Abstract
The fibroblast growth factor (FGF) signaling pathway plays a key role in tumorigenesis and is recognized as a potential therapeutic target. In this study, the authors aimed to assess the impact of serum FGF23 levels in the prognosis of patients with cancer and bone metastases from solid tumors. A cohort of 112 patients with cancer and metastatic bone disease were treated with bone-targeted agents (BTA). Serum baseline FGF23 was quantified by ELISA and dichotomized in FGF23high and FGF23low groups. Additionally, the association between FGF23 and overall survival (OS) and time to skeletal-related events (TTSRE) was investigated. Baseline characteristics were balanced between groups, except for the median urinary N-terminal telopeptide (uNTX) level. After a median follow-up of 26.0 months, a median OS of 34.4 and 12.2 months was found in the FGF23low and FGF23high groups, respectively (multivariate HR 0.18, 95% CI 0.07–0.44, p = 0.001; univariate HR 0.27, p = 0.001). Additionally, TTSRE was significantly longer for patients with FGF23low (13.0 vs. 2.0 months, p = 0.04). Overall, this study found that patients with FGF23low at baseline had longer OS and TTSRE. Further studies are warranted to define its role as a prognostic biomarker and in the use of drugs targeting the FGF axis.
Keywords: FGF23, bone metastases, cancer, prognosis
1. Introduction
The fibroblast growth factor (FGF) protein family includes 22 members with diverse functions [1]. FGF23 is an approximately 32 kDa (251 amino acid) protein with the canonical N-terminal FGF homology domain and a novel 71 amino acid C-terminus [2], which acts as a phosphaturic factor and suppressor of 1α-hydroxylase activity in the kidney [3]. Although FGF23 is additionally present in other biologic structures, it is predominately expressed in osteocytes, which is the most abundant cells in bone [4]. Previous insights on FGF23 function have been mostly derived from mouse models, in which both administration of recombinant FGF23 and implantation of FGF23-overexpressing cells result in phosphaturia and hypophosphatemia [3]. Transgenic mice overexpressing FGF23 have decreased urinary phosphate reabsorption, hypophosphatemia, low serum 1,25-dihydroxyvitamin D [1,25(OH)2D] levels, and hyperparathyroidism [5,6].
FGF23 can bind to target cells via an FGF receptor (FGFR)—probably FGFR1—and its downstream signaling then requires a Klotho co-receptor [7]. Upon FGFR activation, type II sodium-phosphate cotransporters (NaPi-2a and NaPi-2c) in the kidney proximal tubule are down-regulated, leading to renal phosphate excretion [8]. Evidence suggests that the phosphaturic action of FGF23 is, to some extent, parathyroid hormone (PTH)-dependent. Subjects with hypoparathyroidism, typically with very low or undetectable PTH levels, have high serum phosphorus and FGF23, consistently with PTH requirements for a full phosphaturic FGF23 effect [9]. In addition to its action on NaPi-2a and NaPi-2c, FGF23 also regulates 1,25-vitamin D, by downregulating 1α-hydroxylase and up-regulating 24-hydroxylase, resulting in decreased 1,25-dihydroxy vitamin D [10].
Compelling evidence from human tumor sample analyses, in vitro studies, and animal models suggest that FGFRs are also relevant in cancer initiation and progression [10]. Due to its role in regulation of cell proliferation, migration, chemotaxis, morphogenesis, and angiogenesis, aberrant FGF signaling plays an important role in cancer development, tissue repair, and tumorigenesis. FGF23 is overexpressed in prostate cancer tissues and studies in prostate cancer cell lines have shown that it acts as an autocrine, paracrine, and/or endocrine growth factor [10]. Whereas exogenous FGF23 enhanced proliferation, invasion, and anchorage-independent growth in vitro, FGF23 knockdown decreased these phenotypes.
The role of FGF23 in a bone metastases-eliciting microenvironment remains unclear. Furthermore, the association between serum FGF23 and clinical outcomes in patients treated with bone-targeted agents (BTAs; i.e., bisphosphonates and denosumab) to prevent skeletal-related events (SREs) has not been described to date.
In the present study, the authors hypothesize that high levels of circulating FGF23 could negatively contribute to the prognosis of cancer patients with bone metastases, due to FGF23’s ability to promote cancer progression. Therefore, the primary objective of the study was to determine if FGF23 impacts the prognosis of cancer patients with bone metastases, by testing the association between FGF23 serum levels with overall survival (OS). The secondary objective was to investigate a possible association between serum FGF23 levels and time to skeletal-related events (TTSRE).
