. In addition, complement proteins, activated upon contact with the biomaterial, synergistically support platelet adhesion and activation [31,32,33] and the recruitment and adhesion of additional immune cells [34,35], attracted by the local bulk of pro-inflammatory cytokines, chemokines, and growth factors [12,36]. Following the formation of the provisional matrix, the acute and chronic inflammatory responses follow each other and the intensity of these responses is influenced by the wideness of the implantation damage [12] and also by the implanted biomaterial. Extra-cellular matrix (ECM) adhesion proteins, including fibronectin and vitronectin, have also been reported to adhere to biomaterial surfaces [37], and are crucial in modulating the inflammatory reaction to biomaterials, whereas fibrinogen and complement are mainly involved in the activation of the cellular component of inflammation. Fibronectin and vitronectin respectively enhance cell adhesion [38,39,40], promote macrophage fusion, and participate in the FBR chronic phase [41,42,43].
Cell adhesion to protein-coated biomaterials and the subsequent cell activation are mediated by integrin signals [7,44,45]. In addition, leukocytes undergo activation by means of the same systems of surface receptors that detect foreign microorganisms. In particular, some pattern recognition receptors (PRRs) (usually interacting with pathogen-associated molecular patterns—PAMPs, found on microorganisms and primarily expressed on macrophages and dendritic cells) [46,47] are also able to sense dangerous situations. They induce immune responses driven by molecules within the family of damage-associated molecular patterns (DAMPs). Other danger signals participating in leukocyte activation are Alarmins (which include heat shock proteins, high mobility group box 1, ATP—adenosine triphosphate, uric acid). They are endogenous equivalents of PAMPs, and they are similarly recognized by macrophages and DCs, through PRRs (here acting as scavenger receptors), Toll-like receptors (TLR), and C-type lectin [48,49,50].
Activated neutrophils, recruited from the peripheral blood by chemoattractant factors (released from host activated platelets, endothelial cells, and injured tissue cells), adhere to the implantation site (by means of β2 integrins) and attempt to destroy/degrade the biomaterial through phagocytosis, proteolytic enzymes, and reactive oxygen species (ROS) released by cytoplasmic granules [51,52,53,54]. In addition, neutrophils release neutrophil extracellular traps (NETs) [55], a “sticky network” of granular proteins, neutrophil elastase, chromatin DNA, and histones [56], usually involved in trapping pathogens and preventing infection spread [55]. Altered release of neutrophil extracellular traps is involved in sustaining the fibrotic tissue response, leading to the excessive production of a dense fibrotic matrix [57]. The undue production of NETS prevents integration between the tissue and the biomaterial and degrades neutrophil-produced cytokines and chemokines that regulate the healing process [57,58]. This impairs the healing response and the potential for tissue regeneration, and promotes fibrotic encapsulation [59]. Furthermore, NETs release from neutrophils, unable to phagocytose a harmful stimulus [60], may be considered similar to the formation of foreign body giant cells by the fusion of frustrated macrophages [61].
Upon activation, neutrophils synthesize a significant amount of immune-regulatory signals [62]: CXCL(CX chemokine ligand)8 (the most prominent chemokine), whose primary targets are neutrophils themselves, CCL (C chemokine ligand) 2 and CCL4, both potent chemoattractant and activation factors for monocytes, macrophages, immature DCs, and lymphocytes [63]. The progressive increase of these chemokines fosters monocyte infiltration and suppresses that of neutrophils, which, lacking further activating signals, undergo apoptosis and progressively disappear from the implantation site [12]. Concurrently, circulating monocytes respond to chemoattractant (such as CCL2, CCL3, and CCL4) and bind fibrinogen in the biomaterial provisional matrix, thereby undergoing activation [43,64,65] and differentiation into the classically activated or “M1” macrophages [42,66,67,68]. These cells have been classified according to their ability to secrete pro-inflammatory cytokines, (such as interleukin (IL)-1β, IL-6, tumor necrosis factor-TNFα), chemokines [66,69], and enzymes.
Adherent macrophages foster the invasion of additional inflammatory cells by secreting chemokines (CCL2, CCL4, CXCL8) [69] and also attempt to degrade the biomaterial by releasing ROS and degrading enzymes [70,71], before undergoing “frustrated” phagocytosis (since the biomaterial is too large to be internalized), ultimately resulting in an increased release of cytokines [72,73]. Similar to the wound healing process [74], adherent macrophages eventually shift to the “M2” phenotype [75], secreting anti-inflammatory cytokines (such as IL-10) presenting a reduced degradative capacity and achieving tissue remodeling activity, also inducing the migration and proliferation of fibroblasts toward effective tissue regeneration [76]. The overlapping events of the phenotypic M1 to M2 switch (reviewed by [74]), as well as the mechanisms of frustrated phagocytosis, result in macrophage membrane fusion to form a foreign body giant cell (FBGC, an hallmark of chronic inflammation) on the biomaterial surface. This reflects an attempt to increase their phagocytic functionality, to attach and degrade large implants [71,77]. FBGC can adhere to the surface of the biomaterial for a long time, thus forming a barrier between the tissue and device, and eventually ending with implant deterioration and/or loss. Therefore, macrophage plasticity allows their adaptation to immune-regulatory, host defense, tissue repair roles in response to the implant properties. The formation of FBGCs is often a signature component of biomaterial-induced FBR and is fostered through the activation of mast cells, basophils, and T helper (Th) cells that secrete IL-4 and IL-13 shown to enhance macrophage fusion on biomaterials [78,79,80]. Mast cells are consistently reported at the site of implantation [81,82,83], where they degranulate upon activation, releasing histamine (shown to play a role in directing neutrophils and monocytes to implanted biomaterials [82,84] and secreting pro- and anti-inflammatory cytokines, angiogenic, and pro-fibrotic factors (such as vascular endothelial growth factor—VEGF and transforming growth factor—TGF-β) [85,86].
During the chronic inflammatory phase, cytokines are mainly produced by directly or indirectly activated T lymphocytes, mainly CD (cluster of differentiation) 4 helper T cells and their Th1 and Th2 subsets. Their copious cytokine production widely modulates the pro/anti-inflammatory responses [78]. The correlation between the M1/M2 macrophage phenotype and the change in cytokine expression profile from Th1 to Th2 lymphocytes suggests that T lymphocytes are pivotal in promoting the resolution of inflammation and regeneration. With regards to this aspect, some evidence points out the importance of the early neutrophil immune responses for the later modulation of M2 macrophages and Th2 lymphocytes to functional healing [87,88,89]. T cell involvement in the FBR to non-phagocytosable implants has not been fully elucidated; however, T cells have been shown to attach to the biomaterial [90] and become activated through non-canonical pathways [91,92,93]. They also enhance macrophage adhesion and fusion into FBGCs through paracrine actions of secreted cytokines [78,94,95].
The concerted action of immune cells results in the release of pro-fibrogenic factors, such as platelet-derived growth factor (PDGF) [96], VEGF [97,98], and TGF-β [99,100], which recruit fibroblasts. In an attempt to repair the damaged tissue, activated fibroblasts deposit collagen (type I and III); however, excessive secretion results in the undesirable final outcome of fibrotic deposition of ECM (greater ratio of I/III is associated with a greater fibrotic tissue formation) [101], encapsulating the biomaterial [102,103] and compromising implant function [104,105]. The deposition of a new matrix is mainly carried out by fibroblasts and myofibroblasts, the prominent cellular components in the fibrotic reaction. Myofibroblasts (as suggested the “myo” prefix) share proteins of the smooth-muscle cells, whose contraction can concur to fibrotic scar formation [106].
With regards to the pro-regenerative mechanisms, M2 macrophages displaying an anti-inflammatory/anti-fibrotic phenotype contribute to regeneration through crosstalk with a subpopulation of T cells defined as regulatory (Tregs), which play an important role in tissue immune homeostasis. These cells can skew the local immune response from inflammation to a pro-regenerative tissue repair cascade, sustaining the anti-inflammatory/anti-fibrotic phenotype by the secretion of anti-inflammatory cytokines, such as IL-10. Furthermore, Tregs are able to improve healing quality by inducing a type 2 response, including anti-inflammatory macrophages. Following a T cells decrease, resident Treg levels remain elevated, probably because they display an epithelial growth factor receptor (EGF-R) [107,108] whose expression allows the growth factor amphiregulin secreted by mast cells to maintain Tregs at the damaged site [107]. Once present, Tregs proliferate and upregulate the secretion of amphiregulin, which may induce either cell proliferation [109] or differentiation and is necessary for regeneration [110,111,112]. Tregs may also enhance the regenerative capacity of endogenous stem/progenitor cells through the secretion of growth factors.
In addition to the above reported cells and mechanisms, whose involvement in the response to biomaterials is well-described even if not exhaustively explained, other cells have been suggested to play roles in the timely resolution of inflammation and successful regeneration process.
For example, dendritic cells (DCs), similar to macrophages, phagocytize particles and process danger signals at the injury site. Although their exact role during tissue repair and regeneration is still not completely known [113], studies show that they play an important role in the tissue healing process [113,114]. DCs interact with T cells and B cells to initiate and shape the adaptive immune response, they have immuno-regulatory activities, and they influence the development of tolerogenic or anergic T cells, depending on their maturation stage, location, and cytokine environment [115]. Additionally, they induce the activation and growth of regulatory T cells (Treg) (reviewed in [115,116]). The interaction between dendritic cells and biomaterials was mainly studied in the presence of an immunogenic stimulus and the increased immune responses to co-delivered antigens [117] pointed at a novel “adjuvant” role of the biomaterial. However, this role assumes the ability of the biomaterial to activate DCs by direct contact between the material and the cells [118]. It is suggested that biomaterials prime dendritic cells through PRR signaling pathways [48] and depending on which PRR receptor is triggered, maturation or inhibition of DCs can be induced, contributing respectively to the lengthening of the immune response to biomaterials and the delay of wound healing or to the down-regulation of the inflammatory response [119].
Since multiple physio-pathological processes cannot be simply explained by the Th1 and Th2 cell paradigm, this led to the identification of a distinct T cell effector subset, referred to as Th17, the main IL-17 and IL-22 producing cells. Th17 cells, as well as IL-17 and IL-22, are basic components of the mucosal immune system and their alteration is closely linked to autoimmune and inflammatory diseases [120]. A recent report linked IL-17 mediated signaling to the differentiation of monocyte/macrophage populations to a pro-fibrotic phenotype [121]. However, IL-17 is redundantly produced by other immune cell populations, including γδ T cells [122] and innate lymphoid cells type 3 (ILC3) [123].
The γδ T cell subset has a prevalent surveillance role in native tissue [124] and has been widely reported as pro-regenerative, contrary to the αβ T cell subset that displays both pro- and anti-regenerative characteristics [124,125,126]. Few data show the beneficial role of the γδ T cell subset, as described in both mice and humans that do not effectively heal skin wounds in the absence of these cells [127,128]; however, little is known about their potential pro-regenerative contribution in the field of biomaterials.
Another subset, the CD8 cytotoxic T cells (CTL) (responsible for detecting and killing cancer cells and viral infected targets), has been found to influence wound healing, as demonstrated by the improvement of its outcome following CD8 T cell depletion in rats [129] and by the negative impact on bone fractures, following a CD8 T cells increase in humans [130].
In addition to T cell depletion, few available evidence on the role of B cells in tissue healing seems to suggest that their depletion also represents a promising strategy to augment bone regeneration, since adaptive immune system deficient mice exhibit faster bone healing [131]. However, much is still to be discovered on the role of B cells in the repair and regeneration of various tissues.
Recently, innate lymphoid cells (ILCs), defined by the lack of expression of T or B cell receptors, have been subdivided into three classes (ILC1, 2, 3), characterized by their canonical transcription factors and cytokine expression [132]. These subsets somewhat mirror the expression of Th1, Th2, and Th17, respectively. ILC2, similar to Th2 cells, produces IL-4, IL-5, and IL-13 and like Th2 and M2 macrophages, is anti-inflammatory and provides a set of cell signaling, mediators and metabolites that are associated with wound healing. ILC2 also promotes CD4 T cell polarization towards Th2 cells via positive inhibition of Th1 [133,134,135]. Considering that the development of a pro-regenerative response to biomaterials requires type 2 cell populations [136] and that the crosstalk Th2/ILC2 is central to tissue pro-regenerative responses, ILC2 activity may be relevant with biomaterials.
The design of biomaterials targeted to tune the immune response to their benefit must therefore take into account the activation of immune cells and the mutual crosstalk between the different innate and adaptive cellular components [137].
3. Immunological Profile of Biomaterials
Biomaterials have been classified in many different ways, with the most immediate and general one referring to the chemical nature of the material itself. It is thus possible to distinguish metallic materials (ferrous and non-ferrous) and non-metallic materials (organic and inorganic). Among the organic materials, polymers (both synthetic and of natural origin) are of particular importance, whereas among the inorganic ones, ceramic materials must be mentioned [138].
Metal materials are mainly used in the manufacture of prostheses or implants for orthopedics and dentistry, as parts of composite implants. The most used metals are stainless steels, cobalt alloys, titanium and its alloys. However, metals have problems concerning biocompatibility in relationship to bone-metal interface processes and ion release, so metal-based implants must be treated to prevent the onset of inflammatory processes. Moreover, from the point of view of tissue engineering, the use of these materials is very limited.
The most used materials for tissue regeneration with particular attention to bone and cartilage regeneration are ceramics, synthetic polymers, and natural polymers [2,8].
Ceramic materials (such as glass, aluminum oxide, zirconium oxide, calcium phosphate) are mostly applied as orthopedic and dental implants and are also widely used as bone cavity fillers. They are characterized by a high hardness, high temperature resistance, low elasticity, and high fragility. They display an excellent biocompatibility thanks to the chemical and structural formulation analogous to the native bone tissue [139].
Composite materials, consisting of two or more types of materials, are each identifiable for the presence of interfaces between the components. The peculiarity of composite materials is that, being the combination of several components, they can provide better overall properties than individual constituents.
Polymeric materials represent 45% of the biomaterials used in the biomedical field. Generally, they are macromolecules formed by the more or less regular repetition of basic monomeric units. They allow customized architectures with a controllable degradation speed to be realized. However, their composition can lead to inflammatory phenomena and their hydrolytic degradation can release carbon dioxide (CO2) with a consequent decrease in the local pH, thus damaging the cells and inducing surrounding tissue necrosis [140]. Synthetic polymers have been demonstrated to be promising biomaterials for tissue engineering, due to their biomechanical and biodegradability properties. Natural polymer resemblance to the native ECM makes these scaffolds highly biocompatible. The main characteristics and the immunological profiles of both synthetic and natural polymers are summarized in Table 1.
Table 1.
Characteristics of synthetic and natural polymers.
| Characteristics | Synthetic | Natural |
|---|---|---|
| Polymer Types | Poly(anhydride), Poly(propylene fumarate) (PPF), Poly(caprolactone) (PCL), Poly(phosphazene), Poly(lactic acid) (PLA), Poly(ether ether ketone) (PEEK) poly(glycolic acid) (PGA) poly(lactic-co-glycolic acid) (PLGA) |
agarose alginate collagen fibrin, glycosaminoglycans hyaluronic acid, chitosan silk |
| Advantages | inert, high reproducibility, availability on demand, reduced costs, constant quality supporting industrial scale production, possibility to design or tune, mechanical properties, composition adaptable to needs, possibility to fabricate complex shapes, controlled degradation rate, long shelf life, cell attachment improvement, potential to deliver soluble molecules |
readily available, mass producible, large quantities constantly available, cost, low immunogenicity, bioactive properties, binding sites for cells and adhesion molecules |
| Drawbacks | immune response, lower ability to interact with cells, strong inflammasome reaction |
sterilization cost, in vivo source natural variability, lot-to-lot variability, limited mechanical properties, degradation rate difficult to control, unwanted immune reactions due to impurities |
| Host Innate Immune response | high | low |
| Host Adaptive Immune response | not applicable | low |
| Based on data from [2,3,4,5,8,19,141,142] | ||
Among the synthetic polymers, poly(caprolactone) (PCL), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic acid) (PLGA) are currently the most popular for the creation of scaffolds [143,144].
PCL is a bioresorbable polyester material, considered to be both a soft and hard tissue compatible material [145]. PCL is elastic in comparison to other polyesters and is used in various forms, such as films, fibers, and microparticles. PCL also has poor cellular adhesion properties on its own, without some form of functionalization.
PLA has a wide variety of applications, e.g., prosthetics, vascular grafts, skin regeneration, scaffolds, and bone screws [143]. To overcome the possible inflammatory reactions induced by crystalline fragments released during degradation by the lactic acid L isomer form, PLA can be obtained as a blend of L and D monomeric isoforms, thus lacking high crystallinity and improving degradation [143].
PGA degrades almost completely within a few months under an in-vivo condition. It is a good material used for bone tissue engineering applications.
PLGA is among the most widely used synthetic polyesters for scaffold formation in tissue engineering applications [143,146]. The major advantages of PLGA are the biodegradability, adaptability, and customization of different types of formulations or surface modifications. Since lactic acid, the basic component of the polymer, is also a product of cell metabolism, good ‘biocompatible’ properties are attributed to PLGA and its degradation-byproducts. However, one major limitation is the acidity of the degradation products that, if produced in large quantities, can hamper rapid and efficient metabolization by the body: increasing data point out that the frustrated degradation process and/or derived by-products may be able to induce an immune response [147]. The ratio among monomers can influence the tissue response to copolymers like PLGA.
Natural polymers include materials made from many polysaccharides (such as agarose, alginate, chitosan, hyaluronan, glycosaminoglycans) and proteins (such as collagen, fibrin, silk).
Agarose and alginate are natural polysaccharides typically obtained from red and brown seaweed, respectively. Agarose resemblance to the extracellular matrix results in attractive features for tissue engineering. It is an excellent candidate for controlled/localized drug delivery and a suitable biomaterial for cell-encapsulation because of the high water uptake capability [148]. In the biomedical field, alginates are used in medical textiles, hemostatic material, and wound dressing, for controlled drug release, for cells encapsulation, as scaffolds in ligament and tendon tissue engineering, and in preparing molds in dentistry. They are used as a stabilizer in solution and for the dispersion of solid substances by pharmaceutical industries [149].
