Abstract
Objective
The aim of this study was to investigate the protective effect and mechanism of Ginkgo biloba extract-761 (EGb 761) in the rat with myocardial ischemia–reperfusion injury (MIRI).
Materials and methods
Forty Sprague Dawley rats were randomly divided into following four groups: sham group, I/R group and EGb 761 groups (20 and 40 mg/kg). MIRI model was established after 14 days of administration. The myocardial infarct size and myocardial histology were measured and compared. Meanwhile, the levels of creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), troponin T (TnT), TNF-α, IL-6, IL-1β, superoxide dismutase (SOD), malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) were evaluated. Western blot was used to detect the expression of Caspase-3, Bax, Bcl-2, HO-1, Nrf2, Akt, p-Akt and nuclear protein Nrf2.
Results
The levels of infarct size, CK-MB, LDH, TnT, TNF-α, IL-6 and IL-1β in the EGb 761 groups were significantly lower than those in the ischemia/reperfusion (I/R) group. The content of MDA was lower in the myocardium, whereas the activities of SOD and GSH-Px were higher than those in the I/R group. The expressions of Caspase-3 and Bax in the EGb 761 groups were significantly lower than those in the I/R group, whereas the expressions of Bcl-2, p-Akt and HO-1 and nuclear protein Nrf2 in the EGb 761 groups were higher than those in the I/R group.
Conclusion
EGb 761 might inhibit the apoptosis of myocardial cells and protect the myocardium by activating the Akt/Nrf2 pathway, increasing the expression of HO-1, decreasing oxidative stress and repressing inflammatory reaction.
Keywords: Ginkgo biloba extract, myocardial ischemia-reperfusion injury, oxidative stress, superoxide dismutase
Introduction
Myocardial ischemia–reperfusion injury (MIRI) refers to the aggravation of metabolic disorder in the process of reperfusion after myocardial ischemia, causing myocardial ultrastructural damage. It is a common type of myocardial injury in clinic. The pathology and pathogenesis of MIRI are complex, including oxygen-free radical injury, inflammatory reaction, energy metabolism disturbance and microvascular permeability.1,2 In recent years, a large number of evidence showed that the body was in oxidative stress state after MIRI, which increased the formation of reactive oxygen species, further resulting in oxidative stress damage to cells.3,4 Therefore, inhibiting the oxidative stress effectively was an important way to reduce MIRI and protect myocardial tissue. Some research studies indicated that PI3K–Akt signaling pathway played an important role in the expression of nuclear factor Nrf2 and downstream antioxidant gene in the antioxidation process.5,6 And the activities of GSH-Px and SOD were enhanced by the activated Nrf2 pathway, decreasing the oxidative stress and inflammatory response, inhibiting the cell apoptosis and tissue damage.7,8
Ginkgo biloba extract-761 (EGb 761), whose main components are ginkgo flavonoids and ginkgolides, is a natural platelet activation factor antagonist. EGb 761 has many pharmacological effects, such as inhibiting platelet aggregation, scavenging free radicals and protecting micro-vascular endothelial cells. Meanwhile, it could also mediate smooth muscle relaxation in blood vessels and improve ischemia and tissue metabolism.9 Clinical research showed that EGb 761 had been widely used in the treatment of various cardiovascular and cerebrovascular diseases, and obtained significant effect on preventing MIRI.10 However, the molecular mechanism of EGb 761 against MIRI had not been well elucidated. In this study, EGb761 was pretreated MIRI rat model to explore the protective effect of EGb761 on myocardium and whether the effect achieved through regulating the Akt/Nrf2/HO-1 pathway.
Materials and methods
Animals
Forty male Sprague Dawley (SD) rats weighed 200–220 g were provided by the experimental animal center of Zhengzhou University. The animal feeding operations were compliant with the Laboratory animal guideline for the ethical review of animal welfare (GB/T 35892-2018). All animal experiments were examined and approved by the ethics committee of Zhengzhou University.
Main reagents
Ginaton® tablets contained 24% of flavonol glycosides and 6% of terpene trilactones (EGb 761, 40 mg/tablet, registration number: H20140768, Dr Willmar Schwabe GmbH & Co.KG). Troponin T (TnT), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px) assay kits and TNF-α, IL-6 and IL-1β ELISA kits were purchased from American R&D Systems. Antibodies against Casp-3, Bax, Bcl-2, HO-1, Nrf2, Akt and p-Akt were purchased from British Abcam Corporation.
