Abstract
A large proportion (range of 44–75%) of women who experience intimate-partner violence (IPV) have been shown to sustain repetitive mild traumatic brain injuries (mTBIs) from their abusers. Further, despite requests for research on TBI-related health outcomes, there are currently only a handful of studies addressing this issue and only one prior imaging study that has investigated the neural correlates of IPV-related TBIs. In response, we examined specific regions of white matter microstructure in 20 women with histories of IPV. Subjects were imaged on a 3-Tesla Siemens Magnetom TrioTim scanner using diffusion magnetic resonance imaging. We investigated the association between a score reflecting number and recency of IPV-related mTBIs and fractional anisotropy (FA) in the posterior and superior corona radiata as well as the posterior thalamic radiation, brain regions shown previously to be involved in mTBI. We also investigated the association between several cognitive measures, namely learning, memory, and cognitive flexibility, and FA in the white matter regions of interest. We report a negative correlation between the brain injury score and FA in regions of the posterior and superior corona radiata. We failed to find an association between our cognitive measures and FA in these regions, but the interpretation of these results remains inconclusive due to possible power issues. Overall, these data build upon the small but growing literature demonstrating potential consequences of mTBIs for women experiencing IPV, and further underscore the urgent need for larger and more comprehensive studies in this area.
Keywords: corona radiata, diffusion tensor imaging, domestic violence, intimate-partner violence, mild traumatic brain injury
Introduction
Intimate partner violence (IPV) is violence perpetrated against a partner, boyfriend, girlfriend, or spouse. It is the most common form of violence against women, with nearly one in three women over the age of 15 experiencing physical or sexual partner violence.1 It is also the leading cause of homicide for women, with the highest odds of being murdered by a partner occurring while trying to leave or after leaving the abusive relationship.2,3
Although there is a wide range of negative health outcomes related to IPV, traumatic brain injuries (TBIs)—in particular, repetitive mild TBIs (mTBIs)—represent among the most critical yet greatly understudied sequelae. Few studies, for example, have investigated TBIs at all in women experiencing IPV, yet the few that have suggest that IPV-related TBIs are highly prevalent, very often mild and repetitive, and also related to psychological and cognitive functioning.4 We have, in fact, shown that 75% of women with a history of IPV sustained at least one partner-related TBI and nearly 50% sustained repetitive TBIs (44% of which were repetitive mTBIs). These numbers are staggering when one considers that an estimated ∼42,000,000 women over the age of 15 in the United States have experienced physical or sexual abuse.5,6 This would translate into ∼31,500,000 women who have sustained at least one IPV-related TBI and ∼21,000,000 who have sustained repetitive TBIs in the U.S. Even if these are gross over-estimates of women sustaining IPV-related TBIs, these numbers dwarf the combined number of military and National Football League TBIs or concussions reported. Using annual estimates of severe physical violence (totaling ∼3,200,000 women), ∼1,600,000 women can be estimated to sustain repetitive IPV-related TBIs in comparison to the total annual numbers of TBIs reported for the military and NFL at 18,000 and 281 respectively.7,8 Whereas the need to address TBIs in the athletic and military domains has been recognized and is currently addressed with a tremendous amount of resources and research, there are only a handful of studies addressing this issue in women victims of IPV. These data and numbers underscore the critical nature and urgency of increasing our understanding of IPV-related TBIs.
In our previous work attempting to do just this, we demonstrated that a TBI score derived from number, recency, and severity of TBIs was associated with memory, learning, and cognitive flexibility.4 Based on these and other data supporting the high prevalence estimates of IPV-related TBIs, there has been a number of calls for additional research in this highly understudied area.9–11 This is critically important, as the findings from such research would provide the knowledge required for appropriate interactions and interventions with women experiencing IPV-related mTBI. Understanding the psychological or cognitive symptoms reported by women in the context of mTBI, rather than simply “psychological stress,” should prompt providers to incorporate mTBI-sensitive interventions at all levels of support. This would represent a change in the state of most current interventions that fail to take mTBI or its sequelae into account.
One question in dire need of attention pertains to the neural mechanisms of IPV-related TBI and their association with cognitive functioning. There is a growing literature showing that abnormalities in functional and structural neural connectivity are related to cognitive functioning after sustaining non-partner-related mTBIs.12,13 Yet, only one study to date has explicitly examined this question in women experiencing IPV. In this previous study, modeled after replicated findings by Bonelle and colleagues and Jilka and colleagues,14,15 we used resting state functional magnetic resonance imaging (MRI) along with neuropsychological assessment measures to investigate the association between number, recency, and severity of IPV-related TBIs, neural connectivity, and cognitive functioning.16 We found that 75% of the sample sustained repetitive IPV-related mTBIs and more importantly, that our TBI measure was negatively associated with the degree of resting state functional connectivity between nodes of the default mode and salience networks. Additionally, the strength of this network connection was positively associated with memory and learning measures. Notably, emotional disturbances such as post-traumatic stress disorder (PTSD), depression, and anxiety are often reported by women experiencing IPV, and these emotional disturbances themselves have been associated with abnormalities in brain structure and function.17–20 Therefore, we also accounted for the effects of these emotional disturbances in this previous study, and showed the relationship between TBI and functional connectivity to hold strong. This study represented a small first step in understanding the neural mechanisms underlying TBI and cognitive functioning in women experiencing IPV.
