Abstract
Despite recent therapeutic advances, systemic mastocytosis (SM) remains an incurable disease due to limited complete remission (CR) rates even after novel therapies. To date, no study has evaluated the expression on SM bone marrow mast cells (BMMC) of large panel of cell surface suitable for antibody-targeted therapy. In this study, we analyzed the expression profile of six cell-surface proteins for which antibody-based therapies are available, on BMMC from 166 SM patients vs. 40 controls. Overall, variable patterns of expression for the markers evaluated were observed among SM BMMC. Thus, CD22, CD30, and CD123, while expressed on BMMC from patients within every subtype of SM, showed highly variable patterns with a significant fraction of negative cases among advanced SM (aggressive SM (ASM), ASM with an associated clonal non-MC lineage disease (ASM-AHN) and MC leukemia (MCL)), 36%, 46%, and 39%, respectively. In turn, CD25 and FcεRI were found to be expressed in most cases (89% and 92%) in virtually all BMMC (median: 92% and 95%) from both indolent and advanced SM, but with lower/absent levels in a significant fraction of MC leukemia (MCL) and both in MCL and well-differentiated SM (WDSM) patients, respectively. In contrast, CD33 was the only marker expressed on all BMMC from every SM patient. Thus, CD33 emerges as the best potentially targetable cell-surface membrane marker in SM, particularly in advanced SM.
Keywords: hematology, immunology, systemic mastocytosis, monoclonal antibodies, cell therapy and immunotherapy, antibody-targetable cell surface membrane proteins, immuno-phenotyping
1. Introduction
Systemic mastocytosis (SM) consists of a heterogeneous group of clonal mast cell (MC) disorders [1,2] that vary from indolent cases—e.g., indolent SM (ISM)—and that impair the quality of life of patients with a normal life-expectancy, to advanced forms of the disease—e.g., aggressive SM (ASM), SM with an associated clonal non-MC lineage disease (SM-AHN), and mast cell leukemia (MCL)—associated with a significantly shortened survival [3]. In the vast majority of SM patients (>90%), the clonal nature of pathological MC can be demonstrated by the presence of the stem cell factor receptor gene (KIT) D816V mutation [4], except for well-differentiated SM (WDSM) patients [5] and a fraction of MCL [6]. This mutation results in constitutive activation of KIT, which leads to aberrantly sustained proliferative and survival signals in neoplastic MC, that ultimately lead to their expansion and accumulation in distinct tissues, particularly in the skin and bone marrow (BM) [7].
For decades, conventional chemotherapeutic regimens administered to patients with advanced forms of SM have proven to be of limited benefit with low complete remission (CR) rates and only minor improvement on survival [7,8,9]. Wild-type KIT can be currently targeted by a progressively higher number of small tyrosine-kinase inhibitor (TKI) molecules including some that—e.g., midostaurin (PKC412) or imatinib—have proven beneficial for SM [10,11,12,13]. However, overall CR rates, even with these new drugs still remain low, except among the few WDSM patients presenting with mutations at exons 9 and 10 of KIT [14,15,16,17,18]. Altogether, this highlights the need for further improvement in the treatment of SM, particularly for advanced SM [19]. In recent years, immunotherapy, including immunotherapy strategies based on targeting cell-surface membrane proteins, has proven to be of great clinical benefit and has become a cornerstone in the treatment of an increasingly higher number of distinct hematologic malignancies [20]. However, their clinical use in SM remains very limited [21].
At present it is well-known that multiple factors are involved in determining the response to antibody-based therapies. Despite this, a pre-requisite to achieve an optimal response to such therapies is the expression of the targeted protein on the whole tumor MC population in a per patient basis [22,23]. Multiple studies have described the overall patterns of expression of many proteins on the surface membrane of both normal and SM MC, for which distinct therapeutic antibody-based molecules have been designed, evaluated, and approved for their use in tumoral and non-tumoral human diseases [22,24,25]. These antibody-targetable cell surface membrane proteins include CD22, CD25, CD30, CD33, CD123, and FcϵRI, which have all been found in tumor MC from SM patients [22] (Table 1). Some of these markers have even been targeted by therapeutic antibodies outside clinical trials, usually in small series of SM patients and single case reports, with variable responses [26,27,28,29]. However, these immuno-phenotypic studies failed to provide information about the patterns of expression of the involved markers within individual patients and across distinct disease subtypes, particularly among advanced SM cases.
Table 1.
List of monoclonal antibodies directed against mast cell-associated cell surface markers that have been approved by the US Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) for therapeutic use in humans or that are being evaluated in ongoing clinical trials.
| Targeted Protein (Gene Symbol) | Monoclonal Antibody Name | Mechanism of Action | Year of Approval | Approved Indication(s) | References | ORR (CR) Rates in SM |
|---|---|---|---|---|---|---|
|
CD22
(Siglec-2) |
Inotuzumab ozogamicin | Immunotoxin | 2017 ¥§ | B-cell precursor (BCP) acute lymphoblastic leukemia | [30,31] | - |
| Moxetumomab pasudotox | 2018 § | Hairy cell leukemia | [32,33] | - | ||
|
CD25
(IL2RA) |
Daclizumab | ADCC | 1997 §# 1999 ¥# |
Prophylaxis of acute organ rejection in renal transplantation | [34] | 2/5 (40%) (0/5 (0%)) [28] |
| Basiliximab | 1998 ¥§ | Prophylaxis of acute organ rejection in renal transplantation | [35,36] | - | ||
| Daclizumab | 2016 ¥§# | Relapsing forms of multiple sclerosis | [37,38,39,40] | - | ||
|
CD30
( TNFRFS8) |
Brentuximab vedotin | Immunotoxin/ADCP | 2011 § 2012 ¥ |
Hodgkin lymphoma/cutaneous lymphoma/peripheral T-cell lymphoma | [41,42,43,44,45,46] | 2/4 50% (0/4 (0%)) [29] |
|
CD33
(Siglec-3) |
Gemtuzumab ozogamicin | Immunotoxin | 2000 # 2017 ¥§ |
Acute myeloid leukemia | [47,48,49] | (2/2 (100%)) [27,50] |
|
CD123
(IL3RA) |
Talacotuzumab | ADCC | 2018 * | Acute myeloid leukemia | - | - |
|
FcεRI
(High-affinity immunoglobulin ε heavy chain receptor I) |
Omalizumab | ADCC | 2003 § 2005 ¥ |
Moderate to severe persistent asthma | [51,52,53] | 10/21 (48%) (11/21 (52%)) [54,55,56,57,58,59,60,61,62] |
# Marketing discontinued for the first approved indication. ¥ Year of approval in the European Union. § Year of approval in the United States of America. * Undergoing phase III clinical trials. Abbreviations: ADC, antibody-drug conjugate, ADCC, antibody-dependent cell-mediated cytotoxicity, ADCP, antibody-dependent cellular phagocytosis, CAR, chimeric antigen receptor, CR, complete remission, ORR, overall response rate.
In this study, we provide, for the first time, detailed information about the patterns of expression on BMMC from SM patients (n = 166) with distinct World Health Organization (WHO) diagnostic categories of the disease, of six surface proteins known to be expressed on BMMC, and for which the US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA)-approved for safety antibody therapies are available for humans (CD22, CD25, CD30, CD33, CD123 and FcϵRI). Our major goal was to identify, among all the markers, those that would show the highest and broadest expression on BMMC from individual patients across the distinct variants of the disease, particularly in advanced SM, which makes them potentially suitable candidates for currently available antibody-targeted therapies, whenever these are coupled with the appropriate antibody-mediated effector mechanisms.
2. Results
2.1. SM Patients and Samples
A total of 206 BM samples from 116 SM patients and 40 controls (normal/reactive BM) were investigated. In each sample, CD117hi CD45int BMMC were analysed by flow cytometry for the expression of the distinct markers evaluated here: CD22, CD25, CD30, CD33, CD123, and FcϵRI.
2.2. Immuno-Phenotypic Characteristics of Normal/Reactive BMMC
MC from normal vs. reactive BM (control) samples showed overall similar immuno-phenotypic profiles (data not shown). Overall, reactivity for CD22 was found in the majority of control samples investigated (78%) (Figure 1A) with a median percentage of CD22+ MC of 89% (range: 0% to 100%) (Table 2). In turn, normal/reactive BMMC tested systematically positive for CD33 (100%) and FcεRI (100%) (Table 2), whereas CD25, CD30, and CD123 were found to be constantly absent on MC from normal/reactive BM (Table 2).
