HIV-infected individuals with CD4 count >100 cells/μL with cryptococcal meningitis presented more frequently with altered mental status despite having a 10-fold lower fungal burden. The survival by extremes of low and high CD4 are consistent with the damage-response framework theory.
Keywords: cryptococcal meningitis, CD4 T cells, HIV, CSF biomarkers, AIDS
Abstract
Background
Cryptococcal meningitis can occur in persons with less-apparent immunosuppression. We evaluated clinical characteristics and outcomes of persons with HIV-related Cryptococcus presenting with higher CD4 counts.
Methods
We enrolled 736 participants from 2 prospective cohorts in Uganda and South Africa from November 2010 to May 2017. We compared participants with CD4 <50, 50–99, or ≥100 cells/μL by clinical characteristics, cerebrospinal fluid (CSF) parameters, and 18-week survival.
Results
Among first episode of cryptococcosis, 9% presented with CD4 ≥100 cells/μL. Participants with CD4 ≥100 cells/μL presented more often with altered mental status (52% vs 39%; P = .03) despite a 10-fold lower initial median CSF fungal burden of 7850 (interquartile range [IQR] 860–65500) versus 79000 (IQR 7400–380000) colony forming units/mL (P < .001). Participants with CD4 ≥100 cells/μL had higher median CSF levels of interferon-gamma, interleukin (IL)-6, IL-8, and IL-13, and lower monocyte chemokine, CCL2 (P < .01 for each). Death within 18 weeks occurred in 47% with CD4 <50, 35% with CD4 50–99, and 40% with CD4 ≥100 cells/μL (P = .04).
Conclusion
HIV-infected individuals developing cryptococcal meningitis with CD4 ≥100 cells/μL presented more frequently with altered mental status despite having 10-fold lower fungal burden and with greater Th2 (IL-13) immune response. Higher CD4 count was protective despite an increased propensity for immune-mediated damage, consistent with damage-response framework.
Clinical trial registration
NCT01075152 and NCT01802385.
Cryptococcal meningitis is responsible for 15% of AIDS-related mortality and 75% of deaths attributed to cryptococcosis in 2014 were in sub-Saharan Africa [1]. Cryptococcosis typically occurs in human immunodeficiency virus (HIV)-infected individuals with profound immunosuppression caused by quantitative depletion and functional abnormalities of CD4+ T cells. Dysregulation of T helper (Th) cells with high expression of the Th2 phenotype compared to Th1 phenotype appears to contribute to the pathogenesis of cryptococcosis in murine models. Alternatively activated macrophages develop in response to Th2 cytokine stimulation and result in uncontrolled cryptococcosis [2, 3]. However, supportive data in humans hosts are limited and appear to support a more complicated effector T-cell response that differs from homogenous murine models [4, 5]. Additionally, HIV can induce an increase in T regulatory cell frequency, which is only partially reversed by antiretroviral therapy (ART), further impairing protective immune responses [6]. Clinical characteristics of cryptococcosis are commonly known to result from uncontrolled fungal growth due to a weak immune system as evidenced by low CD4 counts. However, more recently, studies suggest that clinical features and outcomes of cryptococcosis may be partially attributed to defective host immune responses, including dysregulated homeostatic and regulatory mechanisms, resulting in exaggerated responses [7]. In one study, CD4+ T-cell–mediated tissue damage was demonstrated in murine models [8].
Historically, Cryptococcus causes infections at very low CD4 counts and, as a result, cryptococcal antigen screening programs are based on a cutoff of CD4 <100 cells/μL. The third quartile of absolute CD4 count from published cryptococcal cohorts from sub-Saharan Africa ranges from 41 to 83 cells/μL [4, 9–11]. This suggests that 75% of individuals with HIV-associated cryptococcal meningitis present with a CD4 count well below 100 cells/μL. However, cryptococcosis does occur with less-apparent immunosuppression, among HIV-negative individuals and HIV-infected individuals with relatively higher CD4 counts. The clinical characteristics and cerebrospinal fluid (CSF) immune profiles of HIV-infected patients with cryptococcal meningitis at relatively higher CD4 counts (>100 cells/μL) are less clear. Describing this population could provide more insight into the role of host immune responses in the immunopathogenesis of cryptococcal meningitis and a possible role of a customized treatment approach for cryptococcosis.
