Abstract
Extracellular vesicles (EVs) are biological nanoparticles comprising exosomes, microvesicles, and other heterogeneous nanoscopic vesicle populations that are produced by most cell types. In addition to their putative roles as critical mediators of intercellular communication, EVs have begun to be harnessed as drug delivery vehicles, with early evidence indicating they may have significant advantages over synthetic nanoparticle delivery systems for particular applications. Targeted delivery of EV-encapsulated cargo has already been realized and may have broad applicability; however, methods for producing and purifying EVs and loading them with therapeutic molecules have yet to be standardized. In this chapter, we outline steps for EV isolation and characterization and compare current methods for active and passive loading of EVs with payloads of short interfering RNA (siRNA) or small molecules, with the results revealing that active loading via electroporation increases loading efficiency of siRNA but not of Rhodamine B, a model for a small molecule drug, in HEK293T-derived EVs. The methods described here may inform future design of targeted delivery of nucleic acids or small molecules via EVs.
Keywords: Extracellular vesicles (EVs), Exosomes, siRNA, Small molecules, Electroporation, Drug delivery, Cancer therapeutics
1. Introduction
Extracellular vesicles (EVs) are natural nanoscopic particles produced by most cells that hold immense promise for utilization as drug carriers. EVs include exosomes (30–120 nm), which are released to the extracellular environment upon fusion of multivesicular endosomes with the plasma membrane, as well as microvesicles (50–1000 nm), which are produced by the outward budding of membrane vesicles from the cell surface [1, 2]. Exosomes and microvesicles have similar properties and are difficult to completely separate with current isolation methods, thus we refer them here as EVs following the recommendations laid out by the International Society for Extracellular Vesicles [3].
EVs play significant roles in intercellular communication via cell-cell transfer of proteins and especially nucleic acids such as microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and mRNAs [4, 5]. The status of EVs as native nucleic acid carriers prompted investigation of their utility for short interfering RNA (siRNA) delivery, and a seminal study by Wood and colleagues demonstrated that exosomes could be targeted to the brain for delivery of therapeutic siRNA [6]. Additional work has shown that EVs can be utilized for targeted delivery of microRNA (miRNA) [7] and small molecule drugs [8] to combat cancer, opening a pathway for EVs to be applied as drug carriers for treatment of numerous diseases as well as in a variety of tissue repair and regeneration applications [9].
However, one limiting factor in further development of EV-based therapeutics is a lack of standardized methods for EV isolation and for loading EVs with therapeutic cargo. This chapter focuses on methods for loading of EVs with siRNA and small molecules; reviews of EV isolation methodologies are available elsewhere [10, 11]. In the study by Wood and colleagues, EVs were loaded with siRNA via electroporation [6, 12], a common molecular biology technique that has been used to deliver DNA, drugs or chemicals into prokaryotic or eukaryotic cells [13, 14]. However, other studies have reported an inability to efficiently load EVs with siRNA [15] or miRNA [7] using this approach, potentially due to electric field-induced aggregation of these short RNA molecules. Here, using HEK293T-derived EVs, we detail an approach for siRNA loading into EVs via electroporation that addresses siRNA aggregation and also define parameters for siRNA loading capacity in EVs.
In addition, we report on methods for loading EVs with small molecules. Small molecule drugs such as curcumin and doxorubicin have been successfully loaded into EVs by different methods [8, 16]. Passive loading, i.e., incubation of EVs with drug in solution with no additional stimulation, is sufficient for EVs to encapsulate curcumin [16]. Alternatively, electroporation has been employed to incorporate doxorubicin into EVs [8]. We have compared these passive and active loading methods using Rhodamine B as a model small molecule drug. Overall, this chapter provides an overview of various methods that could be used to incorporate therapeutic cargo into EVs for a wide variety of targeted delivery applications.
2. Materials
All aqueous solutions of reagents should be prepared using ultrapure water and filter-sterilized through a 0.22 μm filter into sterile container.
2.1. Cell Culture
EV producing cells: HEK293T cells (ThermoScientific HCL4517).