Grouping and modeling
All the SD rats were randomly divided into four groups (ten rats in each group): 1) sham group treated with saline but without ischemia/reperfusion injury (MIRI); 2) MIRI group treated with saline alone; 3) low-dose group treated with 20 mg/kg EGb 761 and 4) high-dose group treated with 40 mg/kg EGb 761. Before modeling, the rats in the sham and MIRI groups were given 1 mL saline (once a day) by gavage for 14 days, whereas the rats in the two EGb 761 groups were administered with 1 mL EGb 761 solution (20 or 40 mg/kg, once a day) by gavage for 14 days, respectively. One hour after the last gavage, the rats were anesthetized with 2% pentobarbital sodium and fixed in supine position. Endotracheal intubation was connected with an animal ventilator. The heart was exposed through the left thoracotomy. Then, the snare encircling the coronary artery was used for occlusion by pulling up on the suture which was clamped with plastic tubes at the left ventricular. Rats were subjected to a coronary artery occlusion for 45 minutes followed by 2 hours reperfusion by releasing the clamp. The rats in the sham group were exposed through the coronary artery for a time-matched normal perfusion without ligation. Observing the changes of the electrocardiogram: Elevated ST segment meant myocardial ischemia. After releasing, the elevated ST segment dropped more than half, the amplitude of R wave decreased, the arrhythmia or accompanied by Q waves was determined successful in reperfusion. At the end of reperfusion, 2 mL of blood was collected from the abdominal aorta and the left ventricle was obtained for detection of myocardial infarction size and other analysis.
Detection of myocardial infarction size
After reperfusion, the hearts of rats were removed rapidly and cryopreserved at -80°C for 10 minutes. Then, the frozen hearts were sliced transversely (perpendicular to the long axis of heart) into 1–1.5 mm sections. The sections were incubated at 37°C for 20 minutes in 1% triphenyltetrazolium chloride in PBS solution, then fixed with 4% poly-formaldehyde solution for 8 hours. The images were captured and analyzed. The size of myocardial infarction was calculated by image analysis software Image Proplus 6.0.
Histological observation of myocardial tissue
After the rats were sacrificed, the hearts were quickly removed and fixed in 10% formalin solution. The left ventricular tissue of the heart was paraffin embedded, sectioned. The sections were stained with H&E, and the histological changes of myocardium were observed using an optical microscope.
Detection of CK-MB, LDH, TnT and pro-inflammatory cytokines in serum
At the end of reperfusion, 2 mL of blood was collected from the abdominal aorta. The serum was separated by centrifugation (2,000 rpm, 5 minutes). To evaluate the myocardial cellular damage, the serum creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) were measured by AU5800 automatic biochemical analyzer (Beckman Coulter), and the level of TnT and pro-inflammatory cytokines, such as IL-6, IL-1β and TNF-α, in the serum was detected by ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Oxidative stress biomarkers
The myocardium tissue of each group was homogenized to detect SOD and GSH-Px activities and MDA contents. The activity of SOD was measured by xanthine oxidase method; the activity of GSH-Px was determined by dithio-dinitrotoluidine method and the content of MDA was determined by thiobarbituric acid method. Detailed process was performed according to the protocol provided by the manufacturer.
Cell apoptosis rate in myocardium tissue by TUNEL
The cell apoptosis rate in myocardium tissue was detected by ApopTag® fluorescein in situ cell death detection kit after being fixed in 4% phosphate-buffered paraformaldehyde overnight. The nucleus was colored blue by DAPI, and the apoptosis cells were colored green. The cell color was observed in spectromicroscope, and the apoptosis rate was the percentage of positive cells in all DAPI-colored cells. Average value was taken from five views.
Related proteins’ detection by Western blot
Nuclear protein and cytoplasmic protein were extracted from heart tissue using nuclear and cytoplasm extraction kits, respectively, according to the manufacturer’s instructions. The protein concentration was determined by bicinchoninic acid methods. Forty micrograms of protein samples was loaded and separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked with skim milk (5%) at room temperature for 2 hours. Then the membranes were incubated with primary antibodies, anti-Bax, anti-Bcl-2, anti-Caps-3, anti-HO-1, anti-Nrf2, anti-Akt, anti-p-Akt and anti-β-actin (1:1,000) at 4°C overnight. Following primary antibody incubations, the secondary antibodies (anti-rabbit or anti-mouse IgG [1:2,000]) were incubated for 1 hour at room temperature. The bands were visualized using enhanced chemiluminescence detection reagents and gel imaging system. The gray value ratio of the target band and the β-actin band was calculated by ImageJ to represent the relative expression of the target protein.
Statistical analyses
All data were analyzed by SPSS 19.0. Measurement data in accordance with the normal distribution were shown as mean ± SD (x ± –s). The comparison between two groups was conducted by independent sample t-test, whereas the comparison among multiple groups was analyzed using one-way ANOVA, P<0.05 represented the significant difference.
