Abstract
Proton-detected solid-state NMR (SSNMR) spectroscopy has attracted much attention due to its excellent sensitivity and effectiveness in the analysis of trace amounts of amyloid proteins and other important biological systems. In this perspective article, we present the recent sensitivity limit of 1H-detected SSNMR using “ultra-fast” magic-angle spinning (MAS) at a spinning rate (νR) of 80–100 kHz. It was demonstrated that the high sensitivity of 1H-detected SSNMR at νR of 100 kHz and fast recycling using the paramagnetic-assisted condensed data collection (PACC) approach permitted “super-fast” collection of 1H-detected 2D protein SSNMR. A 1H-detected 2D 1H–15N correlation SSNMR spectrum for ~27 nmol of a uniformly 13C- and 15N-labeled GB1 protein sample in microcrystalline form was acquired in only 9 s with 50% non-uniform sampling and short recycle delays of 100 ms. Additional data suggests that it is now feasible to detect as little as 1 nmol of the protein in 5.9 h by 1H-detected 2D 1H–15N SSNMR at a nominal signal-to-noise ratio of five. The demonstrated sensitivity is comparable to that of modern solution protein NMR. Moreover, this article summarizes the influence of ultra-fast MAS and 1H-detection on the spectral resolution and sensitivity of protein SSNMR. Recent progress in signal assignment and structural elucidation by 1H-detected protein SSNMR is outlined with both theoretical and experimental aspects.
Keywords: 1H detection, solid-state NMR, ultra-fast MAS, super-fast 2D NMR
Graphical Abstract

Introduction
1H-detected high-resolution solid-state NMR (SSNMR) is gaining increasing attention for its high sensitivity and broad application in the study of biological systems such as amyloid proteins1–5 and membrane-bound protein assemblies,3–7 including ones in native cell membranes.8 1H-indirect detection was originally introduced to solution NMR in the early 1970’s.9–11 Because of its higher sensitivity, 1H detection has become an indispensable tool for biological solution NMR.12–13 In contrast, due to the limited resolution and sensitivity in 1H SSNMR, 1H-detection in high-resolution SSNMR did not provide any substantial sensitivity advantage until the recent development of fast magic angle spinning (MAS), over 30–40 kHz.1, 14–16 The recent introduction of MAS at a spinning speed of 80 kHz or higher, which is often termed “ultra-fast MAS” (UFMAS), has dramatically improved the 1H resolution and sensitivity of SSNMR.2, 17–19 The simplicity of this approach has prompted us to achieve additional sensitivity gains by a series of complementary sensitivity enhancement methods, such as the use of paramagnetic relaxation enhancement,20 a higher static magnetic field,21 more efficient probe circuits with small sample coils, and extensive deuteration.3, 22–23 Although the sensitivity gain achieved by each method is moderate, the cumulative sensitivity gain can reach a couple of orders of magnitude. Thus, despite initial concerns about the limited sample volume (≤ 1 μL) for a fast MAS rotor, the UFMAS approach has been proven to be highly effective for the assignment and structural characterization of a broad range of proteins.2, 18–19, 24
In this perspective article, we explored the current sensitivity limit of 1H-detected protein SSNMR using UFMAS at a spinning rate (νR) of 100 kHz, a high field, and paramagnetic assisted condensed data collection (PACC).20 The high sensitivity of 1H-detected SSNMR and fast recycling in the PACC approach allowed for “super-fast” data collection of multi-dimensional SSNMR for ~27 nmol of a protein within only 9 s. Then, we summarized recent developments in 1H-detected SSNMR methods and other related methods, primarily through introducing recent protein SSNMR data collected at our laboratories. Sequential assignment and structural elucidation strategies in 1H-detected protein SSNMR are also outlined.
Theory
We compare the frequency-domain signal-to-noise (S/N) ratio (per root-square of the time) obtained by the direct detection of a spin-1/2 system (nuclei X) with that obtained by indirect 1H detection. Following ref. 14, the sensitivity enhancement factor ξ for the 2D 1H indirect detection over 1D direct detection of the X nuclei was given by
| [1] |
where γM is a gyromagnetic ratio for the M nuclei (M = H, X), sH is an envelope function of a 1H time-domain signal and wH is a window function for the 1H signal, <a> denotes the time-averaged signal intensity of a, and t2max and t1max are the maximum t2 and t1 periods in the 2D indirect data collection. We assume that the signal length tmax collected in the 1D direct detection is ~t1max and that the same window functions are applied to the indirectly collected X signal in the t1 domain and the directly collected 1D X signal. QM is a circuit Q-factor for the M-channel of the probe, for which the single coil probe circuit double-tuned for H and X is assumed. When a matched window function is applied to sH, eq. [1] is reduced to
| [2] |
with
| [3] |
| [4] |
| [5] |
The expression WM represents a general NMR line width (in units of Hz), which matches with full-width-at-half-height (FWHH) in a Lorentzian line shape or the FWHH times (2πln2)−1/2 or 0.479 in Gaussian line shape. With a matched filter condition, t1max is typically selected to be 1/(2WX) and thus κ = π. In a typical setting using UFMAS at 100 kHz, f ~ 0.6, and QH/QX = 2–3. Assuming the line widths of WX ~ 100 Hz (0.5 ppm) and WH ~160 Hz (0.2 ppm) at a 1H frequency of 800 MHz for 13C (X) and 1H and the Q-ratio of 2, we safely obtain ξ = 2.1 for 1H indirect detection of 13C. For 1H indirect detection of 15N, we obtain ξ = 6.0 assuming that WX ~ 40 Hz (0.5 ppm) and WH ~ 160 Hz (0.2 ppm). As discussed below, 1H line widths of 0.1–0.2 ppm are reported for fully protonated GB1 and other microcrystalline protein with UFMAS.2, 19 Thus, notable sensitivity enhancements are expected, even when 2D 1H detection is compared with 1D X detection. In modern protein SSNMR, matched window functions are rarely used for optimum spectral resolution. However, the factor estimated by eq. [2] is still reasonable in modern SSNMR applications, for which data processing of the indirect dimension is often optimized for better resolution with non-uniform-sampling (NUS). Suppose that we lose some sensitivity by removing the matched window function in the 1H direct dimension for better resolution. The loss is equivalent to a sensitivity gain by applying “matched” NUS25 in the indirect dimension for X. Thus, if the data are adequately collected with NUS, eq. [2] still provides a reasonable estimate for resolution optimized 1H detected data.
When 2D 1H detection is compared with 2D X detection, the sensitivity enhancement factor is given by
| [6] |
For the above-mentioned condition, we obtain enhancement factors of 5.4 and 13.4 for X of 13C and 15N, respectively. Thus, in terms of the required experimental time, notable time saving by a factor of 25–170 is expected.
Estimating the ratio of the Q-factors (QH/QX) in eqs. [1] and [2] from the circuit’s tuning profiles may be sometimes misleading since a probe circuit often involves a parasitic inductance/impedance, especially for a probe with a small sample coil with a long lead wire. Based on the formula by Clark,26–27 the amplitude of the oscillating magnetic field 2B1 from a RF coil is described by
| [7] |
where M = H or X, PM is a transmitter power in watts, Vs is a coil volume in cubic centimeters, and ν0M is the NMR resonance frequency in Hz, and B1M denotes the amplitude of a rotating RF field in tesla. Thus,
| [8] |
where we assume that the coil volume is the same for the circuits of H and X, and ω1M denotes a nutation angular frequency due to B1M, and ω0M = 2πν0M. All the parameters at the right side of the equation can be estimated from the NMR parameters. This relationship suggests that improving a probe-circuit power efficiency for 1H detection also improves sensitivity in the 1H detection.
