Abstract
Macular edema (ME) represents the most common cause for visual loss among uveitis patients. The management of uveitic macular edema (UME) may be challenging, due to its often recalcitrant nature. Corticosteroids remain the mainstay of treatment, through their capability of effectively controlling inflammation and the associated ME. Topical steroids may be effective in milder cases of UME, particularly in edema associated with anterior uveitis. Posterior sub-Tenon and orbital floor steroids, as well as intravitreal steroids often induce rapid regression of UME, although this may be followed by recurrence of the pathology. Intra-vitreal corticosteroid implants provide sustained release of steroids facilitating regression of ME with less frequent injections. Topical nonsteroidal anti-inflammatory drugs may provide a safe alternative or adjuvant therapy to topical steroids in mild UME, predominantly in cases with underlying anterior uveitis. Immunomodulators including methotrexate, mycophenolate mofetil, tacrolimus, azathioprine, and cyclosporine, as well as biologic agents, notably the anti-tumor necrosis factor-α monoclonal antibodies adalimumab and infliximab, may accomplish the control of inflammation and associated ME in refractory cases, or enable the tapering of steroids. Newer biotherapies have demonstrated promising outcomes and may be considered in persisting cases of UME.
Keywords: uveitis, macular edema, treatment, corticosteroids, dexamethasone implant, NSAIDs, anti-TNFα, interferons
Introduction
Macular edema (ME) is a common complication of a broad spectrum of clinical entities. In uveitis patients, it represents the most common cause for permanent visual loss, resulting in remarkable visual disability and compromise of their quality of life.1
ME is defined as a thickening of the macular region, which results from a breakdown of the outer and/or inner blood–retina barrier, leading to increased permeability of the retinal pigment epithelium and the retinal vasculature. The consequent leaking from perifoveal capillaries leads to the accumulation of intracellular and extracellular fluid.
Epidemiology
Uveitis is an inflammatory ocular disease of variable etiology, with an incidence estimated at 17–52 cases per 100,000 persons per year and an annual prevalence of 69.0–114 per 100,000 persons.2–5
Uveitis may be of infectious etiology or associated with autoinflammatory or autoimmune diseases. Inflammation may involve exclusively the ocular tissues or may constitute a manifestation of systemic disorders.6,7
Severe visual impairment has been reported to affect up to 35% of uveitis patients, while it is estimated that uveitis accounts for up to 10% of legal blindness in developed countries.2,3,8,9 Reports indicate that up to 42% of uveitis patients with vision lower than 20/60 have developed clinically significant ME.1,8–12
ME may complicate any type of uveitis, affecting up to 30% of all uveitis patients; however, its prevalence varies depending on the underlying cause.1,8,10 The incidence of ME by anatomical type of uveitis has been summarized in Table 1.
Table 1.
| Anatomical type of uveitis | Incidence (%) |
|---|---|
| Anterior uveitis | 9–11 |
| Intermediate uveitis | 40–60 |
| Posterior uveitis | 28–34 |
| Panuveitis | 53–64 |
Diagnosis and imaging
ME can be detected by slit-lamp biomicroscopy, which may reveal a localized expansion of the retinal space in the macular area. Radially oriented intraretinal cysts in the foveal region may be identified by a reduced central reflex adjacent to a thin, highly reflective edge.13,14 Ancillary imaging tests include, predominantly, fluorescein angiography (FA) and optical coherence tomography (OCT) (Figures 1 and 2). The latter has become the preferred imaging modality for the evaluation and monitoring of uveitic macular edema (UME), due to its noninvasive nature, quantifiable results, and reproducibility. OCT accurately delineates alterations in the macular anatomy and reveals coexisting pathology of the vitreoretinal interface, including vitreomacular traction and epiretinal membranes. FA is the preferred method to evaluate macular ischemia, as well as activity of uveitis. On FA, UME is depicted with the characteristic petaloid pattern of fluorescein leakage.15,16
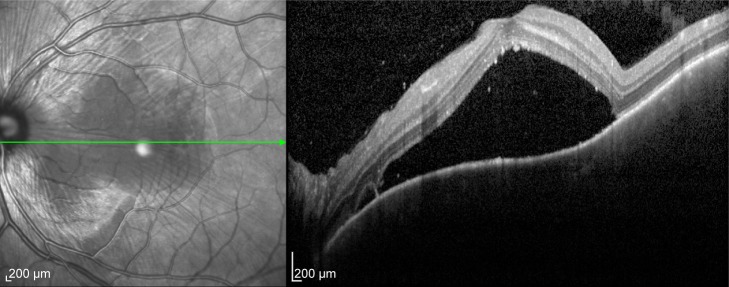
Figure 1.
Optical coherence tomography of a 28-year-old male with Vogt-Koyanagi-Harada choroidopathy showing multilobulated serous retinal detachment involving the macula.
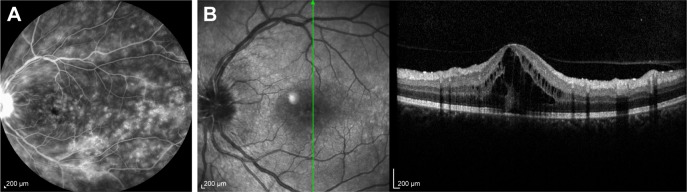
Figure 2.
Fluorescein angiography of a 46-year-old female with sarcoidosis showing diffuse fluorescein pooling and leakage (A) and intraretinal fluid accumulation at the macula on optical coherence tomography (B).
These two methods are very sensitive in presenting different pathologic characteristics of ME, and thus are not interchangeable, but complement each other. Kozak et al and later Kempen et al compared FA with OCT, as well as with the clinical evaluation and noted that the results from the two imaging techniques agreed only moderately, while the evaluation from clinicians was associated with significant number of both false positive and false negative cases. They concluded that ancillary testing may be justified in cases suspicious for ME, regardless of biomicroscopic findings.17,18 In such suspect cases, a negative result of one test (FA or OCT) may be an indication to perform the remaining.15 Indocyanine green angiography may be of some use in ME imaging (Figures 1–5) in conjunction with FA, in an attempt to determine the potential involvement of the choroid in the inflammatory process.15,19 Roesel et al used fundus autofluorescence as a noninvasive test adjacent to OCT, but it was able to identify 50% of all angiographically proven cases of ME.20 Fundus-related microperimetry has also been used in eyes with ME, as a means to monitor the functional damage caused by ME.21
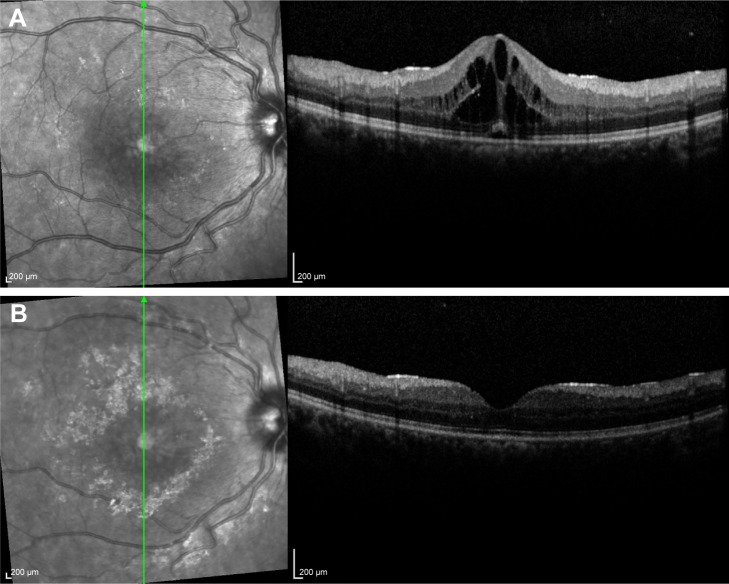
Figure 3.
Thirty-year-old female with Behçet’s disease showing development of macular edema on optical coherence tomography (A) and regression of intraretinal fluid 3 weeks following intravitreal injection of Ozurdex® (B).
Note: The patient was already on systemic treatment with infliximab.
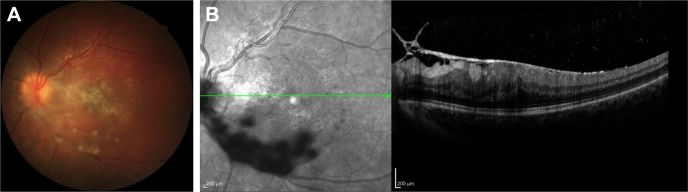
Figure 4.
Color photo of an 18-year-old female with pars planitis showing snowball-like vitreous accumulations over the posterior pole (A) and optical coherence tomography demonstrating vitreoretinal traction with macular thickening (B).
Figure 5.
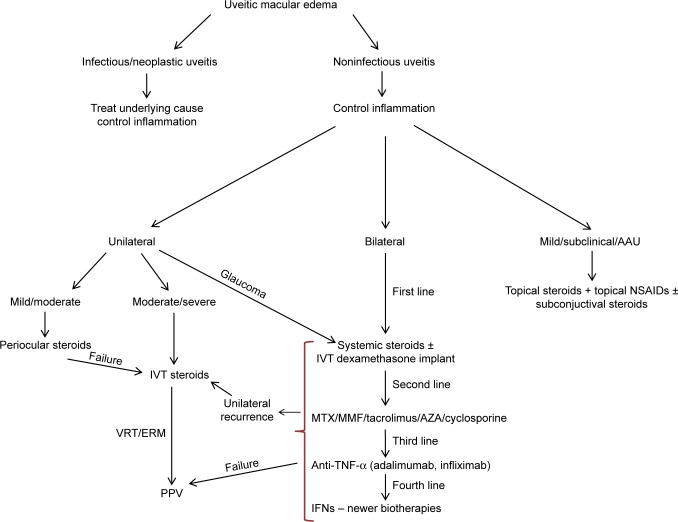
Treatment algorithm for uveitic macular edema.