In the current study, we aimed to extend this extremely limited literature by investigating possible microstructural alterations associated with IPV-related mTBI using diffusion MRI, an imaging methodology sensitive to the movement of water molecules within neural structures. The focus on mild rather than moderate-to-severe TBI is important, as mTBIs have been shown to be the most common and repetitive types of IPV-related TBIs and they typically go unreported and unaddressed.4,16 Additionally, diffusion tensor imaging (DTI), and more specifically fractional anisotropy (FA), has been used extensively to evaluate the microstructure of white matter regions in populations sustaining mTBIs. FA measures the orientation dependency of water molecule diffusion. It is high within white matter, where water molecules diffuse faster along the tracts than perpendicular to the tracts, and is low in gray matter or cerebrospinal fluid (CSF), where water molecules diffuse the same in all directions.12 Previous studies have confirmed FA alterations in individuals who have sustained repetitive non-IPV related mTBIs.13
Many of the diffusion imaging papers examining the effects of repetitive mTBIs have focused on regions comprised of long fiber tracts that are highly susceptible to axonal injury.21–24 Such axonal injuries are induced by biomechanical forces of head impacts, which are often seen in repetitive head injuries. For example, Gajawelli and colleagues25 reported post-season FA changes in regions of the corona radiata (CR) in youth football players. Based in part on these data, Coughlin and colleagues26 examined regions of interest (ROIs) in the posterior (P), superior (S), and anterior (A) CR, as well as in the posterior thalamic radiation (PTR) in current and former National Football League players. With the exception of the ACR, they found medium to large effect sizes (e.g., Cohen's d's range from ∼0.67 to 1.2) for all ROIs, with lower FA values in these regions for the repetitive mTBI athletes relative to controls. Since our current sample was somewhat small, we chose to conduct highly focused analyses on a select group of ROIs that have shown prior associations with repetitive mTBIs. Also, FA is the most commonly used diffusion metric, and of the two used in the Coughlin study, FA was the only one that had large effect sizes. Therefore, we focused our analyses solely on FA. Thus, in our current study we investigated FA within the PCR, SCR, and PTR, in an attempt to replicate the previous findings. We also examined FA within these ROIs in relation to several measures of cognitive functioning (i.e., learning, memory, and cognitive flexibility), that we have previously shown to be related to a score based on the number, recency, and severity of TBIs.16
A limitation of standard diffusion methodologies is the inadvertent inclusion of CSF in the diffusion metric. In order to maximize our chances of detecting effects of repetitive mTBIs in white matter microstructure, we corrected the FA measure for CSF contamination using the free-water imaging methodology.27,28 This method corrects for partial volume with extracellular free-water and calculates a corrected FA measure, thereby increasing its precision.29,30 Free-water imaging has been successfully applied to a range of populations including individuals with mTBIs.31
In summary, we used diffusion MRI with corrected FA metrics to examine the association between IPV-related mTBI and diffusion within select ROIs (e.g., PCR, SCR, and PTR). We then examined possible associations between FA of our ROIs and several facets of cognitive functioning. We hypothesized that the combined number and recency of brain injuries would be negatively associated with FA and that measures of FA within those regions would be positively associated with performance on cognitive tasks.
Methods
Participants were 20 women recruited from women's shelters, domestic violence programs, and word-of-mouth. To be included in the study, at least one incident of physical abuse needed to be reported. Age and race of the women were as follows: mean age = 32.3 (standard deviation [SD] = 10.9); 55% African American, 30% Caucasian, 10% Latina, and 5% mixed race. The mean reading achievement of the group, as assessed with the Wide Range Achievement Test 3 (WRAT-3)32 Reading measure was 89.7 (SD = 12.6). Women reported a mean 11.8 (SD = 2.0) years of education, and 15% were currently employed. (If a woman received a general equivalency diploma, we considered that as 12 years of education. Educational level and vocational status were missing for three subjects.) Functional connectivity and other data (e.g., post-concussive symptom information) were reported on most of this sample in a previous publication.16
Women were phone screened for eligibility criteria and excluded for potentially confounding conditions (e.g., alcohol or drug dependence within the past 6 months, current bipolar disorder, schizophrenia, or autism, autoimmune disorders such as lupus, current pregnancy). Women with non-partner–related mTBIs occurring within the past 3 months or any past history of moderate to severe non-partner related TBI were excluded. Women with questionable substance use issues (n = 3) or who were currently taking psychotropic medications (n = 2) were identified, and primary analyses were conducted with and without these women included. Finally, because we wanted to focus on the effects of mTBIs rather than all TBIs (which could also include moderate or severe TBIs), we also excluded four women who had sustained severe IPV-related TBIs but otherwise met all inclusion/exclusion criteria and had full datasets. Here, we present data without those women included (n = 20) but note that results showed a similar pattern when including them in the analyses (n = 24).
