Abstract
The gene Pm61 that confers powdery mildew resistance has been previously identified on chromosome arm 4AL in Chinese wheat landrace Xuxusanyuehuang (XXSYH). To facilitate the use of Pm61 in breeding practices, the bulked segregant analysis-RNA-Seq (BSR-Seq) analysis, in combination with the information on the Chinese Spring reference genome sequence, was performed in the F2:3 mapping population of XXSYH × Zhongzuo 9504. Two single nucleotide polymorphism (SNP), two Kompetitive Allele Specific PCR (KASP), and six simple sequence repeat (SSR) markers, together with previously identified polymorphic markers, saturated the genetic linkage map for Pm61, especially in the proximal side of the target gene that was short of gene-linked markers. In the newly established genetic linkage map, Pm61 was located in a 0.71 cM genetic interval and can be detected in a high throughput scale by the KASP markers Xicsk8 and Xicsk13 or by the standard PCR-based markers Xicscx497 and Xicsx538. The newly saturated genetic linkage map will be useful in molecular marker assisted-selection of Pm61 in breeding for disease resistant cultivar and in its map-based cloning.
Keywords: Triticum aestivum, Landrace, Powdery mildew, Bulked segregant analysis-RNA-Seq (BSR-Seq), Single nucleotide polymorphism (SNP), Kompetitive Allele Specific PCR (KASP)
1. Introduction
Powdery mildew is one of the most widely epidemic diseases in wheat (Triticum aestivum L.) grown in the temperate and humid regions of the world. The causal agent of powdery mildew, Blumeria graminis f. sp. tritici (Bgt), is an obligate biotrophic fungus, which usually colonizes wheat leaves and develops white pustule symptoms on leaf blades. The penalty in wheat yield caused by infection of powdery mildew has been reported to be from 5% to 40% in various countries, depending on the disease severity [1]. The impact of powdery mildew on grain quality, such as test weight and protein content, has been reported [2]. Changes in grain proteome and composition and grain starch and protein contents caused by the disease were observed using the proteomics analysis [3].
In China, powdery mildew has become an economically important disease since the 1970s. In recent years, the annual areas of powdery mildew infected wheat fields ranged from 6 to 8 million hectares in most winter wheat and parts of spring wheat fields throughout the country (available online: https://www.natesc.org.cn/sites/cb/). Management of wheat powdery mildew relies on growing disease resistant cultivars in accompany with application of fungicides such as triadimefon when necessary.
Breeding for powdery mildew resistant cultivars necessitates the availability of powdery mildew resistance genes (Pm genes). Sources of Pm genes include common wheat and its close or distant relative species. Wheat landraces from China are a class of historically grown and maintained common wheat cultivars, which have provided quite a number of Pm genes. A group of landraces carry allelic genes in the Pm5 locus on chromosome 7BL, for example, Pm5d (IGV1-455) [4], Pm5e (Fuzhuang 30) [5], PmH (Hongquanmang) [6], PmTm4 (Tangmai 4) [7], Mlmz (Mazhamai) [8], Mlxbd (Xiaobaidong) [9], pmHYM (Hongyoumai) [10], PmBYYT (Baiyouyantiao) [11], and PmSGD (Shangeda) [12]. In the Pm24 locus, there are two alleles, Pm24a in Chiyacao [13] and Pm24b in Baihulu [14]. The designated genes Pm2c on 5DS [15], Pm45 on 6DS [16], and Pm47 on 7BS [17] were identified in Niaomai, Wuzhaomai, and Hongyanglazi, respectively. PmX in Xiaohongpi [18] and MlHLT in Hulutou [19] were detected on chromosomes 2AL and 1DS, respectively. Recently, a group of scientists in the USA characterized three Pm genes in wheat landraces: two from Afghanistan, Pm223899 on 1AS in PI 223,899 [20] and Pm59 on 7AL in PI 181,356 [21], and the other one from Iran, Pm63 on 2BL in PI 628,024 [22].
Landraces of wheat are traditionally grown in agriculture until they have been replaced by more productive cultivars since the initiation of modern crop breeding in the mid-20th century. At present, landraces can only be grown in certain marginal lands with less productivity [23]. They are no longer adapting to most of the improved agricultural environments in spite of possessing desirable genes. There is a need to introgress the useful genes, for example, Pm genes, into modern improved genetic backgrounds in order to be used efficiently in modern breeding practices. Molecular approaches facilitate identification and transfer of wheat genes for disease resistance [24]. In fact, many recently characterized Pm genes are identified with the aid of various classes of molecular markers, such as SSR (microsatellite) markers and STS (sequence-tagged site) markers, which are useful in marker-assisted selection (MAS) of target genes.
The breeder-friendly molecular markers associated with resistance genes are useful in the breeding programs during development of disease resistant cultivars. The PCR-based markers are affordable in most wheat breeding programs, so this type of molecular markers can be routinely used in breeding practice. Moreover, high-throughput genotyping is needed in a large scale of population study. The newly improved next-generation sequencing techniques allow the discovery of numerous single nucleotide polymorphism (SNP) markers. The Chinese Spring wheat reference genome sequence has been updated [25], which facilitates the identification of gene-linked molecular markers and map-based cloning of disease resistance genes. The abundance of SNP markers is far greater than the traditional PCR-based markers. The SNP markers can be visualized by converting them into Kompetitive Allele Specific PCR (KASP) markers for establishing high-throughput genotyping platform for MAS of the target genes [26]. They have also been used to detect disease resistance genes, such as Sr26 for resistance to stem rust (caused by Puccinia graminis f. sp. tritici) [27], Yr34 and Yr48 for resistance to stripe rust (caused by Puccinia striiformis f. sp. tritici) [28].
BSR-seq technique, which integrates bulked segregant analysis and RNA-seq [29], has proven to be a rapid and efficient strategy to identify gene-linked molecular markers. It provides a fast and high-throughput method to localize resistance genes in crops with large genome, e.g., wheat. This technique has been used in the molecular characterization of wheat disease resistance genes, such as Yr15 [30], YrZH22 [31], YrMM58 and YrHY1 [32], Yr26 [33], Pm4b [34], and PmSGD [12].
Wheat landrace Xuxusanyuehuang (XXSYH) was resistant to several Bgt isolates from China, and a recessive gene, Pm61, was located on chromosome 4AL [35]. In the genetic linkage map that was developed based on the mapping population of XXSYH × Mingxian 169, Pm61 was mapped in a 0.46 cM genetic interval on 4AL. However, only two linked markers were identified in the proximal side of Pm61. Taking the advantage of BSR-seq and the Chinese Spring reference genome sequence, this study was conducted to (1) saturate genetic linkage map for Pm61, and (2) develop PCR-based markers for breeder-friendly use and KASP markers for large scale and high-throughput detection of Pm61 during its MAS.
2. Results
2.1. Evaluation of Resistance to Bgt Isolates in XXSYH
Thirty Bgt isolates collected from wheat fields in Shandong, Shanxi, Beijing, Hebei, and Sichuan provinces were used to test response of XXSYH to powdery mildew. Twenty isolates were avirulent on XXSYH with ITs 0, 0; 1 or 2. Ten isolates were virulent on XXSYH with ITs 3 or 4 (Table 1). All the Bgt isolates tested were virulent on Zhongzuo 9504, the susceptible control.