2. Results
2.1. Baseline Characteristics
Full data about patients’ characteristics at baseline was available for 103 patients. For the entire cohort, median (interquartile range [IQR]) FGF23 level was 32.09 (19.77–50.72) pg/mL and mean (standard deviation [SD]) FGF23 was 38.17 ± 26.15 pg/mL. When dichotomized into low and high categories, mean FGF23 was 26.15 pg/mL and 38.16 pg/mL, respectively. Overall, 19/112 (16.8%) patients were classified as FGF23high.
Baseline characteristics were balanced between groups (Table 1), except for median urinary N-terminal telopeptide (uNTX) levels, which were higher in the FGF23high group (824.30 vs. 118.02 nmol bone collagen equivalents [BCE]/mmol creatinine, p = 0.040). Median time from beginning of BTA therapy was similar between groups (1.28 vs. 1.10 months, p = 0.161).
Table 1.
Baseline characteristics of the cohort included.
| Characteristic | FGF23low (n = 94) | FGF23high (n = 18) | p |
|---|---|---|---|
| Age, years | |||
| Average ± SD | 57.4 ± 14.1 | 57.9 ± 5.6 | 0.360 |
| Median (IQR) | 57.5 (45.9–68.3) | 58.9 (56.2–61.9) | |
| Tumor type, n (%) | |||
| Breast | 58 (65.2) | 8 (53.3) | 0.096 |
| Prostate | 16 (18.0) | 1 (6.7) | |
| Others (Renal Cell Carcinoma—9; Biliary adenocarcinoma—1; Gastric adenocarcinoma—1; Sarcoma—3; Urothelial—4; Lung adenocarcinoma—1; Neuroendocrine—1; Cervix squamous carcinoma—1) | 15 (16.9) | 6 (40.0) | |
| Extraskeletal metastases, n (%) | |||
| Yes | 51 (60.0) | 12 (80.0) | 0.161 |
| No | 34 (40.0) | 3 (20.0) | |
| Time to BTA, median months | 1.10 | 1.28 | 0.161 |
| uNTX, nmol BCE/mmol creatinine | |||
| Median (IQR) | 118.0 | 824.3 | 0.040 |
| NTXhigh, n (%) | 45 (52.9) | 6 (66.7) | 0.501 |
| NTXlow, n (%) | 40 (47.1) | 3 (33.3) | |
| Calcium, n (%) | |||
| Hypercalcemia | 15 (17.0) | 6 (37.5) | 0.113 |
| Normal range | 66 (75.0) | 10 (62.5) | |
| Hypocalcemia | 7 (8.0) | 0 (0.0) | |
| Median (IQR) | 9.7 (9.4–10.1) | 10.5 (9.9–11.05) | 0.883 |
SD: standard deviation; IQR: interquartile range; BTA: bone-targeted agents; uNTX: urinary N-terminal telopeptide; BCE: bone collagen equivalents; FGF: fibroblast growth factor.
2.2. Survival Analysis
After a median (IQR) follow-up of 26.0 (13.0−47.0) months, 93 deaths (83.0%) were registered; 11 patients were lost to follow-up.
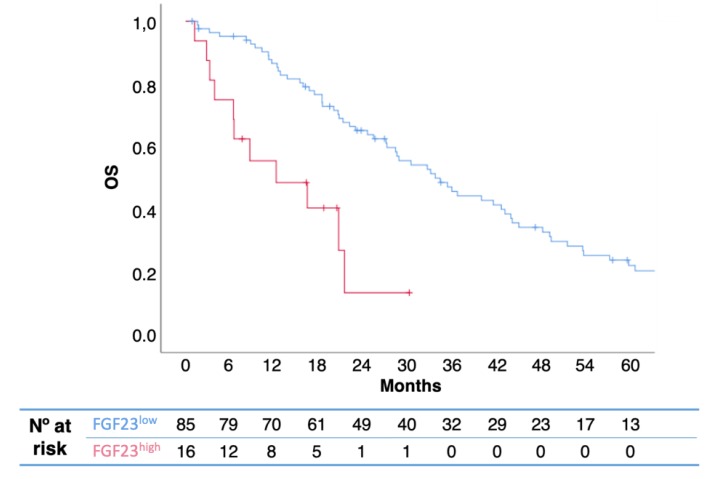
In univariate analysis, FGF23high was associated with poorer OS than FGF23low (median 12.2 vs. 34.4 months; p = 0.001; hazard ratio [HR] 0.27; 95% confidence interval [CI] 0.14−0.55) (Figure 1). In multivariate analysis including pre-therapeutic prognostic factors (extra-bone involvement, uNTX, presence of bone fractures, and serum levels of calcium), FGF23high remained predictive of OS (Table 2). When tumor types were evaluated separately, no statistically significant correlation with prognosis was observed, what can be due to the small sample size of subgroup analyses.
