Abstract
Traumatic brain injury (TBI) is a major cause of disability worldwide. Post-TBI sleep and wake disturbances are extremely common and difficult for patients to manage. Sleep and wake disturbances contribute to poor functional and emotional outcomes from TBI, yet effective therapies remain elusive. A more comprehensive understanding of mechanisms underlying post-TBI sleep and wake disturbance will facilitate development of effective pharmacotherapies. Previous research in human patients and animal models indicates that altered hypocretinergic function may be a major contributor to sleep–wake disturbance after TBI. In this study, we further elucidate the role of hypocretin by determining the impact of TBI on sleep–wake behavior of hypocretin knockout (HCRT KO) mice. Adult male C57BL/6J and HCRT KO mice were implanted with electroencephalography recording electrodes, and pre-injury baseline recordings were obtained. Mice were then subjected to either moderate TBI or sham surgery. Additional recordings were obtained and sleep–wake behavior determined at 3, 7, 15, and 30 days after TBI or sham procedures. At baseline, HCRT KO mice had a significantly different sleep–wake phenotype than control C57BL/6J mice. Post-TBI sleep–wake behavior was altered in a genotype-dependent manner: sleep of HCRT KO mice was not altered by TBI, whereas C57BL/6J mice had more non–rapid eye movement sleep, less wakefulness, and more short wake bouts and fewer long wake bouts. Numbers of hypocretin-positive cells were reduced in C57BL/6J mice by TBI. Collectively, these data indicate that the hypocretinergic system is involved in the alterations in sleep–wake behavior that develop after TBI in this model, and suggest potential therapeutic interventions.
Keywords: brain injury, controlled cortical impact, orexin, sleep, TBI, wake
Introduction
Traumatic brain injury (TBI) is a dramatic public health issue worldwide, with major implications for human health and healthcare cost. In the United States alone, as many as 5.3 million individuals may suffer from long-term disability related to TBI.1 The direct cost of TBI in the U.S. is estimated at $9.2 billion, with more than $50 billion additionally lost due to lost productivity.2
Disorders of sleep and wakefulness are among the most common consequences of TBI, affecting up to 70% of people who suffer a brain injury.3 Sleep disturbances may begin within days after injury4,5 and persist for years.6,7 Sleep and wake disturbance affects other aspects of quality of life: post-TBI sleep disturbance predicts poorer cognitive, social, and emotional outcomes.8–10 In general, sleep disturbances associated with TBI are characterized by fragmentation of sleep–wake states and an inability to sustain long, consolidated periods of wakefulness. TBI patients exhibit excessive daytime sleepiness (EDS), whether assessed objectively (Multiple Sleep Latency Test)11–16 or subjectively (Epworth Sleepiness Scale),6,15,17 which results in high prevalence of daytime napping.11,18 Some studies reveal that TBI patients have greater total sleep time,11,12 with increased light non–rapid eye movement (NREM) sleep16 or in slow wave sleep.11,19–21 Although they sleep more, TBI patients generally report poor sleep quality,17,22–29 and objective measurements demonstrate that sleep of individuals with TBI is more fragmented than controls.5,19,20,30 The majority of studies do not report differences in the time TBI patients spend in rapid eye movement (REM) sleep.11–13,20,21,29 In addition to the amount of sleep and/or characteristic changes in sleep architecture, spectral properties of the electroencephalography (EEG), especially in the delta power frequency band, also may be altered after TBI: delta power may increase during NREM sleep12 or during wakefulness.29 Because delta power is associated with homeostatic sleep pressure (i.e., sleep propensity that builds with increasing time awake),31,32 these increases may be related to EDS and reflect increased sleep need in TBI patients.
Rodent models of TBI produce disturbances of sleep and wakefulness that are similar to those of human patients. TBI decreases wakefulness acutely (within 7 days of injury)33–35 and chronically.36–38 In general, sleep is fragmented and bouts of sleep and wake are shortened.34,38–42 The amount of time spent in REM sleep is not altered, at least as reported in animal models to date.36–39,41,42 After TBI, delta power may be increased during NREM sleep36,37 or during wakefulness.39 Brain-injured mice also have significantly more EEG slow waves during wakefulness, potentially indicating greater sleep pressure.43
In humans, many sleep–wake regulatory neurotransmitters and hormones are altered by TBI, including hypocretin,44–46 histamine,44 acetylcholine,47,48 serotonin,49 noradrenaline,49 and melatonin.20,50 Of these potential transmitter systems, hypocretinergic dysfunction is likely a key contributor to post-TBI sleep–wake disturbance. Most studies demonstrate that hypocretin, a neuropeptide that is essential for promoting wakefulness and stabilizing sleep–wake states, is dysregulated after TBI. In cases of fatal TBI in humans, postmortem brain tissue contains fewer hypocretin neurons than does tissue from patients who died from causes not related to trauma.44,45 Data from postmortem tissue complement those obtained from individuals surviving TBI: during the acute phase, 95% of patients with moderate-to-severe TBI have abnormally low levels of hypocretin in the cerebrospinal fluid (CSF).46 Further, at 6 months post-TBI, CSF hypocretin concentrations are significantly lower in patients with EDS than in the CSF of those without EDS.51
Hypocretinergic dysfunction is also evident in animal models of TBI. Within the first 3 days after TBI, hypocretin dynamics are significantly altered in mice, with reduced hypocretin in the hypothalamus and hippocampus.34 The diurnal rhythm of hypocretin is blunted in brain injured animals, with much less of a peak-to-trough amplitude difference between the light and dark phases.34 We previously demonstrated that at chronic time-points (15 days post-injury and later), there is a significant decrease in the number of hypocretin-producing cells in the hypothalamus of mice subjected to TBI.37,41
To our knowledge, no direct genetic manipulation of the hypocretinergic system has been used in pre-clinical TBI models. In the present study, we used genetically modified mice that lack hypocretin (HCRT KO) to further investigate the role of hypocretin in post-TBI sleep and wake disturbances. We now report that, unlike C57BL/6J control mice, the sleep of HCRT KO mice is not significantly affected by TBI. These new data support the hypothesis that disruption to the hypocretinergic system is necessary for altered sleep–wake behavior after brain injury in this model.
Methods
Animals
Hypocretin knockout mice on a C57BL/6 background (HCRT KO) were kindly provided by Dr. John Peever (University of Toronto; Toronto, Ontario), and adult male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were group housed until surgery (∼3–4 months old at time of use), after which they were single housed. Mice were maintained on a 12:12 light:dark cycle at 29 ± 1° C with food and water provided ad libitum. All procedures involving the use of animals were approved by the University of Washington Institutional Animal Care and Use Committee in accordance with the U.S. Department of Agriculture Animal Welfare Act and the National Institutes of Health policy on Humane Care and Use of Laboratory Animals.
EEG recording and determination of sleep–wake behavior
Sleep–wake behavior of mice was determined based on the electroencephalogram and cage activity patterns. EEG signals were amplified, filtered, and recorded for offline processing using custom software written in LabView for Windows (ICELUS, M. Opp, University of Washington, Seattle, WA; National Instruments, Austin, TX) as previously described.52,53 EEG and cage activity records were visually scored in 10-sec epochs. Artifact-free EEG signals were subjected to fast Fourier transformation, yielding power spectra between 0.5 and 40 Hz in 0.5-Hz frequency bins. Arousal states were determined as previously described and classified as NREM sleep, rapid eye movement (REM) sleep, or wakefulness (WAKE) based upon published criteria.52–54
Experimental design
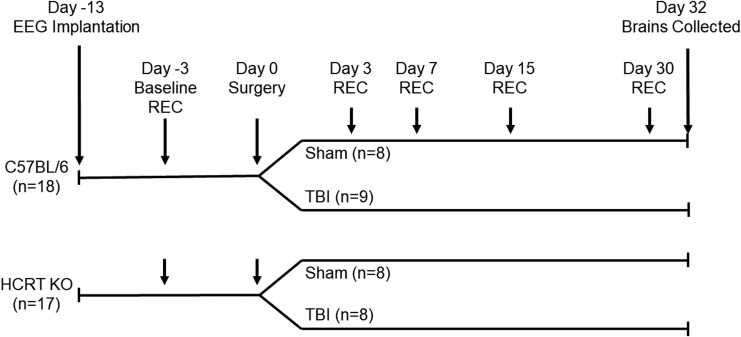
A schematic representation of the protocols used in this study is presented in Figure 1.
FIG. 1.
Schematic representation of the protocols used in the present study. C57BL/6J and hypocretin knockout (HCRT KO) mice were implanted with electroencephalography (EEG) recording electrodes and allowed to recover. Forty-eight hours baseline EEG recordings were obtained from undisturbed mice, after which two mice were dropped from the study due to poor EEG signals. Mice of each genotype were randomized into either control (sham surgeries) or experimental (moderate traumatic brain injury [TBI] surgeries) groups. Moderate TBI was induced using controlled cortical impact with a piston depth of 1.0 mm. Forty-eight–hour recordings were obtained from all mice at 3, 7, 15, and 30 days post-surgery. After the last recording period (32 days post-surgery), animals were perfused and brains were removed for immunohistochemistry.
Experiment 1: Effects of TBI on sleep–wake behavior of hypocretin KO mice
C57BL/6J mice (n = 18) and HCRT KO mice (n = 17) were surgically implanted with EEG recording electrodes under isoflurane anesthesia. These electrodes were implanted in the skull but did not penetrate the dura matter. We37 and others38,42 have previously used this method to assess sleep–wake behavior following TBI. The leads from the screw electrodes were soldered to pins of a plastic connector (Digi-Key; ED85100-ND) to allow coupling to the recording system. Dental acrylic (Integrity Caulk; Dentsply) covered the electrodes and formed a headpiece to which the flexible recording tether could be connected. The section of the skull over the left parietal cortex was not covered with dental acrylic at this time. The incision was closed with sutures, and a subcutaneous injection of an analgesic (0.5 mg/kg buprenorphine) was given at the end of the surgery. Mice were allowed 7 days to recover before they were attached to a flexible tether for habituation to the recording system. After 3 days of habituation to the tether and recording environment, 48-h undisturbed baseline recordings were obtained.