Women provided written informed consent, and the local institutional review board human subjects committee approved the study. The study was conducted in one session, and the brain injury interview was always administered before cognitive testing, which was always administered before scanning. Session length varied but averaged close to 3–4 h. Of note, retrospective histories of abuse and mTBIs are critical to obtain, as most women never seek medical attention and consequently have no medical documentation of their TBIs.16
Measures
Brain injury
Partner- and non-partner–related mTBI was assessed using an identical protocol to that used in previously published reports.4,16 Using a semi-structured interview, women were asked questions about potential traumas to the head that might have resulted in an alteration in consciousness (AIC), consistent with the diagnostic criteria for TBI by the American Congress of Rehabilitation Medicine Special Interest Group on Mild TBI.33 As such, a TBI was defined as: “A traumatically induced physiological disruption of brain function, as manifested by at least one of the following: any loss of consciousness; any loss of memory for events immediately before or after the accident; any alteration in mental state at the time of the accident (e.g., feeling dazed, disoriented, or confused); focal neurologic deficit(s) that may or may not be transient.” For example, “After anything your partner has ever done to you, have you ever lost consciousness or blacked out?” If the subject responded affirmatively, follow-up questions would be used to determine severity of the injury (based on duration of AIC, etc.), as well as the first, last, and number of times the mTBI occurred. Additionally, women subjected to IPV are often strangled. Since the effects of anoxic or hypoxic insults to the brain are uncertain, we also assessed for AIC as a result of strangulation. All women who reported any strangulation-induced AICs also reported a high number of repetitive mTBIs with the exception of one woman. An mTBI was considered mild if a loss of consciousness was ≤30 min and/or the post-traumatic amnesia was ≤24 h.33
Finally, identical to previous work4,16 a brain injury severity score was created based on number, recency, and severity of AICs. The frequency score was the number of TBI-related AICs reported by the women. The recency score was based on the number of weeks since the most recent brain injury. Whether a brain injury was mild versus moderate-to-severe was defined as noted above. A score was then generated based on the following criteria: frequency, 1–5 brain injuries = 1, 6–10 brain injuries = 2, 11–15 brain injuries = 3, 16 or more brain injuries = 4; recency, more than 52 weeks ago = 0, 27–52 weeks ago = 1, 14–26 weeks ago = 2, 0–13 weeks ago = 3; and severity, never sustained a moderate-to-severe brain injury = 0, sustained a moderate-to-severe brain injury = 1. The three scores were added to create the brain injury score. As noted earlier, we excluded women with moderate to severe TBIs so the score for all the women in the current study reflects only number and recency of mTBIs. Non-partner related mTBI information was acquired to ensure exclusion criteria were met.
Cognition
To assess whether cognitive functioning was related to the FA findings that were associated with the brain injury score, we administered a brief neuropsychological battery. We examined cognitive processes, namely learning, memory, and cognitive flexibility, that were associated with mTBIs and functional connectivity in our previous work. Memory and learning were assessed using the Sum of Trials 1–5 and Long Delay Free Recall scores from the California Verbal Learning Test II (CVLT II).34 Women were read a list of words five times and asked to recall as many words as possible immediately following each reading, and after a 20-min delay. The sum of words recalled from trials 1–5 served as a measure of learning, whereas the words recalled after the delay served as a measure of memory. Cognitive flexibility was assessed with the Trail Making Test Part B.35 Women were required to connect a series of numbers and letters in ascending order alternating in numerical and alphabetical sequence.
To account for effects of potential confounds, we also administered questionnaires to assess abuse as an adult and child, as well as symptoms of depression, anxiety, and post-traumatic-stress disorder.
Academic reading achievement, as one index of premorbid cognitive functioning, was assessed with the Reading test of the Wide Range Achievement Test 3 (WRAT-3).32
Adult and childhood abuse
Partner abuse was assessed via the Conflict Tactics Scale (CTS),36 with an additional 10 items from the Severity of Violence Against Women scale37 to assess for more severe forms of violence. The CTS is a 28-item scale that measures the extent to which a participant is exposed to violence within families or in the setting of an intimate relationship. Total score for the past year was used as a measure of partner abuse severity. History of childhood abuse and neglect was assessed with the 34-item Childhood Trauma Questionnaire (CTQ).38 The CTQ is a measure of physical, sexual, and emotional abuse, as well as physical and emotional neglect. Participants are asked to indicate on a 5-point scale (1 = never true; 5 = very often true) the degree to which they experienced a range of traumatic events as a child (e.g., “Someone in my family yelled and screamed at me.”). The CTQ has been associated with interview-based ratings38 and independent corroborations39 of childhood maltreatment.
Psychopathology
Dimensional levels of depression, anxiety, and PTSD symptomatology were assessed with the Mood and Anxiety Symptom Questionnaire (MASQ)40 and the Clinician Administered PTSD Scale IV (CAPS-2),41 respectively. The MASQ is a 62-item questionnaire that assesses non-specific anxiety, depression, and mixed symptomatology, along with anxiety-and depression-specific symptoms. One total MASQ score was computed for each woman. The CAPS-2 is a semi-structured clinical interview used to assess the frequency and intensity of PTSD symptoms. It is a well-established, valid, and reliable measure.41,42 One total severity score was computed for each woman.
Imaging procedures
Diffusion imaging data were acquired on a 3-Tesla Siemens Magnetom TrioTim scanner with a 32-channel head coil (other imaging scans were also acquired and are reported in previous work). A 9-min diffusion-weighted imaging scan was acquired with the following parameters: 68 slices per volume; 1.9 × 1.9 × 1.9 mm3 voxels; b = 700 sec/mm2; 60 diffusion-encoding directions; 10 non-diffusion-weighted B0 images; echo time = 82 msec; repetition time = 7640 msec).