Table 1.
Infection types of Xuxusanyuehuang (XXSYH) and Zhongzuo 9504 to 30 Blumeria graminis f. sp. tritici (Bgt) isolates from different provinces of China.
| Bgt Isolate | XXSYH | Zhongzuo 9504 | Province |
|---|---|---|---|
| 1 | 1 | 4 | Shandong |
| 2 | 1 | 3 | Shandong |
| 3 | 1 | 3 | Shandong |
| 4 | 2 | 3 | Shandong |
| 5 | 1 | 3 | Shandong |
| 6 | 2 | 3 | Shandong |
| 7 | 3 | 3 | Shandong |
| 8 | 3 | 4 | Shanxi |
| 9 | 2 | 3 | Shanxi |
| 10 | 0; | 4 | Shanxi |
| 11 | 3 | 3 | Shanxi |
| 12 | 2 | 3 | Shanxi |
| 13 | 1 | 3 | Beijing |
| 14 | 1 | 3 | Beijing |
| 15 | 1 | 3 | Beijing |
| 16 | 3 | 4 | Beijing |
| 17 | 1 | 3 | Hebei |
| 18 | 3 | 3 | Hebei |
| 19 | 0; | 3 | Hebei |
| 20 | 3 | 4 | Hebei |
| 21 | 3 | 3 | Hebei |
| 22 | 1 | 3 | Hebei |
| 23 | 1 | 3 | Hebei |
| 24 | 3 | 3 | Hebei |
| 25 | 1 | 3 | Hebei |
| 26 | 2 | 4 | Hebei |
| 27 | 3 | 3 | Hebei |
| 28 | 2 | 4 | Hebei |
| 29 | 3 | 3 | Sichuan |
| 30 | 1 | 4 | Sichuan |
| 31 | 2 | 3 | Sichuan |
Note: the infection type on leaves was rated on a 0–4 scale for determine the response of wheat genotypes to powdery mildew, where 0 = immune, no symptom, 0; = hypersensitive necrotic flecks, 1 = highly resistant, necrosis with low sporulation, 2 = moderately resistant, necrosis with moderate sporulation, 3 = moderately susceptible, moderate to high sporulation, and 4 = highly susceptible, no necrosis with full sporulation.
2.2. Genetic Analysis of Powdery Mildew Resistance in XXSYH
When inoculated with isolate Bgt1 from Shandong, province, XXSYH was resistant with an IT 1, while Zhongzuo 9504 was susceptible with an IT 3. Therefore, this Bgt isolate was able to differentiate the phenotypes of the two parents that were crossed to develop the populations for genetic analysis. The IT of the 15 F1 plants from the XXSYH × Zhongzuo 9504 resembled the susceptible parent Zhongzuo 9504 (Figure 1). The 211 F2:3 lines produced 51 homozygous resistant, 115 heterozygous, and 45 homozygous susceptible lines, which agrees to the 1:2:1 segregating ratio (χ2 = 2.05, P = 0.3584). This indicates that the resistance of XXSYH to isolate Bgt1 was in accordance with the single recessive mode of inheritance.
Figure 1.
The phenotypic reactions of resistant parent Xuxusanyuehuang, susceptible parent Zhongzuo 9504, and their F1 plants to isolate Bgt1.
2.3. BSR-Seq Analysis of the Bulked RNA Pools with Distinct Phenotypes to Isolate Bgt1
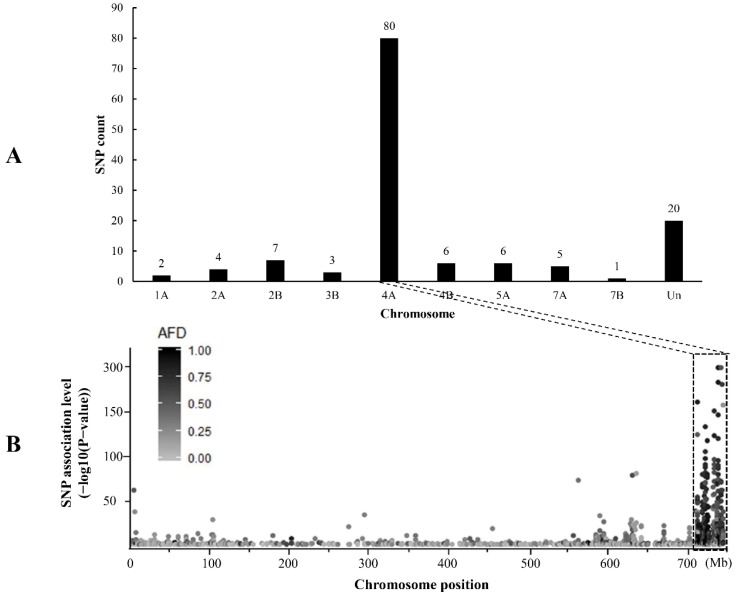
RNA-seq analysis generated a total of 40.0 Gb of raw data from the resistant and susceptible RNA samples and 38.3 Gb (95.77%) of clean data were obtained after quality control. Among the 62,760,214 and 67,764,274 high-quality reads for the two bulks, 58,213,973 (92.76%) and 62,295,839 (91.93%) were uniquely mapped to the Chinese Spring reference genome sequence, respectively (Supplementary Table S1). With the criteria of P < 1 × 10−10 and AFD > 0.6, 134 SNP variants, potentially associated with the target powdery mildew resistance gene, were identified. Further analysis indicated that 80 (59.7%) candidate SNP were distributed on chromosome 4AL (Figure 2A) and corresponded to a 31 Mb interval in the terminal region of 4AL in the reference genome (Figure 2B). This is consistent with previous study in which Pm61 was mapped in a 1.3 Mb genomic region (717,963,176–719,260,469) on chromosome 4AL [35].
Figure 2.
Number of polymorphic single nucleotide polymorphism (SNP) distributed on different wheat chromosomes (A) and distribution of SNP variants on chromosome 4A (B).