Abbreviations: AAU, acute anterior uveitis; AZA, azathioprine; ERM, epiretinal membrane; IFNs, interferons; IVT, intravitreal; MMF, mycophenolate mofetil; MTX, methotrexate; TNF-α, tumor necrosis factor-α; PPV, pars plana vitrectomy; VRT, vitreoretinal traction.
Treatment
Corticosteroids
Corticosteroids are the mainstay of treatment in noninfectious uveitis complicated by ME. Through their remarkable immunosuppressive and anti-inflammatory capabilities and with a variety of administration pathways available, steroids are associated with significant functional and anatomical improvement of ME in the majority of cases.22–24
Topical corticosteroids
The use of topical corticosteroids is largely reserved for milder forms of UME, usually associated with anterior uveitis.
Topical 0.05% difluprednate has been shown to have great ocular tissue penetration and very promising results, similar to prednisolone 1%, for the treatment of UME.25–27 The largest clinical trial for the efficacy of topical 0.05% difluprednate in the treatment of UME was conducted by Schallhorn et al, who reported a decrease of CMT and improvement or resolution of UME in the majority of the patients.28
Although topical corticosteroids appear an acceptable substitute to intraocular corticosteroids, predominantly in milder cases of UME, they are not free of adverse events (AEs). These include cataract formation and intraocular pressure (IOP) elevation, which necessitates close monitoring of patients undergoing this treatment.25,28–30
Periocular corticosteroids
Periocular corticosteroids can be delivered through many routes, including sub-Tenon, subconjunctival, orbital floor, trans-septal, and retrobulbar injections.31 Sen et al, in a multicenter retrospective study, reported similar results between the various administration pathways for periocular steroid injections, most commonly using 40 mg triamcinolone acetonide.23 Improvement of UME may be observed as soon as 4 weeks up to few months following the injections.23,32 Although periocular corticosteroids are usually effective in controlling UME, recurrences are common and repeat injections are frequently required in the course of the disease.
Cataract progression and ocular hypertension may develop following periocular administration of steroids.23,32,33 Although antiglaucoma medication is usually effective for most patients with steroid-induced elevation of IOP, few cases may eventually require surgical intervention.32,33 Upper eyelid ptosis is a common, but under-reported AE, more frequently associated with posterior sub-Tenon delivery of steroids. Despite its commonly self-limiting nature, in rare cases it may persist and require surgery.34 Other more rare AEs include vitreous hemorrhage, retinal detachment, and endophthalmitis.32
Intravitreal corticosteroids
Intravitreal (IVT) corticosteroid administration has been described by many trials as an effective way to control ME in noninfectious uveitis, especially in unilateral cases.
Initial reports evaluated the efficacy of 2 and 4 mg triamcinolone acetonide IVT injections against UME, both in adults and in children.35–40 Shin et al, in a randomized, controlled trial, compared the combination of IVT triamcinolone and systemic treatment vs systemic therapy alone. They noted that the former was associated with faster reduction of CRT, although this was not accompanied with a significant improvement of best-corrected visual acuity (BCVA).41
In a retrospective study comparing the efficacy of IVT vs orbital floor triamcinolone, IVT delivery appeared to be more effective, but was associated with an increased incidence of AEs.42
IVT corticosteroid implants
A variety of slow-release IVT implants has recently become available, assuring prolonged efficacy through more sustained results (Figure 3). Ozurdex® (Allergan, Dublin, Republic of Ireland) is a fully biodegradable implant containing 0.7 mg dexamethasone, which can be administered through the pars plana, by a 22-gage applicator device. Dexamethasone is gradually released for a period of up to 6 months, although real-life studies have showed that in the majority of cases a possible reinjection is required between 4 and 6 months following the initial injection.43,44 The safety and efficacy of the implant in noninfectious uveitis were demonstrated by Lowder et al and the HURON study group, while a number of studies showed that it may effectively control ME secondary to noninfectious uveitis in both adults and children.30,45–47
Ozurdex constitutes an efficacious treatment option against UME in eyes that have undergone pars plana vitrectomy (PPV), as it appears to have similar dissolution rates in vitrectomized and nonvitrectomized eyes.43,48,49
The nonbiodegradable fluocinolone implant, Retisert® (Bausch & Lomb, Rochester, NY, USA), was designed to offer local therapy for noninfectious uveitis, obviating the risk of systemic side-effects. It is surgically implanted through the pars plana and releases steroid into the vitreous cavity for 2½–3 years.50,51 This fluocinolone implant was found to improve UME in the majority of cases, concurrently enabling reduction of systemic and periocular treatment.51–53
Although corticosteroid use is approved for the treatment of noninfectious uveitis, some retrospective studies reported the use of IVT dexamethasone implants for the treatment of persistent ME secondary to infectious uveitis. Fonollosa et al reported a small retrospective series of persisting ME complicating infectious uveitis of various etiologies, including Herpes simplex virus-type 1, Varicela-Zoster virus, Treponema pallidum, Brucella melitensis, Borrelia burgdorferi, Toxoplasma gondii, and Cytomegalovirus. Visual acuity (VA) and CMT were significantly improved in all cases while reactivation of the infection did not occur in none of the participants.54 Similar results were reported by Agarwal et al in a retrospective study of ten eyes with ME secondary to tubercular uveitis. IVT dexamethasone implants were administered as an adjunct to antitubercular therapy, which successfully decreased CMT and improved VA.55
Systemic corticosteroids
Systemic corticosteroids are considered very effective in controlling UME, but burden the patients with systemic AEs, especially in cases of long-term use. The most prominent AEs include Cushing syndrome, hypertension, infection due to immunosuppression, diabetes mellitus, osteoporosis, and atherosclerosis.56 Due to these potential complications, systemic corticosteroids are more commonly used in cases of bilateral UME. Nonetheless, it is recommended that their administration in high doses should be short-term, if possible, while the patients have to be closely monitored for the various AEs of the medication.
NSAIDs
Topical NSAIDs may be used for the treatment of UME, predominantly in milder cases or as adjuncts to steroids. NSAIDs reduce intraocular inflammation through the inhibition of the enzymes cyclooxygenase 1 and 2, which stimulate the production of prostaglandins, increasing vascular permeability and eventually leading to development of ME. A recent retrospective review evaluated the use of topical nepafenac 0.1% for the treatment of ME associated with various forms of uveitis, among which anterior uveitis was the most prominent. The authors concluded that although topical nepafenac 0.1% led to a small functional and anatomical improvement, this did not reach statistical significance, emphasizing the need for larger prospective studies.57
Other topical NSAIDs, including bromfenac and indomethacin 0.5%, have also been investigated in the treatment of UME. Radwan et al showed that topical bromfenac is not effective alone for the treatment of UME, but suggested a potential synergy with steroids and anti-vascular endothelial growth factor (VEGF) agents, the former being associated with slightly better anatomical results.58 The administration of topical indomethacin 0.5% four times daily has shown superior results to the placebo group, improving BCVA, decreasing CMT, while being free of AEs, with only one case of irritation during the first month of administration.59
IVT administration of 500 µg/0.1 mL diclofenac was evaluated for the treatment of UME by a number of prospective clinical studies. Despite the good safety profile, the improvement in VA and CMT was not significant in the majority of studies.60–62
Acetazolamide
Oral acetazolamide, a carbonic anhydrase inhibitor, was first described in 1988 as an alternative treatment for recalcitrant UME predominantly associated with idiopathic uveitis.63 Most studies administered 500 mg once or twice per day, reporting that some patients might need a maintenance dosage ranging from 125 to 500 mg daily. The favorable results presented by older prospective and retrospective studies were also recently reproduced and recorded with the aid of spectral domain OCT.64–66 In contrast, other prospective studies failed to link acetazolamide with an improvement of VA, although a small, significant reduction of the ME was noted.67 Systemic AEs due to acetazolamide include gastroinstestinal disroders, dysgeusia, paresthesia, hypokalemia, and mild depression, which may in few cases lead to discontinuation of the therapy.63,64
Noncorticosteroid, nonbiologic immunomodulatory agents
A number of nonbiologic immunomodulatory agents have been described for the treatment of uveitis, when corticosteroids alone are not able to suppress the inflammation.
