Abstract
The intricate architecture of branched tissues and organs has fascinated scientists and engineers for centuries. Yet―despite their ubiquity―the biophysical and biochemical mechanisms by which tissues and organs undergo branching morphogenesis remain unclear. With the advent of three-dimensional (3D) culture models, an increasingly powerful and diverse set of tools are available for investigating the development and remodeling of branched tissues and organs. In this review, we discuss the application of 3D culture models for studying branching morphogenesis of the mammary gland and the mammalian lung in the context of normal development and disease. While current 3D culture models lack the cellular and molecular complexity observed in vivo, we emphasize how these models can be used to answer targeted questions about branching morphogenesis. We highlight the specific advantages and limitations of using 3D culture models to study the dynamics and mechanisms of branching in the mammary gland and mammalian lung. Finally, we discuss potential directions for future research and propose strategies for engineering the next generation of 3D culture models for studying tissue morphogenesis.
Keywords: Morphodynamics, Organoids, microfabricated tissues, extracellular matrix, epithelium, tissue engineering
Introduction
Branched architectures are observed in tissues and organs as diverse as the lung [1], kidney [2], mammary gland [3], salivary gland [4], pancreas [5], prostate [6], blood vessels [7], and the nervous system [8]. The intricate three-dimensional (3D) geometry of these tissues and organs is generated during normal development through a process known as branching morphogenesis, wherein initially simple tissues undergo recursive rounds of branching and elongation to produce more complex tree-like structures. The resulting branched architectures, which have striking differences in form and function, maximize the surface area of a tissue within a confined volume. While the anatomy and physiology of branched tissues is deeply appreciated, the physical and chemical mechanisms that drive branching morphogenesis remain unclear. Elucidating the mechanisms that generate branched tissues will strengthen our understanding of normal development, disease progression, and regeneration. Moreover, this information will play an integral role in engineering 3D branched tissues and organs that can be used for transplant or the screening of new therapeutics.
The study of biological morphogenesis was formalized more than 100 years ago with the seminal work of D’Arcy Wentworth Thompson [9]. Among other things, Thompson applied principles from physics and chemistry to explain the shape of cells in epithelial tissues [10, 11]. Thompson hypothesized that the same principles that govern the shape of bubbles in two-dimensional (2D) foams govern the shape of cells in an epithelial tissue [10, 11]. The analogy between tissues and foams subsequently led to new concepts in developmental biology [10], which were important for describing cell and tissue geometry. Since these early observations by Thompson and others, our understanding of tissue morphogenesis has improved dramatically as a result of advances in both experimental [12] and computational approaches [13]. However, studying branching morphogenesis remains particularly challenging because branched tissues are not easily observed due to their location and small size (~102 – 104 µm) during development. Animal models such as the mouse have been used successfully to investigate the development of branched tissues and organs, but tissues isolated from embryos can often only be used to probe the earliest stages of their development. Alternatively, cells can be extracted from branched tissues and grown in culture, but cells on 2D surfaces do not maintain the same differentiation or gene expression observed in vivo. In response to these challenges, 3D culture models, which attempt to recapitulate key aspects of the tissue architecture and in vivo microenvironment, have been established to study branching morphogenesis.
The use of 3D culture models to study tissue morphogenesis has been reviewed previously [14, 15]. Here, we focus specifically on the use of 3D culture models to investigate the dynamics and mechanisms of branching morphogenesis in the mammary gland and mammalian lung. While it is widely appreciated that current 3D culture models do not capture the complexity of the in vivo microenvironment, we describe how these simplified model systems can be used to answer key questions about the morphogenesis that builds these two organs. In doing so, we emphasize the advantages and limitations of conventional 3D culture models for studying morphogenesis in these tissues. We also discuss how new advances in biomaterials and cell culture platforms can be used to improve the biological verisimilitude of 3D culture models.
We begin this review with an overview of the development, structure, and function of the mammary gland and the mammalian lung. We highlight their similarities and differences in terms of the timescales of morphogenesis and branching routines as well as the composition of these tissues and their surrounding extracellular matrix (ECM). Next, we discuss state-of-the-art 3D culture models including explants, organoids, microfabricated tissues, and organs-on-a-chip, and provide specific examples wherein each has been used to examine the process of branching. Finally, we summarize new technologies that may be incorporated into 3D culture models to improve their spatial and temporal accuracy and propose strategies for engineering the next generation of models to investigate tissue morphogenesis.
Development and structure of the mammary gland and mammalian lung
Mammary gland
The mammary gland―which is unique to mammals―is located within the fat pad of the breast, and its primary function is to produce and secrete milk. In both mice and humans, the development of the mammary gland occurs in three distinct stages denoted as embryonic, pubertal, and adult. Development of the mouse mammary gland begins in the embryo with the emergence of placodes from the milk lines at embryonic day 11.5 (E11.5) [16]. By E15.5, these placodes transform into mammary epithelial buds through short-range signaling interactions with the surrounding mesenchyme [17]. Cells in these initial mammary epithelial buds proliferate and migrate through the mesenchyme into the developing fat pad. Once in the fat pad, mammary epithelial buds undergo further morphogenesis to form the basic ductal structure that is present at birth, which is comprised of a main duct and 15–20 secondary branches (Figure 1A) [16]. Similar development is not observed in male mice because the mesenchyme condenses around and eventually severs the nascent epithelial buds [18].
Figure 1. Development and structure of the mammary gland.
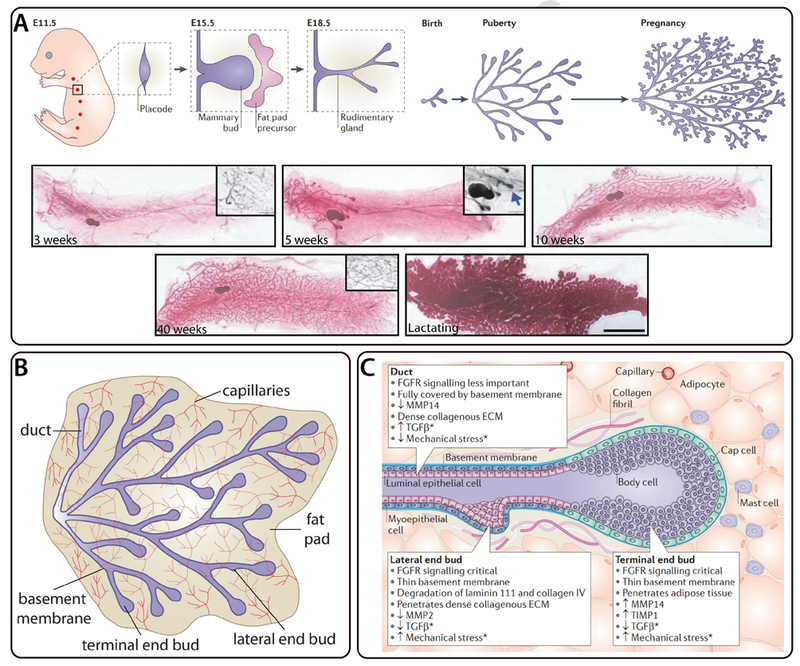
Mammary epithelial branching morphogenesis in the developing mouse mammary gland. A) Mammary gland development begins in the mouse embryo at E11.5 and results in a rudimentary gland at E18.5. The gland remains quiescent until puberty when it undergoes recursive rounds of extension and bifurcation to produce a tree-like epithelial tissue. The tissue undergoes further morphogenesis during pregnancy. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Molecular Cell Biology [20], copyright (2011). Whole-mount inguinal mouse mammary glands are shown at various stages of development after birth; large scale bar represents 1 cm and small scale bar represents 1 mm. Reprinted by permission from Macmillan Publishers Ltd: Nature Genetics [111], copyright (2010). B) Schematic of mouse mammary epithelial tissue labeled for capillaries, duct, basement membrane, fat pad, lateral end bud, and terminal end bud. Figure inspired by [20]. C) Cellular composition of terminal and lateral end buds as well as the surrounding fat pad in the mouse mammary gland. Reprinted by permission from Macmillan Publishers Ltd: Nature Reviews Molecular Cell Biology [20], copyright (2011).
The branched mammary epithelial tissue remains quiescent until puberty (~6–8 week old mice), at which time the mammary epithelium begins to branch in response to increased levels of ovarian hormones. During branching morphogenesis, the tips of the mammary epithelial tissue transform into terminal end buds (TEBs) that invade the surrounding fat pad. TEBs routinely undergo extension and bifurcation, which gives rise to a highly-branched tree-like architecture (Figure 1A). The mammary epithelium grows until the TEBs reach the limit of the fat pad, at which point additional lateral branches continue to form (Figure 1B). In contrast to the highly stereotyped branching morphogenesis observed in other organs such as the lung, branching of the mammary epithelium is a stochastic process, which means each epithelial tissue generates a different number of branches and a random final architecture.