Results
Myocardial infarct size and myocardial injury
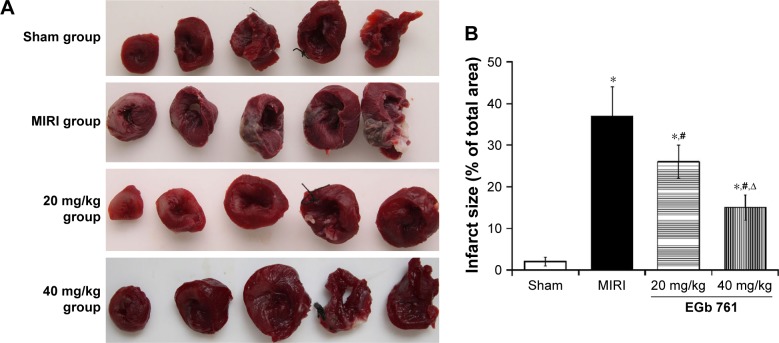
As shown in Figure 1, the myocardial infarct size percentage in the MIRI group was significantly higher than that in the sham group and pretreatment with 20 mg/kg EGb 761 or 40 mg/kg EGb 761 groups could significantly decrease the percentage of myocardial infarct size of MIRI rats (P<0.05). In Table 1, the levels of CK-MB, LDH and TnT in the MIRI group were remarkably higher than those in the sham group. When compared with the MIRI group, the levels of CK-MB, LDH and TnT in the EGb 761 groups were decreased (P<0.05), and the decrease in the high-dose group was more obvious. The results suggested that EGb 761 could reduce myocardial infarct size and protect myocardial tissue.
Figure 1.
Effect of EGb 761 on infarct size.
Notes: (A) TTC staining result of myocardial tissue in each group; (B) comparison of myocardial infarct size among groups. *Compared with the sham group, P<0.05; *compared with the MIRI group, P<0.05, ∆compared with the 20 mg/kg EGb 761 group, P<0.05.
Abbreviations: EGb 761, Ginkgo biloba extract-761; MIRI, myocardial ischemia–reperfusion injury; TTC, triphenyltetrazolium chloride.
Table 1.
The level of myocardial injury serum markers (x±–s)
| Groups | CK-MB (U/L) | LDH (U/L) | TnT (ng/mL) |
|---|---|---|---|
|
| |||
| Sham group | 44.95±10.24 | 117.28±15.31 | 0.07±0.03 |
| MIRI group | 138.51±19.03a | 226.77±29.24a | 1.69±0.17a |
| 20 mg/kg group | 99.82±12.43a,b | 170.63±19.43a,b | 0.96±0.96a,b |
| 40 mg/kg group | 73.54±11.46a,b,c | 142.50±14.27a,b,c | 0.41±0.09a,b,c |
| F | 396.216 | 52.565 | 12.351 |
| P | <0.001 | <0.001 | <0.001 |
Notes:
Compared with the sham group, P<0.05.
Compared with the MIRI group, P<0.05.
Compared with 20 mg/kg group, P<0.05.
Abbreviations: CK-MB, creatine kinase-MB; LDH, lactate dehydrogenase; MIRI, myocardial ischemia–reperfusion injury; TnT, troponin T.
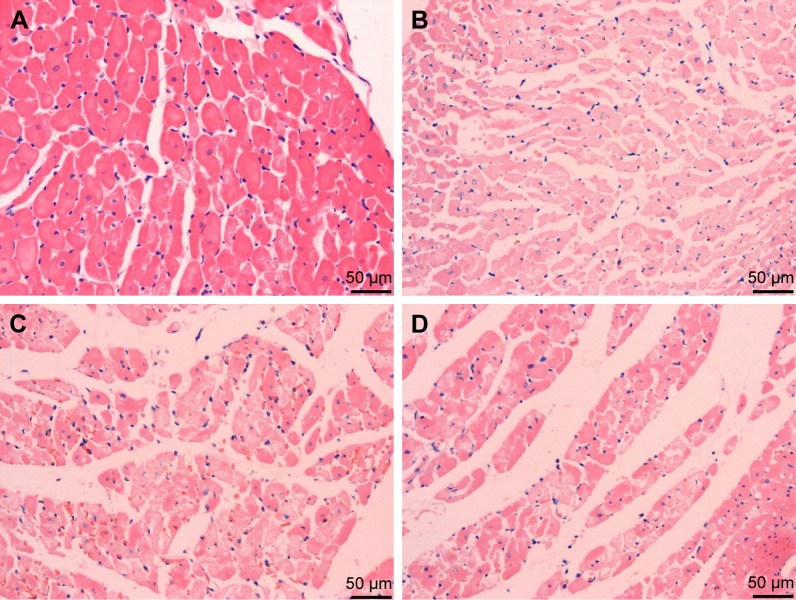
Myocardial histology
H&E staining of myocardial histology is shown in Figure 2. Compared with the sham group, the myocardial tissue damage and infiltration of inflammatory cells were observed in the MIRI group, in which the myocardial cells appeared edema and disorderly arranged. However, the degree of myonecrosis, edema and infiltration of inflammatory cells was reduced in the EGb 761 groups compared with the MIRI group.
