Abstract
Hepatocellular carcinoma (HCC), a primary liver malignancy, is one of the deadliest cancers worldwide. Despite orthotopic liver transplantation and hepatic resection representing the principal lines of treatment for this pathology, only a minority of patients can be resected owing to cirrhosis or late diagnosis. Keeping in mind the end goal of conquering these challenges, new alternative approaches have been proposed. Accumulating evidence has demonstrated that epigallocatechin-3-gallate (EGCG), the principal catechin of green tea with multiple biological properties, is able to modulate different molecular mechanisms underlying HCC, mainly through its antioxidant activity. In this article, we revise these findings reported in the literature, in order to highlight the potential roles of EGCG in the treatment of HCC. The CAMARADES criteria were applied for quality assessment of animal studies, and a narrative synthesis performed. New bits of information available for translational perspectives into clinical practice are addressed.
Keywords: hepatocellular carcinoma, epigallocatechin-3-gallate, tumor progression, preclinical studies
Introduction
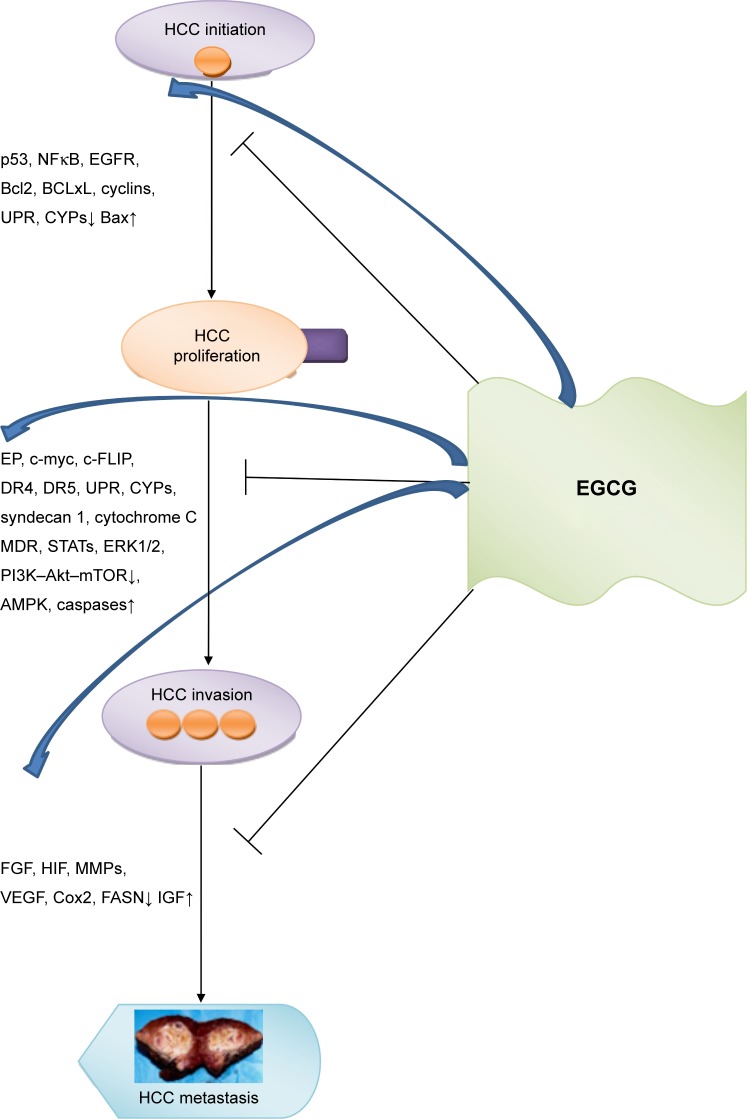
Hepatocellular carcinoma (HCC) is an aggressive liver cancer associated with a high rate of mortality worldwide.1,2 Orthotopic liver transplantation and surgical hepatic resection are considered the principal approaches for HCC treatment, but many patients are precluded from these therapies, due to unavailability of organs, cirrhosis, or late diagnosis.3 Systemic sorafenib-based therapy is an alternative choice for HCC patients in an advanced stage, although a significant number of patients are refractory to this multikinase-inhibitor drug4 and require further approaches, such as regorafenib, which is to date the only systemic treatment associated with survival improvement in HCC patients progressing on sorafenib treatment.5 Based on these premises, new alternative therapies for HCC treatment are much needed. Accumulating evidence has shown that the catechins and flavonoids contained in green tea (Camellia sinensis) confer to this plant cancer-chemopreventive effects. Particularly, epigallocatechin-3-gallate (EGCG), the most abundant active catechin found in green tea, induces apoptosis and inhibits tumor progression through its antioxidant and anti-inflammatory properties by modulating different signaling pathways in several types of cancer, including HCC (Figure 1).6–8 Also, it has been shown that EGCG behaves as a prooxidant in the presence of elevated concentrations of transition metals, mainly copper, thus leading to cytotoxic effects in cancer cells through the generation of reactive oxygen species (ROS), which are responsible for DNA breakage.9–12
Figure 1.
Principal targets modulated by EGCG in HCC progression.
Abbreviations: EGCG, epigallocatechin-3-gallate; HCC, hepatocellular cancer; UPR, unfolded protein response.