During pregnancy, the mammary epithelium undergoes additional remodeling as the luminal epithelium proliferates and differentiates into structures known as alveoli, which produce and secrete milk. This process is referred to as alveologenesis and can occur throughout the reproductive lifetime of a mammal, which spans approximately 8 months for mice and several decades for humans. After pregnancy and lactation, the mammary gland undergoes programmed cell death of milk-producing epithelial cells in a process known as involution. During involution, the stroma surrounding the mammary gland is also remodeled as a result of the secretion of matrix metalloproteinases (MMPs) [19]. Once involution is complete, the mammary epithelium reenters a period of quiescence. As a result of this sustained capacity to undergo dramatic changes in tissue geometry and composition, the mammary epithelium is an ideal system for studying tissue morphogenesis. For a more detailed description of mammary epithelial branching morphogenesis, we refer readers to the following review article [20].
The developing mammary epithelium is comprised of several distinct cell populations including luminal epithelial cells, basal myoepithelial cells, cap cells, and body cells (Figure 1B and C). The internal surface of the gland consists of the luminal epithelium, which surrounds the hollow lumen and is lined basally by the myoepithelium. Luminal epithelial cells are important for lumen integrity and can differentiate into milk-producing alveoli, while myoepithelial cells contract in response to oxytocin to help transport milk [21]. The myoepithelium consists of basal myoepithelial cells that directly contact the basement membrane, which primarily contains type IV collagen and laminin. The basement membrane anchors the mammary epithelium to the surrounding fat pad, which contains type I collagen, adipocytes, capillaries, nerves, and mast cells (Figure 1B and C). In the TEB of the developing mammary epithelium, an outer layer of cap cells surrounds a multi-layered population of body cells (Figure 1C). The cap cells appear to be a population of mammary stem cells that differentiate into the myoepithelium [22], whereas the body cells give rise to luminal epithelial cells [23].
While the development and structure of the mouse mammary gland have been studied extensively, we lack a comprehensive understanding of how signals from the mesenchyme impact epithelial branching and architecture. No single growth factor controls branching of the mammary epithelium [13], but transforming growth factor-β (TGFβ) inhibits branch initiation and fibroblast growth factor (FGF) receptor 2 is required for ductal elongation [25]. In addition, aligned fibers of type I collagen in the fat pad are believed to influence the final geometry of the mammary epithelium [26, 27]. 3D culture models can serve as useful systems to study the role of specific signaling pathways and physical cues in branching morphogenesis of the mammary gland.
Mammalian lung
The mammalian lungs are located in the chest cavity and are responsible for gas exchange with the blood. Early lung development in both human and mouse occurs across four stages referred to as pseudoglandular, canalicular, terminal saccular, and alveolar [28]. In contrast to development of the mammary gland, which occurs in distinct stages, the developmental stages of the mammalian lung overlap [29], and begin with the pseudoglandular stage between E9.5 and E16.5 in the mouse [28]. During this stage, branching morphogenesis of the airway epithelium generates a complex tree-like structure that begins to approximate the architecture of the mature lung (Figure 2A). Branching occurs via three different modes known as domain branching, planar bifurcation, and orthogonal bifurcation (Figure 2A). Domain branches form perpendicular to and around the circumference of the parent branch (Figure 2A). Planar bifurcation occurs at the tips of the developing airway epithelium and generates branches within the same plane as the previous bifurcation event, whereas orthogonal bifurcation generates branches perpendicular to the previous one (Figure 2A). Unlike morphogenesis of the mammary gland, the early stages of airway branching are highly stereotyped. Airway smooth muscle is required for terminal bifurcation of the airway epithelium [30], and mesenchymal expression of FGF10 influences branch initiation [31]. During this stage of development, mesenchymal cell death is also important for branching of the epithelium [32]. By the end of the pseudoglandular stage, the prospective airways and acinar outlines have formed [33].
Figure 2. Development and structure of the mammalian lung.
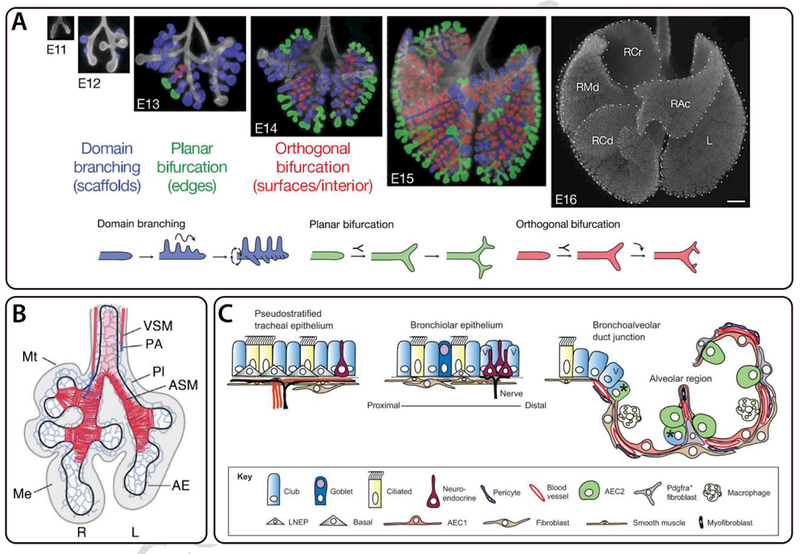
Branching morphogenesis of the airway epithelium in the developing mouse lung. A) Optical images of the ventral side of whole-mount lungs at E11-E16; scale bar represents 500 µm. The left (L) and right cranial (RCr), middle (RMd), caudal (RCd), and accessory (RAc) lobes of the E16 lung are labeled. Branching modes are shown below and highlighted in the images of whole-mount lungs at E12-E15. Reprinted by permission from Macmillan Publishers Ltd: Nature [1], copyright (2008). B) Schematic of an E11.5 developing mouse lung labeled for vascular smooth muscle (VSM), pulmonary arteries (PA), mesothelium (Mt), vascular plexus (Pl), airway smooth muscle (ASM), airway epithelium (AE), and mesenchyme (Me). Figure from [35]. Reprinted with permission from AAAS. C) Cellular composition of the pseudostratified tracheal epithelium, bronchiolar epithelium, and bronchoalveolar duct junction in the mouse lung. Republished with permission of The Company of Biologists Ltd, from [78].
The canalicular stage occurs between E16 and E17 and results in an overall increase in the length and diameter of the lung [28]. Angiogenesis and vascularization also increase during this stage (Figure 2B) [28]. Additional structural changes result in the transformation of terminal bronchioles into both alveolar ducts and respiratory bronchioles [28]. Moreover, airway epithelial cells differentiate into peripheral squamous and proximal cuboidal cells [28]. Squamous cells cover the surface of the alveoli and play an important role in gas exchange, while cuboidal cells line bronchioles and are ciliated in order to help remove debris from the airway.
The next stage of development, known as the terminal saccular stage, spans from E17 to postnatal day 5 (P5) in the mouse [28]. During this stage, apoptosis and differentiation of mesenchymal cells results in thinning of the interstitium [28]. Moreover, capillaries and lymphatics begin to develop rapidly [28]. Alveolar epithelial cells differentiate into mature squamous type I pneumocytes and rounded secretory type II pneumocytes [28]. Type II pneumocytes are important for the synthesis of surfactant, which plays a significant role in stabilizing the alveoli by reducing the surface tension of the air-liquid interface [28, 34]. Lastly, the alveolar stage of development occurs from P5 to P30, and it is during this period that the gas exchange surface matures [28].
The resulting structure consists of a highly-branched network of airways, which are referred to as bronchi if they are surrounded by cartilage and bronchioles if they are not. Bronchioles terminate in the highly vascularized alveoli that are directly responsible for gas exchange with the blood. The key components of the developing lung include the airway epithelium, airway smooth muscle, basement membrane, and other features of the mesenchyme, including the vasculature (Figure 2B). The developing lung is comprised of a rich diversity of cell types, each of which has a specialized function and spatial distribution (Figure 2C). The airways contain basal, ciliated, and secretory epithelial cells, while the alveoli are lined with type I and type II epithelial cells. Basal cells serve as progenitors for ciliated and secretory cells. The airway epithelium surrounds the lumen of the lung and in the adult plays an important role in secreting fluid that can trap and remove inhaled particulates. Airway smooth muscle wraps around the airway epithelium and provides both mechanical support and physical constraints during terminal bifurcation of the branching epithelium [30]. Similar to the mammary gland, the basement membrane anchors the epithelial tissue to the surrounding mesenchyme and is primarily comprised of laminin and type IV collagen. The mesenchyme surrounding the developing lung contains undifferentiated progenitor cells that eventually form components of the stromal tissue including fibroblasts, cartilage, and airway smooth muscle, among others [35]. For a more detailed review of mammalian lung development, readers are referred to the following excellent review articles [28, 29].
