Abstract
Acute leukemia (AL) is the main type of cancer in children worldwide. Mortality by this disease is high in developing countries and its etiology remains unanswered. Evidences showing the role of the long non-coding RNAs (lncRNAs) in the pathophysiology of hematological malignancies have increased drastically in the last decade. In addition to the contribution of these lncRNAs in leukemogenesis, recent studies have suggested that lncRNAs could be used as biomarkers in the diagnosis, prognosis, and therapeutic response in leukemia patients. The focus of this review is to describe the functional classification, biogenesis, and the role of lncRNAs in leukemogenesis, to summarize the evidence about the lncRNAs which are playing a role in AL, and how these genes could be useful as potential therapeutic targets.
Keywords: long non-coding RNAs, cancer, acute leukemia, therapeutic targets
1. Introduction
Leukemia is a group of hematological malignancies characterized by an oligoclonal expansion of abnormally differentiated, and sometimes poorly differentiated hematopoietic cells which infiltrate the bone marrow, and could also invade the blood and other extramedullary tissues. In general, AL can be divided into acute or chronic, and lymphoid or myeloid, according to their progression and affected lineage, respectively. Thus, we can identify the following subtypes: acute lymphoblastic leukemia (ALL), chronic lymphoblastic leukemia (CLL), acute myeloid leukemia (AML), and chronic myeloid leukemia (CML). AL is the main type of cancer in children worldwide [1,2]. In recent years, it has reported a trend of increase in the incidence AL; notwithstanding, the causes are still unclear. Studies conducted to identify the etiology of this disease have reported that a genetic background interacting with environmental factors (i.e., high doses of ionizing radiation, infections, parental occupational exposures, etc.) could explain this phenomenon [3]; however, the molecular mechanisms involved are not fully understood. To date, growing data have shown that different non-coding RNAs (ncRNAs) might be the link between the genome and the environment because they are closely related to normal physiological and pathological processes [4,5]. ncRNAs, also known as non-protein-coding RNAs (npcRNAs), non-messenger RNAs (nmRNAs) or functional RNAs (fRNAs), are functional RNA molecules which are not translated into proteins [6]. These RNAs consist of several distinct families which include microRNAs (miRNAs), small nuclear RNAs (snRNAs), PIWI-interacting RNAs (piRNAs), and long non-coding RNAs (lncRNAs), among others. LncRNAs are one of the most studied ncRNA types, and play an important role as gene expression modulators at the epigenetic, transcriptional, and post-transcriptional level. In fact, it has been suggested that various miRNAs and lncRNAs could act as tumor suppressors genes or oncogenes, because they regulate directly or indirectly the expression of genes involved in molecular mechanisms as cell proliferation/differentiation, apoptosis, and metastasis [4,5]. In comparison with miRNAs, the lncRNAs are more numerous and represents the 41% of the overall ncRNAs. Over the last years, massive technological tools have been useful to increase the knowledge about lncRNAs that are abnormally expressed or mutated in AL and the list of relevant lncRNAs in leukemogenesis is growing rapidly. Moreover, it has reported a distinctive lncRNAs expression signature associated with AL prognosis, suggesting the potential application of these genes to make treatment decisions. Here, we review the most recent findings about lncRNAs in AL pathogenesis and their role as potential biomarkers. We also are pointing out the lncRNAs as promising druggable molecules in the development of new treatments for leukemia [7]. An electronic search strategy using the biomedical database of the National Center for Biotechnology Information (NCBI) was conducted. Studies that combined the keywords lncRNAs with acute leukemia, or acute lymphoblastic leukemia, or acute myeloid leukemia or hematopoiesis were enclosed.
2. Genetic Features of Acute Leukemia
AL has been recognized as a highly genetically heterogeneous disease, where chromosomal abnormalities, either numerical (hyperdiploidy and hypodiploidy) or structural alterations (translocations, amplifications, DNA copy number alterations, insertions/deletions, and punctual mutations) are usually observed; thus, these alterations are the hallmarks of the leukemic cells and represent the major class of oncogenic drivers to the disease. Indeed, due to the fact many childhood ALL cases carry specific fusion genes (MLL gene fusions, ETV6/RUNX1, E2A/PBX1, etc.) and AML (AML1/ETO, PML/RARα, CBFβ/MYH11, etc.), this gives more evidence that childhood AL is initiated in utero during fetal hematopoiesis [8]. In addition to the numerical alterations and common targets of translocations in ALL, this disease is characterized by mutations in transcriptional factors (AML1, ETS, PAX5, IKZF1, EBF1, ETV6, and STAT), suppressor genes (TP53, RB1, CDKN2A/CDKN2B, etc.), oncogenes (ABL1, ABL2, CSF1R, JAK2, PDGFRB, and CRLF2), B lymphoid cell differentiators (IKZF1, TCF3, EBF1, PAX5, and VPREB1), chromatin remodelers, or epigenetic modifiers (DNMT3A, CREBBP, MLL2, NSD2, EP300, ARID1A, TET2, and CHD6) [9,10,11,12]. Data from the St. Jude/Washington Pediatric Cancer Genome Project (PCGP), that has characterized pediatric cancer genomes by whole-genome or whole-exome sequencing, revealed that the somatic mutation rate in childhood ALL ranges from 7.30 × 10−8 per base [13]. In spite of the fact that chromosomal changes detectable by cytogenetic techniques are present in nearly 75% of the precursor B (pre-B) cell ALL cases, the gene expression profiling and genome-wide sequencing analyses have showed that B cell leukemogenesis is more complex [14]. Meanwhile, mutations in nRAS, RUNX1, FLT3, KIT, etc., abnormalities of DNA methylation, biogenesis of ribosomes, activated signaling pathways, myeloid transcription factors, chromatin remodeling, and cohesion complex processes are very common in AML [15].
The discovery of frequent mutations in epigenetic modifiers genes in AL show that epigenetic alterations also play a critical role in leukemogenesis. In this regard, it is known that most of the genes involved in epigenetic process do not code for proteins, and many of them are classified as lncRNAs, which regulate gene expression through different mechanisms.
3. LncRNAs Characteristics
lncRNAs comprise a highly functionally heterogeneous group of RNA molecules with sizes are greater than 200 nucleotides, and, as all the mRNAs usually have more than one exon, most of them are transcribed by RNA polymerase II (RNA pol II), are capped, may be polyadenylate, and can be located within the nucleus or cytoplasm. LncRNAs genes differ from mRNAs because lncRNAs lack protein-coding potential, are mostly expressed in low levels, and show poor species conservation compared to protein-coding genes (mRNAs). Additionally, lncRNAs display tissue-specific and development stage-specific expression showing their important role in cell differentiation mechanisms [16].
The number of lncRNAs is larger than the number of protein-coding RNAs. To date, the GENCODE project lncRNAs catalog consists of 15,779 transcripts (there are potentially more than 28,000 distinct transcripts) in the human genome (https://www.gencodegenes.org); nevertheless, this number could increase, since many primary long non-coding transcripts are often processed into smaller ncRNAs [17]. ncRNA detection led to a solution for the G-value paradox that states that there is no correlation between the amount of coding genes and the complexity of the organism, while we observe a correlation between the complexity of the organism and the ratio of the number of non-coding genes to total genomic DNA. Nowadays, cumulative evidence exhibits that lncRNAs are relevant players in many cellular processes either in physiological as well as pathological conditions. In cancer, the lncRNAs could have oncogenic function and tumor suppressive function since they have been found as upregulated or downregulated in several types of tumors in comparison to healthy tissues [18].
4. Biogenesis and Classification
It has hypothesized that most of lncRNAs are originated from (1) the incorporation of the fragments of original protein-coding genes; (2) juxtaposition of two transcribed and previously well-separated sequence regions of chromosomes giving rise a multi-exon ncRNA; (3) duplication of non-coding genes through retrotransposition; (4) tandem duplication events of neighboring repeats within a ncRNA; and (5) insertion of transcription factor, which is inserted into a sequence.
LncRNAs are transcribed and processed by the RNA pol II transcriptional machinery, thus many of them undergo post-transcriptional modifications such as 5′ capping, splicing, and polyadenylation. Nevertheless, there are also nonpolyadenylated lncRNAs that derive from RNA pol III promoters and snoRNA-related lncRNAs (sno-lncRNAs) expressed from introns via the snoRNP machinery (with the supplementary production of two snoRNAs). LncRNAs have been mapped into a wide range of regions, including coding and non-coding regions (intergenic regions, promoters, enhancers, and introns) [19,20,21,22,23,24,25,26,27].