Cell culture media: DMEM high glucose with sodium pyruvate (110 mg/ml) + L-glutamine (6 mM) + penicillin (100 units/ml) + streptomycin (100 μg/ml) + 10% fetal bovine serum (FBS) as final concentrations.
2.2. EV Isolation and Characterization
Ultracentrifuge with rotor capable of 100,000 × g spins: Beckman Optima L-90K ultracentrifuge with T70i rotor.
Ultracentrifuge tubes: Optiseal tubes (Beckman 41121703).
Nanoparticle imaging apparatus with 50 nm sensitivity: Nanosight LM10 with Nanoparticle Tracking Analysis (NTA) software.
2.3. Cargo Preparation
- siRNA.
- Purified siRNA: sequence: GGUGCCAGUUC UCCAAGAUUdTdT (Dharmacon GE Life Sciences CTM-120916). Resuspend into ultrapure RNase-free water to make a final concentration of 200 pmol/μl (200 μM).
- Small molecules.
- Purified hydrophobic molecules less than 1000 Da: Rhodamine B (Sigma 83689–1G). Prepare 10 mM stock solution in DI H2O and store at room temperature with protection from light.
2.4. Electroporation
Electroporation buffer: 1.15 mM potassium phosphate pH 7.2, 25 mM potassium chloride, 21% Optiprep, as described in Alvarez-Erviti et al. [6].
Electroporator and cuvettes: GenePulser Xcell electroporator (Biorad) with Gene Pulser/Micropulser Cuvettes (Biorad 165–2089).
300 kDa MWCO filter: Pall Nanosep centrifugal device with Omega membrane, MWCO 300 kDa (OD300C33).
2.5. Loaded Cargo Detection
Kit that can detect nucleic acid concentration at picogram sensitivity: Quant-it PicoGreen Assay kit (Life Technologies P7589).
Labeling buffer: 0.5% DMSO in 1× TE (10 mM Tris, pH 7.5 and 1 mM EDTA, pH 8.0.
Black-walled clear bottom non-treated polystyrene 96-well plates.
0.5 ml centrifuge tubes, 0.2 ml thin walled, nuclease-free PCR tubes.
Microplate reader with fluorescence capability (or any other modality needed for small molecule quantification): Molecular Devices SpectraMax M5.
3. Methods
3.1. Measurement of EV Concentration and Size
Grow cells in EV-depleted media (see Note 1) in T150 flasks.
Isolate EVs using differential centrifugation method as described previously [17]. In brief, collect media from cultured cells and centrifuge at 300 × g for 10 min. Transfer supernatant into a new tube and centrifuge again at 2000 × g for 20 min and then 10,000 × g for 30 min to remove larger vesicles and debris. Finally, transfer the supernatant into ultracentrifuge tubes and centrifuge at 100,000 × g for 2 h to pellet EVs consisting primarily of exosome and microvesicle fractions.
Discard the supernatant and resuspend EVs into ice-cold 1× PBS (see Note 2).
Determine size and concentration of EVs (Fig. 1) (see Note 3).
For electroporation, resuspend EVs into electroporation buffer (EB) and, if not immediately used, store EVs either at −20 or −80 °C (see Note 4). For passive loading, resuspend EVs into 1× PBS and, if not immediately used, store EVs either at −20 or −80 °C.
Fig. 1.