Results
Super-fast 2D Protein SSNMR and the Current Sensitivity Limit of 1H Detection
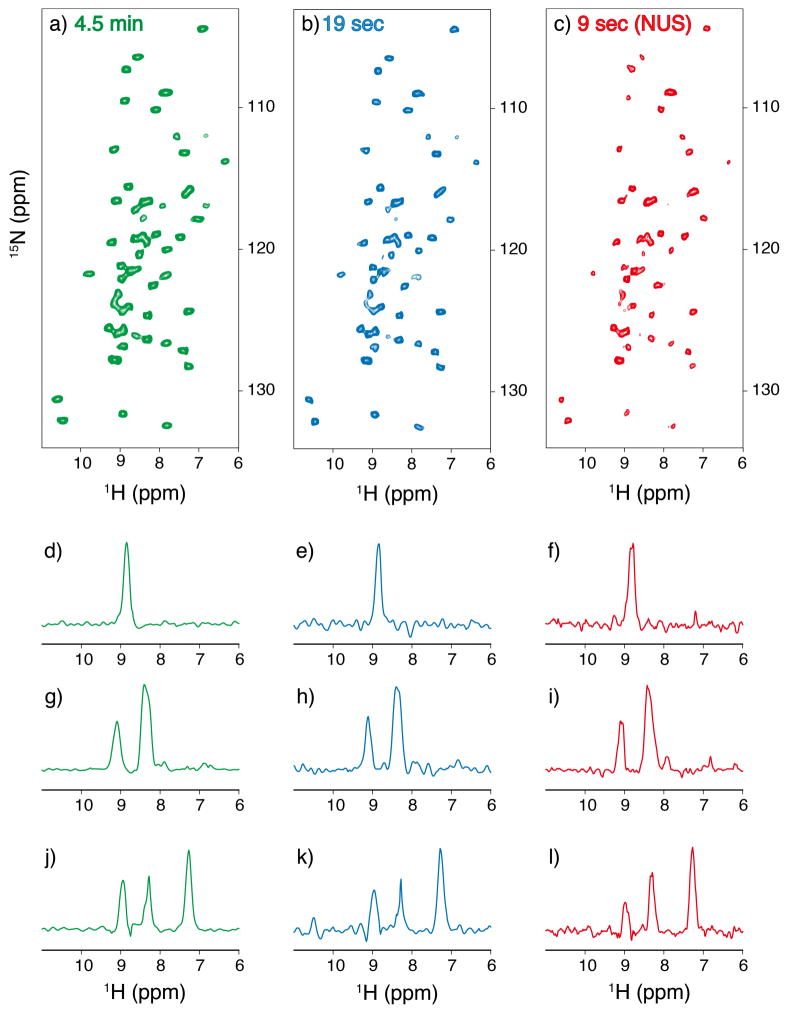
We first discuss the current sensitivity limit of 1H-detected SSNMR and prospects for the UFMAS approach. Figure 1(a) shows a 2D 15N–1H correlation SSNMR spectrum for a microcrystalline sample of uniformly 13C- and 15N-labeled GB1 (ca. 180 μg). With the high sensitivity of UFMAS at a spinning frequency (νR) of 100 kHz and a 1H NMR frequency of 750.2 MHz, the spectrum was obtained in only 5 min for ~27 nmol of the protein sample. Based on the excellent S/N (~16 on average), we estimate that analysis by 1H-detected 2D SSNMR for 1 nmol of the protein sample is feasible within only 5.9 h at a minimal S/N of 5. This is a notable achievement, considering that until a decade ago collecting 13C-detected 2D protein SSNMR data generally required as much as 1 μmol of an isotope labeled sample and a few days of experimental time, even for the very best data in biological SSNMR at that time.28–29
Figure 1.
A comparison of (a) a traditional 1H-detected 2D 15N/1H correlation spectrum (16 scans for each t1 point) with (b, c) super-fast 2D spectra collected (2 scans for each t1 point) at 100 kHz MAS (b) without using NUS and (c) with NUS at 50 % random sampling rate for uniformly 13C- and 15N-labeled GB1 protein in a microcrystal sample (~180 μg) with 20 mM Cu-EDTA. Recycle delay was set to 0.2 s for (a), and 0.1 s for (b) and (c). The experimental times are indicated in the figure. (d–l) 1D 1H slices of the 2D NH spectra obtained at the 15N chemical shifts of (d–f) 107.23 ppm, (g–i) 116.57 ppm, and (j–l) 124.36 ppm.
With this encouraging data, it was appropriate to compare the sensitivity of 1H-detected SSNMR with that of solution NMR. Thus, we performed 2D 1H–15N HSQC for a comparable amount of the labeled GB1 sample (~150 μg) with an 800 MHz NMR equipped with a 5-mm inverse triple-resonance room-temperature probe, at the University of Illinois at Chicago (UIC) the Center for Structural Biology. The sample was dissolved in 200 μL of a 50 mM phosphate buffer with 10% D2O and 0.02 w/v % NaN3 at pH 5.6 in a 3-mm NMR tube. The S/N ratio of the spectrum obtained within 5 min was approximately 10 (data not shown). Thus, the sensitivity of a modern 1H-detected SSNMR has reached a level that is comparable to that of solution NMR.
By taking advantage of the excellent sensitivity of 1H-detected SSNMR, we propose fast data collection of 2D protein SSNMR. In protein solution NMR, fast 2D data collection has been achieved through various creative methods, such as excitation with a short flip angle or simultaneous data collection for different t1 points under a magnetic field gradient.30–32 Here, we combined the benefit of fast recycling (100–200 ms/scan) with the PACC method20 and the high sensitivity of 1H-detected SSNMR, with a view to implementing “super-fast” collection of 2D SSNMR for micro-screening of protein samples in a very straightforward manner. Figure 1(b) and (c) shows super-fast 2D SSNMR spectra collected for the GB1 sample in (b) 19 s and (c) 9 s (b) without and (c) with 50% NUS, respectively. Nearly all of the peaks for directly-bonded amide 15N-1H pairs are observed, except for a few peaks, with a minimal experimental time of 9 s in (c). The average S/N ratio for the resolved signals was ~6 for (c). The spectrum reasonably well reproduced the peak positions, as indicated in the 1D slices for the three spectra (Fig. 1(d–l)). It should be noted that fast 2D NMR in solution protein NMR typically requires ~1 μmol of a protein sample,30, 32 unless an advanced DNP implementation is employed.33–34 Our approach is straightforward and potentially useful for characterizing real-time structural changes in a variety of proteins in solids or semi-solids with minimal sample requirements. Applications for larger protein systems are feasible with additional sensitivity gain, for example, by use of a higher field at a 1H frequency over 1 GHz.35–36
1H Resolution and Spinning Speed
Next, we discuss the prospect of further sensitivity enhancement by 1H-detected SSNMR with faster MAS. Figure 2(a–d) shows MAS frequency (νR) dependence of 1H SSNMR spectra of uniformly 13C- and 15N-labeled alanine (~0.3 mg) obtained at a 1H NMR frequency of 900 MHz with a JEOL 0.75-mm triple-resonance CPMAS probe. Excellent resolution was achieved in (d) at νR of 90 kHz. 1H resonances for the CH and CH3 groups show improved resolution with 13C WALTZ decoupling, as shown in Figure 2(e); the line widths for the CH and CH3 groups were as narrow as 0.27 ppm and 0.36 ppm at a νR of 90 kHz, respectively. The resolution is satisfactory for the analysis of small organic compounds37 without sophisticated homonuclear-decoupling sequences.38–40 For the NH3+ group, there appears to be additional broadening, which is consistent with previous studies.39 Figure 2(f) shows the νR dependence of the signal intensities. The data show linear dependence of the normalized peak intensities on νR with minor deviation for the NH3+ group. These data imply that UFMAS beyond 100 kHz will further improve the resolution and sensitivity of 1H-detected SSNMR. It should be noted that the data were collected without 13C decoupling, for simplicity. For more mobile hydrated proteins in a microcrystal system, sharper lines are often observed, as is discussed below. Table 1 summarizes the sample volume and dimensions of fast MAS rotors provided by Bruker Biospin, JEOL RESONANCE, and Tallinn University of Technology. Various types of fast MAS probes are now available depending on the purpose of experiments.