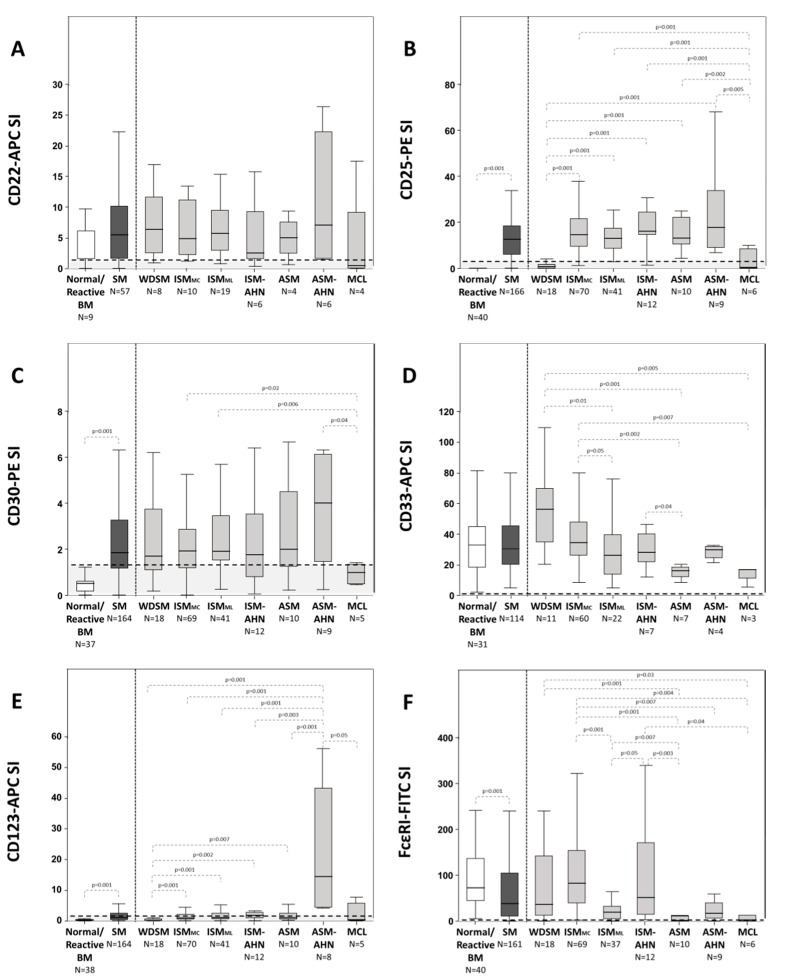
Figure 1.
Immunophenotypic profile of BMMC from healthy subjects vs. SM patients classified according to the distinct diagnostic and prognostic categories of the disease. Results are expressed as stain index (SI, difference in signal width between the positive and the negative population divided by the signal spread of the negative population) for the CD22-APC (panel A), CD25-PE (panel B), CD30-PE (panel C), CD33-APC (panel D), CD123-APC (panel E) and FCεRI-FITC (panel F) markers. The SI threshold for positivity was set at ≥1.5 and is represented by horizontal dotted lines. p-values < 0.05 were considered to be associated with statistical significance (thin dotted lines). Mann Whitney-U test was used for comparisons between continuous variables (SI values) from normal/reactive individuals vs. SM patients or among the different subtypes of SM (SPSS 23 software, IBM, Armonk, NY, USA).
Table 2.
Expression profile for each immunophenotypic marker investigated on mast cells from normal/reactive BM vs. systemic mastocytosis patients distributed according to the diagnostic/prognostic category of the disease.
| Normal BM (N = 40) |
SM (N = 166) |
WDSM (N = 18) |
ISMMC (N = 70) |
ISMML (N = 41) |
ISM-AHN (N = 12) |
ASM (N = 10) |
ASM-AHN (N = 9) |
MCL (N = 6) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| CD22 | N | 9 | 57 | 8 | 10 | 19 | 6 | 4 | 6 | 4 |
| % positive patients | 78 | 77 | 75 | 90 | 79 | 83 | 75 | 83 | 25 | |
| p-value | - | - | - | ¥ p = 0.02 |
¥ p = 0.03 |
- | - | - | §$ p ≤ 0.03 |
|
| % positive cells | 89 (0–100) |
93 (0–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
83 (0–100) |
75 (0–100) |
100 (100–100) |
50 (0–100) |
|
| p-value | - | - | ¥ p = 0.03 |
¥ p = 0.02 |
¶¥ p = 0.03 |
- | $ p = 0.03 |
- | †§$ p ≤ 0.03 |
|
| MFI | 1333 (8–3431) |
2629 (1–11,398) |
2187 (192–6614) |
2363 (424–5081) |
2959 (348–11,398) |
3086 (293–8612) |
3521 (246–6760) |
2841 (455–4435) |
721 (1–2398) |
|
| p-value | - | - | - | - | ¥ p = 0.006 |
- | - | ¥ p = 0.04 |
$‡ p ≤ 0.04 |
|
| CD25 | N | 40 | 166 | 18 | 70 | 41 | 12 | 10 | 9 | 6 |
| % positive patients | 0 | 89 | 33 | 99 | 100 | 92 | 100 | 100 | 33 | |
| p-value | - | * p = 0.001 |
§$‖¶‡ p ≤ 0.002 |
†¥ p = 0.001 |
†¥ p = 0.001 |
†¥ p ≤ 0.009 |
†¥ p ≤ 0.003 |
†¥ p ≤ 0.004 |
§$‖¶‡ p ≤ 0.009 |
|
| % positive cells | 0 (0–0) |
92 (0–100) |
44 (0–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
33 (0–100) |
|
| p-value | - | * p = 0.001 |
§$‖¶‡ p ≤ 0.005 |
†¥ p = 0.001 |
†¥ p = 0.001 |
†¥ p ≤ 0.002 |
†¥ p = 0.003 |
†¥ p ≤ 0.005 |
§$‖¶‡ p ≤ 0.004 |
|
| MFI | 0 (0–0) |
5331 (4–21,943) |
471 (4–4159) |
6448 (806–17,552) |
5427 (2016–14,639) |
6544 (2329–16,474) |
6862 (1406–21,943) |
5371 (2376–11,108) |
1190 (21–4922) |
|
| p-value | - | * p = 0.001 |
§$‖¶‡ p = 0.001 |
†¥ p = 0.001 |
†¥ p = 0.001 |
†¥ p = 0.001 |
†¥ p ≤ 0.005 |
†¥ p ≤ 0.008 |
§$‖¶‡ p ≤ 0.008 |
|
| CD30 | N | 37 | 164 | 18 | 69 | 41 | 12 | 10 | 9 | 5 |
| % positive patients | 5 | 63 | 61 | 61 | 76 | 58 | 70 | 67 | 0 | |
| p-value | - | * p = 0.001 |
¥ p = 0.02 |
¥ p = 0.008 |
¥ p = 0.001 |
¥ p = 0.03 |
¥ p = 0.01 |
¥ p = 0.02 |
†§$‖¶‡ p ≤ 0.03 |
|
| % positive cells | 0 (0–0) |
88 (0–100) |
94 (0–100) |
87 (0–100) |
90 (0–100) |
83 (0–100) |
90 (0–100) |
89 (0–100) |
0 (0–0) |
|
| p-value | - | * p = 0.001 |
¥ p = 0.001 |
¥ p = 0.001 |
¥ p = 0.001 |
¥ p = 0.001 |
¥ p = 0.001 |
¥ p = 0.001 |
†§$‖¶‡ p = 0.001 |
|
| MFI | 129 (1–427) |
887 (0–6261) |
632 (202–1677) |
860 (0–3040) |
993 (199–3038) |
796 (103–2720) |
1095 (74–2847) |
1345 (202–6261) |
297 (153–495) |
|
| p-value | - | * p = 0.001 |
§$¥ p ≤ 0.02 |
†¥ p ≤ 0.02 |
†¥ p ≤ 0.01 |
- | - | ¥ p = 0.02 |
†§$‡ p ≤ 0.02 |
|
| CD33 | N | 31 | 114 | 11 | 60 | 22 | 7 | 7 | 4 | 3 |
| % positive patients | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| p-value | - | - | - | - | - | - | - | - | - | |
| % positive cells | 100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
100 (100–100) |
|
| p-value | - | - | - | - | - | - | - | - | - | |
| MFI | 8163 (2225–16,377) |
19,667 (2308–53,009) |
16,642 (5774–50,767) |
22,673 (4926–53,009) |
20,576 (2922–44,241) |
15,566 (5441–24,537) |
10,063 (2698–18,730) |
8447 (4022–14,916) |
10,917 (2308–23,681) |
|
| p-value | - | * p = 0.001 |
§ p = 0.03 |
†¶‡ p ≤ 0.03 |
¶ p = 0.02 |
- | §$ p ≤ 0.02 |
§ p = 0.002 |
- | |
| CD123 | N | 38 | 164 | 18 | 70 | 41 | 12 | 10 | 8 | 5 |
| % positive patients | 3 | 38 | 11 | 37 | 32 | 67 | 40 | 100 | 40 | |
| p-value | - | * p = 0.001 |
§‖‡ p ≤ 0.03 |
†‡ p ≤ 0.03 |
‖‡ p ≤ 0.03 |
†$ p ≤ 0.03 |
‡ p = 0.007 |
†§$¶¥ p ≤ 0.01 |
‡ p = 0.01 |
|
| % positive cells | 0 (0–0) |
64 (0–100) |
17 (0–100) |
66 (0–100) |
73 (0–100) |
70 (0–100) |
70 (0–100) |
100 (100–100) |
40 (0–100) |
|
| p-value | - | * p = 0.001 |
§$‖¶‡ p = 0.001 |
†‡ p ≤ 0.05 |
† p = 0.001 |
† p = 0.001 |
† p = 0.001 |
†§¥ p ≤ 0.05 |
‡ p = 0.01 |
|
| MFI | 84 (8–221) |
1340 (3–33 861) |
214 (3–2023) |
1024 (17–5425) |
962 (15–4045) |
3685 (24–33,861) |
939 (94–3220) |
5825 (755–18,421) |
903 (14–3085) |
|
| p-value | - | * p = 0.001 |
§$‖¶‡ p = 0.001 |
†‡ p = 0.001 |
†‡ p = 0.001 |
†‡ p ≤ 0.01 |
†‡ p ≤ 0.003 |
†§$‖¶¥ p ≤ 0.03 |
‡ p = 0.03 |
|
| FcεRI | N | 40 | 161 | 18 | 69 | 37 | 12 | 10 | 9 | 6 |
| % positive patients | 100 | 92 | 94 | 99 | 89 | 100 | 60 | 89 | 67 | |
| p-value | - | - | ¶ p = 0.02 |
$¶¥ p ≤ 0.03 |
§¶ p = 0.03 |
¶¥ p ≤ 0.03 |
†§$‖ p ≤ 0.03 |
- | §‖ p ≤ 0.03 |
|
| % positive cells | 100 (100–100) |
95 (0–100) |