2.4. Validation of the Candidate SNP and Development of SNP Markers
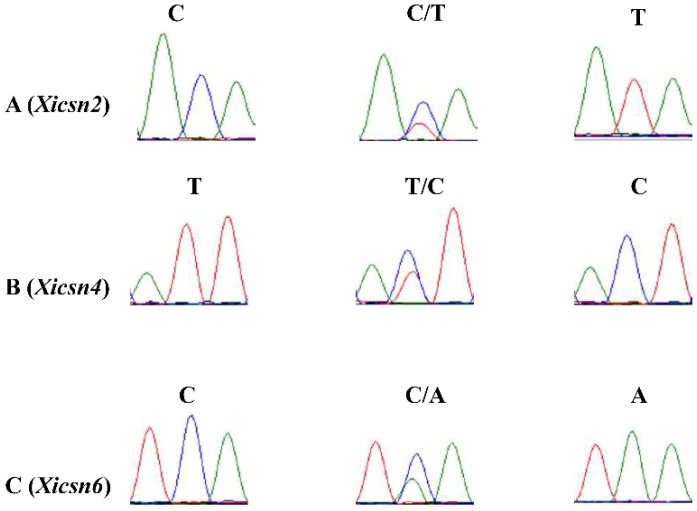
Using the sequences flanking the 80 SNP that were anchored on chromosome 4AL as the queries, Blast analysis against the Chinese spring whole genome assembly (available online: https://urgi.versailles.inra.fr/) produced 24 homologous scaffolds. The 3 kb sequences containing the candidate SNP and corresponding to the above homologous scaffold were used as templates to design 28 pairs of SNP primers on the GSP website (Supplementary Table S2). The amplified products from XXSYH, Zhongzuo 9504, and the resistant and susceptible DNA bulks were sequenced for polymorphism analysis, and seven SNP markers were polymorphic. Based on the linkage analysis with 16 randomly selected F2:3 lines, Xicsn1, Xicsn2, and Xicsn3 were potentially mapped on one side of Pm61, and Xicsn4, Xicsn5, Xicsn6, and Xicsn7, on the other side of target gene. Sequencing analysis of Xicsn2 and Xicsn4 was carried out in 211 F2:3 lines (Figure 3). Pm61 was localized in a 4.5 cM genetic interval between the SNP markers Xicsn2 and Xicsn4 corresponding to a 5.3 Mb physical region (713,523,186–718,866,838) on the distal end of chromosome 4AL. The other five SNP markers were not used in genotyping the mapping population because of poor clustering of the fluorescence signals.
Figure 3.
Sanger sequencing profiles of SNP markers Xicsn2 (A), Xicsn4 (B) and Xicsn6 (C) in the homozygously resistant (R), homozygously susceptible (S), and heterozygous F2:3 lines (H) from the mapping population of the Xuxusanyuehuang × Zhongzuo 9504 cross. Blue, green, and red lines represent bases of cytosine (C), adenine (A), and thymine (T), respectively.
2.5. Development of KASP Markers
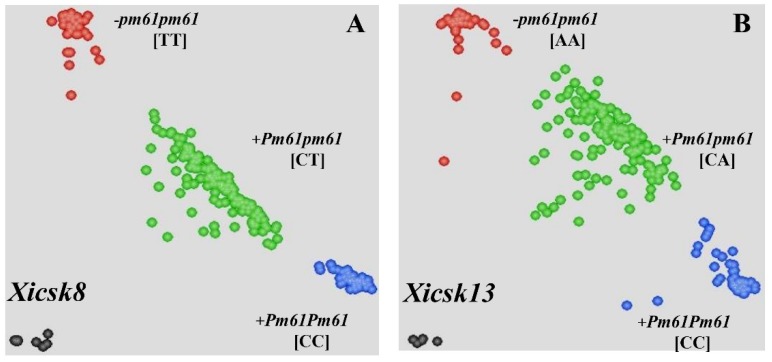
To cost-effectively detect Pm61 in MAS, 15 SNP generated from BSR-Seq analysis between the SNP markers Xicsn1 and Xicsn7 were converted into 13 KASP primer pairs (Supplementary Table S3). They were subjected to polymorphism analysis on the parental cultivars and 15 F2:3 lines (including five homozygous resistant, heterozygous, and homozygous susceptible lines each). Six polymorphic KASP markers, i.e., Xicsk4, Xicsk5, Xicsk7, Xicsk8, Xicsk9, and Xicsk13, were identified. Moreover, the amplicons of 31 SSR primer pairs, which were previously designed based on the genomic sequence corresponding to the genetic interval between markers Xicsn2 and Xicsn4, were sequenced to detect the SNP variants that differed between the two parents. Seven SNP variants were identified in the amplicons of 7 SSR primer pairs and were converted into KASP primer pairs (Supplementary Table S3). The KASP markers Xicsk14 and Xicsk15 showed clear polymorphism between the two parents and 15 F2:3 lines by sequencing analysis. By genotyping the F2:3 mapping population, KASP markers Xicsk8 and Xicsk13 were linked to Pm61 (Figure 4A,B).
Figure 4.
Genotyping results of Xicsk8 (A) and Xicsk13 (B) by Kompetitive Allele Specific PCR (KASP) assay. The scatter plot with the axes x and y represents the allelic discrimination of Xicsk8 or Xicsk13 genotypes. The red, green and blue dots represent the homozygously susceptible, heterozygous, and homozygously resistant F2:3 lines from the mapping population of the Xuxusanyuehuang × Zhongzuo 9504 cross, respectively.
2.6. Development of SSR Markers
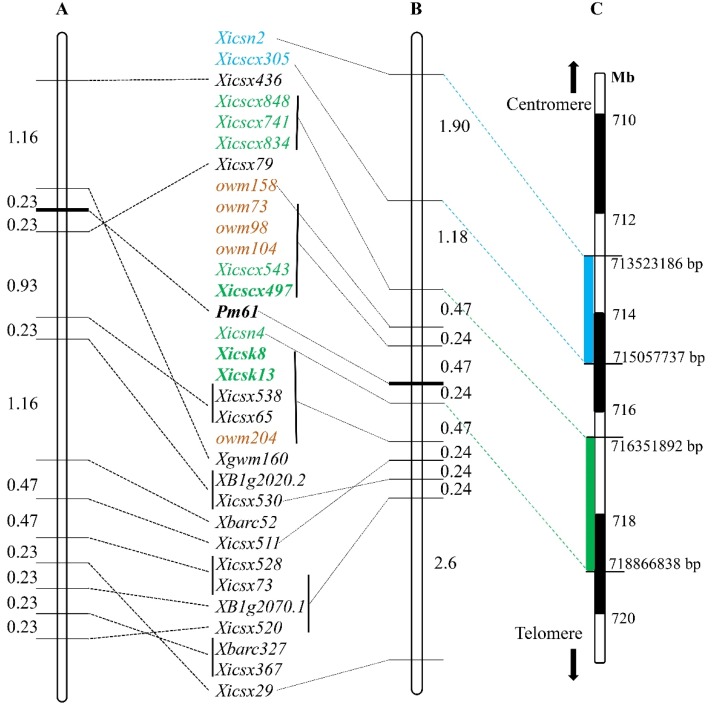
The polymorphism of the eleven SSR markers, previously linked to Pm61 using the mapping population of XXSYH × Mingxian 169 (Figure 5A), was analyzed against the mapping population of XXSYH × Zhongzuo 9504. Seven SSR markers, Xicsx29, Xicsx65, Xicsx73, Xicsx511, Xicsx520, Xicsx530, and Xicsx538, were polymorphic. Linkage analysis indicated that all of these polymorphic markers were located on the distal side of Pm61 (Figure 5B). Markers Xicsx79 and Xicsx436 previously located in the proximal side of Pm61 were not polymorphic in the XXSYH × Zhongzuo 9504 mapping population. To develop more gene-linked markers in the proximal side of Pm61, the 5.3 Mb sequences (713,523,186–718,866,838) of the reference genome corresponding to the Pm61 flanking markers Xicsn2 and Xicsn4 were used as templates to design SSR primer pairs. The 1.5 Mb (713,528,439–715,057,737) genomic sequences extended from marker Xicsn2 toward Pm61 was used to design 347 SSR primer pairs (Figure 5C, Supplementary Table S4). The 2.5 Mb (718,854,012–716,351,892) sequences extended from marker Xicsn4 to Pm61 were used to design 617 SSR primer pairs (Figure 5C, Supplementary Table S5). Five co-dominant SSR markers, Xicscx305, Xicscx497, Xicscx543, Xicscx741, and Xicscx834 and a dominant SSR marker Xicscx848, were polymorphic between the two parents and two DNA bulks, which indicates their possible linkage to Pm61.