Several open questions remain about how airway epithelial branching morphogenesis is regulated throughout the development of the mammalian lung. What specifies the location of domain branches during the pseudoglandular stage of lung development? Moreover, how can a small number of genes regulate the highly stereotyped structure that contains millions of branches? [13]. Attempts have been made to model the lung as a fractal pattern whose development is governed by a simple set of rules, but the airway epithelium does not present as a fractal pattern during the early stages of its development [13]. As with the mammary gland, 3D culture models can test both mechanical and chemical models of lung development and serve as useful tools to investigate the aforementioned questions.
Branched tissues can be remodeled in response to injury and disease
Beyond normal development, the mammary gland and mammalian lung can undergo dramatic remodeling in response to injury or disease progression. Remodeling commonly results in changes to the function and morphology of tissues, differentiation and proliferation of cell populations, and physical or chemical properties of the ECM. In the mammary gland, remodeling primarily occurs in response to aging-related regression and breast cancer. Studies comparing old and young mammary glands found differences in the luminal-to-basal cell ratio, and an increase in irregular ductal morphology in the former [36]. Remodeling of mammary epithelial tissue has been observed in breast cancer where cells may lose or acquire new functions, which can affect tissue organization [37]. Transplantation of cancer stem cells into the mammary fat pad has also been observed to alter the branched architecture of the mammary epithelium [38].
The airways of the mammalian lung are most commonly remodeled in response to injury or disease. Pulmonary cells in the adult lung undergo apoptosis in response to injury [39]. In patients with asthma, the large and small airways can be remodeled through fibrosis, smooth muscle hyperplasia, and changes in vascularization, among others [40]. Airways also remodel in response to diseases including cystic fibrosis [41], chronic obstructive pulmonary disease [42], and idiopathic pulmonary fibrosis [43]. As with the mammary gland, cancer can result in dramatic changes in the organization and architecture of the lung. Moreover, chemotherapeutic drugs such as bleomycin can damage the lung and result in additional tissue remodeling [44]. Taken together, these examples motivate the need for 3D culture models with the flexibility to recapitulate both normal development as well as remodeling in response to injury or disease. In the following section, we describe four state-of-the-art 3D culture models and highlight how each of these models has been used to study normal branching morphogenesis and disease progression of the mammary gland and mammalian lung.
3D culture models for the mammary gland and the mammalian lung
3D culture models are engineered biomimetic systems that are designed to recapitulate key biophysical and biochemical aspects of a tissue and its surrounding microenvironment. As described above, primary components of branched organs include the epithelium, basement membrane, interstitial ECM, muscle, vasculature, connective tissue, and nerves. To date, engineering a culture model that simultaneously incorporates these components has not been achieved. However, the use of simplified model systems can provide several advantages for investigating the dynamics and mechanisms of branching morphogenesis. Above all, one hallmark of 3D culture models is their modular design, which provides experimental flexibility for isolating and studying the role of specific physical or chemical factors in a biological process. 3D culture models also allow for multiple experimental conditions to be rapidly investigated in a controlled environment. In the following subsections, we describe four commonly used 3D culture models and detail their use in studying branching morphogenesis of the mammary gland and mammalian lung.
Organ explants
Organ explants consist of whole organs, slices of organs, or minced organs that are removed from an animal and grown ex vivo (Figure 3A). Explants typically remain viable for several days in culture, which allows researchers to directly observe changes in tissue architecture and cellular composition. In addition to epithelial tissue, whole-organ and sliced-organ explants often include additional components derived from the ECM. Minced organs are often further processed to isolate specific regions of the tissue. One primary advantage of using organ explants is that cells or biological molecules, which are not accessible in vivo, can be labeled and tracked during development in culture. In addition, cells that are normally present in mature branched tissues can be isolated, cultured, and studied. Organ explants can also be cultured in a variety of microenvironments ranging from suspension in cell culture medium alone to embedding within natural ECM proteins, such as type I collagen or Matrigel. These biomaterials allow the bulk physical and chemical properties of the microenvironment surrounding the explant to be modulated.
Figure 3. State-of-the-art 3D culture models for studying the mammary gland and mammalian lung.
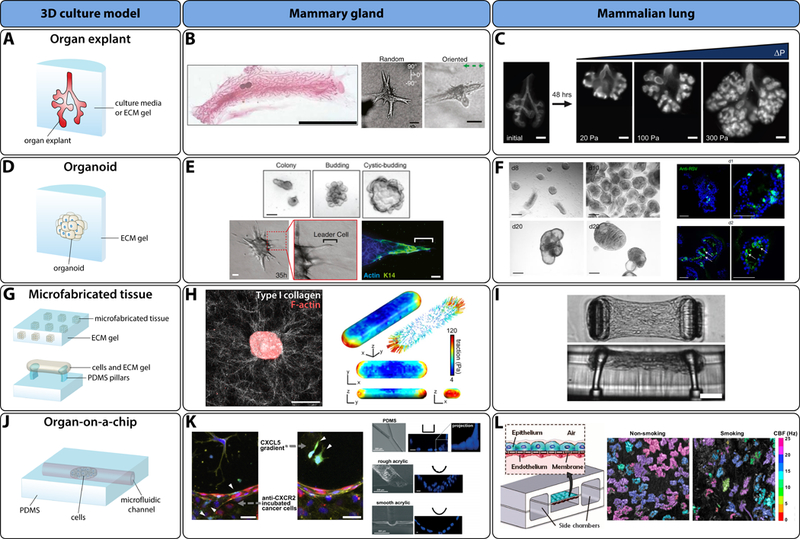
A) Schematic of organ explant. B) Examples of mouse mammary gland explants. Left: whole-mount inguinal mammary gland explant 10 weeks after birth (scale bar 1 cm; reprinted by permission from Macmillan Publishers Ltd: Nature Genetics [111], copyright (2010)). Right: mammary explants branching in collagen gels with random and oriented alignment of collagen fibers (scale bars 50 µm; reprinted with permission from Elsevier, copyright (2013)) [27]. C) Lung explants cultured at various transmural pressures and immunostained (E-cadherin) (scale bars 200 µm; republished with permission of The Company of Biologists Ltd, from [51]). D) Schematic of an organoid. E) Top: examples of mouse mammary basal cell organoids (scale bar 100 µm; republished with permission of The Company of Biologists Ltd, from [66]). Bottom: mammary tumor organoid protrusions in a type I collagen gel (scale bars represent 50 µm for DIC images and 20 µm for immunofluorescence image; reprinted with permission from Elsevier, copyright (2013)) [67]. F) Mammalian lung bud organoids (left: normal (scale bars 250 µm); right: infected with respiratory syncytial virus (scale bars 100 µm) (reprinted by permission from Macmillan Publishers Ltd: Nature Cell Biology [75], copyright (2017)). G) Schematic of square microfabricated tissues embedded in an ECM gel (top) and cell-gel mixture suspended between PDMS pillars (bottom). H) Left: mammary epithelial microfabricated tissue (scale bar 50 µm). Right: average distribution of traction forces around the surface of a microfabricated epithelial tissue (reprinted with permission from Elsevier, copyright (2012)) [84]. I) Microfabricated tissue gauge suspended between PDMS pillars (scale bar 100 µm) [112]. Copyright (2009) National Academy of Sciences. J) Generalized schematic of a microfluidic organ-on-a-chip. K) Left: extravasation of GFP-labelled cancer cells (white arrows) in response to CXCL5 gradient (scale bars 50 µm; reprinted with permission from Elsevier, copyright (2014)) [86]. Right: representative example of organ-on-a-chip systems for the mammary gland (reproduced from [87] with permission of The Royal Society of Chemistry). L) Left: representative example of mammalian lung organ-on-a-chip (Figure from [88]; reprinted with permission from AAAS). Right: time-lapse images representing the ciliary beat frequency (CBF) on the apical surface of the bronchiolar epithelium (reprinted with permission from Elsevier, copyright (2014)) [89].
The mammary gland and mammalian lung have been studied using whole-organ explants [45, 46], organ slices [47, 48], and organ fragments [26, 49]. To investigate branching of the mouse mammary epithelium, whole-gland explants are often obtained from adolescent mice since the most dramatic branching occurs during puberty. Whole-organ explants of the mouse mammary gland have been used to study the dynamics of mammary stem cells, which drive branching morphogenesis [50]. The TEB was found to be comprised of lineage-committed mammary stem cells that contribute to ductal elongation on short timescales and, because of cellular rearrangements, long-term growth of the mammary gland [50]. The role of collagen fiber alignment in mammary epithelial branching morphogenesis has also been investigated using pieces of mammary explants embedded in anisotropic type I collagen gels [27]. Collagen fiber alignment was found to be sufficient to direct mammary epithelial branching in a process modulated by Rac1 GTPase activity (Figure 3B) [27].