94 (0–100) |
100 (100–100) |
95 (0–100) |
100 (100–100) |
70 (0–100) |
100 (100–100) |
67 (0–100) |
|
| p-value | - | - | § p = 0.05 |
†¶¥ p ≤ 0.05 |
¶¥ p = 0.001 |
¶¥ p = 0.04 |
§$‖ p ≤ 0.04 |
- | §$‖ p ≤ 0.03 |
|
| MFI | 29,073 (2181–81,199) |
49,387 (14–239,335) |
40,350 (110–183,969) |
78,922 (1271–239,335) |
24,084 (73–162,320) |
62,685 (1938–177,643) |
4858 (176–26,255) |
11,084 (680–36,688) |
9909 (14–52,514) |
|
| p-value | - | - | §¶¥ p ≤ 0.02 |
†$¶‡¥ p ≤ 0.002 |
§‖¶ p ≤ 0.04 |
$¶‡¥ p ≤ 0.04 |
†§$‖ p ≤ 0.005 |
§‖ p ≤ 0.04 |
†§‖ p ≤ 0.02 |
Results expressed as median values and range between brackets. Abbreviations: BM, bone marrow, WDSM, well-differentiated SM, ISMMC, indolent SM with KIT mutation restricted to mast cells, ISMML, ISM with multi-lineal KIT mutation, ISM-AHN, ISM associated with a clonal non-mast cell lineage hematopoietic disease, ASM, aggressive SM, ASM-AHN, ASM associated with a clonal non-mast cell lineage hematopoietic disease, MCL, mast cell leukemia, SI, stain index is the difference in signal width between the positive and the negative population divided by the signal spread of the negative population, and MFI, median fluorescence intensity (arbitrary values scaled from 0 to 222 144). p-values < 0.05 were considered to be associated with statistical significance for comparisons against: * normal/reactive BM, † WDSM, § ISMMC, $ ISMML, ‖ ISM-AHN, ¶ ASM, ‡ ASM-AHN, and ¥ MCL cases.
2.3. Immuno-Phenotypic Features of BMMC from SM Patients
CD22 was positive on BMMC from SM cases at similar percentages to those observed on normal/reactive BMMC (77% vs. 78%, p > 0.05) (Table 2), with similar median percentages of CD22+ cells (93% vs. 89% in normal BMMC, range: 0–100%, p > 0.05) (Table 2), CD22+ SM and normal/reactive BMMC also showed similar overall levels of expression for this marker (Figure 1A). In contrast, aberrant CD25 expression was found on BMMC from the great majority of SM patients (89% vs. 0% in normal BMMC, p = 0.001) (Table 2 and Figure 1B) with a relatively high median (92% vs. 0%, range: 0 to 100%, p = 0.001) percentage of CD25+ BMMC (Table 2). In addition, BMMC from SM patients also showed a greater rate of positivity for CD30 (63% vs. 5%, p = 0.001) and CD123 (38% vs. 3%, p = 0.001) than normal/reactive BMMC (Table 2, Figure 1C,E). This translated into aberrant CD25, CD30, and CD123 expression levels on BMMC from SM patients (vs. normal/reactive BMMC), both in terms of SI (p = 0.001) (Figure 1B,C,E) and mean fluorescence intensity (MFI) values (p = 0.001) (Table 2).
Similarly to normal/reactive MC, tumor BMMC from SM patients were also systematically positive for CD33 (100%, p > 0.05) on their surface membrane, at similarly high and homogenous levels as evaluated by the stain index (SI) (Figure 1D) and its coefficient of variation within individual cases (data not shown). In addition, reactivity for FcεRI was found in the great majority of SM cases (92% vs. 100% in normal BMMC; p = 0.05) (Table 2 and Figure 1F) with most BMMC (but not always all) typically showing FcεRI+ expression on their surface (95% vs. 100% in normal BMMC, p > 0.05) (Table 2).
Of note, no clearly strong (σ < 0.45) correlation was found between the expresion levels observed for the distinct cell surface proteins analyzed.
2.4. Immuno-Phenotypic Features of BMMC from Patients with Different Diagnostic and Prognostic Categories of SM
Similar percentages of CD22+ cases were found among all distinct diagnostic subtypes of SM (range: 75% to 90% of CD22+ cases) but MCL, which showed a lower rate of positivity (25%, p ≤ 0.03 vs. ISM groups) (Table 2). In line with this observation, the median percentage of CD22+ BMMC per patient, as well as CD22 expression levels per BMMC, were both similar among all patient groups except MCL, which showed significantly lower values (p ≤ 0.03 vs. all other groups of SM) (Table 2).
Regarding CD25, every BMMC from all ISM (100%) and ASM (100%) patients aberrantly expressed this marker on their surface, while it was present in only one third of WDSM and MCL cases, where it was also expressed at significantly lower percentages including a median of CD25+ BMMC of 44% (p ≤ 0.002 vs. ISM and p ≤ 0.005 vs. ASM subtypes) and 33% (p = 0.001 vs. ISM and p ≤ 0.004 vs. ASM), respectively (Table 2). Among all SM groups, ISM and ASM patients displayed the highest levels of CD25 per BMMC (p = 0.001 and p = 0.001 vs. BMMC from WDSM cases and p = 0.001 and p ≤ 0.005 vs. MCL patients) (Table 2 and Figure 1B).
Of note, CD30 expression was aberrantly high in a fraction of patients from every subtype of SM (p < 0.03) (Table 2). Despite this, in many SM patients, CD30 was not expressed by all tumor BMMC, the percentage of CD30+ BMMC in the different diagnostic subtypes of SM ranging from 0% in MCL patients to 94% in WDSM cases (p = 0.001), respectively (Table 2). Among the distinct diagnostic subgroups of SM, similar (but highly variable) CD30 expression levels per BMMC, were observed (p > 0.05) (Figure 1C).
Although CD33 was systematically expressed in all BMMC from SM patients (Table 2), among SM cases, BMMC from WDSM and ISMMC patients displayed greater levels of CD33 (expressed per cell) than ISMML patients (p = 0.01 and p = 0.05), ASM (p = 0.001 and p = 0.002), and MCL (p = 0.005 and p = 0.007) (Figure 1D). In contrast, ASM patients showed lower levels of CD33 compared to ISM-AHN (p = 0.04), WDSM (p = 0.001), and ISMMC (p = 0.002) cases (Figure 1D).