Figure 5.
Genetic linkage maps of Pm61 in previous study [35] (A) and newly developed in the present study (B). The positions of the Pm61-linked molecular markers are indicated on a scale bar based on the Chinese Spring genome sequence (C).
2.7. Construction of the Genetic Linkage Map for Pm61
The polymorphic markers developed, including two SNP markers (Xicsn2 and Xicsn4), two KASP markers (Xicsk8 and Xicsk13), and six SSR markers (Xicscx305, Xicscx497, Xicscx543, Xicscx741, Xicscx834, and Xicscx848), together with seven polymorphic Pm61-linked markers developed in the previous study [35], were used to construct the genetic linkage map after genotyping the F2:3 mapping population of XXSYH × Zhongzuo 9504. In this linkage map, Pm61 was placed in a 0.71 cM genetic interval that corresponded to 0.61 Mb genomic interval (718,257,529–718,866,730) of the genomic region in the reference genome sequence of Chinese Spring. The SSR markers Xicscx543 and Xicscx497 were located in the same locus at the proximal side of Pm61 with genetic distance of 0.47 cM. The SNP marker Xicsn4 was located in the distal side of Pm61 with genetic distance of 0.24 cM. The KASP markers Xicsk8 and Xicsk13 (Figure 4A,B) and SSR markers Xicscx497 and Xicsx538 (Figure 6A,B) produced clear banding patterns and were able to differentiate individuals of the mapping population with distinct phenotypes.
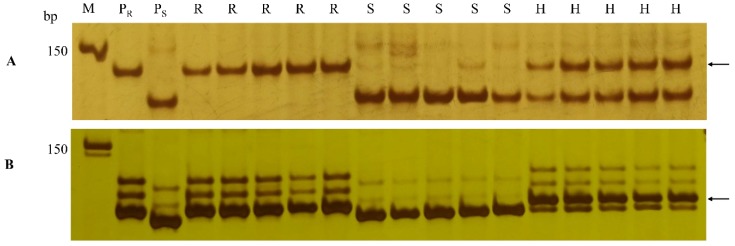
Figure 6.
The banding patterns of Pm61-linked simple sequence repeat (SSR) markers Xicscx497 (A) and Xicsx538 (B) in the parents and the selected F2:3 lines of the Xuxusanyuehuang × Zhongzuo 9504 cross. Lane M, 50 bp DNA ladder; PR, Xuxusanyuehuang; PS, Zhongzuo 9504; R, homozygously resistant F2:3 lines; S, homozygously susceptible F2:3 lines; and H, heterozygous F2:3 lines. Arrows indicate the polymorphic bands specific for Pm61.
2.8. Physical Locations of Pm61, QPm.tut-4A and MlIW30 on Chromosome Arm 4AL
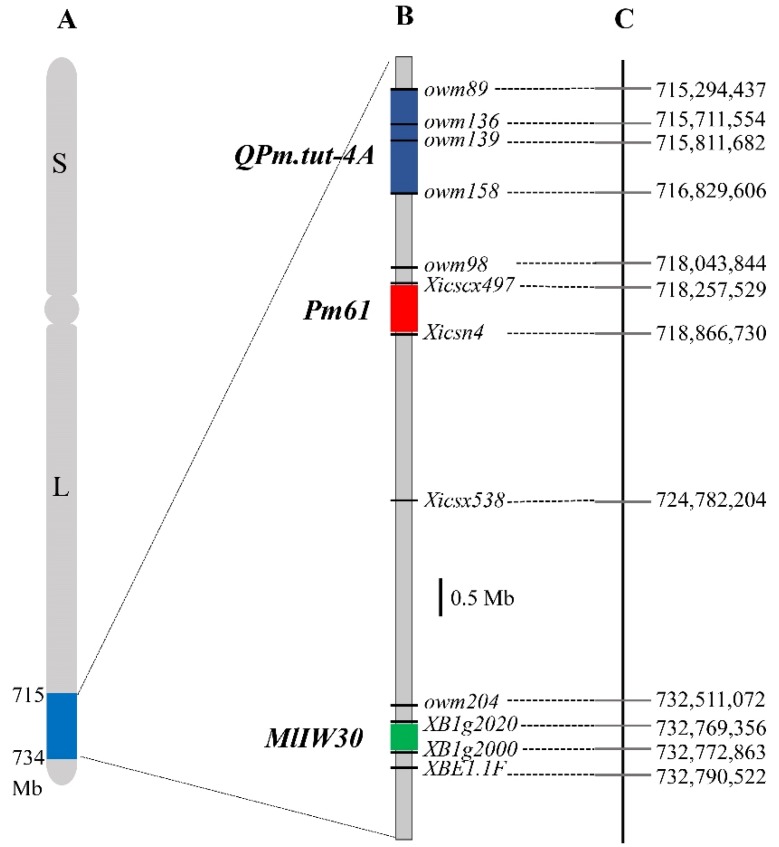
In addition to Pm61, a dominant gene MlIW30 from wild emmer wheat [36] and a QTL QPm.tut-4A from T. militinae Zhuk. et Migusch. [37] were mapped on chromosome arm 4AL. Two MlIW30-linked markers, XB1g2020.2 and XB1g2070.1, were linked to Pm61 using the mapping population of XXSYH × Mingxian 169 [35]. However, only XB1g2070.1 was linked to Pm61 with genetic distance of 1.43 cM in the mapping population of XXSYH × Zhongzuo 9504. Polymorphism of 73 markers linked to QPm.tut-4A [37] were examined against the parents and the contrasting DNA bulks of the XXSYH × Zhongzuo 9504 cross, resulting in 5 polymorphic markers owm73, owm98, owm104, owm158, and owm204 that were linked to Pm61. The closest QPm.tut-4A–linked marker owm158 to Pm61 was 0.95 cM from Pm61. Based on the physical position on the Chinese Spring reference genome sequence, Pm61 was located in 0.61 Mb physical interval (718,257,529–718,866,730) between MlIW30 (21 kb, 732,769,506–732,790,522) and QPm.tut-4A (1.54 Mb, 715,294,437–716,829,606 bp) (Figure 7).
Figure 7.
A sketch physical map of chromosome 4A (A), comparative analysis of the three Pm genes or QTL on chromosome arm 4AL (B), and corresponding physical locations in chromosome 4AL in the Chinese Spring reference genome (C).
