Abstract
Purpose
Traditionally, radiosurgery was considered less effective for patients with cystic brain metastases. However, comparisons of prognosis between cystic and solid brain metastases in cancer patients have been seldom reported. We aimed to compare the survival between cystic and solid brain metastases and assess risk factors for overall survival after brain metastases (BMOS) in patients who underwent radiosurgery treatment.
Patients and methods
The Kaplan–Meier method and multivariate Cox regression analysis were used to compare survival time and evaluate risk factors for BMOS.
Results
A total of 356 patients (including 498 brain metastases) were analyzed in our study, including 67 patients (67/356, 18.8%) with 75 cystic brain metastases. There is no statistical significance in BMOS between patients with cystic (17 months, range: 3–64 months) and solid (17.5 months, range: 1–65 months) brain metastases (P=0.148). However, the volume of cystic brain metastases decreased more slowly than solid brain metastases (P<0.05). The results indicated that high recursive partitioning analysis classification (P=0.006), large volume of brain metastases (P=0.006), and different primary lesion (especially gastrointestinal tract tumor) (P=0.001) were associated with poor prognosis in patients with brain metastases.
Conclusion
There is no difference in prognosis and local control between patients with cystic and solid brain metastases who underwent radiosurgery. However, the rate and speed of tumor shrinkage were lower in cystic brain metastases after radiotherapy. Patients with larger brain metastases had shorter survival time, regardless of cystic or solid brain metastases.
Keywords: cystic brain metastases, radiosurgery treatment, tumor shrinkage, overall survival after brain metastases, risk factors
Introduction
Brain metastases are the most common intracranial tumor in adults and an important factor in shortening the lives of patients with malignant tumor.1–3 In particular, the incidence of brain metastases has increased in recent years due to the developing technologies and prolonged survival time in cancer patients.4 Brain metastases mainly originate from lung cancer, breast cancer, kidney cancer, melanoma, and gastrointestinal tumors. Approximately 15%–20% of patients with non-small-cell lung cancer (NSCLC) and 60%–80% small cell lung cancer (SCLC) patients who have survived more than 2 years will develop brain metastasis.5,6 However, the survival of lung cancer patients with brain metastases is very poor, and they can only survive 1–2 months without treatment.
Developing treatment options, which include surgery, whole brain radiation therapy (WBRT), stereotactic radiotherapy, chemotherapy, and targeted therapy have greatly extended the survival time of patients with brain metastases.7,8 Stereotactic radiosurgery (SRS) has been a routine and effective treatment option for oligometastasis (less than or equal to five brain metastases).9–11 CyberKnife, as a relatively new SRS system, has expanded rapidly worldwide because of its precise positioning, noninvasiveness, and high local control rate.10,12,13 Even so, cystic brain metastases are often deemed not suitable for radiation, which includes CyberKnife, because of cystic lesion insensitivity to radiotherapy and large volume.14,15
Traditionally, radiosurgery was considered less effective for patients with cystic brain metastases, and they had shorter survival time than patients with solid brain metastases. However, the comparisons of prognosis between cystic and solid brain metastases in cancer patients have been seldom reported. In this study, we aimed to analyze the survival difference and treatment response to radiosurgery treatment in these two groups and investigate factors related to local control and overall survival after brain metastases (BMOS).
Patients and methods
Patient characteristics
We reviewed 426 patients with brain metastases who had undergone radiosurgery treatment at the Tianjin Cancer Hospital and Institute from January 2012 to December 2016. All patients were assessed by radiation oncologists and physicists. The inclusion criteria were as follows: 1) definite histopathological diagnosis for primary lesion, 2) newly diagnosed brain metastases, 3) without meningeal metastases, 4) patient received radiosurgery treatment, and 5) integrated clinical and follow-up data. At last, a total of 356 eligible patients were included and analyzed in our study. The study was approved by Tianjin Medical University Cancer Institute and Hospital’s Ethics Committee and was conducted in strict accordance with the principles of the Declaration of Helsinki. We also obtained the waiver for individual patients’ written informed consent for our retrospective study from the committee. To maintain patients’ confidentiality, all clinical data and laboratory results were collected anonymously. All data and records for patients were confidential and individuals outside the research team had no access to them.
Radiosurgical technique
All patients received treatment using CyberKnife (Accuray Inc., Sunnyvale, CA, USA). The CyberKnife is equipped with a 6 MV linear accelerator, which is mounted on a computer-controlled robotic arm. All patients were in the supine position, and a thermoplastic mask was molded to the head. The contrast-enhanced magnetic resonance imaging (MRI) and computed tomography (CT) images of 1.5 mm slice thickness were obtained and fused for treatment planning. We defined gross target volume (GTV) as the enhanced lesion on contrast-enhanced MRI and extended the GTV by 1.25 mm to generate the planning target volume (PTV).