Figure 2.
H&E staining of myocardial histology.
Notes: (A) Sham group; (B) MIRI group; (C) low dose group (EGb 761 20 mg/kg); (D) high dose group (EGb 761 40 mg/kg). Compared with the MIRI group, the degree of myonecrosis, edema and infiltration was reduced pretreatment with EGb 761 (scale bar: 50 µm).
Abbreviations: EGb 761, Ginkgo biloba extract-761; MIRI, myocardial ischemia–reperfusion injury.
Pro-inflammatory cytokines
The levels of pro-inflammatory cytokines in each group are shown in Table 2. The levels of TNF-α, IL-6 and IL-1β in the MIRI group were significantly higher than those in the sham group (P<0.05). However, compared with the MIRI group, the levels of TNF-α, IL-6 and IL-1β in the groups pretreated with EGb 761 were significantly decreased (P<0.05). The levels of pro-inflammatory cytokines in the high-dose group were lower than those in the low-dose group.
Table 2.
The level of serum pro-inflammatory cytokines (x±s)
| Groups | TNF-α (pg/mL) | IL-6 (pg/mL) | IL-1α (pg/mL) |
|---|---|---|---|
|
| |||
| Sham group | 58.80±10.01 | 25.78±5.01 | 23.44±4.30 |
| MIRI group | 96.08±15.36a | 66.09±9.34a | 54.43±9.51a |
| 20 mg/kg group | 85.13±13.57a | 45.89±7.09a,b | 44.98±7.29a,b |
| 40 mg/kg group | 68.37±9.60a,b | 37.83±6.07a,b,c | 36.47±6.96a,b,c |
| F | 16.980 | 61.789 | 39.324 |
| P | <0.001 | <0.001 | <0.001 |
Notes:
Compared with sham group, P<0.05.
Compared with the MIRI group, P<0.05.
Compared with 20 mg/kg group, P<0.05.
Abbreviation: MIRI, myocardial ischemia–reperfusion injury.
Oxidative stress indexes
As shown in Table 3, compared with the sham group, the content of MDA was increased, whereas the activities of antioxidant enzymes SOD and GSH-Px were significantly decreased in the MIRI group. In the groups pretreated with EGb 761, MDA content was lower and the activities of SOD and GSH-Px were higher than those in the MIRI group (P<0.05), and it was more obvious in the high-dose group. The results showed that the groups pretreated with EGb 761 could enhance the antioxidant enzymes activities and decrease the level of oxidative stress in myocardial tissue, and the effect may be dose dependent.
Table 3.
The level of serum oxidative stress indexes (x±–s)
| Groups | SOD (U/mL) | MDA (nmol/mL) | GSH-Px (U/mL) |
|---|---|---|---|
|
| |||
| Sham group | 202.57±12.02 | 2.38±0.41 | 429.51±52.34 |
| MIRI group | 103.12±10.12a | 5.68±0.73a | 326.80±39.51a |
| 20 mg/kg group | 145.06±11.23a,b | 3.79±0.37a,b | 389.51±31.34a,b |
| 40 mg/kg group | 165.36±9.35a,b,c | 3.03±0.34a,b,c | 414.02±37.54b |
| F | 220.200 | 92.629 | 16.857 |
| P | <0.001 | <0.001 | <0.001 |
Notes:
Compared with the sham group, P<0.05.
Compared with the MIRI group, P<0.05.
Compared with the 20 mg/kg group, P<0.05.
Abbreviations: GSH-Px, glutathione peroxidase; MDA, malondialdehyde; MIRI, myocardial ischemia–reperfusion injury; SOD, superoxide dismutase.