Figure 1.
Kaplan-Meier overall survival (OS) curves, according to FGF23 levels. High levels of serum FGRF23 were associated with shorter patient survival. (p = 0.001; HR 0.27; 95% CI 0.14−0.55).
Table 2.
Cox regression models for OS.
| Median (95% CI) | Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p | |
|---|---|---|---|---|---|
| FGF23 | |||||
| FGF23low | 34.4 (26.4–42.3) | 0.27 (0.14–0.55) | 0.001 | 0.18 (0.07–0.44) | 0.001 |
| FGF23high | 12.2 (0.0–25.1) | ||||
| Hypercalcemia | |||||
| No | 34.4 (22.8–45.9) | 0.55 (0.32–0.97) | 0.036 | 0.62 (0.34–1.15) | 0.133 |
| Yes | 18.5 (6.9–30.0) | ||||
| Pathologic fractures | |||||
| No | 28.8 (19.3–38.3) | 1.03 (0.60–1.77) | 0.911 | 1.13 (0.63–2.00) | 0.689 |
| Yes | 34.4 (13.8–55.0) | ||||
| Extraskeletal metastases | |||||
| No | 43.9 (35.6–52.2) | 0.63 (0.39–1.02) | 0.058 | 0.62 (0.37–1.04) | 0.070 |
| Yes | 22.9 (17.5–28.3) |
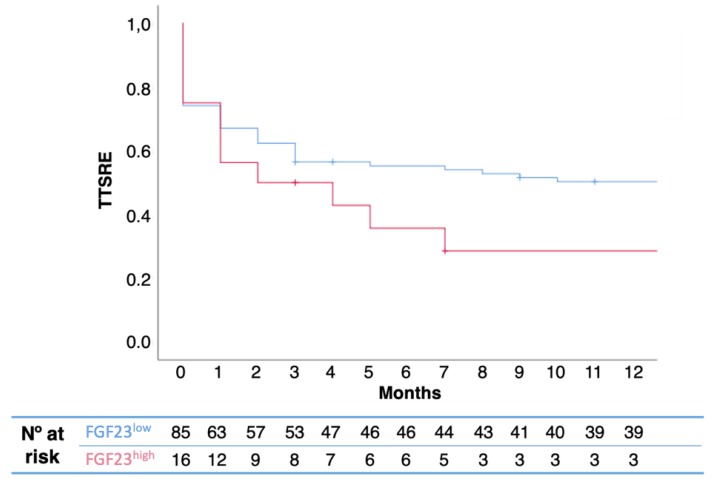
In the Kaplan-Meier model, elevated FGF23 was also predictive of a shorter TTSRE (13.0 vs. 2.0 months, p = 0.04) (Figure 2). The proportion of SRE was not statistically different between groups (Table 3).
Figure 2.
Kaplan-Meier TTSRE curves, according to FGF23 levels. High levels of serum FGRF23 were associated with shorter time to SREs (13.0 vs. 2.0 months, p = 0.04). P-value was calculated using log-rank test.
Table 3.
SRE occurrence by FGF group.
| SRE, n (%) | FGFlow | FGFhigh | p Value |
|---|---|---|---|
| Yes | 54 (57.4) | 6 (33.3) | 0.603 |
| No | 40 (42.6) | 12 (66.7) | |
| Median (IQR) | 1 (0.0–2.0) | 1 (1.0–1.5) | 0.419 |
SRE: skeletal-related events.
3. Discussion
Most biologic prognostic factors derived from bone microenvironment identified in patients with bone metastases are not tumor-type dependent. Indeed, biomarkers as uNTX and alkaline phosphatase (ALP), reflecting both osteoclast and osteoblast activity, are strong predictors of survival in patients with bone metastases across several different tumor types [11].
Interestingly, FGFR signaling has a relevant role in advanced cancer stages, particularly when cancer cells survive and become resistant to hormonal agents, as in androgen deprivation-resistant prostate [12] and endocrine therapy-resistant breast [13] cancers.
Due to the relevance of FGFR signaling when considering endocrine resistance in the most frequent bone metastases-eliciting tumors (as prostate and breast) and the role of FGF23 in bone metabolism, authors of the present work considered that it was relevant to study the correlation of serum FGF23 with survival and TTSREs in patients with bone metastases.
The role of FGF23 in the pathogenesis of tumor-induced osteomalacia (TIO)—also known as oncogenic osteomalacia—is well described [14]. TIO is characterized by decreased renal tubular reabsorption of phosphate, low levels of active vitamin D, and chronic hypophosphatemia, ultimately leading to improper bone mineralization.