After the 48-h baseline recordings, two mice were dropped from the study due to poor quality of the EEG signals. Remaining mice were then randomized into four groups: C57BL/6J sham (n = 8), C57BL/6J TBI (n = 9), HCRT KO sham (n = 8), and HCRT KO TBI (n = 8). TBI was induced using controlled cortical impact (CCI), as previously described.37,55 We previously demonstrated that 1 mm depth CCI produces moderate TBI and significant changes in sleep–wake behavior,37 and we used the same parameters in this study. All mice (sham, TBI) received a craniotomy over the left parietal cortex using a 5 mm trephine, approximately −2 mm relative to bregma and 2.5 mm lateral to the midline. The skull fragment was removed without disrupting the underlying dura, and TBI was induced in the experimental group using the Leica Impact One system (Richmond, IL) equipped with an electrically-driven 3-mm diameter metal piston controlled by a linear velocity displacement transducer. CCI parameters were: 5.0 m/sec impact velocity; 100 msec dwell time; and impact depth of 1.0 mm (moderate TBI). Sham (control) animals received identical anesthesia and craniotomy without the CCI injury. After the CCI, a sterilized polystyrene disc created from a weighing boat was placed over the craniotomy and covered with dental acrylic. We37,55 and others56 have used this or a similar technique to protect the brain after craniotomy. After the incision was closed with sutures, mice received a subcutaneous injection of analgesic (0.5 mg/kg buprenorphine) and were returned to their home cages. Animals were closely monitored after surgery and none displayed overt signs of infection.
Additional 48-h recordings were obtained from all mice on post-craniotomy/TBI Days 3–4, 7–8, 14–15, and 30–31. Sleep–wake behavior was determined and the EEG subjected to fast Fourier transformation to produce power spectra between 0.5 and 40 Hz in 0.5 Hz bins as described previously.52 Power in the delta frequency band (0.5–4.5 Hz) during NREM sleep was analyzed in a subset of mice (n = 5–6 mice per group). NREM delta power was normalized to the total state-specific power (NREM sleep), summed across all frequency bins from 0.5 to 40 Hz for the light and dark periods and expressed as a percent of total power.53
Experiment 2: Effects of TBI on numbers of hypocretin neurons
After the Day 30–31 post-craniotomy/TBI recording, mice were perfused and brains removed for immunohistochemical assessment of TBI effects on hypocretin neurons. Brains were sectioned on a Leica cryostat at 14 μm and mounted on Superfrost Plus slides. Briefly, slides were washed in phosphate-buffered saline (PBS) three times for 5 min each, then blocked for 30 min with a 1:20 dilution of normal donkey serum in PBS with 1% bovine serum albumin. Slides were then incubated overnight at 4°C in rabbit anti-mouse orexin-A (H-003-30; Phoenix Pharmaceuticals, Inc.; 1:1,000 dilution). Slides were then rinsed with PBS six times for 5 min each. Slides were then incubated for 30 min in the secondary antibody solution (Alexa Fluor 488 donkey anti-rabbit; Jackson ImmunoResearch; 711-545-152; 1:400 dilution). Slides were rinsed with PBS six more times for 5 min each and then cover-slipped.
Estimating cell numbers
Cell numbers were estimated using quantitative methods for unbiased stereology.57 Briefly, positively stained cells were visualized on an Olympus BX-51 fluorescent stereoscope using Stereo Investigator 10 (MBF Biosciences, Williston, VT). Hypocretin cell number estimates were obtained from seven sections in a 1:9 series spanning approximately −1.20 mm to −2.10 mm from bregma. The contour for the perifornical-lateral hypothalamic region was outlined using a 4 × objective. Cells were then counted using the 20 × objective and optical fractionator, with a counting frame of 50 × 50 microns and a grid size of 100 × 100 microns. All cell counts were obtained from the hemisphere ipsilateral to injury. TBI-induced changes in cellular and tissue outcomes (cell death, inflammatory cytokine expression, presence of immune cells, etc.) are typically most severe on the side ipsilateral to injury58,59 and previous studies using unilateral CCI have examined hypocretin neuron number and function in the hypothalamus ipsilateral to injury.34
Statistical analysis
To determine the impact of TBI and genotype on sleep–wake behavior, percent time spent in wake, NREM sleep, or REM sleep during the light or dark periods was analyzed using a two-way variance of analysis (ANOVA; with injury condition and genotypes as the two factors) with repeated measures. If there were statistically significant differences observed in the full model, sleep–wake behavior was further examined using a one-way ANOVA within time block to determine differences between the four groups (C57BL/J sham, C57BL/6 TBI, HCRT KO sham, and HCRT KO TBI). If significant differences were detected, post hoc comparisons were made using Tukey's honestly significant difference (HSD) to determine which groups contributed to statistical differences.
We also determined the effect of injury and genotype on the temporal distribution of sleep–wake behavior over the course of 24-h recording periods. The percent time spent in wake, NREM sleep, and REM sleep were determined for each recording hour and then grouped into 4-h time blocks. For each 4-h time block, values were evaluated using a two-way ANOVA (with injury condition and genotype as the two factors) with repeated measures (i.e. across baseline, 3 days post-surgery, etc.). If there were statistically significant differences observed in the full model, a further examination was made using a one-way ANOVA within each 4-h time block to determine differences between the four groups (C57BL/J sham, C57BL/6 TBI, HCRT KO sham, and HCRT KO TBI). If significant differences were detected, post hoc comparisons were made using Tukey's HSD to determine differences between/among groups.
To assess the effects of injury and genotype on sleep architecture, bouts of wakefulness were sorted by length into 1 min bins up to 10-min. Wake bout lengths of 10- 20 min, and wake bout lengths greater than 20 min, were grouped into two separate bins. Light and dark period wake bouts were assessed separately. For each bout length bin, significance was assessed using a two-way ANOVA with repeated measures. If there were statistically significant differences observed in the full model, a further examination was made using a one-way ANOVA within each bout length bin to determine differences between the four groups (C57BL/J sham, C57BL/6 TBI, HCRT KO sham, and HCRT KO TBI). If significant differences were detected, post hoc comparisons were made using Tukey's HSD.
To assess effects of injury and genotype on delta power, normalized NREM delta power was calculated for each hour across the 24-h period. Since animals do not enter NREM sleep every hour, NREM delta power could not be calculated for every hour. Further, each animal did not have the same number of hours in NREM at each time-point. Since a repeated measures ANOVA requires the same number of values at each time-point, the full model could not be statistically analyzed. Statistical analyses using one-way ANOVA (between the four groups) were then conducted separately for 12 h of the light and dark periods for baseline and each post-surgical time-point. If significant differences were revealed within time-point, post hoc comparisons were made using Tukey's HSD to determine which conditions contributed to these effects.
Because HCRT KO mice lack hypocretin, the effect of TBI on numbers of hypocretin neurons was assessed only in C57BL/6J mice. Estimated numbers of hypocretin neurons in mice subjected to sham and TBI were compared using a non-paired Student's t test.
All analyses were performed using SPSS for Windows (IBM Corporation, Armonk, NY). Data are presented as mean ± standard error of the mean (SEM), unless otherwise indicated. An alpha value of p < 0.05 was accepted as indicating a significant difference between or among groups.