In contrast, mouse lung explants are frequently isolated during the embryonic stages of development when the architecture of the airway remains relatively simple. Metzger et al. used mouse lung explants to study the pattern of branching during embryonic lung development [1]; and found that branching of the mouse bronchial tree is highly stereotyped and results from three distinct branching modes: domain branching, planar bifurcation, and orthogonal bifurcation [1]. Mouse lung explants have also been used to study the role of transmural pressure, which controls the rate of lung development and the frequency of contraction of airway smooth muscle (Figure 3C) [51]. In another study of mouse lung explants, Bellusci et al. showed that FGF10 induces morphogenesis of the airway epithelium [31]. Recent advances in live imaging have allowed researchers to study cellular dynamics in domain branching and planar bifurcation of lung explants [52], and have revealed that bifurcation events are regulated by myosin light-chain kinase, while domain branching is not [52].
There are several limitations associated with using organ explants to study branching morphogenesis. Above all, explants cannot be cultured indefinitely, which limits the timescales over which branching morphogenesis and tissue remodeling can be investigated. Explants are typically cultured over the span of several days, but previous studies have cultured mammary gland explants for more than 30 days [53] and mammalian lung explants for up to 27 days [54]. There might also be differences between the branching patterns that emerge from cultured explants and those that develop in vivo. Mammary gland explants cultured in collagen gels fail to form normal end buds unless cultured in direct contact with adipocytes [26]. Similarly, mammalian lung explants are frequently separated from the mesenchyme in culture, which is known to regulate branching through FGF and mechanical signaling [30, 55, 56]. Other studies have observed that the size and extent of airway epithelial branching is lower in lung explants cultured in the absence of exogenous factors than in vivo controls [54]. In order to obtain sufficient power for statistical significance, it is often necessary to isolate several explants for every experiment, which is a time-consuming endeavor. Finally, it is challenging to study the behavior of individual cells within large tissues or whole-organ explants due to light-scattering and other imaging limitations. Nevertheless, organ explants serve as a versatile tool to study the complex tissue geometries that emerge during branching morphogenesis.
Organoids
There are several definitions of the term organoid [57]. Here, we discuss organoids in the context of self-assembling structures that result from isolated primary or stem cells cultured in a 3D gel or ECM-like material (Figure 3D). These structures capture varying degrees of tissue architecture and biological signaling observed in vivo. One advantage of organoids is that the behavior and morphology of specific cells from a tissue or organ can be examined systematically in culture. Moreover, organoids comprised of human cells can be used to investigate aspects of morphogenesis that remain inaccessible in vivo because of technical limitations or ethical considerations. As compared to 2D culture models, organoids more accurately replicate in vivo tissue geometry, which is important for functional differentiation [57] and cell signaling [58]. The composition of organoids is also completely decoupled from the adjacent ECM, which is useful for studies that attempt to determine the role of the physical and chemical properties of the ECM in morphogenesis.
Protocols have been established to generate organoids for both the mammary gland [59, 60] and the mammalian lung [61]. Mammary organoids typically consist of mouse mammary epithelial cells obtained from the thoracic or inguinal mammary glands at the developmental stage of interest. Glands are dissected from mice, minced, treated with collagenase, and fractionated into different cell types. After isolation, cells are embedded in an ECM gel and submerged in culture medium. The resulting mammary organoids can be cultured for timescales on the order of weeks, after which epithelial polarity and functional differentiation may be lost [62, 63]. Alternatively, mammary organoids can be derived from differentiated stem cells. Mammary organoids have been used successfully to study several aspects of mammary epithelial branching morphogenesis. Using primary mammary epithelial organoids and clusters of SCp2 mammary epithelial cells cultured in type I collagen gels, Simian et al. confirmed that MMPs are required for mammary epithelial branching [59]. Another study cultured primary mammary organoids in Matrigel and found that the duration of ERK1/2 activation is important for morphogenesis and can be used to predict both cellular and morphogenetic outcomes [64]. Mammary organoids have also been used to investigate mammary stem cells, which drive branching. For example, whole breast organoids consisting of both basal and luminal cell populations can be derived from a single Lgr5+ cell [65]. Mammary organoids with the bilayered structure observed in vivo have also been generated using single basal mammary epithelial cells [66]. In addition, insight into breast cancer can be gained from studies with mammary organoids. Breast cancer organoids generated from fragments of isolated primary mouse and human mammary tumors have been used to study collective invasion (Figure 3E; bottom) [67]. This study revealed that activation of basal epithelial genes converted luminal cancer cells to invasive leader cells [67]. Mammary organoids thus represent a robust culture model for investigating mammary morphogenesis.
Mammalian lung organoids are typically derived from epithelial cell populations in the developing and adult lung or from differentiated stem cells using the same procedures described above for the mammary gland. To date, lung organoids have been generated from mouse and human basal cells [68], mouse airway secretory cells [69], mouse alveolar type II cells [70], and human pluripotent stem cells [71], but organoids derived from basal cells are the best understood [72]These various organoids have been used to investigate aspects of both human and mouse airway branching morphogenesis, but these models only recapitulate limited aspects of the branching programs observed in vivo [73]. For example, organoids of endothelial and basal-like epithelial cells have been used to show that endothelial cells are required for branching morphogenesis of airway epithelium in culture [15]. Another study found that human bronchial cells cultured in Matrigel form branched structures with a phenotype that depends on signaling from fibroblasts [74]. Lung bud organoids are also promising for capturing changes that occur in response to disease. Lung bud organoids derived from human pluripotent stem cells were infected with respiratory syncytial virus and responded by swelling and shedding infected cells into the organoid lumen, which is similar to the response of human lungs in vivo (Figure 3F; right) [75]. Moreover, organoids comprised of cells with a mutation in HPS1, which can cause an early form of pulmonary fibrosis, accumulated ECM and mesenchymal cells as observed in vivo [75]. Another study used organoids consisting of type II alveolar epithelial cells and found that telomere-mediated lung disease is driven by alveolar stem cell failure in the form of dormancy reminiscent of cellular senescence [76].
Currently available versions of organoids have limited use for studying developmental processes such as branching morphogenesis. The timescales and morphologies of branches observed in organoids are not consistent with the branching morphologies found in vivo. For example, mammary organoids undergo branching over a period of days in culture, but branching takes place over a timescale of weeks in vivo [77]. Mammary organoids can have a range of morphologies and cellular compositions that are reminiscent, but noticeably different, from those found in the mammary gland in vivo [77]. For example, mammary buds can be incompletely covered by myoepithelial cells in mammary organoids cultured in Matrigel, a topology that is only observed in side branches of the mammary epithelium in vivo [77]. Similarly, mammalian lung buds cultured in Matrigel may not undergo branching morphogenesis [71], and even lung bud organoids that do branch are unable to replicate later stages of development [75]. In addition, cultures of type II alveolar epithelial cells do not recapitulate the structure of the alveoli in the lung [78]. The size of organoids in culture is also limited because of the lack of vascular perfusion [79]. Additionally, the efficiency of organoid formation is low, meaning that this approach is not yet amenable to high-throughput screening needed for therapeutic development. It can also be challenging to obtain consistent results from organoid culture due to the variability in isolated cells [78] as well as poorly defined culture media and matrix materials [79]. For example, in the context of mammary epithelial branching morphogenesis, the morphology of the branches that form on mammary organoids depends on the mouse strain, the initial size of the organoid, and the type of growth factors present [77]. Finally, isolating specific populations of cells for organoid studies can be complicated. Despite these limitations, organoids represent a powerful model system for studying the role of cellular and ECM composition in the early stages of branching morphogenesis.
Microfabricated tissues
Microfabricated tissues are microscale model systems generated using soft-lithographic approaches that enable the spatial control of the positions of populations of cells (Figure 3G). Typically, a polydimethylsiloxane (PDMS) elastomeric stamp is generated via soft lithography and used as a mold to generate 3D cavities of defined geometry within hydrogels that can be seeded with cells. These tissues can also take the form of self-assembling cell-gel mixtures that span between two flexible PDMS pillars, which act as a tension sensor [80]. One advantage of microfabricated tissues is that they allow for precise control of tissue geometry, composition, and the bulk properties of the surrounding matrix. These systems also allow for control of the initial geometry of the tissue prior to induction of morphogenesis, which provides a unique advantage relative to other 3D culture models that cannot systematically control initial tissue geometry. Lastly, arrays of hundreds of identical tissues can be fabricated, which provides a large enough sample size to enable statistical analysis.