In this exploratory cohort, patients in the FGF23high group had a shorter OS and TTSRE, which represents the first evidence of the prognostic relevance of serum FGF23 in patients with bone metastases, as far as authors are aware.
This is particularly important as FGFR signaling is currently under investigation as a potential target in cancer. In fact, FGFR antagonists have shown activity in prostate cancer [15] and have been approved to treat clear cell carcinoma and thyroid medullar carcinoma. In breast cancer, results have been less encouraging, possibly due to the lack of biomarkers to select patients that may benefit from FGFR inhibitors [16].
This study has some limitations: (i) it is a small retrospective analysis of a prospective cohort, thus subject to residual confounding; (ii) it included different tumor types (being breast cancer the most predominant); and (iii) expression of FGF23 in metastatic tissue was not assessed. Although not statistically significant, there was a numeric imbalance between the two groups regarding tumor types and presence of extraskeletal metastases. However, its findings suggest that the investigation on the role of FGF23 as a potential prognostic biomarker in patients with bone metastases should be pursued and deepened.
4. Materials and Methods
4.1. Study Population
In this prospective cohort study, 112 consecutive patients were diagnosed and treated at the Medical Oncology Department of Hospital de Santa Maria. Patients had metastatic bone disease from solid tumors and were treated with BTAs (either denosumab or zoledronate). They were consecutively enrolled upon baseline metastatic diagnosis confirmation and prospectively evaluated for disease progression and incidence of SREs every three to four months for one year. Urine and whole blood samples were collected at baseline and during treatment. At the time of diagnosis, overall cancer treatment was provided as per institutional guidelines and in accordance with international oncology society recommendations. Clinical information was retrospectively collected, including tumor type, concomitant therapy, number and timing of SREs, calcium level, date of disease progression (bone and extra-bone progression), and survival. SREs were identified as pathologic fracture, need for radiotherapy to treat bone metastases, spinal cord compression, or surgery to bone. Ethical approval for this study was granted by institutional review boards on 19 December 2013 and compliance with national regulations was observed.
4.2. uNTX and FGF23 Determination
uNTX was quantified using Osteomark NTx Urine enzyme-linked immunosorbent assay (Alere, Waltham, MA) according to the manufacturer’s instructions. Urinary levels of NTX in BCEs were expressed as urine creatinine excretion ratio. FGF23 was quantified by ELISA (Kainos Laboratories Inc., Tokyo, Japan), according to the manufacturer’s instructions.
4.3. Statistical Analysis
FGF23 was dichotomized in two levels (FGF23high and FGF23low) using a sample mean plus one standard deviation as cut-off. Descriptive statistics of baseline clinical and pathologic characteristics according to FGF23 levels were performed. Given the exploratory nature of the study, no specific sample size was predefined.
Median and IQR was reported for continuous variables and absolute and relative frequencies, for categorical variables. To compare categorical variables among groups, χ2 and Fisher exact tests were used when appropriate. The Mann-Whitney test was used to compare medians.
Outcome measures included OS and TTSRE. OS was defined as time between BTA start and death, irrespective of the cause. TTSRE was defined as time between BTA start and first SRE. Patients without a vital status change by January 2018 were right censored.
Median OS and TTSRE were obtained using Kaplan-Meier methods and log-rank test was used to compare group outcomes. Cox regression model was used to perform univariate and multivariate assessment of the effect of investigated parameters in duration of response and prognosis.
All statistical tests were two-tailed and statistical significance was assumed when p values were inferior to 0.05. IBM’s SPSS statistics v.25 was used for the statistical analysis.
5. Conclusions
In this exploratory cohort, patients in the FGF23high group had a shorter OS and TTSRE compared with those on the FGF23low group. Further studies are warranted to define FGF23 role as a prognostic biomarker and potential predictor of response to drugs targeting the FGF23-FGFR axis. This study’s results suggest that new therapeutic agents targeting FGF23 transcription and/or degradation to either increase or lower circulating FGF23 levels, as well as recombinant FGF23- or FGF23-blocking antibodies, could be explored regarding their eventual role in the treatment of bone metastatic disease.
Acknowledgments
The authors would like to acknowledge all the patients included in the analysis and Joana Cavaco Silva for manuscript revision and editing.
