Capsule summary:
This case demonstrates the essential contribution of the p110δ catalytic 21 domain in adaptive immunity function in a patient with expression of a kinase-dead p110δ mutant.
Keywords: PIK3CD, immunodeficiency
To the Editor:
Human class IA phosphoinositide 3-kinases (PI3Ks) are lipid kinases that catalyze the phosphorylation of inactive phosphatitdylinositol-4,5-biphosphonate (PIP2) to biologically active phosphatitdylinositol-3,4,5-trisphonate (PIP3), leading to signaling that regulates cell growth, survival, and metabolism.1 Three catalytic subunits (p110α, p110β, and p110δ) heterodimerize with one of five regulatory subunits.1 The importance of the p110δ in host immunity has been demonstrated by gain-of-function p110δ mutants, resulting in Activated Protein Kinase Delta Syndrome, and much more recently, human deficiency of p110δ.2–4 Here, we report an immunodeficiency due to a kinase-dead PI3Kδ mutant, illustrating the importance of p110δ catalytic activity in adaptive immunity.
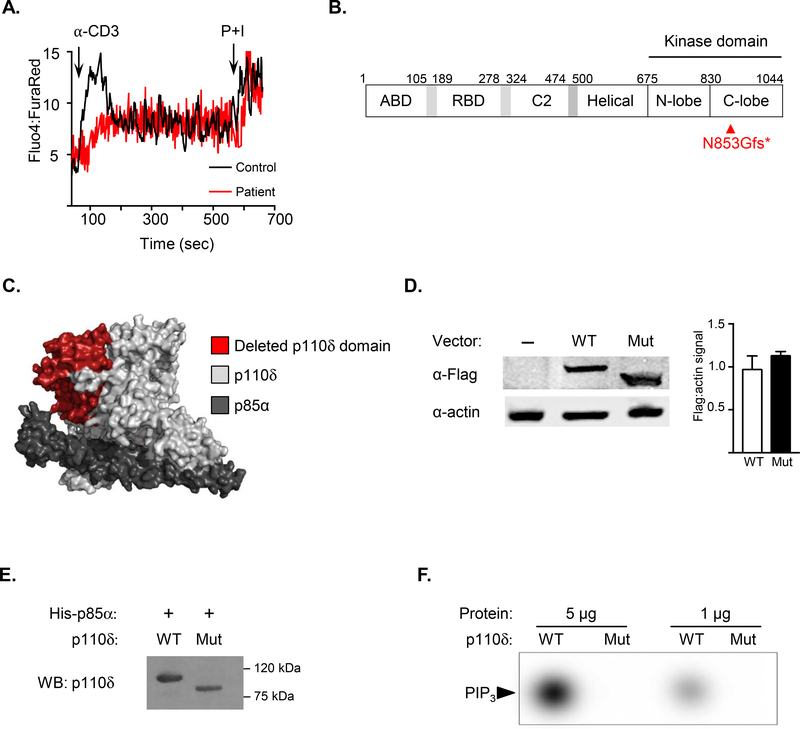
The patient was the son of consanguineous Pakistani parents, whose other son died at six months of age due to sepsis. The patient received the attenuated Bacillus Calmette-Guérin, polio, and measles vaccines without sequelae and was healthy until six years of age, when he presented with chronic diarrhea and polyarticular arthritis affecting his knees and ankles. He was empirically treated with steroids for two months, leading to complete resolution of his symptoms. Over the subsequent five years, he had episodes of colitis treated with prednisone, sulfasalazine, and methotrexate for presumed inflammatory bowel disease. Due to worsening colitis, he underwent an upper endoscopy and colonoscopy that revealed candida esophagitis as well as increased intraepithelial lymphocytes and moderate villous blunting in the duodenum. His laboratory evaluation was notable for leukocytosis, neutrophilia, mild monocytosis, and thrombocytosis (Table 1). He had normal numbers of T, B, and NK cells and normal percentages of T and B cell subsets. His serum levels of IgG and IgA were decreased. Ca2+ flux in response to anti-CD3 crosslinking on T cells was decreased (Fig. 1A), although proliferation to anti-CD3+CD28 stimulation was robust (Table 1). Whole-exome sequencing of the patient revealed a novel homozygous frameshift mutation in PIK3CD (c.2558_2559delAT; p.Asp853Glyfs*20), disrupting exons 20 – 24 encoding 171 amino acids of the ATP binding site within the catalytic domain (Fig. 1B, C). This mutation is not in the 1000 Genomes, ExAC or the NHLBI Exome Sequencing Project databases. The patient was treated with immunoglobulin replacement therapy, antifungal prophylaxis, and prophylactic antibiotics, but died at the age of 14 years due to a severe pneumonia and sepsis shortly after the mutation was identified.
Table 1.