Diffusion imaging data were corrected for motion and eddy currents using affine transformations (FSL).43 Brain masks were edited using Slicer 3D software (www.slicer.org) and manually edited.44 The diffusion MRI data were then processed using a Matlab code that estimates DTI parameters corrected for CSF contamination.45
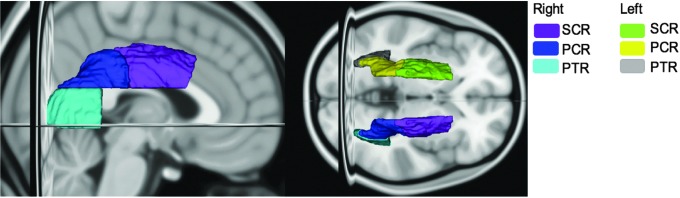
To define our ROIs, the diffusion scans of each subject were non-linearly registered (FSL) to the DTI FA Atlas provided by the Enhanced Neuroimaging Genetics by Meta-Analysis (ENIGMA) DTI Working Group at the University of California,46 in MNI space. Then the FA images were used to generate a white matter skeleton using the Tract Based Spatial Statistics (TBSS) processing pipeline.47 The ICBM-DTI-81 white-matter labels atlas provided by the Johns Hopkins University DTI workgroup,48 was then used to extract and average FA ROI values for the posterior and superior corona radiata (P/SCR) as well as the posterior thalamic radiation (PTR; Fig 1).
FIG. 1.
Regions of interest for fractional anisotropy measures. SCR, superior corona radiata; PCR, posterior corona radiata; PTR, posterior thalamic radiation. Color image is available online.
Statistical analysis
Pearson's correlation analyses were used to examine the association between the brain injury score and FA in our ROIs, namely PCR, SCR, and PTR, and between cognitive functioning and FA in the tracts that showed a significant association to the brain injury score. We then used partial correlational analyses to examine the effects of potential confounds on these associations. Significance levels were set at p < 0.05.
Results
PCR and SCR diffusion linked with IPV related brain injury
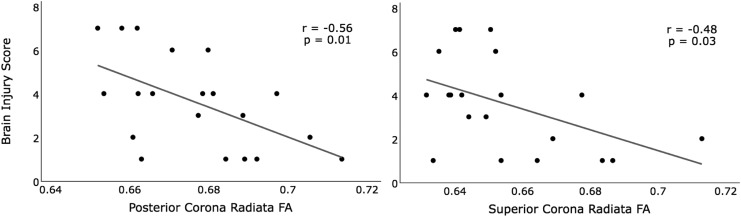
The associations between the brain injury score and FA within our ROIs, (SCR, PCR, and PTR), are summarized in Table 1. The brain injury score was negatively associated with FA in the posterior and superior CR (Table 1; Fig 2). When co-varying for the effects of age, child abuse, partner abuse, levels of anxiety, depression, and PTSD symptomatology, the association between the brain injury score and FA of both the SCR and PCR ROIs remained statistically significant. When covarying for the effects of strangulation, the association with PCR FA held, but dropped to trend level (p = 0.07) for SCR FA. In contrast, the brain injury score showed no statistically significant associations with PTR FA in any analyses.
Table 1.
FA of PCR and SCR Is Negatively Correlated with the Brain Injury Score
| Brain Injury Score | ||||||
|---|---|---|---|---|---|---|
| Controlling for the effects of: | Excluding subjects: | |||||
| FA ROI | Age (n = 20) | Partner and child abuse and psychopathology (n = 20) | Strangulation (n = 20) | Taking psychotropic medications (n = 18) | With questionable substance abuse histories (n = 17) | |
| PCR | −0.56 (0.01) | −0.58 (<0.01) | −0.67 (<0.01) | −0.49 (0.03) | −0.52 (0.03) | −0.49 (0.04) |
| SCR | −0.48 (0.03) | −0.49 (0.03) | −0.60 (0.02) | −0.42 (0.07) | −0.45 (0.06) | −0.45 (0.07) |
| PTR | 0.02 (0.95) | −0.04 (0.87) | −0.13 (0.64) | 0.19 (0.45) | −0.07 (0.78) | 0.07 (0.79) |
Pearson's r (p value).
FA, fractional anisotropy; PCR, posterior corona radiata; SCR, superior corona radiata; PTR, posterior thalamic radiation.
FIG. 2.
Relationship between FA and the brain injury score. Pearson's correlation. n = 20. FA, fractional anisotropy.
When removing subjects currently using psychoactive medications or questionable substance use from the analyses, the association between the brain injury score and PCR FA held, but was reduced to a trend level for SCR (Table 1). In sum, the correlation between the brain injury score and FA in both the PCR and SCR is robust to most potential confounds, but for SCR reduces to a trend level when subjects are removed from analyses—possibly reflecting a loss of power due to the already small sample size.
For exploratory purposes, we examined the relationship between FA and number of mTBIs separately from that of FA and recency of mTBIs. Relative to the originally defined brain injury score (that combines these factors), we found a very similar pattern for the correlations between number of mTBIs and FA within all three ROIs. Specifically, PCR and SCR FA values were significantly correlated with number of mTBIs regardless of covariate or subgroup examined (r's ranged from −0.47 to −0.62; all, p < 0.05), whereas PTR FA showed no significant correlations with number of mTBIs. On the other hand, there was no such similar pattern for the correlation between recency of mTBIs and FA values. The relationship between recency of mTBIs and PCR FA showed a trend for an association (r = -0.40; p = 0.09) when covarying for age, but otherwise there were no significant relationships or trends. These data reveal a highly robust relationship between number of mTBIs and FA in both the PCR and SCR regardless of covariate or subgrouping. They also tentatively suggest that number of mTBIs is a better predictor of these FA values than is recency. However, due to power concerns, we did not separate out chronic versus acute effects of TBIs for our recency variable, which if done with a larger sample, could reveal different findings. Additional research is clearly needed.