Figure 2.
(a–d) Magic angle spinning (MAS) frequency (νR) dependence of 1H spectra of uniformly 13C- and 15N-labeled L-alanine. The spinning frequencies are indicated in the figure. (e) 1H MAS spectrum for the same sample collected at νR of 90 kHz with WALTZ-16 13C decoupling with a RF nutation frequency of 10 kHz. (f) MAS frequency dependence of the 1H signal intensities for the L-alanine sample. The data were obtained without 13C-decoupling. The intensities were normalized to those obtained at νR of 90 kHz.
Table 1.
A comparison of MAS rates and sample volume for different fast MAS rotors
| Outer diameter (mm) | 1.9 | 1.3 | 0.7 | 2.0 | 1.0 | 0.75 | 1.8 | 0.81 |
| Inner diameter (mm) | 1.5 | 0.9 | 0.5 | 1.55 | 0.5 | 0.35 | –e) | –e) |
| Sample volume (μL)a) | 10.4 | 1.5 | 0.37 | 12.6 | 0.55 | 0.19 | 15 | 0.8 |
| Max spinning rate (kHz) | 42 | 67 | 111 | 40 | 80 | 100b) | 50 | 105 |
| Manufacture | Bruker Biospinc) | JEOL RESONANCE | NMR Institute, Tallinn University of Technologyd) | |||||
The sample volume denotes that within a sample coil.
The spinning speed is based on the product specification rather than the maximum rate (~120 kHz).
The information on the Bruker rotors was kindly provided by Dr. Frank Engelke at Bruker Biospin.
The information on Tallin Univ. rotors was kindly provided by Dr. Ago Samoson.
The data were not available.
A Basic Pulse-sequence Scheme for 1H-detected SSNMR
In this section, we review basic pulse sequences of 1H-detected 2D 1H/13C experiments in a fast MAS approach at νR of 40–100 kHz. Figure 3(a) and (b) compares (b) a modern low-power scheme for 1H-detected 2D 1H/13C correlation SSNMR with (a) a traditional high-power scheme for 1D CPMAS experiments with 13C direct detection. In (a), 1H polarization is transferred to 13C spins with a standard adiabatic or ramped cross polarization (CP) block; then, the 13C signals are directly observed under high-power decoupling at a RF field strength of 70–100 kHz. For an efficient CP scheme, the average RF angular nutation frequency for 1H <ωH-CP> should be matched to that of 13C <ωC-CP> so that they satisfy the Hartman-Hahn condition for rotating solids (<ωH-CP> – <ωC-CP>) = nωR, where ωR = 2πνR and n = ±1 or 2. Note that this CP sequence requires high-power RF irradiation under fast MAS because either |<ωH-CP>| or |<ωH-CP>| should exceed ωR. The sequence is typically repeated every 1–5 s so that sample degradation or probe arcing is prevented under high-power RF irradiation. Thus, 95–99 % of SSNMR machine time is typically consumed for long recycle delays that are limited by this factor rather than 1H T1 values of the sample with only 1–5% of the time remaining for collection of spectral information.
Figure 3.
(a) A pulse sequence for a standard CP scheme for direct-detection of spin-1/2 X nuclei such as 13C and 15N. After cross polarization, signals from X spins are detected under a high-power 1H decoupling scheme such as SPINAL-64. Experiments are typically recycled every 1–5 s. (b) A low-power pulse sequence for 1H-detected 2D indirect-detection of the X nuclei. After the DQ-CP period from 1H to X spins, the signals for the X spins are monitored indirectly during the t1 period.
A pulse sequence in (b) shows an example of a 1H-detected SSNMR scheme using the UFMAS approach. Experiments were typically repeated with much shorter recycle delays (100–300 ms) in an approach called paramagnetic-assisted condensed data collection (PACC), which combines the use of a low-power pulse sequence, faster recycling, and paramagnetic T1 relaxation enhancement with dopants such as Cu-EDTA.20 To reduce 1H T1 values of proteins, 10–100 mM Cu-EDTA, or other paramagnetic agents, are doped with hydrated proteins; paramagnetic relaxation enhancement effects with the controlled doping reduce 1H T1 values down to 30–100 ms, allowing for faster recycling without noticeably compromising the spectral resolution.20, 41 The signal acquisition can be as much as 30% of the experimental time. Below, we outline a scheme when the X nucleus is 13C, but the arguments here are generally applicable to most low γ spin-1/2 nuclei, including 15N. In (b), 1H polarization is first transferred to 13C spins with a double-quantum cross polarization (DQ-CP) sequence, in which the sum of <ωH-CP> and <ωX-CP> is matched to nωR. This allows for low-power implementation of cross polarization (that is, |<ωH-CP>|, |<ωX-CP>| < ωR). After DQ-CP, 13C signal evolution during the t1 period is monitored under low-power 1H decoupling, a concept which was originally introduced with original 1H-detected SSNMR.14 At νR ≥ 40 kHz, phase-modulated low-power 1H decoupling schemes such as TPPM,42 XiX,43 and SPINAL-6444 with a 1H RF nutation frequency (νdec) of 5–20 kHz exhibit excellent performance, which is comparable to that of high-power 1H decoupling.42 At νR of 100 kHz, our study indicates that WALTZ-16 1H decoupling at νdec of 5–20 kHz, which was designed for solution NMR, offers superior or comparable resolution to that obtained by the aforementioned 1H decoupling sequences designed for SSNMR, with minimal need for the adjustment of decoupling parameters.24 After the t1 period, the 13C polarization is stored along the Z-axis parallel to the static magnetic field. Any 1H background signal is suppressed by a background-suppression sequence such as the MISSISIPPI scheme45 during the “Z-filter” period. Then, 13C polarization is transferred back to 1H spins by the second DQ-CP scheme. Assuming that a fraction f of the 13C polarization is transferred back to 1H spins, the bulk magnetic moment for 1H is typically enhanced by a factor of fγH/γC over that of 13C. Since the voltage induced across the sample coil caused by the bulk magnetic moment is proportional to fγH/γC, the Larmor frequency ω0H, and the circuit Q-factor,46 the resultant NMR signal is typically much stronger for 1H detection than 13C detection. With UFMAS, a 1H time-domain signal can continue much longer than that with slower MAS. Thus, the overall sensitivity of 1H-detected SSNMR can be greatly enhanced under UFMAS, especially when combined with a PACC approach.20 As discussed above, 1H-detected 2D SSNMR can now be considered feasible for isotope-labeled protein analysis at the nano-mole level.
Sensitivity & Resolution Enhancement by 1H detection
Next, we examine the sensitivity and resolution advantages of 1H-detected protein SSNMR using UFMAS at 80–100 kHz. Figure 4 shows a comparison of 1H-detected 2D 1H/15N correlation spectra obtained at νR values of (a) 80 kHz and (b) 50 kHz for a lysine-reverse-labeled GB1 microcrystalline sample (~0.5 mg or ~80 nmol) doped with 20 mM Cu-EDTA. This fully protonated sample was uniformly 13C- and 15N-labeled, except for six lysine (Lys) residues, as will be discussed later when exploring spectral editing experiments. The pulse sequence detailed in Figure 3(b) was used for the experiments, with recycle delays of 0.2 s. Although the spectrum collected at νR of 50 kHz in (b) displays a modest resolution, the resolution was greatly enhanced in (a) by UFMAS at 80 kHz. The signals were nearly completely resolved in the 2D spectrum. Similar levels of resolution were obtained for a handful of fully protonated proteins with UFMAS over 80 kHz.4
Figure 4.