In this article, we revise these findings reported in the literature in order to highlight the potential roles of EGCG in the prevention and treatment of HCC and to offer a new contribution to conceivable applications into clinical practice. A narrative synthesis is also done for all studies considered, and the level of evidence for each included animal study was assessed by using a modified CAMARADES eleven-item checklist.13
Chemistry of EGCG
EGCG is the most abundant active catechin found in green-tea extract, accounting for 65% of its content. Due to the presence of three heterocyclic rings into EGCG’s chemical structure, as we previously reported,14 electron delocalization is favored, leading to quenching of free radicals. EGCG, like other tea catechins, has redox properties and reacts mainly with ROS. Particularly, the ability of EGCG to chelate free metal ions leads to prevention of ROS formation and the oxidation of catechins.9,15,16 Basically, its chemical structure not only confers antioxidant activities to EGCG but also favors its air oxidation, mainly in the presence of ions, leading to the formation of unstable catechin dimers. Moreover, as a consequence of the catechin structure, EGCG and other catechins are susceptible to several biotransformations, such as methylation, glucuronidation, and degradation, mediated by specific enzymes, mainly in the liver and in the small intestine.17–19 Finally, the hydrogen bonding capacity associated with EGCG’s polyphenolic structures is responsible for reduced EGCG absorption.20
Bioavailability of EGCG: constraints and upgrades
Despite its health-promoting properties, EGCG is characterized by poor bioavailability, mainly due to its elevated instability, lower capacity to permeate the gastrointestinal tract under specific pH conditions, and active efflux.21–25 Therefore, its efficacy and application in clinical practice are very constrained and should be moved forward. For this purpose, several clinical trials have been conducted by controlling pharmacokinetic parameters, and have been reviewed by Mereles and Hunstein,26 in order to select the most appropriate dose and route of administration for EGCG intervention. Results from these studies suggest that adjustments in different parameters (eg, temperature and humidity)27 and improvements in dietary habits (eg, not drinking hard water; EGCG intake on an empty stomach; dietary introduction of vitamins, ω3 polyunsaturated fatty acids, and piperine)28–31 could enhance EGCG bioavailability. Additionally, the application of a nanodelivery system to encapsulate EGCG into specific nanoparticles (eg, chitosan and peptides)32,33 successfully enhance EGCG intestinal absorption, thus improving its bioavailability. In addition, a comparative impact has been likewise depicted by the engineering and blend of EGCG derivatives.34,35
Preclinical assessments of EGCG anticancer effects in hepatocellular carcinoma
In vitro studies on hepatic cancer cell lines
Accumulating preclinical evidence has shown that EGCG inhibits the growth and induces apoptosis of different liver cancer cells by acting via different molecular mechanisms (Table 1 and Figure 1).
Table 1.
A summary of in vitro studies on the role of EGCG in hepatic cancer cell growth
| Cell line | Dose of EGCG | Molecular target(s) | Reference |
|---|---|---|---|
| AH109A | 10 mM, 50 mM | ROS↓ | 37 |
| HCT116, HepG2 | 7.6±0.4 µM in HCT116, 11.2±0.5 µM in HepG2 | – | 38 |
| HepG2 | 12.5, 25, 50, 100 µM | BCL2L1, CDH1, TACSTD1, PTK2↑, BAX, CASP3, HIF1A↓ | 39 |
| HLE, HepG2, Huh7 PLC/PRF/5 | 10, 25, 50, 100 µg/mL | Caspases 8, 9, and 3↑, BCL2α, BCLxL, NFκB↓ | 40 |
| HCCLM6 | 5–100 µg/mL | MMP2, MMP9, FUSE, FUBP1, HSPB1, CH60↓, NPM↑ | 41 |
| HeLa, HepG2 | HIF1α, VEGF, PI3K/Akt, ERK1/2, mTOR↓ | 42 | |
| SK-Hep1 | 2 mg FW/mL, 20 µg/mL | MMP2, MMP9↓ | 43 |
| HepG2 | 1.0 mg/mL | 44 | |
| Huh7, PLC/PRF/5, HLF, HLE, HepG2, and Hep3B | 20 or 40 µg/mL | IGF1, IGF2, p-IGF1R, p-GSK3β, p-ERK, p-Akt, and p-STAT3↓, IGFBP3E↑ | 45 |
| HepG2, SMMC7721 | 6.25, 12.5, 25, 50, 100, 200, and 400 µg/mL | Cox2, BCL2↓, caspases 9 and 3↑ | 46 |
| Hep3B, two primary cultures established from surgically resected HCC | 0–10 µM | PAR1/PAR4, p42, p44, MAPK↓ | 47 |
| HLF, PLC/PRF/5, HepG2, Huh7, HLE, and Hep3B | 0–100 µg/mL | VEGFR2, p-VEGFR2↓ | 48 |
| HepG2, Hep3B | 0–160 µM | AMPK↑, mTOR, FASN, ACC↓, p53 in HepG2↓, p53 in Hep3B↑ | 49 |
| HepG2 | 80, 120, 160 µmol/L | Smad7↓ | 50 |
| BEL7404, BEL7404/Dox | 70, 140, 285, 560, 1,120 µg/mL | Dox chemosensitivity and cytotoxicity↑ | 51 |
| SMMC7721, Hep3B | 0–5 µM | CBR1↓ | 52 |
| Hep3B | 5, 10, 25, and 50 µmol/L in presence or in absence of 5FU | Cox2, Akt, pAkt↓, phospho-ACC, AMPK↑ | 53 |
| HepG2, MHCC97L, MHCC-97H, L02 | 100 µg/mL | PGE2↓, EP1↓ | 54 |
| HCCLM6 | 0, 5, 10, 20, 30, 40, 50, 60, 80, or 100 µg/mL | MMP2, MMP9, FUBP1, HSPB1, CH60↓, NPM↑ | 41 |
| HUVECs, Huh7 | Methyl-EGCG | VEGF, VEGFR2, p42/44 MAPK↓ | 55 |
| BEL-7402, QGY-7703 | 0–350 µM | p-STAT3, cyclin D1, BCLxL, c-Myc, VEGF↓ | 56 |
| HepG2, SMMC7721, SK-Hep1 | 0, 40, 80, 120 µM | PI3K, Akt, NFκB↓ | 57 |
| Hep3B | 0, 10, 20, 40 µg/mL | Autophagic genes↓ | 46 |
| HepG2 | 10, 50, 100, 200 µM | MMP9, syndecan 1, FGF2↓ | 8 |
| HCCLM6, HL7702 | 0, 5, 10, 20, 30, 40, 60, 80, and 100 µg/mL in HL7702, 0, 5, 10, 20, 40, 60, 80, 100, 140, 180, 220, and 260 µg/mL in HCCLM6 | BCL2, NFκB↓, BAX, p53, caspases 9 and 3↑ | 58 |
| HepG2, MHCC97H, Hep3B | 0.02–20 µg/mL | OPN↓ | 59 |
| HepG2, HCT116, HLF, Huh7, HepG2, CSCs | 0–75 µmol | D133, Nanog, ATP-binding cassette transporter genes↓, ABCC1, ABCG2, Nek2, p-Akt in CSCs↓ | 60 |
| HCC-LM3, SMMC7721, Hep3B, HepG2, Huh7, QSG7701, LO2 | 25, 50, 100 µM | PFK↓ | 61 |
| HepG2 | 50 µM | Dox-induced overexpression of Pgp, PI3K/Akt, Mek/ERK↓ | 62 |
| HepG2 | 0–175 µM | AFP↓ | 63 |
Abbreviations: CSCs, cancer stem cells; Dox, doxorubicin; EGCG, epigallocatechin-3-gallate; HCC, hepatocellular carcinoma; HUVECs, human umbilical vein endothelial cells; ROS, reactive oxygen species.