Results
Sleep–wake behavior is altered by TBI in a genotype-dependent manner
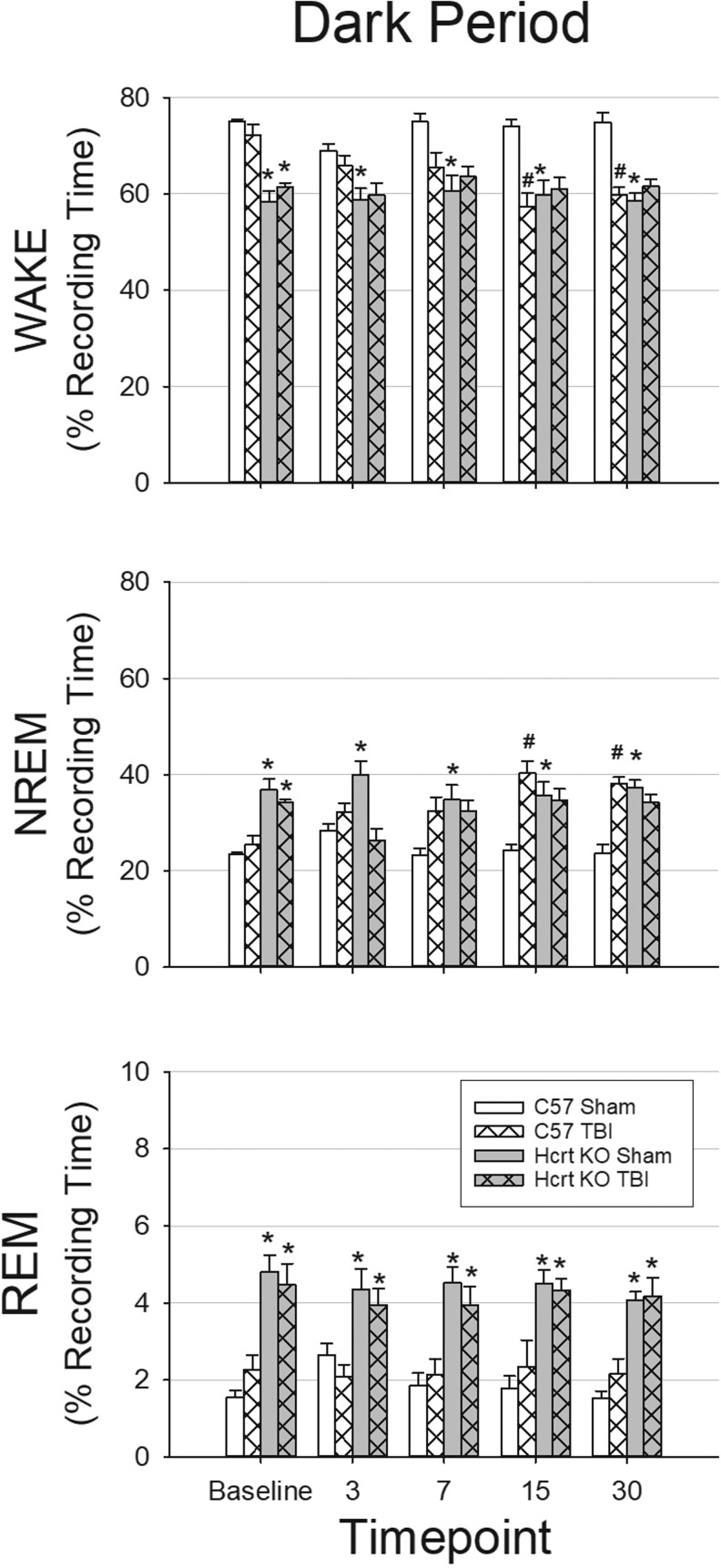
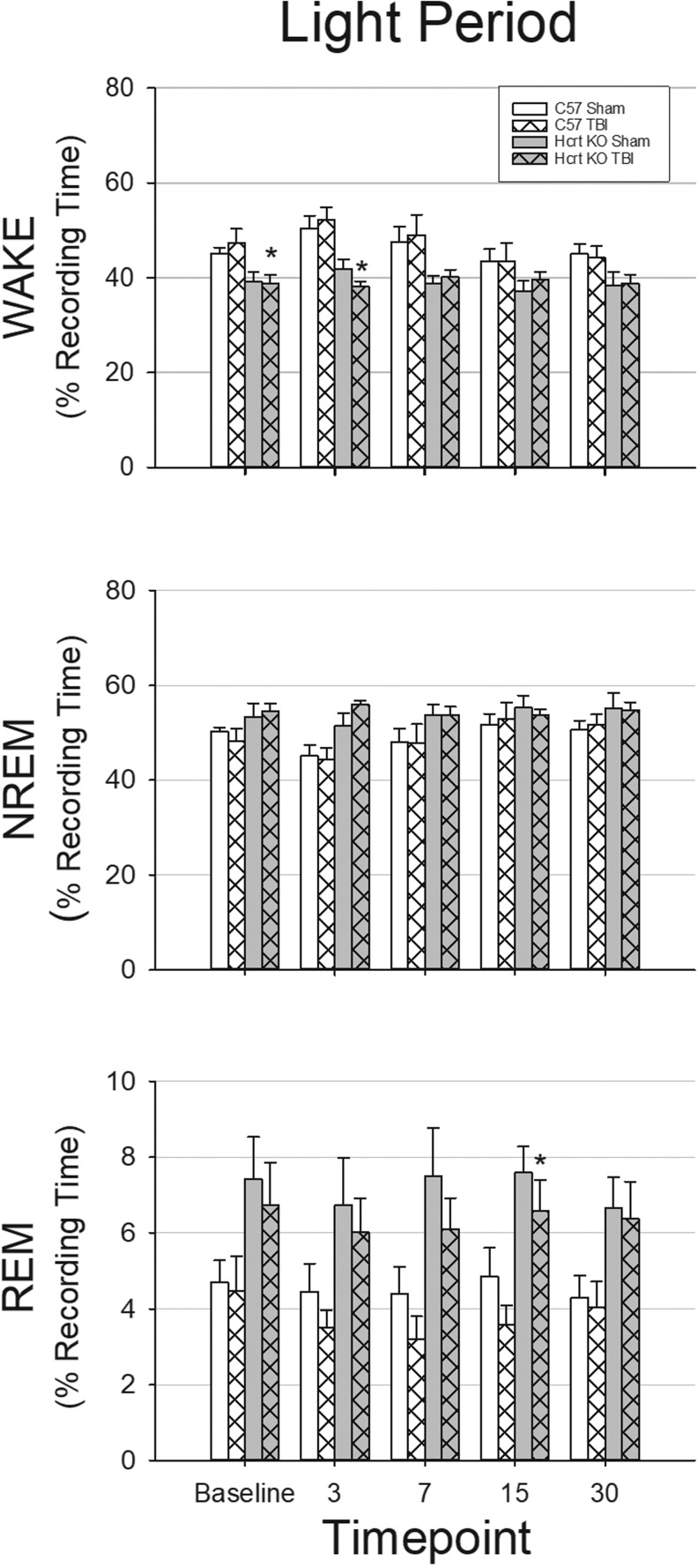
A two-way repeated measures ANOVA revealed that during the dark period, there were significant effects of time-point, genotype, and condition (sham or TBI), as well as significant interaction effects for time-point × genotype, time-point × condition, and time-point × genotype × condition for NREM sleep and wakefulness. During the dark period (Fig. 2) and the light period (Fig. 3), there was a significant genotype effect on the amount of REM sleep. During the light period (Fig. 2), amount of NREM sleep and wakefulness were significantly different based on time-point and genotype, but there were no interaction effects.
FIG. 2.
Traumatic brain injury (TBI) decreases wakefulness and increases non-rapid eye movement sleep during the dark period in C57BL/6J mice but not in hypocretin knockout mice. electroencephalography (EEG) and home cage activity recordings were obtained from four groups of mice: sham surgery C57BL/6J mice (n = 8); TBI surgery C57BL/6J mice (n = 9); sham surgery hypocretin knockout (HCRT KO) mice (n = 8); and TBI surgery HCRT KO mice (n = 8). Values are means (± standard error of the mean) percent of time spent in wakefulness (WAKE), non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep during the 12 h dark periods at baseline, 3, 7, 15, and 30 days post-surgery. * indicates a statistically significant difference (p < 0.05) between genotypes (C57BL/6J vs. HCRT KO) within the same condition. # indicates a statistically significant difference (p < 0.05) between conditions (sham vs. TBI) with the same genotype.
FIG. 3.
Traumatic brain injury (TBI) has little impact on wakefulness and sleep during the light period. EEG and home cage activity recordings were obtained from four groups of mice: sham surgery C57BL/6J mice (n = 8); TBI surgery C57BL/6J mice (n = 9); sham surgery hypocretin knockout (HCRT KO) mice (n = 8); and TBI surgery HCRT KO mice (n = 8). Values are means (± SEM) percent of time spent in wakefulness (WAKE), non-rapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep during the 12 h light period at baseline, 3, 7, 15, and 30 days post-surgery. * indicates a statistically significant difference (p < 0.05) between genotypes (C57BL/6J vs. HCRT KO) within the same condition. # indicates a statistically significant difference (p < 0.05) between conditions (sham vs. TBI) with the same genotype.
As there were significant differences in the full models, we ran ANOVAs within time-point to determine which groups were different within each time-point. Under baseline (undisturbed) conditions prior to craniotomy/TBI surgeries, the amount of time mice spent in wakefulness, NREM, and REM sleep differed by genotype: HCRT KO mice spent significantly less time awake during the dark period and light period, and more time in NREM and REM sleep during the dark period compared with C57BL/6J mice (Fig. 2 and Fig. 3). Sleep–wake behavior of mice of the same genotype that were subsequently randomized into sham or TBI groups did not differ during pre-surgical baseline recordings in terms of total amount of time spent in each state (Fig. 2 and Fig. 3).
TBI did not alter sleep of HCRT KO mice (Fig. 2 and Fig. 3). No differences were detected between HCRT KO sham and TBI groups in the total amount of time spent in wake, NREM, or REM sleep at any post-surgical time-point. Further, the temporal distribution of sleep–wake behavior was not altered by TBI in HCRT KO mice (Supplementary Fig. 1; see online supplementary material at http://www.liebertpub.com).
In contrast to HCRT KO mice, however, TBI altered sleep of C57BL/6J mice (Fig. 2 and Fig. 3) with changes most pronounced during the dark period at 15 and 30 days post-injury. During these chronic post-TBI time-points, relative to control mice that received sham surgeries, the total amount of time C57BL/6J mice spent awake was reduced during the dark period. Reduced wakefulness during the dark period was concomitant with increased time spent in NREM sleep (Fig. 2). The temporal distribution of sleep–wake behavior of C57BL/6J mice was altered by TBI, with effects apparent during the dark period at 7 days post-injury and becoming more profound at 15 and 30 days post-injury. Specifically, reduced wakefulness and increased NREM sleep were first apparent during the early dark period at 7 days post-injury, and these changes progressively extended to encompass the entire 12-h dark period by 30 days post-injury (Supplementary Fig. 1).
There were minimal genotype differences in the total amount of time spent in wakefulness and NREM sleep after TBI, although HCRT KO mice spent less time awake during the light period 3 days after injury (Fig. 3) and during early parts of the dark period (Fig. 2). At chronic time-points, the total amount of NREM sleep and wakefulness of C57BL/6J mice subjected to TBI did not differ from that of HCRT KO mice with TBI (Fig. 2 and Fig. 3).
The total amount of time spent in REM sleep differed between genotype irrespective of whether or not mice had TBI. In general, HCRT KO mice had more REM sleep during any time-point across the 24 h recording periods (Supplementary Fig. 1).
Statistical analyses revealed at least one significant main effect or interaction effect in the temporal distribution of NREM sleep and wakefulness across the 24-h cycle (see Supplementary Fig. 1). There was a significant main effect of post–time-point for blocks 1–4, 5–8, 17–20, and 21–24. There was a significant effect of genotype for all blocks except 9–12 and a significant effect of condition for blocks 13–16 and 17–20. There was a significant interaction effect (time-point × genotype) for blocks 1–4, 9–12, 13–16, and 21–24. There were significant interaction effects (time-point × condition and time-point × genotype × condition) for block 13–16.
For amount of wakefulness, there was a significant main effect of time-point for blocks 1–4, 5–8, 17–20, and 21–24. There was a main effect of genotype for all blocks and a significant effect of condition for blocks 13–16 and 17–20. There was a significant interaction of time-point × genotype for blocks 13–16 and 21–24, a significant interaction of time-point × condition for block 13–16, and a significant interaction of time-point × genotype × condition for block 13–16. For amount of REM, there was a significant effect of genotype for each 4-h time block.