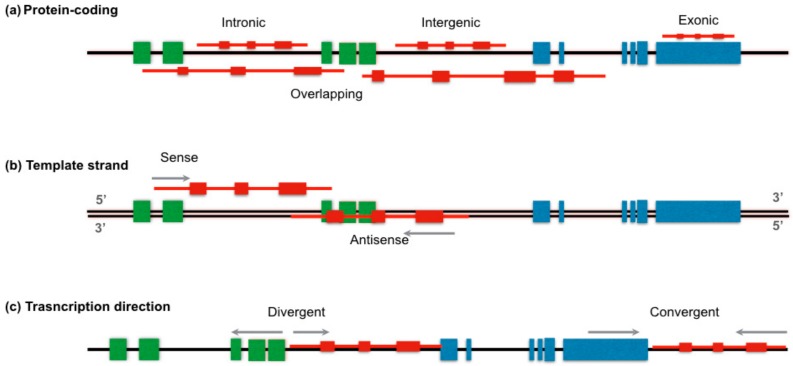
To date, there is not a unique system to classify lncRNAs; however, different classifications have been proposed based on their size, genome localization, RNA mechanism of action, and function [28]. According to their location (Figure 1a), orientation (Figure 1b), and transcription direction (Figure 1c) relative to protein-coding genes, an lncRNA can be placed into one or more broad categories. Thus, lncRNAs can be intronic, when they lie into a intron of a second transcript (COLDAIR, located in the first intron of the flowering repressor locus C or FLC), intergenic (lincRNA) if it is located between two genes without any overlap at least 5 kb from both sides (exemplified by H19, XIST, and lincRNA-p21), exonic if lncRNA is encoded within a exon, or overlapping, which includes those lncRNA located within one or two genes [4,13,29,30]. Based on the orientation, lncRNAs can be transcribed from either the same strand or antisense in a divergent or convergent manner. LncRNAs can be also classified as enhancer-associated RNAs (eRNAs) and promoter-associated long RNAs (or PROMPTs) if they are produced from enhancer or promoter regions, respectively [31].
Figure 1.
Positional classification of the long non-coding RNAs (lncRNA). Carton displays the LncRNA (red) classification base on (a) the location between two coding genes (intronic, exonic, intergenic, or overlapping), (b) the template strand (sense, antisense), and (c) transcription direction when coding genes and lncRNA are transcribed in the same strand (divergent, convergent). Gray arrow indicates in which direction transcription is proceed. Green and blue boxes represent exons of two different genes.
Although lncRNAs show a spatiotemporal expression pattern during proliferation, differentiation, and cell death; these genes are classified based on their function as guide, decoy, signaling, scaffold, or enhancer lncRNAs [32]. Guide lncRNAs interact with transcription factors or proteins and recruit them to their gene target or their genomic loci regulating downstream signaling events and gene expression. Decoy lncRNAs mimic and compete with their consensus DNA-binding motifs for binding nuclear receptors or transcriptional factors in the nucleus, facilitating gene activation or silencing. These genes can also “sponge” proteins such as chromatin modifiers, adding an extra level transcriptome regulation. Signaling lncRNAs are associated with signaling pathways to regulate transcription in response to various stimuli. Scaffold lncRNAs act as a central platform where many protein complexes tying and get directed to specific genomic loci or target gene promoter [17]. Enhancer lnRNAs are cis-encoded DNA elements that bind with mediator complex to regulate transcription genes located within their own chromosome (Table 1) [33]. However, this classification is too simple to cover the whole lncRNAome, cases such as pseudogenes and telomerase RNA (TERC) still lie outside the list [20,32].
Table 1.
Classification of lncRNAs according to their function.
| Functional Type | Cellular Location | Mechanism of Action | Examples | Reference |
|---|---|---|---|---|
| Guide | Nucleus | Essential for the proper localization of proteins to their site-specific reaction. | XIST, ANRIL | [34] |
| Decoys | Plasma membrane, nucleus and cytosol | Sequestering regulatory factors (transcription factors, catalytic proteins subunits, chromatin modifiers, etc.) to modulate transcription | GAS5, MALAT1 | [35,36,37] |
| Scaffold | Nucleus | Providing platforms for assembly of multiple-component complexes such as the polycomb repressive complexes and ribonucleoprotein complex. | CDKN2B-AS1, HOTAIR | [35,36] |
| Signaling | Nucleus | Serving as a molecular signal to regulate transcription in response to various stimuli | TP53COR1, PANDAR | [35,36] |
| Enhancer | Nucleus | Binding with mediator complex to enhance transcription | HOTTIP, CCAT1-L, LUNAR1 | [25,33] |
In terms of size, lincRNAs often range from hundreds of nucleotides to several kilobases [20]. Nevertheless, there are exceptionally long lncRNAs (macroRNAs) and very long intergenic non-coding RNAs (vlincRNAs), stretching 10 kb and 1 Mb, respectively [30].
In addition, lncRNAs have regulatory roles in gene expression at both, the transcriptional, and post-transcriptional levels in mostly biological mechanisms and pathophysiological processes. These molecules can regulate the expression of neighboring genes (cis) or affect genes located at different chromosomes (trans) [38]. In this way, lncRNAs can regulate gene expression via transcription factor and chromatin-modifiers complex recruitment to their DNA targets, acting as enhancers to activate genes, as part of the heterogeneous nuclear ribonucleoprotein (hnRNP) complex, interacting with RNA and DNA by base paring, etc. [38].
5. LncRNAs in Normal Hematopoiesis
Hematopoietic cell lineage differentiation involves the regulation of gene expression at different levels that can occur to activate lineage specific genes and repress those genes that are not specific to that lineage. This activation/suppression is mediated by transcription factors and chromatin remodeling that act as determinants of the intrinsic cell lineage. However, these factors are reactivated in different lines and stages of differentiation, so that the choice of the final lineage reflects the particular combination of elements interacting in a certain stage of cell differentiation [39]. LncRNAs are involved in regulating different steps in hematopoiesis, immune system development, and activation. In fact, several lncRNAs have been identified in the blood cells either in animal models or human samples. For example, over 1109 poliA+ lncRNAs were detected in murine megakaryocytes, erythroblast, and megakaryocyte-erythroid precursors, of which 15% are expressed in humans [40]. The Eosinophil Granule Ontogeny (EGO) was one of the first lncRNAs related with the human normal hematopoiesis process. EGO is nested within an intron of inositol triphosphate receptor type 1 (ITPR1) and was found to be highly expressed in human bone marrow and in mature eosinophils. Despite that the molecular mechanism of their actions is not well known, experimental evidences show that EGO is involved in the eosinophil differentiation of CD34+ hematopoietic progenitor cells by regulating eosinophil granule protein expression at the transcription level [41]. PU.1-As, which is antisense to the master hematopoietic transcriptional factor PU.1, negatively regulates the expression of PU.1, repressing myeloid cells and B cells differentiation [42]. Other examples include dendritic cell-specific lncRNA (lnc-DC), non-coding RNA repressor of NFAT (NRON), and lincRNA-Cox2. lnc-DC was identified from extensive profiling of lncRNAs expression during differentiation of monocytes into dendritic cells (DCs). Mechanistic studies suggest that lnc-DC contributes to prevent STAT3 (signal transducer and activator of transcription 3) dephosphorylation by Src homology region 2 domain-containing phosphatase-1 (SHP1) by directly binding to STAT in the cytoplasm [43]. NRON plays a relevant role in the adaptive immune response through sequestering transcription factors in the cytoplasm, such as the nuclear factor of activated T cells (NFAT). LincRNA-Cox2 contributes with the regulation of the innate immune response by repressing the expression of critical immune-response regulators and by the coordinating the assembly, location and orientation of the complexes that specify the cellular fate [39].
Studying twelve distinct blood cell population purified by multicolor flow cytometry, Schwarzer et al. [44] established a human ncRNA hematopoietic expression atlas per blood cell population, finding LINC00173, LINC000524, RP11-1029J19, and HOTAIRM1 among the lncRNAs that characterize cells of the different human blood lineages. LINC00173 exhibited the most specific expression, with critical regulatory circuits involved in blood homeostasis and myeloid differentiation. In vitro models showed that suppression of LINC00173 in human CD34+ hematopoietic stem and progenitor cells (HSPCs) specifically affects granulocyte differentiation and decreases its phagocytic capacity (which is associated with perturbed maturation). Additional studies reported that LINC00173 is highly expressed in granulocytes [45]. H19, XIST, lncHSC-1, and lncHSC-2, which maintain long-term hematopoietic stem cell (HSC) quiescence and self-renewal, have also been involved in normal hematopoiesis [46].