Additionally, CD123 only tested systematically positive in all BMMC from ASM-AHN cases (100%), the median percentage of CD123+ BMMC in the other subtypes of SM ranging from 17% in WDSM to 73% in ISMML (Table 2). In line with this, CD123 expression levels per BMMC were also higher among ASM-AHN cases vs. all other subtypes of SM (p ≤ 0.05). WDSM patients show the lowest expression levels (p ≤ 0.007 vs. all other groups) (Figure 1E).
Lastly, FcεRI was expressed in virtually all WDSM and ISM subtypes of SM (range: 89% to 100% cases) while showing a lower reactivity (p ≤ 0.03) in more advanced forms of SM (e.g., ASM and MCL), similarly to CD33. ISMML and ASM-AHN displayed intermediate FcεRI expression values (Figure 1F). Similarly, the percentage of FcεRI+ BMMC was high among all subtypes of SM except ASM and MCL (70% and 67%, respectively), that showed significantly lower percentages of FcεRI+ tumor BMMC (Table 2) together with significantly lower levels of this marker per MC (p ≤ 0.04 and p ≤ 0.03 vs. all groups of indolent SM) (Table 2 and Figure 1F).
3. Discussion
Currently, a high number of cytotoxic agents and targeted therapies exists for the treatment of advanced SM. Furthermore, several of them were proven to be of clinical benefit. Despite this, SM still remains an incurable disease with limited CR rates [63,64]. Thus, recent reports show increased overall response rates after treatment with midostaurin (60%) [10] particularly in advanced SM, but in the absence of any CR. Similarly, in a recent trial based on a small number (n = 10) of imatinib-treated SM patients, a high CR rate (40%) was reported, but only for patients carrying mutations in the extracellular region of KIT (K509I) (n = 3) or with wild-type KIT (n = 1), typically with a unique CD25− and CD2− phenotype [27], and that represent a minor fraction (≤5%) of all (indolent and advanced) SM cases [11]. Altogether, these results point out the need to search for new drugs and treatment modalities that might contribute to increased CR rates in SM and to improve the outcome of SM patients with advanced forms of the disease.
In recent years, immunotherapy has emerged as one of the most attractive and efficient cancer treatment strategies, particularly through antibody-mediated tumor-cell targeted therapies [20,65,66]. Despite the highly diverse antibody-based strategies that have been developed so far, with regard to the specific effector mechanisms used, e.g., from complement-mediated, macrophage-mediated, and/or NK cell-mediated antibody-dependent cytotoxicity approaches to chimeric antigen receptor (CAR)-cells, the efficacy of antibody-based immunotherapy requires the expression of enough amounts of the targeted protein on every individual (relevant) tumor cell [65,67,68,69,70,71,72].
Despite a great amount of data that has been generated about the phenotypic profiles of BMMC in SM, no study has specifically focused on the analysis of the patterns of expression of cell surface membrane proteins in every individual tumor cell that might be targeted by currently available antibody-based drugs approved for in vivo use in humans, in a per patient basis. In addition, no studies detailed analysis of the immunophenotypic profile of individual tumor MC within a large series of individual SM patients with distinct diagnostic subtypes of SM have been performed, based on standardized flow cytometry approaches, as seen here. Thus, we performed such an analysis for the first time, by investigating the expression of a broad panel of markers (CD22, CD25, CD30, CD33, CD123, and FcϵRI) that have been reported to be expressed on clonal BMMC from SM patients. Our major goal was to identify the best candidate marker(s) to be targeted by currently available antibody-based therapies approved by the FDA and/or EMA for their use in humans based on its pattern of expression on tumor MC. At this point, evaluation among the available antibody drugs among their specific effector mechanisms was not an aim of this study and requires further investigation.
Overall, our results confirm and extend on previous observations [24,25,73] regarding the existence of variable patterns of expression on BMMC from SM patients for all individual markers evaluated (i.e., CD22, CD25, CD30, CD33, CD123, and FcϵRI). Despite such heterogeneity, CD33 emerged as the only marker that is expressed on the cell surface of virtually every BMMC from individual patients of every subtype of SM even though greater expression levels were found in indolent SM and WDSM vs. advanced SM (i.e., ASM, SM-AHN and MCL). In turn, high levels of FcεRI and aberrant CD25 expression were found on tumor BMMC from the great majority of SM patients. However, FcεRI and CD25-negative BMMC were found in a fraction of the patients, particularly among advanced SM, CD25 is also absent in most WDSM patients.
In contrast, highly variable patterns of expression of CD22, CD30, and CD123 were observed both among distinct patients, and among different BMMC within a patient, pointing out these possibly as less suitable targets for antibody therapy. Despite this, CD123 should still probably be considered as a potentially useful target particularly in ASM-AHN cases where it tested systematically positive in the ASM component in parallel to a fraction of the AHN blast and tumor precursor cells (data not shown). Overall, these results confirm and extend on previous observations about the immunophenotypic profiles of BMMC in distinct diagnostic categories of SM. At the same time, they point out that CD33, and to a less extent also CD25 and FcϵRI, are very well-suited BMMC surface markers for targeted antibody therapies, while CD22, CD30, and CD123 are less suited cell surface targets except for CD123 in ASM-AHN cases. Further studies including quantitative flow cytometric methods with a panel of distinct reagents against CD33 are needed to confirm our results, since CD33 expression levels have also been associated previously with a further response to the anti-CD33 Mylotarg antibody in acute myeloid leukemia (AML) [74,75].
So far, only a few single case reports and small patient series that have been treated with antibody-targeted therapies have been reported in the literature. This includes four ASM patients [28] and one smoldering SM (SSM) case [50] treated with daclizumab (anti-CD25), four patients (three ASM and one ASM) treated with brentuximab-vedotin (anti-CD30) [29], one patient diagnosed with SM-AHN, one with MCL receiving gemtuzumab ozogamicin (anti-CD33) [26,27], and several SM patients with distinct subtypes of SM receiving omalizumab (anti-FcεRI) with significant improvement of symptoms but no objective reduction of tumor cell burden in most tested cases (CR: 52%, 11/21, major response: 33%, 7/21, partial response: 14%, 3/21) [54,55,56,57,58,59,60,61,62]. Moreover, some cutaneous mastocytosis (CM) patients have also been successfully treated with omalizumab with CR in 1/3 treated patients [76,77,78]. In contrast, no SM patients treated with anti-CD22 or anti-CD123 antibody-based immunotherapy have been reported so far.
In contrast to anti-CD30 and FcϵRI therapy, which were both associated with relatively poor responses in SM (e.g., overall response rate of 50% and 100% based on the improvement of symptoms but with only 52% partial responses, respectively) in the absence of any CR [29,54,55,56,57,58,59,60,61,62], anti-CD25, and anti-CD33 have shown more promising results [26,27,28,50]. Thus, a single dose of daclizumab, which is an anti-CD25 monoclonal antibody, was first administered as immunosuppressive therapy to an SSM patient who underwent a heart transplant. After treatment, a dramatic and sustained decrease in serum tryptase levels were observed, which suggests a significant response of her SSM [50]. Subsequently, a few ASM patients (n = 4) were also treated with daclizumab, with partial responses (25%). However, a limited effect on reducing the BMMC burden, which is associated with severe adverse effects (hypotension, tachycardia, and intense pain in back, hips, and femurs due to the massive release of intracellular MC granules) [28], suggests that daclizumab therapy might have some activity in a small subset of patients with advanced SM. In turn, sustained CR has been reported after anti-CD33 (gentuzumab ozogamicin) in an adult MCL patient who did not respond previously to imatinib, interferon, hydroxyurea, midostaurin, and cladribine [27], and in an SM associated with the AML child who finally died from relapsing AML [26]. In contrast, therapy with the humanized anti-CD30 brentuximab-vedotin antibody, that acts via a potent antimitotic agent (auristatin E) emerged as a potential candidate therapy for SM due to its ability (i) to induce apoptosis on neoplastic MC, (ii) to down-regulate of IgE-mediated histamine release, and (iii) to synergize with midostaurin to inhibit neoplastic MC growth in vitro [79]. However, once administered in vivo to SM patients, it was associated with severe adverse effects in the absence of CR [29]. Similarly, omalizumab—a humanized IgG kappa monoclonal antibody that acts via antibody-dependent cellular cytotoxicity (ADCC)—was initially shown to successfully control severe MC mediator-related symptoms in a CM patient [76,77,78]. This led to further administration of the drug to SM patients who displayed refractory symptoms of allergy in whom the drug proved to reduce the frequency of anaphylactic episodes in the absence of CR (or any significant effect on reducing the tumor MC burden), as reflected by the persistence of (stable) increased serum tryptase levels during treatment [54,55,56,57,58,59,60,61,62].