The first report investigating the role of EGCG in hepatoma was from Nishida et al.36 The authors showed that EGCG was able to inhibit the growth and secretion of AFP in human hepatoma-derived PLC/PRF/5 cells without affecting their viability. Also, EGCG inhibited the growth of spontaneous hepatoma in C3H/HeNCrj mice without signs of toxicity. Subsequently, the inhibitory effects of EGCG on the invasion of hepatoma cells were proved in AH109A cells through the ROS-scavenging activity of EGCG.37 Uesato et al38 proved for the first time that EGCG inhibited the growth of HCT116 colorectal and HepG2 HCC cells, although with a lower effect with respect to (–)-epicatechin. A different molecular pathway underlying the effect of EGCG on apoptosis in HCC cells was described by Park et al.39 The authors demonstrated that EGCG diminished hypoxia-incited apoptosis in HepG2 cells and enhanced cell survival. These data support the hypothesis that EGCG could be considered a useful agent for HCC treatment. In fascinating in vitro and in vivo investigations, Nishikawa et al40 demonstrated that EGCG was able to induce apoptosis in HLE cells by inactivation of NFκB alone and in combination with TRAIL. Using in vitro approaches, Zhang et al41 showed that EGCG induced apoptosis of HCCLM6 cells by decreasing the mitochondrial membrane potential and by promoting G0/G1-phase cell-cycle arrest.
In addition to the apoptotic effects, other mechanisms, including the antiangiogenic effect, have clarified the potential role of EGCG on hepatocarcinoma tumor growth. Zhang et al42 showed that green-tea extract and EGCG significantly inhibited the accumulation of HIF1α and its downstream target – VEGF expression – in both human cervical carcinoma (HeLa) and HepG2 cells. Interestingly, they proved that these results were obtained without affecting HIF1α mRNA levels and by acting on PI3K/Akt and/ or ERK1/2 molecular mechanisms. In a study conducted on human HCC cells, SK-Hep1 and Pinus densiflora leaf extract and its phytochemicals, EGCG, EGC, and catechin gallate inhibited cell invasion and metastasis formation by altering the activities of MMPs, particularly gelatinase A (MMP2) and gelatinase B (MMP9), in a dose-dependent manner,43 and EGCG exhibited higher efficacy. Subsequently, Lee et al44 confirmed the ability of EGCG to suppress the cytotoxic effect induced by ethanol at lethal doses in HepG2 cells. In another study, an inhibitory role of EGCG on various types of the tyrosine-kinase receptor IGF1R in HepG2 cells was highlighted.45 Later on, Chen et al showed that EGCG was able to induce apoptosis in HepG2 and SMMC7721 cells by affecting the expression profiles of Cox2 and BCL2 and by activating caspase 9 and caspase 3.46 A potential role of EGCG in HCC treatment was suggested by Kaufmann et al in in vitro studies on the permanent liver carcinoma cell line HEP3B and two primary cultures surgically resected from HCC patients. Data from these studies showed that EGCG had an inhibitory effect on PAR1 and PAR4 (involved in HCC progression) and on p42/p44 MAPKs.47 Shirakami et al48 demonstrated an inhibitory role of EGCG on HCC progression by altering the activities of the tyrosine-kinase receptor VEGFR, which is involved in the angiogenesis of HCC. In vitro experiments were conducted on six human HCC cells – HLF, PLC/PRF/5, HepG2, Huh7, HLE, and Hep3B – and the major EGCG inhibitory effect on HCC-cell growth was observed in Huh7 cells. This result was also confirmed by an in vivo nude mouse xenograft model generated by Huh7 cells injected subcutaneously in mice. Taken together, these results suggest that EGCG can be considered a promising agent for HCC treatment.
A chemopreventive and antilipogenic role of EGCG for HCC was highlighted by Huang et al.49 Using HepG2 (p53-positive cells) and Hep3B (p53-negative cells), the authors showed that EGCG inhibited cell proliferation in a dose-dependent manner, thus suggesting that an additional factor to p53 is involved in promoting the inhibitory effect of EGCG on HCC proliferation. To demonstrate this, levels of AMPK, a sensor of cellular energy levels, was evaluated in both p53-positive and -negative human hepatoma cells. Data showed that EGCG was able to upregulate AMPK activity in HCC cells by inhibiting the mTOR pathway.