We describe the clinical features, outcomes, and CSF immune profiles of HIV infected individuals presenting with an index episode of cryptococcal meningitis stratified by CD4 count. The primary outcome was mortality by 18 weeks.
METHODS
Study Design and Outcomes
We analyzed data from 2 cryptococcal trial cohorts; the Cryptococcal Optimal ART Timing trial (COAT; NCT01075152) and the Adjunctive Sertraline for Treatment of Cryptococcal Meningitis (ASTRO-CM; NCT01802385) pilot study and randomized clinical trials. HIV-infected adults with cryptococcal meningitis were enrolled in Uganda from November 2010 to May 2017 and South Africa from April 2011 to April 2012. The COAT trial was conducted from 2010 to 2013 while the ASTRO-CM study was conducted from 2013 to 2017. All participants in the COAT trial were ART naive whereas the ASTRO-CM trial also included participants who were ART experienced. Follow-up periods for COAT and ASTRO-CM were 52 and 18 weeks, respectively. Written informed consent for enrolment in the clinical trials above was provided. The COAT and ASTRO-CM studies are described in detail elsewhere [11, 12]. Individuals presenting with second episodes or relapse of cryptococcal-related meningitis were excluded from the analyses.
The primary endpoint was 18-week mortality. Secondary endpoints were 2-week mortality and rate of CSF fungal clearance. We compared outcomes for participants by CD4 count category of CD4 ≥100, 50–99, and <50 cells/μL. Absolute CD4 count was measured on anticoagulated whole blood drawn at the time of meningitis diagnosis using the BD FACS Count (BD Biosciences, Erembodegem, Belgium) in Uganda and Beckman Coulter EPICS XL analyzer in South Africa. All laboratories participated in external quality assurance testing for CD4 count measurement via United Kingdom National External Quality Assessment Scheme. Biomarkers were measured on cryopreserved CSF drawn at baseline using the multiplex Luminex assay (Bio-Rad, Hercules, CA) [13]. The biomarker results included herein are from the prior published COAT trial cohort, and include more cytokine data from the ASTRO-CM cohort [14].
Statistical Methods
Clinical characteristics and CSF parameters at the time of meningitis diagnosis were summarized using absolute number (percentages) and medians (interquartile ranges [IQR]). We compared CD4 groups using Χ2, Wilcoxon, and Cochran-Armitage tests for trends as appropriate. CD4 metrics (CD4 ≤100 cells/μL versus CD4 >100 cells/μL and per 50 cell increase in CD4) were used in Cox proportional hazards models to estimate association with mortality at 2 and 18 weeks. Unadjusted models and models adjusted for (1) cohort (study) and use of antiretroviral therapy at baseline and (2) additionally for sterile CSF culture at baseline were considered. A measure of early fungicidal activity (EFA; CSF clearance rate in log10Cryptococcus CFU/mL per day) was calculated with general linear models for each participant [15]. Analyses were conducted with SAS version 9.3 (SAS Institute, Cary, NC) and evaluated against a 2-sided alpha level of 0.05 for clinical outcomes. As cytokine analyses were exploratory, a more stringent alpha of 0.01 was utilized for CSF cytokine comparisons.
RESULTS
Baseline Epidemiological, Clinical, and Laboratory Findings
We included 736 participants with a first episode of HIV-associated cryptococcal meningitis who had a baseline CD4 cell count available, of whom 197 were screened for the COAT trial during 2010–2012 and 539 for the ASTRO-CM pilot during 2013–2014, or ASTRO-CM randomized trial during 2015–2017. The overall median age was 35 years (IQR 30–40 cells/μL) and 59% were men (Table 1). Of the 736 participants, 63 (8.6%) had a baseline CD4 ≥100 cells/μL, and 133 (18%) had a CD4 50–99 cells/μL. The median count for participants with CD4 ≥100 cells/μL was 166 cells/μL (IQR 115–234 cells/μL) whereas participants with CD4 <100 cells/μL had a median CD4 count of 14 cells/μL (IQR 6–40 cells/μL).
Table 1.