EV size distribution. Size distribution of HEK293T־derived EVs as measured by NanoTraoking Analysis reveals a peak at 76 nm in diameter, with the majority of isolated EVs being less than 200 nm in diameter
3.2. Passive and Active Loading
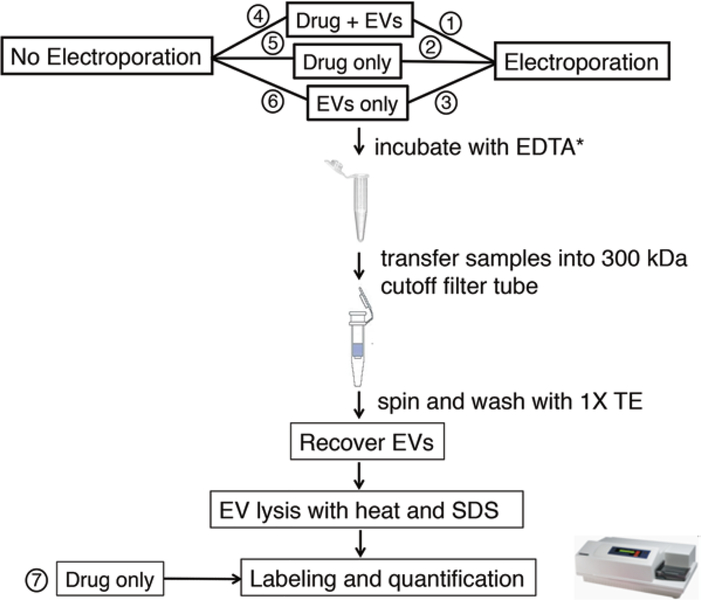
- Prepare samples for loading using the appropriate proportions for each drug molecule type listed below. Examples of appropriate controls are indicated in Figs. 2 and 3 (see Notes 5 and 6).
- siRNA: 10 μl of 1 μg/μl EVs (~3.2 × 108 vesicles for HEK293T) + 5 μl of200 pmol/μl siRNA + 25 μl 2× electroporation buffer (or PBS for passive loading) + 10 μl DI H2O (nuclease free).
- Rhodamine B: 10 μl of 1 μg/μl EVs (~3.2 × 108 vesicles for HEK293T) + 10 μl of 10 mM Rhodamine B (see Note 7) + 25 μl 2× electroporation buffer (or PBS for passive loading) + 5 μl DI H2O (nuclease free).
Transfer each electroporated sample from its cuvette into a 0.5 ml tube.
Recover the residual sample from each cuvette by adding 100 μl of 1× TE, pipetting up and down and transferring to the appropriate 0.5 ml tube.
For siRNA only, add EDTA to a final concentration of 1 mM to alleviate siRNA aggregation and incubate for 15 min at room temperature.
Transfer the sample to a 300 kDa MWCO filter tube. This filter retains EVs but small molecules and buffers that have not been incorporated into EVs will pass through the filter during washing steps.
Centrifuge samples at 5000 × g at 4 °C for 5 min to remove buffer and unincorporated cargo.
Discard flow through, add 500 μl of 1× TE into each tube, and centrifuge again at 5000 × g at 4 °C for 5 min.
Repeat step 8 two times for a total of three washes.
Add 50 μl of 1× TE into the same filter tube, pipette up and down, and transfer the sample into a fresh 0.5 ml tube.
Add additional 45 μl of 1× TE to recover residual amount of sample from filter tube and transfer total volume of sample, i.e., 95 μl into a 0.2 ml thin walled PCR tube (see Note 8).
Fig. 2.
Schematic for controlled experiments to assess active and/or passive loading of EVs. Sample preparation is described in Notes 5 and 6. Model drugs (drug) used included siRNA and Rhodamine B. Asterisk indicates this step is relevant for siRNA loading only
Fig. 3.
siRNA incorporation into EVs. (a) siRNA is detectable from EVs that were electroporated in the presence of siRNA following extensive washing and filtration to remove unassociated molecules. Electroporation of siRNA alone does cause positive signal, potentially as the result of aggregation. EVs incubated passively with siRNA do not retain detectable amounts. (b) siRNA associated with EVs is influenced by the initial loading amount until saturation between 2500 and 5000 pmol loaded. These data were normalized to the background signal generated by electroporated siRNA at each loading amount. Note: 10 μg of EVs corresponds to ~3.2 × 108 vesicles
3.2.1. siRNA Quantification
To lyse EVs and release incorporated siRNA, add 5 μl of 0.4% SDS into 95 μl of EVs mixture, mix well, and incubate in a thermocycler for 15 min at 85 °C (see Note 9).