Azathioprine (AZA) is a purine analog, able to reduce the peripheral T and B lymphocytes and their reactivity, as well as to downregulate IL-2 synthesis and the production of IgM.68 Along with its well-established efficacy in noninfectious uveitis, a protective effect against the development of ME in uveitic eyes has also been described.69–72 The most common AEs include gastrointestinal disturbances, but AZA can also lead to serious hepatotoxicity, a common reason for discontinuation of this agent. Larger doses of AZA may lead to bone marrow suppression, while a potential increase in risk of non-Hodgkin lymphoma has also been reported.56
Methotrexate (MTX) is a folic acid analog, acting as a dihydrofolate reductase inhibitor, exerting immunosuppressive effect by inhibition of leukocyte division. It may effectively control ocular inflammation and reduce ME development in uveitic eyes through oral, subcutaneous (SC), and intramuscular administration.56,73 The main AEs of systemic MTX include gastrointestinal disturbances, hepatotoxicity, cytopenia, and interstitial pneumonia.56
Intravitreally delivered MTX has shown promising results, controlling uveitis and leading to at least partial remission of ME. In both available studies, relapse of ME was delayed up to 18 months and when encountered was controlled through reinjection.74,75
Mycophenolate mofetil (MMF) is an inhibitor of the purine synthesis pathway, which is proven to be very effective in an array of ocular inflammatory conditions, enabling tapering or cessation of steroids.76,77 MMF has also been successfully used for recalcitrant cases of UME, leading to partial or complete resolution in most patients.78,79 However, retrospective studies evaluating its long-term efficacy reported conflicting results. Neri et al described good ME control throughout the 1 year follow-up.79 On the contrary, Doycheva et al presented less favorable outcomes despite standard MMF dosage, with frequent recurrences of ME after the first year of follow-up, as well as cases of newly developed ME during recurrences of inflammation.80 Frequent monitoring of liver function tests and full blood count is required, as MMF may affect the gastrointestinal tract or may be linked to malignancies, leukopenia, and opportunistic infections.56
Cyclosporine A, a natural product from fungi that exerts immunosuppression acting as a T-cell inhibitor, is an effective treatment for noninfectious, sight-threatening uveitis, administered orally at 2–5 up to 10 mg/kg/day.81–83 Its efficacy in uveitic patients was first described in 1983 showing comparable results with systemic steroids in the management of inflammation, improving UME or protecting uveitic patients from the development of macular thickening.72,83,84
The most serious AE of cyclosporine is its nephrotoxicity, while hypertension may also occur.56 Tacrolimus is a macrolide with T lymphocyte inhibiting properties similar to cyclosporine. It is administered orally and constitutes an effective therapeutic alternative for uveitis, even as monotherapy, sparing steroid AEs.56,85 The efficacy of systemic tacrolimus is reportedly similar to cyclosporine with a potentially better safety profile.86 The effect of tacrolimus on UME, although not thoroughly covered in the literature, may be favorable. Many uveitis studies that included eyes with UME showed improved VA, without though specifying the effects of tacrolimus on ME.86,87 It should be noted that systemic use of tacrolimus requires frequent monitoring of serum drug levels in order to ensure that therapeutic levels are reached and maintained. AEs include nephrotoxicity, neurotoxicity, gastrointestinal disturbances, and hyperglycemia.56
Sirolimus is a macrolide and an inhibitor of the mammalian target of rapamycin that exerts immunosuppressive properties similar to cyclosporine and tacrolimus, inhibiting T lymphocytes.88 The evidence available for systemic and regional tacrolimus for the treatment of uveitis and ME is limited. Systemic sirolimus was linked with severe AEs in higher doses, but managed to control inflammation and improve ME in the majority of the remaining patients.89,90 The two SAVE studies reported promising inflammation control through IVT and SC delivery of sirolimus. In contrast, the initial UME improvement was not maintained beyond the second month in most cases, indicating that frequent IVT reinjections may be required. IVT sirolimus was associated with a better safety profile, compared to SC delivery.91,92 The potential AEs of systemic sirolimus are dose-dependent and include anemia, leucopenia, thrombocytopenia, hypercholesterolemia, arthralgias, and extremity edema. Very rarely, pulmonary toxicity, angioedema, and nephrotoxicity were associated with sirolimus.93
Interferons
Type I interferons (IFNs) are intracellular cytokines that exert an important role in the regulation of both innate and adaptive immune response, as well as in the stabilization of the blood–retina barrier.94,95 IFN alpha-2a and IFN alpha-2b subtypes were first described as an adjunctive treatment option for uveitis refractory to conventional treatment in cases of ocular Behçet’s disease. These reports included cases of UME, which regressed within 4 weeks of the initial IFN treatment.96,97 Further studies showed improvement of VA with ME resolution in most patients, which subsequently enabled tapering of systemic steroids.98–101 The administration of IFN-α2a as monotherapy was also compared with systemic corticosteroids and no treatment in the BIRDFERON trial. Both agents resulted in significant anatomic and visual improvement as opposed to baseline and to no treatment, while the outcomes did not differ significantly between the two treatment groups.102 Similar outcomes were presented for IFN alpha-2b against ME associated with a wide range of uveitic conditions, although the cumulative evidence is smaller. The largest study included cases of Behçet’s disease uveitis treated with both IFN alpha-2a and IFN alpha-2b, without differentiating between the two agents. Nonetheless, UME resolved in all reported cases.103–105 Recently, a randomized, controlled trial demonstrated superiority of IFN beta-1a over MTX in the treatment of ME complicating intermediate uveitis. Despite the small sample, IFN results were significantly better regarding macular thickness, whereas functional outcome was marginally superior to MTX.106 AEs are very common among patients, but are dose-dependent and improve in most cases without requiring discontinuation of therapy. These include flu-like syndrome, depression, sometimes requiring treatment, elevation of liver enzymes, leukopenia, and alopecia.107
Biologic agents
Tumor necrosis factor-α antagonists
Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine, which is involved in the pathogenesis of noninfectious uveitis, among other conditions.108 Infliximab is a mouse-human chimeric IgG1 monoclonal antibody (mAb) against TNF-α, administered intravenously, and adalimumab is a human IgG1 mAb against TNF-α administered subcutaneously. Systemic administration of both agents has demonstrated efficacy against uveitis and UME and is usually reserved for recalcitrant cases where steroid and/or other immunosuppressive treatment is ineffective or not tolerated. Treatment is usually maintained for approximately 2 years and most studies report adequate control of inflammation, as well as improvement of VA and CMT sustained throughout the follow-up period.109–115
The evidence for IVT administration of anti-TNF-α agents in UME is limited. A small prospective study reported that despite the initial positive response to IVT infliximab, the effect was transient and ME recurred within 6 months.116 Similarly, IVT administration of adalimumab did not gain acceptance, because its use was associated with mixed results, ranging from no efficacy to significant improvement in some cases.117,118
Other anti-TNF-α agents that have been used with limited evidence include golimumab, which provided successful results in a case report of UME that conventional treatment and adalimumab failed to control, and etanercept, which was found to be ineffective in uveitis treatment.119
Serious AEs from systemic use of anti-TNF-α agents include malignancies, infections (notably tuberculosis), and autoimmune diseases.119
Anti-ILs
IL-1, and its subtypes IL-1α and IL-1β, are cytokines with strong proinflammatory activity. IVT injections of IL-1β resulted in breakdown of the blood–retina barrier, hence anti-IL-1β agents have been considered for the treatment of uveitis and UME.120 Gevokizumab is an anti-IL-1β mAb available for SC use, usually at 60 mg doses per month. Most studies focused on its successful use for the treatment of refractory Behçet’s disease uveitis, with the largest one including cases with coexistent ME. Gevokizumab reduced inflammation and prevented the development of ME, an effect retained throughout the six-month follow-up, albeit without reaching statistical significance as opposed to the control group. No serious AEs were reported.121,122
IL-6 is also a proinflammatory cytokine, which has been implicated in the development of autoimmune diseases, including uveitis.123 The anti-IL-6 mAb tocilizumab, infused intravenously at 8 mg/kg/4 weeks, demonstrated efficacy in retrospective series of UME cases refractive to conventional treatment.124–126 The reported improvement was maintained for up to 24 months in the follow-up, although discontinuation of tocilizumab led to recurrence of ME, which resolved in all cases following reinstitution of treatment. Generally, no major AEs have been reported, with the exception of rare cases of neutropenia and pneumonia.125,127
Daclizumab is an mAb with anti-IL-2 activity. Most studies demonstrated a potential efficacy of SC and intravenous daclizumab against noninfectious uveitis.128–132 In contrast, a double-masked, randomized study on Behçet’s disease uveitis reported nonsignificant benefits from intravenous daclizumab compared to placebo.133 Evidence of its effect on UME is rather limited. A long-term study that included 19 eyes with UME reported mixed results. Intravenous and SC daclizumab managed to slightly reduce CMT, but not FA leakage in the majority of cases. It is important to note that in this study, 4 out of 39 patients developed malignancies, on an average of 26 months after the initiation of treatment.131
Anti-VEGF agents
IVT anti-VEGF therapy has become the mainstay of treatment for various retinal disorders, including neovascular age-related macular degeneration, retinal vein occlusion, and diabetic ME.134 As VEGF is a mediator proven to play a role in the inflammatory process, anti-VEGF agents have been proposed as possible therapeutic alternatives for the management of UME, especially when corticosteroids are contraindicated due to underlying glaucoma or systemic AEs. Nevertheless, particularly in more severe cases, most studies report a suboptimal or transient response of UME, which required multiple injections to maintain the positive outcomes.135–138
Other biologic agents
Rituximab is an mAb that targets the CD20 protein, a molecule found on mature B cells.139 Case reports and small case studies have shown that rituximab may be an effective option for the management of noninfectious uveitis and UME, when conventional or other immunomodulatory treatment fails to control the inflammation.140,141 Despite the mostly positive evidence, some recent case studies reported the development of ME after rituximab administration for the treatment of Wegener’s granulomatosis and an IgG4-related disease of the sinuses.142,143 The use of rituximab is considered relatively safe, with most AEs being transient reactions to the infusion. Although some cases diagnosed with progressive multifocal leukoencephalopathy have been reported, a meta-analysis failed to demonstrate significant predisposition to systemic infections among patients receiving rituximab.144,145
A small series evaluated the efficacy and safety of efalizumab, an anti-CD11a mAb administered subcutaneously, for the treatment of recalcitrant ME associated with noninfectious uveitis. All six patients experienced improvement of BCVA and reduction of CMT at the end of the 16-week follow-up period. AEs were mild and transient, with lymphocytosis being the most frequent one.146
Results of various studies assessing the systemic use of biologic agents in the treatment of UME are summarized in Table 2.