Imaging data
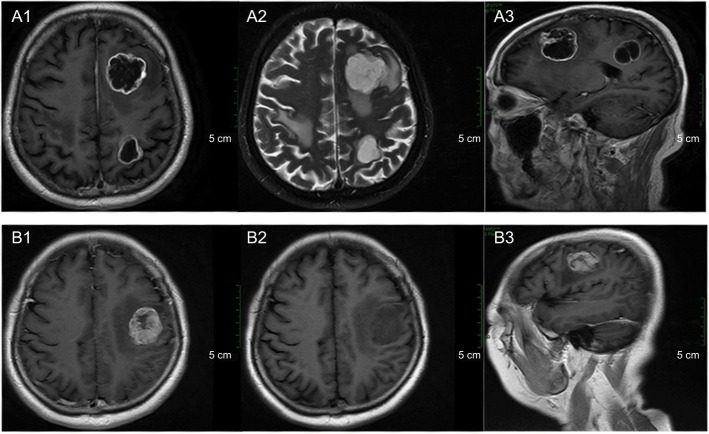
In our study, we defined cystic brain metastasis as the volume of the cystic lesions greater than 50% of the total volume. The typical MRI characteristics were present as shown in Figure 1. Contrast-enhanced MRI is necessary for diagnosing cystic brain metastases. The cystic components revealed hypointense on T1-weighted images, hyperintense on T2-weighted images, and no enhancement.16 Enhanced MRI was performed every 3 months after the initial treatment.
Figure 1.
Typical characteristics of cystic (A1–A3) and solid (B1–B3) brain metastases in magnetic resonance images.
Notes: (A1, B1) Axial contrast-enhanced T1-weighted magnetic resonance images. (A2, B2) Axial T2-weighted magnetic resonance images. (A3, B3) Sagittal contrast-enhanced T1-weighted magnetic resonance images.
Treatment
All patients underwent CyberKnife treatment and a total of 88 (24.72%) patients received prior WBRT. The median timing from WBRT to SRS was 1 month (ranging from 0.3 to 40 months). In our center, large brain metastases usually received supplement SRS treatment again 0.5–1 months after WBRT because the dose of WBRT was not enough for the treatment of lesions of large volume. In our study, most patients (63/88, 71.6%) received prior WBRT before SRS because of multiple brain metastases and large volume of isolated lesion. The rest of the patients (25/88, 28.4%) received SRS after WBRT (range: 3–40 months) because of new brain metastases. Detailed CyberKnife treatment parameters are listed in Table 1.
Table 1.
Characteristics of CyberKnife radiosurgery treatment parameters
| Characteristic | Mean | Median | Range |
|---|---|---|---|
|
| |||
| Prescription dose (Gy) | 24.52 | 22 | 12–42 |
| BED (Gy) | 60.13 | 60 | 26.4–83.2 |
| Conformity index | 1.21 | 1.18 | 1.04–1.99 |
| Target volume (cm3) | 6.87 | 3.073 | 0.056–89.975 |
| Homogeneity index | 1.45 | 1.47 | 1.2–1.92 |
| Prescription isodose (%) | 68 | 67 | 52–83 |
Abbreviation: BED, biological effective dose.
The volume of brain metastases ranged from 0.0565 to 89.975 cm3. And the volume and site of brain metastases determined the dose and fraction schedule (Table 2). A total of 165 patients received SRS, with median dose 20 Gy (range: 12–23 Gy). Two-fraction and three-fraction SRT were given in 136 and 121 patients, with the same median dose 30 Gy (range: 16–32 and 18–36 Gy, respectively). The prescribed dose covered at least 95% of the PTV.
Table 2.
Dose and fraction schedule for patients who underwent CyberKnife treatment
| Prior WBRT | Tumor diameter (cm) | Dose/fraction |
|---|---|---|
| No | ≤1.5 | 21 Gy/1F |
| 1.5–2.5 | 26–28 Gy/2F | |
| 2.5–3.5 | 27–30 Gy/3F | |
| Yes | 12–14 Gy/1F |
Abbreviation: WBRT, whole brain radiotherapy.
Statistical analysis
BMOS was defined as the duration of time from the date of brain metastases diagnosis to the date of death or the last follow-up. The follow-up ended on December 30, 2017. The survival estimates were analyzed by the Kaplan–Meier method, and log-rank test was used for the comparison between patients with solid and cystic brain metastases. Multivariate survival analysis for prognostic factors and cystic brain metastases was performed by using Cox regression and the forward likelihood ratio method. A two-sided test that resulted in P<0.05 was considered to be statistically significant.
Results
Patient characteristics
In our study, data from 356 patients (including 498 brain metastases) were analyzed, and their characteristics are listed in Table 3.
Table 3.