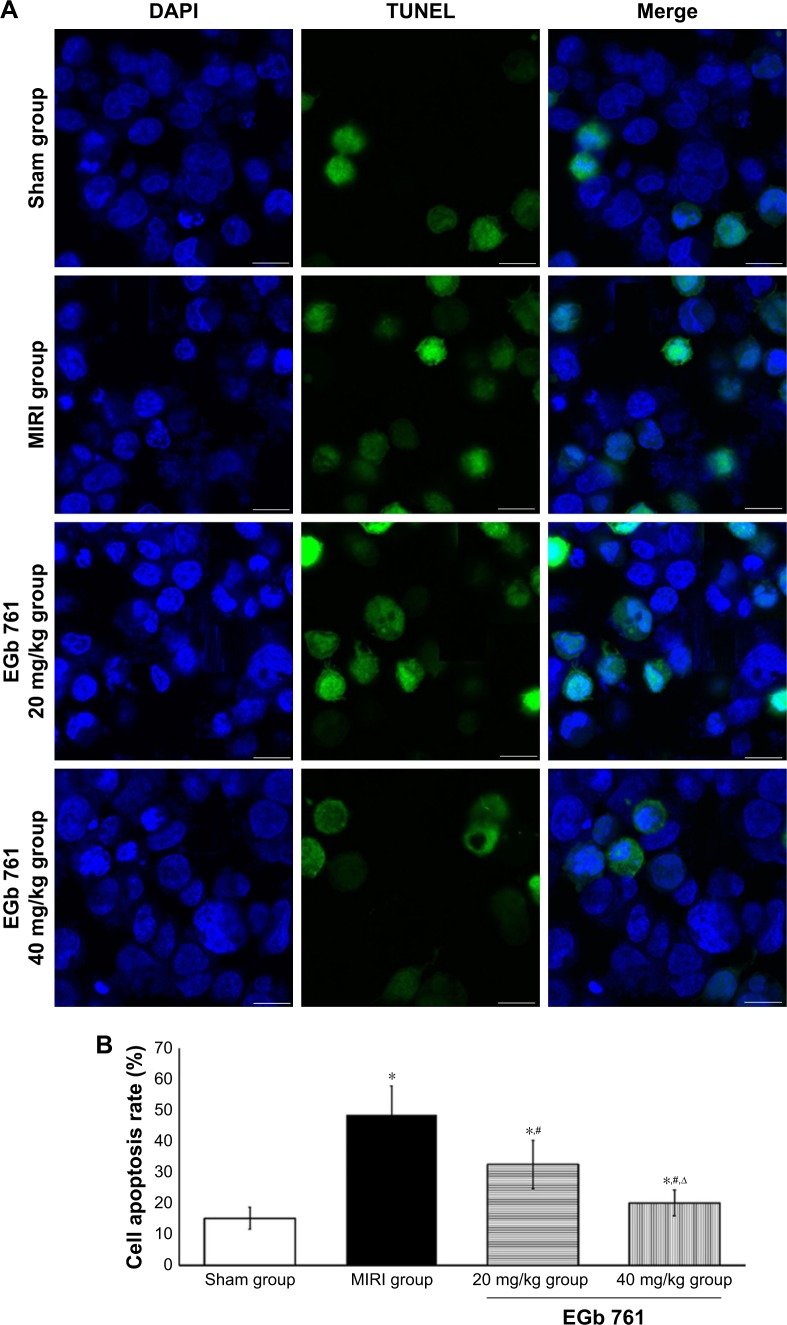
Cell apoptosis rate by TUNEL
The results of cell apoptosis rate in myocardial tissue detected by TUNEL are shown in Figure 3. It could be seen that the apoptosis rate of the MIRI group was higher than those in the sham group, and that of the EGb 761 groups was lower compared with the MIRI group. The results indicated that there was some protective effect of pretreatment with EGb 761 on myocardial tissue.
Figure 3.
Cell apoptosis observation by TUNEL.
Notes: (A) Cell apoptosis detection by TUNEL; scale bar: 25 µm; (B) comparison of apoptosis rate among groups, *compared with the sham group, P<0.05; #compared with the MIRI group, P<0.05, ∆compared with the 20 mg/kg group, P<0.05.
Abbreviations: EGb 761, Ginkgo biloba extract-761; MIRI, myocardial ischemia–reperfusion injury.
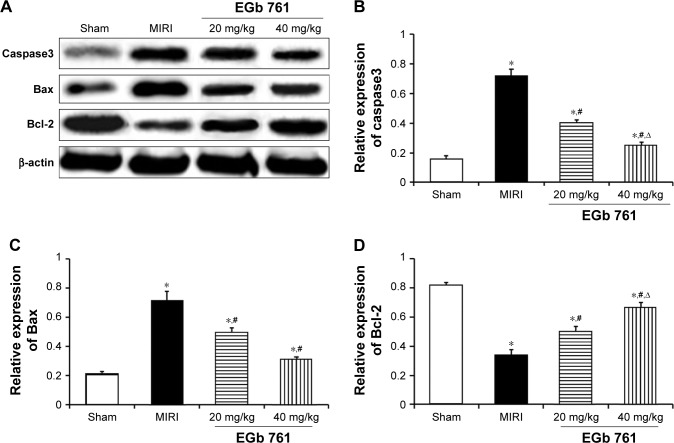
Expression of Caspase3, Bax and Bcl-2 protein
Compared with the sham group, the expression of Caspase3 and Bax was significantly increased in the myocardium tissue of the MIRI group, whereas the expression of Bcl-2 was significantly decreased (Figure 4). The expressions of Caspase3 and Bax in EGb 761 groups were significantly lower than those in the MIRI group, whereas the expression of Bcl-2 in EGb 761 was higher than that in the MIRI group (P<0.05), and were in a dose-dependent manner.