Chitin is a naturally occurring mucopolysaccharide present in the exoskeleton of crustaceans, insects, and fungal cell walls. Chitosan (deacetylated form of chitin) has been reported for its various important pharmacological properties. It is mainly utilized as an excipient for tablets, as a controlled release dosage form, as gels, as an absorption enhancer, for drug delivery, in wound healing products, and in developing micro/nanoparticles. Its role in tissue engineering and regenerative medicine is also well-documented [150].
Glycosaminoglycans (GAGs) are long chains formed by repeated units of disaccharides. These unbranched carbohydrates have a fundamental role in life, being responsible for the coordination of manifold fundamental processes for tissue development and homeostasis, such as biophysical characteristics, cell signaling, and assembly of the extracellular matrix. GAGs are included in various biomaterials used for tissue engineering, drug delivery, and regenerative medicine [151].
Hyaluronic acid (HA) is the only non-sulfated GAG [152]. HA is a major component of the extracellular matrix (ECM) and plays an important role in regulating tissue injury, accelerating tissue repair, and controlling disease outcomes. Improved therapeutic efficacy has been achieved through partial modification and formation of HA-based biomaterials, including HA-scaffolds, sponge-like hydrogels, anti-adhesive sheets, cultured dermal substitutes, thin membranes, and dermal matrix grafts.
Collagen is the most abundant structural protein and is among the most frequently used naturally-derived polymers. It forms a highly organized, three-dimensional architecture and can carry any component due to its network-like structural nature. Collagen is used for different biomedical applications: as biomaterial for tissue engineering and bone substitute and for eye implants, as well as a matrix for drug, gene, and protein delivery and in sponges for burns/wounds [153].
Fibrin is an integral part of the clotting cascade and is formed by polymerization of the soluble plasma protein fibrinogen. Due to its role in hemostasis and tissue repair, fibrin has been used extensively as a tissue sealant. It is particularly interesting because, being fibrinogen and thrombin, obtained from autologous blood, the choice of fibrin allows the production of the patient’s own scaffold, providing the starting support for cell adhesion, migration, growth, and differentiation [154].
Silks are proteins contained in the glands of arthropods. Recently, silk from Bombyx mori (silkworm) larvae cocoons has been extensively discussed. It is a natural protein polymer containing 70–80% fibroin (core protein) and 20–30% sericin (adhesive protein) as major constituents. Silk fibroin (SF) has become a popular biomaterial due to its biocompatibility, robust mechanical performance, adjustable degradation, easy processing, and availability. Due to their paramount achievement, biomaterials based on silk fibroin may be used for different purposes, such as for the regeneration of vascular tissue, bone scaffolds, drug delivery, and dressing of skin wounds [155,156].
As shown in Table 1, well-studied biomaterials (such as alginate, agarose, chitosan, hyaluronic acid, PLGA) that are not or low antigenic in appearance, can differently crosstalk with the immune system, thereby eliciting complex and not fully understood immunomodulating properties [157]. Despite attempts to reduce the immunological response to the material by tuning its chemical and topographical surface characteristics, the synthetic materials trigger the classical foreign body reaction. The need for preventive co-drug treatment of various synthetic polymer surfaces (even if poorly adhesive for proteins), to avoid potential thrombotic or complement mediated reactions, demonstrated that the perfect material is not yet available [158].
Conversely, naturally-derived polymers, together with decellularized tissues, decellularized cellular constructs, and coatings, do not induce the typical foreign body reaction, but a positive innate immune remodeling reaction, associated with an adaptive immune response [158]. A better knowledge of scaffold behavior will have an impact on the control of both natural and synthetic constructs, giving a degree of engineerability to the natural polymers and developing tailored immune modulating constructs using synthetic ones [157,159,160].
Even if silk is less immunogenic, as a natural polymer, it induces a macrophage response [161], mostly mediated by the sericin protein, an adhesive matrix coating that traps fibroin. A controlled release of cytokines from a coated silk polymer induced and reversed M1/M2 macrophage polarization [162]. Moreover, a localized, short-term release from silk biomaterials, of either interferon (IFN)-γ or IL-4, shifted macrophage polarization from M1 to M2 [162]. Large silk biomaterials do not induce peripheral T-cell activation, probably due to markers downregulating the responsiveness of T lymphocytes [163].
Silk can participate in the composition of hydrogels, which are an important class of polymers characterized by highly hydrophilic properties.
Hydrogels are obtained from natural sources, including ECM proteins (collagen, fibrin, gelatins) and polysaccharides (glycosaminoglycans—GAG, dextran, alginate and chitosan), as well as from synthetic sources that are poly(vinyl alcohol)-based. Hydrogels from native tissues can be used as scaffolds for tissue engineering, by sacrificing the original architecture but keeping the native biochemical cues. They can deliver biomolecules and mirror the ECM structure, thus supporting adhesion, proliferation, and differentiation of the embedded cells [138]. High molecular weight hyaluronic acid [164] and chitosan [165] have intrinsic anti-inflammatory properties due to their radical oxygen species-scavenging properties. However, the majority of biomaterials require loading or functionalization.
Natural polymers such as collagen and fibrin, susceptible to enzymatic degradation, are ideal for releasing immune modulating molecules. On the contrary, synthetic biomaterials potentially subjected to degradation and responsible for a possible immune response when implanted, need a close control over degradation and kinetics of molecule release [166].
4. Tunable Properties of Biomaterials
Increasing knowledge on the host responses to biomaterials, the processes of healing, and the potential of biomaterials to modulate immune cells, clearly points out two main developing strategies: the need for testing biomaterial effects on both innate and adaptive immune responses and the design of biomaterials capable of tuning appropriate immune responses at implantation sites [167,168]. Current strategies of such biomaterial design start from the surface properties, shown to be central to the immune response, and intervene either passively on the physicochemical characteristics or actively by incorporating molecules or coating matrices.
4.1. Surface Chemistry: Hydrophobicity, Chemical Moieties, and Charge Characteristics
Biomaterial surface interaction with the adsorbed proteins is crucial for the reaction to the implants, as previously described [6,25]. Different methods of altering surface chemistry have been tested for creating poorly adhesive surfaces, in order to control the amount, composition, and conformational changes of bound proteins [169].
Biomaterial hydrophobicity plays an intrinsic immunogenicity [9,170]. The immune system has evolved to recognize hydrophobic portions of biological molecules as universal damage-associated molecular patterns, thus triggering PRRs and leading to elimination [170]. Therefore, hydrophobicity and hydrophilicity (surface wettability) are important factors affecting protein adsorption. The average unfolding of a protein molecule [171] and the overall spreading [172] are larger on hydrophobic surfaces than on hydrophilic surfaces, on which proteins preserve their native-state secondary structure and show little, if any, adsorption [173,174].
Gold nanoparticles functionalized with increasing hydrophobic chemical groups show a correlation between the degree of hydrophobicity and gene expression of pro-inflammatory cytokines (TNF-α, IFN-γ) by bystander cells [9]. Similarly, silica particle surfaces functionalized with amino acids of increasing hydrophobicity up-regulate IL-1β secretion from DCs and IFNγ secretion from T cells primed by these DCs [175]. Furthermore, hydrophobic particles undergo increased phagocytosis and clearance by the reticuloendothelial system [159].
The lower hydrophilicity of the pristine titanium surfaces induces a higher secretion of several pro-inflammatory cytokines (such as TNF-α, IL-1β, and CCL2) compared to heparin/fibronectin immobilized titanium surfaces [176]. On the contrary, hydrophilic modification of sand-blasted, acid-etched (SLA) surfaces [78] down-regulates the gene expression of several human macrophage pro-inflammatory cytokine genes (TNF, IL-1α, and β; CCL1, 3, 19, and 20; CXCL1 and 8; and IL-1 receptor type 1) and the secretion of the corresponding proteins [177].
With regards to macrophage and lymphocyte adhesion and activation, hydrophilic/anionic surfaces promote higher levels of macrophages and FBGC-adherent lymphocytes. Hydrophilic/neutral surfaces are selective for CD4 T lymphocyte interactions, while hydrophobic surfaces are selective for CD8 T lymphocyte interactions [178].
To counteract the immunogenic effects of hydrophobic surfaces, hydrophilic molecules such as polyethylene oxide (PEO) and polyethylene glycol (PEG) are often added to tissue engineering scaffolds and used as monolayer coatings to reduce surface protein absorption and as delivery vehicles to increase hydrophilicity [179,180,181]. However, an opposite approach has recently been suggested. It privileges the preservation of native three-dimensional protein conformation (in fact, protein unfolding or misfolding and presentation of cryptic bioactive sites can trigger adverse reactions) rather than the exclusion of the implant from all interactions with the surrounding tissue [182].
Another important surface characteristic is represented by chemical groups. The most commonly explored chemical moieties are amino (-NH2), carboxyl (-COOH), hydroxyl (-OH), and methyl (-CH3) groups [183]. Some of the main chemical characteristics and their involvement in the immunological response are summarized in Table 2.
Table 2.
Surface chemistry: commonly explored chemical moieties.
| Groups | -NH2 (Amino) | -OH (Hydroxyl) | -COOH (Carboxyl) | -CH3 (Methyl) |
|---|---|---|---|---|
| Surfaces | hydrophilic | hydrophilic | hydrophilic | hydrophobic |
| Charges | positive | neutral | negative | neutral |
| Focal adhesions | medium | high | medium | low |
| Ability to access fibronectin domains, integrin binding, cell adhesion | medium | high | medium | low |
| Inflammatory cell infiltration | high (in vivo) | high (in vivo) | low | high (in vitro) |
| Macrophage response | anti inflammatory | low inflammatory | inflammatory | |
| low inflammatory | ||||
| low inflammatory promoting regulatory T cell phenotypes (mouse model) | ||||
| Thickness of fibrotic capsules around the implant | high (in vivo) | high (in vivo) | low | high (in vitro) |
| Cell differentiation pathways | medium (osteoblasts) | high (osteoblasts) | medium (osteoblasts) | low (osteoblasts and myoblasts) |
Briefly, amino and hydroxyl groups induce the highest infiltration of inflammatory cells in vivo [185,190,194] and the thicker fibrotic capsules around the functionalized implant [190,195]. Amino terminated nanorods were also described to induce an anti-inflammatory M2 phenotype, whereas carboxyl ones shifted macrophages to an inflammatory M1 phenotype [187]. However, negatively charged carboxylated surfaces smooth inflammatory reactions [186,190,196] and trigger alterations in the migratory behavior and function of circulating macrophages, thus abrogating macrophage-mediated inflammatory activity and promoting regulatory T cell phenotypes [189].
Cell differentiation and focal adhesions [7,197] were maximally induced by hydroxyl moieties, followed by amino and carboxyl groups, and finally by methyl groups that were also able to induce a low myogenic differentiation [7,192].
Contradictory results have been reported on the effects of implants functionalized with hydrophobic, neutral -CH3 moieties; on the thickness of the fibrotic capsule; on the recruitment of inflammatory cells [186]; and on cellular adhesion [185,197,198].
Both cell differentiation and focal adhesions are regulated by integrin binding [44] and their different engagement mirrors conformational changes induced by adsorbed proteins. In fact, accessibility to fibronectin domains, integrin binding, and cell adhesion follow the same chemical moiety order [191,193].
The complement C3b component can covalently link the -OH group [199,200]. Considering that Complement receptor 3 (CR3)(CD11b/CD18) is expressed on leukocytes (mostly neutrophils) and macrophages, this offers an explanation for the increased accumulation of CD11b+ cells at the implant site [196].
The negative charges of both -COOH groups and the cell membrane, as well as the tighter adhesion of proteins (such as albumin and fibrinogen) [201,202,203] to hydrophobic surfaces [204], may account for some of the above results. However, they are not enough to foresee the behavior of bound proteins on these surfaces.
It was shown that proteins adsorbed to -COOH or -NH2 hydrophilic surfaces (negatively and positively charged, respectively) undergo greater conformational changes [203]. These data suggest that the amount of adsorbed proteins and their refolding degree are influenced by surface functional moieties rather than by the hydrophobicity degree. On the contrary, proteins were more prone to retain their native structure on -OH hydrophilic surfaces.
The surface charge of a biomaterial represents another important player in the modulation of the immune responses. Particles with positively charged surfaces lead to activation of the inflammasome (a multi-protein intracellular complex that activates a highly pro-inflammatory signaling cascade of the innate immune system) to greater extents than negatively charged particles [187,205]. Particles with a negative surface charge can inhibit the immune function by preventing uptake of the materials by antigen-presenting cells, thus completely abolishing antibody and T cell responses [206]. Zwitterionic motifs show the ability to activate monocytes and DCs, (stimulated to up-modulate MHC (major histocompatibility complex) class II and costimulatory molecules and to produce cytokines) [207], as well as to induce less inflammatory response and a thinner fibrotic capsule, and to shift macrophages to an M2 phenotype [23].
However, contradictory results are found in the literature given that surface charges have also been described to do the opposite: the negative ones inducing the highest levels of activation and the positive ones (shown above to activate pro-inflammatory responses) inducing lower levels of IL-1β [175].
Alginate and hyaluronic acid are naturally derived, negatively charged biomaterials, that have been highly studied as scaffold materials. The opposite results derived from these studies, on the effect of the negatively charged surface on immune responses, highlight the need for more detailed studies, to fully understand how to take advantage of surface charge and material formulation in supporting regenerative outcomes.
Overall results have revealed that protein binding kinetics and conformations on implant surfaces are dependent on surface chemistry. Hence, the initial cell response is triggered by the adsorbed protein, rather than by the surface itself. The pattern in which adhesion proteins and other bioactive molecules adsorb elicits cellular reactions that are specific to the underlying material physicochemical properties. If on one hand, these aspects point out the difficulty in decoupling related properties, on the other hand, they highlight opportunities to modulate immune cell phenotypes by altering the hydrophobicity, charge, or surface chemical functional groups of materials used for tissue engineering constructs.
4.2. Topography: Size, Shape, and Surface Texture
Medical devices display intrinsic topographical characteristics either suitably introduced or resulting from the manufacturing process. Particle deposition, self-assembled monolayers, soft photolithography, blasting, acid etching, and polymer expansion [208,209,210] are examples of the numerous and different techniques available for modifying material topography. These methods give rise to different size geometries (nano, micron scale), surface protrusions (pillars, posts, gratings, ridges), or dentations (pits and dots) [98,211,212,213].
Topographical characteristics have been identified as important regulators of cellular and physiological processes [188], such as cell adhesion [214,215,216], density spreading and motility [217,218,219,220], proliferation and differentiation [219,221], macrophage fusion [98], and cytokine secretion [222,223].
As described previously, the pattern of adsorbed proteins regulates many phenomena at the nano-bio interface. Fine tuning of the adsorbed protein activity can be achieved by topographical changes at the nanometer scale, reflected by conformational changes of the adsorbed protein [224]. A gold nanoparticle surface (58 nm) is shown to increase serum IgG antibody adsorption by 70%; whereas the C3c complement fraction is decreased by 50% [225].
The increase of nanoscale roughness (from 15 nm to 30 nm) induces a binding affinity decrease of a panel of proteins (< or = 90%) and a relevant increase in adsorbed proteins (> or = 500%) [226].
Further studies have described the effects of nanotopography on the adsorption and modified conformation of fibrinogen [227] that increases with increasing root-mean-square roughness (from 2.0 to 32.9 nm). This is probably due to a change in the geometrical arrangement of the fibrinogen molecules on the surface, and to the adsorption of the cell-binding protein fibronectin [228], which shows a decrease in the thickness of the adsorbed fibronectin layer with decreasing bulk protein concentration. This potentially accounts for differences in cell adhesion and activation on flat versus rough surfaces.
These data point out that the surface nanostructure and nanometric scale represent the relevant morphological characteristics regulating the protein adsorption process and that nanostructures are parameters that must be taken into account in the biomaterial design.
Different nanoscale topographies have also been explored for their interaction with cells [229]. However, larger surface patterns, ranging between 10 and 100 μm, are usually utilized to directly modulate cells [230]. Some of the effects induced on cells by different particle sizes are described in Table 3.
Table 3.
Biomaterial topography: size.
| Size | Cell Types | Findings |
|---|---|---|
| Nano scale | Platelets |
|
| Macrophages |
|
|
| ||
| Dendritic cells |
|
|
| Nano- submicron scale |
Macrophages |
|
| Micron scale | Macrophages |
|
| ||
| Meso scale | Macrophages |
|
Various data are available for titanium implants for orthopedic and dental applications. Titanium, even if more biocompatible than other metals, induces remarkably strong inflammatory responses which represent important aspects of implant failure. The decrease of the immune response to titanium can be obtained by modifying titanium surfaces with nano/micro-structures or with chemical processes changing the surface roughness.
Other studies have demonstrated that cells can “bend” patterned pillars (likely due to filopodia contraction) [237] and a recent observation indicates that nanostructures could penetrate fibroblasts, likely due to failed phagocytosis, resulting in cell thinning and membrane rupture [238].
These examples collectively indicate that initial cellular activation can be modulated by nanoscale surface topography alone and highlight how the multitude of techniques and topographies can differentially affect cell responses.
In addition to the size, the shape of a biomaterial is also an important parameter affecting the interaction with the immune cells, as indicated by some representative results reported in Table 4.
Table 4.
Biomaterial topography: shape.
| Cell Types | Findings |
|---|---|
| Macrophages |
|
| |
| |
| |
| Neutrophils |
|
| Dendritic cells |
|
| |
| T lymphocytes |
|
Overall, these studies reveal that the inflammasome can be modulated by simply altering the particle shape. Since tissue engineering can use biological or synthetic polymers, enabling a variety of shapes, the knowledge of how this parameter promotes or diminishes inflammasome activation could allow biocompatible and tolerated constructs to be produced. However, since these effects occur with some material shapes and not with others, more studies are necessary to fully understand the biology of this behavior.
Recent interesting experimental approaches have used polymeric particles to obtain artificial antigen presenting cells (aAPCs) [243,245,246]. Spherical nanoparticles and various ellipsoidal particles (obtained by mechanical stretch of the spherical ones) were coupled with antigen and co-stimulatory molecules needed for bio-mimicking the correct presentation to T cells and their following activation and proliferation.