Baseline Demographics
| Baseline Characteristic | CD4 <50 cells/μL | CD4 50–99 cells/μL | CD4 ≥100 cells/μL | P valuea | |||
|---|---|---|---|---|---|---|---|
| Cohort, n | 540 | 133 | 63 | .11 | |||
| COAT Trial 2010–13, n (%) | 128 (23.7) | 47 (35.3) | 22 (34.9) | ||||
| ASTRO-CM Trial 2013–17, n (%) | 412 (76.3) | 86 (64.7) | 41 (65.1) | ||||
| Age, y median (IQR) | 540 | 35 (29–40) | 133 | 36 (30–42) | 63 | 35 (30–41) | .14 |
| Men, n (%) | 336 (62.2) | 63 (47.4) | 32 (50.8) | .15 | |||
| Glasgow Coma Scale <15, n (%) | 539 | 208 (38.6) | 133 | 55 (41.4) | 63 | 33 (52.4) | .03 |
| Temperature, ⁰C median (IQR) | 532 | 36.5 (35.9–37.2) | 131 | 36.5 (36.1–37.3) | 63 | 36.8 (36.0–37.7) | .09 |
| Seizures, n (%) | 540 | 22 (4.1) | 133 | 5 (3.8) | 63 | 1 (1.6) | .35 |
| Tuberculosis diagnosis (any site), n (%) | 523 | 87 (16.6) | 130 | 29 (22.3) | 59 | 13 (22.0) | .38 |
| Tuberculosis meningitis, n (%) | 540 | 4 (0.7) | 133 | 2 (1.5) | 63 | 1 (1.6) | .57 |
| CD4 cell count/μL, median (IQR) | 540 | 10 (5–21) | 133 | 72 (59–79) | 63 | 166 (115–234) | |
| On ART, n (%) | 539 | 174 (32.3) | 133 | 52 (39.1) | 62 | 27 (43.5) | .16 |
| Days on ART,b median (IQR) | 174 | 373 (44–1421) | 52 | 109 (34–987) | 27 | 88 (42–517) | .09 |
| CSF (baseline) | |||||||
| Opening pressure, cmH2O median (IQR) | 481 | 27 (18–41) | 110 | 21 (15–34) | 53 | 24 (18–36) | .01 |
| CSF white cells/μL, median (IQR) | 486 | <5 (<5–30) | 122 | 25 (<5–130) | 57 | 45 (<5–235) | <.001 |
| CSF white cells <5/μL, n (%) | 486 | 321 (66.0) | 122 | 49 (40.2) | 57 | 21 (36.8) | <.001 |
| Sterile CSF culture, n (%) | 523 | 14 (2.7) | 129 | 18 (14.0) | 61 | 12 (19.7) | <.001 |
| Quantitative culture,c median (IQR) | 509 | 5.0 (4.1–5.6) | 111 | 4.3 (2.8–5.2) | 49 | 3.9 (3.0–4.7) | <.001 |
Abbreviations: ART, antiretroviral therapy; ASTRO-CM, Adjunctive Sertraline for Treatment of Cryptococcal Meningitis; COAT, Cryptococcal Optimal ART Timing trial; CSF, cerebrospinal fluid; IQR, interquartile range.
a Χ 2 or Wilcoxon test as appropriate.
b Among those currently on ART.
c Among those with a nonsterile CSF culture.
A greater proportion of participants with CD4 ≥100 cells/μL (52%) presented with Glasgow Coma Scale (GCS) score <15 compared to 39% among individuals with CD4< 100 cells/μL (P = .03). The proportion of participants who were receiving antiretroviral therapy at diagnosis did not differ among groups (P = .16). Overall, 6% (44/713) had a sterile fungal culture at baseline with a greater proportion of participants with CD4 ≥100 cells/μL presenting with sterile CSF culture; 20% (12/61) compared with 14% (18/129) of participants with CD4 of 50–99 cells/μL, and 3% (14/523) of participants with CD4 <50 cells/μL (P < .001). Among participants who had fungal growth at baseline, those with CD4 ≥100 cells/μL had a lower median quantitative cryptococcal culture compared to participants with CD4 <100 cells/μL (P < .001).
Of the 736 participants, 345 (47%) had CSF biomarkers measured at time of diagnosis. Participants with CD4 ≥100 cells/μL presented with higher median CSF levels of interleukin-6 (IL-6), IL-8, IL-13, and interferon-gamma (IFN-γ) compared to individuals with CD4 <100 (P < .01 for each; Table 2). Monocyte chemoattractant protein levels (MCP1/CCL2) were lower in participants with CD4 ≥100 cells/μL compared to those with CD4 <100 cells/μL (P < .01). Compared to participants with CD4 <50 cells/μL, those with CD4 ≥100 cells/μL had 2.3-fold higher median levels of IFN-γ and 9-fold higher median levels of IL-13.