A comparison of 2D 15N/1H correlation spectra collected under MAS at (a) 80 kHz and (b) 50 kHz for the lysine-reverse-labeled GB1 microcrystal sample (0.5 mg or ~80 nmol) doped with 20 mM Cu-EDTA. The data were collected with recycle delays of 0.2 s with a total experimental time of 4.5 min each. The pulse sequence is comprised of two adiabatic CP schemes with contact times of 1.5 ms (see Figure 3(b))2. The spin polarization was transferred from 1H to 15N by the first adiabatic CP; then the 15N signal was monitored in the t1 period. After the t1 period, the polarization was transferred back from 15N to 1H spins by the second adiabatic CP scheme for 1H detection. For the data in (a), during the first adiabatic DQ-CP period, the 15N RF field strength was ramped from 18.6 kHz to 31.0 kHz with an average RF field at ~νR/3, while the 1H RF field was kept constant at 55 kHz (~2νR/3). For the second adiabatic CP period, the 15N RF field was ramped down from 33.3 kHz to 19.9 kHz, while the 1H RF field was kept at 55 kHz. For the data in (b), during the first adiabatic CP period, the 15N RF field was swept from 10.6 kHz to 17.7 kHz with an average RF field at ~νR/4, while the 1H RF field strength was kept at 35 kHz (~3νR/4). In the second adiabatic CP period, the 1H RF field was ramped from 9,7 kHz to 16.1 kHz, while the 15N RF field amplitude was kept at 37 kHz. (c) 1D slices of the 2D NH spectra for the spinning speeds of 80 kHz (red) and 50 kHz (blue) for resolution comparison. The spectra were modified from the data in ref. 2.
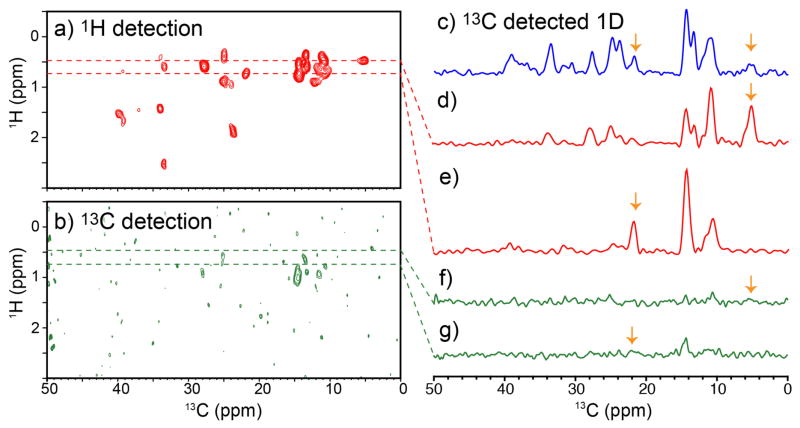
Figure 5(a) and (b) shows (a) 1H-detected and (b) 13C-deteced 2D 13C/1H correction spectra of ubiquitin that was selectively labeled with stereo-array-isotope-labeled (SAIL) Ile (~0.5 mg). A 1D 13C CPMAS spectrum of the same sample is displayed in Figure 5(c). All the data were collected at νR of 80 kHz and a 1H NMR frequency of 750.2 MHz with a JEOL 1-mm quad-resonance probe, using low-power CP and decoupling schemes.18 The recycle delays for (a–c) were set to 0.54 s, which was matched to short 1H T1 values (0.25 s) of this sample, even without paramagnetic dopants. The short 1H T1 values can likely be attributed to the presence of CHD2 groups in Ile and the 1H-1H dipolar network within the residue. For a sensitivity comparison, each of (a–c) was collected in ~5 min. In a SAIL labeling scheme, all the CH2 and CH3 groups of the relevant amino acid residues were replaced with CHD and CHD2, respectively, allowing for improved resolution in the 1H SSNMR spectra.47 The observed line widths WH were in the 0.1–0.2 ppm range. In (a), signals for all seven Ile residues are clearly resolved. Superior sensitivity in the 1H-detected data in (a) over the 13C-detected data is obvious. In a comparison of the peaks (orange arrows) in the slices (d, e) of the 2D 1H-detected with (c), the corresponding peaks (orange arrows) in the 1D 13C CPMAS spectrum indicated a sensitivity enhancement factor of 2.1–5.0.18
Figure 5.
A comparison of (a) 1H-detected and (b) 13C-detected 2D 13C/1H correlation SSNMR spectra of SAIL-Ile labeled ubiquitin obtained at νR of 80 kHz. A comparison of (c) a 1D 13C CPMAS spectrum of the same sample and (d–g) slices from (d, e) 1H-detected and (f, g) 13C-detected 2D correlation spectra. The figures are modified from ref. 18. For a sensitivity comparison, both experiments were performed with low-power pulse sequences and the same recycle delays (0.54 s/scans), without paramagnetic doping.
Signal Assignment and Spectral Editing under Ultra-fast MAS
In this section, we evaluate signal assignment strategies in 1H-detected protein SSNMR. Various approaches using sequential assignments were introduced to assign protein main chain and side chain signals in 1H-detected SSNMR using fast MAS.2, 4, 7, 16, 19 In previous protein SSNMR studies, a suit of 3D 1H-detected experiments such as 3D (H)CANH, (H)CA(CO)NH, (H)CACO(N)H, and (H)CXCA(N)H (polarization transfer diagrams in Figure 6(a) to (d)) were shown to be useful for the sequential assignment of the main chain signals of small or moderate-sized proteins such as GB1.7, 16 In this case, nearly complete resolution is needed in a 2D 15N-1HN correlation spectrum in order to connect 1Hi–15Ni resonances to 13CAi as well as 13CAi-1 resonances for sequential assignments. Alternatively, with sufficient resolution in a 2D 13CA–1HA spectrum, 1HAi–13CAi resonances can be connected to 15Ni and 15Ni-1 for sequential assignments using schemes such as those shown in Figure 6(e) to (h).4 However, NMR signals often substantially overlap when a protein sequence carries repeats of the same amino-acid types in a common secondary structure. In such a case, establishing starting points of sequential assignments or connecting resonances of next-neighboring residues may be difficult. To address this spectral overlap issue in 1H-detected SSNMR, we recently introduced an approach called HIGHLIGHT (HIGHspeed-spinning-optimized Labeled-residue-IGnited Hetero-nuclear Total spectral quenching).2, 48 Figure 7(a) shows a 2D NH correlation spectra of Lys-reverse-labeled GB1 (blue) without and (red) with HIGHLIGHT REDOR quenching. In this HIGHLIGHT experiment, 15N signals of the residues that are next to 13C-labeled residues were nearly completely quenched by recoupled 13CO–15N dipolar couplings; thus, the HIGHLIGHT REDOR experiment highlighted amide 15N–1H signals only for the six residues next to unlabeled lysine residues (see the sequence in (d)), with one Trp-43 side chain signal.
Figure 6.
Diagrams of polarization transfer in (a–d) 1HN-detected and (e–h) 1Hα-detected 3D SSNMR experiments for main-chain protein sequential assignment. (a) (H)CANH, (b) (H)CA(CO)NH, (c) (H)CACO(N)H, (d) (H)CXCA(N)H, (e) (H)NCAH, (f) (H)N(CO)CAH, (g) (H)COCAH, and (h) (H)CXCAH schemes.
Figure 7.