In summary, our results suggest that CD33 might potentially be the best candidate marker for BMMC surface protein targeted antibody therapy, while CD25 and FcεRI are potential alternative targets, whenever adequate effector cytotoxic mechanisms are used. In contrast, CD22, CD30, and CD123 emerge as less suited targets for immunotherapy in SM patients due to a lack of expression in a significant fraction of patients with ISM and particularly advanced forms of SM. The only exception is the potential utility of anti-CD123 therapy in ASM-AHN cases. However, our results are still preliminary since further in vitro and in vivo studies, as well as clinical trials, are required to confirm the potential efficacy and benefits of the specific antibody therapies.
4. Materials and Methods
4.1. Patients, Controls, and Samples
Overall, 206 BM samples from an identical number of adults—40 controls (6 normal BM samples from healthy subjects and 34 reactive BM samples, which correspond to patients undergoing BM aspiration due to a suspicion of a mast cell activation syndrome confirmed to be secondary to distinct underlying non-neoplastic conditions) and 166 patients diagnosed with SM at the reference centers of the Spanish Network on Mastocytosis (REMA, Mast Cell Unit, Hospital Virgen del Valle, Toledo, and, Cancer Research Center, Salamanca, Spain) were studied. According to the WHO criteria [3] and the pattern of BM involvement by the KIT mutation, patients were classified as: ISM, 111 cases (70 showed MC-restricted KIT mutation (ISMMC) and 41 multilineage involvement of BM haematopoiesis by the KIT mutation (ISMML)), ASM, 10 patients, SM-AHN, 21 (12 ISM-AHN and 9 ASM-AHN), and MCL, 6 patients (aleukemic acute (n = 4) and chronic (n = 1) MCL variants, and 1 chronic leukemic MCL variant). The remaining 18 cases corresponded to WDSM variants of the disease [5]. All patients and controls gave their written informed consent to participate prior to entering the study, according to the Declaration of Helsinki, and the study was approved by the local Ethics Committee of the two participating centers.
4.2. Multi-Parameter Flow Cytometry Immuno-Phenotypic Studies
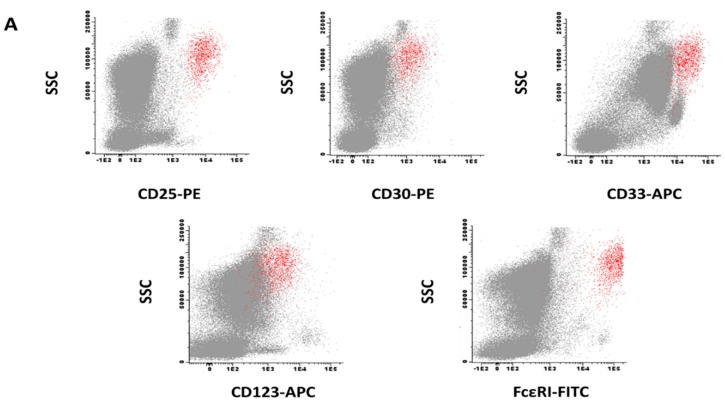
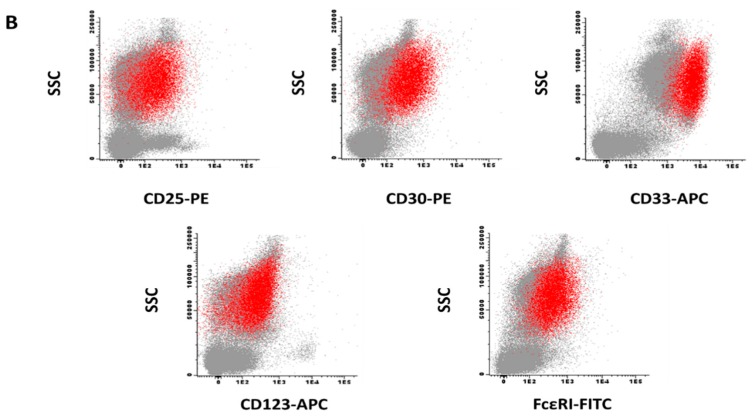
Multi-parameter flow cytometry immunophenotypic studies were performed on aspirated BM samples using a standardized stain-and-then-lyse direct immunofluorescence technique, as described elsewhere [24,80]. Additionally, 200 to 300 μL of BM per tube were stained for 30 min (room temperature) in the darkness with the antibody combinations described below. Then 2 mL/tube of the FACSLysing solution—Becton/Dickinson Biosciences (BD) San José, CA—diluted 1/10 (v/v) in distilled water was added and the stained samples incubated for another 10 min (room temperature) in the darkness. Afterward, non-lysed nucleated cells were centrifuged, washed twice in phosphate buffered saline (PBS), and re-suspended in 0.5 mL of PBS/tube. For staining purposes, two identical seven-color—pacific blue (PacB), pacific orange (PacO), fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin-chlorophyll protein-cyanine 5.5 (PerCPCy5.5), phycoerythrin-cyanine 7 (PeCy7), allophycocyanine-Hilite®7 (APC-H7), which are combinations of monoclonal antibodies were used (Table 3), except for the APC-conjugated reagent that consisted of either CD33-APC or CD22-APC. For each individual marker (stained in ≥66 patient samples), the study only included cases in which the same antibody reagents had been used (antibody clones, fluorochrome conjugates, and manufacturer lots). Flow cytometry data acquisition was performed in a FACSCANTO IITM instrument (BD), using FACS Diva software (BD). For data analysis, the INFINICYTTM software (Cytognos SL, Salamanca, Spain) was used. Expression of individual markers on BMMC was assessed on gated CD117hi CD45int CD34− events BMMC and expressed both as MFI values (arbitrary units scaled from 0 to 262,144) (Table 2) and SI (Figure 1). SI is the difference in signal width between the positive and the negative population divided by the signal spread of the negative population [81,82]. For each individual marker, the SI threshold for positivity was set at ≥1.5, after specifically gating on BMMC (Figure 2). For BMMC gating, a region was drawn to include all CD117hi events after excluding cell debris and doublets in forward light scatter (FSC) vs. sideward light scatter (SSC) and FSCarea vs. FSCwidth dot plots, respectively. Subsequently the CD117hi events were gated on a CD45 vs. CD117 dot plot as CD45int/CD117hi cells. The relative distribution of MC in BM samples analyzed by flow cytometry ranged from 0.001% to 0.5% in control vs. 0.0001% to 54% in SM cases.
Table 3.
List of fluorochrome-conjugated monoclonal antibodies used in this study to characterize BMMCs from SM patients and normal/reactive BM.
| Marker | Clone | Fluorochrome | Source | Specificity |
|---|---|---|---|---|
| CD22 | S-HCL-1 | APC | BD Biosciences * | Sialic acid-binding Ig-like lectin 2 (Siglec-2) |
| CD25 | 2A3 | PE | BD Biosciences * | Interleukin-2 receptor, subunit α |
| CD30 | Ber-H8 | PE | BD Biosciences * | Member 8 of the tumor necrosis factor receptor superfamily |
| CD33 | P67.6 | APC | BD Biosciences * | Sialic acid-binding Ig-like lectin 3 (Siglec-3) |
| CD123 | AC145 | APC | Miltenyi Biotec † | Interleukin-3 receptor, subunit α |
| IgE | Polyclonal | FITC | Invitrogen § | High-affinity immunoglobulin ε heavy chain receptor I |
Abbreviations: APC, allophycocyanine, PE, phycoerythrin, FITC, fluorescein isothiocyanate. * Becton/Dickinson Biosciences (BD, San José, CA, USA), † Miltenyi Biotec (Bergisch Gladbach, Germany), § Invitrogen (Carlsbad, CA, USA).
Figure 2.
Representative bivariate dot plots illustrating the immuno-phenotype of pathological mast cells gated as CD117hi cells (red events) and other residual bone marrow hematopoietic cells (grey events) in a patient with indolent systemic mastocytosis (panel A) and another case of mast cell leukemia (panel B). Abbreviations: SSC, sideward light scatter, PE, phycoerythrin, APC, allophycocyanine, FITC, fluorescein isothiocyanate.
4.3. Statistical Methods
For all phenotypic variables, median, range, mean, and standard deviation values, as well as 95% confidence intervals, were calculated. In order to determine the statistical significance of differences observed among groups, the Fisher χ2 exact test (for categorical variables) and the Mann Whitney-U and Kruskal-Wallis tests (for continuous variables) for comparisons between two or more than two groups were applied, respectively (SPSS 23 software, IBM, Armonk, NY, USA). The degree of correlation between different variables was assessed by the Spearman’s test (SPSS 23 software, IBM, Armonk, NY, USA). p values < 0.05 were considered to be associated with statistical significance.