Prepare working solution (0.5% DMSO in 1× TE buffer). 10 μl of Quant-iT PicoGreen dye is added to 450 μl of working solution to make dye reagent (see Note 10). Protect dye from light by covering with foil or placing in the dark, as the dye is susceptible to photodegradation. Freshly prepare these solutions immediately prior to the labeling reaction.
Add equal volume of dye reagent to each sample prepared from step 1 of this section. Dye reagent (100 μl) is added to 100 μl of each sample to make final volume of 200 μl.
Prepare a control that contains siRNA only (sample 7, Fig. 2). Mix 10 pmol of siRNA (10 μl, 1 pmol/μl) with 85 μl of 1× TE and 5 μl of 0.2% SDS. Finally, combine this solution with 100 μl of dye reagent to make final 200 μl final volume (see Note 11).
Transfer samples into 96-well plates (see Note 12) and incubate at room temperature for 10 min away from light (cover with aluminum foil). Measure the sample fluorescence using a fluorescence microplate reader (excitation ~480 nm, emission ~520 nm).
Measure the fluorescence value from 1× TE with 0.01% SDS as background and subtract this value from all samples (1–7).
After subtraction from background, almost no signal should be detected from samples 3, 5, and 6. No signal from sample 5 means that washing conditions are good enough to remove siRNA from the filter tube. Example data is shown in Fig. 3a (see Note 13).
3.2.2. Small Molecule Quantification
Lyse EVs by following step 1 of Subheading 3.2.1 as described previously.
Prepare a control that contains Rhodamine B only (sample 7, Fig. 2). Mix 10 μl of Rhodamine B (1 nmol, 100 pmol/μl) with 85 μl of 1× TE and 5 μl of 0.2% SDS.
Transfer samples into 96-well plates and measure fluorescence using excitation and emission wavelengths of 540 and 625 nm respectively.
Measure the fluorescence value from 1× TE with 0.01% SDS at these wavelengths as background and subtract this value from all samples (1–7).
After subtraction from background, almost no signal should be detected from samples 3, 5, and 6. No signal from sample 5 means that washing conditions are good enough to remove Rhodamine B from the filter tube. Example data is shown in Fig. 4a (see Note 14).
Fig. 4.
Small molecule incorporation into EVs. (a) Rhodamine B is detectable from EVs that were electroporated or incubated in the presence of Rhodamine B after extensive washing and filtration to remove unassociated molecules. Unlike siRNA, there appears to be no increase in Rhodamine B incorporation into EVs associated with electroporation. (b) Rhodamine B associated with EVs is influenced by the initial loading amount until saturation between 500 and 1000 nmol loaded. These data were normalized to the background signal generated by non- electroporated Rhodamine B at each loading point. Note: 10 μg of EVs corresponds to ~3.2 × 108 vesicles
Acknowledgments
This work was supported by NIH R00 grant HL112905, by an ORAU Ralph E. Power Junior Faculty Enhancement Award, and by two University of Maryland Tier 1 seed grants (all to S.M.J.). The authors thank Rini Pek, Navein Arumugasaamy and Anjana Jeyaram for their helpful contributions.
Footnotes
Prepare EV-depleted media by centrifuging complete media at 100,000 × g for 12 h. EVs included with serum are pelleted out and the supernatant (EV-depleted media) is transferred into a new container and filter sterilized before adding to cells.
EVs that are isolated from cells grown on T150 flasks (collected from 40 ml EV-depleted media) are resuspended into 1 ml of ice-cold, sterile 1× PBS. The concentration of EVs is also determined using the BCA assay.
Dilute EV samples at least 40-fold in 1× PBS to measure by Nanosight. 10 μl of sample is diluted with 390 μl of 1× PBS since approximate 400 μl sample volume is required for Nanosight measurement.
Aliquot EVs into 50 μl (final concentration is ~1 μg/μl) in 0.5 ml tubes to avoid multiple freezing and thawing. Store aliquoted tubes at −80 °C.
- Samples 1 and 4 contain 10 μl of EVs (10 μg, i.e., ~3.2 × 108 vesicles) + 5 μl of siRNA (200 pmol/μl) + 25 μl of 2× electroporation buffer + 10 μl of pure water.