3. Discussion
Using the strategy of BSR-Seq analysis that was performed on the mapping population of XXSYH × Zhongzuo 9504, two Pm61-linked SNP markers (Xicsn2 and Xicsn4) and two KASP markers (Xicsk8 and Xicsk13) were developed. Based on the genomic sequences of the Chinese Spring reference genome that flanked the Pm61 locus, six SSR markers were linked to the target gene. Using these molecular markers, together with previously developed gene-linked markers [35,37], a new saturated genetic linkage map was established, which placed Pm61 in a 0.71 cM genetic interval corresponding to a 0.61 Mb physical interval (718,257,529–718,866,730) on the terminal region of chromosome 4AL. The closest flanking markers of Pm61 were Xicscx497/Xicscx543/owm73/owm98/owm104 and Xicsn4 with genetic distances of 0.47 and 0.24 cM, respectively. Pm61 can be detected with the KASP markers Xicsk8 and Xicsk13 and SSR markers Xicscx497 and Xicsx538. Compared to the previous study [35], the newly developed genetic linkage map was saturated with more molecular markers especially in the proximal side of Pm61. The physical location of Pm61 in the Chinese Spring reference genome between the two genetic linkage maps with different mapping populations is comparable.
Previously identified Pm61-linked markers, Xicsx79 and Xicsx436 [35], the only two markers in the proximal side of the target gene, were not polymorphic between the parents XXSYH and Zhongzuo 9504. Therefore, BSR-seq analysis was performed to find more polymorphic markers that flanked Pm61. Two SNP markers Xicsn2 and Xicsn4 were located on the opposite sides of Pm61. The fragments of genomic sequences from the Chinese Spring reference genome corresponding to the two SNP markers were used to develop SSR markers, which produced 6 markers that were linked to Pm61. After genetic linkage analysis, all these markers were located in the proximal side of Pm61. The newly developed Pm61-linked markers are being used to genotype large scale of F2 and F2:3 population that was derived from XXSYH × Zhongzuo 9504 cross. The saturation of the genetic linkage map for Pm61 facilitates its fine mapping and ultimately map-based cloning.
The race-specific Pm genes inherit either in a dominant mode or in a recessive mode. Many Chinese landraces were identified to possess recessive Pm genes, for example, several genes in the Pm5 locus [4,5,6,8,9,10,11,12], Pm47 [17], and PmX [18]. Pm61 in XXSYH is also a recessive gene [35]. A Pm gene with such a recessive mode of inheritance needs additional generation to allow the expression of resistant phenotype when the target gene is homozygous.
The establishment of the MAS technique for Pm61 will facilitate its application in developing disease resistant wheat cultivars. The KASP markers Xicsk8 and Xicsk13 are able to identify Pm61 in a high throughput scale. KASP assay is also known as tolerance to DNA quality, low cost and high specificity [38]. However, KASP assay may produce a certain proportion of calling errors (0.7~1.6%) [38] and (8.8% ± 5.5%) missing data [39]. The SSR markers Xicscx497 and Xicsx538 are useful to detect Pm61 using a standard DNA amplification method. Many breeders are able to use PCR-based molecular markers in their routine breeding practices, since such type of molecular markers is cost-effective. The combination KASP assay and PCR analysis can provide a more accurate identification of the target gene during the process of MAS.
Three Pm genes or QTL, i.e., MlIW30, Pm61, and QPm.tut-4A, have been detected on a 17.50 Mb genomic region on the terminal part of chromosome arm 4AL (Figure 7). Based on their positions in the Chinese Spring reference genome, MlIW30 was located in a 21 kb (732,769,506–732,790,522) genomic interval [36]. QPm.tut-4A was identified in a 1.54 Mb (715,294,437–716,829,606) genomic interval. MlIW30 and QPm.tut-4A were transferred into common wheat from the wild emmer (T. turgidum ssp. dicoccoides) and T. militinae, respectively [36,37]. Pm61 is derived from a Chinese landrace and is located in a 0.61 Mb (718,257,529–718,866,730) genomic interval between MlIW30 and QPm.uga-4A. It appears that Pm61 was located on different genomic intervals as MlIW30 and QPm.tut-4A. Isolation and functional analysis of these Pm genes will ultimately understand their relationship.
In summary, BSR-seq analysis, in combination with the Chinese Spring reference genome sequence, identified 10 SNP, KASP, and SSR markers, which saturated the genetic linkage map of Pm61, especially in the proximal side of the target gene. The development of KASP markers, Xicsk8 and Xicsk13, and SSR markers, Xicscx497 and Xicsx538, allows the detection of Pm61 in different scales and platforms. Results from this study will facilitate the fine mapping and ultimate map-based cloning, as well as application in breeding and agriculture, of Pm61 gene.
4. Materials and Methods
4.1. Plant Materials
Xuxusanyuehuang as the maternal parent was crossed to powdery mildew susceptible winter wheat Zhongzuo 9504 to generate F1, F2 and F2:3 populations for analyzing the inheritance mode of the resistance gene, determining polymorphism of molecular markers, and establishing linkage relationships between polymorphic markers and Pm61. Zhongzuo 9504 also served as the susceptible control in the powdery mildew tests and provided the host plants to maintain and increase Bgt isolates.
4.2. Assessments of Resistance to Powdery Mildew
Thirty Bgt isolates collected from Shandong, Shanxi, Beijing, Hebei, and Sichuan wheat producing provinces were used to test responses of XXSYH to powdery mildew. These isolates were subjected to three rounds of single-pustule culture on Zhongzuo 9504 plants prior to inoculation. Bgt1 from Shandong province was used in phenotyping the F2:3 mapping population. At least 15 seeds of each F2:3 line and the parents were planted in 8-cm-diameter plastic pots. Inoculation of Bgt isolates was conducted when wheat seedlings were at 2-leaf stage. Seedlings were dusted with freshly increased conidiophores, incubated in a dew chamber with 90% relative humidity, and grown in a greenhouse to allow development of powdery mildew symptoms. The conditions of plant growth were set at 20–22 °C/14 °C (day/night) with 16 h light/8 h dark photoperiod. When the disease symptoms were fully developed on the susceptible control plants 15 day after inoculation, symptom scoring was conducted by determining the infection type (IT) of each plant on a 0–4 scale as described previously [40]. Based on the scores of ITs, plants with ITs 0–2 were categorized into the resistant group, and those with ITs 3–4 into the susceptible group.
4.3. BSR-Seq Analysis
Based on the phenotypic evaluations, 30 homozygous resistant (IT 1) and 30 homozygous susceptible (IT 4) lines in the F2:3 mapping populations of XXSYH × Zhongzuo 9504 were selected to construct the phenotypically contrasting bulks. Each line was represented by a single plant that was free of Bgt inoculation. The leaf segments approximately 3 cm long from each plant two-week old were sampled and pooled as the resistant and susceptible bulks for isolating total RNA following the TRIzol protocol (Invitrogen, Carlsbad, CA, USA). RNA-seq analysis was performed in the platform of Illumina HiSeq 2500 in Beijing Novogene Bioinformatics Technology Co. Ltd. (Beijing, China). To remove the adapter sequences and low-quality sequences, raw reeds of generated were subjected to quality control using the software Trimmomatic v0.36 (available online: http://www.sadellab.org/cms/index.php?page=trimmomatic) [41]. The high-quality reads were aligned to the Chinese Spring reference genome sequence v1.0 and its annotation files [25], which was carried using the software STAR v2.5.1b (available online: http://code.google.com/p/rna-star/.) [42], with the mismatch rate of less than 5%. After removing PCR optical duplicates and spliting the mapped reads spanning introns, the unique and confident alignments were used to call SNP variants using “HaplotypeCaller” module in the software GATK v3.6 (available online: http://www. broadinstitute.org/gsa/wiki/index.php/The_Genome_Analysis_Toolkit) [43]. A Fish Exact Test (FET) and allele frequency for each variant and allele frequency difference (AFD) between the resistant and susceptible bulks were used to identify SNP variants. The SNP variants with P < 1 × 10−10 and AFD > 0.6 were regarded as candidate SNP linked to the target gene and used as templates for developing SNP markers.