Moreover, by activation of AMPK, EGCG is able to decrease the activity and/or expression of lipogenic enzymes, such as ACC and FASN. All these findings support the hypothesis that EGCG could be potentially used as a chemopreventive and antilipogenic compound for HCC. Tong et al50 showed that the cytotoxic effect of EGCG on HepG2 cells was associated with activation of the TGFβ1–Smad signaling pathway. Subsequently, the antitumor effect of EGCG on liver cancer was described in a murine model for chemoresistant HCC using doxo-rubicin (Dox) in combination with epicatechin gallate and EGCG. A synergistic effect between Dox and epicatechin gallate or EGCG administered together in vitro and in vivo was observed. Moreover, it has been proved that catechins enhance chemosensitivity to Dox, increase Dox cytotoxicity in HCC cells, and inhibit Pgp expression in Dox-resistant HCC cells in vitro and in xenograft tumors. An inhibitory effect of catechins on the expression of multidrug-resistance genes was likewise observed.51
A fascinating study supporting the chemopreventive effect of EGCG on HCC, was conducted in SMMC7721 and Hep3B cells. By in vitro and in vivo studies, the authors showed that EGCG was able to inhibit human CBR1 activity, which in turn converted the antitumor drug and anthracycline daunorubicin (Dnr) into the alcohol metabolite daunorubicinol, reducing antitumor activity and cardiotoxicity. Taken together, these data suggest that a combination of EGCG and Dnr might represent a novel approach for HCC chemoprevention.52
Additional studies have confirmed the chemopreventive role of EGCG in HCC treatment. It has been shown that EGCG was able to sensitize HCC cells to 5-fluorouracil antitumor activity. Moreover, a synergistic effect between EGCG and 5-fluorouracil was detected in Hep3B cells, mainly through the downregulation of Cox2 and hyperactivation of AMPK.53 An interesting in vitro study conducted on the HCC cell line HepG2, human hepatoma cell lines MHCC97L, MHCC97H, and the human hepatocyte cell line L02 showed that the anti-HCC effects of EGCG might be mediated by suppression of prostanoid EP1 expression and by the production of PGE2.54 The anticancer effect of EGCG on HCC has also been proven on HCCLM6 cells. Using in vitro approaches, the authors demonstrated that EGCG arrested HCCLM6 cell metastasis by inhibiting MMP2 and MMP9 expression. Moreover, EGCG altered expression levels of FUBP1, HSPB1, CH60, and NPM proteins, strongly related to metastasis formation.41 Despite promising results on the antitumor effect of EGCG on HCC, a report from Hashimoto et al55 described that a new methylated EGC analogue (methyl-EGCG) had a stronger antioxidation effect than EGCG in Huh7 hepatoma cells and corresponding xenografted hepatoma mice. A new molecular mechanism underlying the anticancer effect of EGCG on HCC cells was highlighted by Wang et al.56 By treating BEL7402 and QGY7703 cells with EGCG, they observed suppression of cell proliferation and a reduction in expression levels of phosphorylated STAT3 (p-STAT3), thus suggesting that regulation of STAT3 signaling could be considered as one of the molecular mechanisms underlying the anticancer effect of EGCG on HCC cells.
In another in vitro study, it has been shown that EGCG in HepG2, SMMC7721, and SK-Hep1 cells reduced the secretion of AFP, high levels of which are associated with malignant differentiation of cancer cells, through the modulation of autophagic activities of HCC cells. From these data, it emerged that EGCG inhibited HCC-cell growth by suppressing the Akt pathway.57
Different findings were depicted by Chen et al.46 The authors described a role of autophagy in the inhibitory effects on HCC cells of the synergistic interaction between EGCG and Dox. By using in vitro approaches on hepatoma cells and a subcutaneous Hep3B-cell xenograft tumor model, the authors showed that EGCG acts as a chemotherapeutic agent and acts synergistically by enhancing Dox anticancer effects through the inhibition of autophagy in HCC. The chemopreventive and hepatoprotective effects of EGCG against HCC were studied by Darweish et al in in vitro and Sprague Dawley rats.8 This investigation found that the heparan sulfate proteoglycans pathway was engaged with the chemopreventive and hepatoprotective effects of EGCG. In another study conducted on HCCLM6 cells and noncancerous liver cells (HL7702) treated with EGCG, it was shown that EGCG was able to induce apoptosis, decrease mitochondrial membrane potential, and promoted G0/G1-phase cell-cycle arrest only in HCCLM6 cells.58 An interesting study was conducted on HepG2 and MHCC97H cells to investigate the role of EGCG in modulating OPN, which is a mediator of metastasis and invasion of HCC. Data reported showed that EGCG decreased OPN mRNA and secreted OPN protein levels by decreasing the half-life of OPN mRNA in MHCC97H cells.59 An intriguing study was conducted on the effects of EGCG on human hepatoma and colon cancer stem cells, which are considered responsible for tumor recurrence and chemoresistance after hepatic resection. The authors showed that EGCG was able to inhibit the self-renewal process in cancer stem cells of hepatoma and the colon by modulating the expression of ATP-binding cassette-transporter genes and stem-cell markers and by decreasing the transcription of NEK2 and p-Akt genes, thus resulting in Akt-signaling inhibition.60
An interesting investigation was conducted by Li et al61 on the inhibitory effects of EGCG on HCC-cell growth by involving the glycolysis process. In this study, it was reported that EGCG modulated the oligomeric structure of PFK, a metabolic sensor in the glycolytic pathway, and suppressed PFK activity. Moreover, EGCG enhanced the inhibitory effects of sorafenib on anaerobic HCC-cell growth and in an HCC-xenograft mouse model. Recently, it has been shown that EGCG attenuates the overexpression of Pgp induced by Dox in HepG2 cells by inhibiting the ERK–MAPK and PI3K–Akt signaling pathways.62 Finally, a new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells has been proposed by Zhao et al.63 Taken together, all these findings suggest that EGCG could be conceivably utilized for counteracting and treating HCC by acting on different molecular mechanisms.
Principal targets modulated by EGCG in hepatocellular cancer progression
On the basis of the preclinical results described, it emerges that EGCG is able to regulate HCC progression by modulating principal molecular targets: NFκB and p53; ERK1/2 and PI3K–Akt–mTOR; and FGF and VEGF. As shown in Figure 1, the transition from HCC-tumor initiation to HCC proliferation is blocked by EGCG through downregulation of expression levels of p53, NFκB, EGFR, cyclins, and unfolded protein response and upregulation of BAX. Further, the switch from HCC proliferation to HCC invasion is inhibited by EGCG, mainly through the modulation of the expression levels of ERK1/2, PI3K–Akt–mTOR, STATs, multidrug resistance, AMPK, and caspases. Finally, EGCG greatly reduced HCC metastasis by downregulating expression of FGF, HIF, and MMPs and upregulating expression of IGF.
In vivo investigations: animal models and EGCG effects
The effects of EGCG on HCC have been additionally shown by in vivo experiments, as summarized in Table 2. In a fascinating experiment, Nishida et al36 reported that EGCG, given as 0.05% or 0.1% in drinking water for 7 weeks, reduced the incidence of spontaneous hepatocarcinogenesis in C3H/ HeNCrj mice. More recently, several investigations have been performed on ectopic xenograft murine models. In the aforementioned Nishikawa et al study,40 the oral administration of EGCG (0, 0.8, 2.5, and 7.5 mg/mL ad libitum for 25 days) inhibited tumor growth and induced apoptosis in HLE xenograft tumors (HLE cells injected into the dorsal subcutaneous tissue of nude mice) by the downregulation of BCL2a and BCLxL in a dose-dependent manner. The authors also proved that high EGCG doses did not induce significant adverse effects on the growth or behavior of animals.