6. LncRNAs in Acute Leukemia
Although many studies have implicated lncRNAs in many cancer types, little is known about the functional impact of lncRNAs in AL etiology, progression, and treatment response [44]. Several lncRNAs have been reported to be exclusively involved in specific ALL lineages but few of these are abnormally expressed in ALL and AML [47,48]. For instance, CASC15, involved in cellular survival proliferation and the expression of SOX4 (cis regulation), was detected to be upregulated in t(12;21) (p13;q22) (ETV6/RUNX1) B cell ALL and in AML patients with the (8;21) translocation. In both cases, upregulation of CASC15 was associated with a good prognosis [48]. To date, a large number of lncRNAs have been identified in AL; however, their molecular mechanisms remains elusive. Table 2 includes some examples of lncRNAs which have been reported as implicated in acute leukemia in children [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
Table 2.
Examples of lncRNAs described in acute leukemia.
| LncRNAs | Classification | Function | Target Genes | Expression Level in Leukemia | Reference |
|---|---|---|---|---|---|
| Myeloblastic Leukemia | |||||
| IRAIN | Intronic | Intrachromosomal interactions | IGF1R | Downregulated in leukemia cell lines and in patients with high risk AML | [49] |
| UCA1 | Intergenic | Proliferation of AML cells. Oncofetal gene | CDKN1B | Upregulated | [50,51,52] |
| MEG3 | Intergenic | Tumor suppressor gene | P53 | Downregulated | [52,53] |
| RUNXOR | Sense | Chromosomal translocations | RUNX1 | Upregulated | [54] |
| NEAT1 | Intergenic | Myeloid differentiation cells | Unknown in AML | Downregulated | [50,52,55] |
| LLEST | Tumor suppressor | BCL-2 | Downregulated or even not expressed. | ||
| HOTAIRM1 | Antisense | Myeloid differentiation cells, autophagy mechanisms, chromatin remodeling and architecture | HOXA1, HOXA4, CD11b and CD18 | Upregulated | [52,56,57,58,59,60] |
| HOXA-AS2 | Antisense | Apoptotic repressor in NB4 promyelocytic leukemia cells | Unknown | Upregulated | [61] |
| PU.1-AS | Antisense | Involved in the translation of PU.1 | PU.1 | Downregulated | [62] |
| WT1-AS | Antisense | WT1 expression | WT1 | [63] | |
| EGO | Intronic | MBP and EDN expression | [41] | ||
| BGL3 | Intergenic | Apoptosis and DNA methylation | miR-17, miR-93, miR-20a, miR-20b, miR-106a and miR-106b | Upregulated | [50,52,64] |
| CCAT1 | Intergenic | Monocytic cell differentiation | miR-155 | [9,52,65] | |
| CCDC26 | Intergenic | AML cell proliferation | c-Kit | [66] | |
| HOTAIR | Intergenic | Apoptosis inhibitor | miR-193a and c-Kit | Upregulated | [67] |
| PVT1 | Intergenic | Proliferation of promyelocytes | MYC | Upregulated | [52,68] |
| ZNF571-AS1 | Antisense | Regulator of JAK/STAT signaling pathway | KIT and STAT5 | [69] | |
| Lymphoblastic Leukemia | |||||
| BALR-2 | Uncharacterized | Unknown | Unknown | Overexpressed in prednisone-resistant B-ALL patients | [70] |
| BALR-1 | Unknown | Unknown | Unknown | Upregulated | [70] |
| BARL-6 | Unknown | Promotes cell survival and inhibits apoptosis | Unknown | Upregulated | [70] |
| LINC00958 | Intergenic | Unknown | Unknown | Upregulated in t(12;21) preB cALL | [70,71] |
| DBH-AS1 | Antisense | Cell proliferation and cell survival | Unknown | Upregulated | |
| RP11-137H2.4 | Uncharacterized | Apoptosis, proliferation, cell migration | Unknown | Upregulated. Glucocorticoids resistance |
[72] |
| ANRIL | Antisense | Cellular proliferation and apoptosis | CDKN2A/B. CBX7, SUZ12 | Upregulated | [52] |
| T-ALL-R-LncR1 | Unknown | Promotor of the formation of Par-4/THAP1 protein complex, and the activity of caspase-3 | Unknown | Upregulated in children with T-ALL | [73] |
| LUNAR1 | Enhancer-like | Promotor of T-ALL proliferation by inducing IGF1R expression. | IGF1R | Downregulated | [50,52,74] |
| MALAT1 | Intergenic | Alternative splicing and epigenetic modification | Unknown | Upregulated Downregulated in vincristine-resistant ALL | [50,52,75,76,77] |
| CASC15 | Intergenic | Cellular survival and proliferation | SOX4 | Upregulated | [48] |
7. LncRNAs in Acute Myeloid Leukemia
Regarding the association between lncRNA and hematopoietic cancer, AML has been the most investigated, and has been reported to be an important lncRNA in the biological and pathological processes of the disease. For example, insulin-like growth factor type I receptor antisense imprinted non-protein RNA (IRAIN), which is transcribed antisense to insulin-like growth factor type I receptor (IGF1R) gene, is downregulated in leukemia cell lines and in patients with high-risk AML. IRAIN is involved in the formation of a long-range intrachromosomal interaction between the IGF1R promoter and a distant intragenic enhancer [49]. ZNF571-AS1 is another lncRNA that has been suggested as a relevant player in AML. Based on co-expression correlation analysis across all AML samples with lncRNA–lncRNA pairs, this lncRNA was identified as potential regulator of the Janus Kinase (JAK)/signal transducer and activator of transcription (STAT) 5A and tyrosine-protein kinase Kit (KIT) expression. Thus their participation in AML was suggested via the JAK/STAT signaling pathway [69]. As well, Urothelial carcinoma-associated 1 (UCA1), an oncofetal gene that has been involved in embryonic development and carcinogenesis, was found to be upregulated in myeloid cell lines promoting cell viability, migration, invasion, and apoptosis processes [78,79,80]. A significant upregulation of UCA1 expression in AML with CEBPA (a crucial component during myeloid differentiation) mutations and its relation with chemoresistance in pediatric AML was documented [51,81]. The maternally expressed 3 non-protein-coding gene (MEG3), a tumor suppressor, has also been associated with significantly reduced overall survival rate in AML patients. This gene is related to a variety of human tumors and data point out that directly enhance the anticancer effect through p53 [82,83]. Benetatos et al. [53] evaluated the aberrant promoter methylation of MEG3 in 42 AML patients, and found that MEG3 hypermethylation was present in 47.6% AML cases and might be associated with significantly reduced overall survival rate in these patients [53]. LncRNAs have also been profiled from AML patients cytogenetically normal (CN) and with specific translocation. For example, AML patients carrying NPM1, CEBPA, IDH2, ASXL1, and RUNX1 mutations and internal tandem duplication mutations in FLT3 (FLT3/ITD) gene exhibited specific lncRNA expression signature. As well, Diaz-Beya et al. [84], studying AML cases with t(15;17), t(8;21), inv(16), t(6;9), t(3;3), t(9;11), t(8;16), FLT3/ITD, and monosomal karyotype, found a specific lncRNA profile in t(15;17), t(6;9), and t(8;16) positive cases. That study also revealed a correlation between t(8;16) and linc-HOXA11, HOXA11-AS, HOTTIP, and NR_038120 expression, and suggested that GAT2 is an important transcription factor to these lncRNAs. Otherwise, lncRNAs expression correlated with treatment response and survival. One of the lncRNAs that is specifically upregulated in CN-AML cases with CEBPA mutation is the lncRNA UCA1 [85]. Taurine-upregulated gene 1 (TUG1) expression was reported to be associated with higher white blood cell counts, monosomal karyotype, FLT3/ITD mutation, and worse prognosis in AML adults. In vitro studies in AML cells indicates that TUG1 induces cell proliferation but suppressing cell apoptosis via targeting AURKA [86].
Schwarzer et al. [44] made a high-density reconstruction of the human coding and non-coding hematopoietic landscape to identify an ncRNA fingerprint associated with lineage specification, HSPC maintenance, and cellular differentiation. They define a core ncRNA stem cell signature in normal HSCs and AML blast, which can serve as a prognostic marker in a different cohort of AML patients and may pave the way for novel therapeutic interventions targeting the non-coding transcriptome [44].