Immunological profile of the patient at 11 years of age. Bold values are outside the normal ranges listed in parentheses.
| Hemogram, 103 cells/μL | |
| White blood cells | 15.6 (4 – 10) |
| Neutrophils | 10.5 (2.0 – 7.5) |
| Lymphocytes | 3.2 (1.0 – 4.0) |
| Monocytes | 1.2 (0.2 – 1.0) |
| Platelets | 537 (150 – 400) |
| Lymphocyte subsets, 103 cells/μL | |
| CD3+, 103 cells/μL | 1.7 (1.4 – 3.3) |
| CD3+CD4+, 103 cells/μL | 0.6 (0.53 – 1.3) |
| CD45RA+CCR7+ naive, % CD4+ | 62.7 (57.1–84.8) |
| CD45RA–CCR7+ central memory, % CD4+ | 29.0 (11.2 – 30.0) |
| CD45RA–CCR7– effector memory, % CD4+ | 7.4 (3.3 – 15.2) |
| CD45RA+CCR7– TEMRA, % CD4+ | 0.7 (0.4 – 2.6) |
| Recent thymic emigrants CD45RA+CD31+, % CD4+ | 33.0 (41.2 – 81.5) |
| CD3+CD8+, 103 cells/μL | 0.8 (0.33 – 0.92) |
| CD45RA+CCR7+ naive, % CD8+ | 58.2 (28.4 – 80.0) |
| CD45RA–CCR7+ central memory, % CD8+ | 6.0 (1.0 – 4.5) |
| CD45RA–CCR7– effector memory, % CD8+ | 22.5 (6.2 – 29.3) |
| CD45RA+CCR7– TEMRA, % CD8+ | 13.3 (9.1 – 40.1) |
| CD19+, 103 cells/μL | 0.4 (0.11 – 0.57) |
| CD27 – IgD+ naïve, % CD19+ | 80.5 (51.3 – 82.5) |
| CD27+IgD+ unswitched memory, % CD19+ | 4.4 (4.5 – 18.2) |
| CD27+IgD– switched memory, % CD19+ | 8.4 (8.5 – 25.6) |
| CD3– CD56+, 103 cells/μL | 0.08 (0.07 – 0.48) |
| Immunoglobulins, mg/dL | |
| IgG | 201 (650–1600) |
| IgM | 58 (50–300) |
| IgA | 22 (40–350) |
| Proliferation (% of control) | |
| Anti-CD3+CD28 | 120% |
Figure 1.
A. Calcium flux detected by flow cytometry of patient and control T cells loaded with Fluo-4 and Fura Red, cross-linked with anti-CD3ε antibody, then stimulated with phorbol myristate acetate and ionomycin. Results are representative of three independent experiments. B. Linear schematic of mutant p110δD853Gfs. C. Protein surface modeling of p110δD853Gfs (light gray) bound to the p85α regulatory subunit (dark gray) and the resulting deleted portion of p110δ (red). D. Left: Immunoblotting of HEK293T cells transfected with N-terminal Flag-tagged WT or mutant p110δ. Actin used as a loading control. Right: Densitometry depicting the ratio of Flag to actin signal E. Expi293F™ (Life Technologies) cells co-transfected with untagged WT or mutant p110δ and polyhistidine-tagged p85α. Polyhistidine-tagged p85α was purified using nickel-nitrilotriacetic acid agarose, then eluted in 250 mM imidazole. Immunoblotting of purified PI3K complexes using a p110δ antibody specific to the residues surrounding His481 (Cell Signaling Technologies). WT p110δ and mutant p110δD853Gfs were detected at 110 and 94 kDa, respectively. F. To assess WT and mutant p110δ kinase activity, 1 and 5 μg of purified recombinant PIK3CD-PIK3R1 complexes were combined with [γ−32P]ATP and PIP2:phosphoserine (1:19 molar ratio), followed by thin layer chromatography for detection of PIP2 phosphorylation.
Due to the patient’s death, analysis of p110δD853Gfs protein expression was not possible. Therefore, HEK293T cells were transiently transfected with constructs encoding N-terminal FLAG-tagged WT or mutant p110δ. Immunoblotting revealed expression of mutant PI3KδD853Gfs at a level comparable to that of WT p110δ (Fig. 1D). To assess kinase activity, WT and mutant p110δ were co-transfected in Expi293F™ cells with the polyhistidine-tagged p85α regulatory subunit. Nickel-nitrilotriacetic acid agarose was used to purify PI3K complexes comprised of His-p85α and WT or mutant p110δ. The PI3KδD853Gfs mutant co-purified with His-tagged p85α (Fig. 1E), but lacked kinase activity, as demonstrated by failure of recombinant PI3KδD853Gfs to convert PIP2 to PIP3 (Fig. 1F).