PCR and SCR diffusion and cognitive functioning
The associations between FA in PCR and SCR ROIs with measures of cognitive functioning are summarized in Table 2. There were no statistically significant associations between our cognitive measures and FA in either the SCR or PCR. However, given the potential effects of psychotropic medications on cognitive functioning, we removed from the analyses subjects currently taking psychotropic medications (n = 2) and re-examined these associations. There still were no statistically significant associations with any of the cognitive variables. We note, however, that there were several modest correlation values in the predicted direction (0.27 to 0.30) between PCR and SCR FA with the CVLT measures of memory and learning. Given the small sample in conjunction with the size and direction of the correlation values, we unfortunately lacked power to definitively address this question. Nonetheless, because of the paucity of data in this area, we provide information here to guide future studies.
Table 2.
Correlations between FA and Measures of Cognitive Functioning Excluding Participants Currently Taking Psychotropic Medications
| FA ROI | CVLT LDFR (n = 18) | CVLT Sum of trials 1–5 (n = 18) | Trails B (n = 18) |
|---|---|---|---|
| PCR | 0.27 (0.28) | 0.29 (0.25) | 0.07 (0.78) |
| SCR | 0.30 (0.22) | 0.20 (0.42) | 0.02 (0.94) |
Pearson's r (p value).
FA, fractional anisotropy; ROI, region of interest; CVLT, California Verbal Learning Test; LDFR, long-delay free-recall; PCR, posterior corona radiata; SCR, superior corona radiata.
Discussion
We present the first report of an association between white matter diffusion anisotropy and mild traumatic brain injuries resulting from intimate-partner violence. This association was not accounted for by age or other factors often related to IPV including depression, anxiety, and partner or childhood abuse. These data build upon the small but growing literature demonstrating potential consequences of mTBIs for women experiencing IPV, and underscore the urgent need for larger and more comprehensive studies in this area.
TBI in IPV
There is a growing awareness that TBIs, including repetitive mTBIs, have a host of negative short- and long-term health-related effects. There is also a growing awareness that women experiencing IPV sustain high rates of single and repetitive mTBIs. Despite this awareness, there have been few studies examining the effects of mTBIs on women's behavioral functioning, and only one previous study, from our group, investigating women's neural functioning.16 In the previous work, we revealed an association between partner-related TBIs, functional connectivity, and measures of cognitive functioning. In the current study, we demonstrate associations between white matter microstructure and partner-related mTBIs. Importantly, neither of these effects could be accounted for by IPV per se, past abuse in childhood, or current symptoms of depression, anxiety, or PTSD.
Microstructural abnormalities in the posterior and superior corona radiata
Because of the paucity of data in this area, we took a focused approach in investigating white matter ROIs shown to be susceptible to repetitive mTBIs, as these are the types of TBIs most typically experienced by women abused by their partners. In doing so, we found associations between our brain injury score (as well as number of mTBIs alone) and white matter microstructure within two of our predicted regions of interest, the PCR and SCR. As these are broad white matter regions of the confluence of long fiber tracts, the SCR and PCR are somewhat non-specific in terms of the cortical or subcortical areas they connect. Projections from the SCR region include those of the cingulum, superior longitudinal fascicles I and II, superior fronto-occipital fascicle,49 fibers in callosal sections 3–5, as well as the fibers of the pons, cerebellum, and brainstem.50,51 Projections from the PCR region include those of the cingulum, superior longitudinal fascicles II, arcuate fascicle, inferior-fronto-occipital fascicle, fibers in callosal sections 3–5, as well as the fibers of the pons, cerebellum and brainstem.50,51 Given the high number of projection sites of these regions, damage to these areas has the potential to affect a number of functions such as working memory, learning, attention, or visuospatial or language functions.49,52
Fractional anisotropy and cognitive functioning
In the first study to examine IPV-related TBI in association with neuropsychological test performance, we found associations between our TBI score and measures of memory, learning, and cognitive flexibility.4 Later, using functional connectivity, we showed associations between the degree of salience and default mode internetwork connectivity and the same measures of memory and learning. When investigating these cognitive measures in the current study in association with FA within the SCR and PCR, we failed to find any significant associations. However, as noted earlier, we observed several moderate correlation values (r's = 0.27 to 0.30) of our memory and learning measures (after excluding subjects taking psychotropic medications), despite our sample size. Moreover, these correlation values were of similar direction and magnitude to those we found with a larger sample showing significant associations between our brain injury score and learning.4 As such, although our study lacks the power to determine whether meaningful associations exist, it provides encouraging pilot data for a larger study to examine more comprehensively an association between cognitive functioning and FA in the SCR and PCR.
Clinical implications
These data reveal associations between IPV-related mTBIs and tracts subserving a range of cognitive functions. This is highly important in the context of treatment and interventions for women experiencing IPV, as typically neither mTBI nor its sequelae are taken into consideration by advocates when planning treatment or interventions for women experiencing IPV. Consequently, unrealistic recommendations may inadvertently be made, and as mTBI sequelae interfere with a woman's abilities to successfully follow through, it may appear as if women are uncooperative or unwilling to work towards the treatment/intervention goals. Just as certain steps and/or precautions are taken for other individuals who are diagnosed with suspected or known TBIs, steps need to be taken for women experiencing IPV-related mTBI in order to achieve the most successful intervention. Overlooking these effects will likely undermine even the best attempts.