Patients characteristics and comparison between cystic and solid brain metastases
| Characteristics | No. of all patients (%) | No. of patients with cystic lesions (%) | No. of patients with solid lesions (%) | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 244 (68.54) | 44 (65.67) | 200 (69.2) | 0.579 |
| ≥65 | 112 (31.46) | 23 (34.33) | 89 (30.8) | |
| Sex | ||||
| Male | 195 (54.78) | 37 (55.22) | 158 (54.7) | 0.935 |
| Female | 161 (45.22) | 30 (44.78) | 131 (45.3) | |
| ECOG | ||||
| 0 | 7 (1.97) | 0 (0) | 7 (2.4) | 0.407 |
| 1 | 297 (83.43) | 58 (86.57) | 239 (82.7) | |
| ≥2 | 52 (14.61) | 9 (13.43) | 43 (14.9) | |
| RPA classification | ||||
| 1 | 63 (17.70) | 11 (16.42) | 52 (18) | 0.593 |
| 2 | 280 (78.65) | 55 (82.09) | 226 (78.2) | |
| 3 | 13 (3.65) | 1 (1.49) | 11 (3.8) | |
| Primary tumor | ||||
| Lung | 243 (68.26) | 54 (80.60) | 188 (65.1) | 0.048 |
| Breast | 41 (11.52) | 7 (10.45) | 34 (11.8) | |
| Kidney | 20 (5.62) | 0 (0) | 20 (6.9) | |
| Gastrointestinal tract | 23 (6.46) | 4 (6.0) | 19 (6.6) | |
| Other | 29 (8.15) | 2 (3.0) | 28 (9.7) | |
| Prior WBRT | ||||
| Yes | 88 (24.72) | 24 (35.82) | 64 (22.1) | 0.019 |
| No | 268 (75.28) | 43 (64.18) | 22 (77.9) | |
| No. of brain metastases | ||||
| 1 | 257 (72.19) | 45 (67.2) | 212 (73.4) | 0.758 |
| 2 | 69 (19.38) | 16 (23.9) | 53 (18.3) | |
| 3 | 20 (5.62) | 4 (6.0) | 16 (5.5) | |
| ≥4 lesions | 10 (2.53) | 2 (3.0) | 8 (2.8) | |
| Neurologic symptoms | ||||
| Yes | 206 | 36 (53.7) | 170(58.8) | 0.447 |
| No | 150 | 31(46.3) | 119(41.2) | |
| The volume of brain metastases (cm3) | ||||
| ≤8 | 211(59.3) | 28 (41.8) | 183 (63.3) | 0.005 |
| 8–27 | 123 (34.6) | 34 (50.7) | 89 (30.8) | |
| >27 | 22 (6.2) | 5 (7.5) | 17 (5.9) | |
| ≤27 | 334 (93.8) | 62 (92.5) | 272 (94.1) | 0.628 |
| >27 | 22 (6.2) | 5 (7.5) | 17 (5.9) | |
| Primary tumor status | ||||
| Control | 134 | 23 | 111 | 0.535 |
| Uncontrol | 222 | 44 | 178 | |
| Distribution of brain metastases | ||||
| Frontal | 106 | 12 | 94 | |
| Parietal | 130 | 25 | 105 | |
| Temporal | 88 | 10 | 78 | |
| Occipital | 69 | 14 | 55 | |
| Cerebellum | 58 | 10 | 48 | |
| Other | 47 | 4 | 43 | |
| Cystic brain metastases | ||||
| Yes | 67 | |||
| No | 289 | |||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; RPA, recursive partitioning analysis; WBRT, whole brain radiotherapy.
A total of 67 patients (67/356, 18.8%) developed 75 cystic brain metastases. The median age for all patients was 59 years (range: 24–86 years). In our study, 63 (17.70%) patients had a recursive partitioning analysis (RPA) classification of I, 280 (78.65%) patients were in class II, and only 13 (3.65%) patients were in class III. Lung cancer was the most frequent primary site (243/356, 68.26%); breast cancer (41/356, 11.52%) were the next. Over half of the patients (206/356, 57.87%) had significant neurologic symptoms, including headaches, dizziness, vomiting, hemiplegia, and so on. Most patients had one to three brain metastases and only ten patients had more than three brain metastases.
Overall survival and local control
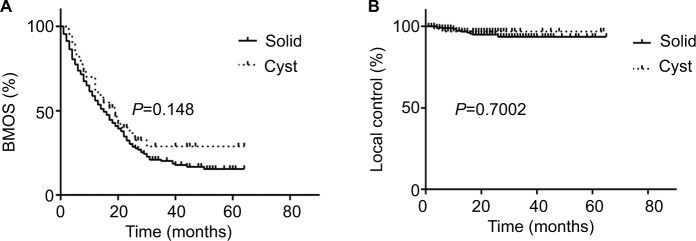
Until the last follow-up time, 93 (93/356, 26.12%) patients were still alive (Figure 2), including 25 (25/67, 37.31%) patients with cystic brain metastases. Most patients died of tumor progression and five patients died from heart disease or severe brain edema. The median BMOS is 17 months (range: 1–65 months). There is no significant difference in BMOS between patients with cystic (17 months, range: 3–64 months) and solid (17.5 months, range: 1–65 months) brain metastases (P=0.148) (Figure 3A). The BMOS ratios at 1, 2, and 3 years in all patients were 64.89%, 33.15%, and 10.96%, respectively, and were 70.15%, 32.83%, and 10.45% for patients with cystic brain metastases. The local control was 96.2% and 97.0% in patients with solid and cystic metastases, respectively, with no significant difference (P=0.7002) (Figure 3B). Definite local failure occurred in four solid brain metastases and one cystic brain metastasis. In addition, the volume of seven solid brain metastases and one cystic brain metastasis increased significantly with obvious edema. We cannot identify necrosis or relapses because these patients rejected further imaging examinations.
Figure 2.
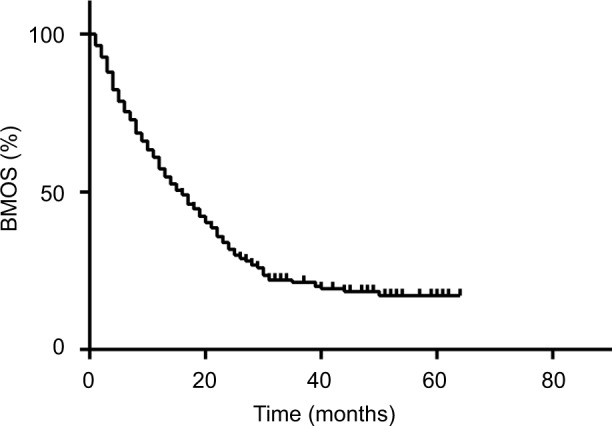
Overall survival after brain metastases curve for 356 patients who underwent CyberKnife treatment.