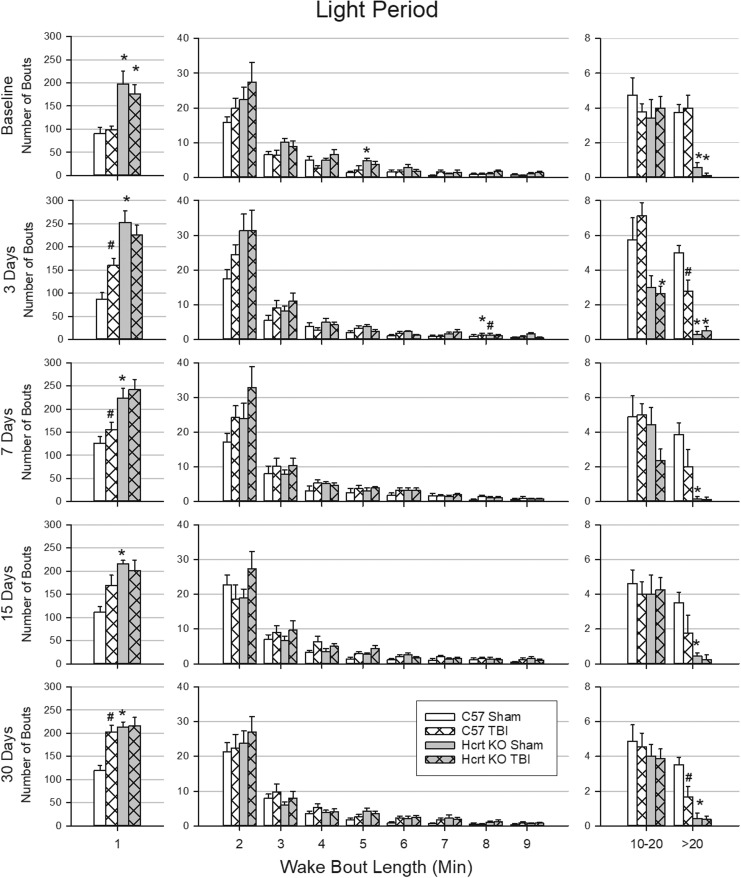
Wake bout length is altered by TBI in a genotype-dependent manner
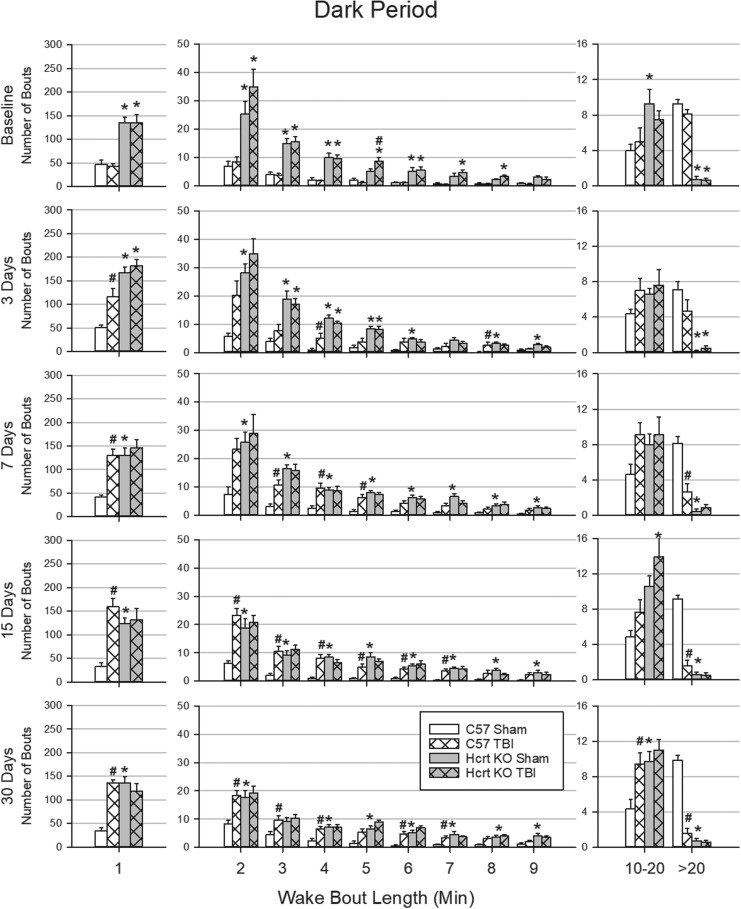
There were genotype differences in the duration of wake bouts during the dark period (Fig. 4) and light period (Fig. 5), which were most consistent for very short (1 min or less) and very long (more than 20 min) bouts. Under baseline conditions during the dark and light periods, HCRT KO mice had more very short bouts and fewer very long bouts than did C57BL/6J mice. TBI did not alter the distribution of wake bout lengths in HCRT KO mice (Fig. 4 and Fig. 5). In contrast, 3 days after TBI, C57BL/6J mice had more very short bouts during the light and dark periods than did sham C57BL/6J mice. At 7 days post-surgery, and at the 15- and 30-day time-points, C57BL/6J mice with TBI had significantly fewer very long wake bouts than did sham C57BL/6J animals during the dark period, and fewer very long wake bouts during the light period at 3 and 30 days post-surgery compared with C57BL/6J sham mice.
FIG. 4.
Duration of wake bouts during the dark period is affected by traumatic brain injury (TBI) in a genotype-dependent manner. Wake bouts were sorted into bins based upon their duration. Values are the mean (± SEM) number of bouts in each bin during the 12 h dark periods at baseline, 3, 7, 15, and 30 days post-surgery for C57BL/6J mice with sham surgery (n = 8); C57BL/6J mice subjected to TBI (n = 9); hypocretin knockout (HCRT KO) mice with sham surgery (n = 8); and HCRT KO mice that had TBI (n = 8). * indicates a statistically significant difference (p < 0.05) between genotypes (C57BL/6J vs. HCRT KO) within the same condition. # indicates a statistically significant difference (p < 0.05) between conditions (sham vs. TBI) within the same genotype.
FIG. 5.
Short- and long duration of wake bouts during the light period are affected by traumatic brain injury in a genotype-dependent manner. Wake bouts were sorted into bins based upon their duration. Values are the mean (± SEM) number of bouts in each bin during the 12 h dark periods at baseline, 3, 7, 15, and 30 days post-surgery for C57BL/6J mice with sham surgery (n = 8); C57BL/6J mice subjected to TBI (n = 9); hypocretin knockout (HCRT KO) mice with sham surgery (n = 8); and HCRT KO mice that had TBI (n = 8). * indicates a statistically significant difference (p < 0.05) between genotypes (C57BL/6J vs. HCRT KO) within the same condition. # indicates a statistically significant difference (p < 0.05) between conditions (sham vs. TBI) within the same genotype.
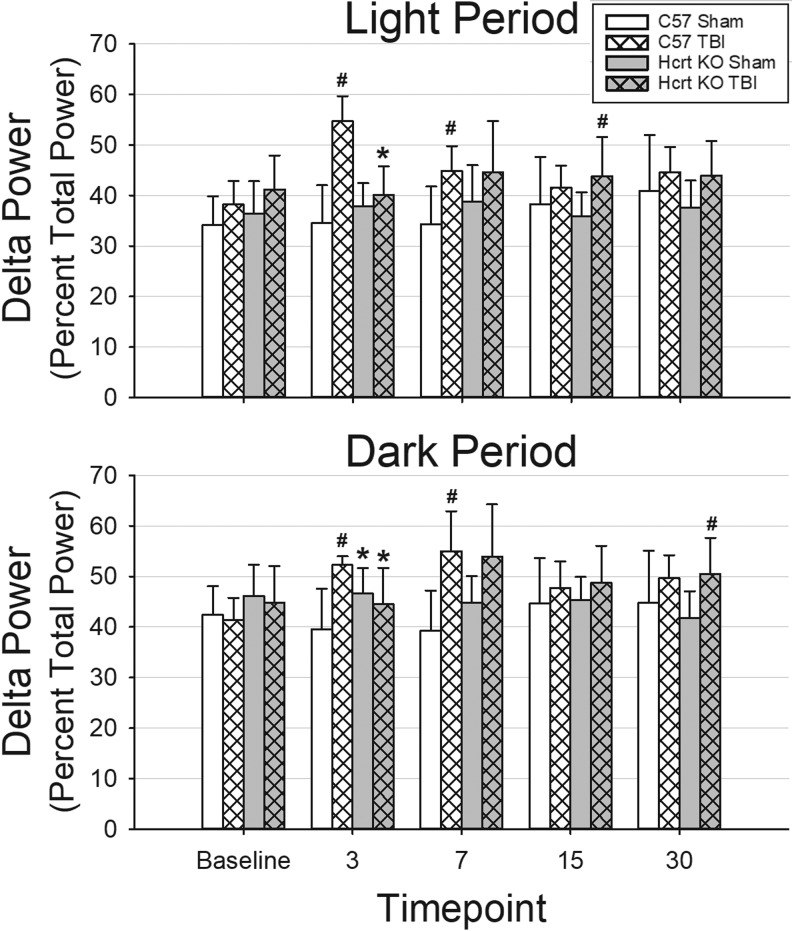
Delta power is transiently altered by TBI
Due to artifacts, some mice were not included in analyses of spectral characteristics of the EEG. However, in these cases the signal was sufficient for manual determination of sleep–wake state. NREM delta power was analyzed separately for the light and dark periods (Fig. 6).
FIG. 6.