8. LncRNAs in Acute Lymphoblastic Leukemia
Data regarding lncRNA playing a role in ALL are still scarce. One of the first clinicopathological correlations with lncRNA expression data in ALL was performed by Fernando et al. [70] who studied 160 children with B-ALL observing that BALR-2 correlates with overall survival and with response to prednisone. These authors also demonstrated a putative mechanism in regulating cell survival in B-ALL that it is downregulated by glucocorticoid receptor engagement, and that its downregulation results in the activation of the glucocorticoid receptor signaling pathway [70]. Loie et al. [71] also reports that lncRNA expression patterns can classify ALL disease by subtypes as well as protein-coding genes. In addition to lncRNA, BARL-2, which is also correlated with resistance to prednisone treatment, these authors found that lncRNAs BALR-1, BRL-6, and LINC0098 were overexpressed in pre-B ALL cases and that all of these genes correlated with cytogenetic abnormalities, disease subtypes, and survivals of B-ALL patients [71]. In that study, they also observed that diverse coding genes adjacent to several of those lncRNAs showed unique overexpression profile in ETV6/RUNX1 positive BCP-ALLS suggesting a possible cis regulatory relationship. Furthermore, Ghazavi et al. [47] identified an ETV6/RUNX1-specific lncRNA signature in a 64 children cohort and in 13 BCP-ALL cell lines. Five-hundred-and-ninty-six lncRNA transcripts (434 up- and 162 downregulated) showed significant differential expression between ETV6/RUNX1-positive BCP-ALL and other genetic BCP-ALL subclasses. However, 16 lncRNAs, of which 14 were upregulated and two were found downregulated, overlapped with the ETV6/RUNX1-specific lncRNA signature, including NKX2-3-1, lncRTN4R-1, lncGIP-1, lnc-LRP8-3, lnc-TCF12-2, lncC8ort4-1, lnc-C8orf4-2, lnc-TINAGL1-1, lnc-LSM11-4, and lnc-SARDH-1 (also known as DBH-AS1). Lnc-SARDH-1 is known to possess an oncogenic role promoting cell proliferation and cell survival through activation of MAPK signaling in the context of hepatocellular carcinoma [87]. Furthermore, the H3K27ac epigenetic mark (associated to enhancers) was found in nine loci of the rest of the lncRNAs and their adjacent coding genes, which, in addition to the finding of a unique expression signature of these coding genes in ETV6/RUNX1 pre-B ALL, suggests a cis interaction between the lncRNAs and their neighboring coding genes [47]. In another study, Ouimet et al. performed a whole transcriptome analysis in a 56 pre-B ALL children cohort finding five lncRNAs specifically overexpressed in pre-B ALL. These genes may have impact in cancer traits such a cell proliferation, migration, apoptosis and treatment response. Specifically, lncRNA RP11-137H2.4 had a considerable impact on apoptosis, proliferation, and cell migration and its silencing is sufficient to restore a NR3C1-independent cellular response to glucocorticoid (GC) in GC-resistant pre-B ALL cells, leading to GC-induced apoptosis [72]. Further to this study, Gioia et al. functionally characterized three lncRNAs—RP-11-624C23.1, RP11-203E8, and RP11-446E9—specifically repressed in pre-B ALL, restoring their expression in a pre-B ALL cell line. All the lncRNAs promoted tumor suppressor-like phenotypes: apoptosis induction in response to DNA damaging agents and a reduction in cell proliferation and migration [88]. Additionally, Garitano-Trojaola et al., while analyzing ALL samples and peripheral blood samples obtained from healthy donors, found 43 lncRNAs abnormally expressed in ALL. Linc-PINT was downregulated both in T- and B-ALL cases [89]. Studies in T-ALL cells found a significant difference in expression of LUNAR1 and lnc-FAM120AOS-1 between NOTCH1 wild type and mutant cases [68]. The use of bioinformatics tools identified that lnc-OAZ3-2:7—located near the RORC gene—was repressed in this leukemia subtype [90]. These studies suggest that lncRNAs might be utilized as diagnostic and prognostic markers in leukemia, but additional analyses are needed.
9. Future Outlooks: Potential Clinical Implications on LncRNAs in Acute Leukemia
It is suggested that more than 97% of the transcribed genome does not encode for proteins. The discovery of the biological role of these non-coding genes took place in 1990, when XIST was reported to be involved in X chromosome inactivation (XCI) and gene dosage compensation. Subsequently, HOTAIR was identified as a repressor of HOX family gene transcription [91]. Most recently, high-throughput expression analyses have been conducted to identify thousands of expressed lncRNA genes either in normal or tumor tissues, showing the potential of lncRNAs as biomarkers for different types of cancer [37,44,52].
Deciphering the molecular mechanisms involved in hematological malignancies addresses new routes to improve diagnosis, prognosis, and treatment of patients with leukemia. In fact, abnormal expression of specific lncRNAs have been reported to be associated with some clinicopathological parameters and molecular subtypes in AL. As example, BALR-1 and LINC0098 have been identified as correlating with poor overall survival and diminished response to prednisone treatment in B cell ALL cases [70,71]. Regarding AML, HOTAIR, IRAIN, and SNHG5 have been suggested as biomarkers for diagnosis [92]; meanwhile, UCA1 overexpression was associated with chemoresistance of pediatric cases [81]. SNHG5 upregulation, which was detected in bone marrow and plasma, was correlated with unfavorable cytogenetics and shorter overall patient survival and was suggested as an independent factor to predict prognosis in AML [93].
Notwithstanding, few of these genes have been replicated across cohorts, probably evidencing biases due to different sample collection and processing techniques, but also as a consequence of AL biological complexity, which is characterized by a wide range of interactions among coding and non-coding genome and spatiotemporal relationships. HOTAIR, a proliferation promotor of leukemic blast and leukemia stem cells [94], is one of the most consistently found in AL. A high-expression level defines a subgroup of AL patients with high white blood cell counts at the time of diagnosis and low survival rates [95,96]. Recently, HOTAIR high-expression was associated with acquired resistance to antileukemic drugs such as doxorubicin and immatinib [97,98], making this gene as a potential therapeutic target molecule that could contribute to solve a tremendous problem in leukemia chemotherapy, the drug-resistance. On the other hand, experimental data suggest that HOTAIR low-expression could be mediated by small interference RNA (siRNA), but still no evidences exist regarding its potential benefit in humans [98]. The development of new molecular strategies as CRISPR/Cas9 to edit the mutated genome or nanotechnology approaches to deliver drugs specifically to leukemia cells prognosticate high applicability of lncRNA as a target to develop new treatments to leukemia [99,100]. Additionally, the high specificity and feasible detection in tissues, serum, plasma, urine, and saliva of the lncRNAs led us to think that lncRNAs could be useful as signals of specific cellular states or read-outs of active cellular pathologies such as leukemia, being promising as predictive biomarkers and potential therapeutic targets in cancer [19].
There is no doubt of the role of lncRNAs in hematopoietic cell transformation, disease evolution, or drug resistance; nevertheless, due to the limited number of studies in hematological entities, these applications are still inconclusive. In fact, before their use as biomarkers in childhood AL, prospective and well-designed cohort studies with adequate sample sizes and further validation of the results in independent cohorts are needed to confirm their clinical usefulness. Therefore, translating this knowledge into the clinical practice still represents a big challenge.
10. Conclusions
At this time, we know that lncRNAs are playing a relevant role in cancer development, including leukemia. However, the knowledge regarding molecular mechanisms underlying the pathogenesis of these diseases remains limited. Massive parallel analysis techniques and, likewise, transcriptome expression analysis and RNA sequencing technologies are increasing the possibility to identify those lncRNAs potentially involved in the pathogenesis of AL and other hematopoietic malignancies. To date, large improvements of the surveillance of AL cases have been achieved; nevertheless, cases still die during the AL treatment. Thus, it is necessary to find suitable biomarkers for early diagnosis and accurate risk stratification in AL patients. The association of lncRNAs with several subtypes of leukemia, such as MEG3, IRAIN, and UCA1 related to AML and ANRIL, LUNAR1, in ALL, increase the possibility to use them as biomarkers for the diagnosis, prognosis, and treatment (to provide a target) for the different subtypes of this disease. In addition, further investigation of the function of aberrant expressed lncRNAs may help to understand the pathogenesis of hematological malignancies and provide an important insight in childhood leukemia therapy.