Abbreviation: BMOS, overall survival after brain metastases.
Figure 3.
Comparison of survival and local control between patients with cystic (A) and solid brain metastases (B) after CyberKnife treatment.
Abbreviation: BMOS, overall survival after brain metastases.
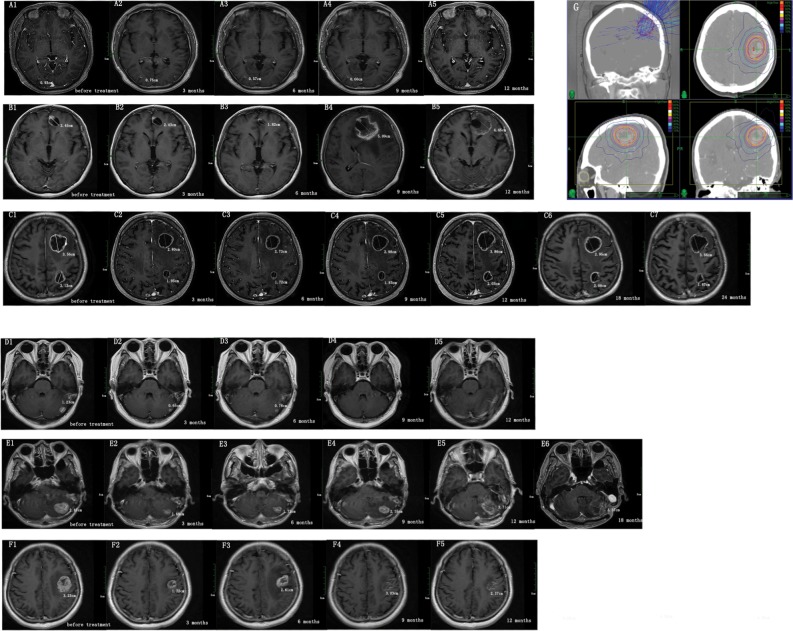
Only 47 patients with cystic brain metastases and 184 patients with solid brain metastases survived for >12 months after brain metastases. Therefore, we measured the volume changes by diameter at 3, 6, 9, and 12 months (Figure 4) after CyberKnife treatment in 39 patients with 41 cystic brain metastases and 153 patients with 174 solid brain metastases. Results showed that the volume of solid brain metastases decreased faster than cystic brain metastases (P<0.05) (Figure 5).
Figure 4.
The volume change in patients with cystic and solid brain metastases after CyberKnife treatment.
Notes: We measured the longest diameter of brain metastases at diagnosis (before treatment) and 3, 6, 9, and 12 months after radiosurgery treatment in axial contrast-enhanced T1-weighted magnetic resonance images. (A–C) Cystic brain metastases images. (D–F) Solid brain metastases images. (A1, B1, C1, D1, E1, F1) Brain metastases images before radiosurgery treatment. The change in volume of brain metastases (A2–A5, B2–B5, C2–C5, D2–D5, E2–E5, F2-F5) over time. (A) A 40-year-old woman with lung cancer received WBRT before radiosurgery treatment. The prescription dose and fraction schedule was 14 Gy/1F. (B) A 44-year-old woman with breast cancer, without prior WBRT. The prescription dose and fraction schedule was 30 Gy/3F. (C) A 74-year-old man with lung cancer, without prior WBRT. The prescription dose and fraction schedule was 32 Gy/4F. (D) A 51-year-old woman with lung cancer, without prior WBRT. The prescription dose and fraction schedule was 20 Gy/1F. (E) A 74-year-old man with lung cancer, without prior WBRT. The prescription dose and fraction schedule was 30 Gy/2F. (F) A 65-year-old woman with endometrial cancer, without prior WBRT. The prescription dose and fraction schedule was 30 Gy/3F. (G) Typical dose distribution images by CyberKnife plans.
Abbreviation: WBRT, whole brain radiotherapy.
Figure 5.
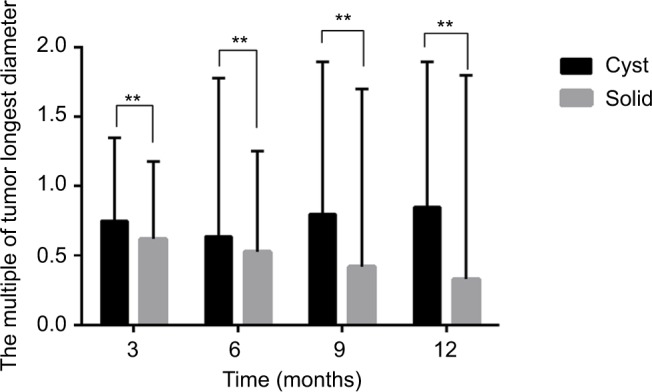
Comparison of volume change between patients with cystic and solid brain metastases after CyberKnife treatment.