Acknowledgments
We thank Elena Blanco, Ignacio Criado, and Luzalba Sanoja for their help in analyzing the data.
Abbreviations
| ADC ADCC ADCP |
Antibody-drug conjugate Antibody-dependent cellular cytotoxicity Antibody-dependent cellular phagocytosis; |
| AML | Acute myeloid leukemia |
| APC-H7 | Allophycocyanine-Hilite®7 |
| ASM | Aggressive systemic mastocytosis |
| BM BMMC |
Bone marrow Bone marrow mast cell |
| CAR | Chimeric antigen receptor |
| CM | Cutaneous mastocytosis |
| CR | Complete remission |
| EMA | European Medicines Agency |
| FDA | US Food and Drug Administration |
| FITC | Fluorescein isothiocyanate |
| ISM | Indolent systemic mastocytosis |
| ISMMC | Indolent systemic mastocytosis with MC-restricted KIT mutation |
| ISMML | Indolent systemic mastocytosis with multilineal KIT-mutated |
| KIT | Stem cell factor receptor |
| MC | Mast cell |
| MCL | Mast cell leukemia |
| MFI ORR |
Mean fluorescence intensity Overall response rate |
| PacB | Pacific blue |
| PacO | Pacific orange |
| PE | Phycoerythrin |
| PeCy7 | Phycoerythrin-cyanine 7 |
| PerCPCy5.5 | Peridinin-chlorophyll protein-cyanine 5.5 |
| PNA | Peptide nucleic acid |
| REMA | Spanish Network on Mastocytosis |
| SI | Stain index |
| SM | Systemic mastocytosis |
| SM-AHN | Systemic mastocytosis with an associated clonal non-MC lineage disease |
| SSM | Smoldering systemic mastocytosis |
| TKI | Tyrosine-kinase inhibitor |
| WDSM | Well-differentiated systemic mastocytosis |
| WHO | World Health Organization |
Author Contributions
N.D.-F. and A.M. performed experiments, analyzed the data, interpreted the results, made the figures, and wrote the paper. C.T. performed experiments and analyzed the data. M.J.-A. and C.C. performed KIT mutation experiments and analyzed/interpreted the results. I.A.-T. and A.M. recruited the patients and controls, followed the patients, and critically reviewed the paper. L.S.-M. and A.H. collected the samples, performed immunophenotyping analysis, and critically reviewed the paper. J.I.M.-G. and J.I.S.-G. contributed with technical support. A.C.G.-M. analyzed data, interpreted the results, and critically reviewed the paper. L.E. supervised the study, performed clinical follow-up of the patients, and critically reviewed the paper. A.O. designed the research, supervised the study, and wrote the paper.
Funding
This work was supported by the Fondo de Investigaciones Sanitarias (FIS) of the Instituto de Salud Carlos III (Ministerio de Economía y Competitividad, Madrid, Spain; grant number PI16/00642, FEDER), the Consejería de Educación (Regional Government of Castilla and León, Spain, grant number SA013U16), Biomedical Research Networking Center on Cancer (CIBERONC CB16/12/00400) of the Instituto de Salud Carlos III (Madrid, Spain) and grants from the Asociación Española de Mastocitosis y Enfermedades Relacionadas (AEDM 2017), Fondos de Investigación para Enfermedades Raras del Ministerio de Sanidad, Servicios Sociales e Igualdad, Ensayo clínico independiente del Ministerio de Salud y Política Social (EC11-287), Hospital Virgen de la Salud Biobank (BioB-HVS, grant PT13/0010/0007; Toledo, Spain). N D-F was supported by a grant from the Consejería de Educación (Junta de Castilla and León, Valladolid, Spain, ORDEN EDU/346/2013). A M was supported by a contract grant from the Biomedical Research Networking Center on Cancer (CIBERONC CB16/12/00400) (contract profile: 170504_CIBERONC_ORFAO_ONCG03/2017) of the Instituto de Salud Carlos III (Madrid, Spain), and JI M-G was supported by a grant from the Consejería de Educación (Junta de Castilla and León, Valladolid, Spain; ORDEN EDU/310/2015).
Ethics Approval and Consent to Participate
All subjects gave their written informed consent to participate prior to entering the study, according to the Declaration of Helsinki, and the study was approved by the local Ethics Committee of the two participating centers (PI16/00642, 4 May 2016).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Valent P., Horny H.P., Escribano L., Longley B.J., Li C.Y., Schwartz L.B., Marone G., Nunez R., Akin C., Sotlar K., et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001;25:603–625. doi: 10.1016/S0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 2.Horny H.P., Akin C., Metcalfe D.D., Escribano L., Bennett J.M., Valent P., Bain B.J. Mastocytosis (mast cell disease) In: Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Vardiman J.W., editors. World Health Organization (WHO) Classification of Tumours. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 54–63. [Google Scholar]
- 3.Valent P., Akin C., Metcalfe D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–1427. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Montero A.C., Jara-Acevedo M., Teodosio C., Sanchez M.L., Nunez R., Prados A., Aldanondo I., Sanchez L., Dominguez M., Botana L.M., et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Twose I., Jara-Acevedo M., Morgado J.M., Garcia-Montero A., Sanchez-Munoz L., Teodosio C., Matito A., Mayado A., Caldas C., Mollejo M., et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 2016;137:168–178. doi: 10.1016/j.jaci.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Georgin-Lavialle S., Lhermitte L., Dubreuil P., Chandesris M.O., Hermine O., Damaj G. Mast cell leukemia. Blood. 2013;121:1285–1295. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 7.Vaes M., Benghiat F.S., Hermine O. Targeted Treatment Options in Mastocytosis. Front. Med. 2017;4:110. doi: 10.3389/fmed.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escribano L., Akin C., Castells M., Orfao A., Metcalfe D.D. Mastocytosis: Current concepts in diagnosis and treatment. Ann. Hematol. 2002;81:677–690. doi: 10.1007/s00277-002-0575-z. [DOI] [PubMed] [Google Scholar]
- 9.Valent P. Diagnosis and management of mastocytosis: An emerging challenge in applied hematology. Hematol. Am. Soc. Hematol. Educ. Program. 2015;2015:98–105. doi: 10.1182/asheducation-2015.1.98. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib J., Kluin-Nelemans H.C., George T.I., Akin C., Sotlar K., Hermine O., Awan F.T., Hexner E., Mauro M.J., Sternberg D.W., et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N. Engl. J. Med. 2016;374:2530–2541. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Twose I., Matito A., Morgado J.M., Sanchez-Munoz L., Jara-Acevedo M., Garcia-Montero A., Mayado A., Caldas C., Teodosio C., Munoz-Gonzalez J.I., et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2017;8:68950–68963. doi: 10.18632/oncotarget.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lortholary O., Chandesris M.O., Bulai Livideanu C., Paul C., Guillet G., Jassem E., Niedoszytko M., Barete S., Verstovsek S., Grattan C., et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: A randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612–620. doi: 10.1016/S0140-6736(16)31403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone R.M., Manley P.W., Larson R.A., Capdeville R. Midostaurin: Its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018;2:444–453. doi: 10.1182/bloodadvances.2017011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akin C., Fumo G., Yavuz A.S., Lipsky P.E., Neckers L., Metcalfe D.D. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Twose I., Gonzalez P., Morgado J.M., Jara-Acevedo M., Sanchez-Munoz L., Matito A., Mollejo M., Orfao A., Escribano L. Complete response after imatinib mesylate therapy in a patient with well-differentiated systemic mastocytosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:e126–e129. doi: 10.1200/JCO.2011.38.9973. [DOI] [PubMed] [Google Scholar]
- 16.Chan E.C., Bai Y., Kirshenbaum A.S., Fischer E.R., Simakova O., Bandara G., Scott L.M., Wisch L.B., Cantave D., Carter M.C., et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J. Allergy Clin. Immunol. 2014;134:178–187. doi: 10.1016/j.jaci.2013.12.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Melo Campos P., Machado-Neto J.A., Scopim-Ribeiro R., Visconte V., Tabarroki A., Duarte A.S., Barra F.F., Vassalo J., Rogers H.J., Lorand-Metze I., et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk. Res. 2014;38:1245–1251. doi: 10.1016/j.leukres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Huang L., Wang S.A., Konoplev S., Bueso-Ramos C.E., Thakral B., Miranda R.N., Jabbour E., Medeiros L.J., Kanagal-Shamanna R. Well-differentiated systemic mastocytosis showed excellent clinical response to imatinib in the absence of known molecular genetic abnormalities: A case report. Medicine. 2016;95:e4934. doi: 10.1097/MD.0000000000004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherber R.M., Borate U. How we diagnose and treat systemic mastocytosis in adults. Br. J. Haematol. 2018;180:11–23. doi: 10.1111/bjh.14967. [DOI] [PubMed] [Google Scholar]