Abbreviations
| 1. ABL1 | 2. ABL protoconcogene 1 |
| 3. ABL2 | 4. ABL protooncogene 2 |
| 5. AL | 6. Acute leukemia |
| 7. ALL | 8. Acute lymphoblastic leukemia |
| 9. AML | 10. Acute myeloblastic leukemia |
| 11. ANRIL | 12. Antisense non-coding RNA in the INK4-ARF locus B-ALL B cell Acute lymphoblastic leukemia |
| 13. ARID1A | 14. AT-rich interaction domain 1A |
| 15. AURKA | 16. Aurora kinase A gene |
| 17. BALR | 18. B-ALL-associated long non-coding RNAs BL Burkitt Lymphoma |
| 19. CAS9 | 20. CRISPR associated protein 9 |
| 21. CBF | 22. Core-binding factor subunit beta |
| 23. CCAT1 | 24. Colon cancer associated transcript 1 ceRNA Competing endogenous RNA |
| 25. CDKN2A | 26. Cyclin dependent kinase inhibitor 2A |
| 27. CDKN2B | 28. Cyclin dependent kinase inhibitor 2B |
| 29. CDKN2B-AS1 | 30. CDKN2B antisense RNA 1 |
| 31. CEBPA | 32. CCAAT enhancer binding protein alpha |
| 33. CHD6 | 34. Chromodomain helicase DNA binding protein 6 |
| 35. circRNA | 36. Circular RNA |
| 37. CLL | 38. Chronic lymphocytic leukemia |
| 39. CML | 40. Chronic myeloblastic leukemia |
| 41. CN | 42. Cytogenetically normal |
| 43. COLDAIR | 44. COLD assisted intronic non-coding RNA |
| 45. CREBBP | 46. CREB binding protein |
| 47. CRISPR | 48. Clustered regularly interspaced short palindromic repeats |
| 49. CRLF2 | 50. Cytokine receptor like factor 2 |
| 51. CSF1R | 52. Colony stimulating factor 1 receptor |
| 53. DCs | 54. Dendritic Cells |
| 55. DNMT3A | 56. DNA methyltransferase 3α |
| 57. EBF1 | 58. Early B cell factor 1 |
| 59. EGO | 60. Eosinophil granule ontogeny |
| 61. EP300 | 62. E1A binding protein P300 |
| 63. eRNAs | 64. Enhancer RNAs |
| 65. ETS1 | 66. ETS proto-oncogene 1 transcription factor |
| 67. ETV6 | 68. ETS Variant6 |
| 69. FLC | 70. Flowering repressor locus |
| 71. FLT3 | 72. Fms related tyrosine kinase 3 |
| 73. fRNAs | 74. Functional RNAs |
| 75. GAS5 | 76. Growth specific 5 |
| 77. GEO | 78. Gene expression omnibus |
| 79. H19 | 80. Imprinted maternally expressed transcript |
| 81. hnRNP | 82. Heterogenous nuclear ribonucleoprotein |
| 83. HOTAIR | 84. The HOX transcript antisense intergenic RNA |
| 85. HOTTIP | 86. HOXA distal transcript antisense RNA |
| 87. IGFR1 | 88. Insuline-like growth factor type 1 |
| 89. IKZF1 | 90. IKAROS family zinc finger 1 |
| 91. IRAIN | 92. IGFR1 antisense imprinted non protein RNA |
| 93. ITPR1 | 94. Inositol1,4,5-triophosphate receptor type 1 |
| 95. JAK2 | 96. Janus kinase 2 |
| 97. KIT | 98. Tyrosine protein kinase |
| 99. LincRNA | 100. Long intergenic non-coding RNA |
| 101. LncRNA | 102. Long non-coding RNA |
| 103. lnc-DC | 104. Dendritic cell-specifict lncRNA |
| 105. lincRNA-p21 | 106. Large intergenic non-coding RNA p21 |
| 107. lncRNA | 108. Long non-coding RNA |
| 109. LUNAR1 | 110. Leukemia-associated non-coding IGF1R |
| 111. MALAT1 | 112. Metastasis associated lung adenocarcinoma transcript 1 MCL Mantle cell lymphoma |
| 113. MEG3 | 114. Maternally expressed 3 |
| 115. miRNA | 116. MicroRNA |
| 117. mRNA | 118. Messenger RNA |
| 119. NCBI | 120. National center of biotechnology information |
| 121. ncRNA | 122. Non-coding RNA |
| 123. NFAT | 124. Nuclear factor activated T cells |
| 125. nmRNA | 126. Non messengers RNA |
| 127. npcRNA | 128. Non protein-coding RNA |
| 129. NRAS | 130. NRAS proto-oncogene |
| 131. NRON | 132. Non-protein-coding RNA Repressor of NFAT |
| 133. NSD2 | 134. Nuclear receptor binding SET domain protein 2 |
| 135. PANDAR | 136. Promoter of CDKN1A antisense DNA damage activated RNA |
| 137. PAX5 | 138. Paired box 5 |
| 139. PBX1 | 140. PBX Homeobox 1 |
| 141. PCGP | 142. Pediatric cancer genome project |
| 143. PDGFRB | 144. Platelet derived growth factor receptor beta |
| 145. piRNAs | 146. PIWI-interacting RNAs |
| 147. PML | 148. Promyelocytic Leukemia gene |
| 149. PROMPTs | 150. Promoter-associated long RNAs |
| 151. RB1 | 152. RB transcriptional corepressor 1 |
| 153. RBPs | 154. RNA-binding proteins |
| 155. RUNX1 | 156. Runt related transcription factor 1 |
| 157. SHP1 | 158. Scr homology region 2 domain containing phosphatase-1 |
| 159. siRNA | 160. Small interference RNA |
| 161. snRNAs | 162. Small nuclear RNA |
| 163. snoRNAs | 164. Small nucleolar RNA |
| 165. STAT3 | 166. Signal transducer and activator of transcription 3 |
| 167. TCF3 | 168. Transcription Factor 3Ç |
| 169. TERC | 170. Telomerase RNA component |
| 171. TET2 | 172. Tet methylcytosine dioxygenase 2 |
| 173. TLR | 174. Tool-like receptor |
| 175. TP53 | 176. Tumor protein P53 |
| 177. TP53COR1 | 178. Tumor protein P53 pathway corepressor 1 |
| 179. TUG1 | 180. Taurine-up regulated gene 1 |
| 181. UCA1 | 182. Urothelial carcinoma associated 1 |
| 183. vlincRNA | 184. Very long intergenic RNA |
| 185. XIST | 186. X inactive specific transcript |
Author Contributions
G.M.C.-M. and S.J.-M. drafted the work. A.H.-M., D.A.B.-L., J.C.N.-E., J.R.-B., and J.M.M.-A. substantively revised the manuscript and contributed intellectually. S.J.-M. conceived the review. All authors read and approved the submitted version.