(a) 2D 1H-detected 15N/1H correlation spectra (blue) without and (red) with HIGHLIGHT REDOR, for the micro-crystalline sample of Lys-reverse-labeled GB1, along with the sequence shown in (d). (b, c) Overlaid 3D strip plots for sequential assignments from (a) Leu-5 through Gly-9 and (b) Gly-14 through Val-21 for a micro-crystalline sample of Lys-reverse-labeled GB1 protein obtained by 3D CANH (green), CA(CO)NH (blue), and 3D CANH with 4.2 ms HIGHLIGHT REDOR mixing data (red). The recycle delays were 0.3 s, 0.4 s, and 1 s for 3D CANH, 3D CA(CO)NH, and 3D CANH with HIGHLIGHT REDOR, respectively. All experiments were performed at a MAS spinning speed of 80 kHz with ~0.5 mg of sample. The total experimental times were 20 min for 3D CANH, 3.7 h for 3D CA(CO)NH, and 1 h for 3D CANH with HIGHLIGHT REDOR (for details, see the Experimental section and Figure S2 of ref. 2). The figure was modified from ref. 2.
With this strategy, we performed sequential assignments using 3D CANH and CA(CO)NH with the HIGLIGHT approach. Figure 7(b) shows overlaid strip plots for 3D CANH (green) and 3D CA(CO)NH (blue) spectra of the Lys-reverse-labeled GB1 sample, along with those for 3D CANH obtained with HIGHLIGHT REDOR (red) for the same sample. The highlighted signals for Leu-5 and Gly-14 (red in Figure 7(b) and (c)) indicate the starting points of the sequential assignments for the residues following lysine residues ((b) Leu-5 to Gly-9, (c) Gly-14 to Val-21) as an excellent example of the approach. Based on these results only from the two data sets, we completed main-chain 1H, 13Cα, and 15N signal assignments, except for lysine and other unassigned residues in the loop region (Ala-23 and Asp-47).2 Torsion angles (ϕ, ψ) predicted by TALOS+ software49 from the obtained 1H, 15N, and 13C shifts showed excellent agreement with those from the crystal structure for most of the residues, except for those around unassigned residues. For complete main-chain assignments, we successfully obtained 3D CONH data. Nominal 3D experimental times needed to obtain average S/N ratios of ~6 were only 2.5 min, 20.5 min, and 28.5 min for 3D CANH, 3D CA(CO)NH and HIGHLIGHT 3D CANH experiments for ~100 nmol of the GB1 protein sample, respectively.
Side-chain signal assignment of a protein by 1H-detected SSNMR is not so straightforward. Although side-chain assignment by 1H-detected SSNMR has been attempted for some fully protonated proteins,4 many amino-acid side-chain groups carry CH2 groups, which often offer limited 1H resolution due to the presence of magnetically non-equivalent vicinal 1H and strong 1H–1H dipolar coupling within the vicinal group, even with UFMAS. For a per-deuterated protein, excellent resolution can be achieved for the back-exchanged amide 1H, especially with fast MAS at 40 kHz or above.3, 7 However, the lack of aliphatic 1H limits the starting point of 3D experiments only to the amide 1H (or other exchangeable 1H in the side chain), making side-chain assignment generally very difficult and less sensitive. To address these problems, we recently introduced the SAIL scheme47 as an isotope-labeling scheme suited for 1H-detected protein SSNMR. Figure 8(a) shows a 2D 13C–13C projection of a 1H-detected 3D CCH correlation of SAIL-Ile labeled ubiquitin. Because of seven Ile residues in the protein sequence, signals overlap substantially in the 2D 13C–13C projection. For example, in the region selected in Figure 8(b), projected 2D signals originating from three Ile residues (Ile-3, -13, and -44) overlap severely. However, these signals can be nicely separated by 1H shifts of isoleucine. For example, in Figure 8(c) to (e), three isoleucine signals can be clearly separated from distinctive 1Hβ resonances for (c) Ile-3, (d) Ile-13, and (e) Ile-44. Figure 8(f) presents assignments for all the side chain resonances of Ile-61. Because of the narrow 1H resonances, all the isoleucine side-chain resonances were well resolved. The data illustrates an excellent example of side-chain assignment by 3D 13C–13C–1H correlation for SAIL-labeled proteins.
Figure 8.
Resolution and side-chain assignments from 3D 13C/13C/1H SSNMR of SAIL-ubiquitin. (a, b) 2D 13C/13C 2D projection spectra from a 1H-detected 3D 13C/13C/1H SSNMR of SAIL-ubiquitin at a MAS frequency of 80 kHz. All peaks, including minor ones in (a), can be attributed to intra-residue cross peaks within the Ile residues. (c–e) Representative 2D 13C/13C slices corresponding to 1H chemical shifts of (c) 1.57 ppm, (d) 1.73 ppm, and (e) 1.41 ppm. The data show clear separation of signals for (c) Ile-3, (d) Ile-13, and (e) Ile-44 by 1H shifts. The spectrum was processed with 45°- and 60°-shifted sine-bell window functions in the 1H and 13C dimensions, respectively. (f) 13C/1H assignments for Ile-61 from the 3D data. The pulse sequence can be found in ref. 18. The figure was modified from ref. 18.
Structure & Distance Restraints in 1H-detected SSNMR
Lastly, we discuss structural determination by 1H-detected SSNMR. Recent SSNMR-based elucidation of high profile amyloid-fibril structures28, 50–54 have ensured that SSNMR is recognized as a powerful tool in structural biology. Among ~100 SSNMR-based structures reported in the Protein Data Bank (PDB), nearly all were determined by traditional 13C-detected SSNMR, which offers a variety of options in elucidating long-range distance restraints. Available methods for this purpose include DARR,55 frequency-selective REDOR,56 PARR,57 and CHHC/CHHN schemes.58 In contrast, for 1H-detected SSNMR using fast MAS, there are limited options for collecting long-range distance restraints.22, 59 For this reason, there are only a handful of recent examples of de-novo protein structural determinations by 1H-detected SSNMR under UFMAS.59–60 An earlier study established the first structural determination on a small model protein, uniformly 2H, 13C, and 15N-labeled protein GB1 with back-exchanged amide 1H.59 Here, we introduce two recent examples, which provide more generic routes for structural determinations of small proteins by 1H-detected SSNMR. The first example was performed on a microcrystalline sample of a model protein ubiquitin at a 1H frequency of 850 MHz.60 The assignments, torsion angles from chemical shifts, and 1H–1H contacts were obtained for uniformly 2H, 13C, 15N-labeled ubiquitin for which amide 2H was back exchanged to 1H. The 1H–1H contacts, including 36 long-range and 50 medium-range contacts, were obtained by 1H-detected 3D 15N-resolved 1H–1H spin diffusion experiments [3D HN(H)–H] at νR of 99.2 kHz, where H–H stands for long-range 1H–1H mixing in the pulse sequence. The 1H–1H rotating-frame spin diffusion involved 20 ms cw spin-lock at 13 kHz for optimum 1H T1ρ values. Additional 1H–1H contacts (68 long-range and 5 medium-range) were collected at a νR of 94.5 kHz for uniformly 2H 15N-labeled ubiquitin, for which one of the methyl groups of deuterated Ile, Leu, and Val were replaced with 13CHD2, while the other was labeled with CD3.61 The 1H–1H contacts were obtained with the 4D HSQC-DREAM-HSQC sequence [4D HC(H)–(H)CH],61 in which a 1HA–13CA correlation was obtained during the t1 and t2 periods for one methyl residue and an additional 1HB–13CB correlation was obtained during the t3 and t4 periods for another methyl residue, after long-range 1HA–1HB polarization transfer using a 8-ms 1H-DREAM mixing sequence at 47.2 kHz. The deuteration method is a practical approach to identify long-range contacts without excess spectral crowding.
More recently, structures of fully protonated proteins were determined for GB1 as well as a microcrystalline Acinetobacter phage 205 coat protein (AP205CP), a 28-kDa dimer in a 2.5MDa viral capside assembly, at a 1H NMR frequency of 1 GHz.19 Long-range 1H–1H contacts were collected for GB1 and AP205CP by 3D (H)CH–H and 3D (H)NH–H sequences. Side-chain assignments were more straightforward for these samples. 1H-1H long-range contacts of 236 and 410 were obtained for GB1 and AP205CP, respectively. Compared with heavily deuterated samples, a much larger number of side-chain long-range contacts were elucidated. It is not clear at this point how this approach can be effective for more structurally heterogeneous or complex biological systems. Nevertheless, considering that a high level of deuteration is very difficult for many such proteins of biological significance, the study presents a promising prospect for protein structural determination by 1H-detected SSNMR.