These studies demonstrate that spherical aAPCs are phagocytosed more quickly and at higher levels compared to ellipsoidal aAPCs, whereas ellipsoidal aAPCs present a greater biodistribution and a lasting presence in the bloodstream when injected intravenously in mice, thus allowing a better interaction with T lymphocytes, confirmed by their increased proliferation [246].
An optimal T lymphocyte proliferative response is obtained when aAPCs are stretched 2–2.5 fold, pointing out the importance of the stretching degree in supporting contact length and the resulting T cell-biomimicking aAPC interactions [245,246].
Titanium dioxide nanotubes, derived by titanium surface anodization, lead to a significant modification of the lipopolysaccharide-induced macrophage inflammatory response, thereby reducing the gene expression of cytokines and chemokines, protein synthesis, the development of FBGCs, and the release of nitric oxide (NO) [247].
A reduced density of macrophages is observed after 24 h culture on nano-textured and nano-tubular anodized titanium samples as a function of anodization voltage increase (10, 15, and 20 V), compared with conventional unmodified samples [248].
Bio-anodized, acid-etched, and machined titanium surfaces (Ti) do not influence macrophage viability and do not induce a macrophage cytokine release (IL1-β, TNF-α and TGF-β1) significantly different compared to the Ti surfaces. Furthermore, the Ti surface characteristics do not induce a typical Th1 or Th2 cytokine profile, suggesting that titanium surfaces are inert to monocytes/macrophages and do not change the characteristics of the cell response [249].
The use of porous materials has been investigated for several decades and has been integrated into areas of dentistry and orthopedics (dental and bone/joint implants) [250].
Although the ideal pore size for osteoblast functionality in implants for bone engineering is still disputed [251], pores ranging within 20–1500 µm [252] have been investigated for cell migration capacity, spreading, proliferation, cartilage and bone formation [253], and angiogenesis [254,255,256]. Many reports indicate optimal pore sizes and validate the model prediction. Others, at constant macroporous characteristics, point out that it is the material processing that influences the biological outcome [257].
Pore size diameters of 300–400 µm represent the optimal dimension for an effective bone formation in porous hydroxyapatite. In fact, straight tunnel structures with diameters of 350 µm allow the direct bone formation, whereas in tunnels with diameters ranging between 90 and 120 µm, cartilage formation precedes bone appearance [257].
The need for carrier geometries able to induce the development of vascular structures represents a further parameter to consider in designing systems for the effective reconstruction of joints and bones. Hydroxyapatite structural characteristics (pore size, geometry, continuity, and straightness) can be exploited for other biomaterials for regenerative medicine applications. In general, the porous nature of these implants is ideal because they allow for tissue integration, vascularization, and the transport of nutrients [250,256]. They are therefore suitable for the fabrication of large engineered constructs. As for the effect on macrophage polarity, porous versus nonporous poly(2-hydroxyethyl methacrylate- methacrylic acid) hydrogels induced iNOS, thus indicating that the biomaterial activates pro-inflammatory pathways. Macrophage mannose receptor positive cells increased significantly at porous implants (suggesting a shift to an M2 phenotype), concomitantly with improved neovascularization for implants with pores >20 μm [258]. A similar study [75] disclosed a positive correlation of increasing pore size with the expression of Arginase 1 (Arg, M2 marker), along with a negative correlation with the expression of inducible nitric oxide synthase (iNOS, M1 marker).
Dealing with expanded polymers characterized by increasing average intranodal distances, the largest distance of 4.4 µm induced an early pro-inflammatory activation of macrophages in vitro, characterized by high levels of IL-1β and TNF-α, together with the increased gene expression of chemokines, leading to the recruitment of monocytes and neutrophils. However, in a mouse model, the same intranodal distance led to a thinner, less organized, and less dense capsule surrounding the implant [211].
Porosity and pore size must also be considered from the perspective of the equilibrium between the scaffold porosity and the structural solidity needed for the implant, to ensure that its strength is not compromised [251]. If on one hand, increased porosity and expanded conformation of constructs influence macrophage function, promoting pro-regenerative environments, on the other hand, changes in scaffold structure may negatively affect mechanical strength. This aspect is particularly important for implants designed to replace anatomical tissue with structural functions, such as bone, for which the mechanical strength of the scaffold is essential. Thus, while scaffold shape and porosity can be handled to switch on inflammation or repair by modulating macrophage phenotype and foreign body cell formation, it is also necessary to better understand the interaction between these immunological outcomes and material properties [75,255].
Last but not least, topographical effects should also be addressed. Parallel line gratings of width ranging between 250 nm–2 µm did not induce a macrophage response distinctive of different grating sizes. On the contrary, different grating topographies were able to modify the macrophage response on every polymer surface, independently of surface chemical properties. Cellular morphology and cytokine production were affected in vitro, whereas cellular adhesion was affected in vivo, particularly on a larger size topography compared to planar controls [98].
Polymeric fibers modified to exhibit different shapes and assembled in scaffolds formed from either random or aligned fibers influenced macrophage behavior. Macrophages were able to penetrate into scaffolds comprised of randomly arranged fibers with expanded thickness (3 or 10 mm), implanted into rat subcutaneous tissue, whereas scaffolds formed from aligned fibers and expanded to a 3 mm thickness supported greater macrophage infiltration and a lower number of giant cells, likely due to the gap distance between the fibers [255]. Overall, published outcomes are often contradictory and difficult to compare due to disparity amongst various surface topographies. This points out the importance of using cell types appropriate for a given implant purpose in order to identify the optimal properties to achieve the desired response in vivo.
5. Immune-Interactive Strategies
For several decades, the design of biomaterials has been specially dedicated to the development of “passive” biomaterials, with the aim of limiting immune adverse reactions. Emphasis on enhancing tissue repair by downregulating an unwanted host inflammatory response to implants has led to the identification of strategies to hide implant surfaces such as immune-isolating coatings to passively prevent/reduce protein adhesion to the implanted biomaterial surface and the resulting leukocyte activation or hydrogels to isolate implants from immune cells and thus to limit the inflammatory response [259]. However, it has now become clear that the immune system is fundamental in orchestrating and defining the nature of the repair process [260,261]. Indeed, the inflammatory response is not an adverse reaction but a crucial gateway in tissue repair and regeneration [262] and allowing specific biological responses is beneficial for both biomaterial integration and performance [105].
The link between the immune response and repair is complex and the current challenge is the development of biomaterials and delivery systems able to modulate the immune system as a way of stimulating the repair of tissues and organs [263]. Accordingly, the concept of ideal biomaterial is moving from ‘‘immune-evasive” aiming at decreasing host responses to ‘‘immune-interactive” triggering desired immunological responses, therefore enabling biomaterial integration and subsequent tissue repair [1,19,264,265]. The numerous studies attempting to modulate biomaterial-immune system interaction by tuning the surface chemical properties and/or changing the topographical characteristics of biomaterials are mostly focused on macrophages, as previously reported. In addition, different strategies, such as the incorporation of bioactive molecules (adhesion sites, drugs, cytokines, growth factors, or pro-resolution mediators either alone or combined) [266], have provided rather interesting results.
The following examples will focus on structures/molecules that are characteristic of the immune response, but are not exhaustive of the entire range of opportunities available for modulating interactions between the implanted biomaterial and the receiving host.
5.1. Immune Modulation by Decellularized ECM
The ECM is the non-cellular milieu spread within all tissues and organs that supplies both the fundamental physical framework for the cellular components and fundamental biochemical and biomechanical signals that regulate morphogenesis, differentiation, and homeostasis of the tissues.
Naturally derived scaffolds such as decellularized extracellular matrices are historically used as frames for reconstruction, for delivering cells and biological factors, and for controlled molecule release [267]. Although the development of biological scaffolds to deliver cells and factors remains an active area, the alternative strategy of using the scaffold to induce therapeutic immune responses or intentional shifts in the immune phenotype is emerging. Data from preclinical studies aiming at the development of ECM scaffolds for therapeutic purposes indicate the pivotal contribution of immune system modulation [268]; however, the basic mechanisms are not yet fully clarified.
Procedures of ECM decellularization collectively remove the tissue immunogenic components, reducing (but not eliminating) the antigen load and retaining only the native architecture. The response to a decellularized implant likely depends on many factors, including the tissue origin, the implant site, and the decellularization process, which influences the degree of immunogenicity of the remaining cell remnants. The structural properties of these natural scaffolds favor the organization and the functional recovery of the tissue by influencing numerous cellular processes that create a pro-regenerative environment and support the host infiltrating cells [269,270]. Indeed, among other properties, decellularized ECM has shown the ability to shift macrophage polarization towards either an M1 or M2 phenotype, thus modulating the wound immune microenvironment.
In agreement, the transplantation of acellular scaffolds has been generally connected to an M2-like response with less scarring and greater constructive remodeling capacities than cellular scaffolds [271] and it has been recently shown that scaffolds obtained from tissue ECM elicit a strong Th2 pro-regenerative immune environment, in turn enhancing M2 macrophage polarization via an IL-4-dependent pathway [136].
Collectively, these findings evidence that the induction of a Th2 environment is an important component of immune-interactive scaffolds in tissue engineering applications. However, the type of immune response induced by a decellularized ECM scaffold highly depends on the tissue from which the ECM is obtained. Since macrophages recognize denatured [272] and strain-damaged [273] collagen, different decellularization procedures may have different effects on macrophages and significantly bias immune activation, depending on the changes they induce in ECM components. Even if the exact underlying mechanism is still not fully clarified [271], it was recently suggested that biologically active microvesicles (MBVs) bound to ECM could be partially responsible for the scaffold dependent effects [274] by means of miRNAs present within MBVs. Although some MBVs miRNA are conserved across different sources, a significant amount are tissue-specific, thus miRNA specificity could be partially responsible for the different effects induced by decellularized scaffolds, depending on tissue origin. Indeed, the comparison of the macrophage response after exposure to ECM from different tissue sources shows very heterogeneous behaviors [275,276]: small intestine submucosa (SIS), urinary bladder, brain, esophageal, and colonic extracellular matrices induced an M2 phenotype similar to the one obtained by control macrophages incubated with IL-4. In contrast, dermal ECM induced an M1 phenotype with increased iNOS expression, while no shift was observed in macrophages treated with liver or skeletal muscle derived ECM.
In addition, since ECM proteins are highly conserved across species, xenografts are usually well-tolerated [269], thus diminishing the risk of undesired inflammatory responses which could interfere with the homeostasis of the immune environment. Interestingly, the capability to modulate the inflammatory response by means of macrophage polarization confers a higher tolerability to xenografts of acellular ECM compared to autologous grafts, in some cases. In different experimental models, decellularized xenogeneic tissues have extensively been shown to provide a better healing response, characterized by a reduced M1 macrophage presence and by shifts to an M2 phenotype, compared to autologous cellular material, as determined by immunohistological evaluations [277,278,279]. Moreover, decellularized ECM also represents an interesting carrier for the delivery of molecules. For instance, the sequential delivery of IFN-γ and IL-4 from decellularized bones switched the macrophage phenotype from M1(IFN-γ) to M2(IL-4) and increased vascularization of the bone scaffolds subcutaneously implanted in mice [280].
Another important characteristic of decellularized ECM is the mechanical properties, which depend on the source and the processing of the tissue. Compounds such as 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and aldehydes are often used as cross-linkers to strengthen tissues and prevent degradation [281]. However, if on the one hand, the crosslinking supplies mechanical stability, on the other hand, the lack of degradation prevents implant remodeling by macrophages and other cells, and prevents replacing by native tissue, thus favoring a stronger FBR [282], further sustained by residual cross-linker macrophage toxicity and inflammation [283]. Even if complete decellularization is important for avoiding an inflammatory response [284], few studies have directly compared the effects of different decellularization protocols on immune activation. Moreover, a recent study of decellularized ECMs from different organs has used high-throughput screening techniques to define scaffold components. Quantitative analysis of tissue-specific responses (such as matrix production, cellular adhesion and growth, culture-dependent modification of morphology) has been found to correlate with tissue proteomics. A network analysis identified several proteins linked to cell function. For example, ECM glycoproteins, but not collagens, have been shown to affect macrophage activity. In particular, cartilage oligomeric matrix proteins and matrilin downregulate M1 function, whereas S100AB induces M2 activity [285]. The biochemical complexity of decellularized matrices is still poorly understood, so a better characterization of the active components of ECM will improve scaffold reproducibility.
5.2. Immunomodulation by Pro-Inflammatory Molecules
Since the inflammatory response is the starting point of the tissue healing program, the use of pro-inflammatory molecules, including danger signals, to treat tissue damage, has been considered. Different studies highlight that targeting specific TLR pathways can force a desired response. Indeed, the HSP (heat shock protein)-70 endogenous agonist of TLR2/TLR4 [286] up-regulated macrophage-mediated phagocytosis [287], thus aiding healing; macrophage-activating lipopeptide-2, a TLR2/6 agonist, increased the vascularization of porous polyethylene without causing any local or systemic side effects [288] and CpG (cytosine-phosphorothioate-guanine) oligodeoxynucleotides designed to trigger human immune cells via TLR9 promoted skin repair [289].
Prostaglandin E2 (PGE2) belonging to a family of pro-inflammatory lipid molecules [290] has been involved in both pro- and anti-regenerative functions and it has also been shown to inhibit proliferation and skew the immune response to Th2 [290] by inhibiting IL-12 [291], IFN-γ, and IL-2 [292] secretion by human lymphocytes. While being beneficial for tissue healing, PGE2 administration requires multiple doses and presents significant side effects [293,294]. Therefore, the biomaterial-mediated local delivery of PGE2 would be better than repeated systemic administrations, as demonstrated in a mouse model [295]. A further improvement has been obtained by using an agonist specifically binding one of the four PGE2 receptors and slowly released via biomaterial [294,296].
Furthermore, the inflammatory and pro-angiogenic CXCL-12 chemokine has been shown to play an important role in the tissue repair process [297], in particular for its ability to mobilize progenitor cells [298] expressing the CXCR (CX chemokine receptor) 4 cognate receptor [299,300]. The usefulness of biomaterials delivering CXCL-12 has been demonstrated in different tissues, such as tendons [301], cardiac muscle [302], skin [303], and liver models [298].
5.3. Immunomodulation by Anti-Inflammatory Molecules
The use of anti-inflammatory factors represents another way to obtain immunomodulatory biomaterials.
Cytokines can be locally delivered either by immobilization into the biomaterial (such as hydrogels) or by nucleic-acid-based strategies that allow prolonged cytokine synthesis and release by the in situ implanted cells [304]. The hydrogel inclusion of TGF-β or IL-10 has been shown to be effective in suppressing the maturation of dendritic cells [305]. The sequential controlled delivery of IFN-γ and IL-4 from scaffolds or double hydrogel layers promoted the transition of M1 to M2 macrophages [280,306]. Besides, for delivering anti-inflammatory agents, polymeric hydrogels can also be designed for sequestering pro-inflammatory signals, as described for TNF-α and CCL2 [307,308]. The inability of the direct delivery system to sustain clinically relevant concentrations of cytokines for a long period of time has fostered the use of gene delivery-based systems which allow a desired concentration of the target cytokine to be maintained in the long term.
Cytokines such as IL-4 and IL-10 are fundamental for correct tissue repair and regeneration, because of their role in M1 to M2 switching [309]. For regenerative applications, IL-10 has been mostly delivered using plasmid DNA and virus vectors [310], or antibodies neutralizing the pro-inflammatory signals [311]. A decrease of the inflammatory response was obtained following scaffold implantation using a gene-therapy approach consisting of the localized delivery of IL-10 [264]. Both IL-4 delivery means (as a protein conjugated to a scaffold [280] or via injectable hydrogel [312]) were effective for inducing/increasing M2 macrophage polarization and tissue repair in a rat model. Delivery of IL-4 and IL-13 via biomaterials has also been extensively explored, with M2-skewed macrophage phenotypes having been observed [280,313]. Novel hydrogel-based gene delivery strategies have also been explored for the release of antisense oligodeoxynucleotides to downregulate local endogenous pro-inflammatory signals at the wound site [314,315].
Overall, it has been shown that introducing IL-4 or analogous anti-inflammatory cytokines into scaffolds may prevent undesired side effects of implanted materials [316] and the delivery may support M2 macrophage driven regeneration.
TNF-α has been shown to positively regulate tissue repair and regeneration in some situations; however, its excessive concentration can be detrimental to the healing process. Thus, strategies aimed at inhibiting the expression of this cytokine have been suggested to decrease the pro-inflammatory macrophage effect. The local administration of common painkillers such as aspirin [317], ibuprofen [318], and pentoxifylline [319] has shown interesting results in TNF-α reduction. The anti-TNF-α molecule conveyed by hyaluronic acid, taking advantage of the specific receptor (CD44) on macrophages, provides the signal directly to the cytokine producing cell [320]. On the other side, taking advantage of the tissue repair characteristics, pre-stimulation of mesenchymal stem cells (MSCs) with TNF-α has been shown to increase their engraftment to myocardial infarct [321] and its administration has been described to enable the mobilization of MSCs into damaged tissues [322].
TGF-β1, a further interesting factor, is required for the early stages of tissue repair [323], although this molecule can exert either inflammatory or anti-inflammatory properties, depending on the cell type it signals. For example, while TGF-β1 can inhibit the activity and proliferation of lymphocytes, at the same time, it can induce regulatory T cells [324]. Nevertheless, TGF-β1 also widely contributes to scar formation [323]. TGF-β3, one of the three isoforms of the cytokine, can be exploited to accelerate regeneration and avoid scarring [323], as demonstrated by prophylactic administration in human studies [325]. The design of a suitable delivery system for the β3 isoform may therefore exploit its anti-fibrotic properties in humans.
An additional molecule IL-33 [326], acting as both a cytokine and a nuclear factor, has been linked to fibrosis through the actions of leukocyte recruitment and modulation of ECM genes [327,328]. Upon secretion, IL-33 has been found to be a chemoattractant for Th2 cells [328], as well as an inducer of the secretion of IL-13 [329,330], an important cytokine involved in FBGC formation, by acting directly on Th2 cells via a constitutively expressed ST (suppression of tumorigenicity) 2 receptor [329,331]. The emerging links between IL-33 and fibrotic disorders [332,333] might suggest that the release of IL-33 at the biomaterial implantation site may induce collagen deposition. Further studies may qualify IL-33 as an additional candidate for a therapeutic blockade to reduce the FBR [334].