Funding
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT), grant numbers: Investigación en Fronteras de la Ciencia (IFC)-2016-01-2119, PDCPN2013-01-215726, SALUD-2010-1-141026, SALUD-2015-1-262190, FONCICYT/37/2018, and CB-2015-1-258042; and by the Instituto Mexicano del Seguro Social, grant numbers: FIS/IMSS/PROT/PRIO/11/017, FIS/IMSS/PROT/G12/1134, FIS/IMSS/PROT/PRIO/14/031, FIS/IMSS/PROT/PRIO/15/048, FIS/IMSS/PROT/MD15/1504, FIS/IMSS/PROT/G15/1477, FIS/IMSS/PROT/895, FIS/IMSS/PROT/1364, FIS/IMSS/PROT/1533, FIS/IMSS/PROT/1782 and FIS/IMSS/PROT/1548. Gabriela Marisol Cruz-Miranda was supported by CONACyT (Scholarship) 2018-000012-01NACF.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mejia-Arangure J.M., McNally R.J.Q. Acute Leukemia in Children. Biomed. Res. Int. 2015 doi: 10.1155/2015/787194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linet M.S., Brown L.M., Mbulaiteye S.M., Check D., Ostroumova E., Landgren A., Devesa S.S. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0–19 years. Int. J. Cancer. 2016;138:1862–1874. doi: 10.1002/ijc.29924. [DOI] [PubMed] [Google Scholar]
- 3.Schuz J., Erdmann F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016;47:607–614. doi: 10.1016/j.arcmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Beltran-Anaya F.O., Cedro-Tanda A., Hidalgo-Miranda A., Romero-Cordoba S.L. Insights into the Regulatory Role of Non-coding RNAs in Cancer Metabolism. Front. Physiol. 2016;7:342. doi: 10.3389/fphys.2016.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Saldivar M.L., Fajardo-Gutierrez A., Bernaldez-Rios R., Martinez-Avalos A., Medina-Sanson A., Espinosa-Hernandez L., Flores-Chapa J.D., Amador-Sanchez R., Penaloza-Gonzalez J.G., Alvarez-Rodriguez F.J., et al. Childhood acute leukemias are frequent in Mexico City: Descriptive epidemiology. BMC Cancer. 2011;11:355. doi: 10.1186/1471-2407-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright M., Bruford E.A. Naming ‘junk’: Human non-protein coding RNA (ncRNA) genome nomenclature. Hum. Genom. 2011;5:90–98. doi: 10.1186/1479-7364-5-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connelly C.M., Moon M.H., Schneekloth J.S. The Emerging Role of RNA as a Therapeutic Target for Small Molecules. Cell Chem. Biol. 2016;23:1077–1090. doi: 10.1016/j.chembiol.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaves M. In utero origins of childhood leukaemia. Early Hum. Dev. 2005;81:123–129. doi: 10.1016/j.earlhumdev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Mullighan C.G. Genomic profiling of B-progenitor acute lymphoblastic leukemia. Best Pract. Res. Clin. Haematol. 2011;24:489–503. doi: 10.1016/j.beha.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janczar S., Janczar K., Pastorczak A., Harb H., Paige A.J.W., Zalewska-Szewczyk B., Danilewicz M., Mlynarski W. The Role of Histone Protein Modifications and Mutations in Histone Modifiers in Pediatric B-Cell Progenitor Acute Lymphoblastic Leukemia. Cancers. 2017;9:2. doi: 10.3390/cancers9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts K.G., Gu Z.H., Payne-Turner D., McCastlain K., Harvey R.C., Chen I.M., Pei D.Q., Iacobucci I., Valentine M., Pounds S.B., et al. High Frequency and Poor Outcome of Philadelphia Chromosome-Like Acute Lymphoblastic Leukemia in Adults. J. Clin. Oncol. 2017;35:394. doi: 10.1200/JCO.2016.69.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordlund J., Kiialainen A., Karlberg O., Berglund E.C., Goransson-Kultima H., Sonderkaer M., Nielsen K.L., Gustafsson M.G., Behrendtz M., Forestier E., et al. Digital gene expression profiling of primary acute lymphoblastic leukemia cells. Leukemia. 2012;26:1218–1227. doi: 10.1038/leu.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson A.K., Ma J., Wang J.M., Chen X., Gedman A.L., Dang J.J., Nakitandwe J., Holmfeldt L., Parker M., Easton J., et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat. Genet. 2015;47:330–337. doi: 10.1038/ng.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X.H., Rastogi P., Shah B., Zhang L. B lymphoblastic leukemia/lymphoma: New insights into genetics, molecular aberrations, subclassification and targeted therapy. Oncotarget. 2017;8:66728–66741. doi: 10.18632/oncotarget.19271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziz H., Ping C.Y., Alias H., Ab Mutalib N.S., Jamal R. Gene Mutations as Emerging Biomarkers and Therapeutic Targets for Relapsed Acute Myeloid Leukemia. Front. Pharmacol. 2017;8:897. doi: 10.3389/fphar.2017.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponting C.P., Oliver P.L., Reik W. Evolution and Functions of Long Noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang X.T., Song X.Y., Glass C.K., Rosenfeld M.G. The Long Arm of Long Noncoding RNAs: Roles as Sensors Regulating Gene Transcriptional Programs. Cold Spring Harb. Perspect. Biol. 2011;3:a003756. doi: 10.1101/cshperspect.a003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi P., Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod. Pathol. 2013;26:155–165. doi: 10.1038/modpathol.2012.160. [DOI] [PubMed] [Google Scholar]
- 19.Kapranov P., St Laurent G., Raz T., Ozsolak F., Reynolds C.P., Sorensen P.H.B., Reaman G., Milos P., Arceci R.J., Thompson J.F., et al. The majority of total nuclear-encoded non-ribosomal RNA in a human cell is ‘dark matter’ un-annotated RNA. BMC Biol. 2010;8:149. doi: 10.1186/1741-7007-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapranov P., Cheng J., Dike S., Nix D.A., Duttagupta R., Willingham A.T., Stadler P.F., Hertel J., Hackermuller J., Hofacker I.L., et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 22.Dieci G., Fiorino G., Castelnuovo M., Teichmann M., Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23:614–622. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Yin Q.F., Yang L., Zhang Y., Xiang J.F., Wu Y.W., Carmichael G.G., Chen L.L. Long Noncoding RNAs with snoRNA Ends. Mol. Cell. 2012;48:219–230. doi: 10.1016/j.molcel.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Hung T., Wang Y.L., Lin M.F., Koegel A.K., Kotake Y., Grant G.D., Horlings H.M., Shah N., Umbricht C., Wang P., et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orom U.A., Derrien T., Beringer M., Gumireddy K., Gardini A., Bussotti G., Lai F., Zytnicki M., Notredame C., Huang Q.H., et al. Long Noncoding RNAs with Enhancer-like Function in Human Cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salzman J., Gawad C., Wang P.L., Lacayo N., Brown P.O. Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L., Duff M.O., Graveley B.R., Carmichael G.G., Chen L.L. Genomewide characterization of non-polyadenylated RNAs. Genome Biol. 2011;12:R16. doi: 10.1186/gb-2011-12-2-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St Laurent G., Wahlestedt C., Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han D., Wang M., Ma N., Xu Y., Jiang Y.T., Gao X. Long noncoding RNAs: Novel players in colorectal cancer. Cancer Lett. 2015;361:13–21. doi: 10.1016/j.canlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Di Gesualdo F., Capaccioli S., Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget. 2014;5:10976–10996. doi: 10.18632/oncotarget.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morlando M., Ballarino M., Fatica A. Long Non-Coding RNAs: New Players in Hematopoiesis and Leukemia. Front. Med. 2015;2:23. doi: 10.3389/fmed.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulitsky I., Bartel D.P. lincRNAs: Genomics, Evolution, and Mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinn J.J., Chang H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016;17:47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 34.Jeon Y., Lee J.T. YY1 Tethers Xist RNA to the Inactive X Nucleation Center. Cell. 2011;146:119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder J., Frankild S., Tsafou K., Stolte C., O’Donoghue S., Schneider R., Jensen L.J. COMPARTMENTS. [(accessed on 11 July 2018)]; Available online: https://compartments.jensenlab.org/Search.