Table 2.
Studies assessing the systemic use of biologic agents in the treatment of macular edema associated with uveitis
| Author | Drug – delivery | Dose | Size | Follow-up | Outcome | Adverse events |
|---|---|---|---|---|---|---|
| Markomichelakis et al, 2011, prospective, observational study115 | IV infliximab vs | 5 mg/kg 1 infusion | 19 eyesa | 4 weeks | IV infliximab was significantly superior to the other groups in clearing retinal vasculitis, resolution of retinitis, and resolution of ME b IV infliximab-induced resolution of ME was significantly faster compared to the other groups |
None |
| IV dexamethasone vs | 1 g/day for 3 days | 8 eyesa | 4 weeks | None | ||
| IVT triamcinolone | 4 mg, single infusion | 8 eyesa | 4 weeks | None | ||
| Wroblewski et al, 2011, structured, retrospective chart review131 | IV daclizumab and | 1 mg/kg/2 weeks for 1 month, then 1 mg/kg/month | 39 patients (19 eyes with ME) | 40.3 months | Mean CMT decreased from 259 to 235 µm in the ME group FA leakage decreased in 32.5% and remained unchanged in 61.76% | Cutaneous reactions, elevated liver function tests, and infections 4/39 patients developed malignancies. Mean time of onset was 26 months |
| SC daclizumab | 2 mg/kg/2 weeks IV for 1 month, then 1 mg/kg/month SC | |||||
| Díaz-Llopis et al, 2012, prospective case series109 | SC adalimumab | 40 mg/2 weeks for 6 months | 131 patients (40 eyes with ME) | 6 months | Complete ME resolutionb with significant mean CMT reduction and BCVA improvement in 70% of patients with ME | Severe relapse of juvenile idiopathic arthritis (1/131) |
| Adán et al, 2013, prospective study126 | IV tocilizumab | 8 mg/kg/4 weeks | 5 patients (8 eyes) | 6 months | Significant CMT reductionb BCVA improvedc in 50%, stabilized in 25%, worsened in 25% | None |
| Dobner et al, 2012, retrospective study110 | SC adalimumab | 40 mg every 2 weeks | 60 patients 32 patients with MEd | 12–255 weeks | ME reductionb in 53.1% | Elevated liver enzyme count (2/60) Furuncolosis (1/60) |
| Al Rashidi et al, 2013, retrospective study111 | IV infliximab | 5 mg/kg at weeks 0, 2, and 6 followed by 5 mg/kg/8 weeks 13–43 infusions | 38 eyes (18 eyes with ME) | 12–112 months | Statistically significant CMTc reduction in the ME group Significant VA improvement compared to baseline (all patients) | Infusion reaction (1/38) |
| Calvo-Río et al, 2017, multicenter retrospective study127 | IV tocilizumab | 8 mg/kg/4 weeks | 25 patients (47 eyes) 9 patients with ME | 12 months (median follow- up) | Significant CMT reductionb in all patients with ME | Autoimmune thrombocytopenia (1/25) and pneumonia, autoimmune anemia and thrombocytopenia (1/25) Viral conjunctivitis and bullous impetigo (1/25) |
| Deuter et al, 2017, retrospective case analysis124 | IV tocilizumab | 8 mg/kg/4 weeks | 5 patients (8 eyes) | ≥3 months | Complete ME resolutionb in 62.5% ME improvement in all remaining cases | None |
| Fardeau et al, 2017, randomized controlled trial102 | SC IFN-α2a vs | 3 MU/3 times per week | 14 patients | 4 months | Intention-to-treat analysis showed no difference in CRT Per-protocol analysis showed significant difference between the corticosteroid and control group, and between the IFN-α2a and control group, but no difference between the IFN-α2a and corticosteroid group | Pancreatitis (1/14) Severe myalgia (1/14) Humor disorders (5/14) |
| Systemic corticosteroids vs | Methylprednisolone 500 mg/ day for 3 days followed by prednisone 1 mg/kg/day and further tapering | 15 patients | Hyperosmolar coma (1/15) Humor disorders (14/15) |
|||
| No treatment | 19 patients | Severe vision loss (2/19) | ||||
| Mesquida et al, 2018, retrospective noncomparative study125 | IV tocilizumab | 8 mg/kg/4 weeks | 12 patients | 24 months | Significant mean CMT reductionb and BCVA improvement compared with baseline | Grade I neutropenia (1/12) Community-acquired pneumonia (1/12) |
| Tugal-Tutkun et al, 2018, randomized, placebo- controlled trial121 | SC gevokizumab | 60 mg/4 weeks | 83 patientsd | 6 months | The emergence of ME was nonsignificantly decreased in the gevokizumab group | Drug hypersensitivity (1 patient) |
Notes:
All cases were diagnosed with Behçet’s disease uveitis.
Evaluated by optical coherence tomography.
Evaluated by optical coherence tomography and fluoroscein angiography.
The purpose of the study was to evaluate the emergence of exacerbations of Behçet’s disease uveitis.
Abbreviations: BCVA, best-corrected visual acuity; CMT, central macular thickness; CRT, central retinal thickness; IFN, interferon; IV, intravenous; IVT, intravitreal; ME, macular edema; SC, subcutaneous; VA, visual acuity.
Pars plana vitrectomy
Despite the amplitude of available pharmacologic treatment options for UME, some cases remain recalcitrant and may warrant surgical intervention. PPV may be indicated for the treatment of uveitis for diagnostic or therapeutic purposes, the latter including the removal of media opacities or epiretinal membranes.147 In cases diagnosed with UME, a standard, three-port PPV is usually performed, while some authors evaluated the effects of internal limiting membrane (ILM) peel. Most studies reported favorable outcomes following PPV in 33%–58% of patients, whereas ILM peel did not appear to differentiate the results.148–151 It has been speculated that the favorable effect of PPV on vision may be a result of media opacities removal. The mechanism of UME regression after PPV is not fully understood, with some evidence pointing toward a decrease of antigen presentation, due to reduction of inflammatory mediators in the vitreous body.152,153
Novel agents under research
Although it has been shown that current treatment practice results in favorable prognosis for a considerable number of patients with UME, the latter remains a potential threat to patients’ vision and a therapeutic challenge for specialists.154 Hence, novel agents are being investigated, while inquiries for the efficacy and safety of many already used agents expand.
ACTHAR gel, a repository adrenocorticotropic hormone injection, is being reevaluated as a treatment option for sarcoidosis.155 An ongoing clinical trial by DA Culver and a retrospective study by JJ Huang aim to assess the effects of this agent in sarcoid uveitis and, as a secondary goal, in associated ME.159,160
The selective janus kinase 1 inhibitor filgotinib, already undergoing Phase III trials for the treatment of rheumatoid arthritis, is being investigated as a possible treatment option for active noninfectious uveitis.156,161 As a secondary outcome, time until development of ME will be evaluated.