Glucocorticoids are potent immunosuppressor drugs of the immune responses [335]. They inhibit inflammatory mediator synthesis (including cytokines, chemokines, prostaglandins, leukotrienes, proteolytic enzymes, free oxygen radicals, and nitric oxide), concomitantly promoting anti-inflammatory cytokine release, Th2-immunity, and tolerance, while suppressing the Th1 response. The delivery of soluble pharmacological anti-inflammatory agents such as dexamethasone and heparin, incorporated onto implants through surface applied coatings, has shown reduced numbers of inflammatory cells and fibrotic capsule formation [336,337,338,339]. However, since the superficial coating only allows a short-term release of the selected factor limiting the length of inflammatory response modulation, the incorporation of nano- and microscale drug delivery systems into implants has been adopted, aimed at prolonging the immunomodulation time [310,340,341].
The combined delivery of glucocorticoids and anti-inflammatory cytokines (IL-6 and IL-10) can attenuate inflammation around implants and promote the repair phase [342]. However, an unwanted side effect of glucocorticoids on the surrounding tissue is the reduction of endogenous angiogenesis and the consequent wound healing delay [343,344]. The combined delivery of dexamethasone together with VEGF (a neo-angiogenetic growth factor) alone or with proportionally shared PDGF has been demonstrated to prevent the FBR, increase angiogenesis, and promote blood vessel maturation, respectively [345,346,347].
Besides VEGF and PDGF, a complex network of growth factors (EGF, FGF—fibroblast growth factor, GM-CSF granulocyte-macrophage colony stimulating factor, TGF-β) have been shown to control adhesion, migration, proliferation, and cell differentiation in wound healing (reviewed in [348]); therefore, biomaterials bearing these molecules can still show immunomodulatory properties. The subcutaneous administration of GW2580 (the inhibitor of CSF1receptor) has been shown to avoid the FBR to the alginate particle implants, by blocking the recruitment of innate and adaptive immune cells [349].
A striking anti-inflammatory effect on both acute and chronic inflammatory responses and a significant inhibition of foreign body giant cell and fibrous capsule formation was obtained using a low molecular weight superoxide dismutase mimic (a new class of drugs which imparts anti-inflammatory property to the material), covalently conjugated to a biomaterial [350]. A durable control of immune responses can be obtained by the loading of biomaterial surfaces with coatings containing NO [351], whose continuous and slow release results in reduced immune cell recruitment, probably due to the down-regulation of inflammatory cytokines, as well as the protein nitrosation [352]. Furthermore, NO may induce macrophages to a self NO production, thus explaining its long-lasting anti-inflammatory activity [353,354].
5.4. Immunomodulation by Integrins, Pro-Resolving Mediators, Cells, and Regulatory Pathways
Functionalization of surfaces with integrin binding sites [355] represents a powerful strategy in initiating distinct intracellular “outside-in” signal pathways mediating specific cell activation elicited by the recognition of integrin adhesion sites [356], thus preventing nonspecific cell–material interaction [357,358]. This technology will play an important role in the future development of biomaterials and scaffolds (reviewed in [359].
The local delivery of specialized pro-resolving mediators (SPMs, a group of endogenous molecules having a fundamental role in triggering signals ending the acute phase of the inflammatory response) [360,361] limiting both the recruitment of neutrophils and their ingestion by macrophages [362] shifted the macrophage phenotypic profile towards a M2 reparative response in vivo [363,364]. These mediators have already proved to be efficient at promoting wound healing [365], improving reepithelization and the formation of granulation tissue, as well as innervation [366], controlling the macrophage polarization induced after a chitosan scaffold implantation [364] in an obese diabetic mouse model.
Cell therapy methods, either by including immune cells as a possible reservoir/producer of a molecules inducer of specific biological events or by inducing their recruitment [1], are other possible strategies used to improve regenerative medicine systems. Thus, employing macrophages as a pro-angiogenic reservoir represents a possible chance of settling one of the basic problems in tissue engineering, i.e., the appropriate vascularization of thick engineered tissues [367]; encapsulated MSCs decreased the fibrotic response of the FBR compared to acellular hydrogels by down-modulating the classically activated macrophages [368] and conditioned medium obtained from macrophages induced by specific biomaterials was able to differentiate other cells [369].
Scaffold-encapsulated cells or 3D printing have gained interest in regenerative medicine since they represent new models of advanced fabrication techniques. These new bio-fabrication approaches mainly contribute to protecting the cells against stressful environmental events, such as exposure to ultraviolet light, reactive chemicals, and mechanical injury, but they also enhance their resistance against in vitro or in vivo perturbations due to inflammatory processes or immune cell interactions. It is documented that the encapsulation of cells limits their accessibility to antibodies, providing an immune-protective barrier [370,371,372], thus representing a promising tool to improve current adoptive T cell therapy strategies [373]. Moreover, it has been shown that the biomaterials (silk or hyaluronan-based) used to encapsulate cells are good systems for local delivery of cytokines [374] or for modulating macrophage polarization [375] by regulating cell-ECM interactions. Recently, it has been shown that the contemporary encapsulation of bone marrow stromal cells and macrophages in a matrix with defined stiffness can influence stromal cell fate both through direct-matrix-associated regulation and indirect macrophage-based modulation [376]. At the same time, encapsulation contributes to delaying clearance of the cells when transplanted in vivo, thus improving their therapeutic effects and protecting the host from the allogeneic transplantation of immune cells and graft versus host disease [377].
The previous examples have been mostly focused on structures/molecules that are characteristic of the immune response, but they are not exhaustive of the whole range of opportunities available for modulating interactions between implanted biomaterial and the receiving host.
Different strategies can intervene by acting on regulatory pathways upstream of protein production [378,379,380], by using Small interfering RNA (siRNA)-mediated gene silencing as a therapeutic approach to control the immune system and to reduce the detrimental effect of excessive inflammation during the tissue healing process [381,382,383,384] or by modulating miRNA signaling either by overexpression or inhibition [385,386,387,388,389,390,391].
6. Concluding Remarks and Open Questions
The immune system, for its central role in strategic regulatory processes, remains the most significant critical issue for the development of tissue engineering. On the one hand, the innate immune system capability to monitor, recognize, and clear foreign bodies activates a response that is unaware of the therapeutic potential of implanted biomaterials; on the other hand, biomaterial possesses characteristics that “irritate” the immune system. Although the inflammatory response is the first step of wound/tissue healing, it is also the underlying reason for the failure of many implanted scaffolds. The failure or the success of this match is determined by the ability of biomaterials to negotiate body sharing.
Many immune cell subpopulations and immuno-modulating factors are involved in the different phases of healing and, despite advancing knowledge and innovative approaches, we are far from having clarified the complex mechanisms by which the immune system orchestrates various organs. In addition, even ongoing studies, targeted to investigate the impact of material properties on immune activation, have yet to fully elucidate the mechanisms through which this activation occurs.
It is important to achieve a detailed understanding of the innate immune inflammatory processes by which neutrophils and monocytes/macrophages can be activated by biomaterial surfaces in the absence of any specific cell surface receptor or cytosolic receptor signaling.
The control of neutrophil mobilization and functions might be an interesting strategy to promote/modulate tissue regeneration. They are the first circulating cells of the innate immune response migrating after tissue injury, likely involved in macrophage polarization, but it is still unknown how exactly this happens.
In addition, the reasons for which macrophages shift from an inflammatory into an anti-inflammatory phenotype in certain types of tissues, while a distinct population of anti-inflammatory macrophages is mobilized in others, are not clarified. Therefore, another key aspect could be to disclose the regulatory processes driving the increase of anti-inflammatory/anti-fibrotic macrophages in vivo and to further bring these mechanisms into regenerative strategies. Immune modulators administered through biomaterials and drug delivery systems promoting M2 (IL-4)-like phenotypes are currently being studied; however, it must be considered that M2-type macrophages are also involved in the development of fibrotic diseases.
The growing evidence that T cell subsets can have both anti-regenerative and pro-regenerative properties indicates these cells as possible cellular targets for intervention, possibly modulating T cell mobilization, activation, and conversion into Tregs.
The limited success of non-fouling coatings and surface functionalization methods, as well as the ECM biology still in its infancy, despite promising results of decellularized materials, together with the pioneering biomimetic strategies, represent further targets of future research in tissue engineering.
Moreover, 3D printing of tissue-engineered constructs is a new flexible system to create biomaterial-based scaffolds. This technique allows the custom “printing” of precise shapes and architectures, eventually encapsulating cells, but the elicited crosstalk between the host immune system and the printed materials has yet to be investigated.
Another important variable not to be underestimated is age. If on the one hand, we can obtain answers from observing newborns whose macrophage populations display pro-regenerative capacities, on the other hand, with increasing frequency, tissue engineering approaches are considered among the therapeutic alternatives for diseases that instead occur more frequently in the elderly. Ageing is accompanied by phenotypic and functional changes of the immune system and by low-grade inflammatory activity reflected by increased circulating levels of pro-inflammatory cytokines. Epidemiological studies have suggested this chronic low-grade inflammation as related to several age-related diseases, sharing an inflammatory pathogenesis. Consequently, the baseline characteristics of immune cells and the surrounding immune environment might be different among these subjects and also different from those of young adult populations, thus adding a further biological variable to the study of the biomaterial design and to the chance of integration and tissue regeneration. However, despite the increasing use of implantable medical devices in aged patients, few studies are available that examine the effects of aging upon the host response to biomaterials and the implications of this response for long-term integration and function.
Increasing knowledge and awareness deriving from biological systems and new structural, chemical, and physical understandings of human-derived biomaterials, together with recent advances in synthesis technology, will open the way to new and more sophisticated biomaterial designs and prospects in the near scaffold technology. The future of this field will continue to grow and evolve with the collaborative development of tissue-engineered products that offer simple solutions to complex problems. Nevertheless, we should also be interested in knowing what the safety of immune-engineered biomaterials and their long-term efficacy will be.
Acknowledgments
The authors thank Patrizia Rappini for her assistance in the preparation of the manuscript and Silvia Bassini (Laboratorio di Biomeccanica e innovazione tecnologica, IRCCS Istituto Ortopedico Rizzoli) for the illustrative contribution to the paper.
Abbreviations
| aAPCs | artificial antigen presenting cells |
| Arg | arginase |
| Arg1 | Arginase 1 |
| CCL | C chemokine ligand |
| CD | cluster of differentiation |
| CH3 | methyl |
| CO2 | carbon dioxide |
| COOH | carboxyl |
| CR | complement receptor |
| CXCL | CX chemokine ligand |
| DAMPs | damage-associated molecular patterns |
| DCs | dendritic cells |
| ECM | extra-cellular matrix |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| FBGC | foreign body giant cell |
| FBR | foreign body reaction |
| GAG | glycosaminoglycans |
| IFN | interferon |
| Ig | immunoglobulin |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| lPS | lipopolysaccharide |
| MBVs | microvesicles |
| MHC | Major histocompatibility complex |
| miRNA | micro RiboNucleic Acid |
| NETs | neutrophil extracellular traps |
| NH2 | amino |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| OH | hydroxyl |
| PAMPs | pathogen-associated molecular patterns |
| PCL | poly(caprolactone) |
| PDGF | platelet-derived growth factor |
| PEEK | poly(ether ether ketone) |
| PEG | poly(ethylene glycol) |
| PEO | poly(ethylene oxide) |
| PGA | poly(glycolic acid) |
| PLA | poly(lactic acid) |
| PLGA | poly(lactic-co-glycolic acid) |
| PPF | poly(propylene fumarate) |
| PRRs | pattern recognition receptors |
| ROS | reactive oxygen species |
| SF | silk fibroin |
| SIS | small intestine submucosa |
| SLA | sand-blasted, acid etched |
| TGF | transforming growth factor |
| Th | T helper |
| Ti | titanium |
| TLR | Toll-like receptors |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
Funding
This paper was partially supported by Italian Health Ministry 5x1000 Fund and Bologna University RFO fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vishwakarma A., Bhise N.S., Evangelista M.B., Rouwkema J., Dokmeci M.R., Ghaemmaghami A.M., Vrana N.E., Khademhosseini A. Engineering Immunomodulatory Biomaterials to Tune the Inflammatory Response. Trends Biotechnol. 2016;34:470–482. doi: 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011;2011:290602. doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- 3.Wiles K., Fishman J.M., De Coppi P., Birchall M.A. The Host Immune Response to Tissue-Engineered Organs: Current Problems and Future Directions. Tissue Eng. Part B Rev. 2016;22:208–219. doi: 10.1089/ten.teb.2015.0376. [DOI] [PubMed] [Google Scholar]
- 4.Roseti L., Parisi V., Petretta M., Cavallo C., Desando G., Bartolotti I., Grigolo B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;78:1246–1262. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Rossi F., Santoro M., Perale G. Polymeric scaffolds as stem cell carriers in bone repair. J. Tissue Eng. Regen. Med. 2015;9:1093–1119. doi: 10.1002/term.1827. [DOI] [PubMed] [Google Scholar]
- 6.Wilson C.J., Clegg R.E., Leavesley D.I., Pearcy M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 7.Keselowsky B.G., Collard D.M., Garcia A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 8.Gasperini L., Mano J.F., Reis R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface. 2014;11:20140817. doi: 10.1098/rsif.2014.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thevenot P., Hu W., Tang L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008;8:270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matlaga B.F., Yasenchak L.P., Salthouse T.N. Tissue response to implanted polymers: The significance of sample shape. J. Biomed. Mater. Res. 1976;10:391–397. doi: 10.1002/jbm.820100308. [DOI] [PubMed] [Google Scholar]
- 11.Delves P.J., Martin S.J., Burton D.R., Roitt I.M. Roitt’s Essential Immunology. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
- 12.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid B., Gibson M., Singh A., Taube J., Furlong C., Murcia M., Elisseeff J. PEG hydrogel degradation and the role of the surrounding tissue environment. J. Tissue Eng. Regen. Med. 2015;9:315–318. doi: 10.1002/term.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson J.M. Inflammatory response to implants. ASAIO Trans. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Boccafoschi F., Mosca C., Cannas M. Cardiovascular biomaterials: When the inflammatory response helps to efficiently restore tissue functionality? J. Tissue Eng. Regen. Med. 2014;8:253–267. doi: 10.1002/term.1526. [DOI] [PubMed] [Google Scholar]
- 16.Brown B.N., Badylak S.F. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013;9:4948–4955. doi: 10.1016/j.actbio.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Christo S.N., Diener K.R., Bachhuka A., Vasilev K., Hayball J.D. Innate Immunity and Biomaterials at the Nexus: Friends or Foes. Biomed. Res. Int. 2015;2015:342304. doi: 10.1155/2015/342304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung L., Maestas D.R., Jr., Housseau F., Elisseeff J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Franz S., Rammelt S., Scharnweber D., Simon J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 20.Ward W.K. A review of the foreign-body response to subcutaneously-implanted devices: The role of Macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2008;2:768–777. doi: 10.1177/193229680800200504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang L., Eaton J.W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 1993;178:2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milleret V., Buzzi S., Gehrig P., Ziogas A., Grossmann J., Schilcher K., Zinkernagel A.S., Zucker A., Ehrbar M. Protein adsorption steers blood contact activation on engineered cobalt chromium alloy oxide layers. Acta Biomater. 2015;24:343–351. doi: 10.1016/j.actbio.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Vitte J., Benoliel A.M., Pierres A., Bongrand P. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at the interface? Eur. Cells Mater. 2004;7:52–63; discussion 63. doi: 10.22203/eCM.v007a06. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Cao Z., Bai T., Carr L., Ella-Menye J.R., Irvin C., Ratner B.D., Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013;31:553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.K., Que R., Wang S.W., Liu W.F. Modification of biomaterials with a self-protein inhibits the macrophage response. Adv. Healthc. Mater. 2014;3:989–994. doi: 10.1002/adhm.201300532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekdahl K.N., Lambris J.D., Elwing H., Ricklin D., Nilsson P.H., Teramura Y., Nicholls I.A., Nilsson B. Innate immunity activation on biomaterial surfaces: A mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 2011;63:1042–1050. doi: 10.1016/j.addr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbet M.B., Sefton M.V. The Biomaterials: Silver Jubilee Compendium. Elsevier Ltd.; Oxford, UK: 2006. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. [DOI] [PubMed] [Google Scholar]