- 20.Helmy K.Y., Patel S.A., Nahas G.R., Rameshwar P. Cancer immunotherapy: Accomplishments to date and future promise. Ther. Deliv. 2013;4:1307–1320. doi: 10.4155/tde.13.88. [DOI] [PubMed] [Google Scholar]
- 21.Ustun C., Arock M., Kluin-Nelemans H.C., Reiter A., Sperr W.R., George T., Horny H.P., Hartmann K., Sotlar K., Damaj G., et al. Advanced systemic mastocytosis: From molecular and genetic progress to clinical practice. Haematologica. 2016;101:1133–1143. doi: 10.3324/haematol.2016.146563. [DOI] [PubMed] [Google Scholar]
- 22.Valent P., Cerny-Reiterer S., Herrmann H., Mirkina I., George T.I., Sotlar K., Sperr W.R., Horny H.P. Phenotypic heterogeneity, novel diagnostic markers, and target expression profiles in normal and neoplastic human mast cells. Best Pract. Res. Clin. Haematol. 2010;23:369–378. doi: 10.1016/j.beha.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Siebenhaar F., Akin C., Bindslev-Jensen C., Maurer M., Broesby-Olsen S. Treatment strategies in mastocytosis. Immunol. Allergy Clin. N. Am. 2014;34:433–447. doi: 10.1016/j.iac.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Teodosio C., Garcia-Montero A.C., Jara-Acevedo M., Sanchez-Munoz L., Alvarez-Twose I., Nunez R., Schwartz L.B., Walls A.F., Escribano L., Orfao A. Mast cells from different molecular and prognostic subtypes of systemic mastocytosis display distinct immunophenotypes. J. Allergy Clin. Immunol. 2010;125:719–726. doi: 10.1016/j.jaci.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Morgado J.M., Perbellini O., Johnson R.C., Teodosio C., Matito A., Alvarez-Twose I., Bonadonna P., Zamo A., Jara-Acevedo M., Mayado A., et al. CD30 expression by bone marrow mast cells from different diagnostic variants of systemic mastocytosis. Histopathology. 2013;63:780–787. doi: 10.1111/his.12221. [DOI] [PubMed] [Google Scholar]
- 26.Jeong D.K., Fauman K., Ross C., Akin C., Mody R. Successful Treatment of Systemic Mastocytosis Associated with AML-M2, t(8:21) in a Child Using MRC-based AML Chemotherapy along with Gemtuzumab. J. Allergy Clin. Immunol. 2007;119:S207. doi: 10.1016/j.jaci.2006.12.180. [DOI] [Google Scholar]
- 27.Alvarez-Twose I., Martinez-Barranco P., Gotlib J., Garcia-Montero A., Morgado J.M., Jara-Acevedo M., Merker J.D., Penalver F.J., Matito A., Hou Y., et al. Complete response to gemtuzumab ozogamicin in a patient with refractory mast cell leukemia. Leukemia. 2016;30:1753–1756. doi: 10.1038/leu.2016.30. [DOI] [PubMed] [Google Scholar]
- 28.Quintas-Cardama A., Amin H.M., Kantarjian H., Verstovsek S. Treatment of aggressive systemic mastocytosis with daclizumab. Leuk. Lymphoma. 2010;51:540–542. doi: 10.3109/10428190903470869. [DOI] [PubMed] [Google Scholar]
- 29.Borate U., Mehta A., Reddy V., Tsai M., Josephson N., Schnadig I. Treatment of CD30-positive systemic mastocytosis with brentuximab vedotin. Leuk. Res. 2016;44:25–31. doi: 10.1016/j.leukres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Kantarjian H.M., DeAngelo D.J., Stelljes M., Martinelli G., Liedtke M., Stock W., Gokbuget N., O’Brien S., Wang K., Wang T., et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantarjian H.M., DeAngelo D.J., Advani A.S., Stelljes M., Kebriaei P., Cassaday R.D., Merchant A.A., Fujishima N., Uchida T., Calbacho M., et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: Results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4:e387–e398. doi: 10.1016/S2352-3026(17)30103-5. [DOI] [PubMed] [Google Scholar]
- 32.Kreitman R.J., Tallman M.S., Robak T., Coutre S., Wilson W.H., Stetler-Stevenson M., Fitzgerald D.J., Lechleider R., Pastan I. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreitman R.J., Dearden C., Zinzani P.L., Delgado J., Karlin L., Robak T., Gladstone D.E., le Coutre P., Dietrich S., Gotic M., et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32:1768–1777. doi: 10.1038/s41375-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vincenti F., Kirkman R., Light S., Bumgardner G., Pescovitz M., Halloran P., Neylan J., Wilkinson A., Ekberg H., Gaston R., et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N. Engl. J. Med. 1998;338:161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 35.Kahan B.D., Rajagopalan P.R., Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation. 1999;67:276–284. doi: 10.1097/00007890-199901270-00016. [DOI] [PubMed] [Google Scholar]
- 36.Nashan B., Moore R., Amlot P., Schmidt A.G., Abeywickrama K., Soulillou J.P. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet. 1997;350:1193–1198. doi: 10.1016/S0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 37.Gold R., Giovannoni G., Selmaj K., Havrdova E., Montalban X., Radue E.W., Stefoski D., Robinson R., Riester K., Rana J., et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): A randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:2167–2175. doi: 10.1016/S0140-6736(12)62190-4. [DOI] [PubMed] [Google Scholar]
- 38.Giovannoni G., Gold R., Selmaj K., Havrdova E., Montalban X., Radue E.W., Stefoski D., McNeill M., Amaravadi L., Sweetser M., et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): A multicentre, randomised, double-blind extension trial. Lancet Neurol. 2014;13:472–481. doi: 10.1016/S1474-4422(14)70039-0. [DOI] [PubMed] [Google Scholar]
- 39.Giovannoni G., Radue E.W., Havrdova E., Riester K., Greenberg S., Mehta L., Elkins J. Effect of daclizumab high-yield process in patients with highly active relapsing-remitting multiple sclerosis. J. Neurol. 2014;261:316–323. doi: 10.1007/s00415-013-7196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold R., Radue E.W., Giovannoni G., Selmaj K., Havrdova E., Stefoski D., Sprenger T., Montalban X., Cohan S., Umans K., et al. Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study. BMC Neurol. 2016;16:117. doi: 10.1186/s12883-016-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pro B., Advani R., Brice P., Bartlett N.L., Rosenblatt J.D., Illidge T., Matous J., Ramchandren R., Fanale M., Connors J.M., et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase II study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 42.Younes A., Gopal A.K., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J., Ramchandren R., Bartlett N.L., Cheson B.D., de Vos S., et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopal A.K., Chen R., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J., Connors J.M., Engert A., Larsen E.K., Chi X., et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R., Gopal A.K., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J., Connors J.M., Engert A., Larsen E.K., Huebner D., et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pro B., Advani R., Brice P., Bartlett N.L., Rosenblatt J.D., Illidge T., Matous J., Ramchandren R., Fanale M., Connors J.M., et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130:2709–2717. doi: 10.1182/blood-2017-05-780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horwitz S., O’Connor O.A., Pro B., Illidge T., Fanale M., Advani R., Bartlett N.L., Christensen J.H., Morschhauser F., Domingo-Domenech E., et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet. 2018 doi: 10.1016/S0140-6736(18)32984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taksin A.L., Legrand O., Raffoux E., de Revel T., Thomas X., Contentin N., Bouabdallah R., Pautas C., Turlure P., Reman O., et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: A prospective study of the alfa group. Leukemia. 2007;21:66–71. doi: 10.1038/sj.leu.2404434. [DOI] [PubMed] [Google Scholar]