Conclusion
1H-detected SSNMR approach has provided unparalleled sensitivity in SSNMR for hydrated protein samples when employed together with UFMAS, a high field, the PACC approach, and selective/extensive deuteration schemes. Tremendous successes using this approach in the past decade have resulted in the sequential assignments and structural elucidation of small/moderate-sized proteins in 1H-detected SSNMR. Nevertheless, the widespread applications of 1H-detected SSNMR to solve serious biological problems remain largely unexplored. Additional efforts are needed to move 1H-detected protein SSNMR schemes to the next level. In the next few decades, further advances in 1H-detected SSNMR are likely to provide additional sensitivity gains though the development of ultra-high field magnets using high-temperature superconductive materials35, faster MAS at 200 kHz or higher, and dynamic nuclear polarization methods.62 A SSNMR analysis of sub-nano-mole- or pico-mole-scale proteins would be within our scope with such advancements.
Experimental Section
The SSNMR experiments for Figure 1 were performed on a Bruker Avance III 750MHz spectrometer using a JEOL 0.75 mm 1H/13C/15N/2H quad-resonance MAS probe at the UIC Center for Structural Biology. The MAS spinning rate was set at 100 kHz ± 50 Hz. The SSNMR experiments for Figure 2 were performed on a JEOL ECZR 900 MHz spectrometer equipped with an Oxford magnet and a JEOL 0.75 mm 1H/13C/15N triple-resonance MAS probe at the Yokohama RIKEN NMR facility. Details of all other data are described in the captions and the relevant references.
For the data in Figure 1, a pulse sequence based on that shown in Figure 3(b) was used with 15N as the X channel and 13C as an additional channel. First, the 1H polarization was excited by a 1H π/2-pulse with a pulse width of 2.5 μs. During the first adiabatic DQ-CP period, the 1H RF field strength was ramped from 59.2 kHz to 98.7 kHz, while the 15N RF field was kept constant at 21.7 kHz. For the second adiabatic DQ-CP period, the 1H RF field was ramped up from 46.5 kHz to 77.5 kHz, while the 15N RF field was kept at 32.5 kHz. During the t1 period, WALTZ-16 1H decoupling was applied with a RF nutation frequency of 10 kHz and a 13C π-pulse with a pulse width of 6 μs was applied in the middle of the t1 period. During the t2 period, 15N and 13C WALTZ-16 decoupling was applied with RF nutation frequencies of 6 kHz and 9 kHz, respectively. As the water signal was removed sufficiently by a minimum phase cycle of 2 scans, no water suppression sequence was employed. The GB1 microcrystal sample was prepared as described previously.2, 63
For the data in Figure 2, a rotor synchronous echo pulse sequence was employed. The sequence started with a 1H excitation π/2-pulse with a pulse width of 0.6 μs. It was followed by an echo period of twice the rotor cycle, in the middle of which a π-pulse with a pulse width of 1.2 μs was applied. Then, a 1H signal was acquired during 8 ms with or without 13C WALTZ-16 decoupling at a nutation frequency of 10 kHz.
Highlights.
First demonstration of super-fast 1H-detected 2D high-resolution solid-state NMR for a nano-mole-scale protein.
First demonstration of collecting a 2D solid-state NMR spectrum for a 1 nmol of protein in less than 6 h.
Perspective review of recent studies of signal assignments and structural elucidation by 1H-detected protein solid-state NMR using ultra-fast MAS
Acknowledgments
This study was supported primarily by the Japanese MEXT Fund for the Promotion of Joint International Research grant-in-aid (Kakenhi 15K21772) and the US National Science Foundation (CHE 1310363) for YI. The instrumentation of the 750 MHz SSNMR at UIC was supported by an NIH HEI grant (1S10 RR025105) and, in part, by an NIH UO1 grant (U01GM098033) for YI. We thank Dr. Hideaki Maeda and staff members at the Yokohama RIKEN NMR facility for accommodating us for the experiments at the facility. We are grateful to Dr. Frank Engelke at Bruker Biospin for kindly providing the information on the Bruker rotor dimensions. We also thank Dr. Ben Ramirez at the UIC Center for Structural Biology for assistance for solution NMR measurements.
References
- 1.Ishii Y, Yesinowski JP, Tycko R. Sensitivity Enhancement in Solid-State C-13 Nmr of Synthetic Polymers and Biopolymers by H-1 Nmr Detection with High-Speed Magic Angle Spinning. J Am Chem Soc. 2001;123:2921–2922. doi: 10.1021/ja015505j. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Parthasarathy S, Xiao Y, Nishiyama Y, Endo Y, Nemoto T, Matsuda I, Long F, Yamauchi K, Asakura T, et al. Nano-Mole Scale Sequential Signal Assignment by 1h-Detected Protein Solid-State Nmr Using Ultra-Fast Magic-Angle Spinning and Highlight Spectral Editing. Chem Comm. 2015;51:15055–15058. doi: 10.1039/c5cc04618a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linser R, Dasari M, Hiller M, Higman V, Fink U, del Amo JML, Markovic S, Handel L, Kessler B, Schmieder P, et al. Proton-Detected Solid-State Nmr Spectroscopy of Fibrillar and Membrane Proteins. Angew Chem Int Edit. 2011;50:4508–4512. doi: 10.1002/anie.201008244. [DOI] [PubMed] [Google Scholar]
- 4.Stanek J, Andreas LB, Jaudzems K, Cala D, Lalli D, Bertarello A, Schubeis T, Akopjana I, Kotelovica S, Tars K, et al. Nmr Spectroscopic Assignment of Backbone and Side-Chain Protons in Fully Protonated Proteins: Microcrystals, Sedimented Assemblies, and Amyloid Fibrils. Angew Chem Int Edit. 2016;55:15503–15509. doi: 10.1002/anie.201607084. [DOI] [PubMed] [Google Scholar]
- 5.Zhou DH, Nieuwkoop AJ, Berthold DA, Comellas G, Sperling LJ, Tang M, Shah GJ, Brea EJ, Lemkau LR, Rienstra CM. Solid-State Nmr Analysis of Membrane Proteins and Protein Aggregates by Proton Detected Spectroscopy. J Biomol NMR. 2012;54:291–305. doi: 10.1007/s10858-012-9672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward ME, Wang SL, Krishnamurthy S, Hutchins H, Fey M, Brown LS, Ladizhansky V. High-Resolution Paramagnetically Enhanced Solid-State Nmr Spectroscopy of Membrane Proteins at Fast Magic Angle Spinning. J Biomol NMR. 2014;58:37–47. doi: 10.1007/s10858-013-9802-2. [DOI] [PubMed] [Google Scholar]
- 7.Barbet-Massin E, Pell AJ, Retel JS, Andreas LB, Jaudzems K, Franks WT, Nieuwkoop AJ, Hiller M, Higman V, Guerry P, et al. Rapid Proton-Detected Nmr Assignment for Proteins with Fast Magic Angle Spinning. J Am Chem Soc. 2014;136:12489–12497. doi: 10.1021/ja507382j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros-Silva J, Mance D, Daniels M, Jekhmane S, Houben K, Baldus M, Weingarth M. H-1-Detected Solid-State Nmr Studies of Water-Inaccessible Proteins Invitro and Insitu. Angew Chem Int Edit. 2016;55:13606–13610. doi: 10.1002/anie.201606594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller L. Sensitivity Enhanced Detection of Weak Nuclei Using Heteronuclear Multiple Quantum Coherence. J Am Chem Soc. 1979;101:4481–4484. [Google Scholar]