- 28.Chiumiento A., Lamponi S., Barbucci R. Role of fibrinogen conformation in platelet activation. Biomacromolecules. 2007;8:523–531. doi: 10.1021/bm060664m. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Simonovsky F.I., Ratner B.D., Horbett T.A. The role of adsorbed fibrinogen in platelet adhesion to polyurethane surfaces: A comparison of surface hydrophobicity, protein adsorption, monoclonal antibody binding, and platelet adhesion. J. Biomed. Mater. Res. A. 2005;74:722–738. doi: 10.1002/jbm.a.30381. [DOI] [PubMed] [Google Scholar]
- 30.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 31.Andersson J., Ekdahl K.N., Lambris J.D., Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–1485. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Hed J., Johansson M., Lindroth M. Complement activation according to the alternate pathway by glass and plastic surfaces and its role in neutrophil adhesion. Immunol. Lett. 1984;8:295–299. doi: 10.1016/0165-2478(84)90013-0. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson B., Ekdahl K.N., Mollnes T.E., Lambris J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Flick M.J., Du X., Witte D.P., Jirouskova M., Soloviev D.A., Busuttil S.J., Plow E.F., Degen J.L. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Investig. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szaba F.M., Smiley S.T. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99:1053–1059. doi: 10.1182/blood.V99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esche C., Stellato C., Beck L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 37.McFarland C.D., Thomas C.H., DeFilippis C., Steele J.G., Healy K.E. Protein adsorption and cell attachment to patterned surfaces. J. Biomed. Mater. Res. 2000;49:200–210. doi: 10.1002/(SICI)1097-4636(200002)49:2<200::AID-JBM7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 38.Groth T., Zlatanov I., Altankov G. Adhesion of Human Peripheral Lymphocytes on Biomaterials Preadsorbed with Fibronectin and Vitronectin. J. Biomater. Sci. Polym. Ed. 1995 doi: 10.1163/156856295X00111. [DOI] [PubMed] [Google Scholar]
- 39.Jenney C.R., Anderson J.M. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J. Biomed. Mater. Res. 2000;49:435–447. doi: 10.1002/(SICI)1097-4636(20000315)49:4<435::AID-JBM2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.McNally A.K., Jones J.A., Macewan S.R., Colton E., Anderson J.M. Vitronectin is a critical protein adhesion substrate for IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. A. 2008;86:535–543. doi: 10.1002/jbm.a.31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keselowsky B.G., Bridges A.W., Burns K.L., Tate C.C., Babensee J.E., LaPlaca M.C., Garcia A.J. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials. 2007;28:3626–3631. doi: 10.1016/j.biomaterials.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikh Z., Brooks P.J., Barzilay O., Fine N., Glogauer M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials. 2015;8:5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen M., Garcia I., Maier R.V., Horbett T.A. Effects of adsorbed proteins and surface chemistry on foreign body giant cell formation, tumor necrosis factor alpha release and procoagulant activity of monocytes. J. Biomed. Mater. Res. A. 2004;70:533–541. doi: 10.1002/jbm.a.30069. [DOI] [PubMed] [Google Scholar]
- 44.Keselowsky B.G., Collard D.M., Garcia A.J. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc. Natl. Acad. Sci. USA. 2005;102:5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNally A.K., Macewan S.R., Anderson J.M. alpha subunit partners to beta1 and beta2 integrins during IL-4-induced foreign body giant cell formation. J. Biomed. Mater. Res. A. 2007;82:568–574. doi: 10.1002/jbm.a.31161. [DOI] [PubMed] [Google Scholar]
- 46.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 48.Babensee J.E. Interaction of dendritic cells with biomaterials. Semin. Immunol. 2008;20:101–108. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 50.Grandjean-Laquerriere A., Tabary O., Jacquot J., Richard D., Frayssinet P., Guenounou M., Laurent-Maquin D., Laquerriere P., Gangloff S. Involvement of toll-like receptor 4 in the inflammatory reaction induced by hydroxyapatite particles. Biomaterials. 2007;28:400–404. doi: 10.1016/j.biomaterials.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Labow R.S., Meek E., Santerre J.P. Neutrophil-mediated biodegradation of medical implant materials. J. Cell. Physiol. 2001;186:95–103. doi: 10.1002/1097-4652(200101)186:1<95::AID-JCP1008>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Nimeri G., Majeed M., Elwing H., Ohman L., Wettero J., Bengtsson T. Oxygen radical production in neutrophils interacting with platelets and surface-immobilized plasma proteins: Role of tyrosine phosphorylation. J. Biomed. Mater. Res. A. 2003;67:439–447. doi: 10.1002/jbm.a.10081. [DOI] [PubMed] [Google Scholar]
- 53.Nimeri G., Ohman L., Elwing H., Wettero J., Bengtsson T. The influence of plasma proteins and platelets on oxygen radical production and F-actin distribution in neutrophils adhering to polymer surfaces. Biomaterials. 2002;23:1785–1795. doi: 10.1016/S0142-9612(01)00305-2. [DOI] [PubMed] [Google Scholar]
- 54.Wettero J., Bengtsson T., Tengvall P. Complement activation on immunoglobulin G-coated hydrophobic surfaces enhances the release of oxygen radicals from neutrophils through an actin-dependent mechanism. J. Biomed. Mater. Res. 2000;51:742–751. doi: 10.1002/1097-4636(20000915)51:4<742::AID-JBM24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 55.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 56.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 57.Hahn J., Schauer C., Czegley C., Kling L., Petru L., Schmid B., Weidner D., Reinwald C., Biermann M.H.C., Blunder S., et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2018 doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schauer C., Janko C., Munoz L.E., Zhao Y., Kienhofer D., Frey B., Lell M., Manger B., Rech J., Naschberger E., et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 59.Selders G.S., Fetz A.E., Radic M.Z., Bowlin G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017;4:55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Branzk N., Lubojemska A., Hardison S.E., Wang Q., Gutierrez M.G., Brown G.D., Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNally A.K., Anderson J.M. Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol. 2011;713:97–111. doi: 10.1007/978-94-007-0763-4_7. [DOI] [PubMed] [Google Scholar]
- 62.Scapini P., Lapinet-Vera J.A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M.A. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 63.Yamashiro S., Kamohara H., Wang J.M., Yang D., Gong W.H., Yoshimura T. Phenotypic and functional change of cytokine-activated neutrophils: Inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J. Leukoc. Biol. 2001;69:698–704. [PubMed] [Google Scholar]
- 64.Altieri D.C., Mannucci P.M., Capitanio A.M. Binding of fibrinogen to human monocytes. J. Clin. Investig. 1986;78:968–976. doi: 10.1172/JCI112687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trezzini C., Jungi T.W., Kuhnert P., Peterhans E. Fibrinogen association with human monocytes: Evidence for constitutive expression of fibrinogen receptors and for involvement of Mac-1 (CD18, CR3) in the binding. Biochem. Biophys. Res. Commun. 1988;156:477–484. doi: 10.1016/S0006-291X(88)80866-0. [DOI] [PubMed] [Google Scholar]
- 66.Mesure L., De Visscher G., Vranken I., Lebacq A., Flameng W. Gene expression study of monocytes/macrophages during early foreign body reaction and identification of potential precursors of myofibroblasts. PLoS ONE. 2010;5:e12949. doi: 10.1371/journal.pone.0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Badylak S.F., Gilbert T.W. Immune response to biologic scaffold materials. Semin. Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sridharan R., Cameron A.R., Kelly D.J., Kearney C.J., O’Brien F.J. Biomaterial Based Modulation of macrophage polarization: A review and suggested design principles. Mater. Today. 2015;18:313–325. doi: 10.1016/j.mattod.2015.01.019. [DOI] [Google Scholar]
- 69.Jones J.A., Chang D.T., Meyerson H., Colton E., Kwon I.K., Matsuda T., Anderson J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A. 2007;83:585–596. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 70.Lynn A.D., Kyriakides T.R., Bryant S.J. Characterization of the in vitro macrophage response and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A. 2010;93:941–953. doi: 10.1002/jbm.a.32595. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Q., Topham N., Anderson J.M., Hiltner A., Lodoen G., Payet C.R. Foreign-body giant cells and polyurethane biostability: In vivo correlation of cell adhesion and surface cracking. J. Biomed. Mater. Res. 1991;25:177–183. doi: 10.1002/jbm.820250205. [DOI] [PubMed] [Google Scholar]
- 72.Labrousse A.M., Meunier E., Record J., Labernadie A., Beduer A., Vieu C., Ben Safta T., Maridonneau-Parini I. Frustrated phagocytosis on micro-patterned immune complexes to characterize lysosome movements in live macrophages. Front. Immunol. 2011;2:51. doi: 10.3389/fimmu.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Underhill D.M., Goodridge H.S. Information processing during phagocytosis. Nat. Rev. Immunol. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garg K., Pullen N.A., Oskeritzian C.A., Ryan J.J., Bowlin G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34:4439–4451. doi: 10.1016/j.biomaterials.2013.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Broughton G., 2nd, Janis J.E., Attinger C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 77.Kyriakides T.R., Foster M.J., Keeney G.E., Tsai A., Giachelli C.M., Clark-Lewis I., Rollins B.J., Bornstein P. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am. J. Pathol. 2004;165:2157–2166. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brodbeck W.G., Macewan M., Colton E., Meyerson H., Anderson J.M. Lymphocytes and the foreign body response: Lymphocyte enhancement of macrophage adhesion and fusion. J. Biomed. Mater. Res. A. 2005;74:222–229. doi: 10.1002/jbm.a.30313. [DOI] [PubMed] [Google Scholar]
- 79.Burd P.R., Thompson W.C., Max E.E., Mills F.C. Activated mast cells produce interleukin 13. J. Exp. Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venkayya R., Lam M., Willkom M., Grunig G., Corry D.B., Erle D.J. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell Mol. Biol. 2002;26:202–208. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- 81.Rezzani R., Rodella L., Tartaglia G.M., Paganelli C., Sapelli P., Bianchi R. Mast cells and the inflammatory response to different implanted biomaterials. Arch. Histol. Cytol. 2004;67:211–217. doi: 10.1679/aohc.67.211. [DOI] [PubMed] [Google Scholar]
- 82.Tang L., Jennings T.A., Eaton J.W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. USA. 1998;95:8841–8846. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christenson L., Wahlberg L., Aebischer P. Mast cells and tissue reaction to intraperitoneally implanted polymer capsules. J. Biomed. Mater. Res. 1991;25:1119–1131. doi: 10.1002/jbm.820250906. [DOI] [PubMed] [Google Scholar]
- 84.Zdolsek J., Eaton J.W., Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 2007;5:31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kanbe N., Kurosawa M., Nagata H., Saitoh H., Miyachi Y. Cord blood-derived human cultured mast cells produce transforming growth factor beta1. Clin. Exp. Allergy. 1999;29:105–113. doi: 10.1046/j.1365-2222.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 86.Ribatti D., Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Tacchini-Cottier F., Zweifel C., Belkaid Y., Mukankundiye C., Vasei M., Launois P., Milon G., Louis J.A. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 88.Ma Y., Yabluchanskiy A., Iyer R.P., Cannon P.L., Flynn E.R., Jung M., Henry J., Cates C.A., Deleon-Pennell K.Y., Lindsey M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016;110:51–61. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen F., Wu W., Millman A., Craft J.F., Chen E., Patel N., Boucher J.L., Urban J.F., Jr., Kim C.C., Gause W.C. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 2014;15:938–946. doi: 10.1038/ni.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yokoyama M., Nakahashi T., Nishimura T., Maeda M., Inoue S., Kataoka K., Sakurai Y. Adhesion behavior of rat lymphocytes to poly(ether)-poly(amino acid) block and graft copolymers. J. Biomed. Mater. Res. 1986;20:867–878. doi: 10.1002/jbm.820200702. [DOI] [PubMed] [Google Scholar]
- 91.Curtsinger J.M., Mescher M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Curtsinger J.M., Schmidt C.S., Mondino A., Lins D.C., Kedl R.M., Jenkins M.K., Mescher M.F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 93.Rodriguez A., Voskerician G., Meyerson H., MacEwan S.R., Anderson J.M. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J. Biomed. Mater. Res. A. 2008;85:556–565. doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- 94.Chang D.T., Colton E., Anderson J.M. Paracrine and juxtacrine lymphocyte enhancement of adherent macrophage and foreign body giant cell activation. J. Biomed. Mater. Res. A. 2009;89:490–498. doi: 10.1002/jbm.a.31981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson J.M. Biological Interactions on Materials Surfaces. Springer; New York, NY, USA: 2009. In Vitro and In Vivo Monocyte, Macrophage, Foreign Body Giant Cell, and Lymphocyte Interactions with Biomaterials. [Google Scholar]
- 96.Shen E.C., Chou T.C., Gau C.H., Tu H.P., Chen Y.T., Fu E. Releasing growth factors from activated human platelets after chitosan stimulation: A possible bio-material for platelet-rich plasma preparation. Clin. Oral Implants Res. 2006;17:572–578. doi: 10.1111/j.1600-0501.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- 97.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen S., Jones J.A., Xu Y., Low H.Y., Anderson J.M., Leong K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 2010;31:3479–3491. doi: 10.1016/j.biomaterials.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garg K., Sell S.A., Madurantakam P., Bowlin G.L. Angiogenic potential of human macrophages on electrospun bioresorbable vascular grafts. Biomed. Mater. 2009;4:031001. doi: 10.1088/1748-6041/4/3/031001. [DOI] [PubMed] [Google Scholar]
- 100.Mustafa K., Rubinstein J., Lopez B.S., Arvidson K. Production of transforming growth factor beta1 and prostaglandin E2 by osteoblast-like cells cultured on titanium surfaces blasted with TiO2 particles. Clin. Oral Implants Res. 2003;14:50–56. doi: 10.1034/j.1600-0501.2003.140107.x. [DOI] [PubMed] [Google Scholar]
- 101.Diegelmann R.F., Evans M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 102.Castro P.R., Marques S.M., Campos P.P., Cardoso C.C., Sampaio F.P., Ferreira M.A., Andrade S.P. Kinetics of implant-induced inflammatory angiogenesis in abdominal muscle wall in mice. Microvasc. Res. 2012;84:9–15. doi: 10.1016/j.mvr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Oviedo-Socarras T., Vasconcelos A.C., Barbosa I.X., Pereira N.B., Campos P.P., Andrade S.P. Diabetes alters inflammation, angiogenesis, and fibrogenesis in intraperitoneal implants in rats. Microvasc. Res. 2014;93:23–29. doi: 10.1016/j.mvr.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 104.Ratner B.D. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J. Control. Release. 2002;78:211–218. doi: 10.1016/S0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- 105.Ratner B.D., Bryant S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 106.Rockey D.C., Bell P.D., Hill J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 107.Zaiss D.M., van Loosdregt J., Gorlani A., Bekker C.P., Grone A., Sibilia M., van Bergen en Henegouwen P.M., Roovers R.C., Coffer P.J., Sijts A.J. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arpaia N., Green J.A., Moltedo B., Arvey A., Hemmers S., Yuan S., Treuting P.M., Rudensky A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zaiss D.M.W., Gause W.C., Osborne L.C., Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burzyn D., Kuswanto W., Kolodin D., Shadrach J.L., Cerletti M., Jang Y., Sefik E., Tan T.G., Wagers A.J., Benoist C., et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weirather J., Hofmann U.D., Beyersdorf N., Ramos G.C., Vogel B., Frey A., Ertl G., Kerkau T., Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 112.Nosbaum A., Prevel N., Truong H.A., Mehta P., Ettinger M., Scharschmidt T.C., Ali N.H., Pauli M.L., Abbas A.K., Rosenblum M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016;196:2010–2014. doi: 10.4049/jimmunol.1502139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vinish M., Cui W., Stafford E., Bae L., Hawkins H., Cox R., Toliver-Kinsky T. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen. 2016;24:6–13. doi: 10.1111/wrr.12388. [DOI] [PubMed] [Google Scholar]
- 114.Anzai A., Anzai T., Nagai S., Maekawa Y., Naito K., Kaneko H., Sugano Y., Takahashi T., Abe H., Mochizuki S., et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–1245. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 115.Rutella S., Danese S., Leone G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 116.Lutz M.B., Schuler G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/S1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 117.Babensee J.E., Stein M.M., Moore L. Interconnections between inflammatory and immune responses in tissue engineering. Ann. N. Y. Acad. Sci. 2002;961:360–363. doi: 10.1111/j.1749-6632.2002.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 118.Yoshida M., Babensee J.E. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J. Biomed. Mater. Res. A. 2004;71:45–54. doi: 10.1002/jbm.a.30131. [DOI] [PubMed] [Google Scholar]
- 119.Keselowsky B.G., Lewis J.S. Dendritic cells in the host response to implanted materials. Semin. Immunol. 2017;29:33–40. doi: 10.1016/j.smim.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 121.Wu L., Ong S., Talor M.V., Barin J.G., Baldeviano G.C., Kass D.A., Bedja D., Zhang H., Sheikh A., Margolick J.B., et al. Cardiac fibroblasts mediate IL-17A-driven inflammatory dilated cardiomyopathy. J. Exp. Med. 2014;211:1449–1464. doi: 10.1084/jem.20132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martin B., Hirota K., Cua D.J., Stockinger B., Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 123.Housseau F., Wu S., Wick E.C., Fan H., Wu X., Llosa N.J., Smith K.N., Tam A., Ganguly S., Wanyiri J.W., et al. Redundant Innate and Adaptive Sources of IL17 Production Drive Colon Tumorigenesis. Cancer Res. 2016;76:2115–2124. doi: 10.1158/0008-5472.CAN-15-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramirez K., Witherden D.A., Havran W.L. All hands on DE(T)C: Epithelial-resident gammadelta T cells respond to tissue injury. Cell. Immunol. 2015;296:57–61. doi: 10.1016/j.cellimm.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W.L. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 126.Ono T., Okamoto K., Nakashima T., Nitta T., Hori S., Iwakura Y., Takayanagi H. IL-17-producing gammadelta T cells enhance bone regeneration. Nat. Commun. 2016;7:10928. doi: 10.1038/ncomms10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z., Burns A.R., Miller S.B., Smith C.W. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang W., Magadi S., Li Z., Smith C.W., Burns A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017;154:22–29. doi: 10.1016/j.exer.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis P.A., Corless D.J., Aspinall R., Wastell C. Effect of CD4(+) and CD8(+) cell depletion on wound healing. Br. J. Surg. 2001;88:298–304. doi: 10.1046/j.1365-2168.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- 130.Reinke S., Geissler S., Taylor W.R., Schmidt-Bleek K., Juelke K., Schwachmeyer V., Dahne M., Hartwig T., Akyuz L., Meisel C., et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci. Transl. Med. 2013;5:177ra136. doi: 10.1126/scitranslmed.3004754. [DOI] [PubMed] [Google Scholar]