Limitations and future directions
Although we uncovered several robust effects, our sample was small and therefore we chose focused hypotheses based on previous literature. As a result, effects outside our a priori regions could be present but not detected. It also is likely that a larger sample (with subsequently greater power) would have brought more clarity to our questions regarding associations between FA in the PCR and SCR, and measures of learning and memory. Future work using larger samples is critical to addressing these issues. Additionally, we were interested in investigating whether number and recency, rather than presence or absence alone, of mTBIs were related to FA, and therefore we did not employ the use of a control group. However, a control group would have been helpful to establish baseline measures and should be used in future studies. It is important to note that the goal of this work should have a focus of understanding the impact of repetitive mTBIs in women experiencing IPV rather than to understand the impact of IPV per se in women. As such, it is critical to select a control group that is matched to the IPV-mTBI group nearly identically with the exception of sustaining repetitive mTBIs, namely women who have experienced IPV but no mTBIs. Finally, retrospective self-reports were used to acquire information about TBIs. Ideally, medical records or other documentation of mTBIs would be used to confirm TBI history. However, as has been frequently reported,4,16,53,54 most women do not seek medical treatment for mTBIs, and therefore reliance on medical records would result in acquiring data from only a small and unrepresentative sample of women who have experienced IPV. Going forward, it is also critical that we conduct longitudinal studies that will allow us to assess potential changes over time and begin to understand the potential long-term sequelae of repetitive IPV-related mTBIs.
Impact summary
It has been estimated that the number of women subjected to partner-related TBIs far exceeds the combined number of Iraq and Afghanistan military personnel and National Football League football players sustaining mTBIs.55 Given the potential impact repetitive mTBIs may have on such a large number of women in our society, it is imperative that we increase our understanding of the effects of mTBIs in women experiencing IPV. This paper represents a small but critical step toward reaching that goal.
Acknowledgments
We thank all of the women who participated in this study. We thank Brittany LeBlanc for data management assistance, Drs. Michael Alexander, Margaret O'Connor, and Gregory Sorensen for helpful advice in developing this project. This work was supported by a Harvard Medical School Center of Excellence grant from the HMS Fund for Women's Health (EMV), and grants provided to the Athinoula A. Martinos Center for Biomedical Imaging, NCRR P41RR14075 and P41 EB015896. This work also involved the use of instrumentation supported by the National Institutes of Health (NIH) Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant numbers 1S10RR023043 and 1S10RR023401. The study was also supported by the following grants: NIH R21AT008865, NIH R01 AG042512, NIH R01 MH102377, NIH R01 MH112748, NIH K24 MH110807 to MK; NIH P41 EB015902 to OP; NIH R01MH111917 and R01MH112748 to NM; VA Merit Award I01 RX00928 to MS; and National Natural Science Foundation of China, grant 81401131, China Postdoctoral Science Foundation, grant 2015M572049, and China State Scholarship Fund to AC.
Author Disclosure Statement
Dr. Valera has been provided with honoraria and travel funds to present this research. For the remaining authors, no competing financial interests exist.
References
- 1. Devries K.M., Mak J.Y., Garcia-Moreno C., Petzold M., Child J.C., Falder G., Lim S., Bacchus L.J., Engell R.E., Rosenfeld L., Pallitto C., Vos T., Abrahams N., and Watts C.H. (2013). Global health. The global prevalence of intimate partner violence against women. Science 340, 1527–1528 [DOI] [PubMed] [Google Scholar]
- 2. Campbell J.C., Webster D., Koziol-McLain J., Block C., Campbell D., Curry M.A., Gary F., Glass N., McFarlane J., Sachs C., Sharps P., Ulrich Y., Wilt S.A., Manganello J., Xu X., Schollenberger J., Frye V., and Laughon K. (2003). Risk factors for femicide in abusive relationships: results from a multisite case control study. Am. J. Public Health 93, 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson M. and Daly M. (1994). Spousal homicide. Juristat 14, 1–14 [PubMed] [Google Scholar]
- 4. Valera E.M. and Berenbaum H. (2003). Brain injury in battered women. J. Consult. Clin. Psychol. 71, 797–804 [DOI] [PubMed] [Google Scholar]
- 5. The National Intimate Partner and Sexual Violence Survey 2010: Summary Report. (2011). Available at: www.cdc.gov/violenceprevention/pdf/nisvs_report2010-a.pdf Accessed July15, 2018
- 6. U.S. Census Bureau. (2011). Age and Sex Composition: 2010. Available at: www.census.gov/prod/cen2010/briefs/c2010br-03.pdf Accessed July15, 2018
- 7. Defense and Veterans Brain Injury Center. (2017). DoD Worldwide Numbers for TBI. Available at: http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi Accessed July15, 2018
- 8. National Football League. (2018). NFL releases injury data for 2017 season. Available at: www.nfl.com/news/story/0ap3000000911123/article/nfl-releases-injury-data-for-2017-season Accessed July15, 2018