Microfabricated tissues have been used extensively to study the role of tissue geometry and matrix properties in epithelial morphogenesis [81]. Microfabricated mammary epithelial tissues embedded in a type I collagen matrix revealed that the position of branch initiation is dictated by tissue geometry [24]. Branches were observed to initiate in regions with a local minimum concentration of TGFβ, which acts as an autocrine inhibitory morphogen in the mammary gland [24]. Using the same system to investigate the importance of adipose stroma in mammary epithelial morphogenesis, branching was found to be induced by paracrine signals secreted by the adipocytes such as hepatocyte growth factor [82]. Additional studies have investigated interactions between microfabricated mammary epithelial tissues and their surrounding fibrous ECM. Mammary epithelial tissues align their surrounding collagen fibers prior to branching in a primarily physical process, and tissue geometry dictates the pattern of aligned collagen fibers [83]Microfabricated tissues have also been used to quantify the mechanical strain applied by mammary epithelial tissues on their surrounding matrix during the branching process [84]. The spatial distribution of mechanical stresses is also dictated by tissue geometry, and contraction of multicellular tissues can result in regions of compression of the matrix (Figure 3H; right) [84].
Microfabricated lung tissues have not been used extensively because it is difficult to obtain primary embryonic lung epithelial cells, which are necessary to study the earliest stages of branching that take place before epithelial differentiation. One recent study generated microtissues using airway smooth muscle cells (Figure 3I), and found that fibroblasts were important for tissue survival time whereas substratum geometry appeared to regulate tissue contractility [80].
Current microfabrication-based approaches can be used to generate small tissues (~102 – 103 cells) of relatively simple initial geometries. These systems are smaller than those that undergo branching morphogenesis in vivo, which typically commences in tissues comprised of thousands of cells. In their current form, only the bulk properties of the surrounding matrix can be tuned and microfabricated culture models do not incorporate vasculature, which means that the size of tissues are currently limited to the order of 102 µm. It can also be challenging to replicate tissue-tissue interactions, which are required for morphogenetic processes such as branching. For example, interactions between the airway epithelium and its surrounding mesenchyme are essential for airway branching, but these interactions cannot be recapitulated using microfabricated tissues in their current form. Nonetheless, microfabricated tissues can be used to provide important information about the role of tissue geometry as well as the ECM and tissue composition in the early stages of branching morphogenesis.
Organs-on-a-chip
Organ-on-a-chip systems include organoids, explants, cell aggregates, or cell monolayers cultured on a micropatterned (often microfluidic) device (Figure 3J). These systems often incorporate vascular (or vascular-like) perfusion, which helps to maintain cell viability for relatively long periods of time (~ 1 month) [85]. In addition, these models are designed in a modular fashion, which means that interactions between distinct tissues and organs can be studied by integrating multiple organ-on-a-chip systems. This is particularly helpful for the screening of potential therapeutics, which can impact multiple organs due to non-specific or off-target effects.
Despite their many advantages, organ-on-a-chip models have not yet been used to study branching of any tissue to the best of our knowledge. However, these culture models have been used to investigate factors that are known or suspected to be important in branching morphogenesis or remodeling during disease. In the context of the mammary gland, organ-on-a-chip models have been used to study diseases such as breast cancer, the progression of which affects mammary epithelial tissue morphology. The specificity of breast cancer metastasis to bone was examined using a microfluidic chip model consisting of human endothelial, breast cancer, and osteo-differentiated bone marrow-derived mesenchymal stem cells [86]. This study identified a breast cancer cell receptor (CXCR2) and a bone-secreted chemokine (CXCL5) that impacted the rate of extravasation of tumor cells and subsequent distance traveled (Figure 3K; left) [86]. In another study, mammary epithelial tumor growth was recapitulated using a semicircular acrylic support to mimic the geometry of the mammary duct (Figure 3K; right) This proof-of-concept study found that cells had different morphologies and responses to anticancer drugs when cultured on curved surfaces as compared to flat surfaces [87].
The current lung-on-a-chip model incorporates human alveolar epithelial and pulmonary microvascular endothelial cell layers separated by a semipermeable boundary [88]. While the lung-on-a-chip model is not designed to investigate tissue morphogenesis per se, it can be used in studies related to the lung remodeling that occurs during asthma or chronic obstructive pulmonary disease. A small airway-on-a-chip model comprised of normal or diseased human bronchiolar epithelium was used to examine the effect of smoking on the epithelium [89]. This study found new ciliary micropathologies induced by smoking, which could not have been accomplished with other systems because they do not recapitulate physiological breathing conditions (Figure 3L; right) [89]. Other lung-on-a-chip systems have been created to study lung inflammation [90]. In particular, the role of eosinophil cationic protein in lung inflammation was investigated and it was determined that fibrocyte extravasation was mediated by the CXCL12-CXCR4 axis [90]. Lung-on-a-chip systems have also been used to recapitulate orthotopic growth of non-small-cell lung cancer [91]. In this model, lung cancer cell growth, invasion, and response to therapy were found to be influenced by physical cues that arise from breathing motions, which appear to be mediated by epidermal growth factor receptor and MET protein kinase [91].
Despite the added advantage of incorporating vascular perfusion, organ-on-a-chip culture models also suffer from some unique limitations. Most importantly, organ-on-a-chip systems are currently limited in their ability to replicate the remodeling of epithelial tissues that occurs in response to disease progression. For example, asthma is associated with airway stiffening, but current lung-on-a-chip studies of asthma have not yet shown the ability to recapitulate this change in mechanical properties. However, it is possible that smooth muscle cells and pulmonary fibroblasts, which contribute to ECM remodeling, can be incorporated into future lung-on-a-chip systems [92]. Another limitation is that the mass of tissues generated by these systems is low compared to alternative culture systems such as bioreactors [85], which limits the types of analysis that can be performed. It is also technically challenging to fabricate these devices consistently and without defects. Despite these limitations, organs-on-a-chip represent a promising avenue for future investigations of branching morphogenesis.
Strategies for designing the next generation of 3D culture models
3D culture models have dramatically improved our ability to study the dynamics and mechanisms of tissue morphogenesis, including branching. However, the current design of these models and the associated analysis techniques still suffer from several limitations. In this section, we discuss overarching limitations of conventional 3D culture models and propose strategies based on new or existing technologies that can be used to overcome these limitations.
One theme common amongst state-of-the-art culture models is that the tissues generated have a limited size and viability because they lack vascular perfusion [93]. Without a system to deliver oxygen and nutrients to cells, it is not possible to reproduce the complex branched architectures reminiscent of the later stages of development of the mammary gland or mammalian lung. It also becomes challenging to study tissue remodeling in response to aging or disease progression, which occur over long timescales (>1 month). The small tissue sizes that can be produced limits the techniques that can be used to analyze them. For example, analysis of gene expression using quantitative polymerase chain reaction (qPCR) requires a minimum number of cells (~10 – 105 depending on gene expression levels). A promising solution to overcome this limitation is that of microfabricated [94] or 3D-printed [95] blood vessels, which could be used to incorporate vascular perfusion into existing 3D culture models (Figure 4). Microfabricated vascular channels can be used to generate simple endothelial networks that can be perfused, which is a promising first step in the production of vasculature [94]. In addition, a proof-of-concept study showed that 3D-printed vasculature can be used to culture large human tissues (>1 cm) for long durations (>6 weeks) [95].
Figure 4. Engineering the next generation of 3D culture models for branching morphogenesis.
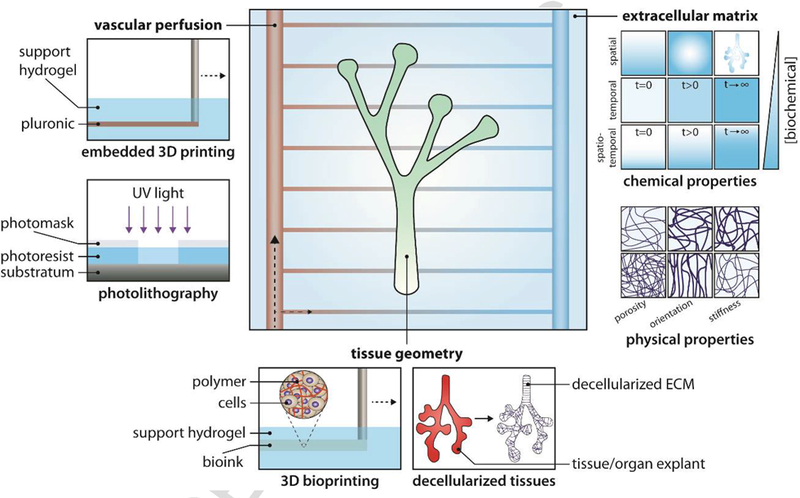
Three key components of culture models, including vascular perfusion, tissue geometry, and ECM are highlighted. Specific examples of techniques or types of materials that could potentially be used to incorporate vascular perfusion, complex tissue geometries, and materials that replicate the dynamic properties of the ECM into a 3D culture model are provided.