- 37.Fang K., Han B.W., Chen Z.H., Lin K.Y., Zeng C.W., Li X.J., Li J.H., Luo X.Q., Chen Y.Q. A distinct set of long non-coding RNAs in childhood MLL-rearranged acute lymphoblastic leukemia: Biology and epigenetic target. Hum. Mol. Genet. 2014;23:3278–3288. doi: 10.1093/hmg/ddu040. [DOI] [PubMed] [Google Scholar]
- 38.Chen L.L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016;41:761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Xia F., Dong F.L., Yang Y., Huang A.F., Chen S., Sun D., Xiong S.D., Zhang J.P. Dynamic Transcription of Long Non-Coding RNA Genes during CD4+ T Cell Development and Activation. PLoS ONE. 2014;9:e101588. doi: 10.1371/journal.pone.0101588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paralkar V.R., Mishra T., Luan J., Yao Y., Kossenkov A.V., Anderson S.M., Dunagin M., Pimkin M., Gore M., Sun D., et al. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood. 2014;123:1927–1937. doi: 10.1182/blood-2013-12-544494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner L.A., Christensen C.J., Dunn D.M., Spangrude G.J., Georgelas A., Kelley L., Esplin M.S., Weiss R.B., Gleich G.J. EGO, a novel, noncoding RNA gene, regulates eosinophil granule protein transcript expression. Blood. 2007;109:5191–5198. doi: 10.1182/blood-2006-06-027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imperato M.R., Cauchy P., Obier N., Bonifer C. The RUNX1-PU.1 axis in the control of hematopoiesis. Int. J. Hematol. 2015;101:319–329. doi: 10.1007/s12185-015-1762-8. [DOI] [PubMed] [Google Scholar]
- 43.Wang P., Xue Y.Q., Han Y.M., Lin L., Wu C., Xu S., Jiang Z.P., Xu J.F., Liu Q.Y., Cao X.T. The STAT3-Binding Long Noncoding RNA lnc-DC Controls Human Dendritic Cell Differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 44.Schwarzer A., Emmrich S., Schmidt F., Beck D., Ng M., Reimer C., Adams F.F., Grasedieck S., Witte D., Kabler S., et al. The non-coding RNA landscape of human hematopoiesis and leukemia. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hon C.C., Ramilowski J.A., Harshbarger J., Bertin N., Rackham O.J., Gough J., Denisenko E., Schmeier S., Poulsen T.M., Severin J., et al. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berg J.S., Lin K.K., Sonnet C., Boles N.C., Weksberg D.C., Nguyen H., Holt L.J., Rickwood D., Daly R.J., Goodell M.A. Imprinted Genes That Regulate Early Mammalian Growth Are Coexpressed in Somatic Stem Cells. PLoS ONE. 2011;6:e26410. doi: 10.1371/journal.pone.0026410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghazavi F., De Moerloose B., Van Loocke W., Wallaert A., Helsmoortel H.H., Ferster A., Bakkus M., Plat G., Delabesse E., Uyttebroeck A., et al. Unique long non-coding RNA expression signature in ETV6/RUNX1-driven B-cell precursor acute lymphoblastic leukemia. Oncotarget. 2016;7:73769–73780. doi: 10.18632/oncotarget.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernando T.R., Contreras J.R., Zampini M., Rodriguez-Malave N.I., Alberti M.O., Anguiano J., Tran T.M., Palanichamy J.K., Gajeton J., Ung N.M., et al. The lncRNA CASC15 regulates SOX4 expression in RUNX1-rearranged acute leukemia. Mol. Cancer. 2017;16:126. doi: 10.1186/s12943-017-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J.N., Li W., Sun Y.P., Yu D.H., Wen X., Wang H., Cui J.W., Wang G.J., Hoffman A.R., Hu J.F. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014;42:9588–9601. doi: 10.1093/nar/gku549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S.Y., Liang H.R., Yang H., Zhou K.R., Xu L.M., Liu J.X., Lai B., Song L., Luo H., Peng J.M., et al. Long non-coding RNAs: The novel diagnostic biomarkers for leukemia. Environ. Toxicol. Pharmacol. 2017;55:81–86. doi: 10.1016/j.etap.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Hughes J.M., Legnini I., Salvatori B., Masciarelli S., Marchioni M., Fazi F., Morlando M., Bozzoni I., Fatica A. C/EBPα-p30 protein induces expression of the oncogenic long non-coding RNA UCA1 in acute myeloid leukemia. Oncotarget. 2015;6:18534–18544. doi: 10.18632/oncotarget.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhan A., Soleimani M., Mandal S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benetatos L., Hatzimichael E., Dasoula A., Dranitsaris G., Tsiara S., Syrrou M., Georgiou I., Bourantas K.L. CpG methylation analysis of the MEG3 and SNRPN imprinted genes in acute myeloid leukemia and myelodysplastic syndromes. Leuk. Res. 2010;34:148–153. doi: 10.1016/j.leukres.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Wang H., Li W., Guo R., Sun J., Cui J., Wang G., Hoffman A.R., Hu J.F. An intragenic long noncoding RNA interacts epigenetically with the RUNX1 promoter and enhancer chromatin DNA in hematopoietic malignancies. Int. J. Cancer. 2014;135:2783–2794. doi: 10.1002/ijc.28922. [DOI] [PubMed] [Google Scholar]
- 55.Zeng C., Xu Y., Xu L., Yu X., Cheng J., Yang L., Chen S., Li Y. Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer. 2014;14:693. doi: 10.1186/1471-2407-14-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X., Lian Z., Padden C., Gerstein M.B., Rozowsky J., Snyder M., Gingeras T.R., Kapranov P., Weissman S.M., Newburger P.E. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood. 2009;113:2526–2534. doi: 10.1182/blood-2008-06-162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X., Weissman S.M., Newburger P.E. Long intergenic non-coding RNA HOTAIRM1 regulates cell cycle progression during myeloid maturation in NB4 human promyelocytic leukemia cells. RNA Biol. 2014;11:777–787. doi: 10.4161/rna.28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Z.H., Wang W.T., Huang W., Fang K., Sun Y.M., Liu S.R., Luo X.Q., Chen Y.Q. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017;24:212–224. doi: 10.1038/cdd.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X.Q., Dostie J. Reciprocal regulation of chromatin state and architecture by HOTAIRM1 contributes to temporal collinear HOXA gene activation. Nucleic Acids Res. 2017;45:1091–1104. doi: 10.1093/nar/gkw966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Díaz-Beyá M., Brunet S., Nomdedéu J., Pratcorona M., Cordeiro A., Gallardo D., Escoda L., Tormo M., Heras I., Ribera J.M., et al. The lincRNA HOTAIRM1, located in the HOXA genomic region, is expressed in acute myeloid leukemia, impacts prognosis in patients in the intermediate-risk cytogenetic category, and is associated with a distinctive microRNA signature. Oncotarget. 2015;6:31613–31627. doi: 10.18632/oncotarget.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H., Zhang X., Frazão J.B., Condino-Neto A., Newburger P.E. HOX antisense lincRNA HOXA-AS2 is an apoptosis repressor in all trans retinoic acid treated NB4 promyelocytic leukemia cells. J. Cell. Biochem. 2013;114:2375–2383. doi: 10.1002/jcb.24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebralidze A.K., Guibal F.C., Steidl U., Zhang P., Lee S., Bartholdy B., Jorda M.A., Petkova V., Rosenbauer F., Huang G., et al. PU.1 expression is modulated by the balance of functional sense and antisense RNAs regulated by a shared cis-regulatory element. Genes Dev. 2008;22:2085–2092. doi: 10.1101/gad.1654808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarty G., Loeb D.M. Hypoxia-sensitive epigenetic regulation of an antisense-oriented lncRNA controls WT1 expression in myeloid leukemia cells. PLoS ONE. 2015;10:e0119837. doi: 10.1371/journal.pone.0119837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo G., Kang Q., Zhu X., Chen Q., Wang X., Chen Y., Ouyang J., Zhang L., Tan H., Chen R., et al. A long noncoding RNA critically regulates Bcr-Abl-mediated cellular transformation by acting as a competitive endogenous RNA. Oncogene. 2015;34:1768–1779. doi: 10.1038/onc.2014.131. [DOI] [PubMed] [Google Scholar]