Ustekinumab is an mAb targeting the p40 subunit of IL-12 and IL-23 and it is considered a potent and safe treatment option for psoriatic arthritis and Crohn’s disease.157,158 Subcutaneously delivered ustekinumab is currently undergoing a Phase II open-label clinical trial by HN Sen, which aims to assess efficacy as well as effect on ME and CMT, if present.162
Conclusion
ME is a common and potentially sight-threatening complication of acute and chronic uveitis that may persist despite the regression of the inflammation. Corticosteroids, either systemic or regional, remain the mainstay of treatment. However, their AEs and the occasionally recalcitrant nature of UME may lead to a need for more therapeutical options. Nonsteroidal immunomodulators and predominantly the biologic agents are becoming increasingly popular in the therapeutic scheme, reinforcing efficacy and enabling discontinuation or reduction of steroids to maintenance doses. Real-life evidence has demonstrated that currently available treatment modalities with the aid of treatment algorithms (Figure 5) ensure favorable long-term prognosis in the majority of patients with UME.154
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. 2008;146(6):890.e8–896.e8. doi: 10.1016/j.ajo.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific ocular inflammation study. JAMA Ophthalmol. 2013;131(11):1405–1412. doi: 10.1001/jamaophthalmol.2013.4237. [DOI] [PubMed] [Google Scholar]
- 5.Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23(5):705–717. doi: 10.5301/ejo.5000278. [DOI] [PubMed] [Google Scholar]
- 6.de Smet MD, Taylor SR, Bodaghi B, et al. Understanding uveitis: the impact of research on visual outcomes. Prog Retin Eye Res. 2011;30(6):452–470. doi: 10.1016/j.preteyeres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Fardeau C, Champion E, Massamba N, Lehoang P. OEdème maculaire Au cours des uvéites [Uveitic macular edema] J Fr Ophtalmol. 2015;38(1):74–81. doi: 10.1016/j.jfo.2014.09.001. French. [DOI] [PubMed] [Google Scholar]
- 8.Rothova A, Suttorp-van Schulten MSA, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suttorp-Schulten MSA, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol. 1996;80(9):844–848. doi: 10.1136/bjo.80.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothova A. Inflammatory cystoid macular edema. Curr Opin Ophthalmol. 2007;18(6):487–492. doi: 10.1097/ICU.0b013e3282f03d2e. [DOI] [PubMed] [Google Scholar]
- 11.Fardeau C, Champion E, Massamba N, Lehoang P. Uveitic macular edema. Eye (Lond) 2016;30(10):1277–1292. doi: 10.1038/eye.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–1162. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gass JD, Norton EW. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. Arch Ophthalmol. 1966;76(5):646–661. doi: 10.1001/archopht.1966.03850010648005. [DOI] [PubMed] [Google Scholar]
- 14.Staurenghi G, de Polo L, Pellegrini M. Macular edema. Diagnosis and detection. Dev Ophthalmol. 2010;47:27–48. doi: 10.1159/000320072. [DOI] [PubMed] [Google Scholar]
- 15.Ossewaarde-van Norel A, Rothova A. Imaging methods for inflammatory macular edema. Int Ophthalmol Clin. 2012;52(4):55–66. doi: 10.1097/IIO.0b013e318266bf14. [DOI] [PubMed] [Google Scholar]
- 16.Skondra D, Hunter R, Papakostas TD, Vavvas DG. Near infrared autofluorescence imaging of retinal diseases. Semin Ophthalmol. 2012;27(5–6):202–208. doi: 10.3109/08820538.2012.708806. [DOI] [PubMed] [Google Scholar]
- 17.Kempen JH, Sugar EA, Jaffe GJ, et al. Fluorescein angiography versus optical coherence tomography for diagnosis of uveitic macular edema. Ophthalmology. 2013;120(9):1852–1859. doi: 10.1016/j.ophtha.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozak I, Morrison VL, Clark TM, et al. Discrepancy between fluorescein angiography and optical coherence tomography in detection of macular disease. Retina. 2008;28(4):538–544. doi: 10.1097/IAE.0b013e318167270b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe L, Stanford M, Graham E, Marshall J. Indocyanine green angiography in inflammatory eye disease. Eye. 1998;12(5):761–767. doi: 10.1038/eye.1998.199. [DOI] [PubMed] [Google Scholar]
- 20.Roesel M, Heinz C, Dietzel M, Spital G, Henschel A, Heiligenhaus A. Fundus autofluorescence and spectral domain optical coherence tomography in uveitic macular edema. Graefes Arch Clin Exp Ophthalmol. 2009;247(12):1685–1689. doi: 10.1007/s00417-009-1149-8. [DOI] [PubMed] [Google Scholar]
- 21.Roesel M, Heimes B, Heinz C, Henschel A, Spital G, Heiligenhaus A. Comparison of retinal thickness and fundus-related microperimetry with visual acuity in uveitic macular oedema. Acta Ophthalmol. 2011;89(6):533–537. doi: 10.1111/j.1755-3768.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- 22.Kempen JH, Altaweel MM, Holbrook JT, Jabs DA, Louis TA, Sugar EA, Thorne JE, Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Writing Committee Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: The Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118(10):1916–1926. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen HN, Vitale S, Gangaputra SS, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121(11):2275–2286. doi: 10.1016/j.ophtha.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowder C, Belfort R, Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129(5):54510. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 25.Sheppard JD, Toyos MM, Kempen JH, Kaur P, Foster CS. Difluprednate 0.05% versus prednisolone acetate 1% for endogenous anterior uveitis: a phase III, multicenter, randomized study. Investig Ophthalmol Vis Sci. 2014;55(5):2993–3002. doi: 10.1167/iovs.13-12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tajika T, Waki M, Tsuzuki M, Kida T, Sakaki H. Pharmacokinetic features of difluprednate ophthalmic emulsion in rabbits as determined by glucocorticoid receptor-binding bioassay. J Ocul Pharmacol Ther. 2011;27(1):29–34. doi: 10.1089/jop.2010.0106. [DOI] [PubMed] [Google Scholar]
- 27.Symes RJ, Forooghian F. Topical difluprednate monotherapy for uveitic macular edema. Can J Ophthalmol. 2016;51(1):47–49. doi: 10.1016/j.jcjo.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Schallhorn JM, Niemeyer KM, Browne EN, Chhetri P, Acharya NR. Difluprednate for the treatment of uveitic cystoid macular edema. Am J Ophthalmol. 2018;191:14–22. doi: 10.1016/j.ajo.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012;153(5):932–938. doi: 10.1016/j.ajo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Feiler DL, Srivastava SK, Pichi F, Bena J, Lowder CY. Resolution of noninfectious uveitic cystoid macular edema with topical difluprednate. Retina. 2017;37(5):844–850. doi: 10.1097/IAE.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 31.Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007;52(5):503–522. doi: 10.1016/j.survophthal.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Leder HA, Jabs DA, Galor A, Dunn JP, Thorne JE. Periocular triamcinolone acetonide injections for cystoid macular edema complicating noninfectious uveitis. Am J Ophthalmol. 2011;152(3):441–448. doi: 10.1016/j.ajo.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Iwao K, Inatani M, Kawaji T, Koga T, Mawatari Y, Tanihara H. Frequency and risk factors for intraocular pressure elevation after posterior sub-Tenon capsule triamcinolone acetonide injection. J Glaucoma. 2007;16(2):251–256. doi: 10.1097/IJG.0b013e31802d696f. [DOI] [PubMed] [Google Scholar]
- 34.Ferrante P, Ramsey A, Bunce C, Lightman S. Clinical trial to compare efficacy and side-effects of injection of posterior sub-Tenon triamcinolone versus orbital floor methylprednisolone in the management of posterior uveitis. Clin Exp Ophthalmol. 2004;32(6):563–568. doi: 10.1111/j.1442-9071.2004.00902.x. [DOI] [PubMed] [Google Scholar]
- 35.Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005;112(11):1916.e1–1916.e7. doi: 10.1016/j.ophtha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Androudi S, Letko E, Meniconi M, Papadaki T, Ahmed M, Foster CS. Safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema. Ocul Immunol Inflamm. 2005;13(2–3):205–212. doi: 10.1080/09273940590933511. [DOI] [PubMed] [Google Scholar]
- 37.Angunawela RI, Heatley CJ, Williamson TH, et al. Intravitreal triamcinalone acetonide for refractory uveitic cystoid macular oedema: long-term management and outcome. Acta Ophthalmol Scand. 2005;83(5):595–599. doi: 10.1111/j.1600-0420.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- 38.Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Exp Ophthalmol. 2001;29(1):2–6. doi: 10.1046/j.1442-9071.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 39.Sallam A, Comer RM, Chang JH, et al. Short-term safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema in children. Arch Ophthalmol. 2008;126(2):200. doi: 10.1001/archophthalmol.2007.59. [DOI] [PubMed] [Google Scholar]
- 40.Sallam A, Taylor SRJ, Habot-Wilner Z, et al. Repeat intravitreal triamcinolone acetonide injections in uveitic macular oedema. Acta Ophthalmol. 2012;90(4):e323–e325. doi: 10.1111/j.1755-3768.2011.02247.x. [DOI] [PubMed] [Google Scholar]
- 41.Shin JY, Yu HG. Intravitreal triamcinolone injection for uveitic macular edema: a randomized clinical study. Ocul Immunol Inflamm. 2015;23(6):430–436. doi: 10.3109/09273948.2015.1025982. [DOI] [PubMed] [Google Scholar]