Current 3D culture models also exhibit high intrinsic variability of tissue composition and behavior. This might result from variability in components of culture media such as fetal bovine serum and naturally derived matrix materials such as Matrigel and type I collagen, which have significant lot-to-lot variability and spatial heterogeneity [79]. Naturally derived materials are also susceptible to additional limitations such as pathogen transmission and immunogenicity [96]. To address these concerns, synthetic biopolymers can be used to reduce variability. In addition, culture models that rely on patient-derived cells are a source of significant variability, which has been discussed previously for human lung samples [78]. The careful selection of human samples or the use of carefully controlled stem cells may help to address this problem. With more consistent results, high-throughput screening could be realized for systems such as organoids, which have significant potential to be used for the testing of therapeutic efficacy and toxicity.
To date, the generation of replacement organs or tissues with branched architectures that have identical cellular composition and function of in vivo equivalents remains challenging. Specifically, there is a need to simulate dynamic tissue-tissue interactions and account for key features of the in vivo microenvironment such as interstitial flow, among others. Whereas organoids, microfabricated tissues, and organ-on-a-chip approaches allow the incorporation of multiple cell types, these approaches are largely unable to replicate the complex tissue-tissue interactions found in the mammary gland and mammalian lung. One promising approach would be to stack layers of tissues to generate models that recapitulate multiscale tissue architecture found in vivo. This approach has been developed as a commercial lung airway model, which consists of a three-layer system containing a ciliated apical surface, mucociliary epithelium, and a microporous membrane. This culture model contains keratin 5+ basal cells and goblet cells for mucus production and has been shown to respond to stress from exposure to tobacco smoke [97]. Branched tissues in culture also lack the genetic variability found in human tissues and organs in vivo. Moreover, the cellular composition of tissues and organs may differ between humans and animal models. For example, basal cells are broadly distributed in human lungs [98, 99], while they are located specifically in the trachea of rodent lungs [68]; this spatial distribution could be incorporated into studies using 3D models.
Replicating the intricate in vivo architecture of tissues as well as their surrounding ECM remains challenging for conventional 3D culture models. One promising approach that could address this problem is the use of decellularized and recellularized tissues and organs (Figure 4). The decellularization procedure uses physical and chemical agents to remove cells from a tissue or organ while preserving the composition and structure of the underlying ECM [100]. After removing cells, the decellularized construct can be recellularized to recover the native architecture of a tissue or organ. This approach has not been applied to human or mouse mammary glands, but porcine mammary glands have been decellularized in order to isolate the ECM for subsequent breast tissue engineering [101]. In another study, the stroma from a mouse mammary gland was altered to more closely match that of the human mammary gland, which contains more fibrous type I collagen, by incorporating human stromal fibroblasts [102]. Primary mammary epithelial organoids were subsequently injected into this modified gland, which can be used to study human mammary epithelial branching in a microenvironment more reminiscent of the human mammary gland [102]. Several studies have investigated decellularized and recellularized lung tissues. Gilpin et al. seeded differentiated human induced pluripotent stem cells into whole or sliced decellularized rat and human lungs and found that these decellularized scaffolds support both the culture and lineage commitment of seeded cells [103]. Another study seeded type II human and rat epithelial cells into decellularized rat lungs and cultured the lungs for up to 7 days [104]. This study found that epithelial markers were not present in seeded cells after 7 days and suggested that lung epithelial phenotypes might be obtained using different medium components or physiological stimuli [104].
An alternative approach for recapitulating tissue and matrix architecture with greater accuracy is 3D bioprinting. 3D structures can be generated by printing mixtures of live cells and hydrogels onto a flat surface or into a hydrogel support (Figure 4). To date, no publications have described the use of 3D bioprinting to generate ECM or tissue architecture observed in the mammary gland. One thesis (protected by embargo) focused on reconstructing tissue culture models of the mammary ductal epithelium using rapid prototyping [105]. Similarly, few studies have investigated the application of 3D bioprinting to generate tissue or ECM architecture found in the mammalian lung. One proof-of-concept study bioprinted a human air-blood tissue barrier containing a Matrigel basement membrane as well as human endothelial and type II alveolar epithelial cells [106]. The macroscopic architecture of the lung has also been replicated in the form of a 3D-printed tracheal splint made from polycaprolactone [107]. Taken together, these preliminary studies suggest that 3D bioprinting has potential for spatially controlling cellular composition and ECM architecture in a manner that could improve the utility of 3D culture models in studies of tissue morphogenesis.
Conventional culture models are further limited in their ability to tune the properties of the matrix over multiple length scales, which is important to recapitulate ECM remodeling. This limitation arises because it is challenging to modulate local properties of conventional self-assembling materials such as Matrigel or type I collagen. One promising direction for future research is the incorporation of synthetic biomaterials with stimuli-responsive properties [96]. Incorporating hydrogels with light-mediated spatial and temporal control of stiffness could improve the ability of 3D culture models to recapitulate dynamic changes in the ECM (Figure 4). For example, Stowers et al. designed an alginate gel with tunable stiffness that is driven by the light-induced release of calcium or a chelator [108]. In addition to the physical properties of the ECM, it is also important to modulate chemical properties such as the release of growth factors, which play an integral role in tissue development. To that end, Richardson et al. fabricated a poly(lactide-co-glycolide)-based polymeric material that can deliver both vascular endothelial growth factor and platelet-derived growth factor [109]. Poly(ethylene glycol)-based hydrogels have also been developed to mimic the response of the ECM to adhesion-dependent proteolytic matrix remodeling [110]. Taken together, these preliminary studies show that key properties of the native ECM can be recapitulated with synthetic materials. Improved control of the ECM will allow 3D culture models to be used for the investigation of aspects of tissue morphogenesis that rely on spatiotemporal changes in mechanical or chemical properties of the ECM.
Concluding remarks
Several 3D culture models have been used successfully to investigate tissue morphogenesis. Here, we described specific examples of how organ explants, organoids, microfabricated tissues, and organs-on-a-chip can be used to study branching morphogenesis of the mammary gland or the mammalian lung. While these systems are limited in their ability to recapitulate the intricate tissue architecture and physical and chemical complexity of the ECM observed in vivo, they have been used to reveal several mechanistic details about morphogenesis. However, many more questions about the mechanisms of branching morphogenesis in the mammary gland and mammalian lung remain unanswered. Moving forward, the incorporation of additional components such as synthetic materials and vasculature will improve our ability to study tissue morphogenesis in engineered culture models over longer timescales and with greater relevance to the process in vivo.
Acknowledgements
Work from the authors’ lab was supported in part by grants from the National Institutes of Health (HL110335, HL118532, HL120142, CA187692, CA214292), the David & Lucile Packard Foundation, the Alfred P. Sloan Foundation, the Camille & Henry Dreyfus Foundation, the Burroughs Wellcome Fund, and a Faculty Scholars Award from the Howard Hughes Medical Institute. B.A.N. was supported in part by a postgraduate scholarship-doctoral (PGS-D) from the Natural Sciences and Engineering Research Council of Canada.