Table 2.
In vivo studies on the antitumor effects of EGCG on HCC
| Animal models | Drug | Dose of EGCG | Effects | Reference |
|---|---|---|---|---|
| Spontaneous hepatoma in C3H/HeNCrj mice | EGCG | 0.05% (w:w) or 0.1% EGCG in drinking water | EGCG reduced the incidence of hepatoma-bearing mice and the average number of hepatomas | 36 |
| Xenograft mouse model (HLE cells injected into the dorsal subcutaneous tissue of mice) | EGCG | 0, 0.8, 2.5, and 7.5 mg/mL ad libitum for 25 days | EGCG inhibited tumor growth, induced apoptosis, and downregulated BCL2α and BCLxL expression, EGCG sensitized HLE cells to TRAIL (100 ng/mL)-mediated apoptosis | 40 |
| Xenograft mouse model (BEL7404/Dox HCC cells subcutaneously injected into the right axilla of mice) | EGCG + Dox | Dox alone (2 mg/kg IP), EGCG alone (80 mg/kg IP), Dox + EGCG (40, 80, 160 mg/kg OG) | EGCG enhanced Dox anticancer activity, downregulated HIF1α, and upregulated MDR1 expression | 51 |
| Xenograft mouse model (SMMC7721 or Hep3B cells subcutaneously injected in mice) | EGCG | 20 mg/kg IP three times a week for 4 weeks | EGCG enhanced anticancer activity of Dnr and reduced cardiotoxicity | 52 |
| Xenograft mouse model (Huh7 cells subcutaneously injected in mice) | Methyl-EGCG | 1.1 mg/kg IP 8.3 mg/kg PO | Methyl-EGCG suppressed tumor growth in Huh7 hepatoma cells via inhibition of angiogenesis | 55 |
| Xenograft mouse model (Hep3B cells per mouse in the right fossa axillaries) | EGCG + Dox | EGCG 50 mg/kg, OG; Dox 2 mg/kg IP | EGCG synergistically enhanced Dox anticancer effects involving autophagy inhibition | 46 |
| SD rats with HCC induced by thioacetamide (200 mg/kg) | EGCG | EGCG 20 mg/kg or sodium ascorbate 100 mg/kg IP twice per week for 16 weeks | EGCG blocked HCC-induced elevation of oxidative stress markers and induced HSPG activity | 8 |
| Xenograft model (HCC-LM3 cells subcutaneously injected in mice) | EGCG | EGCG (10 mg/kg/BW/day) or sorafenib (10 mg/kg/BW/day) | EGCG inhibited HCC-tumor growth and induced apoptosis in combination with sorafenib | 62 |
Abbreviations: BW, body weight; Dnr, daunorubicin; Dox, doxorubicin; EGCG, epigallocatechin-3-gallate; HCC, hepatocellular carcinoma; IP, intraperitoneally; OG, oral gavage; PO, per os (orally).
The efficacy of EGCG has been also investigated in vivo in combination with anticancer drugs. Liang et al51 evaluated the effect of EGC and EGCG on the antitumor activity of Dox in a murine xenograft model for chemoresistant HCC. In their experiments, the author found that the coadministration of Dox and low doses of EGCG significantly inhibited hepatoma growth. In addition, EGCG was able to increase the intracellular accumulation of Dox, due to the inhibition of Pgp and mRNA expression of several drug-resistance genes (eg, MDR1). Subsequently, the same authors proved that the synergistic effect of the combination of EGCG + Dox involved autophagy inhibition in a dose- and time-dependent manner.46
In another murine xenograft model obtained with SMMC7721 or Hep3B cells, EGCG enhanced the anticancer activity of the anthracycline Dnr through the inhibition of human CBR1, which is involved in its catabolism.52 Starting from the results of a previous experiment proving the biological activity of the methyl-EGCG analogue, Hashimoto et al55 demonstrated that oral administration of low-dose (8.3 mg/kg) methyl-EGCG inhibited cell growth in xenografted Huh7 hepatoma mice. In particular, methyl-EGCG was able to inhibit angiogenesis activity. EGCG-induced inhibition of vascular invasion was also studied by Darweish et al.8 In an animal model of HCC, the authors demonstrated that EGCG was able to induce restoration of HSPG receptors. In a murine xenograft model of HCC, Li et al62 demonstrated that EGCG inhibited glycolysis and induced apoptosis in HCC cells through inhibition of the expression and activity of PFK, a limiting enzyme of glycolysis. As a consequence of this effect, EGCG improved the resistance of aerobic glycolytic HCC cells to sorafenib and enhanced cell-growth inhibition.
In the CAMARADES analysis (Table 3), we found that all the studies considered were published in peer-reviewed journals and showed detailed biochemical/tissue evaluations and professional statistical analysis. The newest studies8,62 obtained the highest score (9/11) despite the lack of any allocation-concealment technique, mandatory to prevent selection bias. This criterion is often underestimated, as demonstrated in our previous CAMARADES evaluations.64 Despite this gap, the results of all considered investigations were suggestive of a good-quality methodological approach in the research on these topics.
Table 3.
Quality of evidence obtained using a modified CAMARADES checklist
| References | ||||||||
|---|---|---|---|---|---|---|---|---|
| 36 | 40 | 51 | 46 | 52 | 55 | 8 | 62 | |
| Publication in a peer-reviewed journal | √ | √ | √ | √ | √ | √ | √ | √ |
| Number of experiments and control-groups | √ | √ | √ | √ | √ | √ | √ | |
| Housing and husbandry conditions | √ | √ | √ | √ | ||||
| Details of intervention/exposure group procedures | √ | √ | √ | √ | √ | √ | √ | |
| Random allocation to groups | √ | √ | √ | √ | ||||
| Allocation concealment | ||||||||
| Blinded assessment of outcome | √ | √ | ||||||
| Biochemical/molecular evaluations | √ | √ | √ | √ | √ | √ | √ | |
| Tissue evaluations | √ | √ | √ | √ | √ | √ | √ | √ |
| Statistical analysis | √ | √ | √ | √ | √ | √ | √ | √ |
| Statement regarding possible conflict of interest | √ | √ | √ | √ | √ | √ | ||
| Total (of 11) | 7 | 8 | 8 | 8 | 5 | 7 | 9 | 9 |
Note: √, criterion satisfied.