Table 2.
Baseline CSF Immune Profiles by CD4 Groups
| Baseline CSF Biomarker | Baseline CD4 < 50 cells/μL | Baseline CD4 50–99 cells/μL | Baseline CD4 ≥ 100 cells/μL | P valuea | |||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||
| IL-1β | 249 | 0.5 (≤0.4–1.7) | 67 | 0.7 (≤0.4–1.6) | 29 | 1.1 (≤0.4–2.7) | .12 |
| IL-2 | 249 | 2.1 (≤0.6–8.0) | 67 | 2.3 (≤0.6–8.2) | 29 | 2.2 (≤0.6–6.3) | .91 |
| IL-4 | 249 | 1.1 (≤0.4–2.5) | 67 | 0.8 (≤0.3–1.7) | 29 | 1.1 (≤0.5–2.4) | .12 |
| IL-5 | 249 | 0.5 (≤0.4–1.8) | 67 | 1.4 (≤0.4–3.4) | 29 | 1.1 (≤0.4–3.7) | .002 |
| IL-6 | 249 | 168 (46.7–622) | 67 | 270 (75.1–1543) | 29 | 519 (303–4062) | .004 |
| IL-7 | 249 | 4.1 (1.9–10.7) | 67 | 4.6 (2.3–10.8) | 29 | 6.2 (2.7–17.6) | .42 |
| IL-8 | 249 | 430 (211–1051) | 67 | 768 (272–1292) | 29 | 1069 (402–1717) | .006 |
| IL-10 | 249 | 12.5 (6.6–23.3) | 67 | 13.3 (5.2–25.2) | 29 | 19.6 (5.8–51.8) | .10 |
| IL-13 | 249 | 7.3 (2.0–39.9) | 67 | 22.8 (9.6–95.6) | 29 | 65.6 (10.7–130) | <.001 |
| IL-17 | 249 | 16.3 (5.5–37.5) | 67 | 15.9 (1.3–37.8) | 29 | 14.5 (8.2–29.6) | .91 |
| G-CSF | 249 | 51.8 (26.1–127) | 67 | 70.1 (32.7–153) | 29 | 87.8 (39.5–209) | .06 |
| GM-CSF | 249 | 283 (228–435) | 67 | 242 (197–363) | 29 | 340 (267–424) | .02 |
| IFN-γ | 249 | 38.3 (16.2–87.6) | 67 | 45.9 (14.6–118.0) | 29 | 89.0 (31.7–218) | .008 |
| IFN-γ/IL-13 ratio | 249 | 5.1 (1.0–15.1) | 67 | 1.4 (0.3–10.3) | 29 | 2.7 (0.6–8.7) | .008 |
| MCP1 (CCL2) | 249 | 680 (261–1854) | 67 | 495 (210–1027) | 29 | 213 (132–598) | .002 |
| TNF-α | 249 | 9.5 (3.9–21.5) | 67 | 13.1 (6.8–40.5) | 29 | 10.2 (4.8–44.8) | .06 |
Abbreviations: CSF, cerebrospinal fluid; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IFN-γ, interferon-gamma; IL, interleukin; MCP1, monocyte chemotaxis protein-1 (CCL2 chemokine); TNF-α, tumor necrosis factor-alpha.
aWilcoxon Test. Values in pg/mL. Supplemental Table 1 provides distribution by 18-week survival or mortality outcomes.