Figure 4.
Effect of EGb 761 on myocardium Caspase3, Bax, Bcl-2 protein expression.
Notes: (A) Result of Western blot; (B) comparison of Caspase3 expression level among groups; (C) comparison of Bax expression level among groups; (D) comparison of Bcl-2 expression level among groups. *Compared with the sham group, P<0.05; #compared with the MIRI group, P<0.05; ∆compared with the 20 mg/kg group, P<0.05.
Abbreviations: EGb 761, Ginkgo biloba extract-761; MIRI, myocardial ischemia–reperfusion injury.
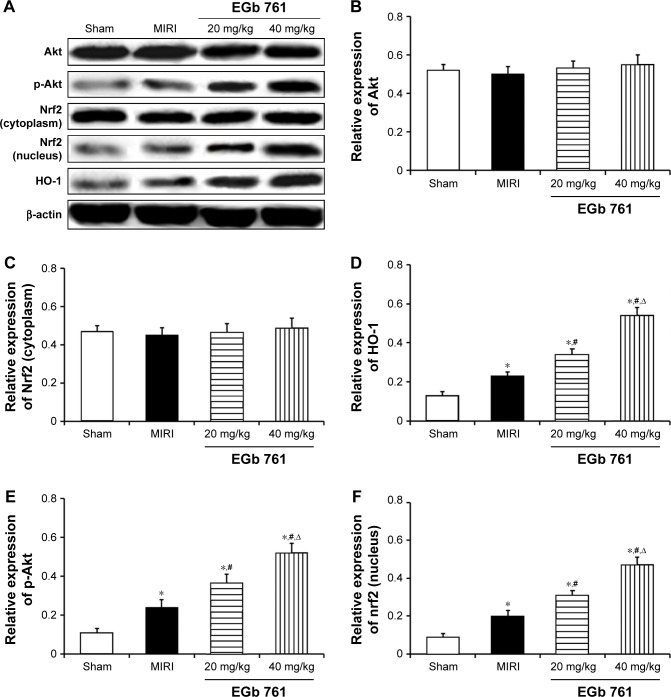
Expression of protein related to Akt/Nrf2 signaling pathway
The expression of Akt/Nrf2/HO-1 pathway-related proteins was detected to explore the molecular mechanism of EGb 761 in myocardium protection effect. There was no significant difference in the expression of cytoplasmic proteins Akt and Nrf2 in the sham group, MIRI group and EGb 761 groups (Figure 5). Compared with the sham group, the expressions of cytoplasmic proteins p-Akt, HO-1 and nuclear protein Nrf2 were significantly decreased in the MIRI group, which were regulated by oxidative stress. When compared with the MIRI group, pretreatment with EGb 761 (20 or 40 mg/kg) showed that the expressions of cytoplasmic proteins p-Akt, HO-1 and nuclear protein Nrf2 were gradually increased and the differences were statistically significant (P<0.05). The results indicated that EGb 761 could protect the myocardium by activating the Akt/Nrf2 pathway, and the expression of downstream antioxidant-associated protein HO-1.
Figure 5.
Effect of EGb 761 on myocardium Akt, p-Akt, Nrf2 and HO-1 protein expression.
Notes: (A) Result of Western blot; (B) comparison of Akt expression level among groups; (C) comparison of p-Akt expression level among groups; (D) comparison of Nrf2 (cytoplasm) expression level among groups; (E) comparison of Nrf2 (nucleus) expression level among groups; (F) comparison of HO-1 expression level among groups. *Compared with the sham group, P<0.05; #compared with the MIRI group, P<0.05; ∆compared with the 20 mg/kg group, P<0.05.
Abbreviations: EGb 761, Ginkgo biloba extract-761; MIRI, myocardial ischemia–reperfusion injury.
Discussion
MIRI is closely related to the occurrence and development of various clinical cardiovascular diseases and its pathophysiological is complex. Research studies showed that oxidative stress and inflammatory reactions were the main causes of MIRI. Under normal conditions, the inflammatory and anti-inflammatory reactions, the oxidation and anti-oxidant system of the myocardium were in the dynamic balance state. During myocardial ischemia–reperfusion, a large amount of pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β, were released with the aggravated inflammatory reaction and a large amount of reactive oxygen species and MDA were produced, decreasing the activities of endogenous antioxidants SOD and GSH-Px. The balance state was broken, which ultimately damaged the myocardial cell, inducing apoptosis and necrosis.11–13 The present results found that the myocardial tissue in the MIRI group was seriously damaged compared with the sham group. The levels of TNF-α, IL-6, IL-1β and MDA in the MIRI group were significantly higher than those in the sham group, whereas the activities of SOD and GSH-Px were significantly lower than those in the sham group. The results suggested that myocardial ischemia–reperfusion aggravated the inflammatory reaction, weakened the antioxidant capacity and led to myocardial tissue damage and necrosis.