- 42.Roesel M, Gutfleisch M, Heinz C, Heimes B, Zurek-Imhoff B, Heiligenhaus A. Intravitreal and orbital floor triamcinolone acetonide injections in noninfectious uveitis: a comparative study. Ophthalmic Res. 2009;42(2):81–86. doi: 10.1159/000220600. [DOI] [PubMed] [Google Scholar]
- 43.Adán A, Pelegrín L, Rey A, et al. Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina. 2013;33(7):1435–1440. doi: 10.1097/IAE.0b013e31827e247b. [DOI] [PubMed] [Google Scholar]
- 44.Bezatis A, Spital G, Höhn F, et al. Functional and anatomical results after a single intravitreal Ozurdex injection in retinal vein occlusion: a 6-month follow-up – the solo study. Acta Ophthalmol. 2013;91(5):e340–e347. doi: 10.1111/aos.12020. [DOI] [PubMed] [Google Scholar]
- 45.Tsang AC, Virgili G, Abtahi M, Gottlieb CC. Intravitreal dexamethasone implant for the treatment of macular edema in chronic noninfectious uveitis. Ocul Immunol Inflamm. 2017;25(5):685–692. doi: 10.3109/09273948.2016.1160130. [DOI] [PubMed] [Google Scholar]
- 46.Cao JH, Mulvahill M, Zhang L, Joondeph BC, Dacey MS. Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation. Ophthalmology. 2014;121(10):1871–1876. doi: 10.1016/j.ophtha.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Lam WC, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the chrome study. Clin Ophthalmol. 2015;9:1255–1268. doi: 10.2147/OPTH.S80500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intra-vitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25(5):556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Investig Ophthalmol Vis Sci. 2011;52(7):4605–4609. doi: 10.1167/iovs.10-6387. [DOI] [PubMed] [Google Scholar]
- 50.Pavesio C, Zierhut M, Bairi K, Comstock TL, Usner DW, Fluocinolone Acetonide Study Group Evaluation of an intravitreal fluocinolone acetonide implant versus standard systemic therapy in noninfectious posterior uveitis. Ophthalmology. 2010;117(3):567–575. doi: 10.1016/j.ophtha.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 51.Callanan DG, Jaffe GJ, Martin DF, Pearson PA, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126(9):1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 52.Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: thirty-four-week results of a multicenter randomized clinical study. Ophthalmology. 2006;113(6):1020. doi: 10.1016/j.ophtha.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 53.Tomkins-Netzer O, Lightman S, Drye L, et al. Multicenter Uveitis Steroid Treatment Trial Research Group Outcome of treatment of uveitic macular edema: The Multicenter Uveitis Steroid Treatment Trial 2-year results. Ophthalmology. 2015;122(11):2351–2359. doi: 10.1016/j.ophtha.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonollosa A, Llorenç V, Artaraz J, et al. Safety and efficacy of intravitreal dexamethasone implants in the management of macular edema secondary to infectious uveitis. Retina. 2016;36(9):1778–1785. doi: 10.1097/IAE.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal A, Handa S, Aggarwal K, et al. The role of dexamethasone implant in the management of tubercular uveitis. Ocul Immunol Inflamm. 2018;26(6):884–892. doi: 10.1080/09273948.2017.1400074. [DOI] [PubMed] [Google Scholar]
- 56.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130(4):492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 57.Petrushkin H, Rogers D, Pavesio C. The use of topical non-steroidal anti-inflammatory drugs for uveitic cystoid macular edema. Ocul Immunol Inflamm. 2018;26(5):795–797. doi: 10.1080/09273948.2016.1269931. [DOI] [PubMed] [Google Scholar]
- 58.Radwan AE, Arcinue CA, Yang P, Artornsombudh P, Abu Al-Fadl EM, Foster CS. Bromfenac alone or with single intravitreal injection of bevacizumab or triamcinolone acetonide for treatment of uveitic macular edema. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1801–1806. doi: 10.1007/s00417-013-2309-4. [DOI] [PubMed] [Google Scholar]
- 59.Allegri P, Murialdo U, Peri S, et al. Randomized, double-blind, placebo-controlled clinical trial on the efficacy of 0.5% indomethacin eye drops in uveitic macular edema. Invest Ophthalmol Vis Sci. 2014;55(3):146310. doi: 10.1167/iovs.13-13202. [DOI] [PubMed] [Google Scholar]
- 60.Soheilian M, Eskandari A, Ramezani A, Rabbanikhah Z, Soheilian R. A pilot study of intravitreal diclofenac versus intravitreal triamcinolone for uveitic cystoid macular edema. Ocul Immunol Inflamm. 2013;21(2):124–129. doi: 10.3109/09273948.2012.745883. [DOI] [PubMed] [Google Scholar]
- 61.Kianersi F, Rezaeian-Ramsheh A, Pourazizi M, Kianersi H. Intravitreal diclofenac for treatment of refractory uveitis-associated cystoid macular oedema: a before and after clinical study. Acta Ophthalmol. 2018;96(3):e355–e360. doi: 10.1111/aos.13604. [DOI] [PubMed] [Google Scholar]
- 62.Esmaeilpour NF, Rabbanikhah Z, Soheilian R, Soheilian M. Intravitreal diclofenac for refractory uveitic cystoid macular edema. J Ophthalmic Vis Res. 2013;8(1):47–52. [PMC free article] [PubMed] [Google Scholar]
- 63.Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106(9):1190–1195. doi: 10.1001/archopht.1988.01060140350030. [DOI] [PubMed] [Google Scholar]
- 64.Pepple KL, Nguyen MH, Pakzad-Vaezi K, et al. Response of inflammatory cystoid macular edema to treatment using oral acetazolamide. Retina (Philadelphia, Pa) 2018 Jan 16; doi: 10.1097/IAE.0000000000002044. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schilling H, Heiligenhaus A, Laube T, Bornfeld N, Jurklies B. Long-term effect of acetazolamide treatment of patients with uveitic chronic cystoid macular edema is limited by persisting inflammation. Retina. 2005;25(2):182–188. doi: 10.1097/00006982-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Farber MD, Lam S, Tessler HH, Jennings TJ, Cross A, Rusin MM. Reduction of macular oedema by acetazolamide in patients with chronic iridocyclitis: a randomised prospective crossover study. Br J Ophthalmol. 1994;78(1):4–7. doi: 10.1136/bjo.78.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitcup SM, Csaky KG, Podgor MJ, et al. A randomized, masked, crossover trial of acetazolamide for cystoid macular edema in patients with uveitis. Ophthalmology. 1996;103(7):1054–1063. doi: 10.1016/s0161-6420(96)30567-8. [DOI] [PubMed] [Google Scholar]
- 68.Bacon PA, Salmon M. Modes of action of second-line agents. Scand J Rheumatol. 1987;16(Suppl 64):17–24. doi: 10.3109/03009748709096717. [DOI] [PubMed] [Google Scholar]
- 69.Pato E, Muñoz-Fernández S, Francisco F, et al. Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Semin Arthritis Rheum. 2011;40(4):314–323. doi: 10.1016/j.semarthrit.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Mérida S, Palacios E, Navea A, Bosch-Morell F. New immunosuppressive therapies in uveitis treatment. Int J Mol Sci. 2015;16(8):18778–18795. doi: 10.3390/ijms160818778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pacheco PA, Taylor SR, Cuchacovich MT, Diaz GV. Azathioprine in the management of autoimmune uveitis. Ocul Immunol Inflamm. 2008;16(4):161–165. doi: 10.1080/09273940802204519. [DOI] [PubMed] [Google Scholar]
- 72.Thorne JE, Jabs DA, Peters GB, Hair D, Dunn JP, Kempen JH. Bird-shot retinochoroidopathy: ocular complications and visual impairment. Am J Ophthalmol. 2005;140(1):45–51. doi: 10.1016/j.ajo.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 73.Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. 2009;116(11):2188–2198. doi: 10.1016/j.ophtha.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor SR, Habot-Wilner Z, Pacheco P, Lightman SL. Intraocular methotrexate in the treatment of uveitis and uveitic cystoid macular edema. Ophthalmology. 2009;116(4):797–801. doi: 10.1016/j.ophtha.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 75.Taylor SRJ, Banker A, Schlaen A, et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina. 2013;33(10):2149–2154. doi: 10.1097/IAE.0b013e31828ac07d. [DOI] [PubMed] [Google Scholar]
- 76.Thorne JE, Jabs DA, Qazi FA, Nguyen QD, Kempen JH, Dunn JP. Mycophenolate mofetil therapy for inflammatory eye disease. Ophthalmology. 2005;112(8):1472–1477. doi: 10.1016/j.ophtha.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 77.Goldberg NR, Lyu T, Moshier E, Godbold J, Jabs DA. Success with single-agent immunosuppression for multifocal choroidopathies. Am J Ophthalmol. 2014;158(6):1310–1317. doi: 10.1016/j.ajo.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doycheva D, Jägle H, Zierhut M, et al. Mycophenolic acid in the treatment of birdshot chorioretinopathy: long-term follow-up. Br J Ophthalmol. 2015;99(1):87–91. doi: 10.1136/bjophthalmol-2014-305535. [DOI] [PubMed] [Google Scholar]
- 79.Neri P, Mariotti C, Cimino L, Mercanti L, Giovannini A. Long-term control of cystoid macular oedema in noninfectious uveitis with myco-phenolate mofetil. Int Ophthalmol. 2008;29(3):127–133. doi: 10.1007/s10792-008-9200-z. [DOI] [PubMed] [Google Scholar]
- 80.Doycheva D, Zierhut M, Blumenstock G, Stuebiger N, Deuter C. Mycophenolate mofetil in the therapy of uveitic macular edema – long-term results. Ocul Immunol Inflamm. 2012;20(3):203–211. doi: 10.3109/09273948.2012.665562. [DOI] [PubMed] [Google Scholar]