Traumatic brain injury (TBI) transiently increases delta power during non-rapid eye movement sleep in C57BL/6J mice. Power in the electroencephalogram (EEG) delta frequency band (0.5–4.5 Hz) was calculated by fast Fourier transform from artifact-free, state-specific epochs during the light and dark periods. Animals with the least artifact from each group were used: C57BL/6J mice with sham surgery (n = 5); C57BL/6J mice subjected to TBI (n = 5); hypocretin knockout (HCRT KO) mice that had sham surgeries (n = 6); and HCRT KO mice with TBI (n = 6). Delta power was normalized as the percent of the total power and is plotted as mean ± standard error of the mean. * indicates a statistically significant difference (p < 0.05) between genotypes (C57BL/6J vs. HCRT KO) within the same condition, whereas # indicates a statistically significant difference (p < 0.05) between conditions (sham vs. TBI) within the same genotype.
During baseline recordings, there were no significant differences between groups in NREM delta power. At 3- and 7 days post-surgery, TBI increased NREM delta power during both the light and dark periods in C57BL/6J mice compared with C57BL/6J mice that had sham surgeries. Increased NREM delta power was not detected at 15 or 30 days post-surgery in C57BL/6J mice. NREM delta power was increased after TBI in HCRT KO mice relative to HCRT KO sham mice only at 15 days post-surgery (light period) and 30 days post-surgery (dark period). Differences between genotypes in NREM delta power after TBI were transient, only observed at 3 days post-surgery, and as such may be residual effects of anesthesia and surgery (Fig. 6).
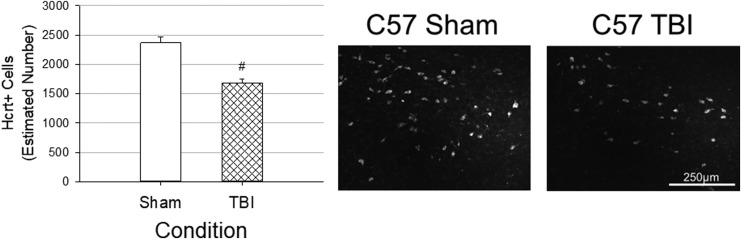
Estimated numbers of hypocretin neurons are reduced by TBI
At 32 days post-injury, C57BL/6J animals with TBI had significantly fewer hypocretin neurons in the lateral hypothalamus than did sham-operated C57BL/6J mice (Fig. 7). As expected, hypocretin immunoreactivity was not detected in HCRT KO mice.
FIG. 7.
Traumatic brain injury (TBI) reduces numbers of hypocretin neurons in C57BL/6J mice. Numbers of hypocretin neurons in the lateral hypothalamus ipsilateral to the injury site were estimated using unbiased stereology and the optical fractionator method. Values are means ± standard error of the mean obtained from C57BL/6J mice with sham surgery (n = 8) or TBI (n = 9). Counts were obtained at the end of the study, 32 days post-surgery. # indicates a statistically significant difference (p < 0.05) between conditions (sham vs. TBI). Representative immunofluorescent images of the lateral hypothalamus ipsilateral to the injury site are presented.
Discussion
Sleep–wake disturbances after TBI have been documented extensively in humans.6,10,16,51,60,61 In spite of the large body of literature on post-TBI sleep and wake disturbances, there is a lack of effective treatments. Stimulants like methylphenidate, modafinil, and armodafinil have mixed efficacy in normalizing sleep–wake behavior in TBI patients.62–66 Antidepressants and melatonin agonists are not always effective in improving nighttime sleep quality.62,67,68 Sleep hygiene interventions are ineffective,69 and although small trials of blue light therapy70 or modified cognitive behavioral therapy71,72 have shown some promise, few of these interventions have been attempted, and these treatments are expensive and time-consuming.
The lack of effective therapeutics is likely due, at least in part, to a lack of understanding of the precise causes of post-TBI sleep and wake disturbances. Many have hypothesized that changes in neurotransmitters, neuropeptides, and hormones that regulate sleep and wakefulness are responsible for post-TBI disturbances. Hypocretin,37,38,45,46 histamine,36,44 serotonin,49 noradrenaline,49 acetylcholine,37,47,73 and melatonin50 are the transmitters for which data suggest potential roles as mediators of post-TBI sleep and wake disturbances. Melatonin is relatively easy to assay in saliva or plasma, but determining levels of neurotransmitters in living human patients is much more difficult. Although neurotransmitters can be measured in the CSF with a lumbar puncture as a proxy for levels in brain, this process is invasive and not without risk.
Given the limitations in obtaining appropriate samples from living patients, some studies have determined neurotransmitter concentrations in cases of fatal TBI.44,45,49 Although postmortem collection provides access to samples for the assay of neurotransmitters, the extent to which a brain that has undergone a fatal injury resembles a brain that has been subjected to mild or moderate injury and then survived is not known. Utilizing appropriate animal models to identify potential neurotransmitters or other systems that are potential contributors to, or mediators of post-TBI sleep and wake disturbances ameliorates some of the limitations that are inherent in the study of patients subjected to brain injury. Animal models are especially useful in the context of TBI as injury location and severity can be precisely controlled and the brain can be assessed at selected time-points after injury.74–78 Further, genomic manipulation in animal models is a powerful tool that can be used to determine the functional role of transmitter substances in exacerbating or ameliorating the secondary injury or reparative processes that occur after TBI.
To our knowledge, this study is the first to use genetic ablation of hypocretin to determine its role in responses to TBI. In this study, we subjected HCRT KO mice to moderate TBI, and now report that hypocretin plays a critical role in sleep responses to brain injury in this model. Sleep–wake behavior of HCRT KO mice is not altered by TBI relative to values obtained from HCRT KO mice subjected to sham surgery. Further, although TBI reduces wakefulness and increases NREM sleep of C57BL/6J (control) mice at later post-injury time-points (15 and 30 days), the amount of time spent in these arousal states does not differ from those of HCRT KO mice.
Similarly, although they have significantly more very short wake bouts and fewer very long wake bouts during baseline conditions than do C57BL/6J mice, TBI does not change the distribution of wake bouts in HCRT KO mice. In contrast, after TBI, C57BL/6J mice have significantly more very short and fewer very long wake bouts than do mice of the same genotype that had sham surgeries. Further, at chronic time-points (15 and 30 days), the distribution of wake bout lengths of C57BL/6J mice and HCRT KO mice do not differ after TBI.
Although the majority of studies report sleep disturbance after TBI, the exact nature of altered sleep is highly variable, even in animal models. Our present results agree with other pre-clinical studies of TBI that report increased NREM sleep36–38 and increased sleep fragmentation34,38,39,41 during the dark period without changes in the amount of REM sleep.36–39,41,42 Although the timing is variable, our results are also in agreement with other studies that found increased NREM delta power at some times after TBI.36,37
Our results are also consistent with some studies in humans that found increases in slow wave sleep11,19–21 and no differences in time spent in REM sleep.11–13,20,21,29 In our model, shown in the present study and in previous research,37 TBI has little impact on REM sleep. Although HCRT KO mice normally spend more time in REM sleep than do genetically-intact control mice, there are no consistent changes in this sleep stage after TBI in either genotype. Loss of hypocretin neurons in narcoleptic humans79–82 and KO mice83–85 consistently produces REM sleep abnormalities (increased time in REM sleep and sleep-onset REM periods). However, acute modulation of hypocretin neurons has less impact on REM sleep. For example, optogenetic silencing of hypocretin neurons in mice decreases wakefulness and increases NREM sleep, and increases sleep–wake state fragmentation86 without altering REM sleep.86,87 Similarly, pharmacogenetic suppression of hypocretin neuron activity decreases wakefulness and increases NREM sleep with no change in REM sleep time.88 Studies of hypocretin antagonists in humans and animals consistently reveal reduced wakefulness and increased NREM sleep, and although some studies also report increased REM sleep,89–91 others do not.92–94
One possible explanation for inconsistent findings of hypocretin function on REM sleep is that there may be dose-related effects. For example, at low doses, hypocretin antagonists increase NREM sleep, but not REM sleep, whereas high doses of the same antagonist increase NREM and REM sleep.95 Even at high doses, hypocretin antagonists generally do not induce sleep-onset REM periods, which are a common feature of narcolepsy (i.e., a state of hypocretin loss).96,97 Further, homozygous HCRT KO mice display an animal form a cataplexy, whereas heterozygous HCRT KO mice do not.83 Thus, it is possible that the partial loss of hypocretin neurons observed in genetically-intact mice in this and our previous study37 is sufficient to decrease wakefulness and increase NREM sleep, but not enough to increase REM sleep. Increased REM sleep is only evident when hypocretin is completely absent, as in the HCRT KO mice.
The reduction in the number of very long wake bouts observed in control mice subjected to TBI in this present study suggests an inability to maintain long periods of wakefulness during their active period. This observation is consistent with the high rates of daytime napping observed in humans with TBI.11,18
Our results also generally agree with other research on sleep–wake behavior in animals and humans that lack hypocretin. Similar to other reports, HCRT KO mice in our current study exhibit less wakefulness,83,84 more NREM sleep,83 more REM sleep,83,84,98 and fragmented sleep and wake bouts85,99,100 under pre-injury baseline conditions compared with genetically intact mice. Like HCRT KO mice, humans with narcolepsy exhibit more NREM sleep,82 more REM sleep,82 and more fragmented sleep–wake behavior.82,101,102 The reduced wakefulness observed in HCRT KO mice during their active period may be analogous to the excessive daytime sleepiness observed in narcolepsy patients.103–105
Lastly, we found that numbers of hypocretin-positive cells in genetically intact mice subjected to TBI were reduced at the end of the study relative to sham-injured animals. These data extend findings from our previous work in which hypocretin neurons were significantly reduced by TBI at 7 and 15 days post-injury.37 These new results suggest that hypocretin neurons are not recovered with time, at least within the 1-month post-injury period evaluated in this study. These results are consistent with previous human44,45 and animal studies41 that report reduced numbers of hypocretin neurons after TBI. Other aspects of hypocretin function also are altered by TBI. For example, hypocretin release34 and hypocretin neuronal activation38 are reduced after TBI. Why some studies show that hypocretin neurons disappear while others find that they are merely dysfunctional remains to be determined.