4.4. DNA Isolation, Amplification and Electrophoresis
The leaf tissues of each F2:3 line were used for DNA extraction after disease resistance test following the CTAB protocol [44]. DNA concentration was determined using the Napdrop One (Thermo Fisher Scientific Inc, Madison, WI, USA) and was adjusted to 50 ng·mL−1. DNA bulks with resistant or susceptible phenotype were constructed by pooling equal amounts of DNA from 10 resistant and susceptible F2:3 lines each. DNA was amplified in a Biometra T3000 Thermocycler (ABI, New York, NY, USA). Each reaction mixture (10 μL) was composed of 5 μL mixture (including Taq polymerase, dNTPs, and 10× PCR buffer and Mg2+), 2 μL ddH2O, 1 μL DNA, and 1 μL 10 μM each of the forward and reverse primers. The profile of DNA amplification was set at 98 °C for 3 min; 35 cycles of 98 °C for 10 s, 55 °C–60 °C (depending on the specific primers) for 10 s and 72 °C for 25 s; 72 °C for 10 min. Products amplified were separated by 2% agarose gel or 8% non-denaturing polyacrylamide gel (Acr:Bis = 29:1).
4.5. Development and Validation of SNP Markers
Approximately 3 kb sequences extracted from the RefSeqv1.0 Chinese Spring genome sequence [25] containing the candidate SNP linked to the target gene were used as queries in searching the Chinese Spring whole genome assembly (available online: https://urgi.versailles.inra.fr/) to acquire the homologous scaffolds. The sequences containing candidate SNP that corresponded to the above homologous scaffolds were used as templates to design SNP primers on the GSP website (available online: https://probes.pw.usda.gov/GSP/) [45]. The primers designed contained at least one variant site at the 3′ ends and were anticipated to amplify products in a range of 300~1000 bp in length. The polymorphism of SNP primers was validated in the parents and the two DNA bulks by analyzing the sequences of the amplicons (Invitrogen Trading Co., Ltd., Shanghai, China), and the polymorphic SNP primers were used to genotype the F2:3 mapping population.
4.6. Conversion of SNP Markers to KASP Markers
The polymorphic SNP primers were converted to KASP markers using the PolyMarker software (available online: http://polymarker.tgac.ac.uk) [46]. Each KASP reaction was carried out using a 5 μL reaction mixture consisting of 2.2 μL DNA, 2.5 μL 2× KASP master mix, 0.056 μL primer mix (12 mM of each allele-specific primer and 30 mM of the common primer), 0.039 μL Mg2+, and 0.205 μL ddH2O. Amplification was performed in the ABI 7500 device (Applied Biosystems, Foster City, CA, USA) with the program of 94 °C for 15 min; 35 cycles of 94 °C for 20 s and 60 °C for 1 min. The FLUOstar Omega microplate reader (BMG Labtech, Durham, NC, USA) was used to read the green (521 nm) and pink (556 nm) fluorescence signals at 25 °C for 2 min after the reactions were completed. The fluorescence signals were transformed into different genotypes, i.e., FAM homozygotes, HEX homozygotes, and FAM/HEX heterozygotes using the Klustering Caller software (available online: http://www.lgcgroup.com/).
4.7. Development of SSR Markers
The sequences of Pm61-linked SNP markers designed were used to search for the Chinese Spring reference genome sequence v1.0 [25]. The corresponding genomic sequences were used as templates to design SSR markers with BatchPrimer3 software (available online: https://wheat.pw.usda.gov/demos/batchprimer3). Polymorphism of SSR markers were examined using the parents and the contrasting DNA bulks. DNA amplification and visualization of banding patterns were carried out as described previously [35]. The polymorphic markers were used to establish a genetic linkage map of Pm61 with the F2:3 mapping population.
4.8. Construction of High-Density Genetic Linkage Map
The Pm61-linked markers, including previously developed SSR markers and the SNP markers, KASP markers, and SSR markers developed in present studies [35,37], were used to construct the high-density genetic linkage map of Pm61 using the software Mapdraw v.2.1 (Huazhong Agricultural Sciences, Wuhan, China) [47]. The genetic distance was measured by the Kosambi function. Linkage relationship between markers and Pm61 was established with the software Mapmaker/Exp Version 3.0b and a logarithm of the odd ratio (LOD) score threshold of 3.0 [48].
Acknowledgments
Financial support of this research by the National Natural Science Foundation of China (31501310 and 31471491), the National Key Research and Development Program of China (2017YFD0101000), Scientific and Technological Research Project of Henan Province of China (172102110110), the CAAS Innovation Team, Henan Province Young College Key Teacher Subsidy Program (2017GGJS177) are gratefully appreciated.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/3/750/s1. Table S1 Sequencing quality assessment details; Table S2 A list of SNP marker primers used in this study; Table S3 A list of KASP marker primers used in this study; Table S4 A list of 347 SSR primer pairs based on the 1.5 Mb sequences extended from marker Xicsn2 toward Pm61; Table S5 A list of 617 SSR primer pairs based on the 2.5 Mb sequences extended from marker Xicsn4 toward Pm61.