Absolute CD4 Count and Clinical Outcomes
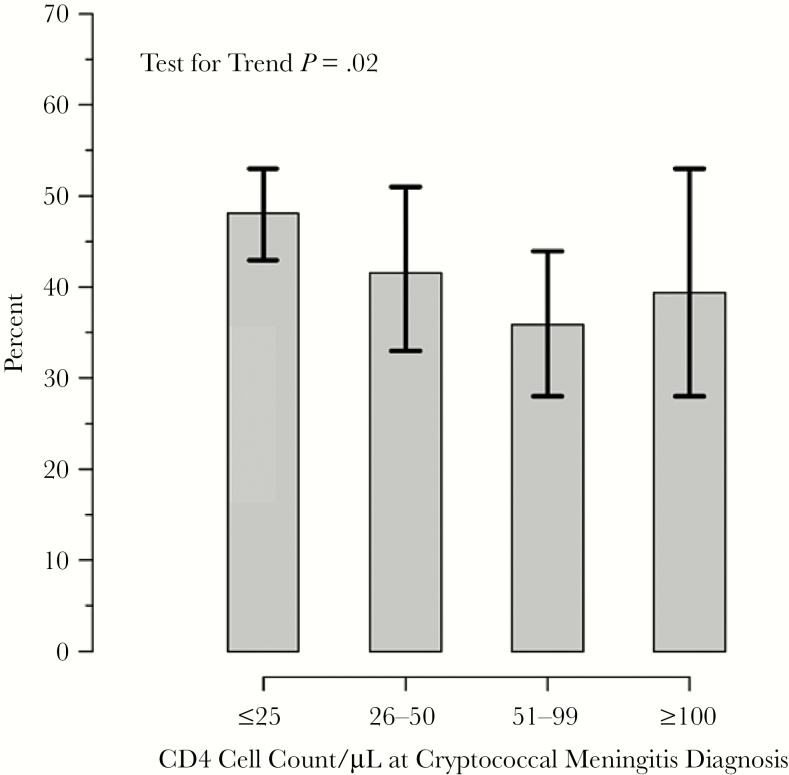
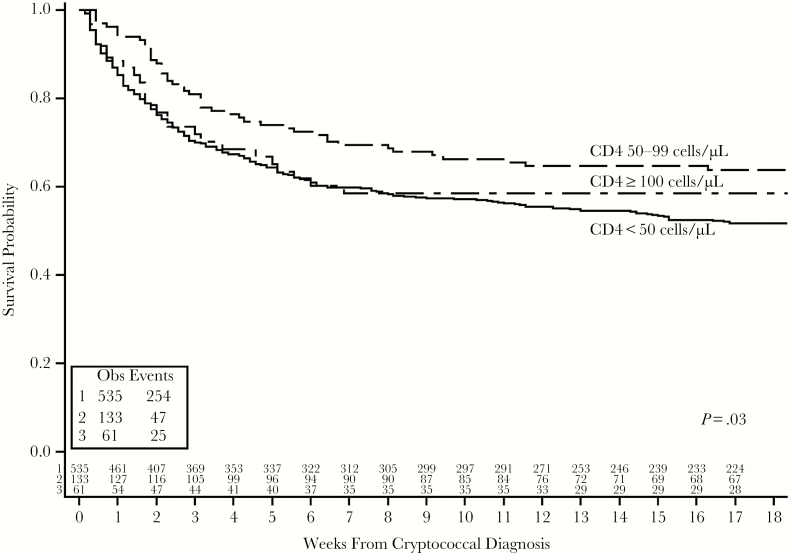
Survival was associated with baseline CD4 count (Table 3). Overall, 21% of participants died by 2 weeks and 44% by 18 weeks. The proportions of deaths at 2 weeks was 2-fold lower among participants with CD4 50–99 cells/μL (P = .02). By 18 weeks, a greater proportion of participants with CD4 <50 cells/μL died (47%, compared to 40% of participants with CD4 ≥100 cells/μL and 35% with CD4 50–99 cells/μL; P = .04). Figure 1 presents the percentages with 18-week mortality for the 4 CD4 groups. The Cochran-Armitage test for trend showed a significant association with decreasing mortality with higher CD4 counts (P = .02). When adjusting for other potential confounders, lower CD4 remained associated with higher mortality through 18 weeks (Table 4). Overall, a decreased risk of death by 18 weeks was observed per 50 cells/μL increase in baseline absolute CD4 count (unadjusted hazard ratio = 0.90; 95% confidence interval [CI], 0.81–0.99; P = .04). Results were similar with adjusted models. In a similar model, fully adjusted for study, use of antiretroviral therapy, Glasgow Coma Scale <15, and sterile CSF culture at baseline, the results are more significant (hazard ratio = 0.86; 95% CI, 0.77–0.97; P = .01). However, the mortality is lowest in the CD4 50–99 cells/μL group with nonstatistically higher mortality in the CD4>100 cells/μL group in comparison (Figure 2).
Table 3.