EGb 761 as an effective medicinal ingredient is widely used against ischemia injury in lung, brain, kidney and other organ diseases. Some studies showed that EGb 761 could promote vasodilation, improve microcirculation, regulate blood lipids, reduce the aggregation of platelets mediated by inflammatory mediators and affect the thrombosis.14–16 The reductive group of EGb 761 could directly scavenge oxygen-free radicals, reduce the content of MDA and improve the activity of antioxidant enzyme SOD. Meanwhile, it also upregulated the ability of oxygen-free radical scavenging system, making the formation/clearance of oxygen-free radical balance, reducing ischemia–reperfusion injury.17,18 In the study, the myocardial infarct size and the levels of CK-MB, LDH and TnT were significantly reduced in EGb 761 groups, which were in a dose-dependent manner. The levels of TNF-α, IL-6, IL-1β and MDA were decreased, whereas the activities of SOD and GSH-Px were significantly increased in the EGb 761 groups (P<0.05), compared with the MIRI group. The results indicated that EGb 761 could reduce the inflammatory response and oxidative stress reaction, improve the body’s antioxidant capacity, reduce myocardial ischemia–reperfusion injury and protect the myocardial tissue in MIRI rats. In addition, the effect of the high-dose group was more obvious. The result of expression of apoptosis-related proteins also confirmed that EGb 761 could inhibit the apoptosis of cardiac myocytes, which was consistent with the existing research results.19,20
Nrf2 pathway, as an important endogenous antioxidant stress pathway, played a key role in oxidative stress response.21 The PI3K/Akt signaling pathway was involved in the activation of nuclear factor Nrf2 and the expression of downstream antioxidant genes in oxidative stress.22,23 The Nrf2 was phosphorylated by Akt to activate and release from Keap1 and transferred to the nucleus, combining with the antioxidant reaction element, upregulating the expression of the Phase II detoxifying enzyme and antioxidant gene, improving the activity of antioxidant enzymes GSH-Px and SOD, responding to antioxidant stress.24,25 HO-1 was a small heat shock protein that could catalyze the degradation of hemoglobin to produce bilirubin, CO and Fe2+. The four factors formed the endogenous protective system for the antioxidative stress damage, which weakened the oxidative stress and inflammatory response, and inhibited the expression of the apoptotic protein, playing a role in antiapoptosis.26 In this study, the expression of nuclear protein Nrf2 and cytoplasmic protein Nrf2, Akt, p-Akt and HO-1 was detected to explore the molecular mechanism of EGb 761-protecting myocardium. Results showed that EGb 761 could somehow induce phosphorylation of Akt to activate Akt, promoting the Nrf2 transferred into nuclear to upregulate the expression of HO-1, inhibiting oxidative stress response and protecting the myocardium.
Conclusion
EGb 761 had an obvious protective effect against myocardial ischemia–reperfusion injury in the rat model. The mechanism may be that EGb 761 upregulated the expression of HO-1 by activating the Akt/Nrf2 pathway, reducing oxidative stress and inflammatory reaction and inhibiting the myocardial cell apoptosis to protect the myocardium.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell RM, Yellon DM. There is more to life than revascularization: therapeutic targeting of myocardial ischemia/reperfusion injury. Cardiovasc Ther. 2011;29(6):e67–e79. doi: 10.1111/j.1755-5922.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- 3.Ji L, Fu F, Zhang L, et al. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/nitrative stress. Am J Physiol Endocrinol Metab. 2010;298(4):E871–E880. doi: 10.1152/ajpendo.00623.2009. [DOI] [PubMed] [Google Scholar]
- 4.Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):RA209–RA219. [PubMed] [Google Scholar]