Abbreviations:
- 2D
two-dimensional
- 3D
three-dimensional
- AE
airway epithelium
- ASM
airway smooth muscle
- E
embryonic day
- ECM
extracellular matrix
- FGF
fibroblast growth factor
- Me
mesenchyme
- MMP
matrix metalloproteinase
- Mt
mesothelium
- P
postnatal day
- PA
pulmonary artery
- Pl
vascular plexus
- TEB
terminal end bud
- TGF
transforming growth factor
- VSM
vascular smooth muscle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Metzger RJ, Klein OD, Martin GR, Krasnow MA. (2008) The branching programme of mouse lung development. Nature 453: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costantini F, Kopan R. Patterning a Complex Organ: Branching Morphogenesis and Nephron Segmentation in Kidney Development. Developmental Cell 18: 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennighausen L, Robinson GW. (2001) Signaling Pathways in Mammary Gland Development. Developmental Cell 1: 467–475. [DOI] [PubMed] [Google Scholar]
- 4.Patel VN, Rebustini IT, Hoffman MP. (2006) Salivary gland branching morphogenesis. Differentiation 74: 349–364. [DOI] [PubMed] [Google Scholar]
- 5.Villasenor A, Chong DC, Henkemeyer M, Cleaver O. (2010) Epithelial dynamics of pancreatic branching morphogenesis. Development 137: 4295–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson AA, Marker PC. (2006) Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation 74: 382–392. [DOI] [PubMed] [Google Scholar]
- 7.Dor Y, Djonov V, Keshet E. (2003) Making vascular networks in the adult: branching morphogenesis without a roadmap. Trends in Cell Biology 13: 131–136. [DOI] [PubMed] [Google Scholar]
- 8.Kollins K, Davenport R. Branching Morphogenesis in Vertebrate Neurons 2000–2013; Available from: https://www.ncbi.nlm.nih.gov/books/NBK6520/. [Google Scholar]
- 9.Thompson DW, On Growth and Form 1 ed. 1917, Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Graner F, Riveline D. (2017) ‘The Forms of Tissues, or Cell-aggregates ’: D’Arcy Thompson’s influence and its limits. Development 144: 4226–4237. [DOI] [PubMed] [Google Scholar]
- 11.Thompson DW, On Growth and Form 2 ed. 1942, Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Shamir ER, Ewald AJ. (2014) Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nature Reviews Molecular Cell Biology 15: 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iber D, Menshykau D. (2013) The control of branching morphogenesis. Open Biology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada KM, Cukierman E. (2007) Modeling Tissue Morphogenesis and Cancer in 3D. Cell 130: 601–610. [DOI] [PubMed] [Google Scholar]
- 15.Franzdóttir SR, Axelsson IT, Arason AJ, Baldursson Ó, Gudjonsson T, et al. (2010) Airway branching morphogenesis in three dimensional culture. Respiratory Research 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hens JR, Wysolmerski JJ. (2005) Key stages of mammary gland development: Molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Research 7: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson GW. (2007) Cooperation of signalling pathways in embryonic mammary gland development . Nature Reviews Genetics 8: 963–972. [DOI] [PubMed] [Google Scholar]
- 18.Kratochwil K, Schwartz P. (1976) Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone . Proceedings of the National Academy of Sciences of the United States of America 73: 4041–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson CJ. (2006) Key stages in mammary gland development - Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Research 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gjorevski N, Nelson CM. (2011) Integrated morphodynamic signalling of the mammary gland. Nat Rev Mol Cell Biol 12: 581–593. [DOI] [PubMed] [Google Scholar]
- 21.Sopel M. The myoepithelial cell: its role in normal mammary glands and breast cancer. [PubMed] [Google Scholar]
- 22.Williams JM, Daniel CW. (1983) Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Developmental Biology 97: 274–290. [DOI] [PubMed] [Google Scholar]
- 23.Hennighausen L, Robinson GW. (2005) Information networks in the mammary gland. Nature Reviews Molecular Cell Biology 6: 715–725. [DOI] [PubMed] [Google Scholar]
- 24.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA, Bissell MJ. (2006) Tissue Geometry Determines Sites of Mammary Branching Morphogenesis in Organotypic Cultures. Science 314: 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu P, Ewald AJ, Martin GR, Werb Z. (2008) Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Developmental Biology 321: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel CW, Berger JJ, Strickland P, Garcia R. (1984) Similar growth pattern of mouse mammary epithelium cultivated in collagen matrix in vivo and in vitro. Developmental Biology 104: 57–64. [DOI] [PubMed] [Google Scholar]
- 27.Brownfield DG, Venugopalan G, Lo A, Mori H, Tanner K, et al. (2013) Patterned Collagen Fibers Orient Branching Mammary Epithelium through Distinct Signaling Modules. Current biology 23: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, et al. (2010) Lung Organogenesis. Current Topics in Developmental Biology 90: 73–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schittny JC. (2017) Development of the lung. Cell and Tissue Research 367: 427–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HY, Pang M-F, Varner VD, Kojima L, Miller E, et al. (2015) Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Developmental Cell 34: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. (1997) Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867–4878. [DOI] [PubMed] [Google Scholar]
- 32.Wongtrakool C, Roman J. (2008) Apoptosis of mesenchymal cells during the pseudoglandular stage of lung development affects branching morphogenesis Experimental Lung Research 34: 481–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burri PH. (1984) Fetal and Postnatal Development of the Lung. Annual Review of Physiology 46: 617–628. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuizen EJA, Haagsman HP. (2000) Role of pulmonary surfactant components in surface film formation and dynamics. Biochimica et Biophysica Acta (BBA) - Biomembranes 1467: 255–270. [DOI] [PubMed] [Google Scholar]
- 35.Kumar ME, Bogard PE, Espinoza FH, Menke DB, Kingsley DM, et al. (2014) Defining a mesenchymal progenitor niche at single-cell resolution. Science 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Q, Gao H, Shi Y, Zhang F, Gu X, et al. (2016) Aging is associated with an expansion of CD49f(hi) mammary stem cells that show a decline in function and increased transformation potential. Aging (Albany NY) 8: 2754–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen RK, Bissell MJ. (2000) Tissue architecture and breast cancer: the role of extracellular matrix and steroid hormones. Endocrine-related cancer 7: 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parashurama N, Lobo NA, Ito K, Mosley AR, Habte FG, et al. (2012) Remodeling of Endogenous Mammary Epithelium by Breast Cancer Stem Cells. Stem cells (Dayton, Ohio) 30: 2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polunovsky VA, Chen B, Henke C, Snover D, Wendt C, et al. (1993) Role of mesenchymal cell death in lung remodeling after injury. The Journal of Clinical Investigation 92: 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergeron C, Tulic MK, Hamid Q. (2010) Airway remodelling in asthma: From benchside to clinical practice. Canadian Respiratory Journal : Journal of the Canadian Thoracic Society 17: e85–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regamey N, Jeffery PK, Alton EWFW, Bush A, Davies JC. (2011) Airway remodelling and its relationship to inflammation in cystic fibrosis. Thorax 66: 624–629. [DOI] [PubMed] [Google Scholar]
- 42.Jones RL, Noble PB, Elliot JG, James AL. (2016) Airway remodelling in COPD: It’s not asthma! Respirology 21: 1347–1356. [DOI] [PubMed] [Google Scholar]
- 43.Parra ER, David YR, Costa LRSd, Ab’Saber A, Sousa R, et al. (2005) Heterogeneous Remodeling of Lung Vessels in Idiopathic Pulmonary Fibrosis. Lung 183: 291–300. [DOI] [PubMed] [Google Scholar]
- 44.Reinert T, Baldotto CSdR, Nunes FAP, Scheliga AAdS. (2013) Bleomycin-Induced Lung Injury. Journal of Cancer Research 2013. [Google Scholar]
- 45.Ichinose RR, Nandi S. (1964) Lobuloalveolar Differentiation in Mouse Mammary Tissues in vitro. Science 145: 496–497. [DOI] [PubMed] [Google Scholar]
- 46.Chen JM. (1954) The cultivation in fluid medium of organised liver, pancreas and other tissues of foetal rats. Experimental Cell Research 7: 518–529. [DOI] [PubMed] [Google Scholar]
- 47.Grant GA. (1936) The metabolism of galactose: 1. Lactose synthesis from (a) a glucose-galactose mixture, (b) phosphoric esters, by slices of the active mammary gland in vitro. 2 The effect of prolactin on lactose synthesis by the mammary gland. Biochemical Journal 30: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerrero RR, Rounds DE, Booher J. (1977) An improved organ culture method for adult mammalian lung. In Vitro 13: 517–524. [DOI] [PubMed] [Google Scholar]
- 49.Brigham KL, Meyrick B, Christman B, Conary JT, King G, et al. (1993) Expression of Human Growth Hormone Fusion Genes in Cultured Lung Endothelial Cells and in the Lungs of Mice. American Journal of Respiratory Cell and Molecular Biology 8: 209–213. [DOI] [PubMed] [Google Scholar]
- 50.Scheele CLGJ, Hannezo E, Muraro MJ, Zomer A, Langedijk NSM, et al. (2017) Identity and dynamics of mammary stem cells during branching morphogenesis. Nature 542: 313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson CM, Gleghorn JP, Pang M-F, Jaslove J, Goodwin K, et al. (2017) Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 144: 4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnatwinkel C, Niswander L. (2013) Multiparametric image analysis of lung-branching morphogenesis. Developmental Dynamics 242: 622–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harbell JW, Bowman PD, Shannon JM, Daniel CW. (1977) Long-term Organ Culture of Mouse Mammary Gland. In Vitro 13: 490–496. [DOI] [PubMed] [Google Scholar]
- 54.Jaskoll TF, Don-Wheeler G, Johnson R, Slavkin HC. (1988) Embryonic mouse lung morphogenesis and type II cytodifferentiation in serumless, chemically defined medium using prolonged in vitro cultures. Cell Differentiation 24: 105–117. [DOI] [PubMed] [Google Scholar]
- 55.Nogawa H, Ito T. (1995) Branching morphogenesis of embryonic mouse lung epithelium in mesenchyme-free culture. Development 121: 1015. [DOI] [PubMed] [Google Scholar]