- 9. Kwako L.E., Glass N., Campbell J., Melvin K.C., Barr T., and Gill J.M. (2011). Traumatic brain injury in intimate partner violence: a critical review of outcomes and mechanisms. Trauma Violence Abuse 12, 115–126 [DOI] [PubMed] [Google Scholar]
- 10. Zieman G., Bridwell A., and Cardenas J.F. (2017). Traumatic brain injury in domestic violence victims: a retrospective study at the Barrow Neurological Institute. J. Neurotrauma 34, 876–880 [DOI] [PubMed] [Google Scholar]
- 11. Hunnicutt G., Lundgren K., Murray C.E., and Olson L.N. (2016). Practice update: what professionals who are not brain injury specialists need to know about intimate partner violence-related traumatic brain injury. Trauma Violence Abuse 17, 298–305 [DOI] [PubMed] [Google Scholar]
- 12. Hayes J.P., Bigler E.D., and Verfaellie M. (2016). Traumatic brain injury as a disorder of brain connectivity. J. Int. Neuropsychol. Soc. 22, 120–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonnelle V., Ham T.E., Leech R., Kinnunen K.M., Mehta M.A., Greenwood R.J., and Sharp D.J. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 109, 4690–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jilka S.R., Scott G., Ham T., Pickering A., Bonnelle V., Braga R.M., Leech R., and Sharp D.J. (2014). Damage to the salience network and interactions with the default mode network. J. Neurosci. 34, 10798–10807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valera E. and Kucyi A. (2016). Brain injury in women experiencing intimate partner-violence: neural mechanistic evidence of an “invisible” trauma. Brain Imaging Behav. 11, 1664–1677 [DOI] [PubMed] [Google Scholar]
- 17. Averill C.L., Satodiya R.M., Scott J.C., Wrocklage K.M., Schweinsburg B., Averill L.A., Akiki T.J., Amoroso T., Southwick S.M., Krystal J.H., and Abdallah C.G. (2017). Posttraumatic stress disorder and depression symptom severities are differentially associated with hippocampal subfield volume loss in combat veterans. Chronic Stress (Thousand Oaks) 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wrocklage K.M., Averill L.A., Cobb Scott J., Averill C.L., Schweinsburg B., Trejo M., Roy A., Weisser V., Kelly C., Martini B., Harpaz-Rotem I., Southwick S.M., Krystal J.H., and Abdallah C.G. (2017). Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur. Neuropsychopharmacol. 27, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murrough J.W., Abdallah C.G., Anticevic A., Collins K.A., Geha P., Averill L.A., Schwartz J., DeWilde K.E., Averill C., Jia-Wei Yang G., Wong E., Tang C.Y., Krystal J.H., Iosifescu D.V., and Charney D.S. (2016). Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum. Brain Mapp. 37, 3214–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kircanski K., White L.K., Tseng W.L., Wiggins J.L., Frank H.R., Sequeira S., Zhang S., Abend R., Towbin K.E., Stringaris A., Pine D.S., Leibenluft E., and Brotman M.A. (2018). A latent variable approach to differentiating neural mechanisms of irritability and anxiety in youth. JAMA Psychiatry. 75, 631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Catalano S. (2013). Intimate Partner Violence: Attributes of Victimization, 1993–2011. Available at: www.bjs.gov/content/pub/pdf/ipvav9311.pdf Accessed July15, 2018
- 22. National Center for Injury Prevention and Control. (2003). Costs of Intimate Partner Violence Against Women in the United States. Centers for Disease Control and Prevention: Atlanta, GA; [Google Scholar]
- 23. McAllister T. and McCrea M. (2017). Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. J. Athl. Train. 52, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finkbeiner N.W., Max J.E., Longman S., and Debert C. (2016). Knowing what we don't know: long-term psychiatric outcomes following adult concussion in sports. Can. J. Psychiatry 61, 270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gajawelli N., Lao Y., Apuzzo M.L., Romano R., Liu C., Tsao S., Hwang D., Wilkins B., Lepore N., and Law M. (2013). Neuroimaging changes in the brain in contact versus noncontact sport athletes using diffusion tensor imaging. World Neurosurg. 80, 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coughlin J.M., Wang Y., Minn I., Bienko N., Ambinder E.B., Xu X., Peters M.E., Dougherty J.W., Vranesic M., Koo S.M., Ahn H.H., Lee M., Cottrell C., Sair H.I., Sawa A., Munro C.A., Nowinski C.J., Dannals R.F., Lyketsos C.G., Kassiou M., Smith G., Caffo B., Mori S., Guilarte T.R., and Pomper M.G. (2017). Imaging of glial cell activation and white matter integrity in brains of active and recently retired National Football League players. JAMA Neurol. 74, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pasternak O., Sochen N., Gur Y., Intrator N., and Assaf Y. (2009). Free water elimination and mapping from diffusion MRI. Magn. Reson. Med. 62, 717–730 [DOI] [PubMed] [Google Scholar]
- 28. Baumgartner C., Michailovich O., Levitt J., Pasternak O., Bouix S., and Westin C.F. (2012). A unified tractography framework for comparing diffusion models on clinical scans. Available at: http://cmic.cs.ucl.ac.uk/cdmri12/pdfs/o1_3.pdf Accessed July15, 2018
- 29. Metzler-Baddeley C., O'Sullivan M.J., Bells S., Pasternak O., and Jones D.K. (2012). How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 59, 1394–1403 [DOI] [PubMed] [Google Scholar]