- 131.Konnecke I., Serra A., El Khassawna T., Schlundt C., Schell H., Hauser A., Ellinghaus A., Volk H.D., Radbruch A., Duda G.N., et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone. 2014;64:155–165. doi: 10.1016/j.bone.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 132.Klose C.S., Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 133.Halim T.Y., Steer C.A., Matha L., Gold M.J., Martinez-Gonzalez I., McNagny K.M., McKenzie A.N., Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mirchandani A.S., Besnard A.G., Yip E., Scott C., Bain C.C., Cerovic V., Salmond R.J., Liew F.Y. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 135.Huang Y., Paul W.E. Inflammatory group 2 innate lymphoid cells. Int. Immunol. 2016;28:23–28. doi: 10.1093/intimm/dxv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sadtler K., Estrellas K., Allen B.W., Wolf M.T., Fan H., Tam A.J., Patel C.H., Luber B.S., Wang H., Wagner K.R., et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016;352:366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderson J.M., McNally A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011;33:221–233. doi: 10.1007/s00281-011-0244-1. [DOI] [PubMed] [Google Scholar]
- 138.Matassi F., Nistri L., Chicon Paez D., Innocenti M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011;8:21–24. [PMC free article] [PubMed] [Google Scholar]
- 139.Gao C., Deng Y., Feng P., Mao Z., Li P., Yang B., Deng J., Cao Y., Shuai C., Peng S. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 2014;15:4714–4732. doi: 10.3390/ijms15034714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vroman I., Tighzert L. Biodegradable polymers. Materials. 2009;2:307–344. doi: 10.3390/ma2020307. [DOI] [Google Scholar]
- 141.Bai X., Gao M., Syed S., Zhuang J., Xu X., Zhang X.Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018;3:401–417. doi: 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jafari M., Paknejad Z., Rad M.R., Motamedian S.R., Eghbal M.J., Nadjmi N., Khojasteh A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:431–459. doi: 10.1002/jbm.b.33547. [DOI] [PubMed] [Google Scholar]
- 143.Stratton S., Shelke N.B., Hoshino K., Rudraiah S., Kumbar S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016;1:93–108. doi: 10.1016/j.bioactmat.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Janouskova O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018;67:S335–S348. doi: 10.33549/physiolres.933983. [DOI] [PubMed] [Google Scholar]
- 145.Malikmammadov E., Tanir T.E., Kiziltay A., Hasirci V., Hasirci N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018;29:863–893. doi: 10.1080/09205063.2017.1394711. [DOI] [PubMed] [Google Scholar]
- 146.Li W.J., Laurencin C.T., Caterson E.J., Tuan R.S., Ko F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 147.Nair A., Tang L. Influence of scaffold design on host immune and stem cell responses. Semin. Immunol. 2017;29:62–71. doi: 10.1016/j.smim.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 148.Zarrintaj P., Manouchehri S., Ahmadi Z., Saeb M.R., Urbanska A.M., Kaplan D.L., Mozafari M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018;187:66–84. doi: 10.1016/j.carbpol.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 149.Lee K.Y., Mooney D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 151.Rnjak-Kovacina J., Tang F., Whitelock J.M., Lord M.S. Glycosaminoglycan and Proteoglycan-Based Biomaterials: Current Trends and Future Perspectives. Adv. Healthc. Mater. 2018;7:e1701042. doi: 10.1002/adhm.201701042. [DOI] [PubMed] [Google Scholar]
- 152.Collins M.N., Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013;92:1262–1279. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 153.Wlodarczyk-Biegun M.K., Del Campo A. 3D bioprinting of structural proteins. Biomaterials. 2017;134:180–201. doi: 10.1016/j.biomaterials.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 154.Sproul E., Nandi S., Brown A. Fibrin biomaterials for tissue regeneration and repair. In: Barbosa M.A., Martins M.C.L., editors. Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair. Woodhead Publishing; Sawston, Cambridge, UK: 2018. pp. 151–173. [Google Scholar]
- 155.Melke J., Midha S., Ghosh S., Ito K., Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16. doi: 10.1016/j.actbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 156.Qi Y., Wang H., Wei K., Yang Y., Zheng R.Y., Kim I.S., Zhang K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017;18:237. doi: 10.3390/ijms18030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park J., Gerber M.H., Babensee J.E. Phenotype and polarization of autologous T cells by biomaterial-treated dendritic cells. J. Biomed. Mater. Res. A. 2015;103:170–184. doi: 10.1002/jbm.a.35150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morris A.H., Stamer D.K., Kyriakides T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017;29:72–91. doi: 10.1016/j.smim.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Hotaling N.A., Tang L., Irvine D.J., Babensee J.E. Biomaterial Strategies for Immunomodulation. Annu. Rev. Biomed. Eng. 2015;17:317–349. doi: 10.1146/annurev-bioeng-071813-104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mora-Solano C., Collier J.H. Engaging adaptive immunity with biomaterials. J. Mater. Chem. B. 2014;2:2409–2421. doi: 10.1039/C3TB21549K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Panilaitis B., Altman G.H., Chen J., Jin H.J., Karageorgiou V., Kaplan D.L. Macrophage responses to silk. Biomaterials. 2003;24:3079–3085. doi: 10.1016/S0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 162.Reeves A.R., Spiller K.L., Freytes D.O., Vunjak-Novakovic G., Kaplan D.L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials. 2015;73:272–283. doi: 10.1016/j.biomaterials.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bhattacharjee M., Schultz-Thater E., Trella E., Miot S., Das S., Loparic M., Ray A.R., Martin I., Spagnoli G.C., Ghosh S. The role of 3D structure and protein conformation on the innate and adaptive immune responses to silk-based biomaterials. Biomaterials. 2013;34:8161–8171. doi: 10.1016/j.biomaterials.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 164.Nakamura K., Yokohama S., Yoneda M., Okamoto S., Tamaki Y., Ito T., Okada M., Aso K., Makino I. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J. Gastroenterol. 2004;39:346–354. doi: 10.1007/s00535-003-1301-x. [DOI] [PubMed] [Google Scholar]
- 165.Je J.Y., Kim S.K. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg. Med. Chem. 2006;14:5989–5994. doi: 10.1016/j.bmc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 166.Orenstein S.B., Qiao Y., Klueh U., Kreutzer D.L., Novitsky Y.W. Activation of human mononuclear cells by porcine biologic meshes in vitro. Hernia. 2010;14:401–407. doi: 10.1007/s10029-010-0634-7. [DOI] [PubMed] [Google Scholar]
- 167.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 168.Maitra R., Clement C.C., Scharf B., Crisi G.M., Chitta S., Paget D., Purdue P.E., Cobelli N., Santambrogio L. Endosomal damage and TLR2 mediated inflammasome activation by alkane particles in the generation of aseptic osteolysis. Mol. Immunol. 2009;47:175–184. doi: 10.1016/j.molimm.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Seong S.Y., Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 170.Moyano D.F., Goldsmith M., Solfiell D.J., Landesman-Milo D., Miranda O.R., Peer D., Rotello V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012;134:3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Song S., Ravensbergen K., Alabanza A., Soldin D., Hahm J.I. Distinct adsorption configurations and self-assembly characteristics of fibrinogen on chemically uniform and alternating surfaces including block copolymer nanodomains. ACS Nano. 2014;8:5257–5269. doi: 10.1021/nn5013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kumar N., Parajuli O., Gupta A., Hahm J.I. Elucidation of protein adsorption behavior on polymeric surfaces: Toward high-density, high-payload protein templates. Langmuir. 2008;24:2688–2694. doi: 10.1021/la7022456. [DOI] [PubMed] [Google Scholar]
- 173.Absolom D.R., Zingg W., Neumann A.W. Protein adsorption to polymer particles: Role of surface properties. J. Biomed. Mater. Res. 1987;21:161–171. doi: 10.1002/jbm.820210202. [DOI] [PubMed] [Google Scholar]
- 174.Ouberai M.M., Xu K., Welland M.E. Effect of the interplay between protein and surface on the properties of adsorbed protein layers. Biomaterials. 2014;35:6157–6163. doi: 10.1016/j.biomaterials.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kakizawa Y., Lee J.S., Bell B., Fahmy T.M. Precise manipulation of biophysical particle parameters enables control of proinflammatory cytokine production in presence of TLR 3 and 4 ligands. Acta Biomater. 2017;57:136–145. doi: 10.1016/j.actbio.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 176.Li G., Yang P., Guo X., Huang N., Shen R. An in vitro evaluation of inflammation response of titanium functionalized with heparin/fibronectin complex. Cytokine. 2011;56:208–217. doi: 10.1016/j.cyto.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 177.Alfarsi M.A., Hamlet S.M., Ivanovski S. Titanium surface hydrophilicity modulates the human macrophage inflammatory cytokine response. J. Biomed. Mater. Res. A. 2014;102:60–67. doi: 10.1002/jbm.a.34666. [DOI] [PubMed] [Google Scholar]
- 178.Chang D.T., Colton E., Matsuda T., Anderson J.M. Lymphocyte adhesion and interactions with biomaterial adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A. 2009;91:1210–1220. doi: 10.1002/jbm.a.32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Drury J.L., Mooney D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 180.Peppas N.A., Kavimandan N.J. Nanoscale analysis of protein and peptide absorption: Insulin absorption using complexation and pH-sensitive hydrogels as delivery vehicles. Eur. J. Pharm. Sci. 2006;29:183–197. doi: 10.1016/j.ejps.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 181.Tiller J.C., Bonner G., Pan L.C., Klibanov A.M. Improving biomaterial properties of collagen films by chemical modification. Biotechnol Bioeng. 2001;73:246–252. doi: 10.1002/bit.1057. [DOI] [PubMed] [Google Scholar]
- 182.Blaszykowski C., Sheikh S., Thompson M. Biocompatibility and antifouling: Is there really a link? Trends Biotechnol. 2014;32:61–62. doi: 10.1016/j.tibtech.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 183.Maitra R., Clement C.C., Crisi G.M., Cobelli N., Santambrogio L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PloS ONE. 2008;3:e2438. doi: 10.1371/journal.pone.0002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andorko J.I., Jewell C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017;2:139–155. doi: 10.1002/btm2.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barbosa J.N., Barbosa M.A., Aguas A.P. Inflammatory responses and cell adhesion to self-assembled monolayers of alkanethiolates on gold. Biomaterials. 2004;25:2557–2563. doi: 10.1016/j.biomaterials.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 186.Barbosa J.N., Madureira P., Barbosa M.A., Aguas A.P. The influence of functional groups of self-assembled monolayers on fibrous capsule formation and cell recruitment. J. Biomed. Mater. Res. A. 2006;76:737–743. doi: 10.1002/jbm.a.30602. [DOI] [PubMed] [Google Scholar]
- 187.Bartneck M., Keul H.A., Singh S., Czaja K., Bornemann J., Bockstaller M., Moeller M., Zwadlo-Klarwasser G., Groll J. Rapid uptake of gold nanorods by primary human blood phagocytes and immunomodulatory effects of surface chemistry. ACS Nano. 2010;4:3073–3086. doi: 10.1021/nn100262h. [DOI] [PubMed] [Google Scholar]
- 188.Christo S.N., Bachhuka A., Diener K.R., Mierczynska A., Hayball J.D., Vasilev K. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. Adv. Healthc. Mater. 2016;5:956–965. doi: 10.1002/adhm.201500845. [DOI] [PubMed] [Google Scholar]
- 189.Getts D.R., Terry R.L., Getts M.T., Deffrasnes C., Muller M., van Vreden C., Ashhurst T.M., Chami B., McCarthy D., Wu H., et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 2014;6:219ra217. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kamath S., Bhattacharyya D., Padukudru C., Timmons R.B., Tang L. Surface chemistry influences implant-mediated host tissue responses. J. Biomed. Mater. Res. A. 2008;86:617–626. doi: 10.1002/jbm.a.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Keselowsky B.G., Collard D.M., Garcia A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 192.Lan M.A., Gersbach C.A., Michael K.E., Keselowsky B.G., Garcia A.J. Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials. 2005;26:4523–4531. doi: 10.1016/j.biomaterials.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 193.Lee M.H., Ducheyne P., Lynch L., Boettiger D., Composto R.J. Effect of biomaterial surface properties on fibronectin-alpha5beta1 integrin interaction and cellular attachment. Biomaterials. 2006;27:1907–1916. doi: 10.1016/j.biomaterials.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 194.Sperling C., Schweiss R.B., Streller U., Werner C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials. 2005;26:6547–6557. doi: 10.1016/j.biomaterials.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 195.Tang L., Wu Y., Timmons R.B. Fibrinogen adsorption and host tissue responses to plasma functionalized surfaces. J. Biomed. Mater. Res. 1998;42:156–163. doi: 10.1002/(SICI)1097-4636(199810)42:1<156::AID-JBM19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 196.Nair A., Zou L., Bhattacharyya D., Timmons R.B., Tang L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir. 2008;24:2015–2024. doi: 10.1021/la7025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sastry S.K., Burridge K. Focal adhesions: A nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 198.Kalltorp M., Oblogina S., Jacobsson S., Karlsson A., Tengvall P., Thomsen P. In vivo cell recruitment, cytokine release and chemiluminescence response at gold, and thiol functionalized surfaces. Biomaterials. 1999;20:2123–2137. doi: 10.1016/S0142-9612(99)00115-5. [DOI] [PubMed] [Google Scholar]
- 199.Benesch J., Svedhem S., Svensson S.C., Valiokas R., Liedberg B., Tengvall P. Protein adsorption to oligo(ethylene glycol) self-assembled monolayers: Experiments with fibrinogen, heparinized plasma, and serum. J. Biomater. Sci. Polym. Ed. 2001;12:581–597. doi: 10.1163/156856201316883421. [DOI] [PubMed] [Google Scholar]
- 200.Hirata I., Hioki Y., Toda M., Kitazawa T., Murakami Y., Kitano E., Kitamura H., Ikada Y., Iwata H. Deposition of complement protein C3b on mixed self-assembled monolayers carrying surface hydroxyl and methyl groups studied by surface plasmon resonance. J. Biomed. Mater. Res. A. 2003;66:669–676. doi: 10.1002/jbm.a.10067. [DOI] [PubMed] [Google Scholar]
- 201.Kim J., Somorjai G.A. Molecular packing of lysozyme, fibrinogen, and bovine serum albumin on hydrophilic and hydrophobic surfaces studied by infrared-visible sum frequency generation and fluorescence microscopy. J. Am. Chem. Soc. 2003;125:3150–3158. doi: 10.1021/ja028987n. [DOI] [PubMed] [Google Scholar]
- 202.Szott L.M., Horbett T.A. Protein interactions with surfaces: Cellular responses, complement activation, and newer methods. Curr. Opin. Chem. Biol. 2011;15:677–682. doi: 10.1016/j.cbpa.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 203.Sivaraman B., Fears K.P., Latour R.A. Investigation of the effects of surface chemistry and solution concentration on the conformation of adsorbed proteins using an improved circular dichroism method. Langmuir. 2009;25:3050–3056. doi: 10.1021/la8036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vieira E.P., Rocha S., Carmo Pereira M., Mohwald H., Coelho M.A. Adsorption and diffusion of plasma proteins on hydrophilic and hydrophobic surfaces: Effect of trifluoroethanol on protein structure. Langmuir. 2009;25:9879–9886. doi: 10.1021/la9009948. [DOI] [PubMed] [Google Scholar]
- 205.Neumann S., Burkert K., Kemp R., Rades T., Rod Dunbar P., Hook S. Activation of the NLRP3 inflammasome is not a feature of all particulate vaccine adjuvants. Immunol. Cell Biol. 2014;92:535–542. doi: 10.1038/icb.2014.21. [DOI] [PubMed] [Google Scholar]
- 206.Wen Y., Waltman A., Han H., Collier J.H. Switching the Immunogenicity of Peptide Assemblies Using Surface Properties. ACS Nano. 2016 doi: 10.1021/acsnano.6b03409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gallorini S., Berti F., Parente P., Baronio R., Aprea S., D’Oro U., Pizza M., Telford J.L., Wack A. Introduction of zwitterionic motifs into bacterial polysaccharides generates TLR2 agonists able to activate APCs. J. Immunol. 2007;179:8208–8215. doi: 10.4049/jimmunol.179.12.8208. [DOI] [PubMed] [Google Scholar]
- 208.Betancourt T., Brannon-Peppas L. Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. Int. J. Nanomed. 2006;1:483–495. doi: 10.2147/nano.2006.1.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Refai A.K., Textor M., Brunette D.M., Waterfield J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. A. 2004;70:194–205. doi: 10.1002/jbm.a.30075. [DOI] [PubMed] [Google Scholar]
- 210.Soskolne W.A., Cohen S., Sennerby L., Wennerberg A., Shapira L. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin. Oral Implants Res. 2002;13:86–93. doi: 10.1034/j.1600-0501.2002.130111.x. [DOI] [PubMed] [Google Scholar]
- 211.Bota P.C., Collie A.M., Puolakkainen P., Vernon R.B., Sage E.H., Ratner B.D., Stayton P.S. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J. Biomed. Mater. Res. A. 2010;95:649–657. doi: 10.1002/jbm.a.32893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Collie A.M., Bota P.C., Johns R.E., Maier R.V., Stayton P.S. Differential monocyte/macrophage interleukin-1beta production due to biomaterial topography requires the beta2 integrin signaling pathway. J. Biomed. Mater. Res. A. 2011;96:162–169. doi: 10.1002/jbm.a.32963. [DOI] [PubMed] [Google Scholar]
- 213.Mohiuddin M., Pan H.A., Hung Y.C., Huang G.S. Control of growth and inflammatory response of macrophages and foam cells with nanotopography. Nanoscale Res. Lett. 2012;7:394. doi: 10.1186/1556-276X-7-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fink J., Fuhrmann R., Scharnweber T., Franke R.P. Stimulation of monocytes and macrophages: Possible influence of surface roughness. Clin. Hemorheol. Microcirc. 2008;39:205–212. [PubMed] [Google Scholar]