Author Contributions
H.J.L., J.L., and Z.L. conceived and designed the study. J.H., P.W. and D.Q. conducted the experiments. J.H., Y.L., Y.Q., H.Z., T.Z., and L.Y. participated the disease tests and sample preparation. Y.Y., M.L. and T.F. helped with electrophoresis. J.H., J.L. and J.X. analyzed data. J.H., J.L. and H.J.L. wrote the manuscript with contributions of Z.L. and Y.Z. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Singh R.P., Singh P.K., Rutkoski J., Hodson D.P., He X., Jørgenssen L.N., Huertaespino J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016;54:303–322. doi: 10.1146/annurev-phyto-080615-095835. [DOI] [PubMed] [Google Scholar]
- 2.Samobor V., Vukobratović M., Jošt M. Effect of powdery mildew attack on quality parameters and experimental bread baking of wheat. Acta Agric. Slov. 2006;87:381–391. [Google Scholar]
- 3.Li J., Liu X.H., Yang X.W., Li Y.C., Wang C.Y., He D.X. Proteomic analysis of the impacts of powdery mildew on wheat grain. Food Chem. 2018;261:30–35. doi: 10.1016/j.foodchem.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Hsam S.L.K., Huang X.Q., Zeller F.J. Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em. Thell.). 6. Alleles at the Pm5 locus. Theor. Appl. Genet. 2001;102:127–133. doi: 10.1007/s001220051627. [DOI] [Google Scholar]
- 5.Huang X.Q., Wang L.X., Xu M.X., Röder M.S. Microsatellite mapping of the powdery mildew resistance gene Pm5e in common wheat (Triticum aestivum L.) Theor. Appl. Genet. 2003;106:858–865. doi: 10.1007/s00122-002-1146-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R.H., Zhu Z.D., Kong X.Y., Huo N.X., Tian Q.Z., Li P., Jin C.Y., Dong Y.C., Jia J.Z. Development of wheat near-isogenic lines for powdery mildew resistance. Theor. Appl. Genet. 2005;110:640–648. doi: 10.1007/s00122-004-1889-0. [DOI] [PubMed] [Google Scholar]
- 7.Hu T.Z., Li H.J., Xie C.J., You M.S., Yang Z.M., Sun Q.X., Liu Z.Y. Molecular mapping and chromosomal location of powdery mildew resistance gene in wheat cultivar Tangmai 4. Acta Agron. Sin. 2008;34:1193–1198. [Google Scholar]
- 8.Zhai W.W., Duan X.Y., Zhou Y.L., Ma H.Q. Inheritance of resistance to powdery mildew in four Chinese landraces. Plant Protect. 2008;34:37–40. [Google Scholar]
- 9.Xue F., Zhai W.W., Duan X.Y., Zhou Y.L., Ji W.Q. Microsatellite mapping of powdery mildew resistance gene in wheat landrace Xiaobaidong. Acta Agron. Sin. 2009;35:1806–1811. doi: 10.1016/S1875-2780(08)60109-1. [DOI] [Google Scholar]
- 10.Fu B.S., Zhang Z.L., Zhang Q.F., Wu X.Y., Wu J.Z., Cai S.B. Identification and mapping of a new powdery mildew resistance allele in the Chinese wheat landrace Hongyoumai. Mol. Breed. 2017;37:133. doi: 10.1007/s11032-017-0728-3. [DOI] [Google Scholar]
- 11.Xu X.D., Jing F., Fan J.R., Liu Z.Y., Li Q., Zhou Y.L., Ma Z.H. Identification of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao. J. Integr. Agric. 2018;17:37–45. doi: 10.1016/S2095-3119(16)61610-6. [DOI] [Google Scholar]
- 12.Xu X.D., Li Q., Ma Z.H., Fan J.R., Zhou Y.L. Molecular mapping of powdery mildew resistance gene PmSGD in Chinese wheat landrace Shangeda using RNA-seq with bulk segregant analysis. Mol. Breed. 2018;38:23. doi: 10.1007/s11032-018-0783-4. [DOI] [Google Scholar]
- 13.Huang X.Q., Hsam S.L.K., Zeller F.J., Wenzel G., Mohler V. Molecular mapping of the wheat powdery mildew resistance gene Pm24 and marker validation for molecular breeding. Theor. Appl. Genet. 2000;101:407–414. doi: 10.1007/s001220051497. [DOI] [Google Scholar]
- 14.Xue F., Wang C.Y., Li C., Duan X.Y., Zhou Y.L., Zhao N.J., Wang Y.J., Ji W.Q. Molecular mapping of a powdery mildew resistance gene in common wheat landrace Baihulu and its allelism with Pm24. Theor. Appl. Genet. 2012;125:1425–1432. doi: 10.1007/s00122-012-1923-6. [DOI] [PubMed] [Google Scholar]
- 15.Xu H.X., Yi Y.J., Ma P.T., Qie Y.M., Fu X.Y., Xu Y.F., Zhang X.T., An D.G. Molecular tagging of a new broad-spectrum powdery mildew resistance allele Pm2c in Chinese wheat landrace Niaomai. Theor. Appl. Genet. 2015;128:2077–2084. doi: 10.1007/s00122-015-2568-z. [DOI] [PubMed] [Google Scholar]
- 16.Ma H.Q., Kong Z.X., Fu B.S., Li N., Zhang L.X., Jia H.Y., Ma Z.Q. Identification and mapping of a new powdery mildew resistance gene on chromosome 6D of common wheat. Theor. Appl. Genet. 2011;123:1099–1106. doi: 10.1007/s00122-011-1651-3. [DOI] [PubMed] [Google Scholar]
- 17.Xiao M.G., Song F.J., Jiao J.F., Wang X.M., Xu H.X., Li H.J. Identification of the gene Pm47 on chromosome 7BS conferring resistance to powdery mildew in the Chinese wheat landrace Hongyanglazi. Theor. Appl. Genet. 2013;126:1397–1403. doi: 10.1007/s00122-013-2060-6. [DOI] [PubMed] [Google Scholar]
- 18.Fu B.S., Chen Y., Li N., Ma H.Q., Kong Z.X., Zhang L.X., Jia H.Y., Ma Z.Q. pmX: A recessive powdery mildew resistance gene at the Pm4 locus identified in wheat landrace Xiaohongpi. Theor. Appl. Genet. 2013;126:913–921. doi: 10.1007/s00122-012-2025-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z.Z., Li H.W., Zhang D.Y., Guo L., Chen J.J., Chen Y.X., Wu Q.H., Xie J.Z., Zhang Y., Sun Q.X., et al. Genetic and physical mapping of powdery mildew resistance gene MlHLT in Chinese wheat landrace Hulutou. Theor. Appl. Genet. 2015;128:365–373. doi: 10.1007/s00122-014-2436-2. [DOI] [PubMed] [Google Scholar]
- 20.Li G., Carver B.F., Cowger C., Bai G., Xu X. Pm223899, a new recessive powdery mildew resistance gene identified in Afghanistan landrace PI 223899. Theor. Appl. Genet. 2018;131:2775–2783. doi: 10.1007/s00122-018-3199-y. [DOI] [PubMed] [Google Scholar]
- 21.Tan C.C., Li G.Q., Cowger C., Carver B.F., Xu X.Y. Characterization of Pm63, a powdery mildew resistance gene in Iranian landrace PI 628024. Theor. Appl. Genet. 2018 doi: 10.1007/s00122-018-3265-5. [DOI] [PubMed] [Google Scholar]
- 22.Tan C.C., Li G.Q., Cowger C., Carver B.F., Xu X.Y. Characterization of Pm59, a novel powdery mildew resistance gene in Afghanistan wheat landrace PI 181356. Theor. Appl. Genet. 2018;131:1145–1152. doi: 10.1007/s00122-018-3067-9. [DOI] [PubMed] [Google Scholar]
- 23.Newton A.C., Akar T., Baresel J.P., Bebeli P.J., Bettencourt E., Blandenopoulos K.V., Czembor J.H., Fasoula D.A., Katsiotis A., Koutis K., et al. Cereal landraces for sustainable agriculture. A review. Agron. Sustain. Dev. 2010;20:237–269. doi: 10.1051/agro/2009032. [DOI] [Google Scholar]