Future research needs and translational perspectives in clinical practice
Although experimental studies on the roles of EGCG in the prevention and treatment of HCC have demonstrated promising findings, further investigations are needed in order to understand the molecular basis underlying these effects. An intriguing issue concerns the mechanisms underlying cell-cycle arrest and apoptosis in HCC. For instance, in contrast with results found in differentiated cell lines (eg, HepG2 cells), it has been reported that EGCG failed to induce cell-cycle arrest and had no effect on Fas expression in an undifferentiated cell line (HLE), whereas in these latter cells apoptosis was obtained via caspase 9 activation through inhibition of BCL2a and BCLxL. The translational point of view of these discoveries is exceptionally fascinating, as they suggest that in HCC – featuring rapid cell proliferation and strong expression of antiapoptotic genes – EGCG ought to work by affecting different apoptotic mechanisms in different phases of cancer development and progression. Additional investigations should be focused on better understanding the antiangiogenic effects of EGCG and the mechanisms involved in the activation of the immunoresponse against tumors. Positive responses in this field could stimulate preclinical investigation – and subsequently translational approaches in clinical practice – based on the combination of EGCG with tumor-targeted and immunotargeted therapies in the treatment of HCC.
Liang et al’s51 results on the combination of EGCG and Dox could stimulate research on the synergy between EGCG and chemotherapy drugs on cell killing and potentially sensitizing chemoresistant HCC cells to the latter. Furthermore, combinations of EGCG and anticancer drugs can represent a useful strategy to prevent and limit different side effects associated with antineoplastic drug therapy.65,66 In this regard, Huang et al52 showed us that EGCG could be tested in a clinical trial in order to prevent the cardiotoxicity of the anticancer drug Dnr.
Several clinical reports have indicated the use of green-tea-based supplements as agents of liver toxicity.67 In addition, animal studies have indicated that EGCG-induced hepatotoxicity was the effect of increased IL6 activity combined with oxidative stress activation involving hepatic lipid peroxidation, plasma 8-isoprostane, and increased hepatic metallothionein and γ-histone 2AX protein expression.68 On the other hand, decreased superoxide dismutase and glutathione peroxidase levels suggest the potential role of mitochondria-function inhibition due to high doses of EGCG.69 Probably, these alarming data restrain translational approaches. Preclinical research could offer suggestions to overcome these obstacles by the identification of the optimum therapeutic dosage of EGCG for intervention trials in patients suffering from HCC and by investigation into effective strategies to prevent EGCG-induced toxicity. For instance, Hashimoto et al’s study indicated that modification of the 3-position by methylation in EGCG (methyl-EGCG) was effective in cell-growth inhibition at very low oral dosages.55 Finally, according to Garcia et al,70 we believe that a great limitation of the translation of preclinical data on EGCG and other flavonoids into clinical practice is the lack of standardized animal models.
Conclusion
Several preclinical studies reviewed here provide evidence that EGCG induces apoptosis and inhibits HCC progression by acting on different molecular pathways. These promising results encourage the use of EGCG in clinical practice. Despite the EGCG compound being currently used in clinical trials for the treatment of various type of cancer and other diseases,7 no clinical trials have been conducted for patients suffering from HCC. For these reasons, more studies are urgently needed. These studies should be focused on understanding molecular mechanisms regulated by EGCG in HCC, identification of the optimum therapeutic dosage of EGCG to be used in clinical trials, and the identification of more effective strategies to prevent EGCG-induced toxicity.
Acknowledgments
We are grateful to Dr Alessandra Trocino and Mrs Cristina Romano from the National Cancer Institute of Naples for providing excellent bibliographic service and assistance.
Footnotes
Author contributions
This review was written mainly by SB and MC. RP and MC are co-last authors. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Byam J, Renz J, Millis JM. Liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2013;2(1):22–30. doi: 10.3978/j.issn.2304-3881.2012.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention (CDC) hepatocellular carcinoma – United States 2001–2006. MMWR Morb Mortal Wkly Rep. 2010;59(17):517–520. [PubMed] [Google Scholar]
- 3.Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi: 10.2147/JHC.S61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niu L, Liu L, Yang S, Ren J, Lai PBS, Chen GG. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim Biophys Acta. 1868;2017(2):564–570. doi: 10.1016/j.bbcan.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Cascella M, Bimonte S, Muzio MR, Schiavone V, Cuomo A. The efficacy of epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: an overview of pre-clinical studies and translational perspectives in clinical practice. Infect Agent Cancer. 2017;12(1):36. doi: 10.1186/s13027-017-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bimonte S, Leongito M, Barbieri A, et al. Inhibitory effect of (-)-epi-gallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect Agent Cancer. 2015;10(1):22. doi: 10.1186/s13027-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darweish MM, Ebrahim MA, Al-Gayyar MM, Abbas A. Anticancer activity of EGCG against HCC. J Pharm Pharmacol. 2014;66:1032–1045. doi: 10.1111/jphp.12229. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Cao D, Cui W, Ji M, Qian X, Zhong L. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2010;49(12):2010–2018. doi: 10.1016/j.freeradbiomed.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Elias RJ. Antioxidant and pro-oxidant activity of (-)-epigal-locatechin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem. 2013;138(2–3):1503–1509. doi: 10.1016/j.foodchem.2012.09.132. [DOI] [PubMed] [Google Scholar]
- 11.Farhan M, Rizvi A, Naseem I, Hadi SM, Ahmad A. Targeting increased copper levels in diethylnitrosamine induced hepatocellular carcinoma cells in rats by epigallocatechin-3-gallate. Tumor Biol. 2015;36(11):8861–8867. doi: 10.1007/s13277-015-3649-y. [DOI] [PubMed] [Google Scholar]
- 12.Farhan M, Khan HY, Oves M, et al. Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins. 2016;8(2):37. doi: 10.3390/toxins8020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macleod MR, O’Collins T, Howells DW, Donnan GA. Stroke pooling of animal experimental data reveals influence of study design and publication bias. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 14.Bimonte S, Cascella M, Schiavone V, Mehrabi-Kermani F, Cuomo A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: an update on preclinical in vivo studies and future perspectives. Drug Des Devel Ther. 2017;11:2737–2742. doi: 10.2147/DDDT.S142475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan HY, Zubair H, Ullah MF, Ahmad A, Hadi SM. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: implications for a cancer preventive mechanism. Biometals. 2011;24(6):1169–1178. doi: 10.1007/s10534-011-9475-9. [DOI] [PubMed] [Google Scholar]
- 16.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35(1):32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Lambert JD, Lee MJ, Lu H, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003;133(12):4172–4177. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 18.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab Dispos. 2003;31(5):572–579. doi: 10.1124/dmd.31.5.572. [DOI] [PubMed] [Google Scholar]
- 19.Liu AB, Tao S, Lee MJ, et al. Effects of gut microbiota and time of treatment on tissue levels of green tea polyphenols in mice. BioFactors. 2018;44(4):348–360. doi: 10.1002/biof.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Liu Q, Liu L, Hu YY, Feng Q. Potential biological effects of (-)-epigallocatechin-3-gallate on the treatment of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2018;62(1):1700483. doi: 10.1002/mnfr.201700483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MJ, Maliakal P, Chen L. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 22.Altland K, Schreiner R, Hunstein W. Of green tea, black pepper, and amyloidoses. 2010. [Accessed July 13, 2011]. Available from: http://www.ukgm.de/ugm_2/deu/ugi_hum/EGCG_Piperin_engl.pdf.