Risk factors for BMOS
Factors that may be related to BMOS were included in correlational analyses (Table 4). Univariate analysis suggested that age (P=0.032), Eastern Cooperative Oncology Group score (P=0.002), RPA classification (P=0.001), primary tumor (P=0.000), neurologic symptoms (P=0.015), the volume of brain metastases (P=0.011), and extracranial metastases (P=0.006) were the independent influencing factors for BM OS. These factors were included in the multivariate analysis, and the results indicated that high RPA classification (P=0.006) (Figure 6) and large volume of brain metastases (P=0.006) (Figure 7) were associated with poor prognosis in patients with brain metastases. Different primary tumors were also related to prognosis (P=0.001). Compared to lung cancer, gastrointestinal tract cancer had poorer BMOS (HR=2.339, P=0.000).
Table 4.
Prognostic factors for overall survival after brain metastases in all patients
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| X2 | P-value | HR | 95% CI | P-value | ||
| Age (years) | ||||||
| <65 | 4.602 | 0.032 | ||||
| ≥65 | ||||||
| Sex | ||||||
| Male | 0.04 | 0.841 | ||||
| Female | ||||||
| ECOG | ||||||
| 0 | 12.347 | 0.002 | ||||
| 1 | ||||||
| ≥2 | ||||||
| RPA classification | ||||||
| 1 | 14.645 | 0.001 | 1 | 0.006 | ||
| 2 | 1.646 | 1.164–2.329 | 0.005 | |||
| 3 | 2.441 | 1.232–4.835 | 0.011 | |||
| Primary tumor | ||||||
| Lung | 28.581 | 0.000 | 1 | 0.001 | ||
| Breast | 1.133 | 0.771–1.665 | 0.526 | |||
| Gastrointestinal tract | 2.339 | 1.476–3.706 | 0.000 | |||
| Kidney | 1.476 | 0.894–2.437 | 0.128 | |||
| Other | 0.731 | 0.456–1.173 | 0.194 | |||
| Prior WBRT | ||||||
| Yes | 0.842 | 0.359 | ||||
| No | ||||||
| No. of brain metastases | ||||||
| 1 | 3.945 | 0.267 | ||||
| 2 | ||||||
| 3 | ||||||
| ≥4 lesions | ||||||
| Neurologic symptoms | ||||||
| Yes | 5.884 | 0.015 | ||||
| No | ||||||
| Cystic brain metastases | ||||||
| Yes | 2.091 | 0.148 | ||||
| No | ||||||
| Extracranial metastases | ||||||
| Yes | 7.669 | 0.006 | ||||
| No | ||||||
| The volume of total BM (cm3) | ||||||
| ≤8 | 9.001 | 0.011 | 1 | 0.006 | ||
| 8–27 | 1.243 | 0.958–1.613 | 0.102 | |||
| >27 | 2.146 | 1.314–3.505 | 0.002 | |||
| Primary tumor status | ||||||
| Control | 3.046 | 0.081 | ||||
| Uncontrol | ||||||
Abbreviations: BM, brain metastases; ECOG, Eastern Cooperative Oncology Group; RPA, recursive partitioning analysis; WBRT, whole brain radiotherapy.
Figure 6.
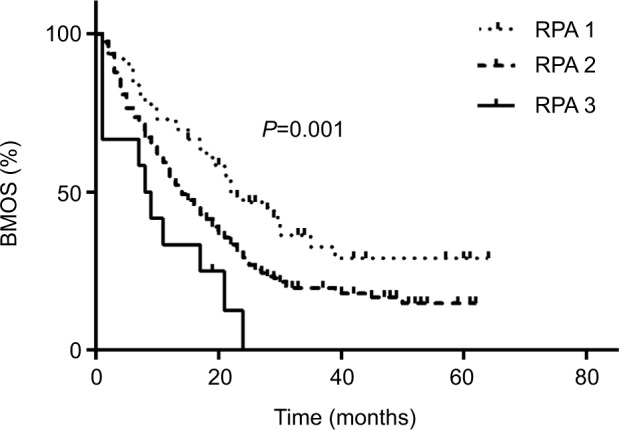
Comparison of survival after brain metastases in patients with different RPA classification.
Abbreviations: BMOS, overall survival after brain metastases; RPA, recursive partitioning analysis.
Figure 7.
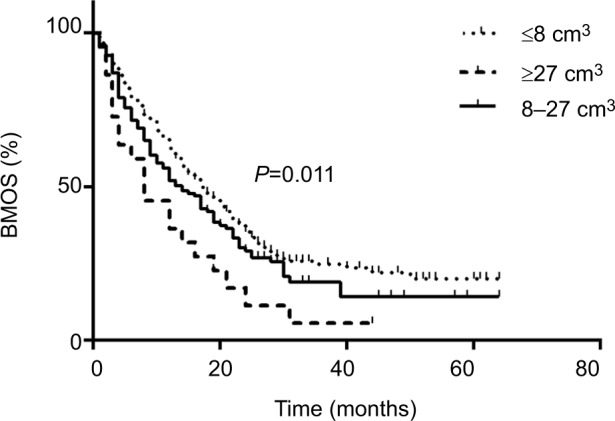
Comparison of survival after brain metastases in patients with different volume of brain metastases.
Abbreviation: BMOS, overall survival after brain metastases.
Because lung cancer accounts for the most of the primary disease, we analyzed risk factors in patients with lung cancer separately (Table 5). RPA classification (P=0.038), extra-cranial metastases (P=0.049), the volume of brain metastases (P=0.004), and primary tumor condition (P=0.019) were included in the multivariate analysis, and the results indicated that the volume of brain metastases (P=0.001) and primary tumor (P=0.003) condition were independent prognostic factors for the overall survival in patients with brain metastases.