Survival Outcomes by CD4 Cells/μL Groups
| Event | CD4 <50 cells/μL n = 540 | CD4 50–99 cells/μL n = 133 |
CD4 ≥ 100 cells/μL n = 63 | P Value |
|---|---|---|---|---|
| Death within 2 weeks, n (%) | 126 (23.3) | 16 (12.0) | 14 (22.2) | .02 |
| Death within 18 weeks, n (%) | 254 (47.0) | 47 (35.3) | 25 (39.7) | .04 |
| CSF clearance rate, median (IQR) | −0.33 (−0.47 to −0.20) | −0.35 (−0.45 to −0.26) | −0.38 (−0.65 to −0.31) | .05 |
P values are via Χ2 or Wilcoxon tests.
Cerebrospinal fluid (CSF) clearance rate in log10Cryptococcus CFU/mL calculated by general linear models for each participant.
Figure 1.
Proportions of deaths (95% confidence interval) by 18 weeks for 4 CD4 groups (CD4 ≤25, 25–50, 51–99, and ≥100 cells/μL).
Table 4.
Hazard Ratios for 18-Week Mortality
| CD4 Group | Model 1 Univariate | Model 2 Adjusted for Cohort and ART Use | Model 3 Fully Adjusteda | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| CD4 < 50 cells/μL | 1.51 (1.10–2.06) | 0.01 | 1.46 (1.07–2.00) | 0.02 | 1.57 (1.14–2.17) | 0.006 |
| CD4 50–99 cells/μL | Ref | Ref | Ref | |||
| CD4 ≥ 100 cells/μL | 1.27 (0.78–2.07) | 0.33 | 1.20 (0.73–1.96) | 0.47 | 1.15 (0.70–1.90) | 0.58 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio.
aAdditionally adjusted for Glasgow Coma Scale < 15 and sterile culture at baseline.
Figure 2.
Kaplan-Meier curve for cryptococcal meningitis survival by baseline CD4 group.
Additionally, the rate of CSF fungal clearance was marginally, approximately15% better (steeper) in the group with baseline CD4 >100 cells/μL (median EFA −0.38 log10 CFU/mL versus −0.35 log10 CFU/mL in the CD4 50–99 cells/μL group and −0.33 log10 CFU/mL in the CD4 <50 cells/μL group; P = .05).
DISCUSSION
The results demonstrate that 9% of persons diagnosed with a first episode of cryptococcal meningitis in these 2 cohorts presented with absolute CD4 count above 100 cells/μL. Individuals with a relatively higher CD4 count presented more often with altered mental status despite having a 10-fold lower initial fungal burden. While the estimated hazard of death at 18 weeks was 10% lower for every 50 cells/μL increment in absolute CD4 count at diagnosis, the lowest 18-week mortality was among those with CD4 50–99 cells/μL (35% mortality) as compared to CD4 <50 cells/μL (47% mortality) or CD4 ≥100 cells/μL (40% mortality). These results are consistent with the damage-response framework theory, whereby the extremes of low and high CD4 had worse survival than the middle 50–99 cells/μL group [16].
While the pathogenesis of cryptococcal meningitis is partially understood, there is a growing body of evidence to suggest a fundamental role of the host immune response to Cryptococcus in the manifestations and prognosis of cryptococcosis [7, 8, 16, 17]. Our previous findings suggest that altered mentation, which is a hallmark manifestation of central nervous system disease, may be largely a function of host immune response rather than simply virulence from Cryptococcus alone [18]. One might expect that the higher CD4 group that presented with lower fungal burden would present less often with altered mental status. Paradoxically, our observation was contrary. One plausible explanation for this could be CD4 T-cell mediated neuronal damage, which has been demonstrated in murine models [8]. Furthermore, Lofgren and others have suggested that dysfunctional immune responses rather than high fungal burden could explain poor outcomes in some patients presenting with altered mental status [18]. It is also interesting to note that the higher CD4 group, in which the majority of participants presented with altered mental status, did not have worse prognosis compared to the lower CD4 group. On the contrary, patients with higher CD4 counts in general appeared to have better prognosis than the lowest CD4 group (<50 cells/μL).