Hypocretin is an attractive therapeutic candidate because, in addition to sleep and wake disturbances, hypocretinergic dysfunction also may be involved in several other sequelae, including impaired cognition,106 depression/depressive-like behavior,41,107 and microglial dysregulation.108,109 Hypocretin is implicated in memory processes110,111 and hippocampal cell proliferation.112 In humans113 and animals,114 low hypocretin is associated with depression or depressive-like behaviors. Hypocretin is also involved in microglial regulation, steering them towards a less inflammatory phenotype.115 Thus, therapeutics that attempt to normalize hypocretin might address several behavioral and neuroinflammatory symptoms of TBI.
In conclusion, the present study is in agreement with our previous work and that of others, which report TBI-induced alterations in sleep. TBI in this model decreases wakefulness, increases NREM sleep, decreases the length of wake bouts, and reduces the number of hypocretin neurons. We extend these observations by providing convincing evidence that hypocretin cell loss, or hypocretin dysfunction, is responsible for post-TBI sleep and wake disturbances. First, sleep–wake behavior of HCRT KO mice is not altered by TBI in this model. Second, although sleep–wake behavior of genetically-intact C57BL/6J mice differs from that of HCRT KO mice prior to injury, after TBI the time spent in wake and NREM sleep does not differ, and the distribution of very long or very short wake bouts is the same between genotypes. Thus, as genetically-intact mice lose hypocretin cells after TBI, their sleep patterns become similar to HCRT KO mice. As such, the decrease in cell numbers, or change in cell function of hypocretin neurons that occurs after TBI is necessary for the altered sleep–wake behavior observed in this model. Findings from this study indicate that the hypocretinergic system is a major contributor to post-TBI sleep and wake disturbances and suggest that hypocretin agonists may be a useful therapeutic intervention for ameliorating these disturbances.
Supplementary Material
Acknowledgments
We thank Dr. John Peever for providing the hypocretin knockout mice used in these experiments, and Dr. Maria Pavlova for overseeing their breeding. The technical assistance of Ms. Phoebe Domingo is greatly appreciated. This study was supported, in part, by the Department of Anesthesiology and Pain Medicine and the Graduate Program in Neuroscience of the University of Washington, Seattle, Washington, as well as the National Institutes of Health grant AI115706 (MRO).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Thurman D., Alverson C., and Dunn K. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 2. Ma V.Y., Chan L., and Carruthers K.J. (2014). Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, meultiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 95, 986–995 .e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumann C.R. (2016). Sleep and traumatic brain injury. Sleep Med. Clin. 11, 19–23 [DOI] [PubMed] [Google Scholar]
- 4. Wiseman-Hakes C., Duclos C., Blais H., Dumont M., Bernard F., Desautels A., Menon D., Gilbert D. and Carrier J. (2016). Sleep in the acute phase of severe traumatic brain injury: a snapshot of polysomnography. Neurorehabil. Neural Repair 30, 713–721 [DOI] [PubMed] [Google Scholar]
- 5. Chiu H.Y., Chen P.Y., Chen N.H., Chuang L.P., and Tsai P.S. (2013). Trajectories of sleep changes during the acute phase of traumatic brain injury: a 7-day actigraphy study. J. Formos. Med. Assoc. 112, 545–553 [DOI] [PubMed] [Google Scholar]
- 6. Kempf J., Werth E., Kaiser P.R., Bassetti C.L., and Baumann C.R. (2010). Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 81, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 7. Beetar J.T., Guilmette T.J., and Sparadeo F.R. (1996). Sleep and pain complaints in symptomatic traumatic brain injury and neurologic populations. Arch. Phys. Med. Rehabil. 77, 1298–1302 [DOI] [PubMed] [Google Scholar]
- 8. Chan L.G. and Feinstein A. (2015). Persistent sleep disturbances independently predict poorer functional and social outcomes 1 year after mild traumatic brain injury. J. Head Trauma Rehabil. 30, E67–E75 [DOI] [PubMed] [Google Scholar]
- 9. Duclos C., Beauregard M.P., Bottari C., Ouellet M.C., and Gosselin N. (2015). The impact of poor sleep on cognition and activities of daily living after traumatic brain injury: a review. Aust. Occup. Ther. J. 62, 2–12 [DOI] [PubMed] [Google Scholar]
- 10. Theadom A., Cropley M., Parmar P., Barker-Collo S., Starkey N., Jones K., Feigin V.L., McPherson K., Jones A., Ao B. Te. Brown P., Fairbairn-Dunlop P., Kydd R., Alan Barber P., Parag V., Ameratunga S., Dowell T., Kahan M., Christey G., and Hardaker N. (2015). Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 16, 926–932 [DOI] [PubMed] [Google Scholar]
- 11. Sommerauer M., Valko P.O., Werth E., and Baumann C.R. (2013). Excessive sleep need following traumatic brain injury: a case-control study of 36 patients. J. Sleep Res. 22, 634–639 [DOI] [PubMed] [Google Scholar]
- 12. Imbach L.L., Valko P.O., Li T., Maric A., Symeonidou E.R., Stover J.F., Bassetti C.L., Mica L., Werth E., and Baumann C.R. (2015). Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain 138, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imbach L.L., Büchele F., Valko P.O., Li T., Maric A., Stover J.F., Bassetti C.L., Mica L., Werth E., and Baumann C.R. (2016). Sleep-wake disorders persist 18 months after traumatic brain injury but remain underrecognized. Neurology 86, 1945–1949 [DOI] [PubMed] [Google Scholar]
- 14. Castriotta R.J., Atanasov S., Wilde M.C., Masel B.E., Lai J.M., and Kuna S.T. (2009). Treatment of sleep disorders after traumatic brain injury. J. Clin. Sleep Med. 5, 137–144 [PMC free article] [PubMed] [Google Scholar]
- 15. Verma A., Anand V., and Verma N.P. (2007). Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 3, 357–62 [PMC free article] [PubMed] [Google Scholar]
- 16. Schreiber S., Barkai G., Gur-Hartman T., Peles E., Tov N., Dolberg O.T., and Pick C.G. (2008). Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med. 9, 481–487 [DOI] [PubMed] [Google Scholar]
- 17. Sinclair K.L., Ponsford J., and Rajaratnam S.M.W. (2014). Actigraphic assessment of sleep disturbances following traumatic brain injury. Behav. Sleep Med. 12, 13–27 [DOI] [PubMed] [Google Scholar]
- 18. Parcell D.L., Ponsford J.L., Rajaratnam S.M., and Redman J.R. (2006). Self-reported changes to nighttime sleep after traumatic brain injury. Arch. Phys. Med. Rehabil. 87, 278–285 [DOI] [PubMed] [Google Scholar]
- 19. Parcell D.L., Ponsford J.L., Redman J.R., and Rajaratnam S.M. (2008). Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch. Phys. Med. Rehabil. 89, 843–850 [DOI] [PubMed] [Google Scholar]
- 20. Shekleton J.A., Parcell D.L., Redman J.R., Phipps-Nelson J., Ponsford J.L., and Rajaratnam S.M.W. (2010). Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 74, 1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mantua J., Mahan K., Henry O., and Spencer R.M.C. (2015). Altered sleep composition after traumatic brain injury does not affect declarative sleep-dependent memory consolidation. Front. Hum. Neurosci. 9, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mani A., Dastgheib S.A., Chanor A., Khalili H., Ahmadzadeh L., and Ahmadi J. (2015). Sleep quality among patients with mild traumatic brain injury: a cross-sectional study. Bull. Emerg. Trauma 3, 93–96 [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt A.T., Li X., Hanten G.R., McCauley S.R., Faber J., and Levin H.S. (2015). A longitudinal investigation of sleep quality in adolescents and young adults after mild traumatic brain injury. Cogn. Behav. Neurol. 28, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheng P., Hou L., Wang X., Wang X., Huang C., Yu M., Han X., and Dong Y. (2013). Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: A systematic review and meta-analysis. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang T.Y., Ma H.-P., Tsai S.H., Chiang Y.H., Hu C.J., and Ou J. (2015). Sleep duration and sleep quality following acute mild traumatic brain injury: a propensity score analysis. Behav. Neurol. 2015, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arbour C., Khoury S., Lavigne G.J., Gagnon K., Poirier G., Montplaisir J.Y., Carrier J., and Gosselin N. (2015). Are NREM sleep characteristics associated to subjective sleep complaints after mild traumatic brain injury? Sleep Med. 16, 534–539 [DOI] [PubMed] [Google Scholar]
- 27. Ponsford J.L., Ziino C., Parcell D.L., Shekleton J.A., Roper M., Redman J.R., Phipps-Nelson J., and Rajaratnam S.M.W. (2012). Fatigue and sleep disturbance following traumatic brain injury–their nature, causes, and potential treatments. J. Head Trauma Rehabil. 27, 224–233 [DOI] [PubMed] [Google Scholar]
- 28. Towns S.J., Silva M.A., and Belanger H.G. (2015). Subjective sleep quality and postconcussion symptoms following mild traumatic brain injury. Brain Inj. 7, 1–5 [DOI] [PubMed] [Google Scholar]
- 29. Gosselin N., Lassonde M., Petit D., Leclerc S., Mongrain V., Collie A., and Montplaisir J. (2009). Sleep following sport-related concussions. Sleep Med. 10, 35–46 [DOI] [PubMed] [Google Scholar]
- 30. Mollayeva T., Colantonio A., Cassidy J.D., Vernich L., Moineddin R., and Shapiro C.M. (2017). Sleep stage distribution in persons with mild traumatic brain injury: a polysomnographic study according to American Academy of Sleep Medicine standards. Sleep Med. 34, 179–192 [DOI] [PubMed] [Google Scholar]
- 31. Borbely A.A., Baumann F., Brandeis D., Strauch I., and Lehmann D. (1981). Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 51, 483–495 [DOI] [PubMed] [Google Scholar]