Table 5.
Prognostic factors for overall survival after brain metastases in patients with lung cancer
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| X2 | P-value | HR | 95% CI | P-value | |
| Age (years) | |||||
| <65 | 2.397 | 0.122 | |||
| ≥65 | |||||
| Sex | |||||
| Male | 0.017 | 0.896 | |||
| Female | |||||
| ECOG | |||||
| 0 | 3.529 | 0.171 | |||
| 1 | |||||
| ≥2 | |||||
| RPA classification | |||||
| 1 | 6.557 | 0.038 | |||
| 2 | |||||
| 3 | |||||
| Prior WBRT | 0.044 | 0.834 | |||
| Yes | |||||
| No | |||||
| No. of brain metastases | 3.356 | 0.340 | |||
| 1 | |||||
| 2 | |||||
| 3 | |||||
| ≥4 | |||||
| Neurologic symptoms | 0.389 | 0.533 | |||
| Yes | |||||
| No | |||||
| Cystic brain metastases | 1.33 | 0.249 | |||
| Yes | |||||
| No | |||||
| Extracranial metastases | |||||
| Yes | 3.875 | 0.049 | |||
| No | |||||
| The volume of total BM (cm3) | |||||
| ≤8 | 10.937 | 0.004 | 1 | 0.001 | |
| 8–27 | 1.108 | 0.802–1.532 | 0.533 | ||
| >27 | 3.637 | 1.879–7.038 | 0.000 | ||
| Primary tumor status | |||||
| Control | 5.500 | 0.019 | 1 | 0.003 | |
| Uncontrol | 1.679 | 1.186–2.378 | |||
Abbreviations: BM, brain metastases; ECOG, Eastern Cooperative Oncology Group; RPA, recursive partitioning analysis; WBRT, whole brain radiotherapy.
Discussion
Brain metastasis is usually regarded as an important sign of poor prognosis in malignancies, especially in cystic brain metastases, and there still exist controversy about radiotherapy treatment for cystic cerebral metastases.17 However, in the few studies comparing prognosis between cystic and solid brain metastases, we can only find one retrospective study associated with cystic brain metastases in breast metastases showing that patients with cystic brain metastases have shorter survival time than the patients with solid brain metastases.18 Apparently, as we have known, our study is the first to compare the prognosis between cystic and solid brain metastases and assess the risk factors for BMOS in patients who had received radiosurgery treatment.
The formation mechanism of cystic brain metastases remains poorly understood; many researchers have come up with hypotheses to explain this phenomenon. A study from the United States proposed that the breakdown of the blood–brain barrier, which could probably cause accumulation of fluids, was the cause of cystic masses.19 Another study assumed that the primary cancer with abundant mucus had higher risk of developing cystic metastases.20 Rapid growth could be another possible cause for generating cystic components. One study also showed that patients with poor histological grade had higher risk of developing cystic brain metastases in breast cancer.18
In our study, the local control rate was higher than reported data from several studies related to brain metastases (Table 6),10,15,21–27 which may be caused by the differences in treatments and primary lesions. In our view, different definitions of local failure were also the main factor that resulted in different outcomes. Many authors defined local tumor control only according to tumor volume change.25 But sometimes, it is difficult to identify tumor progression and necrosis after treatment. Especially, tumor progression and necrosis could both cause peritumoral edema. All patients in our study would be advised further magnetic resonance spectroscopy screening and treatment for relieving the cerebral edema once they developed enlarged, inconclusive brain lesions. The results showed that most of them were necrosis caused by radiation treatment and the lesions would gradually shrink with symptomatic treatment. Traditional treatments for necrosis include corticosteroids, mannitol agents, anticoagulants, and so on. In recent years, endothelial cell dysfunction followed by production of vascular endothelial growth factor has been proved to play an important role in necrosis after irradiation. Some relevant researches, which included randomized controlled trials indicated that bevacizumab could offer improved symptomatic relief, especially for patients with poor response to other treatments.28–30
Table 6.
Summary of studies of patients with brain metastases who underwent radiotherapy (stereotactic radiosurgery)
| Author and publication year | Primary tumor and characteristic of brain metastases | No. of patients | Treatment | Median survival time (months) | Local control |
|---|---|---|---|---|---|
|
| |||||
| Gerosa et al. (2002)21 | Multiple primary tumor | 804 | GK | 13.5 | 1 year: 94% |
| Pan et al. (2005)15 | Lung cancer | 191 | GK | GK alone: 15; WBRT + GK: 14 | 84.4% (<0.5 cm3); 94% (0.5–2 cm3); 89.1% (2–4 cm3); 93.4% (4–8 cm3); 85.7% (8–14 cm3; 87.5% (>14 cm3) |
|
| |||||
| Franzin et al. (2008)22 | Multiple primary tumor | 30 | GK | 15 | 91.3% |
|
| |||||
| Park et al. (2009)23 | Multiple primary tumor (cystic BM) | 24 | GK | 17.8 | 79.2% |
|
| |||||
| Fahed et al. (2014)24 | NSCLC | 89 | GK | 24 | 1 year: 91.5 2 years: 85.5% |
|
| |||||
| Keisuke et al. (2015)10 | NSCLC: 64 SCLC: 3 | 67 | CK | 13.1 | 1 year: 83.3% 2 years: 78.5% |
|
| |||||
| Sang et al. (2016)24 | Multiple primary tumor (cystic BM) | 28 | GK | 17.7 | 1 year: 82.3% |
|
| |||||
| Wang et al. (2016)26 | Multiple primary tumor (cystic BM) | 48 | GK | 19.5 | 91.7% |
|
| |||||
| Antonio et al. (2016)27 | Multiple primary tumor | 223 | CK | 11 | 1 year: 85% |
Abbreviations: BM, brain metastases; CK, CyberKnife; GK, Gamma Knife; NSCLC, non-small-cell lung cancer; SCLC, small cell lung cancer; WBRT, whole brain radiotherapy.