- Samples 2 and 5 are siRNA only, i.e., no EVs and the composition of mixture is 5 μl of siRNA (200 pmol/μl) + 25 μl of 2× electroporation buffer +20 μl of pure water.
- Samples 3 and 6 are EVs only, i.e., without siRNA, the composition of mixture is 10 μl of EVs (10 μg, i.e., ~3.2 × 108 vesicles) + 25 μl of 2× electroporation buffer + 15 μl of pure water.
Sample 2 is used to determine if electroporation causes any siRNA aggregation and sample 5 is used to determine background siRNA levels after the filtration procedure.
- Samples 1 and 4 contain 10 μl of EVs (10 μg, i.e., ~3.2 × 108 vesicles) + 10 μl of Rhodamine B + 25 μl of 2× electroporation buffer +5 μl of water.
- Samples 2 and 5 do not contain EVs and the composition of the solutions is 10 μl of Rhodamine B + 25 μl of 2× electroporation buffer +15 μl of water.
- Samples 3 and 6 do not contain Rhodamine B and the composition of the solutions is 10 μl of EVs (10 μg, i.e., ~3.2 × 108 vesicles) + 25 μl of 2× electroporation buffer +15 μl of water.
Sample 2 is used to determine if electroporation causes any Rhodamine B aggregation or other positive signal and sample 5 is used to determine background Rhodamine B levels after the filtration procedure.
Stock solution of Rhodamine B is 10 mM. Higher concentration of Rhodamine B may result in significant adhesion with filter tips and might result in inaccurate concentration measurement.
Sample is mixed by pipetting up and down multiple times with 1× TE to ensure that EVs are completely retrieved from the membrane of filter tube.
The concentration of SDS will be 0.02% during lysis, which is not inhibitory for the subsequent labeling reaction.
The ratio of dye to working solution is 1:50, modified from a suggested 1:200 ratio from the kit instructions.
SDS is also added to siRNA alone (sample 7, Fig. 2) to make the same final concentration of SDS as with other samples. The value obtained here is used in the determination of the amount of siRNA associated with EVs.
Transfer samples gently into 96-well plates because the presence of SDS can create bubbles easily that hinders fluorescence measurement.
The amount of siRNA loaded can be controlled up to a saturation point by varying the initial amount loaded, as indicated in Fig. 3b.
The amount of Rhodamine B loaded can be controlled up to a saturation point by varying the initial amount loaded, as indicated in Fig. 4b.
References
- 1.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI (2011) Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 68:2667–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289 [DOI] [PubMed] [Google Scholar]
- 3.Gould SJ, Raposo G (2013) As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2:eCollection 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659 [DOI] [PubMed] [Google Scholar]
- 5.Ludwig AK, Giebel B (2012) Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol 44:11–15 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345 [DOI] [PubMed] [Google Scholar]
- 7.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35:2383–2390 [DOI] [PubMed] [Google Scholar]
- 9.Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM (2015) Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 21:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor DD, Zacharias W, Gercel-Taylor C (2011) Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol 728:235–246 [DOI] [PubMed] [Google Scholar]
- 11.Rani S, O’Brien K, Kelleher FC, Corcoran C, Germano S, Radomski MW, Crown J, O’Driscoll L (2011) Isolation of exosomes for subsequent mRNA, MicroRNA, and protein profiling. Methods Mol Biol 784:181–195 [DOI] [PubMed] [Google Scholar]
- 12.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ (2012) Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc 7:2112–2126 [DOI] [PubMed] [Google Scholar]
- 13.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH (1982) Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J 1:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugar IP, Neumann E (1984) Stochastic model for electric field-induced membrane pores electroporation. Biophys Chem 19:211–225 [DOI] [PubMed] [Google Scholar]
- 15.Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJ, Schiffelers Rm , Raemdonck K, Vader P (2013) Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release 172:229–238 [DOI] [PubMed] [Google Scholar]
- 16.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ (2012) Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56:293–304 [DOI] [PubMed] [Google Scholar]