- 24.Kaur N., Street K., Mackay M., Yahiaoui N., Keller B. Molecular approaches for characterization and use of natural disease resistance in wheat. Eur. J. Plant Pathol. 2008;121:387–397. doi: 10.1007/s10658-007-9252-3. [DOI] [Google Scholar]
- 25.International Wheat Genome Sequencing Consortium (IWGSC) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:eaar7191. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- 26.Rasheed A., Wen W., Gao F., Zhai S., Jin H., Liu J., Guo Q., Zhang Y., Dreisigacker S., Xia X., et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016;129:1843–1860. doi: 10.1007/s00122-016-2743-x. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi N., Kandiah P., Gessese M.K., Nsabiyera V., Wells V., Babu P., Wong D., Hayden M.J., Bariana H., Bansal U. Development of co-dominant KASP markers co-segregating with Ug99 effective stem rust resistance gene Sr26 in wheat. Mol. Breed. 2018;38:97. doi: 10.1007/s11032-018-0854-6. [DOI] [Google Scholar]
- 28.Qureshi N., Bariana H.S., Zhang P., McIntosh R., Bansal U.K., Wong D., Hayden M.J., Dubcovsky J., Shankar M. Genetic relationship of stripe rust resistance genes Yr34 and Yr48 in wheat and identification of linked KASP markers. Plant Dis. 2018;102:413–420. doi: 10.1094/PDIS-08-17-1144-RE. [DOI] [PubMed] [Google Scholar]
- 29.Trick M., Adamski N.M., Mugford S.G., Jiang C., Febrer M., Uauy C. Combining SNP discovery from next-generation sequencing data with bulked segregant analysis (BSA) to fine-map genes in polyploid wheat. BMC Plant Biol. 2012;12:14. doi: 10.1186/1471-2229-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez-Gonzalez R.H., Segovia V., Bird N., Fenwick P., Holdgate S., Berry S., Jack P., Caccamo M., Uauy C. RNA-Seq bulked segregant analysis enables the identification of high-resolution genetic markers for breeding in hexaploid wheat. Plant Biotechnol. J. 2015;13:613–624. doi: 10.1111/pbi.12281. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Xie J.Z., Zhang H.Z., Guo B.M., Ning S.Z., Chen Y.X., Lu P., Wu Q.H., Li M.M., Zhang D.Y., et al. Mapping stripe rust resistance gene YrZH22 in Chinese wheat cultivar Zhoumai 22 by bulked segregant RNA-Seq (BSR-Seq) and comparative genomics analyses. Theor. Appl. Genet. 2017;130:2191–2201. doi: 10.1007/s00122-017-2950-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., Zhang H.Z., Xie J.Z., Guo B.M., Chen Y.X., Zhang H.Y., Lu P., Wu Q.H., Li M.M., Zhang D.Y., et al. Mapping stripe rust resistance genes by BSR-Seq, YrMM58 and YrHY1 on chromosome 2AS in Chinese wheat lines Mengmai 58 and Huaiyang 1 are Yr17. Crop J. 2018;6:91–98. doi: 10.1016/j.cj.2017.03.002. [DOI] [Google Scholar]
- 33.Wu J.H., Zeng Q.D., Wang Q.L., Liu S.J., Yu S.Z., Mu J.M., Huang S., Sela H., Distelfeld A., Huang L.L., et al. SNP-based pool genotyping and haplotype analysis accelerate fine-mapping of the wheat genomic region containing stripe rust resistance gene Yr26. Theor. Appl. Genet. 2018;131:1481–1496. doi: 10.1007/s00122-018-3092-8. [DOI] [PubMed] [Google Scholar]
- 34.Wu P.P., Xie J.Z., Hu J.H., Qiu D., Liu Z.Y., Li J.T., Li M.M., Zhang H.J., Yang L., Liu H.W., et al. Development of molecular markers linked to powdery mildew resistance gene Pm4b by combining SNP discovery from transcriptome sequencing data with bulked segregant analysis (BSR-Seq) in wheat. Front. Plant Sci. 2018;9:95. doi: 10.3389/fpls.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H.G., Hu J.H., Song W., Qiu D., Cui L., Wu P.P., Zhang H.J., Liu H.W., Yang L., Qu Y.F., et al. Pm61: A recessive gene for resistance to powdery mildew in wheat landrace Xuxusanyuehuang identified by comparative genomics analysis. Theor. Appl. Genet. 2018;131:2085–2097. doi: 10.1007/s00122-018-3135-1. [DOI] [PubMed] [Google Scholar]
- 36.Geng M.M., Zhang J., Peng F.X., Liu X., Lv X.D., Mi Y.Y., Li Y.H., Li F., Xie C.J., Sun Q.X. Identification and mapping of MlIW30, a novel powdery mildew resistance gene derived from wild emmer wheat. Mol. Breed. 2016;36:130. doi: 10.1007/s11032-016-0553-0. [DOI] [Google Scholar]
- 37.Janáková E., Jakobson I., Peusha H., Abrouk M., Škopová M., Šimková H., Šafář J., Jan Vrána J., Doležel J., Järve K., et al. Divergence between bread wheat and Triticum militinae in the powdery mildew resistance QPm.tut-4A locus and its implications for cloning of the resistance gene. Theor. Appl. Genet. 2018 doi: 10.1007/s00122-018-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semagn K., Babu R., Hearne S., Olsen M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014;33:1–14. doi: 10.1007/s11032-013-9917-x. [DOI] [Google Scholar]
- 39.Allen A.M., Barker G.L., Wilkinson P., Burridge A., Winfield M., Coghill J., Uauy C., Griffiths S., Jack P., Berry S., et al. Discovery and development of exome-based, co-dominant single nucleotide polymorphism markers in hexaploid wheat (Triticum aestivum L.) Plant Biotechnol. J. 2013;11:279–295. doi: 10.1111/pbi.12009. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z., Sun Q., Ni Z., Yang T., McIntosh R.A. Development of SCAR markers linked to the Pm21 gene conferring resistance to powdery mildew in common wheat. Plant Breed. 1999;118:215–219. doi: 10.1046/j.1439-0523.1999.118003215.x. [DOI] [Google Scholar]
- 41.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saghai-Maroof M.A., Soliman K.M., Jorgensen R.A., Allard R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Tiwari V.K., Rawat N., Gill B.S., Huo N.X., You F.M., Coleman-Derr D., Gu Y.Q. GSP: A web-based platform for designing genome-specific primers in polyploids. Bioinformatics. 2016;32:2382–2383. doi: 10.1093/bioinformatics/btw134. [DOI] [PubMed] [Google Scholar]
- 46.Ramirez-Gonzalez R.H., Uauy C., Caccamo M. PolyMarker: A fast polyploid primer design pipeline. Bioinformatics. 2015;31:2038–2039. doi: 10.1093/bioinformatics/btv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R.H., Meng J.L. MapDraw: A Microsoft excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas. 2003;25:317–321. [PubMed] [Google Scholar]
- 48.Lincoln S.E., Daly M.J., Lander E.S. Constructing Genetic Linkage Maps with MAPMAKER/EXP Version 3.0: A Tutorial and Reference Mannual. 3rd ed. Whitehead Institute for Medical Research; Cambridge, MA, USA: 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.