- 65.Chen L., Wang W., Cao L., Li Z., Wang X. Long Non-Coding RNA CCAT1 Acts as a Competing Endogenous RNA to Regulate Cell Growth and Differentiation in Acute Myeloid Leukemia. Mol. Cells. 2016;39:330–336. doi: 10.14348/molcells.2016.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirano T., Yoshikawa R., Harada H., Harada Y., Ishida A., Yamazaki T. Long noncoding RNA, CCDC26, controls myeloid leukemia cell growth through regulation of KIT expression. Mol. Cancer. 2015;14:90. doi: 10.1186/s12943-015-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xing C.Y., Hu X.Q., Xie F.Y., Yu Z.J., Li H.Y., Bin-Zhou, Wu J.B., Tang L.Y., Gao S.M. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589:1981–1987. doi: 10.1016/j.febslet.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 68.Zeng C., Yu X., Lai J., Yang L., Chen S., Li Y. Overexpression of the long non-coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J. Hematol. Oncol. 2015;8:126. doi: 10.1186/s13045-015-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan J.Q., Zhang Y.Q., Wang J.H., Xu P., Wang W. lncRNA co-expression network model for the prognostic analysis of acute myeloid leukemia. Int. J. Mol. Med. 2017;39:663–671. doi: 10.3892/ijmm.2017.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernando T.R., Rodriguez-Malave N.I., Waters E.V., Yan W.H., Casero D., Basso G., Pigazzi M., Rao D.S. LncRNA Expression Discriminates Karyotype and Predicts Survival in B-Lymphoblastic Leukemia. Mol. Cancer Res. 2015;13:839–851. doi: 10.1158/1541-7786.MCR-15-0006-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lajoie M., Drouin S., Caron M., St-Onge P., Ouimet M., Gioia R., Lafond M.H., Vidal R., Richer C., Oualkacha K., et al. Specific expression of novel long non-coding RNAs in high-hyperdiploid childhood acute lymphoblastic leukemia. PLoS ONE. 2017;12:e174124. doi: 10.1371/journal.pone.0174124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ouimet M., Drouin S., Lajoie M., Caron M., St-Onge P., Gioia R., Richer C., Sinnett D. A childhood acute lymphoblastic leukemia-specific lncRNA implicated in prednisolone resistance, cell proliferation, and migration. Oncotarget. 2017;8:7477–7488. doi: 10.18632/oncotarget.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L., Xu H.G., Lu C. A novel long non-coding RNA T-ALL-R-LncR1 knockdown and Par-4 cooperate to induce cellular apoptosis in T-cell acute lymphoblastic leukemia cells. Leuk. Lymphoma. 2014;55:1373–1382. doi: 10.3109/10428194.2013.829574. [DOI] [PubMed] [Google Scholar]
- 74.Melo C.P.D., Campos C.B., Rodrigues J.D., Aguirre-Neto J.C., Atalla A., Pianovski M.A.D., Carbone E.K., Lares L.B.Q., Moraes-Souza H., Octacilio-Silva S., et al. Long non-coding RNAs: Biomarkers for acute leukaemia subtypes. Br. J. Haematol. 2016;173:318–320. doi: 10.1111/bjh.13588. [DOI] [PubMed] [Google Scholar]
- 75.Romero-Barrios N., Legascue M.F., Benhamed M., Ariel F., Crespi M. Splicing regulation by long noncoding RNAs. Nucleic Acids Res. 2018;46:2169–2184. doi: 10.1093/nar/gky095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akbari Moqadam F., Lange-Turenhout E.A., Ariës I.M., Pieters R., Den Boer M.L. MiR-125b, miR-100 and miR-99a co-regulate vincristine resistance in childhood acute lymphoblastic leukemia. Leuk. Res. 2013;37:1315–1321. doi: 10.1016/j.leukres.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X., Hamblin M.H., Yin K.J. The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biol. 2017;14:1705–1714. doi: 10.1080/15476286.2017.1358347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan Y., Shen B., Tan M., Mu X., Qin Y., Zhang F., Liu Y. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281:1750–1758. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 79.Han Y., Yang Y.N., Yuan H.H., Zhang T.T., Sui H., Wei X.L., Liu L., Huang P., Zhang W.J., Bai Y.X. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46:396–401. doi: 10.1097/PAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 80.Sun M.D., Zheng Y.Q., Wang L.P., Zhao H.T., Yang S. Long noncoding RNA UCA1 promotes cell proliferation, migration and invasion of human leukemia cells via sponging miR-126. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2233–2245. doi: 10.26355/eurrev_201804_14809. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y., Liu Y., Xu X. Knockdown of LncRNA-UCA1 suppresses chemoresistance of pediatric AML by inhibiting glycolysis through the microRNA-125a/hexokinase 2 pathway. J. Cell. Biochem. 2018;119:6296–6308. doi: 10.1002/jcb.26899. [DOI] [PubMed] [Google Scholar]
- 82.Miyoshi N., Wagatsuma H., Wakana S., Shiroishi T., Nomura M., Aisaka K., Kohda T., Surani M.A., Kaneko-Ishino T., Ishino F. Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells. 2000;5:211–220. doi: 10.1046/j.1365-2443.2000.00320.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang P., Ren Z., Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J. Cell. Biochem. 2012;113:1868–1874. doi: 10.1002/jcb.24055. [DOI] [PubMed] [Google Scholar]
- 84.Diaz-Beya M., Navarro A., Cordeiro A., Pratcorona M., Castellano J., Torrente M.A., Nomdedeu M., Risueño R., Rozman M., Monzo M., et al. Exploring the Expression Profile of Long Non-Coding RNA (lncRNA) in Different Acute Myeloid Leukemia (AML) Subtypes: t(8;16)(p11;p13)/MYST3-Crebbp AML Harbors a Distinctive LncRNA Signature. Blood. 2015;126:1397. [Google Scholar]
- 85.Garzon R., Volinia S., Papaioannou D., Nicolet D., Kohlschmidt J., Yan P.S., Mrozek K., Bucci D., Carroll A.J., Baer M.R., et al. Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2014;111:18679–18684. doi: 10.1073/pnas.1422050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X., Zhang L., Zhao F., Xu R., Jiang J., Zhang C., Liu H., Huang H. Long non-coding RNA taurine-upregulated gene 1 correlates with poor prognosis, induces cell proliferation, and represses cell apoptosis via targeting aurora kinase A in adult acute myeloid leukemia. Ann. Hematol. 2018;97:1375–1389. doi: 10.1007/s00277-018-3315-8. [DOI] [PubMed] [Google Scholar]
- 87.Huang J.L., Ren T.Y., Cao S.W., Zheng S.H., Hu X.M., Hu Y.W., Lin L., Chen J., Zheng L., Wang Q. HBx-related long non-coding RNA DBH-AS1 promotes cell proliferation and survival by activating MAPK signaling in hepatocellular carcinoma. Oncotarget. 2015;6:33791–33804. doi: 10.18632/oncotarget.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gioia R., Drouin S., Ouimet M., Caron M., St-Onge P., Richer C., Sinnett D. LncRNAs downregulated in childhood acute lymphoblastic leukemia modulate apoptosis, cell migration, and DNA damage response. Oncotarget. 2017;8:80645–80650. doi: 10.18632/oncotarget.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garitano-Trojaola A., José-Enériz E.S., Ezponda T., Unfried J.P., Carrasco-León A., Razquin N., Barriocanal M., Vilas-Zornoza A., Sangro B., Segura V., et al. Deregulation of linc-PINT in acute lymphoblastic leukemia is implicated in abnormal proliferation of leukemic cells. Oncotarget. 2018;9:12842–12852. doi: 10.18632/oncotarget.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ngoc P.C.T., Tan S.H., Tan T.K., Chan M.M., Li Z., Yeoh A.E.J., Tenen D.G., Sanda T. Identification of novel lncRNAs regulated by the TAL1 complex in T-cell acute lymphoblastic leukemia. Leukemia. 2018;32:2138–2151. doi: 10.1038/s41375-018-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ransohoff J.D., Wei Y.N., Khavari P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018;19:143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sayad A., Hajifathali A., Hamidieh A.A., Roshandel E., Taheri M. HOTAIR Long Noncoding RNA is not a Biomarker for Acute Myeloid Leukemia (AML) in Iranian Patients. Asian Pac. J. Cancer Prev. 2017;18:1581–1584. doi: 10.22034/APJCP.2017.18.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J., Sun C.K. Long noncoding RNA SNHG5 is up-regulated and serves as a potential prognostic biomarker in acute myeloid leukemia. Eur. Rev. Med. Pharmacol. Sci. 2018;22:3342–3347. doi: 10.26355/eurrev_201806_15154. [DOI] [PubMed] [Google Scholar]
- 94.Gao S., Zhou B., Li H., Huang X., Wu Y., Xing C., Yu X., Ji Y. Long noncoding RNA HOTAIR promotes the self-renewal of leukemia stem cells through epigenetic silencing of p15. Exp. Hematol. 2018 doi: 10.1016/j.exphem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 95.Wu S.H., Zheng C.P., Chen S.Y., Cai X.P., Shi Y.J., Lin B.J., Chen Y.M. Overexpression of long non-coding RNA HOTAIR predicts a poor prognosis in patients with acute myeloid leukemia. Oncol. Lett. 2015;10:2410–2414. doi: 10.3892/ol.2015.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y.Y., Huang S.H., Zhou H.R., Chen C.J., Tian L.H., Shen J.Z. Role of HOTAIR in the diagnosis and prognosis of acute leukemia. Oncol. Rep. 2016;36:3113–3122. doi: 10.3892/or.2016.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H., Li Q., Tang S., Li M., Feng A., Qin L., Liu Z., Wang X. The role of long noncoding RNA HOTAIR in the acquired multidrug resistance to imatinib in chronic myeloid leukemia cells. Hematology. 2017;22:208–216. doi: 10.1080/10245332.2016.1258152. [DOI] [PubMed] [Google Scholar]
- 98.Shang C., Guo Y., Zhang H., Xue Y.X. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother. Pharmacol. 2016;77:507–513. doi: 10.1007/s00280-016-2964-3. [DOI] [PubMed] [Google Scholar]
- 99.Tabassum N., Verma V., Kumar M., Kumar A., Singh B. Nanomedicine in cancer stem cell therapy: From fringe to forefront. Cell Tissue Res. 2018 doi: 10.1007/s00441-018-2928-5. [DOI] [PubMed] [Google Scholar]
- 100.Sakuma T., Yamamoto T. Acceleration of cancer science with genome editing and related technologies. Cancer Sci. 2018 doi: 10.1111/cas.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]