- 215.Gamboa J.R., Mohandes S., Tran P.L., Slepian M.J., Yoon J.Y. Linear fibroblast alignment on sinusoidal wave micropatterns. Colloids Surf. B Biointerfaces. 2013;104:318–325. doi: 10.1016/j.colsurfb.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hulander M., Lundgren A., Faxalv L., Lindahl T.L., Palmquist A., Berglin M., Elwing H. Gradients in surface nanotopography used to study platelet adhesion and activation. Colloids Surf. B Biointerfaces. 2013;110:261–269. doi: 10.1016/j.colsurfb.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 217.Gilchrist C.L., Ruch D.S., Little D., Guilak F. Micro-scale and meso-scale architectural cues cooperate and compete to direct aligned tissue formation. Biomaterials. 2014;35:10015–10024. doi: 10.1016/j.biomaterials.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lopacinska J.M., Gradinaru C., Wierzbicki R., Kobler C., Schmidt M.S., Madsen M.T., Skolimowski M., Dufva M., Flyvbjerg H., Molhave K. Cell motility, morphology, viability and proliferation in response to nanotopography on silicon black. Nanoscale. 2012;4:3739–3745. doi: 10.1039/c2nr11455k. [DOI] [PubMed] [Google Scholar]
- 219.Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 220.Yim E.K., Reano R.M., Pang S.W., Yee A.F., Chen C.S., Leong K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26:5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gittens R.A., McLachlan T., Olivares-Navarrete R., Cai Y., Berner S., Tannenbaum R., Schwartz Z., Sandhage K.H., Boyan B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395–3403. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ma Q.L., Zhao L.Z., Liu R.R., Jin B.Q., Song W., Wang Y., Zhang Y.S., Chen L.H., Zhang Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials. 2014;35:9853–9867. doi: 10.1016/j.biomaterials.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 223.Tan K.S., Qian L., Rosado R., Flood P.M., Cooper L.F. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials. 2006;27:5170–5177. doi: 10.1016/j.biomaterials.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 224.Hulander M., Lundgren A., Berglin M., Ohrlander M., Lausmaa J., Elwing H. Immune complement activation is attenuated by surface nanotopography. Int. J. Nanomed. 2011;6:2653–2666. doi: 10.2147/IJN.S24578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Roach P., Eglin D., Rohde K., Perry C.C. Modern biomaterials: A review—bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 2007;18:1263–1277. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- 226.Scopelliti P.E., Borgonovo A., Indrieri M., Giorgetti L., Bongiorno G., Carbone R., Podesta A., Milani P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE. 2010;5:e11862. doi: 10.1371/journal.pone.0011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rechendorff K., Hovgaard M.B., Foss M., Zhdanov V.P., Besenbacher F. Enhancement of protein adsorption induced by surface roughness. Langmuir. 2006;22:10885–10888. doi: 10.1021/la0621923. [DOI] [PubMed] [Google Scholar]
- 228.Hovgaard M.B., Rechendorff K., Chevallier J., Foss M., Besenbacher F. Fibronectin adsorption on tantalum: The influence of nanoroughness. J. Phys. Chem. B. 2008;112:8241–8249. doi: 10.1021/jp801103n. [DOI] [PubMed] [Google Scholar]
- 229.Meyer U., Buchter A., Wiesmann H.P., Joos U., Jones D.B. Basic reactions of osteoblasts on structured material surfaces. Eur. Cells Mater. 2005;9:39–49. doi: 10.22203/eCM.v009a06. [DOI] [PubMed] [Google Scholar]
- 230.Mrksich M., Whitesides G.M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 1996;25:55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 231.Da Silva C.A., Chalouni C., Williams A., Hartl D., Lee C.G., Elias J.A. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J. Immunol. 2009;182:3573–3582. doi: 10.4049/jimmunol.0802113. [DOI] [PubMed] [Google Scholar]
- 232.Fernandez T.D., Pearson J.R., Leal M.P., Torres M.J., Blanca M., Mayorga C., Le Guevel X. Intracellular accumulation and immunological properties of fluorescent gold nanoclusters in human dendritic cells. Biomaterials. 2015;43:1–12. doi: 10.1016/j.biomaterials.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 233.Lee S., Choi J., Shin S., Im Y.M., Song J., Kang S.S., Nam T.H., Webster T.J., Kim S.H., Khang D. Analysis on migration and activation of live macrophages on transparent flat and nanostructured titanium. Acta Biomater. 2011;7:2337–2344. doi: 10.1016/j.actbio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 234.Lu J., Webster T.J. Reduced immune cell responses on nano and submicron rough titanium. Acta Biomater. 2015;16:223–231. doi: 10.1016/j.actbio.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 235.McWhorter F.Y., Wang T., Nguyen P., Chung T., Liu W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Veiseh O., Doloff J.C., Ma M., Vegas A.J., Tam H.H., Bader A.R., Li J., Langan E., Wyckoff J., Loo W.S., et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Baker D.W., Liu X., Weng H., Luo C., Tang L. Fibroblast/fibrocyte: Surface interaction dictates tissue reactions to micropillar implants. Biomacromolecules. 2011;12:997–1005. doi: 10.1021/bm1013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jahed Z., Molladavoodi S., Seo B.B., Gorbet M., Tsui T.Y., Mofrad M.R. Cell responses to metallic nanostructure arrays with complex geometries. Biomaterials. 2014;35:9363–9371. doi: 10.1016/j.biomaterials.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 239.Hornung V., Bauernfeind F., Halle A., Samstad E.O., Kono H., Rock K.L., Fitzgerald K.A., Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y., Kawaguchi A., Hasegawa H., Kajino K., Ninomiya T., et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 241.Padmore T., Stark C., Turkevich L.A., Champion J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. Biochim. Biophys. Acta Gen Subj. 2017;1861:58–67. doi: 10.1016/j.bbagen.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schanen B.C., Karakoti A.S., Seal S., Drake D.R., 3rd, Warren W.L., Self W.T. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3:2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sunshine J.C., Perica K., Schneck J.P., Green J.J. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials. 2014;35:269–277. doi: 10.1016/j.biomaterials.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vaine C.A., Patel M.K., Zhu J., Lee E., Finberg R.W., Hayward R.C., Kurt-Jones E.A. Tuning innate immune activation by surface texturing of polymer microparticles: The role of shape in inflammasome activation. J. Immunol. 2013;190:3525–3532. doi: 10.4049/jimmunol.1200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kosmides A.K., Meyer R.A., Hickey J.W., Aje K., Cheung K.N., Green J.J., Schneck J.P. Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials. 2017;118:16–26. doi: 10.1016/j.biomaterials.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Meyer R.A., Sunshine J.C., Perica K., Kosmides A.K., Aje K., Schneck J.P., Green J.J. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–1525. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Neacsu P., Mazare A., Cimpean A., Park J., Costache M., Schmuki P., Demetrescu I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014;55:187–195. doi: 10.1016/j.biocel.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 248.Rajyalakshmi A., Ercan B., Balasubramanian K., Webster T.J. Reduced adhesion of macrophages on anodized titanium with select nanotube surface features. Int. J. Nanomed. 2011;6:1765–1771. doi: 10.2147/IJN.S22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Moura C.C., Zanetta-Barbosa D., Dechichi P., Carvalho V.F., Soares P.B. Effects of titanium surfaces on the developmental profile of monocytes/macrophages. Braz. Dent. J. 2014;25:96–103. doi: 10.1590/0103-6440201302260. [DOI] [PubMed] [Google Scholar]
- 250.Mahyudin F., Widhiyanto L., Hermawan H. Advanced Structured Materials. Springer Publishing; New York, NY, USA: 2016. Biomaterials in orthopaedics. [Google Scholar]
- 251.Loh Q.L., Choong C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Matena J., Petersen S., Gieseke M., Kampmann A., Teske M., Beyerbach M., Murua Escobar H., Haferkamp H., Gellrich N.C., Nolte I. SLM produced porous titanium implant improvements for enhanced vascularization and osteoblast seeding. Int. J. Mol. Sci. 2015;16:7478–7492. doi: 10.3390/ijms16047478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kuboki Y., Jin Q., Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Joint Surg. Am. 2001;83(Suppl. 1):S105–S115. doi: 10.2106/00004623-200100002-00005. [DOI] [PubMed] [Google Scholar]
- 254.Artel A., Mehdizadeh H., Chiu Y.C., Brey E.M., Cinar A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A. 2011;17:2133–2141. doi: 10.1089/ten.tea.2010.0571. [DOI] [PubMed] [Google Scholar]
- 255.Jiang J., Li Z., Wang H., Wang Y., Carlson M.A., Teusink M.J., MacEwan M.R., Gu L., Xie J. Expanded 3D Nanofiber Scaffolds: Cell Penetration, Neovascularization, and Host Response. Adv. Healthc. Mater. 2016;5:2993–3003. doi: 10.1002/adhm.201600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Muller D., Chim H., Bader A., Whiteman M., Schantz J.T. Vascular guidance: Microstructural scaffold patterning for inductive neovascularization. Stem Cells Int. 2010;2011:547247. doi: 10.4061/2011/547247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kim H.J., Kim U.J., Vunjak-Novakovic G., Min B.H., Kaplan D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005;26:4442–4452. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 258.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., Ratner B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Bridges A.W., Singh N., Burns K.L., Babensee J.E., Andrew Lyon L., Garcia A.J. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials. 2008;29:4605–4615. doi: 10.1016/j.biomaterials.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Eming S.A., Hammerschmidt M., Krieg T., Roers A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009;20:517–527. doi: 10.1016/j.semcdb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 261.Badylak S.F. TISSUE REGENERATION. A scaffold immune microenvironment. Science. 2016;352:298. doi: 10.1126/science.aaf7587. [DOI] [PubMed] [Google Scholar]
- 262.Mescher A.L., Neff A.W., King M.W. Inflammation and immunity in organ regeneration. Dev. Comp. Immunol. 2017;66:98–110. doi: 10.1016/j.dci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 263.Julier Z., Park A.J., Briquez P.S., Martino M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 264.Gower R.M., Boehler R.M., Azarin S.M., Ricci C.F., Leonard J.N., Shea L.D. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials. 2014;35:2024–2031. doi: 10.1016/j.biomaterials.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 266.Boontheekul T., Mooney D.J. Protein-based signaling systems in tissue engineering. Curr. Opin. Biotechnol. 2003;14:559–565. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 267.Wolf M.T., Dearth C.L., Sonnenberg S.B., Loboa E.G., Badylak S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv. Drug Deliv. Rev. 2015;84:208–221. doi: 10.1016/j.addr.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Badylak S.F., Dziki J.L., Sicari B.M., Ambrosio F., Boninger M.L. Mechanisms by which acellular biologic scaffolds promote functional skeletal muscle restoration. Biomaterials. 2016;103:128–136. doi: 10.1016/j.biomaterials.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 269.Badylak S.F., Freytes D.O., Gilbert T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 270.Turner N.J., Badylak S.F. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv. Wound Care. 2015;4:490–500. doi: 10.1089/wound.2014.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brown B.N., Ratner B.D., Goodman S.B., Amar S., Badylak S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gowen B.B., Borg T.K., Ghaffar A., Mayer E.P. Selective adhesion of macrophages to denatured forms of type I collagen is mediated by scavenger receptors. Matrix Biol. 2000;19:61–71. doi: 10.1016/S0945-053X(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 273.Veres S.P., Brennan-Pierce E.P., Lee J.M. Macrophage-like U937 cells recognize collagen fibrils with strain-induced discrete plasticity damage. J. Biomed. Mater. Res. A. 2015;103:397–408. doi: 10.1002/jbm.a.35156. [DOI] [PubMed] [Google Scholar]
- 274.Huleihel L., Hussey G.S., Naranjo J.D., Zhang L., Dziki J.L., Turner N.J., Stolz D.B., Badylak S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016;2:e1600502. doi: 10.1126/sciadv.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dziki J.L., Wang D.S., Pineda C., Sicari B.M., Rausch T., Badylak S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J. Biomed. Mater. Res. A. 2017;105:138–147. doi: 10.1002/jbm.a.35894. [DOI] [PubMed] [Google Scholar]
- 276.Keane T.J., Dziki J., Sobieski E., Smoulder A., Castleton A., Turner N., White L.J., Badylak S.F. Restoring Mucosal Barrier Function and Modifying Macrophage Phenotype with an Extracellular Matrix Hydrogel: Potential Therapy for Ulcerative Colitis. J. Crohns Colitis. 2017;11:360–368. doi: 10.1093/ecco-jcc/jjw149. [DOI] [PubMed] [Google Scholar]
- 277.Brown B.N., Valentin J.E., Stewart-Akers A.M., McCabe G.P., Badylak S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Fishman J.M., Lowdell M.W., Urbani L., Ansari T., Burns A.J., Turmaine M., North J., Sibbons P., Seifalian A.M., Wood K.J., et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA. 2013;110:14360–14365. doi: 10.1073/pnas.1213228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Keane T.J., Dziki J., Castelton A., Faulk D.M., Messerschmidt V., Londono R., Reing J.E., Velankar S.S., Badylak S.F. Preparation and characterization of a biologic scaffold and hydrogel derived from colonic mucosa. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:291–306. doi: 10.1002/jbm.b.33556. [DOI] [PubMed] [Google Scholar]
- 280.Spiller K.L., Nassiri S., Witherel C.E., Anfang R.R., Ng J., Nakazawa K.R., Yu T., Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Deeken C.R., Eliason B.J., Pichert M.D., Grant S.A., Frisella M.M., Matthews B.D. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann. Surg. 2012;255:595–604. doi: 10.1097/SLA.0b013e3182445341. [DOI] [PubMed] [Google Scholar]
- 282.Delgado L.M., Bayon Y., Pandit A., Zeugolis D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015;21:298–313. doi: 10.1089/ten.teb.2014.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.McDade J.K., Brennan-Pierce E.P., Ariganello M.B., Labow R.S., Michael Lee J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: Influence of crosslinking treatment. Acta Biomater. 2013;9:7191–7199. doi: 10.1016/j.actbio.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 284.Keane T.J., Londono R., Turner N.J., Badylak S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 285.Beachley V.Z., Wolf M.T., Sadtler K., Manda S.S., Jacobs H., Blatchley M.R., Bader J.S., Pandey A., Pardoll D., Elisseeff J.H. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat. Methods. 2015;12:1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Asea A., Rehli M., Kabingu E., Boch J.A., Bare O., Auron P.E., Stevenson M.A., Calderwood S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 287.Kovalchin J.T., Wang R., Wagh M.S., Azoulay J., Sanders M., Chandawarkar R.Y. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006;14:129–137. doi: 10.1111/j.1743-6109.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 288.Laschke M.W., Augustin V., Kleer S., Tschernig T., Menger M.D. Locally applied macrophage-activating lipopeptide-2 (MALP-2) promotes early vascularization of implanted porous polyethylene (Medpor(R)) Acta Biomater. 2014;10:4661–4669. doi: 10.1016/j.actbio.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 289.Yamamoto M., Sato T., Beren J., Verthelyi D., Klinman D.M. The acceleration of wound healing in primates by the local administration of immunostimulatory CpG oligonucleotides. Biomaterials. 2011;32:4238–4242. doi: 10.1016/j.biomaterials.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Noguchi K., Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontology 2000. 2007;43:85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 291.Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 292.Katamura K., Shintaku N., Yamauchi Y., Fukui T., Ohshima Y., Mayumi M., Furusho K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J. Immunol. 1995;155:4604–4612. [PubMed] [Google Scholar]
- 293.Paralkar V.M., Borovecki F., Ke H.Z., Cameron K.O., Lefker B., Grasser W.A., Owen T.A., Li M., DaSilva-Jardine P., Zhou M., et al. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc. Natl. Acad. Sci. USA. 2003;100:6736–6740. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kamolratanakul P., Hayata T., Ezura Y., Kawamata A., Hayashi C., Yamamoto Y., Hemmi H., Nagao M., Hanyu R., Notomi T., et al. Nanogel-based scaffold delivery of prostaglandin E(2) receptor-specific agonist in combination with a low dose of growth factor heals critical-size bone defects in mice. Arthritis Rheumatol. 2011;63:1021–1033. doi: 10.1002/art.30151. [DOI] [PubMed] [Google Scholar]
- 295.Kato N., Hasegawa U., Morimoto N., Saita Y., Nakashima K., Ezura Y., Kurosawa H., Akiyoshi K., Noda M. Nanogel-based delivery system enhances PGE2 effects on bone formation. J. Cell Biochem. 2007;101:1063–1070. doi: 10.1002/jcb.21160. [DOI] [PubMed] [Google Scholar]
- 296.Toyoda H., Terai H., Sasaoka R., Oda K., Takaoka K. Augmentation of bone morphogenetic protein-induced bone mass by local delivery of a prostaglandin E EP4 receptor agonist. Bone. 2005;37:555–562. doi: 10.1016/j.bone.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 297.Kucia M., Jankowski K., Reca R., Wysoczynski M., Bandura L., Allendorf D.J., Zhang J., Ratajczak J., Ratajczak M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004;35:233–245. doi: 10.1023/B:HIJO.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 298.Lau T.T., Wang D.A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011;11:189–197. doi: 10.1517/14712598.2011.546338. [DOI] [PubMed] [Google Scholar]
- 299.Son B.R., Marquez-Curtis L.A., Kucia M., Wysoczynski M., Turner A.R., Ratajczak J., Ratajczak M.Z., Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 300.Kim Y.H., Tabata Y. Recruitment of mesenchymal stem cells and macrophages by dual release of stromal cell-derived factor-1 and a macrophage recruitment agent enhances wound closure. J. Biomed. Mater. Res. A. 2016;104:942–956. doi: 10.1002/jbm.a.35635. [DOI] [PubMed] [Google Scholar]
- 301.Shen W., Chen X., Chen J., Yin Z., Heng B.C., Chen W., Ouyang H.W. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–7249. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 302.Projahn D., Simsekyilmaz S., Singh S., Kanzler I., Kramp B.K., Langer M., Burlacu A., Bernhagen J., Klee D., Zernecke A., et al. Controlled intramyocardial release of engineered chemokines by biodegradable hydrogels as a treatment approach of myocardial infarction. J. Cell. Mol. Med. 2014;18:790–800. doi: 10.1111/jcmm.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kimura Y., Tabata Y. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J. Biomater. Sci. Polym. Ed. 2010;21:37–51. doi: 10.1163/156856209X410193. [DOI] [PubMed] [Google Scholar]