- 48.Renneville A., Abdelali R.B., Chevret S., Nibourel O., Cheok M., Pautas C., Dulery R., Boyer T., Cayuela J.M., Hayette S., et al. Clinical impact of gene mutations and lesions detected by SNP-array karyotyping in acute myeloid leukemia patients in the context of gemtuzumab ozogamicin treatment: Results of the ALFA-0701 trial. Oncotarget. 2014;5:916–932. doi: 10.18632/oncotarget.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amadori S., Suciu S., Selleslag D., Aversa F., Gaidano G., Musso M., Annino L., Venditti A., Voso M.T., Mazzone C., et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 50.Moro J.A., Almenar L., Jarque I., Martinez-Dolz L., Hernandez M.D., Crespo M., Salvador A. Heart transplantation in a patient with systemic mastocytosis. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2008;27:689–691. doi: 10.1016/j.healun.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Busse W., Corren J., Lanier B.Q., McAlary M., Fowler-Taylor A., Cioppa G.D., van As A., Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 52.Corren J., Casale T., Deniz Y., Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J. Allergy Clin. Immunol. 2003;111:87–90. doi: 10.1067/mai.2003.49. [DOI] [PubMed] [Google Scholar]
- 53.Finn A., Gross G., van Bavel J., Lee T., Windom H., Everhard F., Fowler-Taylor A., Liu J., Gupta N. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J. Allergy Clin. Immunol. 2003;111:278–284. doi: 10.1067/mai.2003.54. [DOI] [PubMed] [Google Scholar]
- 54.Carter M.C., Robyn J.A., Bressler P.B., Walker J.C., Shapiro G.G., Metcalfe D.D. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J. Allergy Clin. Immunol. 2007;119:1550–1551. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Douglass J.A., Carroll K., Voskamp A., Bourke P., Wei A., O’Hehir R.E. Omalizumab is effective in treating systemic mastocytosis in a nonatopic patient. Allergy. 2010;65:926–927. doi: 10.1111/j.1398-9995.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 56.Kontou-Fili K., Filis C.I., Voulgari C., Panayiotidis P.G. Omalizumab monotherapy for bee sting and unprovoked “anaphylaxis” in a patient with systemic mastocytosis and undetectable specific IgE. Ann. Allergyasthma Immunol. 2010;104:537–539. doi: 10.1016/j.anai.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Pitt T.J., Cisneros N., Kalicinsky C., Becker A.B. Successful treatment of idiopathic anaphylaxis in an adolescent. J. Allergy Clin. Immunol. 2010;126:415–416. doi: 10.1016/j.jaci.2010.05.043. author reply 416. [DOI] [PubMed] [Google Scholar]
- 58.Paraskevopoulos G., Sifnaios E., Christodoulopoulos K., Mantopoulou F., Papakonstantis M., Sabaziotis D. Successful treatment of mastocytic anaphylactic episodes with reduction of skin mast cells after anti-IgE therapy. Eur. Ann. Allergy Clin. Immunol. 2013;45:52–55. [PubMed] [Google Scholar]
- 59.Kibsgaard L., Skjold T., Deleuran M., Vestergaard C. Omalizumab induced remission of idiopathic anaphylaxis in a patient suffering from indolent systemic mastocytosis. Acta Derm. Venereol. 2014;94:363–364. doi: 10.2340/00015555-1687. [DOI] [PubMed] [Google Scholar]
- 60.Lieberoth S., Thomsen S.F. Cutaneous and gastrointestinal symptoms in two patients with systemic mastocytosis successfully treated with omalizumab. Case Rep. Med. 2015;2015:903541. doi: 10.1155/2015/903541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broesby-Olsen S., Vestergaard H., Mortz C.G., Jensen B., Havelund T., Hermann A.P., Siebenhaar F., Moller M.B., Kristensen T.K., Bindslev-Jensen C., et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. Allergy. 2018;73:230–238. doi: 10.1111/all.13237. [DOI] [PubMed] [Google Scholar]
- 62.Chen M., Kim A., Zuraw B., Doherty T.A., Christiansen S. Mast cell disorders: Protean manifestations and treatment responses. Ann. Allergyasthma Immunol. 2018;121:120–130. doi: 10.1016/j.jaci.2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valent P., Ghannadan M., Akin C., Krauth M.T., Selzer E., Mayerhofer M., Sperr W.R., Arock M., Samorapoompichit P., Horny H.P., et al. On the way to targeted therapy of mast cell neoplasms: Identification of molecular targets in neoplastic mast cells and evaluation of arising treatment concepts. Eur. J. Clin. Investig. 2004;34(Suppl. 2):41–52. doi: 10.1111/j.0960-135X.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 64.Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2015;90:250–262. doi: 10.1002/ajh.23931. [DOI] [PubMed] [Google Scholar]
- 65.Wang M., Yin B., Wang H.Y., Wang R.F. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6:1265–1278. doi: 10.2217/imt.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicodemus C.F. Antibody-based immunotherapy of solid cancers: Progress and possibilities. Immunotherapy. 2015;7:923–939. doi: 10.2217/imt.15.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Firor A.E., Jares A., Ma Y. From humble beginnings to success in the clinic: Chimeric antigen receptor-modified T-cells and implications for immunotherapy. Exp. Biol. Med. 2015;240:1087–1098. doi: 10.1177/1535370215584936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Golay J., Introna M. Mechanism of action of therapeutic monoclonal antibodies: Promises and pitfalls of in vitro and in vivo assays. Arch. Biochem. Biophys. 2012;526:146–153. doi: 10.1016/j.abb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Anikeeva N., Steblyanko M., Fayngerts S., Kopylova N., Marshall D.J., Powers G.D., Sato T., Campbell K.S., Sykulev Y. Integrin receptors on tumor cells facilitate NK cell-mediated antibody-dependent cytotoxicity. Eur. J. Immunol. 2014;44:2331–2339. doi: 10.1002/eji.201344179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herter S., Birk M.C., Klein C., Gerdes C., Umana P., Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J. Immunol. 2014;192:2252–2260. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiskopf K., Weissman I.L. Macrophages are critical effectors of antibody therapies for cancer. mAbs. 2015;7:303–310. doi: 10.1080/19420862.2015.1011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beck A., Goetsch L., Dumontet C., Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017;16:315–337. doi: 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- 73.Krauth M.T., Bohm A., Agis H., Sonneck K., Samorapoompichit P., Florian S., Sotlar K., Valent P. Effects of the CD33-targeted drug gemtuzumab ozogamicin (Mylotarg) on growth and mediator secretion in human mast cells and blood basophils. Exp. Hematol. 2007;35:108–116. doi: 10.1016/j.exphem.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Van der Velden V.H., Boeckx N., Jedema I., te Marvelde J.G., Hoogeveen P.G., Boogaerts M., van Dongen J.J. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia. 2004;18:983–988. doi: 10.1038/sj.leu.2403350. [DOI] [PubMed] [Google Scholar]
- 75.Velden V., van Dongen J.J.M. Effectiveness of Gemtuzumab Ozogamicin (Mylotarg) Treatment: Cellular and Systemic Determinants. EJHP Sci. 2006;12:118–122. [Google Scholar]
- 76.Siebenhaar F., Kuhn W., Zuberbier T., Maurer M. Successful treatment of cutaneous mastocytosis and Meniere disease with anti-IgE therapy. J. Allergy Clin. Immunol. 2007;120:213–215. doi: 10.1016/j.jaci.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Matito A., Blazquez-Goni C., Morgado J.M., Alvarez-Twose I., Mollejo M., Sanchez-Munoz L., Escribano L. Short-term omalizumab treatment in an adolescent with cutaneous mastocytosis. Ann. Allergyasthma Immunol. 2013;111:425–426. doi: 10.1016/j.anai.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Sokol K.C., Ghazi A., Kelly B.C., Grant J.A. Omalizumab as a desensitizing agent and treatment in mastocytosis: A review of the literature and case report. J. Allergy Clin. Immunol. Pract. 2014;2:266–270. doi: 10.1016/j.jaip.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Blatt K., Cerny-Reiterer S., Schwaab J., Sotlar K., Eisenwort G., Stefanzl G., Hoermann G., Mayerhofer M., Schneeweiss M., Knapp S., et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood. 2015;126:2832–2841. doi: 10.1182/blood-2015-03-637728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Escribano L., Orfao A., Diaz Agustin B., Cervero C., Herrero S., Villarrubia J., Bravo P., Torrelo A., Montero T., Valdemoro M., et al. Human bone marrow mast cells from indolent systemic mast cell disease constitutively express increased amounts of the CD63 protein on their surface. Cytometry. 1998;34:223–228. doi: 10.1002/(SICI)1097-0320(19981015)34:5<223::AID-CYTO3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 81.Maecker H., Trotter J. Selecting reagents for multicolor flow cytometry with BD™ LSR II and BD FACSCanto™ systems. Nat. Methods. 2008;5:A6. doi: 10.1038/nmeth.f.229. [DOI] [Google Scholar]
- 82.Kantor A.B., Moore W.A., Meehan S., Parks D.R. A Quantitative Method for Comparing the Brightness of Antibody-dye Reagents and Estimating Antibodies Bound per Cell. Curr. Protoc. Cytom. 2016;77:1.30.1–1.30.23. doi: 10.1002/cpcy.6. [DOI] [PubMed] [Google Scholar]