Pirofski and Casadevall suggest a damage-response framework parabola with a weak immune response resulting in fungal proliferation and little or no inflammation at one end and a strong cellular host immune response resulting in excessive inflammation and tissue damage at the other end [16, 19]. Here, we observed highest mortality (and baseline fungal burden) in the group with CD4<50 cells/μL essentially depicting failure of the immune response to limit fungal growth. The slightly higher mortality observed in the higher CD4 group (≥100 cells/μL) in comparison to the intermediate CD4 group (50–99 cells/μL) probably depicts detrimental effects of excessive inflammation stemming from a greater host immune response. Higher mortality among patients with CD4 ≥100 cells/μL is unlikely to be attributable to immune reconstitution inflammatory syndrome given that the proportion of patients at diagnosis receiving ART and the duration of ART use was similar across the 3 groups. Furthermore, the hazard ratio was unchanged after adjusting for ART use.
Additionally, we observed that more than a third of patients in the higher CD4 group had sterile cultures at the time of cryptococcal meningitis diagnosis (diagnosed by CSF cryptococcal antigen only). This could be explained as early meningoencephalitis in patients whose clinical presentation, such as altered mentation, is related primarily to host immune response and less with the seemingly low fungal burden in CSF. Jarvis and Bicanic have reported that more proinflammatory responses, particularly IFN-γ, correlate positively with increasing CD4 count [4, 15], and more Th1 inflammation correlates with a faster rate of cryptococcal clearance from CSF [15, 20]. Yet despite this high rate of baseline sterile cultures coupled with better rates of fungal clearance, the CD4 >100 cells/μL group did not have the best survival. Thus, some of the greater immune response present in the CD4 >100 cells/μL group did not translate into a better immune response.
In the context of cryptococcosis, animal models demonstrate that a type 1 T helper cell (Th1) immune response is protective while a predominantly type 2 T helper cell (Th2) response is detrimental [2, 3]. Th2 cytokine responses in these models render the host vulnerable to infection with pathogens such as Cryptococcus where macrophage activation and killing functions are required. We observed that persons with relatively higher CD4 count presented with a more vigorous proinflammatory response compared to persons with baseline CD4 <50 cells/μL. Whereas most of the differences in measured CSF biomarker levels were by a factor of 1 or 2, median IL-13 levels were 9-fold higher in persons who presented with CD4 >100 versus CD4 <50 cells/μL, whereas IFN-γ only increased 2-fold. This may suggest a disproportionate Th2 response. We hypothesize that persons presenting with CD4 count >100 cells/μL are susceptible to cryptococcal meningitis not only as a result of CD4 depletion, but also due to a dysregulated proinflammatory T-cell response. Nevertheless, these patients are able to mount an effective immune response to contain fungal growth as evidenced by lower fungal burden at baseline and later, better rates of fungal clearance. Yet, this greater immune response may result in more frequent altered mental status.
The main limitation of this study is that CSF biomarkers were measured in only a subset of participants (47%) analyzed from a combined cohort enrolled over a 7-year timespan. We acknowledge that the results are exploratory and are primarily hypothesis generating. Additionally, biomarker levels were considered only at baseline, and how immune responses evolve over time is not known.
In summary, our findings demonstrate that HIV-associated cryptococcal meningitis does occur in persons with CD4 >100 cells/μL who have previously not been the focus of public health cryptococcal screening programs in low-resource settings. Patients with higher CD4 cell counts at cryptococcal meningitis diagnosis appear to have better prognosis at 18 weeks from diagnosis than persons with CD4 <50 cells/μL. The more frequent altered mental status in this CD4 >100cells/μL subgroup appears to be associated with the greater host immune response. Whether Th2 skewed immune response is a risk factor for cryptococcosis in patients with higher CD4 counts should be assessed in future pathogenesis studies. Further research into customized adjunctive treatment approaches for this subgroup is also warranted. Options could include adjunctive IFN-γ or anti-IL13 monoclonal therapy (eg, dupilumab, lebrikizumab, and tralokinumab). By better understanding host immunology, we may enable rationale customization of therapy. Promoting Th1 IFN-γ response in persons with low CD4 counts <50 cells/μL and blocking nonprotective Th2 responses (eg, IL-13) in persons with high CD4 counts >100 cells/μL may be a logical customized, precision medicine approach to cryptococcal care in this critically ill population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
COAT and ASTRO-CM team members. Henry W. Nabeta, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Alice Namudde, James Mwesigye, Tadeo Kiiza Kandole, Richard Kwizera, Paul Kirumira, Michael Okirwoth, Andrew Akampurira, Tony Luggya, Julian Kaboggoza, Eva Laker, Leo Atwine, Davis Muganzi, Emily E. Evans, Sarah C. Bridge, Sruti S. Velamakanni, Radha Rajasingham, Katelyn Pastick, Anna Stadelman, Andrew Flynn, A. Wendy Fujita, Liliane Mukaremera, Sarah M. Lofgren, Bozena M. Morawski, Ananta Bangdiwala, Kirsten Nielsen, Paul R. Bohjanen, and Andrew Kambugu.