- 5.Hamdulay SS, Wang B, Birdsey GM, et al. Celecoxib activates PI-3K/Akt and mitochondrial redox signaling to enhance heme oxygenase-1-mediated anti-inflammatory activity in vascular endothelium. Free Radic Biol Med. 2010;48(8):1013–1023. doi: 10.1016/j.freeradbiomed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Sun Q, Shen ZY, Duan WN, Meng QT, Xia ZY. Mechanism of myocardial ischemia/reperfusion-induced acute kidney injury through DJ-1/Nrf2 pathway in diabetic rats. Exp Ther Med. 2017;14(5):4201–4207. doi: 10.3892/etm.2017.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erkens R, Suvorava T, Sutton TR, et al. Nrf2 deficiency unmasks the significance of nitric oxide synthase activity for cardioprotection. Oxid Med Cell Longev. 2018;2018(3):1–15. doi: 10.1155/2018/8309698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi C, Liu J, Wu F, Yew DT. Ginkgo biloba extract in Alzheimer’s disease: from action mechanisms to medical practice. Int J Mol Sci. 2010;11(1):107–123. doi: 10.3390/ijms11010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanter M. Protective effects of Ginkgo biloba (EGb 761) on testicular torsion/detorsion-induced ischemia-reperfusion injury in rats. Exp Mol Pathol. 2011;91(3):708–713. doi: 10.1016/j.yexmp.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Jin Z, Zhao R, Ren K, Deng C, Yu S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med. 2015;8(7):10420–10428. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Chen J, Zhao J, Meng M. Etanercept attenuates myocardial ischemia/reperfusion injury by decreasing inflammation and oxidative stress. PLoS One. 2014;9(9):e108024. doi: 10.1371/journal.pone.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawaguchi M, Takahashi M, Hata T, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 14.Gargouri B, Carstensen J, Bhatia HS, Huell M, Dietz GPH, Fiebich BL. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine. 2018;44:45–55. doi: 10.1016/j.phymed.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Liu MY, Zhang LJ. Role of Ginkgo biloba extract in regulating 5-hydroxytrytamine and its receptor in heart failure mice. Zhonghua Yi Xue Za Zhi. 2018;98(25):2024–2029. doi: 10.3760/cma.j.issn.0376-2491.2018.25.012. Chinese. [DOI] [PubMed] [Google Scholar]
- 16.Qin Y, Zhang Y, Tomic I. Ginkgo biloba extract EGb 761 and its specific components elicit protective protein clearance through the autophagy-lysosomal pathway in Tau-Transgenic mice and cultured neurons. J Alzheimers Dis. 2018;65(1):243–263. doi: 10.3233/JAD-180426. [DOI] [PubMed] [Google Scholar]
- 17.Mansour SM, Bahgat AK, El-Khatib AS, Khayyal MT. Ginkgo biloba extract (EGb 761) normalizes hypertension in 2K, 1c hypertensive rats: role of antioxidant mechanisms, ACE inhibiting activity and improvement of endothelial dysfunction. Phytomedicine. 2011;18(8–9):641–647. doi: 10.1016/j.phymed.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Z, Liu N, Huang J, Lu PH, Xu XM. Inhibition of cPLA2 activation by Ginkgo biloba extract protects spinal cord neurons from glutamate excitotoxicity and oxidative stress-induced cell death. J Neurochem. 2011;116(6):1057–1065. doi: 10.1111/j.1471-4159.2010.07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Zhou G, Yao L, et al. Protective effect of Ginkgo biloba leaves extract, EGb761, on myocardium injury in ischemia reperfusion rats via regulation of TLR-4/NF-κB signaling pathway. Oncotarget. 2017;8(49):86671–86680. doi: 10.18632/oncotarget.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ran K, Yang DL, Chang YT, et al. Ginkgo biloba extract postconditioning reduces myocardial ischemia reperfusion injury. Genet Mol Res. 2014;13(2):2703–2708. doi: 10.4238/2014.April.8.14. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Peng L, Yu X. Protective effect of hydrogen sulfide on rats with myocardial ischemia/reperfusion injury and its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(3):316–320. Chinese. [PubMed] [Google Scholar]
- 22.Yin X, Wang X, Fan Z, et al. Hyperbaric oxygen preconditioning attenuates myocardium ischemia-reperfusion injury through upregulation of heme oxygenase 1 expression. J Cardiovasc Pharmacol Ther. 2015;20(4):428–438. doi: 10.1177/1074248414568196. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen CN, Kim HE, Lee SG. Caffeoylserotonin protects human keratinocyte HaCaT cells against H2O2-induced oxidative stress and apoptosis through upregulation of HO-1 expression via activation of the PI3K/Akt/Nrf2 pathway. Phytother Res. 2013;27(12):1810–1818. doi: 10.1002/ptr.4931. [DOI] [PubMed] [Google Scholar]
- 24.Hu T, Wei G, Xi M, et al. Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway. Int J Mol Med. 2016;38(1):83–94. doi: 10.3892/ijmm.2016.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan D, Dong J, Sulik KK, Chen SY. Induction of the Nrf2-driven antioxidant response by tert-butylhydroquinone prevents ethanol-induced apoptosis in cranial neural crest cells. Biochem Pharmacol. 2010;80(1):144–149. doi: 10.1016/j.bcp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He M, Pan H, Chang RC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014;9(1):e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]