- 56.Varner VD, Gleghorn JP, Miller E, Radisky DC, Nelson CM. (2015) Mechanically patterning the embryonic airway epithelium. Proceedings of the National Academy of Sciences 112: 9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simian M, Bissell MJ. (2016) Organoids: A historical perspective of thinking in three dimensions. The Journal of Cell Biology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, et al. (1998) Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proceedings of the National Academy of Sciences 95: 14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, et al. (2001) The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 128: 3117–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirai Y, Lochter A, Galosy S, Koshida S, Niwa S, et al. (1998) Epimorphin Functions as a Key Morphoregulator for Mammary Epithelial Cells. The Journal of Cell Biology 140: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y-W, Ahmed A, Snoeck H-W. (2017) Generation of three-dimensional lung bud organoid and its derived branching colonies. Protocol Exchange [Google Scholar]
- 62.Krause S, Maffini MV, Soto AM, Sonnenschein C. (2008) A Novel 3D In Vitro Culture Model to Study Stromal–Epithelial Interactions in the Mammary Gland. Tissue Engineering Part C: Methods 14: 261–271. [DOI] [PubMed] [Google Scholar]
- 63.Campbell JJ, Watson CJ. (2009) Three-dimensional culture models of mammary gland. Organogenesis 5: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, et al. (2007) The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFα and FGF7 in morphogenesis of mouse mammary epithelium. Developmental biology 306: 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Adileh M, Martin ML, Klingler S, White J, et al. (2017) Establishing estrogen-responsive mouse mammary organoids from single Lgr5(+) cells. Cellular signalling 29: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jamieson PR, Dekkers JF, Rios AC, Fu NY, Lindeman GJ, et al. (2017) Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 67.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. (2013) Collective Invasion in Breast Cancer Requires a Conserved Basal Epithelial Program. Cell 155: 1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, et al. (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences 106: 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, et al. (2009) The Role of Scgb1a1+ Clara Cells in the Long-Term Maintenance and Repair of Lung Airway, but Not Alveolar, Epithelium. Cell Stem Cell 4: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, et al. (2013) Type 2 alveolar cells are stem cells in adult lung. The Journal of Clinical Investigation 123: 3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dye BR, Hill DR, Ferguson MAH, Tsai Y-H, Nagy MS, et al. (2015) In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikolić MZ, Rawlins EL. (2017) Lung Organoids and Their Use To Study Cell-Cell Interaction. Current Pathobiology Reports 5: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan Q, Choi KM, Sicard D, Tschumperlin DJ. (2017) Human airway organoid engineering as a step toward lung regeneration and disease modeling. Biomaterials 113: 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaisani A, Delgado O, Fasciani G, Kim SB, Wright WE, et al. (2014) Branching morphogenesis of immortalized human bronchial epithelial cells in three-dimensional culture. Differentiation 87: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y-W, Huang SX, de Carvalho ALRT, Ho S-H, Islam MN, et al. (2017) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nature Cell Biology 19: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, et al. (2015) Telomere dysfunction causes alveolar stem cell failure. Proceedings of the National Academy of Sciences 112: 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen-Ngoc K-V, Shamir ER, Huebner RJ, Beck JN, Cheung KJ, et al. (2015) 3D Culture Assays of Murine Mammary Branching Morphogenesis and Epithelial Invasion. Methods in molecular biology (Clifton, NJ) 1189: 135–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barkauskas CE, Chung M-I, Fioret B, Gao X, Katsura H, et al. (2017) Lung organoids: current uses and future promise. Development 144: 986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.(2017) Advances in Organoid Technology: Hans Clevers, Madeline Lancaster, and Takanori Takebe. Cell Stem Cell 20: 759–762. [Google Scholar]
- 80.West AR, Zaman N, Cole DJ, Walker MJ, Legant WR, et al. (2012) Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. American Journal of Physiology-Lung Cellular and Molecular Physiology 304: L4–L16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nerger BA, Siedlik MJ, Nelson CM. (2017) Microfabricated tissues for investigating traction forces involved in cell migration and tissue morphogenesis. Cellular and Molecular Life Sciences 74: 1819–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pavlovich AL, Manivannan S, Nelson CM. (2010) Adipose Stroma Induces Branching Morphogenesis of Engineered Epithelial Tubules. Tissue Engineering Part A 16: 3719–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piotrowski-Daspit AS, Nerger BA, Wolf AE, Sundaresan S, Nelson CM. (2017) Dynamics of Tissue-Induced Alignment of Fibrous Extracellular Matrix. Biophysical Journal 113: 702–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gjorevski N, Nelson Celeste M. (2012) Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues. Biophysical Journal 103: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhatia SN, Ingber DE. (2014) Microfluidic organs-on-chips. Nature Biotechnology 32: 760–762. [DOI] [PubMed] [Google Scholar]
- 86.Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, et al. (2014) A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 35: 2454–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vidi P-A, Maleki T, Ochoa M, Wang L, Clark SM, et al. (2014) Disease-on-a-chip: mimicry of tumor growth in mammary ducts. Lab on a Chip 14: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, et al. (2010) Reconstituting Organ-Level Lung Functions on a Chip. Science 328: 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benam KH, Novak R, Nawroth J, Hirano-Kobayashi M, Ferrante TC, et al. (2016) Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Systems 3: 456–466.e454. [DOI] [PubMed] [Google Scholar]
- 90.Punde TH, Wu W-H, Lien P-C, Chang Y-L, Kuo P-H, et al. (2015) A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation. Integrative Biology 7: 162–169. [DOI] [PubMed] [Google Scholar]
- 91.Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, et al. (2017) Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Reports 21: 508–516. [DOI] [PubMed] [Google Scholar]
- 92.Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, et al. (2015) Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nature Methods 13: 151–157. [DOI] [PubMed] [Google Scholar]
- 93.Kaully T, Kaufman-Francis K, Lesman A, Levenberg S. (2009) Vascularization—The Conduit to Viable Engineered Tissues. Tissue Engineering Part B: Reviews 15: 159–169. [DOI] [PubMed] [Google Scholar]
- 94.Golden AP, Tien J. (2007) Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab on a Chip 7: 720–725. [DOI] [PubMed] [Google Scholar]
- 95.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. (2016) Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences 113: 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lutolf MP, Hubbell JA. (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology 23: 47–55. [DOI] [PubMed] [Google Scholar]
- 97.Balharry D, Sexton K, BéruBé KA. (2008) An in vitro approach to assess the toxicity of inhaled tobacco smoke components: Nicotine, cadmium, formaldehyde and urethane. Toxicology 244: 66–76. [DOI] [PubMed] [Google Scholar]
- 98.Boers JE, Ambergen AW, Thunnissen FBJM. (1998) Number and Proliferation of Basal and Parabasal Cells in Normal Human Airway Epithelium. American Journal of Respiratory and Critical Care Medicine 157: 2000–2006. [DOI] [PubMed] [Google Scholar]
- 99.Nakajima Y, Kawanami O, Jin E, Ghazizadeh M, Honda M, et al. (1998) Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathology International 48: 944–953. [DOI] [PubMed] [Google Scholar]
- 100.Crapo PM, Gilbert TW, Badylak SF. (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32: 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giatsidis G, Venezia ED, De Stefani D, Rizzuto R, Bassetto F. (2015) Breast Tissue Engineering: Decellularized Scaffolds Derived from Porcine Mammary Glands. Plastic and Reconstructive Surgery 136. [Google Scholar]
- 102.Proia DA, Kuperwasser C. (2006) Reconstruction of human mammary tissues in a mouse model. Nature Protocols 1: 206. [DOI] [PubMed] [Google Scholar]
- 103.Gilpin SE, Ren X, Okamoto T, Guyette JP, Mou H, et al. (2014) Enhanced Lung Epithelial Specification of Human Induced Pluripotent Stem Cells on Decellularized Lung Matrix. The Annals of Thoracic Surgery 98: 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Calle EA, Mendez JJ, Ghaedi M, Leiby KL, Bove PF, et al. (2015) Fate of Distal Lung Epithelium Cultured in a Decellularized Lung Extracellular Matrix. Tissue Engineering Part A 21: 1916–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hinton T, Rapid Prototyping Tissue Models of Mammary Duct Epithelium, in Biomedical Engineering. 2017, Carnegie Mellon University. [Google Scholar]
- 106.Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, et al. (2015) Engineering an in vitro air-blood barrier by 3D bioprinting. Scientific Reports 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. (2013) Bioresorbable Airway Splint Created with a Three-Dimensional Printer. New England Journal of Medicine 368: 2043–2045. [DOI] [PubMed] [Google Scholar]
- 108.Stowers RS, Allen SC, Suggs LJ. (2015) Dynamic phototuning of 3D hydrogel stiffness. Proceedings of the National Academy of Sciences of the United States of America 112: 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richardson TP, Peters MC, Ennett AB, Mooney DJ. (2001) Polymeric system for dual growth factor delivery. Nature Biotechnology 19: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 110.Raeber GP, Lutolf MP, Hubbell JA. (2005) Molecularly Engineered PEG Hydrogels: A Novel Model System for Proteolytically Mediated Cell Migration. Biophysical Journal 89: 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PAB, et al. (2010) miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nature Genetics 42: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 112.Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, et al. (2009) Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proceedings of the National Academy of Sciences 106: 10097–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]