There is no difference in survival time and volume between patients with cystic and solid brain tumor; however, we discovered that the volume of cystic brain metastases decreased more slowly than solid brain metastases. We also found that large volume was an independent risk factor for prognosis. Another study, which included 290 breast cancer cases got different results in that patients with cystic brain metastases had poorer survival time (P<0.001) and heavier tumor burden (P=0.005).18 The difference indicated that the volume of tumor was real risk factor for prognosis and patients with cystic tumor had poorer BMOS just because of its larger volume. Especially, there was also no significant difference between different proportions of cystic components in prognosis (50%–75% contrast with 75%–100%; P=0.9358) in our analysis. Other studies that have got the similar conclusions offer strong support for our hypothesis.31,32 Besides, in our study, patients received different treatment schedule according to the volume and characteristics of tumor, usually more fractions for larger volume. Multifraction SRS could improve tumor hypoxia effectively, which was an important factor for resistance to radiation in cystic brain tumor. It could also explain the reason why cystic metastases responded well to radio surgery similar to solid tumors. Cystic component was absorbed more slowly compared with solid tumor, and it could explain the difference in volume shrink rate. Further prospective study and longer follow-up are necessary to prove the results.
Conclusion
Our study overturned the previous perception that patients with cystic brain metastases had poorer prognosis, and we advise that radiosurgery could be a suitable treatment option for cystic brain metastases. However, the rate and speed of tumor shrinkage was lower in cystic brain metastases after radiotherapy. Patients with larger brain metastases had shorter survival time, regardless of cystic or solid brain metastases. Of course, further prospective randomized trials are needed to prove the results.
Acknowledgments
This study was supported by research grants from National Natural Science Foundation of China (No. 81472797) and Tianjin Municipal Science and Technology Commission (No. 16JCQNJC10000).
Footnotes
Author contributions
HW and ZYY designed the study. XGW, YCS, and ZYY recruited patients. XYL, XCJ, HW, JSW, YD, ZQW, and FTL were involved in acquisition of data, follow-up, and statistical analysis. HW wrote the paper with ZYY and YHZ. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Klos KJ, O’Neill BP. Brain metastases. Neurologist. 2004;10(1):31–46. doi: 10.1097/01.nrl.0000106922.83090.71. [DOI] [PubMed] [Google Scholar]
- 2.Lowery FJ, Yu D. Brain metastasis: unique challenges and open opportunities. Biochim Biophys Acta Rev Cancer. 2017;1867(1):49–57. doi: 10.1016/j.bbcan.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. histology, multiplicity, surgery, and survival. Cancer. 1996;78(8):1781–1788. [PubMed] [Google Scholar]
- 4.Nabors LB, Portnow J, Ammirati M, et al. Central nervous system cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(10):1191–1202. doi: 10.6004/jnccn.2015.0148. [DOI] [PubMed] [Google Scholar]
- 5.Kawabe T, Phi JH, Yamamoto M, Kim DG, Barfod BE, Urakawa Y. Treatment of brain metastasis from lung cancer. Prog Neurol Surg. 2012;25:148–155. doi: 10.1159/000331188. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Sun Y, Yu J, et al. China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version) Zhongguo Fei Ai Za Zhi. 2017;20(1):1–13. doi: 10.3779/j.issn.1009-3419.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abd-El-Barr MM, Rahman M, Rao G. Investigational therapies for brain metastases. Neurosurg Clin N Am. 2011;22(1):87–96. doi: 10.1016/j.nec.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Bulbul A, Forde PM, Murtuza A, et al. Systemic treatment options for brain metastases from non-small-cell lung cancer. Oncology. 2018;32(4):156–163. [PubMed] [Google Scholar]
- 9.Li B, Yu J, Suntharalingam M, et al. Comparison of three treatment options for single brain metastasis from lung cancer. Int J Cancer. 2000;90(1):37–45. [PubMed] [Google Scholar]
- 10.Tamari K, Suzuki O, Hashimoto N, et al. Treatment outcomes using CyberKnife for brain metastases from lung cancer. J Radiat Res. 2015;56(1):151–158. doi: 10.1093/jrr/rru092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocher M, Maarouf M, Bendel M, Voges J, Müller RP, Sturm V. Linac radiosurgery versus whole brain radiotherapy for brain metastases. A survival comparison based on the RTOG recursive partitioning analysis. Strahlenther Onkol. 2004;180(5):263–267. doi: 10.1007/s00066-004-1180-y. [DOI] [PubMed] [Google Scholar]