During the preparation of this manuscript, two kindreds with loss-of-function (LOF) variants in PIK3CD were published, all of whom had hypogammaglobulinemia and recurrent sinopulmonary infections (Table E1).3,4 Protein expression of PI3Kδ was absent in one kindred, which also harbored a pathogenic mutation in the gene encoding the small kinetochore-associated protein, and was not investigated in the other kindred.3,4 This study is therefore the first report of a patient with a combined immunodeficiency due to a kinase-dead p110δ mutant. Pik3cd−/− mice lacking PIK3CD as well as the kinase-dead p110δD910A mutant have indicated the importance of PI3Kδ in B cell development and function.5,6 Specifically, the Pik3cd−/− and p110δD910A mice have absent marginal zone B cells and B-1 peritoneal B cells, hypogammaglobulinemia, impaired proliferation to IgM or CD40 ligation, and defective responses to T-independent and T-dependent antigen stimulation. Although p110δ is essential for B cell function, it does not appear to be essential for the maintenance of total B cell numbers. Normal B cells numbers have been found in the Pik3cd−/− mice, the previously reported kindred lacking p110δ expression,3 and our patient with the kinase-dead p110δD853Gfs mutant. This may reflect the redundancy between p110δ and p110α in the antigen-independent, tonic B cell receptor signaling important for the development and survival of follicular B cells.7 In contrast, MZ and B1 cell development depends on antigen-driven BCR signaling that requires intact p110δ.
The p110δD910A mice as well as three of the four previously reported patients with LOF variants in PIK3CD have susceptibility to colitis, which is also a potential adverse effect associated with p110δ inhibitors used to treat solid and hematopoietic malignancies. This has been attributed to reduced secretion of IL-10 and increased secretion of IL-12/23 from colonic macrophages in response to enteric microbiota.8 However, colitis is not a universal feature shared by all patients with LOF PIK3CD variants or p110δ inhibition, and has not been reported in Pik3cd−/− mice. Therefore, the susceptibility to colitis may reflect differences in microbiota, genetic background, or environmental factors.
Our patient experienced no adverse sequelae from live vaccines and had normal T cell proliferation to anti-CD3+CD28 stimulation. However, the p110δD853Gfs mutant impaired Ca2+ flux in T cells after anti-CD3 crosslinking (Fig. 1B). Prior reports of human PI3K deficiency have not assessed Ca2+ flux in patient lymphocytes; reduced Ca2+ flux has been shown in T cells from p110δD910A mice. As the magnitude and frequency of Ca2+ flux oscillations in T cells correlates with the strength of downstream TCR signaling,9 our patient’s reduced Ca2+ flux reflects a component of impaired cellular immunity in this disease. Of note, opportunistic infections with Pneumocystic jiroveci and Klebsiella aerogenes were reported in one patient with the p110δQ73X1 variant who also had a homozygous mutation in SKAP. Two of the five reported patients (40%) with LOF PIK3CD variants are deceased due to sepsis, indicating the severity of this immunodeficiency.
Precise regulation of p110δ activity is required for the maintenance of host immunity. Our patient demonstrates the essential contribution of the p110δ catalytic domain. Additional patients and studies are needed to determine the outcomes of hematopoietic stem cell transplantation for treatment of this immunodeficiency.
Supplementary Material
Acknowledgments
Supported by: 5T32AI007512-33 (S.B.C), 5K08AI116979-04 (J.C.), 1R01AI139633-01 (RSG) 18 and the Perkin Fund (RSG)
Abbreviations:
- PIK3CD or p110δ
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta
- PI3K
phosphoinositide 3-kinase
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- MZ
marginal zone
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell. 2017;170:605–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kδ and primary immunodeficiencies. Nat Rev Immunol. 2016;16:702–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharfe N, Karanxha A, Dadi H, Merico D, Chitayat D, Herbrick J-A, et al. Dual loss of p110δ PI3-kinase and SKAP (KNSTRN) expression leads to combined immunodeficiency and multisystem syndromic features. J Allergy Clin Immunol. 2018;142:618–29. [DOI] [PubMed] [Google Scholar]
- 4.Sogkas G, Fedchenko M, Dhingra A, Jablonka A, Schmidt RE, Atschekzei F. Primary immunodeficiency disorder caused by phosphoinositide 3–kinase δ deficiency. J Allergy Clin Immunol [Internet]. 2018. [cited 2018 Aug 23]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S0091674918310510 [DOI] [PubMed] [Google Scholar]
- 5.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T Cell Antigen Receptor Signaling in p110δ PI 3-Kinase Mutant Mice. Science. 2002;297:1031–4. [DOI] [PubMed] [Google Scholar]
- 6.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A Crucial Role for the p110δ Subunit of Phosphatidylinositol 3-Kinase in B Cell Development and Activation. J Exp Med. 2002;196:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramadani F, Bolland DJ, Garcon F, Emery JL, Vanhaesebroeck B, Corcoran AE, et al. The PI3K Isoforms p110α and p110δ Are Essential for Pre–B Cell Receptor Signaling and B Cell Development. Sci Signal. 2010;3:ra60–ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinbach EC, Kobayashi T, Russo SM, Sheikh SZ, Gipson GR, Kennedy ST, et al. Innate PI3K p110δ regulates Th1/Th17 development and microbiota-dependent colitis. J Immunol Baltim Md 1950. 2014;192:3958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dura B, Dougan SK, Barisa M, Hoehl MM, Lo CT, Ploegh HL, et al. Profiling lymphocyte interactions at the single-cell level by microfluidic cell pairing. Nat Commun [Internet]. 2015. [cited 2018 Aug 14];6 Available from: http://www.nature.com/articles/ncomms6940 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.