- 10.Bodenhausen G, Ruben DJ. Natural Abundance Nitrogen-15 Nmr by Enhanced Heteronuclear Spectroscopy. Chem Phys Lett. 1980;69:185–189. [Google Scholar]
- 11.Bax A, Griffy RH, Hawkins BL. Correlation of Proton and Nitrogen-15 Chemical Shifts by Multiple Quantum Nmr. J Magn Reson. 1983;55:301–315. [Google Scholar]
- 12.Bax A, Ikura M, Lewis EK, Torchia DA, Tschudin R. Comparison of Different Modes of Two-Dimensional Reverse-Correlation Nmr for the Study of Proteins. J Magn Reson. 1990;86:304–318. [Google Scholar]
- 13.Cavanagh J, Fairbrother WJ, Palmer AG, Skelton NJ. Protein Nmr Spectroscopy. Academic Press; San Diegp: 1996. [Google Scholar]
- 14.Ishii Y, Tycko R. Sensitivity Enhancement in Solid State 15n Nmr by Indirect Detection with High-Speed Magic Angle Spinning. J Magn Reson. 2000;142:199–204. doi: 10.1006/jmre.1999.1976. [DOI] [PubMed] [Google Scholar]
- 15.Zhou DH, Shah G, Cormos M, Mullen C, Sandoz D, Rienstra CM. Proton-Detected Solid-State Nmr Spectroscopy of Fully Protonated Proteins at 40 Khz Magic Angle Spinning. J Am Chem Soc. 2007;129:11791–11801. doi: 10.1021/ja073462m. [DOI] [PubMed] [Google Scholar]
- 16.Zhou DH, Shah G, Cormos M, Mullen C, Sandoz D, Rienstra CM. Proton-Detected Solid-State Nmr Spectroscopy of Fully Protonated Proteins at 40 Khz Magic-Angle Spinning. J Am Chem Soc. 2007;129:11791–11801. doi: 10.1021/ja073462m. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal V, Penzel S, Szekely K, Cadalbert R, Testori E, Oss A, Past J, Samoson A, Ernst M, Boeckmann A, et al. De Novo 3d Structure Determination from Sub-Milligram Protein Samples by Solid-State 100 Khz Mas Nmr Spectroscopy. Angew Chem Int Edit. 2014;53:12253–12256. doi: 10.1002/anie.201405730. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Parthasarathy S, Xiao Y, Nishiyama Y, Endo Y, Nemoto T, Matsuda I, Long F, Yamauchi K, Asakura T, et al. Nano-Mole Scale Side-Chain Signal Assignment by 1h-Detected Protein Solid-State Nmr by Ultra-Fast Magic-Angle Spinning and Stereo-Array Isotope Labeling. Plos One. 2015;10:e0122714. doi: 10.1371/journal.pone.0122714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreas LB, Jaudzems K, Stanek J, Lalli D, Bertarello A, Le Marchand T, Paepe DCD, Kotelovica S, Akopjana I, Knott B, et al. Structure of Fully Protonated Proteins by Proton-Detected Magic-Angle Spinning Nmr. Proc Natl Acad Sci U S A. 2016;113:9187–9192. doi: 10.1073/pnas.1602248113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickramasinghe NP, Parthasarathy S, Jones CR, Bhardwaj C, Long F, Kotecha M, Mehboob S, Fung LWM, Past J, Samoson A, et al. Nanomole-Scale Protein Solid-State Nmr by Breaking Intrinsic H-1 T-1 Boundaries. Nature Methods. 2009;6:215–218. doi: 10.1038/nmeth.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti A, Jehle S, Felletti M, Knight MJ, Wang Y, Xu ZQ, Park AY, Otting G, Lesage A, Emsley L, et al. Backbone Assignment of Fully Protonated Solid Proteins by 1h Detection and Ultrafast Magic-Angle-Spinning Nmr Spectroscopy. Angew Chem Int Edit. 2012;51:10756–10759. doi: 10.1002/anie.201203124. [DOI] [PubMed] [Google Scholar]
- 22.Reif B, Jaroniec CP, Rienstra CM, Hohwy M, Griffin RG. H-1-H-1 Mas Correlation Spectroscopy and Distance Measurements in a Deuterated Peptide. J Magn Reson. 2001;151:320–327. doi: 10.1006/jmre.2001.2354. [DOI] [PubMed] [Google Scholar]
- 23.Asami S, Szekely K, Schanda P, Meier BH, Reif B. Optimal Degree of Protonation for H-1 Detection of Aliphatic Sites in Randomly Deuterated Proteins as a Function of the Mas Frequency. J Biomol NMR. 2012;54:155–168. doi: 10.1007/s10858-012-9659-9. [DOI] [PubMed] [Google Scholar]
- 24.Wickramasinghe A, Wang S, Matsuda I, Nishiyama Y, Nemoto T, Endo Y, Ishii Y. Evolution of Cpmas under Fast Magic-Angle-Spinning at 100 Khz and Beyond. Solid-state Nuclear Magnetic Resonance. 2015;72:9–16. doi: 10.1016/j.ssnmr.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer MR, Suiter CL, Henry GE, Rovnyak J, Hoch JC, Polenova T, Rovnyak D. Sensitivity of Nonuniform Sampling Nmr. J Phys Chem B. 2015;119:6502–6515. doi: 10.1021/jp5126415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark WG. Pulsed Nuclear Resonance Apparatus. Review of Scientific Instruments. 1964;35:316. [Google Scholar]
- 27.Wheeler DD, Conradi MS. Practical Exercises for Learning to Construct Nmr/Mri Probe Circuits. Concepts in Magnetic Resonance Part A. 2012;40A:1–13. [Google Scholar]
- 28.Petkova A, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. A Structural Model for Alzheimer’s B-Amyloid Peptide Fibrils Based on Experimental Constraints from Solid-State Nmr Spectroscopy. Proc Natl Acad Sci U S A. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Igumenova TI, McDermott AE, Zilm KW, Martin RW, Paulson EK, Wand AJ. Assignments of Carbon Nmr Resonances for Microcrystalline Ubiquitin. J Am Chem Soc. 2004;126:6720–6727. doi: 10.1021/ja030547o. [DOI] [PubMed] [Google Scholar]
- 30.Schanda P, Brutscher B. Very Fast Two-Dimensional Nmr Spectroscopy for Real-Time Investigation of Dynamic Events in Proteins on the Time Scale of Seconds. J Am Chem Soc. 2005;127:8014–8015. doi: 10.1021/ja051306e. [DOI] [PubMed] [Google Scholar]
- 31.Frydman L, Lupulescu A, Scherf T. Principles and Features of Single-Scan Two-Dimensional Nmr Spectroscopy. J Am Chem Soc. 2003;125:9204–9217. doi: 10.1021/ja030055b. [DOI] [PubMed] [Google Scholar]
- 32.Frydman L, Scherf T, Lupulescu A. The Acquisition of Multidimensional Nmr Spectra within a Single Scan. Proc Natl Acad Sci U S A. 2002;99:15858–15862. doi: 10.1073/pnas.252644399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frydman L, Blazina D. Ultrafast Two-Dimensional Nuclear Magnetic Resonance Spectroscopy of Hyperpolarized Solutions. Nature Physics. 2007;3:415–419. [Google Scholar]
- 34.Shapira B, Morris E, Muszkat KA, Frydman L. Sub-Second 2d Nmr Spectroscopy at Sub-Millimolar Concentrations. J Am Chem Soc. 2004;126:11756–11757. doi: 10.1021/ja0461668. [DOI] [PubMed] [Google Scholar]