- 81.Mathews D, Mathews J, Jones NP. Low-dose cyclosporine treatment for sight-threatening uveitis: efficacy, toxicity, and tolerance. Indian J Ophthalmol. 2010;58(1):55. doi: 10.4103/0301-4738.58472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaçmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117(3):576–584. doi: 10.1016/j.ophtha.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporin a therapy in the treatment of intraocular inflammatory disease resistant to systemic corticosteroids and cytotoxic agents. Am J Ophthalmol. 1983;96(3):275–282. doi: 10.1016/s0002-9394(14)77814-6. [DOI] [PubMed] [Google Scholar]
- 84.Nussenblatt RB, Palestine AG, Chan CC, Stevens G, Mellow SD, Green SB. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. Am J Ophthalmol. 1991;112(2):138–146. doi: 10.1016/s0002-9394(14)76692-9. [DOI] [PubMed] [Google Scholar]
- 85.Jeon S, Lee WK, Jung Y. Changes in the intraocular cytokine levels after intravitreal bevacizumab in uveitic macular edema. Ocul Immunol Inflamm. 2012;20(5):360–364. doi: 10.3109/09273948.2012.709576. [DOI] [PubMed] [Google Scholar]
- 86.Murphy CC, Greiner K, Plskova J, et al. Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Arch Ophthalmol. 2005;123(5):634–641. doi: 10.1001/archopht.123.5.634. [DOI] [PubMed] [Google Scholar]
- 87.Hogan AC, McAvoy CE, Dick AD, Lee RW. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology. 2007;114(5):1000–1006. doi: 10.1016/j.ophtha.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal A, Rajagopalan N, Hassan M, et al. Sirolimus for retinal and uveitic diseases. Dev Ophthalmol. 2016;55:276–281. doi: 10.1159/000438951. [DOI] [PubMed] [Google Scholar]
- 89.Shanmuganathan VA, Casely EM, Raj D, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol. 2005;89(6):666–669. doi: 10.1136/bjo.2004.048199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips BN, Wroblewski KJ. A retrospective review of oral low-dose sirolimus (rapamycin) for the treatment of active uveitis. J Ophthalmic Inflamm Infect. 2010;1(1):29–34. doi: 10.1007/s12348-010-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen QD, Ibrahim MA, Watters A, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J Ophthalmic Inflamm Infect. 2013;3(1):32. doi: 10.1186/1869-5760-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nguyen QD, Sadiq MA, Soliman MK, Agarwal A, do DV, Sepah YJ. The effect of different dosing schedules of intravitreal sirolimus, a mammalian target of rapamycin (mTOR) inhibitor, in the treatment of non-infectious uveitis (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2016;114 [PMC free article] [PubMed] [Google Scholar]
- 93.Buhaescu I, Izzedine H, Covic A. Sirolimus – challenging current perspectives. Ther Drug Monit. 2006;28(5):577–584. doi: 10.1097/01.ftd.0000245377.93401.39. [DOI] [PubMed] [Google Scholar]
- 94.Gillies MC, Su T. Interferon-alpha 2B enhances barrier function of bovine retinal microvascular endothelium in vitro. Microvasc Res. 1995;49(3):277–288. doi: 10.1006/mvre.1995.1024. [DOI] [PubMed] [Google Scholar]
- 95.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 96.Kötter I, Eckstein AK, Stübiger N, Zierhut M. Treatment of ocular symptoms of Behçet’s disease with interferon alpha 2A: a pilot study. Br J Ophthalmol. 1998;82(5):488–494. doi: 10.1136/bjo.82.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feron EJ, Rothova A, van Hagen PM, Baarsma GS, Suttorp-Schulten MS. Interferon-alpha 2b for refractory ocular Behçet’s disease. Lancet. 1994;343(8910):1428. doi: 10.1016/s0140-6736(94)92549-6. [DOI] [PubMed] [Google Scholar]
- 98.Kötter I, Zierhut M, Eckstein AK, et al. Human recombinant interferon alfa-2a for the treatment of Behçet’s disease with sight threatening posterior or panuveitis. Br J Ophthalmol. 2003;87(4):423–431. doi: 10.1136/bjo.87.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deuter CME, Koetter INA, Guenaydin I, Stuebiger N, Zierhut M. Interferon alfa-2a: a new treatment option for long lasting refractory cystoid macular edema in uveitis? A pilot study. Retina. 2006;26(7):786–791. doi: 10.1097/01.iae.0000244265.75771.71. [DOI] [PubMed] [Google Scholar]
- 100.Bodaghi B, Gendron G, Wechsler B, et al. Efficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: a retrospective monocentric study of 45 patients. Br J Ophthalmol. 2007;91:335–339. doi: 10.1136/bjo.2006.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deuter CM, Kötter I, Günaydin I, Stübiger N, Doycheva DG, Zierhut M. Efficacy and tolerability of interferon alpha treatment in patients with chronic cystoid macular oedema due to non-infectious uveitis. Br J Ophthalmol. 2009;93(7):906–913. doi: 10.1136/bjo.2008.153874. [DOI] [PubMed] [Google Scholar]
- 102.Fardeau C, Simon A, Rodde B, et al. Interferon-alpha2a and systemic corticosteroid in monotherapy in chronic uveitis: results of the randomized controlled BIRDFERON study. Am J Ophthalmol. 2017;177:182–194. doi: 10.1016/j.ajo.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Diwo E, Gueudry J, Saadoun D, Weschler B, Lehoang P, Bodaghi B. Long-term efficacy of interferon in severe uveitis associated with Behçet disease. Ocul Immunol Inflamm. 2017;25(1):76–84. doi: 10.1080/09273948.2016.1206204. [DOI] [PubMed] [Google Scholar]
- 104.Plskova J, Greiner K, Forrester JV. Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol. 2007;144(1):55–61. doi: 10.1016/j.ajo.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 105.Butler NJ, Suhler EB, Rosenbaum JT. Interferon alpha 2b in the treatment of uveitic cystoid macular edema. Ocul Immunol Inflamm. 2012;20(2):86–90. doi: 10.3109/09273948.2011.645989. [DOI] [PubMed] [Google Scholar]
- 106.Mackensen F, Jakob E, Springer C, et al. Interferon versus methotrexate in intermediate uveitis with macular edema: results of a randomized controlled clinical trial. Am J Ophthalmol. 2013;156(3):478–486. doi: 10.1016/j.ajo.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 107.Mackensen F, Max R, Becker MD. Interferons and their potential in the treatment of ocular inflammation. Clin Ophthalmol. 2009;3:559. doi: 10.2147/opth.s3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dick AD, Forrester JV, Liversidge J, Cope AP. The role of tumour necrosis factor (TNF-alpha) in experimental autoimmune uveoretinitis (EAU) Prog Retin Eye Res. 2004;23(6):617–637. doi: 10.1016/j.preteyeres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Díaz-Llopis M, Salom D, Garcia-de-Vicuña C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–1581. doi: 10.1016/j.ophtha.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 110.Dobner BC, Max R, Becker MD, et al. A three-centre experience with adalimumab for the treatment of non-infectious uveitis. Br J Ophthalmol. 2012;97(2):134–138. doi: 10.1136/bjophthalmol-2011-301401. [DOI] [PubMed] [Google Scholar]
- 111.Al Rashidi S, Al Fawaz A, Kangave D, Abu El-Asrar AM. Long-term clinical outcomes in patients with refractory uveitis associated with Behçet disease treated with infliximab. Ocul Immunol Inflamm. 2013;21(6):468–474. doi: 10.3109/09273948.2013.779727. [DOI] [PubMed] [Google Scholar]
- 112.Schaap-Fogler M, Amer R, Friling R, Priel E, Kramer M. Anti-TNF-α agents for refractory cystoid macular edema associated with noninfectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):633–640. doi: 10.1007/s00417-013-2552-8. [DOI] [PubMed] [Google Scholar]
- 113.Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP. Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol. 2004;138(4):648–650. doi: 10.1016/j.ajo.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 114.Maleki A, Sahawneh HF, Ma L, Meese H, He Y, Foster CS. Infliximab therapy in patients with noninfectious intermediate uveitis resistant to conventional immunomodulatory therapy. Retina. 2017;37(5):836–843. doi: 10.1097/IAE.0000000000001269. [DOI] [PubMed] [Google Scholar]
- 115.Markomichelakis N, Delicha E, Masselos S, Fragiadaki K, Kaklamanis P, Sfikakis PP. A single infliximab infusion vs corticosteroids for acute panuveitis attacks in Behcet’s disease: a comparative 4-week study. Rheumatology. 2011;50(3):593–597. doi: 10.1093/rheumatology/keq366. [DOI] [PubMed] [Google Scholar]
- 116.Farvardin M, Afarid M, Shahrzad S. Long-term effects of intravitreal infliximab for treatment of sight-threatening chronic noninfectious uveitis. J Ocul Pharmacol Ther. 2012;28(6):628–631. doi: 10.1089/jop.2011.0199. [DOI] [PubMed] [Google Scholar]
- 117.Androudi S, Tsironi E, Kalogeropoulos C, Theodoridou A, Brazitikos P. Intravitreal adalimumab for refractory uveitis-related macular edema. Ophthalmology. 2010;117(8):1612–1616. doi: 10.1016/j.ophtha.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 118.Hamam RN, Barikian AW, Antonios RS, et al. Intravitreal adalimumab in active noninfectious uveitis: a pilot study. Ocul Immunol Inflamm. 2016;24(3):319–326. doi: 10.3109/09273948.2014.990041. [DOI] [PubMed] [Google Scholar]
- 119.Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18(6):481–486. doi: 10.1097/ICU.0b013e3282f03d42. [DOI] [PubMed] [Google Scholar]
- 120.Bamforth SD, Lightman SL, Greenwood J. Ultrastructural analysis of interleukin-1 beta-induced leukocyte recruitment to the rat retina. Invest Ophthalmol Vis Sci. 1997;38(1):25–35. [PubMed] [Google Scholar]