- 23.Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of poly-phenon E flavan-3-ols in humans with an ileostomy. J Nutr. 2008;138(8):1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 24.Stalmach A, Troufflard S, Serafini M, Crozier A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol Nutr Food Res. 2009;53(Suppl 1):S44–S53. doi: 10.1002/mnfr.200800169. [DOI] [PubMed] [Google Scholar]
- 25.Roowi S, Stalmach A, Mullen W, Lean ME, Edwards CA, Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J Agric Food Chem. 2010;58(2):1296–1304. doi: 10.1021/jf9032975. [DOI] [PubMed] [Google Scholar]
- 26.Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int J Mol Sci. 2011;12(9):5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Taylor LS, Mauer LJ. Degradation kinetics of catechins in green tea powder: effects of temperature and relative humidity. J Agric Food Chem. 2011;59(11):6082–6090. doi: 10.1021/jf200203n. [DOI] [PubMed] [Google Scholar]
- 28.Ishii T, Ichikawa T, Minoda K, et al. Human serum albumin as an antioxidant in the oxidation of (-)-epigallocatechin gallate: participation of reversible covalent binding for interaction and stabilization. Biosci Biotechnol Biochem. 2011;75(1):100–106. doi: 10.1271/bbb.100600. [DOI] [PubMed] [Google Scholar]
- 29.Hunstein W. AL-amyloidosis and epigallocatechin-3-gallate: 4 years and 5 months later. 2011. [Accessed July 13, 2011]. Available from: http://www.hunstein-egcg.de.
- 30.Peters CM, Green RJ, Janle EM, Ferruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int. 2010;43(1):95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giunta B, Hou H, Zhu Y, et al. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci Lett. 2010;471(3):134–138. doi: 10.1016/j.neulet.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur J Pharm Sci. 2010;41(2):219–225. doi: 10.1016/j.ejps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Hu B, Ting Y, Yang X, Tang W, Zeng X, Huang Q. Nanochemoprevention by encapsulation of (-)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun. 2012;48(18):2421–2423. doi: 10.1039/c2cc17295j. [DOI] [PubMed] [Google Scholar]
- 34.Vyas S, Sharma M, Sharma PD, Singh TV, Design STV. Design, semi-synthesis, and evaluation of O-acyl derivatives of (-)-epigallocatechin-3-gallate as antitumor agents. J Agric Food Chem. 2007;55(15):6319–6324. doi: 10.1021/jf070519f. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka H, Miyoshi H, Chuang YC, Ando Y, Takahashi T. Solid-phase synthesis of epigallocatechin gallate derivatives. Angew Chem Int Ed. 2007;46(31):5934–5937. doi: 10.1002/anie.200701276. [DOI] [PubMed] [Google Scholar]
- 36.Nishida H, Omori M, Fukutomi Y, et al. Inhibitory effects of (-)-epigallocatechin gallate on spontaneous hepatoma in C3H/HeNCrj mice and human hepatoma-derived PLC/PRF/5 cells. Jpn J Cancer Res. 1994;85(3):221–225. doi: 10.1111/j.1349-7006.1994.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang G, Miura Y, Yagasaki K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000;159(2):169–173. doi: 10.1016/s0304-3835(00)00545-0. [DOI] [PubMed] [Google Scholar]
- 38.Uesato S, Kitagawa Y, Kamishimoto M, Kumagai A, Hori H, Nagasawa H. Inhibition of green tea catechins against the growth of cancerous human colon and hepatic epithelial cells. Cancer Lett. 2001;170(1):41–44. doi: 10.1016/s0304-3835(01)00571-7. [DOI] [PubMed] [Google Scholar]
- 39.Park HJ, Shin DH, Chung WJ, et al. Epigallocatechin gallate reduces hypoxia-induced apoptosis in human hepatoma cells. Life Sci. 2006;78(24):2826–2832. doi: 10.1016/j.lfs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa T, Nakajima T, Moriguchi M, et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of BCL2 family proteins. J Hepatol. 2006;44(6):1074–1082. doi: 10.1016/j.jhep.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Owusu L, Duan W, et al. Anti-metastatic and differential effects on protein expression of epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma cells. Int J Mol Med. 2013;32(4):959–964. doi: 10.3892/ijmm.2013.1446. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5(5):1227–1238. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Lee KW, Hur HJ, Chun JY, Kim SY, Lee HJ. Phenolic phytochemicals derived from red pine (Pinus densiflora) inhibit the invasion and migration of SK-HEP-1 human hepatocellular carcinoma cells. Ann N Y Acad Sci. 2007;1095(1):536–544. doi: 10.1196/annals.1397.058. [DOI] [PubMed] [Google Scholar]
- 44.Lee SI, Kim HJ, Boo YC. Effect of green tea and (-)-epigallocatechin gallate on ethanol-induced toxicity in HepG2 cells. Phytother Res. 2008;22(5):669–674. doi: 10.1002/ptr.2390. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu M, Shirakami Y, Sakai H, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262(1):10–18. doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Ye HL, Zhang G, et al. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PLoS One. 2014;9(1):e85771. doi: 10.1371/journal.pone.0085771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufmann R, Henklein P, Henklein P, Settmacher U. Green tea poly-phenol epigallocatechin-3-gallate inhibits thrombin-induced hepatocellular carcinoma cell invasion and p42/p44-MAPKinase activation. Oncol Rep. 2009;21(5):1261–1267. doi: 10.3892/or_00000349. [DOI] [PubMed] [Google Scholar]