- 35.Hashi K, Ohki S, Matsumoto S, Nishijima G, Goto A, Deguchi K, Yamada K, Noguchi T, Sakai S, Takahashi M, et al. Achievement of 1020 Mhz Nmr. J Magn Reson. 2015;256:30–33. doi: 10.1016/j.jmr.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Pandey MK, Zhang R, Hashi K, Ohki S, Nishijima G, Matsumoto S, Noguchi T, Deguchi K, Goto A, Shimizu T, et al. 1020 Mhz Single-Channel Proton Fast Magic Angle Spinning Solid-State Nmr Spectroscopy. J Magn Reson. 2015;261:1–5. doi: 10.1016/j.jmr.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishiyama Y. Fast Magic-Angle Sample Spinning Solid-State Nmr at 60–100 Khz for Natural Abundance Samples. Solid State Nucl Magn Reson. 2016;78:24–36. doi: 10.1016/j.ssnmr.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Mote KR, Agarwal V, Madhu PK. Five Decades of Homonuclear Dipolar Decoupling in Solid-State Nmr: Status and Outlook. Prog Nucl Magn Reson Spectrosc. 2016;97:1–39. doi: 10.1016/j.pnmrs.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Paul S, Thakur RS, Madhu PK. H-1 Homonuclear Dipolar Decoupling at High Magic-Angle Spinning Frequencies with Rotor-Synchronised Symmetry. Chem Phys Lett. 2008;456:253–256. [Google Scholar]
- 40.Brauckmann JO, Janssen JWG, Kentgens APM. High Resolution Triple Resonance Micro Magic Angle Spinning Nmr Spectroscopy of Nanoliter Sample Volumes. Physical Chemistry Chemical Physics. 2016;18:4902–4910. doi: 10.1039/c5cp07857a. [DOI] [PubMed] [Google Scholar]
- 41.Wickramasinghe NP, Kotecha M, Samoson A, Past J, Ishii Y. Sensitivity Enhancement in 13c Solid-State Nmr of Protein Microcrystals by Use of Paramagnetic Metal Ions for Optimizing 1h T1 Relaxation. J Magn Reson. 2007;184:350–356. doi: 10.1016/j.jmr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotecha M, Wickramasinghe NP, Ishii Y. Efficient Low-Power Heteronuclear Decoupling in 13c High-Resolution Solid-State Nmr under Fast Magic Angle Spinning. Magn Reson Chem. 2007;45:S221–230. doi: 10.1002/mrc.2151. [DOI] [PubMed] [Google Scholar]
- 43.Ernst M, Samoson A, Meier BH. Low-Power Xix Decoupling in Mas Nmr Experiments. J Magn Reson. 2003;163:332–339. doi: 10.1016/s1090-7807(03)00155-1. [DOI] [PubMed] [Google Scholar]
- 44.Fung BM, Khitrin AK, Ermolaev K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J Magn Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 45.Zhou DH, Rienstra CM. High-Performance Solvent Suppression for Proton Detected Solid-State Nmr. J Magn Reson. 2008;192:167–172. doi: 10.1016/j.jmr.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abragam A. Principles of Nuclear Magnetism. Oxford University Press; New York: 1961. [Google Scholar]
- 47.Kainosho M, Guentert P. Sail - Stereo-Array Isotope Labeling. Q Rev Biophys. 2009;42:247–300. doi: 10.1017/S0033583510000016. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Matsuda I, Long F, Ishii Y. Spectral Editing at Ultra-Fast Magic-Angle-Spinning in Solid-State Nmr: Facilitating Protein Sequential Signal Assignment by Highlight Approach. J Biomol NMR. 2016;64:131–141. doi: 10.1007/s10858-016-0014-4. [DOI] [PubMed] [Google Scholar]
- 49.Shen Y, Delaglio F, Cornilescu G, Bax A. Talos Plus: A Hybrid Method for Predicting Protein Backbone Torsion Angles from Nmr Chemical Shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petkova AT, Yau WM, Tycko R. Experimental Constraints on Quaternary Structure in Alzheimer’s Beta-Amyloid Fibrils. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH. Amyloid Fibrils of the Het-S(218–289) Prion Form a Beta Solenoid with a Triangular Hydrophobic Core. Science. 2008;319:1523–1526. doi: 10.1126/science.1151839. [DOI] [PubMed] [Google Scholar]
- 52.Helmus JJ, Surewicz K, Nadaud PS, Surewicz WK, Jaroniec CP. Molecular Conformation and Dynamics of the Y145stop Variant of Human Prion Protein. Proc Natl Acad Sci U S A. 2008;105:6284–6289. doi: 10.1073/pnas.0711716105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular Structure of Beta-Amyloid Fibrils in Alzheimer’s Disease Brain Tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao Y, Ma B, McElheny D, Parathasarathy S, Hoshi M, Nussinov R, Ishii Y. Aβ(1–42) Fibril Structure Illuminates Self-Recognition and Replication of Amyloid in Alzheimer’s Disease. Nature Structural and Molcular Biology. 2015;22:499–505. doi: 10.1038/nsmb.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takegoshi K, Nakamura S, Terao T. C-13-H-1 Dipolar-Driven C-13-C-13 Recoupling without C-13 Rf Irradiation in Nuclear Magnetic Resonance of Rotating Solids. J Chem Phys. 2003;118:2325–2341. [Google Scholar]
- 56.Jaroniec CP, Tounge BA, Herzfeld J, Griffin RG. Frequency Selective Heteronuclear Dipolar Recoupling in Rotating Solids: Accurate C-13-N-15 Distance Measurements in Uniformly C-13,N-15-Labeled Peptides. J Am Chem Soc. 2001;123:3507–3519. doi: 10.1021/ja003266e. [DOI] [PubMed] [Google Scholar]
- 57.De Paepe G, Lewandowski JR, Loquet A, Bockmann A, Griffin RG. Proton Assisted Recoupling and Protein Structure Determination. J Chem Phys. 2008;129 doi: 10.1063/1.3036928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lange A, Seidel K, Verdier L, Luca S, Baldus M. Analysis of Proton-Proton Transfer Dynamics in Rotating Solids and Their Use for 3d Structure Determination. J Am Chem Soc. 2003;125:12640–12648. doi: 10.1021/ja034555g. [DOI] [PubMed] [Google Scholar]
- 59.Zhou DH, Shea JJ, Nieuwkoop AJ, Franks WT, Wylie BJ, Mullen C, Sandoz D, Rienstra CM. Solid-State Protein-Structure Determination with Proton-Detected Triple-Resonance 3d Magic-Angle-Spinning Nmr Spectroscopy. Angew Chem Int Edit. 2007;46:8380–8383. doi: 10.1002/anie.200702905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber M, Boeckmann A, Hiller S, Meier BH. 4d Solid-State Nmr for Protein Structure Determination. Physical Chemistry Chemical Physics. 2012;14:5239–5246. doi: 10.1039/c2cp23872a. [DOI] [PubMed] [Google Scholar]
- 61.Huber M, Hiller S, Schanda P, Ernst M, Boeckmann A, Verel R, Meier BH. A Proton-Detected 4d Solid-State Nmr Experiment for Protein Structure Determination. Chemphyschem: a European journal of chemical physics and physical chemistry. 2011;12:915–918. doi: 10.1002/cphc.201100062. [DOI] [PubMed] [Google Scholar]
- 62.Ni QZ, Daviso E, Can TV, Markhasin E, Jawla SK, Swager TM, Temkin RJ, Herzfeld J, Griffin RG. High Frequency Dynamic Nuclear Polarization. Acc Chem Res. 2013;46:1933–1941. doi: 10.1021/ar300348n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM. Magic-Angle Spinning Solid-State Nmr Spectroscopy of the Beta 1 Immunoglobulin Binding Domain of Protein G (Gb1): N-15 and C-13 Chemical Shift Assignments and Conformational Analysis. J Am Chem Soc. 2005;127:12291–12305. doi: 10.1021/ja044497e. [DOI] [PubMed] [Google Scholar]