Disclaimer. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Financial support. This work was supported by the National Institute of Neurologic Diseases and Stroke (grant number R01NS086312); the Fogarty International Center (grant numbers K01TW010268, R25TW009345); the National Institute of Allergy and Infectious Diseases (grant numbers T32AI055433, U01AI089244); the Wellcome Trust (grant number 098316 to G. M.); and the Department of Science and Technology and National Research Foundation of South Africa Research Chairs Initiative (grant number 64787) and incentive funding (grant number UID: 85858).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
COAT and ASTRO-CM teams:
Henry W Nabeta, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Carolyne Namuju, Alisat Sadiq, Alice Namudde, James Mwesigye, Tadeo Kiiza Kandole, Richard Kwizera, Paul Kirumira, Michael Okirwoth, Andrew Akampurira, Tony Luggya, Julian Kaboggoza, Eva Laker, Leo Atwine, Davis Muganzi, Emily E Evans, Sarah C Bridge, Sruti S Velamakanni, Radha Rajasingham, Katelyn Pastick, Anna Stadelman, Andrew Flynn, A Wendy Fujita, Liliane Mukaremera, Sarah M Lofgren, Bozena M Morawski, Ananta Bangdiwala, Kirsten Nielsen, Paul R Bohjanen, and Andrew Kambugu
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. . Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stenzel W, Müller U, Köhler G, et al. . IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am J Pathol 2009; 174:486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Müller U, Stenzel W, Köhler G, et al. . IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol 2007; 179:5367–77. [DOI] [PubMed] [Google Scholar]
- 4. Jarvis JN, Meintjes G, Bicanic T, et al. . Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog 2015; 11:e1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scriven JE, Graham LM, Schutz C, et al. . The CSF immune response in HIV-1-associated cryptococcal meningitis: macrophage activation, correlates of disease severity, and effect of antiretroviral therapy. J Acquir Immune Defic Syndr 2017; 75:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Presicce P, Orsborn K, King E, Pratt J, Fichtenbaum CJ, Chougnet CA. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One 2011; 6:e28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meya DB, Manabe YC, Boulware DR, Janoff EN. The immunopathogenesis of cryptococcal immune reconstitution inflammatory syndrome: understanding a conundrum. Curr Opin Infect Dis 2016; 29:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neal LM, Xing E, Xu J, et al. . CD4+ T cells orchestrate lethal immune pathology despite fungal clearance during Cryptococcus neoformans meningoencephalitis. mBio 2017; 8: pii:e01415–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beardsley J, Wolbers M, Kibengo FM, et al. ; CryptoDex Investigators Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarvis JN, Bicanic T, Loyse A, et al. . Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhein J, Morawski BM, Hullsiek KH, et al. ; ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scriven JE, Rhein J, Hullsiek KH, et al. ; COAT Team Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis 2015; 212:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scriven JE, Rhein J, Hullsiek KH, et al. ; COAT Team Early ART after cryptococcal meningitis is associated with cerebrospinal fluid pleocytosis and macrophage activation in a multisite randomized trial. J Infect Dis 2015; 212:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bicanic T, Muzoora C, Brouwer AE, et al. . Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis 2009; 49:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pirofski LA, Casadevall A. Immune-mediated damage completes the parabola: Cryptococcus neoformans pathogenesis can reflect the outcome of a weak or strong immune response. mBio 2017; 8: pii:e02063–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panackal AA, Williamson KC, van de Beek D, Boulware DR, Williamson PR. Fighting the monster: applying the host damage framework to human central nervous system infections. MBio 2016; 7: pii:e01906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lofgren S, Hullsiek KH, Morawski BM, et al. ; COAT and ASTRO-CM Trial Teams Differences in immunologic factors among patients presenting with altered mental status during cryptococcal meningitis. J Infect Dis 2017; 215:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pirofski LA, Casadevall A. The damage-response framework of microbial pathogenesis and infectious diseases. Adv Exp Med Biol 2008; 635:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jarvis JN, Meintjes G, Rebe K, et al. . Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 2012; 26:1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.