- 48.Shirakami Y, Shimizu M, Adachi S, et al. (-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100(10):1957–1962. doi: 10.1111/j.1349-7006.2009.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53(9):1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 50.Tong JL, Nie F, Ran ZH, et al. Epigallocatechin gallate induces apoptosis in human hepatocellular carcinoma HepG2 cells via TGF/Smad signaling pathway. Zhonghua Zhong Liu Za Zhi. 2009;31(9):646–650. Chinese. [PubMed] [Google Scholar]
- 51.Liang G, Tang A, Lin X, et al. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int J Oncol. 2010;37(1):111–123. [PubMed] [Google Scholar]
- 52.Huang W, Ding L, Huang Q, et al. Carbonyl reductase 1 as a novel target of (-)-epigallocatechin gallate against hepatocellular carcinoma. Hepatology. 2010;52(2):703–714. doi: 10.1002/hep.23723. [DOI] [PubMed] [Google Scholar]
- 53.Yang XW, Wang XL, Cao LQ, et al. Green tea polyphenol epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell growth inhibition of hepatocellular carcinoma cells. Hepatol Res. 2012;42(5):494–501. doi: 10.1111/j.1872-034X.2011.00947.x. [DOI] [PubMed] [Google Scholar]
- 54.Jin J, Chang Y, Wei W, et al. Prostanoid EP1 receptor as the target of (-)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacologica Sinica. 2012;33(5):701–709. doi: 10.1038/aps.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto O, Nakamura A, Nakamura T, et al. Methylated-(3″)-epigallocatechin gallate analog suppresses tumor growth in Huh7 hepatoma cells via inhibition of angiogenesis. Nutr Cancer. 2014;66(4):728–735. doi: 10.1080/01635581.2013.783601. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Ren X, Deng C, et al. Mechanism of the inhibition of the STAT3 signaling pathway by EGCG. Oncol Rep. 2013;30(6):2691–2696. doi: 10.3892/or.2013.2743. [DOI] [PubMed] [Google Scholar]
- 57.Shen X, Zhang Y, Feng Y, et al. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the Akt pathway. Int J Oncol. 2014;44(3):791–796. doi: 10.3892/ijo.2014.2251. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Duan W, Owusu L, Wu D, Xin Y. Epigallocatechin-3-gallate induces the apoptosis of hepatocellular carcinoma LM6 cells but not non-cancerous liver cells. Int J Mol Med. 2015;35(1):117–124. doi: 10.3892/ijmm.2014.1988. [DOI] [PubMed] [Google Scholar]
- 59.Zapf MA, Kothari AN, Weber CE, et al. Green tea component epigal-locatechin-3-gallate decreases expression of osteopontin via a decrease in mRNA half-life in cell lines of metastatic hepatocellular carcinoma. Surgery. 2015;158(4):1039–1048. doi: 10.1016/j.surg.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wubetu GY, Shimada M, Morine Y, et al. Epigallocatechin gallate hinders human hepatoma and colon cancer sphere formation. J Gastroenterol Hepatol. 2016;31(1):256–264. doi: 10.1111/jgh.13069. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Wu L, Feng J, et al. In vitro and in vivo study of epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic hepatocellular carcinoma cells involving inhibition of phosphofructokinase activity. Sci Rep. 2016;6(1):28479. doi: 10.1038/srep28479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satonaka H, Ishida K, Takai M, et al. (-)-Epigallocatechin-3-gallate Down-regulates Doxorubicin-induced Overexpression of P-glycoprotein Through the Coordinate Inhibition of PI3K/Akt and Mek/ERK Signaling Pathways. Anticancer Res. 2017;37(11):6071–6077. doi: 10.21873/anticanres.12055. [DOI] [PubMed] [Google Scholar]
- 63.Zhao L, Liu S, Xu J, et al. A new molecular mechanism underlying the EGCG-mediated autophagic modulation of AFP in HepG2 cells. Cell Death Dis. 2017;8(11):e3160. doi: 10.1038/cddis.2017.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cascella M, Palma G, Barbieri A, et al. Role of Nigella sativa and its constituent thymoquinone on chemotherapy-induced nephrotoxicity: Evidences from experimental animal studies. Nutrients. 2017;9(6):625. doi: 10.3390/nu9060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cascella M. Chemotherapy-induced peripheral neuropathy: limitations in current prophylactic strategies and directions for future research. Curr Med Res Opin. 2017;33(6):981–984. doi: 10.1080/03007995.2017.1284051. [DOI] [PubMed] [Google Scholar]
- 66.Cascella M. Preoperative cardiac evaluation and anesthetic considerations for cancer patients who underwent chemotherapy. Trends Anaesth Crit Care. 2017;14:9–18. [Google Scholar]
- 67.Jimenez-Saenz M, Martinez-Sanchez MC. Acute hepatitis associated with the use of green tea infusions. J Hepatol. 2006;44(3):616–617. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 68.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48(1):409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.James KD, Kennett MJ, Lambert JD. Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2018;111:302–309. doi: 10.1016/j.fct.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.García ER, Gutierrez EA, Fcsade Melo, Novaes RD, Gonçalves RV. Flavonoids effects on hepatocellular carcinoma in murine models: a systematic review. Evid Based Complement Alternat Med. 2018;2018:6328970. doi: 10.1155/2018/6328970. [DOI] [PMC free article] [PubMed] [Google Scholar]