Abbreviations
| ALP | alkaline phosphatase |
| BCE | bone collagen equivalent |
| BTA | bone-targeted agents |
| ELISA | enzyme-linked immunosorbent assay |
| FGF | fibroblast growth factor |
| FGF | fibroblast growth factor receptor |
| HR | hazard ratio |
| IQR | interquartile range |
| OS | overall survival |
| PTH | parathyroid hormone |
| SRE | skeletal-related event |
| TIO | tumor-induced osteomalacia |
| TTSRE | time to skeletal-related events |
| uNTX | urinary N-terminal telopeptide |
Author Contributions
Conception/Design: A.M., I.A., S.C., and L.C.; Provision of study material or patients: A.M., A.R.F., S.C., I.A., I.V., A.L.C., R.S., C.A., C.P., D.M., T.R.P., L.Cor., and L.C.; Collection and/or assembly of data: A.M., A.F., I.V., A.L.C., R.S., C.A., C.P., D.M., and T.R.P.; Data analysis and interpretation: A.M., S.C., I.A., and L.C.; Manuscript writing: A.M., S.C., I.A., and L.C.; Final approval of manuscript: A.M., A.F., S.C., I.A., I.V., A.L.C., R.S., C.A., C.P., D.M., T.R.P., L.Cor., and L.C.
Funding
This research received funding from an anonymous non-pharma related individual in the form of donation. This study was supported by project UID/BIM/50005/2019 of Fundação para a Ciência e a Tecnologia (FCT)/Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) through State Budget funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Itoh N., Ornitz D.M. Evolution of the FGF and FGFR gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita T., Yoshioka M., Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 3.Shimada T., Mizutani S., Muto T., Yoneya T., Hino R., Takeda S., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S., Guo R., Simpson L.G., Xiao Z.S., Burnham C.E., Quarles L.D. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 5.Larsson T., Marsell R., Schipani E., Ohlsson C., Ljunggren O., Tenenhouse H.S., Jüppner H., Jonsson K.B. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 6.Bai X., Miao D., Li J., Goltzman D., Karaplis A.C. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology. 2004;145:5269–5279. doi: 10.1210/en.2004-0233. [DOI] [PubMed] [Google Scholar]
- 7.Razzaque M.S. The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A., Winer K., Econs M.J., Marx S.J., Collins M.T. FGF-23 is elevated by chronic hyperphosphatemia. J. Clin. Endocrinol. Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 10.Feng S., Wang J., Zhang Y., Creighton C.J., Ittmann M. FGF23 promotes prostate cancer progression. Oncotarget. 2015;6:17291–17301. doi: 10.18632/oncotarget.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipton A., Smith M.R., Fizazi K., Stopeck A.T., Henry D., Brown J.E., Shore N.D., Saad F., Spencer A., Zhu L., et al. Changes in Bone Turnover Marker Levels and Clinical Outcomes in Patients with Advanced Cancer and Bone Metastases Treated with Bone Antiresorptive Agents. Clin. Cancer Res. 2016;22:5713–5721. doi: 10.1158/1078-0432.CCR-15-3086. [DOI] [PubMed] [Google Scholar]
- 12.Bluemn E.G., Coleman I.M., Lucas J.M., Coleman R.T., Hernandez-Lopez S., Tharakan R., Bianchi-Frias D., Dumpit R.F., Kaipainen A., Corella A.N., et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell. 2017;32:474–489.e6. doi: 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formisano L., Stauffer K.M., Young C.D., Bhola N.E., Guerrero-Zotano A.L., Jansen V.M., Estrada M.M., Hutchinson K.E., Giltnane J.M., Schwarz L.J., et al. Association of FGFR1 with ERα Maintains Ligand-Independent ER Transcription and Mediates Resistance to Estrogen Deprivation in ER+ Breast Cancer. Clin. Cancer Res. 2017;23:6138–6150. doi: 10.1158/1078-0432.CCR-17-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minisola S., Peacock M., Fukumoto S., Cipriani C., Pepe J., Tella S.H., Collins M.T. Tumour-induced osteomalacia. Nat. Rev. Dis. Primers. 2017;3:17044. doi: 10.1038/nrdp.2017.44. [DOI] [PubMed] [Google Scholar]
- 15.Feng S., Shao L., Castro P., Coleman I., Nelson P.S., Smith P.D., Davies B.R., Ittmann M. Combination treatment of prostate cancer with FGF receptor and AKT kinase inhibitors. Oncotarget. 2017;8:6179–6192. doi: 10.18632/oncotarget.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Luca A., Frezzetti D., Gallo M., Normanno N. FGFR-targeted therapeutics for the treatment of breast cancer. Expert Opin. Investig. Drugs. 2017;26:303–311. doi: 10.1080/13543784.2017.1287173. [DOI] [PubMed] [Google Scholar]