- 12.Leeman JE, Clump DA, Wegner RE, Heron DE, Burton SA, Mintz AH. Prescription dose and fractionation predict improved survival after stereotactic radiotherapy for brainstem metastases. Radiat Oncol. 2012;7(1):107. doi: 10.1186/1748-717X-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson AC, Wegner RE, Rwigema JC, Heron DE, Burton SA, Mintz AH. Clinical outcomes of reirradiation of brain metastases from small cell lung cancer with Cyberknife stereotactic radiosurgery. J Cancer Res Ther. 2012;8(3):411–416. doi: 10.4103/0973-1482.103522. [DOI] [PubMed] [Google Scholar]
- 14.Jung TY, Kim IY, Jung S, et al. Alternative treatment of stereotactic cyst aspiration and radiosurgery for cystic brain metastases. Stereotact Funct Neurosurg. 2014;92(4):234–241. doi: 10.1159/000362935. [DOI] [PubMed] [Google Scholar]
- 15.Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L. Gamma knife surgery for brain metastases from lung cancer. J Neurosurg. 2005;102(Suppl):128–133. doi: 10.3171/jns.2005.102.s_supplement.0128. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V, Prabhash K, Noronha V, Tandon N, Joshi A. A systematic approach to diagnosis of cystic brain lesions. South Asian J Cancer. 2013;2(2):98–101. doi: 10.4103/2278-330X.110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchino M, Nagao T, Seiki Y, Shibata I, Terao H, Kaneko I. Radiosurgery for cystic metastatic brain tumor. No Shinkei Geka. 2000;28(5):417–421. [PubMed] [Google Scholar]
- 18.Sun B, Huang Z, Wu S, Hz SB, Ding L, et al. Cystic brain metastasis is associated with poor prognosis in patients with advanced breast cancer. Oncotarget. 2016;7(45):74006–74014. doi: 10.18632/oncotarget.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner WJ, Collis JS, Lewis LA. Cystic brain tumors and the blood-brain barrier. Comparison of protein fractions in cyst fluids and sera. Arch Neurol. 1963;8:291–298. doi: 10.1001/archneur.1963.00460030075007. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi H, Okamoto I, Tanizaki J, et al. Cystic brain metastasis in non-small-cell lung cancer with ALK rearrangement. J Clin Oncol. 2014;32(36):e122–e124. doi: 10.1200/JCO.2012.48.2141. [DOI] [PubMed] [Google Scholar]
- 21.Gerosa M, Nicolato A, Foroni R, et al. Gamma knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002;97(5 Suppl):515–524. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 22.Franzin A, Vimercati A, Picozzi P, et al. Stereotactic drainage and gamma knife radiosurgery of cystic brain metastasis. J Neurosurg. 2008;9(2):259–267. doi: 10.3171/JNS/2008/109/8/0259. [DOI] [PubMed] [Google Scholar]
- 23.Park WH, Jang IS, Kim CJ, Kwon DH. Gamma knife radiosurgery after stereotactic aspiration for large cystic brain metastases. J Korean Neurosurg Soc. 2009;46(4):360–364. doi: 10.3340/jkns.2009.46.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zairi F, Ouammou Y, Le Rhun E, et al. Relevance of gamma knife radiosurgery alone for the treatment of non-small cell lung cancer brain metastases. Clin Neurol Neurosurg. 2014;125:87–93. doi: 10.1016/j.clineuro.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Lee Sr OJ, Kim SH. Gamma knife radiosurgery for cystic brain metastases. Br J Neurosurg. 2016;30(1):43–48. doi: 10.3109/02688697.2015.1039489. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Qi S, Dou C, et al. Gamma knife radiosurgery combined with stereotactic aspiration as an effective treatment method for large cystic brain metastases. Oncol Lett. 2016;12(1):343–347. doi: 10.3892/ol.2016.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pontoriero A, Conti A, Iatì G, et al. Prognostic factors in patients treated with stereotactic image-guided robotic radiosurgery for brain metastases: a single-center retrospective analysis of 223 patients. Neurosurg Rev. 2016;39(3):495–504. doi: 10.1007/s10143-016-0718-7. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Rong X, Hu W, et al. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101(5):1087–1095. doi: 10.1016/j.ijrobp.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Zheng C, Feng Y, Xu Q. Bevacizumab for the treatment of Gammaknife radiosurgery-induced brain radiation necrosis. J Craniofac Surg. 2017;28(6):e569–e571. doi: 10.1097/SCS.0000000000003874. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang H, Yuan X, Yuan Z, Wang P. Indication of bevacizumab for cerebral radiation necrosis. Recent Pat Anticancer Drug Discov. 2017;12(3):272–277. doi: 10.2174/1574892812666170425124430. [DOI] [PubMed] [Google Scholar]
- 31.Baschnagel AM, Meyer KD, Chen PY, et al. Tumor volume as a predictor of survival and local control in patients with brain metastases treated with gamma knife surgery. J Neurosurg. 2013;119(5):1139–1144. doi: 10.3171/2013.7.JNS13431. [DOI] [PubMed] [Google Scholar]
- 32.Ebinu JO, Lwu S, Monsalves E, et al. Gamma knife radiosurgery for the treatment of cystic cerebral metastases. Int J Radiat Oncol Biol Phys. 2013;85(3):667–671. doi: 10.1016/j.ijrobp.2012.06.043. [DOI] [PubMed] [Google Scholar]