- 121.Tugal-Tutkun I, Pavesio C, de Cordoue A, Bernard-Poenaru O, Gül A. Use of gevokizumab in patients with Behçet’s disease uveitis: an international, randomized, double-masked, placebo-controlled study and open-label extension study. Ocular Immunol Inflamm. 2018;26(7):1023–1033. doi: 10.1080/09273948.2017.1421233. [DOI] [PubMed] [Google Scholar]
- 122.Fabiani C, Vitale A, Emmi G, et al. Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2016;36(1):183–189. doi: 10.1007/s10067-016-3480-x. [DOI] [PubMed] [Google Scholar]
- 123.Tanaka T, Kishimoto T. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci. 2012;8(9):1227–1236. doi: 10.7150/ijbs.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deuter CME, Zierhut M, Igney-Oertel A, et al. Tocilizumab in uveitic macular edema refractory to previous immunomodulatory treatment. Ocul Immunol Inflamm. 2017;25(2):215–220. doi: 10.3109/09273948.2015.1099680. [DOI] [PubMed] [Google Scholar]
- 125.Mesquida M, Molins B, Llorenç V, et al. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema. Retina. 2018;38(7):1361–1370. doi: 10.1097/IAE.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 126.Adán A, Mesquida M, Llorenç V, et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(11):2627–2632. doi: 10.1007/s00417-013-2436-y. [DOI] [PubMed] [Google Scholar]
- 127.Calvo-Río V, Santos-Gómez M, Calvo I, et al. Anti-interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthritis Rheumatol. 2017;69(3):668–675. doi: 10.1002/art.39940. [DOI] [PubMed] [Google Scholar]
- 128.Nussenblatt RB, Peterson JS, Foster CS, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology. 2005;112(5):764–770. doi: 10.1016/j.ophtha.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 129.Papaliodis GN, Chu D, Foster CS. Treatment of ocular inflammatory disorders with daclizumab. Ophthalmology. 2003;110(4):786–789. doi: 10.1016/S0161-6420(02)01932-2. [DOI] [PubMed] [Google Scholar]
- 130.Bhat P, Castañeda-Cervantes RA, Doctor PP, Cs F, Foster CS. Intravenous daclizumab for recalcitrant ocular inflammatory disease. Graefe’s Arch Clin Exp Ophthalmol. 2009;247(5):687–689. doi: 10.1007/s00417-009-1043-4. [DOI] [PubMed] [Google Scholar]
- 131.Wroblewski K, Sen HN, Yeh S, et al. Long-term daclizumab therapy for the treatment of noninfectious ocular inflammatory disease. Can J Ophthalmol. 2011;46(4):322–328. doi: 10.1016/j.jcjo.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sobrin L, Huang JJ, Christen W, Kafkala C, Choopong P, Foster CS. Daclizumab for treatment of birdshot chorioretinopathy. Arch Ophthalmol. 2008;126(2):186. doi: 10.1001/archophthalmol.2007.49. [DOI] [PubMed] [Google Scholar]
- 133.Buggage RR, Levy-Clarke G, Sen HN, et al. A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behçet’s disease. Ocul Immunol Inflamm. 2007;15(2):63–70. doi: 10.1080/09273940701299370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Comyn O, Lightman SL, Hykin PG. Corticosteroid intravitreal implants vs. ranibizumab for the treatment of vitreoretinal disease. Curr Opin Ophthalmol. 2013;24(3):248–254. doi: 10.1097/ICU.0b013e32835fab27. [DOI] [PubMed] [Google Scholar]
- 135.Bae JH, Lee CS, Lee SC. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina. 2011;31(1):111–118. doi: 10.1097/IAE.0b013e3181e378af. [DOI] [PubMed] [Google Scholar]
- 136.Cervantes-Castañeda RA, Giuliari GP, Gallagher MJ, et al. Intravitreal bevacizumab in refractory uveitic macular edema: one-year follow-up. Eur J Ophthalmol. 2009;19(4):622–629. doi: 10.1177/112067210901900417. [DOI] [PubMed] [Google Scholar]
- 137.Weiss K, Steinbrugger I, Weger M, et al. Intravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumab. Eye. 2009;23(9):1812–1818. doi: 10.1038/eye.2008.388. [DOI] [PubMed] [Google Scholar]
- 138.Kozak I, Shoughy SS, Stone DU. Intravitreal antiangiogenic therapy of uveitic macular edema: a review. J Ocul Pharmacol Ther. 2017;33(4):235–239. doi: 10.1089/jop.2016.0118. [DOI] [PubMed] [Google Scholar]
- 139.Perosa F, Favoino E, Caragnano MA, Prete M, Dammacco F. CD20: a target antigen for immunotherapy of autoimmune diseases. Autoimmun Rev. 2005;4(8):526–531. doi: 10.1016/j.autrev.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 140.Davatchi F, Shams H, Rezaipoor M, et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study) Int J Rheum Dis. 2010;13(3):246–252. doi: 10.1111/j.1756-185X.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 141.Tappeiner C, Heinz C, Specker C, Heiligenhaus A. Rituximab as a treatment option for refractory endogenous anterior uveitis. Ophthalmic Res. 2007;39(3):184–186. doi: 10.1159/000103239. [DOI] [PubMed] [Google Scholar]
- 142.Bussone G, Kaswin G, de Menthon M, Delair E, Brézin AP, Guillevin L. Macular oedema following rituximab infusion in two patients with Wegener’s granulomatosis. Clin Exp Rheumatol. 2010;28(1 Suppl 57):90–92. [PubMed] [Google Scholar]
- 143.Gilca M, Merrill PT. Cystoid macular edema secondary to rituximab. Retin Cases Brief Rep. 2017 Mar 6; doi: 10.1097/ICB.0000000000000556. Epub. [DOI] [PubMed] [Google Scholar]
- 144.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68(1):25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Miserocchi E, Modorati G. Rituximab for noninfectious uveitis. Dev Ophthalmol. 2012;51:98–109. doi: 10.1159/000336188. [DOI] [PubMed] [Google Scholar]
- 146.Faia LJ, Nida Sen H, Li Z, et al. Treatment of inflammatory macular edema with humanized anti-CD11a antibody therapy. Investig Ophthalmol Vis Sci. 2011;52(9):6919–6924. doi: 10.1167/iovs.10-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Golan S, Loewenstein A. Surgical treatment for macular edema. Semin Ophthalmol. 2014;29(4):242–256. doi: 10.3109/08820538.2013.796394. [DOI] [PubMed] [Google Scholar]
- 148.Gutfleisch M, Spital G, Mingels A, Pauleikhoff D, Lommatzsch A, Heiligenhaus A. Pars plana vitrectomy with intravitreal triamcinolone: effect on uveitic cystoid macular oedema and treatment limitations. Br J Ophthalmol. 2007;91(3):345–348. doi: 10.1136/bjo.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tranos P, Scott R, Zambarakji H, et al. The effect of pars plana vitrectomy on cystoid macular oedema associated with chronic uveitis: a randomised, controlled pilot study. Br J Ophthalmol. 2006;90(9):1107–1110. doi: 10.1136/bjo.2006.092965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Henry CR, Becker MD, Yang Y, Davis JL. Pars plana vitrectomy for the treatment of uveitis. Am J Ophthalmol. 2018;190:142–149. doi: 10.1016/j.ajo.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 151.Radetzky S, Walter P, Fauser S, Koizumi K, Kirchhof B. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefe’s Arch Clin Exp Ophthalmol. 2004;242(4):273–278. doi: 10.1007/s00417-003-0731-8. [DOI] [PubMed] [Google Scholar]
- 152.Muhaya M, Calder VL, Towler HM, Jolly G, McLauchlan M, Lightman S. Characterization of phenotype and cytokine profiles of T cell lines derived from vitreous humour in ocular inflammation in man. Clin Exp Immunol. 1999;116(3):410–414. doi: 10.1046/j.1365-2249.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liversidge J, Dick A, Cheng YF, Scott GB, Forrester JV. Retinal antigen specific lymphocytes, TCR-gamma delta T cells and CD5+ B cells cultured from the vitreous in acute sympathetic ophthalmitis. Autoimmunity. 1993;15(4):257–266. doi: 10.3109/08916939309115747. [DOI] [PubMed] [Google Scholar]
- 154.Tranos PG, Tsaousis KT, Vakalis AN, Asteriades S, Pavesio CE. Long-term follow-up of inflammatory cystoid macular edema. Retina. 2012;32(8):1624–1628. doi: 10.1097/IAE.0b013e3182483348. [DOI] [PubMed] [Google Scholar]
- 155.Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66–72. doi: 10.1016/j.rmed.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 156.Taylor PC, Abdul Azeez M, Kiriakidis S. Filgotinib for the treatment of rheumatoid arthritis. Expert Opin Investig Drugs. 2017;26(10):1181–1187. doi: 10.1080/13543784.2017.1372422. [DOI] [PubMed] [Google Scholar]
- 157.Feagan BG, Sandborn WJ, Gasink C, et al. UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 158.López-Ferrer A, Laiz A, Puig L. The safety of ustekinumab for the treatment of psoriatic arthritis. Exp Opin Drug Saf. 2017;16(6):733–742. doi: 10.1080/14740338.2017.1323864. [DOI] [PubMed] [Google Scholar]
- 159.The Cleveland Clinic, Mallinckrodt Ocular Sarcoidosis Open Label Trial of ACTHAR Gel. [Accessed 15 October 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT02725177?term=macular+edema&cond=Uveitis&rank=58. ClinicalTrials.gov Identifier: NCT02725177.
- 160.John Huang MD. (ACTH) for the Treatment of Sarcoid Uveitis (ACTH) [Accessed 15 October 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT03473964?term=macular+edema&cond=Uveitis&rank=40. ClinicalTrials.gov Identifier: NCT03473964.
- 161.Gilead Sciences Efficacy and Safety of Filgotinib in Adults With Active Noninfectious Uveitis (Humboldt) [Accessed 15 October 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT03207815?term=macular+edema&cond=Uveitis&rank=45. ClinicalTrials.gov Identifier: NCT03207815.
- 162.National Eye Institute (NEI) Ustekinumab (STELARA) for the Treatment of Active Sight-Threatening Uveitis (STAR Study) [Accessed 15 October 2018]. Available from: https://clinicaltrials.gov/ct2/show/NCT02911116?term=ustekinumab&cond=uveitis&rank=1. ClinicalTrials.gov Identifier: NCT02911116.