- 32. Cajochen C., Knoblauch V., Kräuchi K., Renz C., and Wirz-Justice A. (2001). Dynamics of frontal EEG activity, sleepiness and body temperature under high and low sleep pressure. Neuroreport 12, 2277–2281 [DOI] [PubMed] [Google Scholar]
- 33. Rowe R.K., Striz M., Bachstetter A.D., Van Eldik L.J., Donohue K.D., O'Hara B.F., and Lifshitz J. (2014). Diffuse brain injury induces acute post-traumatic sleep. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Willie J.T., Lim M.M., Bennett R.E., Azarion A.A., Schwetye K.E., and Brody D.L. (2012). Controlled cortical impact traumatic brain injury acutely disrupts wakefulness and extracellular orexin dynamics as determined by intracerebral microdialysis in mice. J. Neurotrauma 29, 1908–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rowe R.K., Harrison J.L., O'Hara B.F., and Lifshitz J. (2014). Diffuse brain injury does not affect chronic sleep patterns in the mouse. Brain Inj. 28, 504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noain D., Büchele F., Schreglmann S.R., Valko P.O., Gavrilov Y., Morawska M.M., Imbach L.L., and Baumann C.R. (2017). Increased sleep need and reduction of tuberomammillary histamine neurons after rodent traumatic brain injury. J. Neurotrauma 35, 85–93 [DOI] [PubMed] [Google Scholar]
- 37. Thomasy H.E., Febinger H.Y., Ringgold K.M., Gemma C., and Opp M.R. (2017). Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury. Neurobiol. Sleep Circadian Rhythm 2, 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lim M.M., Elkind J., Xiong G., Galante R., Zhu J., Zhang L., Lian J., Rodin J., Kuzma N.N., Pack A.I., and Cohen A.S. (2013). Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury. Sci. Transl. Med. 5, 215ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabir M., Gaudreault P.O., Freyburger M., Massart R., Blanchet-Cohen A., Jaber M., Gosselin N., and Mongrain V. (2015). Impact of traumatic brain injury on sleep structure, electrocorticographic activity and transcriptome in mice. Brain. Behav. Immun. 47, 118–130 [DOI] [PubMed] [Google Scholar]
- 40. Hazra A., Macolino C., Elliott M.B., and Chin J. (2014). Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J. Neurosci. Res. 92, 1434–1445 [DOI] [PubMed] [Google Scholar]
- 41. Skopin M.D., Kabadi S. V, Viechweg S.S., Mong J.A., and Faden A.I. (2015). Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury. J. Neurotrauma 32, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petraglia A.L., Plog B.A., Dayawansa S., Chen M., Dashnaw M.L., Czerniecka K., Walker C.T., Viterise T., Hyrien O., Iliff J.J., Deane R., Nedergaard M., and Huang J.H. (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: a novel mouse model of chronic traumatic encephalopathy. J. Neurotrauma 31, 1211–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Modarres M.H., Kuzma N.N., Kretzmer T., Pack A.I., and Lim M.M. (2017). EEG slow waves in traumatic brain injury: convergent findings in mouse and man. Neurobiol. Sleep Circadian Rhythm. 2, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valko P.O., Gavrilov Y. V., Yamamoto M., Finn K., Reddy H., Haybaeck J., Weis S., Scammell T.E., and Baumann C.R. (2015). Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann. Neurol. 77, 177–182 [DOI] [PubMed] [Google Scholar]
- 45. Baumann C.R., Bassetti C.L., Valko P.O., Haybaeck J., Keller M., Clark E., Stocker R., Tolnay M., and Scammell T.E. (2009). Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann. Neurol. 66, 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baumann C.R., Stocker R., Imhof H.G., Trentz O., Hersberger M., Mignot E., and Bassetti C.L. (2005). Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology 65, 147–149 [DOI] [PubMed] [Google Scholar]
- 47. Murdoch I., Perry E.K., Court J.A., Graham D.I., and Dewar D. (1998). Cortical cholinergic dysfunction after human head injury. J. Neurotrauma 15, 295–305 [DOI] [PubMed] [Google Scholar]
- 48. Murdoch I., Nicoll J.A.R., Graham D.I., and Dewar D. (2002). Nucleus basalis of Meynert pathology in the human brain after fatal head injury. J. Neurotrauma 19, 279–284 [DOI] [PubMed] [Google Scholar]
- 49. Valko P.O., Gavrilov Y. V, Yamamoto M., Noaín D., Reddy H., Haybaeck J., Weis S., Baumann C.R., and Scammell T.E. (2016). Damage to arousal-promoting brainstem neurons with traumatic brain injury. Sleep, 1249–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grima N.A., Ponsford J.L., St Hilaire M.A., Mansfield D., and Rajaratnam S.M. (2016). Circadian melatonin rhythm following traumatic brain injury. Neurorehabil. Neural Repair 30, 972–977 [DOI] [PubMed] [Google Scholar]
- 51. Baumann C.R., Werth E., Stocker R., Ludwig S., and Bassetti C.L. (2007). Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 130, 1873–1883 [DOI] [PubMed] [Google Scholar]
- 52. Baracchi F. and Opp M.R. (2008). Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav Immun 22, 982–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ingiosi A.M., Raymond R.M., Pavlova M.N., and Opp M.R. (2015). Selective contributions of neuronal and astroglial interleukin-1 receptor 1 to the regulation of sleep. Brain. Behav. Immun. 48, 244–257 [DOI] [PubMed] [Google Scholar]
- 54. Sutton B.C., and Opp M.R. (2014). Musculoskeletal sensitization and sleep: chronic muscle pain fragments sleep of mice without altering its duration. Sleep 37, 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Febinger H.Y., Thomasy H.E., Pavlova M.N., Ringgold K.M., Barf P.R., George A.M., Grillo J.N., Bachstetter A.D., Garcia J.A., Cardona A.E., Opp M.R., and Gemma C. (2015). Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J. Neuroinflammation 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miller D.M., Wang J.A., Buchanan A.K., and Hall E.D. (2014). Temporal and spatial dynamics of nrf2-antioxidant response elements mediated gene targets in cortex and hippocampus after controlled cortical impact traumatic brain injury in mice. J. Neurotrauma 31, 1194–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. West M.J., Slomianka L., and Gundersen H.J. (1991). Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 [DOI] [PubMed] [Google Scholar]
- 58. Hall E.D., Sullivan P.G., Gibson T.R., Pavel K.M., Thompson B.M., and Scheff S.W. (2005). Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma 22, 252–265 [DOI] [PubMed] [Google Scholar]
- 59. Timaru-Kast R., Luh C., Gotthardt P., Huang C., Schäfer M.K., Engelhard K., and Thal S.C. (2012). Influence of age on brain edema formation, secondary brain damage and inflammatory response after brain trauma in mice. PLoS One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gosselin N. and Tellier M. (2010). Patients with traumatic brain injury are at high risk of developing chronic sleep-wake disturbances. J. Neurol. Neurosurg. Psychiatry 81, 1297. [DOI] [PubMed] [Google Scholar]
- 61. Shekleton J.A., Parcell D.L., Redman J.R., Phipps-Nelson J., Ponsford J.L., and Rajaratnam S.M. (2010). Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 74, 1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee H., Kim S.W., Shin I.S., Yang S.J., and Yoon J.S. (2005). Comparing effects of methylphenidate, sertraline and placebo on neuropsychiatric sequelae in patients with traumatic brain injury. Hum. Psychopharmacol. 20, 97–104 [DOI] [PubMed] [Google Scholar]
- 63. Al-Adawi S., Burke D.T., and Dorvlo A.S.S. (2006). The effect of methylphenidate on the sleep-wake cycle of brain-injured patients undergoing rehabilitation. Sleep Med. 7, 287–291 [DOI] [PubMed] [Google Scholar]
- 64. Kaiser P.R., Valko P.O., Werth E., Thomann J., Meier J., Stocker R., Bassetti C.L., and Baumann C.R. (2010). Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology 75, 1780–1785 [DOI] [PubMed] [Google Scholar]
- 65. Jha A., Weintraub A., Allshouse A., Morey C., Cusick C., Kittelson J., Harrison-Felix C., Whiteneck G., and Gerber D. (2008). A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J. Head Trauma Rehabil. 23, 52–63 [DOI] [PubMed] [Google Scholar]
- 66. Menn S.J., Yang R., and Lankford A. (2014). Armodafi nil for the treatment of excessive sleepiness associated with mild or moderate closed traumatic brain injury: a 12-week, randomized, double-blind study followed by a 12-month open-label extension. J. Clin. Sleep Med. 10, 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kemp S., Biswas R., Neumann V., and Coughlan A. (2004). The value of melatonin for sleep disorders occurring post-head injury: a pilot RCT. Brain Inj. 18, 911–919 [DOI] [PubMed] [Google Scholar]
- 68. Lequerica A., Jasey N., Portelli Tremont J.N., and Chiaravalloti N.D. (2015). Pilot study on the effect of ramelteon on sleep disturbance after traumatic brain injury: preliminary evidence from a clinical trial. Arch. Phys. Med. Rehabil. 96, 1802–1809 [DOI] [PubMed] [Google Scholar]
- 69. De La Rue-Evans L., Nesbitt K., and Oka R.K. (2013). Sleep hygiene program implementation in patients with traumatic brain injury. Rehabil. Nurs. 38, 2–10 [DOI] [PubMed] [Google Scholar]
- 70. Sinclair K.L., Ponsford J.L., Taffe J., Lockley S.W., and Rajaratnam S.M.W. (2014). Randomized controlled trial of light therapy for fatigue following traumatic brain injury. Neurorehabil. Neural Repair 28, 303–313 [DOI] [PubMed] [Google Scholar]
- 71. Nguyen S., McKay A., Wong D., Rajaratnam S.M., Spitz G., Williams G., Mansfield D., and Ponsford J.L. (2017). Cognitive behavior therapy to treat sleep disturbance and fatigue After traumatic brain injury: a pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 98, 1508–1517 .e2 [DOI] [PubMed] [Google Scholar]
- 72. Ouellet M.C., and Morin C.M. (2007). Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch. Phys. Med. Rehabil. 88, 1581–1592 [DOI] [PubMed] [Google Scholar]