- 304.Boehler R.M., Graham J.G., Shea L.D. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51:239–240, 242, 244 passim. doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hume P.S., He J., Haskins K., Anseth K.S. Strategies to reduce dendritic cell activation through functional biomaterial design. Biomaterials. 2012;33:3615–3625. doi: 10.1016/j.biomaterials.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chen J., Li M., Yang C., Yin X., Duan K., Wang J., Feng B. Macrophage phenotype switch by sequential action of immunomodulatory cytokines from hydrogel layers on titania nanotubes. Colloids Surf. B Biointerfaces. 2018;163:336–345. doi: 10.1016/j.colsurfb.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 307.Lin C.C., Boyer P.D., Aimetti A.A., Anseth K.S. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. J. Control. Release. 2010;142:384–391. doi: 10.1016/j.jconrel.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Lin C.C., Metters A.T., Anseth K.S. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wynn T.A., Vannella K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Boehler R.M., Kuo R., Shin S., Goodman A.G., Pilecki M.A., Gower R.M., Leonard J.N., Shea L.D. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol. Bioeng. 2014;111:1210–1221. doi: 10.1002/bit.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gonzalez R., Glaser J., Liu M.T., Lane T.E., Keirstead H.S. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp. Neurol. 2003;184:456–463. doi: 10.1016/S0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 312.Mokarram N., Merchant A., Mukhatyar V., Patel G., Bellamkonda R.V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hachim D., LoPresti S.T., Yates C.C., Brown B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials. 2017;112:95–107. doi: 10.1016/j.biomaterials.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mori R., Shaw T.J., Martin P. Molecular mechanisms linking wound inflammation and fibrosis: Knockdown of osteopontin leads to rapid repair and reduced scarring. J. Exp. Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Holladay C., Power K., Sefton M., O’Brien T., Gallagher W.M., Pandit A. Functionalized scaffold-mediated interleukin 10 gene delivery significantly improves survival rates of stem cells in vivo. Mol. Ther. 2011;19:969–978. doi: 10.1038/mt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Rao A.J., Nich C., Dhulipala L.S., Gibon E., Valladares R., Zwingenberger S., Smith R.L., Goodman S.B. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J. Biomed. Mater. Res. A. 2013;101:1926–1934. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.De Oliveira C.P., Magolbo N., De Aquino R., Weller C. Oral aspirin for treating venous leg ulcers. Cochrane Database Syst Rev. 2016;2:CD009432. doi: 10.1002/14651858.CD009432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Canton I., McKean R., Charnley M., Blackwood K.A., Fiorica C., Ryan A.J., MacNeil S. Development of an Ibuprofen-releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration. Biotechnol. Bioeng. 2010;105:396–408. doi: 10.1002/bit.22530. [DOI] [PubMed] [Google Scholar]
- 319.Varatharajan L., Thapar A., Lane T., Munster A.B., Davies A.H. Pharmacological adjuncts for chronic venous ulcer healing: A systematic review. Phlebology. 2016;31:356–365. doi: 10.1177/0268355515587194. [DOI] [PubMed] [Google Scholar]
- 320.Friedrich E.E., Sun L.T., Natesan S., Zamora D.O., Christy R.J., Washburn N.R. Effects of hyaluronic acid conjugation on anti-TNF-alpha inhibition of inflammation in burns. J. Biomed. Mater. Res. A. 2014;102:1527–1536. doi: 10.1002/jbm.a.34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kim Y.S., Park H.J., Hong M.H., Kang P.M., Morgan J.P., Jeong M.H., Cho J.G., Park J.C., Ahn Y. TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front. Biosci. 2009;14:2845–2856. doi: 10.2741/3417. [DOI] [PubMed] [Google Scholar]
- 322.Bocker W., Docheva D., Prall W.C., Egea V., Pappou E., Rossmann O., Popov C., Mutschler W., Ries C., Schieker M. IKK-2 is required for TNF-alpha-induced invasion and proliferation of human mesenchymal stem cells. J. Mol. Med. 2008;86:1183–1192. doi: 10.1007/s00109-008-0378-3. [DOI] [PubMed] [Google Scholar]
- 323.Penn J.W., Grobbelaar A.O., Rolfe K.J. The role of the TGF-beta family in wound healing, burns and scarring: A review. Int. J. Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 324.Johnston C.J., Smyth D.J., Dresser D.W., Maizels R.M. TGF-beta in tolerance, development and regulation of immunity. Cell. Immunol. 2016;299:14–22. doi: 10.1016/j.cellimm.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ferguson M.W., Duncan J., Bond J., Bush J., Durani P., So K., Taylor L., Chantrey J., Mason T., James G., et al. Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet. 2009;373:1264–1274. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- 326.Yang D., Wei F., Tewary P., Howard O.M., Oppenheim J.J. Alarmin-induced cell migration. Eur J. Immunol. 2013;43:1412–1418. doi: 10.1002/eji.201243138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rankin A.L., Mumm J.B., Murphy E., Turner S., Yu N., McClanahan T.K., Bourne P.A., Pierce R.H., Kastelein R., Pflanz S. IL-33 induces IL-13-dependent cutaneous fibrosis. J. Immunol. 2010;184:1526–1535. doi: 10.4049/jimmunol.0903306. [DOI] [PubMed] [Google Scholar]
- 328.Lopetuso L.R., Scaldaferri F., Pizarro T.T. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenes. Tissue Repair. 2012;5:18. doi: 10.1186/1755-1536-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T.K., Zurawski G., Moshrefi M., Qin J., Li X., et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 330.Li D., Guabiraba R., Besnard A.G., Komai-Koma M., Jabir M.S., Zhang L., Graham G.J., Kurowska-Stolarska M., Liew F.Y., McSharry C., et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J. Allergy Clin. Immunol. 2014;134:1422–1432. doi: 10.1016/j.jaci.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Smithgall M.D., Comeau M.R., Yoon B.R., Kaufman D., Armitage R., Smith D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 332.Gao Q., Li Y., Li M. The potential role of IL-33/ST2 signaling in fibrotic diseases. J. Leukoc. Biol. 2015;98:15–22. doi: 10.1189/jlb.3RU0115-012R. [DOI] [PubMed] [Google Scholar]
- 333.Luzina I.G., Kopach P., Lockatell V., Kang P.H., Nagarsekar A., Burke A.P., Hasday J.D., Todd N.W., Atamas S.P. Interleukin-33 potentiates bleomycin-induced lung injury. Am. J. Respir. Cell Mol. Biol. 2013;49:999–1008. doi: 10.1165/rcmb.2013-0093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.McHedlidze T., Waldner M., Zopf S., Walker J., Rankin A.L., Schuchmann M., Voehringer D., McKenzie A.N., Neurath M.F., Pflanz S., et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Franchimont D., Kino T., Galon J., Meduri G.U., Chrousos G. Glucocorticoids and inflammation revisited: The state of the art. NIH clinical staff conference. Neuroimmunomodulation. 2002;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 336.Crossley G.H., Brinker J.A., Reynolds D., Spencer W., Johnson W.B., Hurd H., Tonder L., Zmijewski M. Steroid elution improves the stimulation threshold in an active-fixation atrial permanent pacing lead. A randomized, controlled study. Model 4068 Investigators. Circulation. 1995;92:2935–2939. doi: 10.1161/01.CIR.92.10.2935. [DOI] [PubMed] [Google Scholar]
- 337.Zhong Y., Bellamkonda R.V. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Morais J.M., Papadimitrakopoulos F., Burgess D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Patil S.D., Papadimitrakopoulos F., Burgess D.J. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol. Ther. 2004;6:887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 340.Minardi S., Corradetti B., Taraballi F., Byun J.H., Cabrera F., Liu X., Ferrari M., Weiner B.K., Tasciotti E. IL-4 Release from a Biomimetic Scaffold for the Temporally Controlled Modulation of Macrophage Response. Ann. Biomed. Eng. 2016;44:2008–2019. doi: 10.1007/s10439-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 341.Kim D.H., Martin D.C. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 342.Tsianakas A., Varga G., Barczyk K., Bode G., Nippe N., Kran N., Roth J., Luger T.A., Ehrchen J., Sunderkoetter C. Induction of an anti-inflammatory human monocyte subtype is a unique property of glucocorticoids, but can be modified by IL-6 and IL-10. Immunobiology. 2012;217:329–335. doi: 10.1016/j.imbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 343.Luo J.C., Shin V.Y., Liu E.S., Ye Y.N., Wu W.K., So W.H., Chang F.Y., Cho C.H. Dexamethasone delays ulcer healing by inhibition of angiogenesis in rat stomachs. Eur. J. Pharmacol. 2004;485:275–281. doi: 10.1016/j.ejphar.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 344.Koedam J.A., Smink J.J., van Buul-Offers S.C. Glucocorticoids inhibit vascular endothelial growth factor expression in growth plate chondrocytes. Mol. Cell. Endocrinol. 2002;197:35–44. doi: 10.1016/S0303-7207(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 345.Patil S.D., Papadmitrakopoulos F., Burgess D.J. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J. Control. Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 346.Norton L.W., Koschwanez H.E., Wisniewski N.A., Klitzman B., Reichert W.M. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J. Biomed. Mater. Res. A. 2007;81:858–869. doi: 10.1002/jbm.a.31088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Kastellorizios M., Papadimitrakopoulos F., Burgess D.J. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J. Control. Release. 2015;214:103–111. doi: 10.1016/j.jconrel.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 348.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 349.Doloff J.C., Veiseh O., Vegas A.J., Tam H.H., Farah S., Ma M., Li J., Bader A., Chiu A., Sadraei A., et al. Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates. Nat. Mater. 2017;16:671–680. doi: 10.1038/nmat4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Udipi K., Ornberg R.L., Thurmond K.B., 2nd, Settle S.L., Forster D., Riley D. Modification of inflammatory response to implanted biomedical materials in vivo by surface bound superoxide dismutase mimics. J. Biomed. Mater. Res. 2000;51:549–560. doi: 10.1002/1097-4636(20000915)51:4<549::AID-JBM2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 351.Hetrick E.M., Prichard H.L., Klitzman B., Schoenfisch M.H. Reduced foreign body response at nitric oxide-releasing subcutaneous implants. Biomaterials. 2007;28:4571–4580. doi: 10.1016/j.biomaterials.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Schwentker A., Vodovotz Y., Weller R., Billiar T.R. Nitric oxide and wound repair: Role of cytokines? Nitric Oxide. 2002;7:1–10. doi: 10.1016/S1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 353.Bogdan C. Regulation of lymphocytes by nitric oxide. Methods Mol. Biol. 2011;677:375–393. doi: 10.1007/978-1-60761-869-0_24. [DOI] [PubMed] [Google Scholar]
- 354.Cha K.H., Wang X., Meyerhoff M.E. Nitric Oxide Release for Improving Performance of Implantable Chemical Sensors—A Review. Appl. Mater. Today. 2017;9:589–597. doi: 10.1016/j.apmt.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Hersel U., Dahmen C., Kessler H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/S0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 356.Kao W.J., Lee D. In vivo modulation of host response and macrophage behavior by polymer networks grafted with fibronectin-derived biomimetic oligopeptides: The role of RGD and PHSRN domains. Biomaterials. 2001;22:2901–2909. doi: 10.1016/S0142-9612(01)00037-0. [DOI] [PubMed] [Google Scholar]
- 357.VandeVondele S., Voros J., Hubbell J.A. RGD-grafted poly-l-lysine-graft-(polyethylene glycol) copolymers block non-specific protein adsorption while promoting cell adhesion. Biotechnol. Bioeng. 2003;82:784–790. doi: 10.1002/bit.10625. [DOI] [PubMed] [Google Scholar]
- 358.Zhu J., Tang C., Kottke-Marchant K., Marchant R.E. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug. Chem. 2009;20:333–339. doi: 10.1021/bc800441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Karimi F., O’Connor A.J., Qiao G.G., Heath D.E. Integrin Clustering Matters: A Review of Biomaterials Functionalized with Multivalent Integrin-Binding Ligands to Improve Cell Adhesion, Migration, Differentiation, Angiogenesis, and Biomedical Device Integration. Adv. Healthc. Mater. 2018;7:e1701324. doi: 10.1002/adhm.201701324. [DOI] [PubMed] [Google Scholar]
- 360.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Schwab J.M., Chiang N., Arita M., Serhan C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vasconcelos D.P., Costa M., Amaral I.F., Barbosa M.A., Aguas A.P., Barbosa J.N. Development of an immunomodulatory biomaterial: Using resolvin D1 to modulate inflammation. Biomaterials. 2015;53:566–573. doi: 10.1016/j.biomaterials.2015.02.120. [DOI] [PubMed] [Google Scholar]
- 364.Vasconcelos D.P., Costa M., Amaral I.F., Barbosa M.A., Aguas A.P., Barbosa J.N. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials. 2015;37:116–123. doi: 10.1016/j.biomaterials.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 365.Tang Y., Zhang M.J., Hellmann J., Kosuri M., Bhatnagar A., Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hong S., Tian H., Lu Y., Laborde J.M., Muhale F.A., Wang Q., Alapure B.V., Serhan C.N., Bazan N.G. Neuroprotectin/protectin D1: Endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol. Cell Physiol. 2014;307:C1058–C1067. doi: 10.1152/ajpcell.00270.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Dohle E., Bischoff I., Bose T., Marsano A., Banfi A., Unger R.E., Kirkpatrick C.J. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur. Cells Mater. 2014;27:149–164; discussion 164-145. doi: 10.22203/eCM.v027a12. [DOI] [PubMed] [Google Scholar]
- 368.Swartzlander M.D., Blakney A.K., Amer L.D., Hankenson K.D., Kyriakides T.R., Bryant S.J. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials. 2015;41:79–88. doi: 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Chen Z., Wu C., Gu W., Klein T., Crawford R., Xiao Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35:1507–1518. doi: 10.1016/j.biomaterials.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 370.Mansouri S., Merhi Y., Winnik F.M., Tabrizian M. Investigation of layer-by-layer assembly of polyelectrolytes on fully functional human red blood cells in suspension for attenuated immune response. Biomacromolecules. 2011;12:585–592. doi: 10.1021/bm101200c. [DOI] [PubMed] [Google Scholar]
- 371.Kim J.Y., Lee H., Park T., Park J., Kim M.H., Cho H., Youn W., Kang S.M., Choi I.S. Artificial Spores: Cytocompatible Coating of Living Cells with Plant-Derived Pyrogallol. Chem. Asian J. 2016;11:3183–3187. doi: 10.1002/asia.201601237. [DOI] [PubMed] [Google Scholar]
- 372.Hasturk O., Kaplan D.L. Cell armor for protection against environmental stress: Advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2018 doi: 10.1016/j.actbio.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Weiden J., Voerman D., Dolen Y., Das R.K., van Duffelen A., Hammink R., Eggermont L.J., Rowan A.E., Tel J., Figdor C.G. Injectable Biomimetic Hydrogels as Tools for Efficient T Cell Expansion and Delivery. Front. Immunol. 2018;9:2798. doi: 10.3389/fimmu.2018.02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kumar M., Coburn J., Kaplan D.L., Mandal B.B. Immuno-Informed 3D Silk Biomaterials for Tailoring Biological Responses. ACS Appl. Mater. Interfaces. 2016;8:29310–29322. doi: 10.1021/acsami.6b09937. [DOI] [PubMed] [Google Scholar]
- 375.Wang H., Morales R.T., Cui X., Huang J., Qian W., Tong J., Chen W. A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Macrophage Immunomodulation. Adv. Healthc. Mater. 2018:e1801234. doi: 10.1002/adhm.201801234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.He X.T., Wu R.X., Xu X.Y., Wang J., Yin Y., Chen F.M. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018;71:132–147. doi: 10.1016/j.actbio.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 377.Zhao S., Zhang L., Han J., Chu J., Wang H., Chen X., Wang Y., Tun N., Lu L., Bai X.F., et al. Conformal Nanoencapsulation of Allogeneic T Cells Mitigates Graft-versus-Host Disease and Retains Graft-versus-Leukemia Activity. ACS Nano. 2016;10:6189–6200. doi: 10.1021/acsnano.6b02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ishida Y., Kondo T., Kimura A., Matsushima K., Mukaida N. Absence of IL-1 receptor antagonist impaired wound healing along with aberrant NF-kappaB activation and a reciprocal suppression of TGF-beta signal pathway. J. Immunol. 2006;176:5598–5606. doi: 10.4049/jimmunol.176.9.5598. [DOI] [PubMed] [Google Scholar]
- 379.Chang J., Wang Z., Tang E., Fan Z., McCauley L., Franceschi R., Guan K., Krebsbach P.H., Wang C.Y. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Chang J., Liu F., Lee M., Wu B., Ting K., Zara J.N., Soo C., Al Hezaimi K., Zou W., Chen X., et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc. Natl. Acad. Sci. USA. 2013;110:9469–9474. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Mountziaris P.M., Tzouanas S.N., Sing D.C., Kramer P.R., Kasper F.K., Mikos A.G. Intra-articular controlled release of anti-inflammatory siRNA with biodegradable polymer microparticles ameliorates temporomandibular joint inflammation. Acta Biomater. 2012;8:3552–3560. doi: 10.1016/j.actbio.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Leuschner F., Dutta P., Gorbatov R., Novobrantseva T.I., Donahoe J.S., Courties G., Lee K.M., Kim J.I., Markmann J.F., Marinelli B., et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sager H.B., Dutta P., Dahlman J.E., Hulsmans M., Courties G., Sun Y., Heidt T., Vinegoni C., Borodovsky A., Fitzgerald K., et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci. Transl. Med. 2016;8:342ra380. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 385.Mehta A., Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016;16:279–294. doi: 10.1038/nri.2016.40. [DOI] [PubMed] [Google Scholar]
- 386.Li D., Wang A., Liu X., Meisgen F., Grunler J., Botusan I.R., Narayanan S., Erikci E., Li X., Blomqvist L., et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J. Clin. Investig. 2015;125:3008–3026. doi: 10.1172/JCI79052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Baumjohann D., Ansel K.M. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat. Rev. Immunol. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Frith J.E., Porrello E.R., Cooper-White J.J. Concise review: New frontiers in microRNA-based tissue regeneration. Stem Cells Transl. Med. 2014;3:969–976. doi: 10.5966/sctm.2014-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Gori M., Trombetta M., Santini D., Rainer A. Tissue engineering and microRNAs: Future perspectives in regenerative medicine. Expert Opin. Biol. Ther. 2015;15:1601–1622. doi: 10.1517/14712598.2015.1071349. [DOI] [PubMed] [Google Scholar]
- 390.Li J., Kooger R., He M., Xiao X., Zheng L., Zhang Y. A supramolecular hydrogel as a carrier to deliver microRNA into the encapsulated cells. Chem. Commun. (Camb.) 2014;50:3722–3724. doi: 10.1039/C4CC00156G. [DOI] [PubMed] [Google Scholar]
- 391.Morishita Y., Imai T., Yoshizawa H., Watanabe M., Ishibashi K., Muto S., Nagata D. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int. J. Nanomed. 2015;10:3475–3488. doi: 10.2147/IJN.S82587. [DOI] [PMC free article] [PubMed] [Google Scholar]