- 30. Albi A., Pasternak O., Minati L., Marizzoni M., Bartres-Faz D., Bargallo N., Bosch B., Rossini P.M., Marra C., Muller B., Fiedler U., Wiltfang J., Roccatagliata L., Picco A., Nobili F.M., Blin O., Sein J., Ranjeva J.P., Didic M., Bombois S., Lopes R., Bordet R., Gros-Dagnac H., Payoux P., Zoccatelli G., Alessandrini F., Beltramello A., Ferretti A., Caulo M., Aiello M., Cavaliere C., Soricelli A., Parnetti L., Tarducci R., Floridi P., Tsolaki M., Constantinidis M., Drevelegas A., Frisoni G., and Jovicich J; PharmaCog Consortium. (2017). Free water elimination improves test-retest reproducibility of diffusion tensor imaging indices in the brain: a longitudinal multisite study of healthy elderly subjects. Hum. Brain Mapp. 38, 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasternak O., Koerte I.K., Bouix S., Fredman E., Sasaki T., Mayinger M., Helmer K.G., Johnson A.M., Holmes J.D., Forwell L.A., Skopelja E.N., Shenton M.E., and Echlin P.S. (2014). Hockey Concussion Education Project, Part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. J. Neurosurg. 120, 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilkinson G.S. (1993). WRAT3 Wide Range Achievement Test: Administration Manual. Wide Range, Inc.: Wilmington, DE; [Google Scholar]
- 33. Committee on Mild Traumatic Brain Injury and American Congress of Rehabilitation Medicine. (1993). Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 48–59 [Google Scholar]
- 34. Delis D.C., Kramer J.H., Kaplan E., and Ober B.A. (2000). CVLT-II, California Verbal Learning Test- Second Edition Manual. Harcourt: San Diego, CA; [Google Scholar]
- 35. Army Individual Test Battery. (1944) Manual of directions and scoring. War Department, Adjunct General's Office: Washington, D.C: [Google Scholar]
- 36. Straus M. (1979). Measuring intrafamily conflict and violence: The conflict tactics (CT) scales. J. Marriage Fam. 75–88 [Google Scholar]
- 37. Marshall L. (1992). Development of the severity of violence against women scales. J. Fam. Violence. 7, 103–121 [Google Scholar]
- 38. Bernstein D.P., Fink L., Handelsman L., Foote J., Lovejoy M., Wenzel K., Sapareto E. and Ruggiero J. (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136 [DOI] [PubMed] [Google Scholar]
- 39. Bernstein D.P., Ahluvalia T., Pogge D., and Handelsman L. (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36, 340–348 [DOI] [PubMed] [Google Scholar]
- 40. Clark L. and Watson D. (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 100, 316–336 [DOI] [PubMed] [Google Scholar]
- 41. Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S. and Keane T.M. (1995). The development of a Clinician-Administered PTSD Scale. J. Trauma Stress 8, 75–90 [DOI] [PubMed] [Google Scholar]
- 42. Weathers F.W., Keane T.M., and Davidson J.R. (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depress. Anxiety 13, 132–156 [DOI] [PubMed] [Google Scholar]
- 43. Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., and Smith S.M. (2012). Fsl. Neuroimage 62, 782–790 [DOI] [PubMed] [Google Scholar]
- 44. Fedorov A., Beichel R., Kalpathy-Cramer J., Finet J., Fillion-Robin J.C., Pujol S., Bauer C., Jennings D., Fennessy F., Sonka M., Buatti J., Aylward S., Miller J.V., Pieper S., and Kikinis R. (2012). 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 30, 1323–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pasternak O., Shenton M.E., and Westin C.F. (2012). Estimation of extracellular volume from regularized multi-shell diffusion MRI. Med. Image Comput. Comput. Assist. Interv. 15, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jahanshad N., Kochunov P.V., Sprooten E., Mandl R.C., Nichols T.E., Almasy L., Blangero J., Brouwer R.M., Curran J.E., de Zubicaray G.I., Duggirala R., Fox P.T., Hong L.E., Landman B.A., Martin N.G., McMahon K.L., Medland S.E., Mitchell B.D., Olvera R.L., Peterson C.P., Starr J.M., Sussmann J.E., Toga A.W., Wardlaw J.M., Wright M.J., Hulshoff Pol H.E., Bastin M.E., McIntosh A.M., Deary I.J., Thompson P.M., and Glahn D.C. (2013). Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage 81, 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., and Behrens T.E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 48. Mori S., Wakana S., van Zijl P.C.M., and Nagae-Poetscher L.M. (2005). MRI Atlas of Human White Matter. Elsevier Science, Amsterdam: [Google Scholar]
- 49. Makris N., Papadimitriou G.M., Sorg S., Kennedy D.N., Caviness V.S., and Pandya D.N. (2007). The occipitofrontal fascicle in humans: a quantitative, in vivo, DT-MRI study. Neuroimage 37, 1100–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Makris N., Meyer J.W., Bates J.F., Yeterian E.H., Kennedy D.N., and Caviness V.S. (1999). MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage 9, 18–45 [DOI] [PubMed] [Google Scholar]
- 51. Meyer J.W., Makris N., Bates J.F., Caviness V.S., and Kennedy D.N. (1999). MRI-Based topographic parcellation of human cerebral white matter. Neuroimage 9, 1–17 [DOI] [PubMed] [Google Scholar]
- 52. Makris N., Kennedy D.N., McInerney S., Sorensen A.G., Wang R., Caviness V.S., Jr., and Pandya D.N. (2005). Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex 15, 854–869 [DOI] [PubMed] [Google Scholar]
- 53. Lipsky S. and Caetano R. (2007). Impact of intimate partner violence on unmet need for mental health care: results from the NSDUH. Psychiatr Serv. 58, 822–829 [DOI] [PubMed] [Google Scholar]
- 54. Plichta S.B. and Falik M. (2001). Prevalence of violence and its implications for women's health. Womens Health Issues 11, 244–258 [DOI] [PubMed] [Google Scholar]
- 55. St Ivany A. and Schminkey D. (2016). Intimate partner violence and traumatic brain injury: state of the science and next steps. Fam. Community Health 39, 129–137 [DOI] [PubMed] [Google Scholar]