- 73. Pepeu G. and Grazia Giovannini M. (2017). The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 1670, 173–184 [DOI] [PubMed] [Google Scholar]
- 74. Sandsmark D.K., Elliott J.E., and Lim M.M. (2017). Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lifshitz J., Rowe R.K., Griffiths D.R., Evilsizor M.N., Thomas T.C., Adelson P.D., and McIntosh T.K. (2016). Clinical relevance of midline fluid percussion brain injury: acute deficits, chronic morbidities and the utility of biomarkers. Brain Inj. 30, 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brody D.L., Benetatos J., Bennett R.E., Klemenhagen K.C., and Mac Donald C.L. (2015). The pathophysiology of repetitive concussive traumatic brain injury in experimental models; new developments and open questions. Mol. Cell. Neurosci. 66, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Osier N.D., and Dixon C.E. (2016). The controlled cortical impact model: Applications, considerations for researchers, and future directions. Front. Neurol. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Petraglia A.L., Dashnaw M.L., Turner R.C., and Bailes J.E. (2014). Models of mild traumatic brain injury: translation of physiological and anatomic injury. Neurosurgery 75, S34–S49 [DOI] [PubMed] [Google Scholar]
- 79. Scammell T.E. (2015). Narcolepsy. N. Engl. J. Med. 373, 2654–2662 [DOI] [PubMed] [Google Scholar]
- 80. Goldbart A., Peppard P., Finn L., Ruoff C.M., Barnet J., Young T., and Mignot E. (2014). Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. Sleep 37, 1043–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pizza F., Vandi S., Iloti M., Franceschini C., Liguori R., Mignot E., and Plazzi G. (2015). Nocturnal sleep dynamics identify narcolepsy type 1. Sleep 38, 1277–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xu X., Wu H., Zhuang J., Chen K., Huang B., Zhao Z., and Zhao Z. (2017). Sleep-wake patterns, non-rapid eye movement, and rapid eye movement sleep cycles in teenage narcolepsy. Sleep Med. 33, 47–56 [DOI] [PubMed] [Google Scholar]
- 83. Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C., Richardson J.A., Clay Williams S., Xiong Y., Kisanuki Y., Fitch T.E., Nakazato M., Hammer R.E., Saper C.B., and Yanagisawa M. (1999). Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98, 437–451 [DOI] [PubMed] [Google Scholar]
- 84. Mori T., Uzawa N., Iwase Y., Masukawa D., Rahmadi M., Hirayama S., Hokazono M., Higashiyama K., Shioda S., and Suzuki T. (2016). Narcolepsy-like sleep disturbance in orexin knockout mice are normalized by the 5-HT1A receptor agonist 8-OH-DPAT. Psychopharmacology (Berl). 233, 2343–2353 [DOI] [PubMed] [Google Scholar]
- 85. Hunsley M.S., Curtis W.R., and Palmiter R.D. (2006). Behavioral and sleep/wake characteristics of mice lacking norepinephrine and hypocretin. Genes, Brain Behav. 5, 451–457 [DOI] [PubMed] [Google Scholar]
- 86. Tsunematsu T., Tabuchi S., Tanaka K.F., Boyden E.S., Tominaga M., and Yamanaka A. (2013). Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav. Brain Res. 255, 64–74 [DOI] [PubMed] [Google Scholar]
- 87. Tsunematsu T., Kilduff T.S., Boyden E.S., Takahashi S., Tominaga M., andYamanaka a. (2011). Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J. Neurosci. 31, 10529–10539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sasaki K., Suzuki M., Mieda M., Tsujino N., Roth B., and Sakurai T. (2011). Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gotter A.L., Garson S.L., Stevens J., Munden R.L., Fox S. V, Tannenbaum P.L., Yao L., Kuduk S.D., McDonald T., Uslaner J.M., Tye S.J., Coleman P.J., Winrow C.J., and Renger J.J. (2014). Differential sleep-promoting effects of dual orexin receptor antagonists and GABA receptor modulators. BMC Neurosci. 15, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brisbare-Roch C., Dingemanse J., Koberstein R., Hoever P., Aissaoui H., Flores S., Mueller C., Nayler O., van Gerven J., de Haas S.L., Hess P., Qiu C., Buchmann S., Scherz M., Weller T., Fischli W., Clozel M., and Jenck F. (2007). Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat. Med. 13, 150–155 [DOI] [PubMed] [Google Scholar]
- 91. Mang G.M., Dürst T., Bürki H., Imobersteg S., Abramowski D., Schuepbach E., Hoyer D., Fendt M., and Gee C.E. (2012). The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep 35, 1625–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tannenbaum P.L., Tye S.J., Stevens J., Gotter A.L., Fox S. V, Savitz A.T., Coleman P.J., Uslaner J.M., Kuduk S.D., Hargreaves R., Winrow C.J., and Renger J.J. (2016). Inhibition of orexin signaling promotes sleep yet preserves salient arousability in monkeys. Sleep 39, 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morairty S.R., Wilk A.J., Lincoln W.U., Neylan T.C., and Kilduff T.S. (2014). The hypocretin/orexin antagonist almorexant promotes sleep without impairment of performance in rats. Front. Neurosci. 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Betschart C., Hintermann S., Behnke D., Cotesta S., Fendt M., Gee C.E., Jacobson L.H., Laue G., Ofner S., Chaudhari V., Badiger S., Pandit C., Wagner J., and Hoyer D. (2013). Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J. Med.Chem. 56, 7590–7607 [DOI] [PubMed] [Google Scholar]
- 95. Yoshida Y., Naoe Y., Terauchi T., Ozaki F., Doko T., Takemura A., Tanaka T., Sorimachi K., Beuckmann C.T., Suzuki M., Ueno T., Ozaki S., and Yonaga M. (2015). Discovery of (1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): a potent and efficacious oral orexin receptor antagonist. J. Med. Chem. 58, 4648–4664 [DOI] [PubMed] [Google Scholar]
- 96. Cao M. and Guilleminault C. (2011). Hypocretin and its emerging role as a target for treatment of sleep disorders. Curr. Neurol. Neurosci. Rep. 11, 227–234 [DOI] [PubMed] [Google Scholar]
- 97. Kishi T., Matsunaga S., and Iwata N. (2015). Suvorexant for primary insomnia: A systematic review and meta-analysis of randomized placebo-controlled trials. PLoS One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Anaclet C., Parmentier R., Ouk K., Guidon G., Buda C., Sastre J.-P., Akaoka H., Sergeeva O.A., Yanagisawa M., Ohtsu H., Franco P., Haas H.L., and Lin J.S. (2009). Orexin/hypocretin and histamine: distinct roles in the control of wakefulness eemonstrated using knock-out mouse models. J. Neurosci. 29, 14423–14438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scammell T.E., Crocker A., McCormack S., Yanagisawa M., Sakurai T., and Mochizuki T. (2004). Behavioral state instability in orexin knockout mice, in: Sleep Biol. Rhythms 2, S13–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Blumberg M.S., Coleman C.M., Johnson E.D., and Shaw C. (2007). Developmental divergence of sleep-wake patterns in orexin knockout and wild-type mice. Eur. J. Neurosci. 25, 512–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Roth T., Dauvilliers Y., Mignot E., Montplaisir J., Paul J., Swick T., and Zee P. (2013). Disrupted nighttime sleep in narcolepsy. J. Clin. Sleep Med. 9, 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Plazzi G., Pizza F., Vandi S., Aricò D., Bruni O., Dauvilliers Y., and Ferri R. (2014). Impact of acute administration of sodium oxybate on nocturnal sleep polysomnography and on multiple sleep latency test in narcolepsy with cataplexy. Sleep Med. 15, 1046–1054 [DOI] [PubMed] [Google Scholar]
- 103. Wu H., Zhuang J., Stone W.S., Zhang L., Zhao Z., Wang Z., Yang Y., Li X., Zhao X., and Zhao Z. (2014). Symptoms and occurrences of narcolepsy: a retrospective study of 162 patients during a 10-year period in Eastern China. Sleep Med. 15, 607–613 [DOI] [PubMed] [Google Scholar]
- 104. Benca R.M. (2007). Narcolepsy and excessive daytime sleepiness: Diagnostic considerations, epidemiology, and comorbidities. J. Clin. Psychiatry 68, 5–8 [PubMed] [Google Scholar]
- 105. Jiménez-Correa U., Haro R., González R.O., and Velázquez-Moctezuma J. (2009). Correlations between subjective and objective features of nocturnal sleep and excessive diurnal sleepiness in patients with narcolepsy. Arq. Neuropsiquiatr. 67, 995–1000 [DOI] [PubMed] [Google Scholar]
- 106. Schretlen D.J., and Shapiro A.M. (2003). A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int. Rev. Psychiatry 15, 341–349 [DOI] [PubMed] [Google Scholar]
- 107. Bombardier C.H., Fann J.R., Temkin N.R., Esselman P.C., Barber J., and Dikmen S.S. (2010). Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 303, 1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., Liou A.K.F., Leak R.K., Gao Y., and Chen J. (2013). Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang L., Zou B., Xiong X., Pascual C., Xie J., Malik A., Xie J., Sakurai T., and Xie X.S. (2013). Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. Ann. Intern. Med. 158, 5275–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Aitta-aho T., Pappa E., Burdakov D., and Apergis-Schoute J. (2016). Cellular activation of hypothalamic hypocretin/orexin neurons facilitates short-term spatial memory in mice. Neurobiol. Learn. Mem. 136, 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ito N., Yabe T., Gamo Y., Nagai T., Oikawa T., Yamada H., and Hanawa T. (2008). I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience 157, 720–732 [DOI] [PubMed] [Google Scholar]
- 113. Brundin L., Björkqvist M., Petersén Å., and Träskman-Bendz L. (2007). Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur. Neuropsychopharmacol. 17, 573–579 [DOI] [PubMed] [Google Scholar]
- 114. Deats S.P., Adidharma W., Lonstein J.S., and Yan L. (2014). Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience 272, 252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Duffy C.M., Yuan C., Wisdorf L.E., Billington C.J., Kotz C.M., Nixon J.P., and Butterick T.A. (2015). Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci. Lett. 606, 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.